Abstract
Adenosine triphosphate (ATP), an indispensable molecule that provides energy for essentially all cellular processes, has been shown to be affected by some magnetic fields (MFs). Although people are frequently exposed to various static and power frequency MFs in their daily lives, the exact effects of these MFs of different frequencies have not been systematically investigated. Here, we tested 6-mT MFs with 0, 50, and 120 Hz for their effects on cellular ATP levels in 11 different cell lines. We found that the 6-mT static magnetic field (SMF) either does not affect or increase cellular ATP levels, while 6-mT 50-Hz MF either does not affect or decrease cellular ATP levels. In contrast, 6-mT 120-Hz MF has variable effects. We examined the mitochondrial membrane potential (MMP) as well as reactive oxygen species (ROS) in four different cell lines, but did not find their direct correlation with ATP levels. Although none of the ATP level changes induced by these three different frequencies of 6-mT MFs are dramatic, these results may be used to explain some differential cellular responses of various cell lines to different frequency MFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adenosine triphosphate (ATP) is the most commonly used form of energy for essentially all cellular activities, such as cell proliferation, division, migration, autophagy, and cell death. Mitochondria are the main sources of ATP production, in which a series of protein complexes embedded in the inner membrane of mitochondria, called the electron transport chain and ATP synthase, perform oxidative phosphorylation to create ATP, the major energy currency of the cell.
Although whether static magnetic field (SMF) could directly affect the enzymatic ATP synthesis in vitro has been debated in the literature (Buchachenko and Kuznetsov 2008; Crotty et al. 2012; Hore 2012), there are some cellular works showing that cellular ATP levels and the mean activities of Na(+)–K+ ATPase and Ca2+ ATPase could be affected by 0.2–9.0 T SMFs in a magnetic field (MF) intensity- and cell type-dependent manner (Itegin et al. 1995; Kurzeja et al. 2013; Wang et al. 2018; Zhao et al. 2011). In fact, people are frequently exposed to SMFs generated by magnets on household items such as refrigerator magnets and some accessories, which usually generate SMFs of a few millitesla (mT) on human bodies adjacent to them. However, the effect of SMFs below 0.2 T on cellular ATP level has never been reported.
Besides SMFs, people are also frequently exposed to various alternating MFs, especially low-frequency MFs generated by electric current, which works at 50 or 60 cycles per second, also called hertz (Hz). It should be known that the MF intensity for both SMFs and alternating MFs diminishes with increased distance from the source. Although the MFs can be as high as a few hundred of millitesla when getting close to some home appliances, the average power frequency MFs in residential homes are usually lower than 1 mT. Nevertheless, people still raise many concerns about the effects of these power frequency MFs on human health. However, there are very limited studies about these low-frequency MFs for their effects on ATP, the vital energy source of cells. Moreover, the limited reported results are variable between individual studies. For example, 50-Hz 5-mT MF could increase the ATP level in sperm (Iorio et al. 2011), but 50-Hz 45-μT MF did not affect cellular ATP levels in human breast cancer SKBR3 cells or human colorectal cancer HT29 cells (Destefanis et al. 2015). The ATP level of rat skeletal muscle was not affected by 50-Hz 10-mT whole body exposure (Stefl et al. 2006) but 75-Hz 150-μT MF significantly decreased cellular ATP level in rod outer segment of bovine retina (Ravera et al. 2004). Since these studies are sporadic and the experimental evidences are very limited, it is not clear whether these different effects of MFs on cellular ATP levels were due to MF intensity, frequency, or cell type differences.
To systematically investigate the effects of SMF and low-frequency MFs on cellular ATP levels, we compared 11 different cell lines, including five human cancer cell lines, three rat cancer cell lines, and three non-cancer cell lines for their cellular ATP responses to MFs of 0 Hz, 50 Hz, and 120 Hz in three different time points. We chose 6 mT because it is within the intensity range that people have many chances to be exposed to, such as refrigerator magnets, power station, some home appliances, and workplaces. Our results show that although the overall effects of 6-mT MFs (0 Hz, 50 Hz, and 120 Hz) on cellular ATPs are not dramatic (the changes are all within 20%), the experimental outcomes are influenced by MF frequency, cell types, and exposure time.
Materials and methods
Cell exposure to 6-mT low-frequency magnetic fields and static magnetic field
We set up a 6-mT SMF by placing two 126 mm × 85 mm × 22 mm 12-well plates and a 118 mm × 85 mm × 78 mm plastic box on the top center of a 60 mm × 50 mm × 35 mm neodymium N38 permanent magnet (Jiangsu Zhongxin magnetoelectricity, Dafeng, China) with the North pole up (facing the cell sample) and South pole down (away from the cell sample). The magnetic field intensity on the surface of the magnet is 0.4–0.5 T (Fig. 1a), and the magnetic field intensities on the top of the 12-well plate were measured by Gaussmeter (LakeShore 410, Lake Shore Cryotronics, Westerville, OH) to locate the positions of 6 mT, where we placed the cell culture plates.
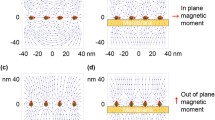
Cells exposed to 6-mT static magnetic field (SMF) and low-frequency electromagnetic fields. a Schematic illustration and b picture of the experimental setup for cells exposed to 6-mT static magnetic field. c Schematic illustration and d picture of the experimental setup for cells exposed to 6-mT 50-Hz and 120-Hz low-frequency electromagnetic fields
In order to maintain the same culture conditions, the whole set of 6-mT SMF group using permanent magnet was kept in the 37 °C cell incubator all the time (BC-J160S, Shanghai Boxun, Shanghai, China) (37.0 ± 0.1 °C, 5% CO2) (Fig. 1b). On the first day, 4 × 105 cells/ml cells were seeded in the cell culture plates. On the second day, the cell plates were either placed at the location of 6 mT as the experimental group, or kept at 30 cm away from the magnet in the same cell incubator as control (the magnetic field at the control group was measured to be 0.925 ± 0.206 Gs, which is 65 times lower than the experimental group).
The electromagnetic field setup was manufactured by Shanghai LIONCEL electromagnetic Co., Ltd. (HLY10-50, shanghai, China), which provides adjustable MF intensity ranging from 0 to 6 mT and frequency of 45–120 Hz at the cell sample area (Fig. 1c). It was composed of electromagnetic field generator and Helmholtz coil, which was wound into a tightly packed helix and placed in a plastic box. The cell culture dish was placed in the center of a plastic drawer, which is then inserted into the center of the MF exposure apparatus. The whole box containing the Helmholtz coil and the cell drawer was placed in a CO2 cell incubator, which has proper gas, temperature, and humidity control (Fig. 1d). For experiments in this paper, we used 6 mT 50 Hz and 6 mT 120 Hz. The MF intensity was measured by LakeShore 410 Gaussmeter. For the sham control group, we put them into another CO2 cell incubator that had the same conditions with treated group, except for the magnetic field. The magnetic field at the control group incubator was 0.875 ± 0.096 Gs. Temperatures inside the two incubators were measured, and the differences were less than 0.1 °C.
Cell culture
We tested 11 cell lines including five human cancer cell lines (HeLa, HCT116, MCF7, A549, and GIST-T1), three rat cancer cell lines (differentiated PC12, undifferentiated PC12, and C6), and three non-cancer cell lines (RPE1, CHO, and 293T). HeLa, HCT116, MCF7, A549, GIST-T1, differentiated PC12 cells, C6, RPE1, CHO, and 293T cells were cultured in Dulbecco’s Modification of Eagle’s Medium (15-017-CVR, Corning, Corning, NY) with no l-glutamine, supplemented with 10% (v/v) FBS (fetal bovine serum, FB25015, CLARK Bioscience, Richmond, VA), 2 mM GlutaMAX (35050-061, Gibco, Carlsbad, CA), and 1% of P/S (penicillin/streptomycin,SV30010, HyClone, Logan, UT). The undifferentiated PC-12 cells were maintained in RPMI 1640 medium (11875-093, Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated horse serum (26050-070, Gibco, Carlsbad, CA), 5% FBS, and 1% P/S. The undifferentiated PC-12 cells were provided by Stem Cell Bank (Chinese Academy of Sciences, Shanghai, China) while other cell lines were from American Type Culture Collection (Manassas, VA).
Measurement of cellular ATP level
To measure the cellular ATP levels, we first compared two commonly used methods, the ATP Determination Kit (A22066, Molecular Probes, Eugene, Oregon) and the Cell Titer-Glo Luminescent Cell Viability Assay (G7572, Promega, Madison, WI) (Supplementary Fig. 1).
Molecular Probesʼ ATP Determination Kit (A22066) can measure the ATP levels quantitatively with recombinant firefly luciferase and d-luciferin. 4 × 105/well cells were plated one night in advance on a 12-well plate (712001, NEST, Wuxi, China). On the second day, all cells were treated with 0.5-T or 1.0-T SMF for 24 h. After exposure, all cells were lysed by M-PER buffer (78505, Thermo Fisher, Shanghai, China) supplemented with protease inhibitor (04693116001, Roche, Basel, Switzerland) and phosphatase inhibitor cocktail (04906837001, Roche, Basel, Switzerland) at 4 °C for 20 min. In the meantime, we prepared a standard reaction solution and obtained the standard curve according the manufacturer’s instructions. After 20 min, the lysate was added to the reaction solution. Then, we utilized Multimode Plate Reader (EnVision, PerkinElmer, Waltham, MA) to measure the luminescence and calculated the amount of ATP based on the standard curve.
The other cellular ATP-measuring method, the Cell Titer-Glo Luminescent Cell Viability Assay, is based on the principle that the luminescence value is proportional to the cellular ATP levels. Therefore, higher luminescence value indicates more ATP. Due to the space limitation of the electromagnetic field exposure device, the whole 96-well plate (3917, Corning, Corning, NY) could not fit in. We have to cut them using an electrothermal knife (S20, Brand, Suzhou, China) into smaller plates with nine wells and sterilize them with ethanol. On the first day, 4 × 104 cells/well were plated on the pre-cut 9-well plate. On the second day, cells were treated with 6-mT MFs for 2 h, 4 h, and 6 h before adding the Cell Titer-Glo reagent. Finally, ATP content was measured by PerkinElmer Multimode Plate Reader (EnVision, Waltham, MA). Since our data show that there is no difference between these two methods for monitoring the ATP level (Supplementary Fig. 1), we chose the Cell Titer-Glo assay for the rest of the experiments for easy handling.
Measurement of cellular MMP
The JC-1 dye (C2006, Beyotime, Shanghai, China) was used to sense MMP (mitochondrial membrane potential) level based on the green fluorescence emission (~ 529 nm) and red fluorescence emission (~ 590 nm). The ratio of red/green fluorescence represents MMP changes. After the JC-1 dye was added according to the manufacturer’s instruction, cells were incubated at 37 °C for another 20 min before the fluorescence was analyzed using flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA).
Intracellular reactive oxygen species measurement
The intracellular ROS (reactive oxygen species) level of HCT116, GIST-T1, 293T, and RPE1 were monitored by 1–4-μM 2′,7′-dichlorofluorescin diacetate (D6883, Sigma-Aldrich, St. Louis, MO). 2′,7′-Dichlorofluorescin diacetate itself is a cell-permeable non-fluorescent probe, which becomes highly fluorescent upon oxidation by ROS. Therefore, it can be used to quantify cellular ROS levels. After magnetic field exposure for 2 h, 2′,7′-dichlorofluorescin diacetate was added to the cells. The cells were incubated at 37 °C for another 20 min before their green fluorescence (~ 535 nm) was detected using flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA).
Statistical analysis
To get unbiased experimental results, all experiments in this paper were repeated for at least three times by two researchers independently. The experimental results were quantified by GraphPad Prism 5 (version 5.01, GraphPad Software, La Jolla, CA). Comparisons between treatments were analyzed by Student’s t test. P values were labeled in figures, and standard deviations are shown. All the control groups have the value “1” because each independent experiment has been normalized to the control. Raw data is provided in the supplementary materials.
Results
We first compared two different methods for measuring cellular ATP levels (Supplementary Fig. 1), and our results show that there was no difference between them. 0.5-T or 1.0-T SMF exposure for 1 day did not affect cellular ATP levels in multiple cell lines (Supplementary Fig. 1). However, it has been shown previously that 0.5-T–1.0-T SMFs generally increase cellular ATP levels in shorter time points, and the ATP levels fluctuate over time (Wang et al. 2018); we chose to use Cell Titer-Glo for the rest of the experiments for easy handling and examined three different time points (2 h, 4 h, and 6 h) to get more complete information.
To examine the effects of 6-mT MFs of different frequencies, we set up a 6-mT SMF using a neodymium N38 permanent magnet (Fig. 1a, b), and 6-mT 50-Hz and 120-Hz low-frequency MFs with an electromagnetic fields generator and Helmholtz coil (Fig. 1c, d). To get reproducible results, each experiment was repeated by at least two researchers for multiple times and their results were pooled together for statistical analysis.
We first chose undifferentiated and differentiated PC12 cells; the cellular ATP level of which was increased by 0.25–1.0-T SMF and reduced by 9.0-T SMF in previous studies (Wang et al. 2018; Wang et al. 2010). We compared 6-mT SMF and 50-Hz and 120-Hz MFs exposure for 0–6 h and found that the cellular ATP levels were not affected in most conditions (Fig. 2, Supplementary Fig. 2). However, it is interesting that although 6-mT SMF and 50-Hz MF did not change cellular ATP levels, 6-mT 120-Hz MF increased the ATP level in undifferentiated PC12 cells after 4-h and 6-h exposures (Fig. 2c, Supplementary Fig. 2c).
Effects of 6-mT SMF and 50-Hz and 120-Hz magnetic fields on cellular ATP levels in undifferentiated and differentiated PC12 cells. Undifferentiated and differentiated PC12 cells were exposed to 6-mT SMF and 50-Hz and 120-Hz MFs for 2 h, 4 h, or 6 h before their ATP levels were measured. Each experiment was repeated for at least three times by two researchers independently. “Ns” not significant. “*”p < 0.05. Green color indicates that the increase is statistically significant
Since it has been shown that the MF effects are usually cell type-dependent (Zhang et al. 2017b; Zhang et al. 2016), we further tested nine more cell lines to test the effect of 6-mT SMF on their ATP levels (Fig. 3, Supplementary Fig. 3). Previous studies indicated that cancer cells and non-cancer cells often have differential responses to MFs (Aldinucci et al. 2003; Ghibelli et al. 2006; Tofani et al. 2001; Zhang et al. 2017b; Zhang et al. 2016), we included eight cancer cell lines and three non-cancer cell lines in our test. Our results show that 6-mT SMF generally does not affect or only increases the cellular ATP levels around only 2.3–9.1%. Moreover, there was no apparent correlation between cancer or non-cancer cells with MF-induced ATP level changes, and the time point-induced variations are also different between various cell lines.
Effects of 6-mT SMF (0 Hz) on cellular ATP levels in nine different cell lines. Cells were exposed to 6-mT SMF for 2 h, 4 h, or 6 h before their ATP levels were measured. Each experiment was repeated for at least three times by two researchers independently. “Ns” not significant. “*”p < 0.05; “**”p < 0.01. Green color indicates that the increase is statistically significant
Next, we used the same nine cell lines to test the effect of 6-mT 50-Hz MF on their cellular ATP levels (Fig. 4, Supplementary Fig. 4). It is interesting that 6 mT 50 Hz does not affect or only decreases the cellular ATP levels in these cell lines around 1.7–16.1%. In fact, the cellular ATP levels in most cell lines were not affected (8 out of 11 cell lines, 72.7%).
Effects of 6-mT 50-Hz electromagnetic field on cellular ATP levels in nine different cell lines. Cells were exposed to 6-mT 50-Hz electromagnetic field for 2 h, 4 h, or 6 h before their ATP levels were measured. Each experiment was repeated for at least three times by two researchers independently. “Ns” not significant. “*”p < 0.05. Red color indicates that the decrease is statistically significant
To further examine the influence of MF frequencies, we used the same nine cell lines to test the effect of 6-mT 120-Hz MF on their cellular ATP levels (Fig. 5, Supplementary Fig. 5). Unlike the 6-mT SMF or 50-Hz MF, which only affected cellular ATP levels in one way, 120-Hz MF have variable impacts on cellular ATP levels (Fig. 5, Supplementary Fig. 5). Again, for cells with altered ATP levels, the change of percentages is only 0.9–14.3%. We summarized all the ATP level changes in Table 1.
Effects of 6-mT 120-Hz electromagnetic field on cellular ATP levels in nine different cell lines. Cells were exposed to 6-mT 120-Hz electromagnetic field for 2 h, 4 h, or 6 h before their ATP levels were measured. Each experiment was repeated for at least three times by two researchers independently. “Ns” not significant. “*”p < 0.05. Green color indicates that the increase is statistically significant, and red color indicates that the decrease is statistically significant
Since mitochondrial membrane potential (MMP) can affect ATP synthesis (Bornhovd et al. 2006; Buchet and Godinot 1998; Zorova et al. 2018), it is possible that the MF-induced ATP level changes were mediated by altered MMP. To test this possibility, we examined the MMP in four of the above tested cell lines, which have differential responses to these 6-mT MFs. We did the 6-mT SMF and 6-mT 50-Hz MF exposure side-by-side. It is interesting that although the cellular ATP levels of HCT116 were increased by 6-mT SMF and decreased by 50-Hz MF, their MMP was not affected (Fig. 6a, Supplementary Fig. 6). Similarly, the MMP responses of the other three cell lines are not correlated with their ATP level changes either (Fig. 6b, c, d; Supplementary Fig. 6). We further examined the ROS (reactive oxygen species) levels in these four cell lines, which is mainly produced in mitochondria and frequently correlated with MMP and ATP levels, but did not find their correlation with 6-mT MF-induced ATP changes either (Fig. 7, Supplementary Fig. 7).
MMP level changes induced by 6-mT SMF and 50-Hz electromagnetic field in four different cell lines. a–d Relative MMP levels of HCT116, 293T, GIST-T1, and RPE1 cells with or without 6-mT SMF or 50-Hz EMF treatment for 2 h. Each experiment was repeated for three independent times by two researchers (n = 3). “Ns” not significant. “*”p < 0.05. Green color indicates that the increase is statistically significant, and red color indicates that the decrease is statistically significant
ROS level changes induced by 6-mT SMF and 50-Hz electromagnetic field in four different cell lines. Relative ROS levels of HCT116, 293T, GIST-T1, and RPE1 cells with or without 6-mT SMF or 50-Hz EMF treatment for 2 h. Each experiment was repeated for three independent times by two researchers (n = 3). “Ns” not significant. “*”p < 0.05; “**”p < 0.01. Green color indicates that the increase is statistically significant, and red color indicates that the decrease is statistically significant
Discussion
It is already known that the parameters of MFs directly influence the effects of MFs on cells. MF intensity, gradient, direction, and exposure time are all key factors that impact the biological effects of MFs (Bras et al. 2014; Chionna et al. 2005; Denegre et al. 1998; Kiss et al. 2013; Milovanovich et al. 2016; Morris and Skalak 2008; Sullivan et al. 2011; Tian et al. 2018; Zhang et al. 2017a, c). Here, we focus on MF frequency by comparing 0 Hz, 50 Hz, and 120 Hz at three different time points in 11 different cell lines. We found that cell types, MF frequencies, and exposure time all influence the effect of MFs on cellular ATP levels. It is interesting that the 6-mT SMF generally does not affect (5 out of 11 cell lines) or only increases (6 out of 11 cell lines) cellular ATP levels, while 6-mT 50-Hz MF does not affect (8 out of 11 cell lines) or decrease (3 out of 11 cell lines) cellular ATP levels. In contrast, 6-mT 120-Hz electromagnetic field has variable effects. The underlying mechanism of these frequency-induced variations are still unclear, but we think it might be related to the proposed hypothesis that cells have their specific electromagnetic frequency. Further investigations are necessary to unravel this mystery.
Since ATP level is tightly regulated in cells, 6 mT is a relatively low MF intensity and 50/120 Hz belongs to low-frequency MFs; it is not too surprising that all changes in this study are less than 16.1%. In fact, for SMFs of much higher intensity, such as 1 T and 9 T, the ATP level change percentages in multiple cell lines are also very small, usually within 20% (Wang et al. 2018). However, for 1-T and 9-T SMFs, the MMP changes seem to be correlated with the ATP level changes (Wang et al. 2018), while in this study, there is no direct correlation between ATP level changes and the MMP or ROS. Although MMP, ATP, and ROS are all related to mitochondria, their relative levels are controlled in a complex way (Korshunov et al. 1997; Ramzan et al. 2010; Sun et al. 2011; Suski et al. 2012). It is possible that other ATP generation pathways are affected during 6-mT MF exposure, such as glycolysis or oxidative phosphorylation. It is also possible that the ATP consumption, but not ATP production, is affected by 6-mT MFs.
In conclusion, although the ATP level changes induced by 6-mT MFs are not dramatic, the exact effects are cell type-, MF frequency-, and time-dependent. These may help to explain some observed cellular phenomenon of low-frequency MFs. Given the prevalence of power frequency and SMF exposure for human bodies, such as the MFs generated by electric power lines, home appliance, and household items, people should be aware of their potential effects on cellular ATP, which is the foundation of many cellular processes.
References
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003) The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 24:109–117. https://doi.org/10.1002/bem.10071
Bornhovd C, Vogel F, Neupert W, Reichert AS (2006) Mitochondrial membrane potential is dependent on the oligomeric state of F1F0-ATP synthase supracomplexes. J Biol Chem 281:13990–13998. https://doi.org/10.1074/jbc.M512334200
Bras W, Torbet J, Diakun GP, Rikken GL, Diaz JF (2014) The diamagnetic susceptibility of the tubulin dimer. J Biophys 2014:985082–985085. https://doi.org/10.1155/2014/985082
Buchachenko AL, Kuznetsov DA (2008) Magnetic field affects enzymatic ATP synthesis. J Am Chem Soc 130:12868–12869. https://doi.org/10.1021/ja804819k
Buchet K, Godinot C (1998) Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho degrees cells. J Biol Chem 273:22983–22989. https://doi.org/10.1074/jbc.273.36.22983
Chionna A, Tenuzzo B, Panzarini E, Dwikat MB, Abbro L, Dini L (2005) Time dependent modifications of Hep G2 cells during exposure to static magnetic fields. Bioelectromagnetics 26:275–286. https://doi.org/10.1002/bem.20081
Crotty D, Silkstone G, Poddar S, Ranson R, Prina-Mello A, Wilson MT, Coey JM (2012) Reexamination of magnetic isotope and field effects on adenosine triphosphate production by creatine kinase. Proc Natl Acad Sci U S A 109:1437–1442. https://doi.org/10.1073/pnas.1117840108
Denegre JM, Valles JM Jr, Lin K, Jordan WB, Mowry KL (1998) Cleavage planes in frog eggs are altered by strong magnetic fields. Proc Natl Acad Sci U S A 95:14729–14732. https://doi.org/10.1073/pnas.95.25.14729
Destefanis M, Viano M, Leo C, Gervino G, Ponzetto A, Silvagno F (2015) Extremely low frequency electromagnetic fields affect proliferation and mitochondrial activity of human cancer cell lines. Int J Radiat Biol 91:964–972. https://doi.org/10.3109/09553002.2015.1101648
Ghibelli L, Cerella C, Cordisco S, Clavarino G, Marazzi S, De Nicola M, Nuccitelli S, D'Alessio M, Magrini A, Bergamaschi A, Guerrisi V, Porfiri LM (2006) NMR exposure sensitizes tumor cells to apoptosis. Apoptosis 11:359–365. https://doi.org/10.1007/s10495-006-4001-1
Hore PJ (2012) Are biochemical reactions affected by weak magnetic fields? Proc Natl Acad Sci U S A 109:1357–1358. https://doi.org/10.1073/pnas.1120531109
Iorio R, Delle Monache S, Bennato F, Di Bartolomeo C, Scrimaglio R, Cinque B, Colonna RC (2011) Involvement of mitochondrial activity in mediating ELF-EMF stimulatory effect on human sperm motility. Bioelectromagnetics 32:15–27. https://doi.org/10.1002/bem.20602
Itegin M, Gunay I, Logoglu G, Isbir T (1995) Effects of static magnetic field on specific adenosine-5′- triphosphatase activities and bioelectrical and biomechanical properties in the rat diaphragm muscle. Bioelectromagnetics 16:147–151. https://doi.org/10.1002/bem.2250160302
Kiss B, Gyires K, Kellermayer M, Laszlo JF (2013) Lateral gradients significantly enhance static magnetic field-induced inhibition of pain responses in mice--a double blind experimental study. Bioelectromagnetics 34:385–396. https://doi.org/10.1002/bem.21781
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18. https://doi.org/10.1016/S0014-5793(97)01159-9
Kurzeja E, Synowiec-Wojtarowicz A, Stec M, Glinka M, Gawron S, Pawlowska-Goral K (2013) Effect of a static magnetic fields and fluoride ions on the antioxidant defense system of mice fibroblasts. Int J Mol Sci 14:15017–15028. https://doi.org/10.3390/ijms140715017
Milovanovich ID, Cirkovic S, De Luka SR, Djordjevich DM, Ilic AZ, Popovic T, Arsic A, Obradovic DD, Opric D, Ristic-Djurovic JL, Trbovich AM (2016) Homogeneous static magnetic field of different orientation induces biological changes in subacutely exposed mice. Environ Sci Pollut Res Int 23:1584–1597. https://doi.org/10.1007/s11356-015-5109-z
Morris CE, Skalak TC (2008) Acute exposure to a moderate strength static magnetic field reduces edema formation in rats. Am J Physiol Heart Circ Physiol 294:H50–H57. https://doi.org/10.1152/ajpheart.00529.2007
Ramzan R, Staniek K, Kadenbach B, Vogt S (2010) Mitochondrial respiration and membrane potential are regulated by the allosteric ATP-inhibition of cytochrome c oxidase. Biochim Biophys Acta 1797:1672–1680. https://doi.org/10.1016/j.bbabio.2010.06.005
Ravera S, Repaci E, Morelli A, Pepe IM, Botter R, Beruto D (2004) Effects of extremely low frequency electromagnetic fields on the adenylate kinase activity of rod outer segment of bovine retina. Bioelectromagnetics 25:545–551. https://doi.org/10.1002/bem.20031
Stefl B, Vojtisek M, Synecka L, Zurmanova J (2006) Whole body exposure to low frequency magnetic field: no provable effects on the cellular energetics of rat skeletal muscle. Mol Cell Biochem 284:111–115. https://doi.org/10.1007/s11010-005-9025-2
Sullivan K, Balin AK, Allen RG (2011) Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 32:140–147. https://doi.org/10.1002/bem.20624
Sun RC, Board PG, Blackburn AC (2011) Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol Cancer 10:142. https://doi.org/10.1186/1476-4598-10-142
Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR (2012) Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol 810:183–205. https://doi.org/10.1007/978-1-61779-382-0_12
Tian X, Wang D, Zha M, Yang X, Ji X, Zhang L, Zhang X (2018) Magnetic field direction differentially impacts the growth of different cell types. Electromagn Biol Med 37:114–125. https://doi.org/10.1080/15368378.2018.1458627
Tofani S, Barone D, Cintorino M, de Santi MM, Ferrara A, Orlassino R, Ossola P, Peroglio F, Rolfo K, Ronchetto F (2001) Static and ELF magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics 22:419–428. https://doi.org/10.1002/bem.69
Wang Z, Che PL, Du J, Ha B, Yarema KJ (2010) Static magnetic field exposure reproduces cellular effects of the Parkinson's disease drug candidate ZM241385. PLoS One 5:e13883. https://doi.org/10.1371/journal.pone.0013883
Wang D, Wang Z, Zhang L, Li Z, Tian X, Fang J, Lu Q, Zhang X (2018) Cellular ATP levels are affected by moderate and strong static magnetic fields. Bioelectromagnetics 39:352–360. https://doi.org/10.1002/bem.22122
Zhang L, Wang J, Wang H, Wang W, Li Z, Liu J, Yang X, Ji X, Luo Y, Hu C, Hou Y, He Q, Fang J, Wang J, Liu Q, Li G, Lu Q, Zhang X (2016) Moderate and strong static magnetic fields directly affect EGFR kinase domain orientation to inhibit cancer cell proliferation. Oncotarget 7:41527–41539. https://doi.org/10.18632/oncotarget.9479
Zhang J, Meng X, Ding C, Xie L, Yang P, Shang P (2017a) Regulation of osteoclast differentiation by static magnetic fields. Electromagn Biol Med 36:8–19. https://doi.org/10.3109/15368378.2016.1141362
Zhang L, Ji X, Yang X, Zhang X (2017b) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 8:13126–13141. https://doi.org/10.18632/oncotarget.14480
Zhang X, Yarema K, Xu A (2017c) Biological effects of static magnetic fields. Springer, Berlin
Zhao G, Chen S, Wang L, Zhao Y, Wang J, Wang X, Zhang W, Wu R, Wu L, Wu Y, Xu A (2011) Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: role of reactive oxygen species. Bioelectromagnetics 32:94–101. https://doi.org/10.1002/bem.20617
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59. https://doi.org/10.1016/j.ab.2017.07.009
Acknowledgments
This work was supported by the National Natural Science Foundation of China [Grant Number U1532151], the Major/Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology [Grant Numbers 2016FXCX004, 2016HSC-IU007], and the Chinese Academy of Sciences “Hundred Talent Program” to Xin Zhang.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 4148 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Zhang, L., Shao, G. et al. 6-mT 0–120-Hz magnetic fields differentially affect cellular ATP levels. Environ Sci Pollut Res 25, 28237–28247 (2018). https://doi.org/10.1007/s11356-018-2868-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2868-3