Abstract
Aging is a multifactorial process that encompasses a wide range of physiological implications including the onset of age-associated diseases and eventually death. Although recent years have seen a surge in the number of anti-aging interventions after the elucidation of the hallmarks of aging, a special attraction for gerontologists is the dietary restriction interventions which comprise caloric restriction (CR) and intermittent fasting (IF) strategies. CR is the reduction in calorie intake by 30–40% without causing malnutrition and improves health and increases lifespan in many model organisms. An alternative to CR is Intermittent Fasting, another DR intervention widely popular since ancient times in the form of religious fasting that is now being scientifically explored for its ability to impact metabolism in a way that is beneficial in reducing age-associated ailments and overall health and physiology. IF exerts its action through the activation of bioenergetics sensors and genes associated with longevity like AMPK and sirtuins. Some of the health benefits of fasting include reduction in body weight and obesity, and reduced incidence of diseases like cancer, cardiovascular disorders, neurodegeneration, inflammation, and metabolic syndrome. The ketogenic diet and Mediterranean diet are among the variants that are widely popular as IF regimen today.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Aging is a complex and gradual physiological change occurring in every organism eventually leading to death. The main characteristics of aging include steady accumulation of damage in macromolecules, and disruption in physiology and metabolism according to various observational and descriptive studies conducted on a wide range of aging model organisms (Rattan 2006). As a result, aging has become a substantial problem, serving as a key risk factor for a wide range of human diseases, including diabetes, cardiovascular disease, neurological disorder, and cancer (Niccoli and Partridge 2012). Although scientific breakthroughs in recent decades have resulted in effective treatment approaches that have drastically increased human life expectancy.
In recent years, aging research has progressed at an unforeseen rate, and nine hallmarks of aging have been identified at the molecular and cellular levels that are stated as follows: genomic instability, epigenetic alterations, loss of proteostasis, telomere attrition, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and impaired intercellular communication (López-Otín et al. 2013). Researchers have been captivated by possible treatments that could slow the aging process since the dawn of time, and recent improvements in our understanding of the mechanism(s) of aging have resulted in a surge in anti-aging methods. The identification of the gene (daf-2) that influences longevity in Caenorhabditis elegans (Klass 1983) urged scientists to discover a number of pathways (such as mTOR, AMPK, sirtuins, and insulin/IGF-1) have been found that regulate the aging phenotype. These discoveries have almost always resulted in the identification of novel potential therapeutic molecules that target various signaling pathways by activating or inhibiting specific intermediary proteins (Johnson et al. 2013; Salminen and Kaarniranta 2012).
Several proposed interventions have been used to delay the onset of aging in various organisms over the years, including caloric restriction (CR), antioxidant supplementation, autophagy induction, hormonal therapies, epigenetic regulation, and telomerase activation (Saraswat and Rizvi 2017). This chapter discusses the DR interventions specifically caloric restriction and more broadly intermittent fasting, both of which are promising strategies to achieve healthy aging in near future.
10.1.1 Dietary Restriction as an Anti-Aging Intervention
The dietary restriction (DR) intervention is presently widely popular worldwide due to its efficiency in inducing weight loss in obese individuals and scientifically is a well-established non-genetic, non-pharmacological method that has been shown to extend active and healthy lifespan in a variety of organisms (Katewa and Kapahi 2010). CR, genetic modifications, and pharmacological delivery have been the most common therapies of DR (Liang et al. 2018). DR is defined as a reduction in nutrient intake, either specific or total, that does not result in malnutrition. CR, in which total calorie intake is lowered, as well as research including the limitation of main dietary components (protein, fat, or carbohydrates) or temporal fluctuations of food consumption are all examples of dietary restriction. In recent years, research into the molecular pathways by which DR slows aging and age-related disorders has accelerated (Fig. 10.1) (Bartke et al. 2001). This is largely due to the use of sophisticated genetic tools in simple and short-lived model organisms such as Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster to figure out the core mechanisms of DR’s protective effects. These researches set the groundwork for studying mammals’ conserved basic systems. Insightful research in mammalian systems is bringing closer the aim of using knowledge of DR to delay aging and age-related pathologies in humans (Fontana 2007).
10.1.2 Types of Dietary Restriction
10.1.2.1 Caloric Restriction
CR is the reduction in total calorie consumption (by 10–40%) without causing malnutrition. CR, in combination with intermittent fasting (a type of CR in which bouts of ad libitum food are alternated with periods of up to no calorie consumption), is the only known approach for improving health and lifespan in most, of the living creatures (Madeo et al. 2019). Remarkable findings were observed from CR studies that revealed its influence on health span, which in turn, was associated with a considerable reduction in age-related disorders such as cardiovascular diseases, diabetes, neurodegenerative disorders, and malignancies (Balasubramanian et al. 2017). The positive effects of CR are mediated by a diverse set of biological mechanisms, many of which overlap with the prevention of the hallmarks of aging (Gensous et al. 2019).
Several factors, including dietary composition, the duration of the imposed dietary regimen, and the timing of diet commencement, appear to influence the overall efficiency of CR. Several scientific findings on the effects of CR on the first and second halves of life have also been examined, with the conclusion that CR administered in the first or second half of life is more effective than CR applied in both halves. An increase in the length of CR to more than the median age of the animal enhances the lifespan by reducing lifetime reproduction, according to a study utilizing rodents. As a result, CR has been advocated as a promising method that should be researched further in order to promote healthy aging (Erbaba et al. 2021; Weithoff 2007).
10.1.2.1.1 Caloric Restriction Mimetics (CRMs)
The term “caloric restriction mimetics” was coined recently to characterize pharmacologically active drugs that replicate some of the effects of caloric restriction. Many studies claim that prospective CR-mimicking chemicals could be a prime factor in achieving a healthy lifespan and improve age-related disorders in model organisms. CRMs ought to be able to induce autophagy, a complex degradative process that maintains cellular homeostasis by destroying damaged or unnecessary proteins or cellular structures by reducing protein acetylation via depletion of acetyl-CoA, activation of deacetylases, or inhibition of acetylases (Yin et al. 2016; Madeo et al. 2019).
CRMs also have the ability to imitate more general metabolic, physiological, and hormonal changes caused by CR, as well as the activation of stress response pathways and greater stress resistance. Several chemically varied CRM candidates have been found, with potential sources and work as either inhibitors of lipid and carbohydrate metabolism, mammalian target of rapamycin (mTOR), glycolysis, or act as activators of AMP-activated protein kinase (AMPK), sirtuin, and polyphenols (Ingram et al. 2006).
Rapamycin, a molecule originally discovered from Streptomyces bacteria and used to reduce organ transplant rejection due to its immunosuppressive effects, is probably the most well-known CR mimic. Rapamycin stops mTOR from controlling protein synthesis and cell proliferation in response to nutrition and growth hormones. Autophagy, a cellular recycling process that is thought to renew cells and underlie the anti-aging benefits of rapamycin, is activated when mTOR is inhibited (Johnson et al. 2013).
Metformin, an anti-diabetic agent, is also considered a CRM. Metformin was discovered to improve insulin sensitivity through a variety of mechanisms, including inhibition of complex I of the electron transport chain, activation of AMPK, and modulation of the gut microbiota (Rena et al. 2017). Resveratrol, a polyphenol present in low amounts in red wine, blueberries, raspberries, and peanuts, is another CR mimetic substance that can disrupt the electron transport chain and activate AMPK (Martel et al. 2021). Human cartilage contains glucosamine, an amino monosaccharide that inhibits glycolysis, activates AMPK, and stimulates mitochondrial biogenesis to exert its action (Weimer et al. 2014). Spermidine (found in soybean and mushroom) extends lifespan of model rats by inhibition of acetyltransferase and by the activation of autophagy (Hofer et al. 2021; Madeo et al. 2018).
10.2 Intermittent Fasting
IF is a potential non-genetic, non-pharmacological strategy known for granting several health benefits which significantly improves overall health and confers increased lifespan. IF is defined as the umbrella term for the different eating patterns comprising cycles of feeding and fasting. IF serves as an anti-aging strategy and can be categorized under the broad spectrum of dietary restriction interventions. IF relies on the rhythmic or arrhythmic pattern of eating and abstaining from food maintained in regular intervals or on a specific routine based on the type of fasting practiced. The feeding phase during IF may comprise ad libitum intake of food or restricting food intake to a mere 25–30% with regular fasting intervals. There are several types of IF that can be followed based on one’s need and ability to fast.
IF is a commonly practiced intervention mainly by individuals who choose to fast for religious reasons such as the Ramadan fasting among Muslims during the holy month of Ramadan, and among Christians, Buddhists, Orthodox, Jews, and Hindus to name a few. The religious fasts rely on the timing and duration of the fast according to their calendar year and the type of food consumed during this period. These are the widely discussed religious fasts whose detailed effects and benefits have been elucidated (Persynaki et al. 2017; Trepanowski and Bloomer 2010).
The various types and patterns of IF are as follows:
-
(i)
Alternate day fasting (ADF): The ADF method consists of alternate days of feeding and fasting, i.e., feeding ad libitum (no energy restrictions) on a particular day and fasting (no caloric intake, total energy restriction) the subsequent day following repeated cycles. Some individuals may acquire some food (20–30% of one’s energy needs or more) on the eating day as per one’s preferences and needs. ADF confers several health benefits aside from weight loss like glucose and insulin resistance, cardio-protection, and improvement in metabolism and mental well-being.
-
(ii)
Periodic fasting (PF): 5:2 or other Periodic fasting refers to an arrhythmic pattern of feeding and fasting where 2 days of complete fasting or fasting with the intake of fewer calories is followed by the remaining 5 days of normal feeding in a week. The days of fasting may be any day of the week or 2 consecutive days followed by 5 days of ad libitum eating.
-
(iii)
Time-restricted feeding (TRF): While IF relies on the cycles of feeding and fasting, time-restricted eating crucially entails the time period of fasting and feeding. TRF generally implies 8:12 h of feeding and fasting, respectively. TRF is based on the ability of the suprachiasmatic nucleus (SCN) to regulate the synchronization of the peripheral clocks and the circadian cycle of the organism. TRF presses on the intake of food during the active phase of the organism since this confers various metabolic and physiological benefits to the organism.
-
(iv)
Fasting-mimicking diets (FMD): FMDs tend to mimic fasting-like effects and comprise diets such as ketogenic diets (KD’s), very low carbohydrate diet (VLCD), low carbohydrate diet (LCD), or high-fat plant-based diets. This is a hypocaloric diet plan which intends to cause no micronutrient deficiency and observe fasting-like benefits.
-
(v)
Long-term fasting (LF): LF refers to prolonged durations of fasting lasting from a few days to a few weeks (21 days or more). It is followed by taking very low/reduced amounts of calorie intake (<1000 calories in a day) which is given in appropriate amounts for a specified duration of the day (Wilhelmi de Toledo et al. 2020).
10.2.1 Metabolic Switch During Fasting and Its Mechanism
The basic difference between IF and CR is that during CR organism relies on eating at regular periods but with a reduced amount of calories (restriction in calories of about 25–30% in food) whereas during IF, the fasting period requires complete abstinence from food in the majority of the types of fasting. Therefore, the initial response of the body to both of these methods of DR is a slightly different approach, but gradually these strategies almost share the same mechanism of action and functions (Hofer et al. 2022). When an organism initiates a fast, the body responds to the starting phase by breaking down the stored glycogen in the liver by glycogenolysis. The hepatic glycogen store serves as the glucose reservoir during the first 12–36 h of the fasting cycle. Post all the glycogen depletion, the organism undergoes a process generally referred to as “flipping the metabolic switch” or “flipping the switch” which refers to the breakdown of reserved fat to free fatty acids (FFAs) and utilization of ketone bodies (β-hydroxybutyrate, acetoacetate, and acetic acid). Most of the β-hydroxybutyrate generated is utilized by the brain whereas the ketone bodies also serve as an energy source in other organs requiring energy for functioning like muscles. Therefore, flipping the switch mechanism transfers the functioning of metabolic machinery from glycogen to ketone bodies and FFAs from adipocytes which supply the required energy to neurons and other organs for maintenance.
10.2.2 Molecular Mechanisms of Intermittent Fasting
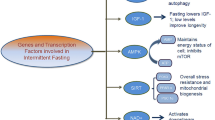
IF is a widely demonstrated DR strategy with various evidence-based studies reporting its role in retarding aging and associated diseases. The basic mechanism of IF involves a network of genes, transcription factors (TFs), hormones, and biochemical markers in an organism. During IF, the fasting period erupts a wave of changes in the functioning of the metabolic pathways which helps the body to cope with the starvation induced in the body. IF basically affects the following factors: (1) reduces oxidative damage caused by ROS and inflammation, (2) induces autophagy, (3) reduces blood glucose and insulin levels and initiates body weight loss, (4) reduces the production of advanced glycation end-products (AGEs), (5) releases adiponectin and ghrelin and reduces leptin, and (6) reduces inflammation and generation of lipid and protein oxidized products.
IF activates a few energy sensors which help in stress resistance and impacts organismal health span. The activity of the mTOR pathway is downregulated in response to lowered amino acids and the availability of growth factors. The inhibition of mTOR also reduces ribosomal biogenesis and induces autophagy which is a major factor for IF-induced protection against cancer and tumor formation (Fig. 10.2). The oscillations arising due to changes in the ratio of ATP/AMP, NAD+/NADH, and acetyl-CoA/CoA is the cause of the activation of the AMPK pathway and Sirtuins (SIRTs). The SIRTs activate a network of transcription factors (TFs) FOXO, PGC-1α, NRF-2, and FGF-21, all involved in coordinating stress resistance, mitochondrial biogenesis, cell survival, proteostasis, and glucose and lipid metabolism (de Cabo and Mattson 2019).
-
1.
The peroxisome proliferator-activated receptor-α (PPAR-α) is a transcription factor which binds to fatty acid derivatives and regulates genes related to ketogenesis, fatty acid oxidation, gluconeogenesis, and amino acid utilization (Goldstein and Hager 2015).
-
2.
The Forkhead box factor (FOXO) is activated during fasting as a metabolic adaptation to regulate energy homeostasis and glucose metabolism. The FOXO plays a crucial role in upregulating several longevity controlling factors most likely conferring stress resistance, cellular proliferation, autophagy, and antioxidant activity (Fontana and Partridge 2015).
-
3.
The PPAR Ɣ coactivator (PGC-1α) is activated via the upstream energy sensing pathways (AMPK and Sirtuins) and mainly regulates fatty acid oxidation, mitochondrial biogenesis, and antioxidant defenses.
-
4.
The nuclear factor-erythroid factor 2-related factor 2 (Nrf-2) pathway plays a major role in resistance against stress by upregulating NRF 2-ARE (antioxidant response element) activation and promoting antioxidant defenses against oxidative stress.
-
5.
Collectively, major changes occurring in an organism during intermittent fasting are the inhibition of anabolic pathways involving growth and reproduction and activation of catabolic processes like DNA repair, cell survival and recycling of damaged organelles, autophagy, maintenance of antioxidant activity, and stress resistance.
10.2.3 Fasting and Circadian Rhythm
The coordinated regulation of the metabolic pathways, biological activities, and behavioral processes occurring in an organism is possible due to the presence of the circadian clock machinery. The circadian clock is the rhythmic oscillations taking place within a 24 h cycle and is primarily regulated by the suprachiasmatic nucleus located in the hypothalamus. The circadian rhythms in an organism arise due to the sleep-wake cycle, exposure to light and darkness, fluctuations in body temperature, hormones, and changes in pattern of feeding and fasting. The circadian cycle influences the rhythms of the sleep-wake cycle and the physiological functioning of the digestive system, the endocrine, reproductive, and cardiovascular systems, the rhythmicity of the brain and immune systems, the renal system, and hepatic metabolism (Froy and Miskin 2010; Manoogian and Panda 2017). The SCN controls the biological functioning of the various peripheral secondary clocks located in various organs like the liver, adipose tissue, heart, retina, intestine, pancreas, skeletal muscle, and parts of the brain (Queiroz et al. 2021).
10.2.4 Time-Restricted Feeding and Circadian Rhythm
The circadian cycle is regulated by a systematic network of transcription and translational feedback loops which involves the circadian clock genes and their transcription factors CLOCK (Circadian Locomotor Output Cycles Kaput) and BMAL1 (Brain and muscle ARNT-like protein 1). The downstream effects of these clock-controlled genes synchronize the interplay of the circadian and peripheral clocks with metabolism which influences changes in glucose tolerance, redox state, memory, and lipid functions (Challet 2019). Erratic schedules of eating and fasting in modern lifestyle hampers the coordination of the circadian cycle with the metabolic clock giving rise to diseases which can well be an indicator of aging. Therefore, the eating window in TRF must be scheduled in such a way that maximizes the beneficial effects imposed on organismal physiology and slows aging. TRF is the provision of eating within a <12 h timespan and the rest of the time period is the fasting timespan. The beneficial effects of TRF can mitigate the negative impact associated with chronodisruption. TRF during the active phase of an organism helps in initiating body weight loss, lowering glucose and insulin in the morning, and also peak other hormones related to aging like cortisol and growth hormone. Therefore, TRF following a robust feeding/fasting regimen in rodents helps in reprogramming the smooth synchronization of the circadian cycle which enables the harmonious functioning of the metabolic pathways which leads to extended longevity.
10.2.5 Beneficial Effects of Intermittent Fasting
10.2.5.1 Hormonal Changes During Intermittent Fasting
Intermittent energy restriction (IER), commonly practiced as ADF or PF reduces levels of blood glucose and insulin. It also lowers insulin resistance which corresponds to improved insulin sensitivity (Hofer et al. 2022). Nutrient deprivation during fasting causes the reduction in plasma insulin and glucose which leads to decline in Insulin-like growth factor (IGF-1) and IGFBP-1. This occurs due to the G- to- K switch which activates the gluconeogenesis cycle in the liver. The adiponectin levels rise during this metabolic switch and this plays a crucial role in fasting-induced improved longevity and lowered stress (Golbidi et al. 2017). The dramatic increase in adiponectin also mediates the cardioprotective effects during fasting.
The adipose tissue largely affects the body’s metabolism because it secretes hormonal adipokines and uncoupling proteins. ADF exerts beneficial effects on adipose tissue by increasing the secretion of serum adiponectin and ghrelin hormones. Adiponectin and ghrelin are protein-derived hormones indicator of hunger and satiety which peak during the fasting state, adiponectin in response to hunger and ghrelin enhances feeding and promotes energy conservation after a brief duration of fasting (Challet 2019). Whereas leptin secretion from the fat cells is reduced in response to the metabolic switch, signaling the brain of reduction in appetite. Hunger levels remain elevated during fasting due to reduced leptin, a hormone which signals the body and brain about condition related to appetite.
10.2.5.2 Obesity and Body Weight
IF is an immensely popular dietary protocol for reducing body weight and, in turn, obesity and related causes. Intermittent calorie restriction (ICR) is an efficient phenomenon in rats and humans to decrease visceral fat from adipocytes and initiate weight loss in overweight individuals along with reduction in waist circumference, fat mass, fat-free mass, and body structure (Seimon et al. 2015). A number of studies have reported ADF to be a standard method for weight loss studies. Intermittent fasting accompanying restricted meal intake (or ad libitum) is effective in promoting weight loss and deduction in body fat (Tinsley and La Bounty 2015). IER-led organisms show some adaptive responses in return of energy restriction most likely which include a decline in physical activity, increases appetite, conservation of body energy, and hormonal stimulations which reserve fat tissues and dispense loss of lean mass. TRF with a feeding window of <10 h a day during the active phase has a significant role in weight loss and fat mass reduction in humans (Hoddy et al. 2020).
10.2.5.3 Fasting and Cardiovascular Diseases
IF immensely affects the functioning of the heart and exerts cardioprotective effects. The increase in BDNF in brain during fasting influences neurons that respond to heart and stabilizes the heart rate (Mattson et al. 2017). ADF reduces the rate of myocardial infarction in humans and rats. ADF reduces the risk of blood pressure associated with increasing age. ADF protects the heart against age-associated risk factors like inflammation, oxidative stress, and hyperlipidemia which are the main cause of heart attacks and stroke. Rise in adiponectin and reduced inflammation help in reducing the risk of heart diseases.
10.2.5.4 Fasting and Cancer
Fasting is helpful in reducing tumor incidence since 2 days or more of periodic fasting initiates autophagy, a cellular conservation process which helps eradicate dead and dysfunctional proteins and organelles. Fasting promotes glycolytic inhibition and decline in IGF-1 level which induces protective effects by inhibiting carcinogenesis and DNA damage. IF-induced protection against cancer is also attributed to the sirtuin genes. Sirtuins activate downstream transcription factors FOXO and Nrf2 which are concerned with inducing apoptosis during fasting and block the inflammasome pathway (Lee et al. 2020).
10.2.5.5 Fasting and Neurodegeneration
Aging increases manifold the vulnerability of the brain to neurodegenerative diseases and oxidative stress-associated damages. An aging brain is highly susceptible to neurodegenerative disorders like Alzheimer’s disease (AD) and Parkinson’s disease. Accumulation of oxidatively damaged molecules (lipid peroxidation products and carbonyl-modified proteins) damages portions of the brain and accelerates aging.
Fasting elicits major beneficial changes in brain and enhances cognitive power and neuronal functions. IF increases mitochondrial biogenesis in neurons, increases stress resistance, reduces markers of inflammation Interleukin-6 (IL-6) and Tumor necrosis factor (TNF-α), improves synaptic plasticity, and stimulates neurogenesis. The rise in BDNF (Brain-derived neurotropic factors) during fasting presses its protective role in enhancing overall cognitive functioning. BDNF signaling during IF activates the antioxidant enzymes superoxide dismutase (SOD) and catalase in the brain which protects it from various neurodegenerative diseases. IF elicits these protective effects due to decreased oxidative damage and an overall improvement in cellular bioenergetics (Longo and Mattson 2014). BDNF stimulates neuron regeneration and synaptic plasticity and regulates cognitive functions like learning and memory. The mitochondrial bioenergetics is improved and an overall reduction in oxidative damage is seen (Kalsi 2015; Longo and Mattson 2014).
10.2.5.6 Fasting and Metabolic Syndrome
Metabolic syndrome is a combination of various metabolic complications associated with aging health, i.e., abdominal obesity, insulin resistance, elevated levels of triglycerides, and hypertension. The cumulative effect of these parameters is the cause of cardiovascular diseases, stroke, and diabetes (Anton et al. 2018). IF reverses the negative features which cause metabolic syndrome like inflammation, elevated blood pressure, and reduced adipocyte fat deposits. IF increases insulin sensitivity, reduces total cholesterol, and visceral fat mass nullifying the risk factors associated with heart disorders and diabetes (Longo and Mattson 2014).
The hormonal changes induced by fasting which include elevation in adiponectin and ghrelin levels and reduction in leptin are correlated to lowered inflammation and increased sensitivity to insulin. Overall fasting reduces all conditions corresponding to metabolic syndrome in rodents and humans like cardiovascular diseases (CVDs), AD, blood pressure, and diabetes.
10.2.5.7 Fasting and Inflammation
Lifestyle changes in the present era have given rise to a number of metabolic disorders like diabetes, metabolic syndrome, cancer, cardiovascular disorders, and neurodegenerative diseases. The disturbance caused by these disorders also gives rise to chronic inflammation due to the activation of certain harmful metabolic pathways in an organism. The molecular pathways which cause an increase in inflammatory biomarkers in blood and other organs are the STAT3 (signal inducer and activator of transcription 3), COX2 (cyclooxygenase 2), NF-κβ (Nuclear factor kappa light chain enhancer of activated B cells), MMP9 (matrix metallopeptidase 9), and MAPK (mitogen-activated protein kinase) (Margină et al. 2020). Whereas, IF induces activation of the NRF2, AMPK, mTOR pathways, and sirtuin genes that coordinate in a systematic way during fasting to reduce the level of inflammatory cytokines and molecules in body.
The rise in inflammation is known by a significant increase in cytokines including TNF-α, IL-6, CRP (C-reactive protein) in blood, liver, and adipocytes. In addition to these, the adipose tissue also produces other adipokines such as resistin, angiotensinogen, and plasminogen activator inhibitor-1 (PAI-1).
10.3 Intermittent Fasting in Model Organisms
The laboratory-based study of CR and IF requires the use of certain model organisms which basically are treated as models for trying and testing the efficacy of the DR routine upon them. Animal models are widely used since they are reliable to study and be experimented upon for longer periods than humans. Laboratory rats and mice are widely used model animals for longevity studies. However, several other model organisms are also extensively used to help determine the molecular pathways of action and its metabolic regulation. The most extensive laboratory-used model organisms are yeast, flies, worms, rodents, non-human primates (monkeys), and mammals too. In bacteria Escherichia coli the transfer of E. coli culture from a normal nutrient medium to a deficient one may cause an increase in lifespan asserting the belief that nutrient/dietary restriction is a strong determinant of lifespan extension even in lower organisms like bacteria (Lushchak et al. 2018).
In budding yeast Saccharomyces cerevisiae, nematode Caenorhabditis elegans, and fruit fly Drosophila melanogaster restriction of calories either by transfer to a low nutrient medium or alternate day fasting has proven to be effective in inducing healthy lifespan. C. elegans lifespan increase can be attributed to a reduction in levels of Daf-2, 16 transcription factor, whose similarity corresponds to the IGF-1 pathway in rodents and humans (López-Lluch and Navas 2016). The sensory abilities in Drosophila and C. elegans including gustatory and olfactory sensing neurons tend to increase lifespan. Any sensation of alterations in the nutrient-sensing pathways specifically IGF-1 network in C. elegans and of steroidal and growth hormonal signaling pathways in mice plays a stimulatory role in the longevity process (Fontana and Partridge 2015). Fasting in Drosophila has shown a positive effect on health with an increase in autophagy and higher stress resistance. Moreover, lowered IIS pathway activity and dietary restriction promote longevity rate and survival in Drosophila (Fontana et al. 2010).
The preliminary evidences of IF being a successful DR anti-aging approach was found in rodents. Rats were kept on 20–50% of calorie restriction for CR studies or were maintained on 1–3 days of fasting on alternative days or periodically for IF studies. The genetic makeup of rodents is most closely similar to humans and therefore rodents are widely used model organisms for dietary restriction studies. Studies have also been conducted on non-human primates like rhesus monkeys and primates including humans (Most et al. 2017). Overall, it is the reduction in the incidence of age-related diseases and hence longer health span that defines the purpose of dietary restriction interventions in longevity studies because these anti-aging interventions have produced successful results in few cases of human studies as well (Green et al. 2022; Longo et al. 2015).
10.3.1 Mediterranean Diet
The Mediterranean diet is popular because of its cardiovascular benefits offering properties. Mediterranean diet comprises a high intake of fruits and vegetables along with fish and unsaturated fats with restricted consumption of saturated fat and processed food (Voglhuber et al. 2021). Mediterranean diet has mainly effects on markers of cardiovascular risk like lowering of HDL and LDL cholesterol and atheroprotective effect. The Mediterranean diet has shown to lower the risk of CVDs by about 30% and also includes reduced incidence of heart diseases, metabolic syndrome, and diabetes.
10.3.2 Ketogenic Diet
Intermittent fasting causes a metabolic transition in the body which induces conversion of the fatty acids to ketone bodies. This change in metabolic state is now adapted in a dietary form referred to as the ketogenic diet (KD). KD involves the complete reduction in carbohydrate intake and excess consumption of fats (>70%) and scarce protein (<20%) content which forcefully generates ketone bodies. The generation of a ketogenic state is being adapted as it is useful in the treatment of a number of maladies including diabetes, cancer, CVDs, obesity, multiple sclerosis, neurological effects, and others (Green et al. 2022). KD produces several benefits as it imitates some molecular effects of IF such as downregulation of IGF-1, inhibition of mTOR, and stimulation of autophagy. KD lowers the generation of ROS and oxidative stress by causing fatty acid oxidation in the liver and cessation of fatty acid synthesis (Bhoumik and Rizvi 2021).
10.4 Conclusion
Aging as a biological problem has attracted attention from gerontologists to find a possible way to improve the duration of healthy lifespan. The cumulative effort of the worldwide aging research has therefore given us diverse approaches to target aging which are broadly classified under “anti-aging strategies.” Dietary restriction interventions have been a fruitful approach to delay aging of which intermittent fasting is a modern dietary intervention that provides metabolic and physiological benefits to organisms. IF is further classified into its types based on time period and duration of fast. IF studies conducted on model organisms have revealed interesting results in improving longevity. Moreover, modifications of diet based on its constituents are a trending dietary pattern that induces each of its own beneficial impact on human health and metabolism. In conclusion, Intermittent Fasting may prove to be beneficial in reducing the age-associated implications and improving health span.
References
Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, Leeuwenburgh C, Mattson MP (2018) Flipping the metabolic switch: understanding and applying the health benefits of fasting: flipping the metabolic switch. Obesity 26(2):254–268. https://doi.org/10.1002/oby.22065
Balasubramanian P, Howell PR, Anderson RM (2017) Aging and caloric restriction research: A biological perspective with translational potential. EBioMedicine 21:37–44. https://doi.org/10.1016/j.ebiom.2017.06.015
Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS (2001) Extending the lifespan of long-lived mice. Nature 414(6862):412–412. https://doi.org/10.1038/35106646
Bhoumik S, Rizvi SI (2021) Anti-aging effects of intermittent fasting: A potential alternative to calorie restriction? Biologia 76(8):2329–2336. https://doi.org/10.1007/s11756-021-00770-5
Challet E (2019) The circadian regulation of food intake. Nat Rev Endocrinol 15(7):393–405. https://doi.org/10.1038/s41574-019-0210-x
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381(26):2541–2551. https://doi.org/10.1056/NEJMra1905136
Erbaba B, Arslan-Ergul A, Adams MM (2021) Effects of caloric restriction on the antagonistic and integrative hallmarks of aging. Ageing Res Rev 66:101228. https://doi.org/10.1016/j.arr.2020.101228
Fontana L (2007) Aging, adiposity, and calorie restriction. JAMA 297(9):986. https://doi.org/10.1001/jama.297.9.986
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span—from yeast to humans. Science 328(5976):321–326. https://doi.org/10.1126/science.1172539
Fontana L, Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161(1):106–118. https://doi.org/10.1016/j.cell.2015.02.020
Froy O, Miskin R (2010) Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging 2(1):7–27. https://doi.org/10.18632/aging.100116
Gensous N, Franceschi C, Santoro A, Milazzo M, Garagnani P, Bacalini MG (2019) The impact of caloric restriction on the epigenetic signatures of aging. Int J Mol Sci 20(8):2022. https://doi.org/10.3390/ijms20082022
Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I (2017) Health benefits of fasting and caloric restriction. Curr Diab Rep 17(12):123. https://doi.org/10.1007/s11892-017-0951-7
Goldstein I, Hager GL (2015) Transcriptional and chromatin regulation during fasting – the genomic era. Trends Endocrinol Metab 26(12):699–710. https://doi.org/10.1016/j.tem.2015.09.005
Green CL, Lamming DW, Fontana L (2022) Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol 23(1):56–73. https://doi.org/10.1038/s41580-021-00411-4
Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E (2020) Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity 28(S1):S29–S23. https://doi.org/10.1002/oby.22829
Hofer SJ, Davinelli S, Bergmann M, Scapagnini G, Madeo F (2021) Caloric restriction mimetics in nutrition and clinical trials. Front Nutr 8:717343. https://doi.org/10.3389/fnut.2021.717343
Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F (2022) The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med 14(1):e14418. https://doi.org/10.15252/emmm.202114418
Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R (2006) Calorie restriction mimetics: an emerging research field. Aging Cell 5(2):97–108. https://doi.org/10.1111/j.1474-9726.2006.00202.x
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) MTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345. https://doi.org/10.1038/nature11861
Kalsi DS (2015) What is the effect of fasting on the lifespan of neurons? Ageing Res Rev 24:160–165. https://doi.org/10.1016/j.arr.2015.07.010
Katewa SD, Kapahi P (2010) Dietary restriction and aging, 2009. Aging Cell 9(2):105–112. https://doi.org/10.1111/j.1474-9726.2010.00552.x
Klass MR (1983) A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev 22(3–4):279–286. https://doi.org/10.1016/0047-6374(83)90082-9
Lee JH, Verma N, Thakkar N, Yeung C, Sung H-K (2020) Intermittent fasting: physiological implications on outcomes in mice and men. Physiology 35(3):185–195. https://doi.org/10.1152/physiol.00030.2019
Liang Y, Liu C, Lu M, Dong Q, Wang Z, Wang Z, Xiong W, Zhang N, Zhou J, Liu Q, Wang X, Wang Z (2018) Calorie restriction is the most reasonable anti-ageing intervention: A meta-analysis of survival curves. Sci Rep 8(1):5779. https://doi.org/10.1038/s41598-018-24146-z
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19(2):181–192. https://doi.org/10.1016/j.cmet.2013.12.008
Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG et al (2015) Interventions to slow aging in humans: are we ready? Aging Cell 14(4):497–510. https://doi.org/10.1111/acel.12338
López-Lluch G, Navas P (2016) Calorie restriction as an intervention in ageing: calorie restriction and ageing. J Physiol 594(8):2043–2060. https://doi.org/10.1113/JP270543
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Lushchak O, Strilbyska O, Piskovatska V, Koliada A, Storey KB (2018) Intermittent fasting. In: Reference module in biomedical sciences. Elsevier, Amsterdam, p B9780128012383621000. https://doi.org/10.1016/B978-0-12-801238-3.62133-5
Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018) Spermidine in health and disease. Science 359(6374):eaan2788. https://doi.org/10.1126/science.aan2788
Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G (2019) Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab 29(3):592–610. https://doi.org/10.1016/j.cmet.2019.01.018
Manoogian ENC, Panda S (2017) Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev 39:59–67. https://doi.org/10.1016/j.arr.2016.12.006
Margină D, Ungurianu A, Purdel C, Tsoukalas D, Sarandi E, Thanasoula M, Tekos F, Mesnage R, Kouretas D, Tsatsakis A (2020) Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int J Environ Res Public Health 17(11):4135. https://doi.org/10.3390/ijerph17114135
Martel J, Chang S-H, Wu C-Y, Peng H-H, Hwang T-L, Ko Y-F, Young JD, Ojcius DM (2021) Recent advances in the field of caloric restriction mimetics and anti-aging molecules. Ageing Res Rev 66:101240. https://doi.org/10.1016/j.arr.2020.101240
Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39:46–58. https://doi.org/10.1016/j.arr.2016.10.005
Most J, Tosti V, Redman LM, Fontana L (2017) Calorie restriction in humans: an update. Ageing Res Rev 39:36–45. https://doi.org/10.1016/j.arr.2016.08.005
Niccoli T, Partridge L (2012) Ageing as a risk factor for disease. Curr Biol 22(17):R741–R752. https://doi.org/10.1016/j.cub.2012.07.024
Persynaki A, Karras S, Pichard C (2017) Unraveling the metabolic health benefits of fasting related to religious beliefs: A narrative review. Nutrition 35:14–20. https://doi.org/10.1016/j.nut.2016.10.005
Queiroz J d N, Macedo RCO, Tinsley GM, Reischak-Oliveira A (2021) Time-restricted eating and circadian rhythms: the biological clock is ticking. Crit Rev Food Sci Nutr 61(17):2863–2875. https://doi.org/10.1080/10408398.2020.1789550
Rattan SIS (2006) Theories of biological aging: genes, proteins, and free radicals. Free Radic Res 40(12):1230–1238. https://doi.org/10.1080/10715760600911303
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z
Salminen A, Kaarniranta K (2012) AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11(2):230–241. https://doi.org/10.1016/j.arr.2011.12.005
Saraswat K, Rizvi SI (2017) Novel strategies for anti-aging drug discovery. Expert Opin Drug Discovery 12(9):955–966. https://doi.org/10.1080/17460441.2017.1349750
Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, Wood RE, King NA, Byrne NM, Sainsbury A (2015) Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol 418:153–172. https://doi.org/10.1016/j.mce.2015.09.014
Tinsley GM, La Bounty PM (2015) Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev 73(10):661–674. https://doi.org/10.1093/nutrit/nuv041
Trepanowski JF, Bloomer RJ (2010) The impact of religious fasting on human health. Nutr J 9(1):57. https://doi.org/10.1186/1475-2891-9-57
Voglhuber J, Ljubojevic-Holzer S, Abdellatif M, Sedej S (2021) Targeting cardiovascular risk factors through dietary adaptations and caloric restriction mimetics. Front Nutr 8:758058. https://doi.org/10.3389/fnut.2021.758058
Weimer S, Priebs J, Kuhlow D, Groth M, Priebe S, Mansfeld J, Merry TL, Dubuis S, Laube B, Pfeiffer AF, Schulz TJ, Guthke R, Platzer M, Zamboni N, Zarse K, Ristow M (2014) D-glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun 5(1):3563. https://doi.org/10.1038/ncomms4563
Weithoff G (2007) Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia 153(2):303–308. https://doi.org/10.1007/s00442-007-0739-6
Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M (2020) Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann Med 52(5):147–161. https://doi.org/10.1080/07853890.2020.1770849
Yin Z, Pascual C, Klionsky D (2016) Autophagy: machinery and regulation. Microbial Cell 3(12):588–596. https://doi.org/10.15698/mic2016.12.546
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhoumik, S., Yadawa, A.K., Srivastava, P., Rizvi, S.I. (2023). Intermittent Fasting as an Anti-Aging Strategy. In: Rizvi, S.I. (eds) Emerging Anti-Aging Strategies. Springer, Singapore. https://doi.org/10.1007/978-981-19-7443-4_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-7443-4_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7442-7
Online ISBN: 978-981-19-7443-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)