Abstract
Old age is one of the major determinants of neurodegenerative diseases. There have been major advancements in understanding the biology of aging along with various interventions that may promote healthy aging. Many nutritional interventions such as caloric restriction, periodic fasting, and alternate day fasting have been proposed that may hamper age-associated cognitive decline. Among the various regimens, intermittent fasting-dietary restriction (IF-DR) seems to be most promising as it has been well documented to provide neuroprotection by enhancing synaptic plasticity and neurogenesis. It is also known to prolong life span and delay the onset of age-associated disorders by reducing inflammation and oxidative stress. IF-DR regimen is known to possibly work by establishing a conditioning response which maintains survival mode in organisms by focusing on energy conservation, thereby causing a metabolic shift from growth to maintenance activities and hence promoting anti-aging effects. IF-DR regimen is also known to improve many physiological indicators such as reduced levels of leptin, insulin, amount of body fat, reduced blood pressure, and increase in resistance to stress. Thus, IF-DR regimen initiated in middle or old age has the ability to impede age-associated neurodegeneration and cognitive decline and may be a potential intervention to abrogate age-related impairment of brain functions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Aging is a naturally occurring, inexorable process which is characterized by progressive loss of physiological integrity, leading to impaired functioning of the body. Aging has been characterized by many hallmark features, such as genomic instability, cellular senescence, attrition of telomeres, mitochondrial dysfunction, epigenetic alterations, and altered intercellular communication (reviewed in López-Otín et al. 2013). Deterioration of physiological functions with aging makes a person prone to many pathological conditions such as diabetes, cancer, cardiovascular diseases, and neurodegenerative disorders. Most age-associated diseases have been closely linked to persistence of chronic inflammatory milieu as evidenced by infiltration of inflammatory mediators such as macrophages and higher circulation levels of adhesion molecules, pro-inflammatory cytokines, and components of complement system (Sarkar and Fischer 2006). Further, various age-related neuropathologies, such as dementia, Alzheimer’s disease, and Parkinson’s disease along with cognitive decline, have been attributed to enhanced oxidative stress, neuronal degeneration, neuroinflammation, glutamate excitotoxicity, and various other factors (Hamilton et al. 2001).
Several theories have been proposed to understand underlying mechanism of aging including free radical and oxygen stress-mitochondrial theories. Free radical theory of aging explains that damage induced by free radicals to biological macromolecules and inability of cellular endogenous antioxidant mechanisms to counterbalance this stress leads to enhanced oxidative stress, aging, and related pathologies (initially proposed by Harman 1956). On the other hand, mitochondrial theory of aging says that increased oxidative stress induces mutations in mitochondrial DNA resulting in deregulated and disrupted mitochondrial biogenesis and bioenergetics (Loeb et al. 2005). Increased ROS are reported to induce mutations and deterioration of DNA, oxidation, and damage to proteins and lipids. Modification of DNA such as formation of 8-hydroxydeoxyguanosine, protein modifications such as carbonyl formation, nitration, glycation, lipid peroxidation generating 4-hydroxynonenal, and mitochondrial membrane potential are the common parameters which undergo changes during oxidative stress (Sohal and Weindruch 1996; Munch et al. 2000; Lopez-Lluch et al. 2006; Johnson et al. 2007; Mattson 2009; Singh et al. 2015). These modifications of DNA, lipids, proteins, and other biomolecules have been reported to be involved in various neurodegenerative diseases (Martin et al. 2006). Aging is also associated with impairments in learning and memory functions, which occurs due to changes in hippocampal plasticity. Accumulation of oxidative stress in aging hippocampus is the main driving force for these synaptic impairments (Serrano and Klann 2004).
Aging research has witnessed prodigious advancements in recent years, and various interventions have been proposed to encourage healthy aging. These interventions aim to improve physical and psychological well-being and to promote health among aging citizens by delaying the onset of age-associated pathological conditions. Hormesis is an adaptive response of cells and organisms to a moderate, usually intermittent stress (reviewed in Mattson 2008). Hormetins have been characterized as physical hormetins, such as exercise; mental hormetins, such as intense brain activity and meditation; and nutritional hormetins, such as flavonoids and polyphenols (Rattan 2017). Various hormetins have been described which can be used as agents to promote healthy aging and enhance life span. Caloric restriction is one of the nutritional hormetins, which has gained attention of many researchers worldwide.
Caloric restriction (CR) is an extremely popular approach to slow down the degenerative effects of aging. CR has been defined as 20–40% less intake of calories than is normally required by the body (Mattson et al. 2003). Intermittent fasting-dietary restriction (IF-DR) is another variation of CR, which involves alternate day fasting while maintaining complete nutritional intake in the intervening day. IF-DR is also referred to as “every other day feeding” (Martin et al. 2006). In comparison to CR, IF-DR is a better approach as the compliance with IF-DR regimen may be greater than CR regimen. Owing to the periodic nature of fasting, IF-DR regimen mitigates the constant hunger effect experienced by CR practitioners (reviewed in Horne et al. 2015). Moreover, persistent CR may lead to malnutrition, the possibility of which is ruled out in IF-DR regimen due to ad libitum access to food every alternate day. Further, a pilot study comparing the effects of daily CR and IF-DR regimens on weight loss has reported reversion of weight loss after stopping CR regimen (Catenacci et al. 2016). IF-DR regimen was shown to produce greater energy deficit from weight maintenance requirements than daily CR. Furthermore, IF-DR regimen has been shown to produce similar beneficial effects as CR (Varady 2011; Anton and Leeuwenburgh 2013).
The beneficial effects of IF-DR have been extensively studied in middle age and old age animal model systems by our lab (Singh et al. 2012, 2015) as well as others (Lara-Padilla et al. 2015; Vasconcelos et al. 2015). IF-DR regimen is known to possibly work by establishing a conditioning response which maintains survival mode in organisms by focusing on energy conservation, thereby causing a metabolic shift from growth to maintenance activities and hence promoting antiaging effects (Kaur and Lakhman 2012). IF-DR regimen has been reported to delay the onset of neurodegenerative disorders in experimental models of Alzheimer’s disease, Parkinson’s disease, and stroke by increasing resistance of hippocampal neurons to degeneration (Mattson 2003). IF-DR regimen is also known to improve many physiological indicators such as reduced amount of body fat (Tinsley and La Bounty 2015), reduction in inflammation (Johnson et al. 2007; Castello et al. 2010), increase in resistance to stress (Vasconcelos et al. 2015), and reduced levels of insulin and leptin (Duan et al. 2003). IF-DR regimen has also been recently reported to exert antitumor effects (reviewed in Mattson et al. 2017).
The architecture of aging brain is prone to modifications by various nutritional and metabolic stimuli. Research in the recent past has tried to unveil various mechanisms of neuroprotection posed by IF-DR. The valuable insights gained from a plethora of studies have helped in better understanding of relationship between energy metabolism and brain functioning. It has also expanded our knowledge regarding various interventions which may be beneficial in improving brain health and providing resistance to age-associated neurological disorders. As already mentioned, brain aging is characterized by reduced synaptic plasticity, cognitive decline, increase in oxidative stress, and inflammatory milieu. Keeping in view these characteristics, we have discussed the effects of IF-DR regimen on age-associated changes in brain architecture and functions in this review.
13.2 IF-DR and Brain Plasticity
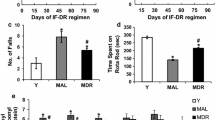
Over many years, various lines of evidence have suggested that dietary restriction (IF-DR or daily CR) can enhance brain plasticity and cognitive performance in different age group of rats by counteracting molecular and cellular changes that impair cognition (Idrobo et al. 1987; Komatsu et al. 2008; Stranahan et al. 2009). Various preclinical studies have shown the beneficial effects of IF-DR regimen in preventing the cognitive decline associated with aging. The behavioral responses to IF-DR are associated with increased brain plasticity and neurogenesis. For instance, young mice maintained on IF-DR regimen for 11 months performed significantly better on tasks of learning and memory (fear conditioning and Barnes maze), and these behavioral outcomes were associated with increased size of pyramidal neurons of CA1 region of the hippocampus (Li et al. 2013). In line with this, another study has also demonstrated that IF-DR regimen enhances hippocampal neurogenesis by promoting the survival of newly synthesized neurons (Lee et al. 2002). Studies from our lab have also shown that IF-DR regimen initiated in middle age and old age rats for 3 months exhibited improved motor performance on rotarod and task of spatial learning and memory (morris water maze) than their ad libitum (AL) fed counterparts (Singh et al. 2012, 2015). The studies suggested that these behavioral outcomes could be due to enhanced synaptic plasticity and reduced mitochondrial oxidative stress.
The cellular and molecular mechanisms by which dietary restriction enhances brain plasticity and improves cognitive functions during aging include increase in synaptic activity that causes production of neurotrophic factors (Amigo and Kowaltowski 2014), which in turn, stimulate the formation of new synapses and promote neurogenesis and potentiation (Anton and Leeuwenburgh 2013). Other factors include activation of cellular stress-responsive machinery against oxidative and metabolic stress (Bruce-Keller et al. 1999), and activation of immune and inflammatory mediators (reviewed in Mattson 2015). Brain-derived neurotrophic factor (BDNF) is one of the most notably produced neurotrophic factors in response to fasting in discrete brain regions with most robust production in hippocampus region of the brain (Cotman et al. 2007). BDNF promotes various aspects of synaptogenesis, neurogenesis, migration, and plasticity (Greenberg et al. 2009; Park and Poo 2013). In addition it is critical for synaptic plasticity implicated in optimization of various domains of cognitive functions (Kuipers and Bramham 2006). Highlighting the relevance of this neurotrophic factor in brain function, individuals carrying mutation in this gene exhibited decreased secretion and deficits in memory and increased anxiety and depression (Egan et al. 2003; Hariri et al. 2003). Evidence suggests that BDNF is produced and released at or near to synapses in response to synaptic activity and thus plays a pivotal role in synapse formation and learning and memory (Marosi and Mattson 2014). Mild metabolic stress and increased neuronal activity can induce BDNF production and its downstream signaling to enhance brain plasticity.
BDNF signaling activates the protein translational machinery which is critical for neural transmission and potentiation (Lu et al. 2008). Direct application of BDNF to the hippocampus was observed to upregulate the expression of markers critical in synapse formation and plasticity, viz., postsynaptic density protein 95 (PSD95) and glutamate receptor subunit (GluR2) (Robinet and Pellerin 2011). In addition, BDNF production is known to promote the survival of neurons under conditions of oxidative and metabolic stress (Mattson 2015). IF-DR has shown its beneficial role in protecting hippocampal neurons from seizure-induced excitotoxicity (Bruce-Keller et al. 1999), and another study has speculated that this protection is in part mediated by BDNF signaling (Duan et al. 2001). Further the beneficial effects of IF-DR following excitotoxic stimulus are associated with lower levels of corticosterone, leading to decreased hippocampal cell death, increased plasticity by activation of BDNF and phosphorylated CREB, and reversal of learning deficits (Qiu et al. 2012). Such effects of BDNF signaling likely contribute to the processes by which IF-DR regimen may enhance cognitive function and prevent brain damage. For instance, in a recent study from our lab, we have shown that IF-DR regimen activated the production of neurotrophic factors BDNF and NT-3 (neurotrophic factor 3) and immature neuronal marker PSA-NCAM (polysialylated neural cell adhesion molecule) in hippocampus, hypothalamus, and piriform cortex (PC) regions of rat brain in response to pilocarpine-induced excitotoxic insult (Kumar et al. 2009). Further the study showed that proliferation rate of neural progenitor cells was also increased in response to dietary restriction as evident from the BrdU immunostaining. These observations highlight the beneficial role of IF-DR as an effective intervention to protect and enhance the resistance of the brain to excitotoxic insult. Studies from our lab have also proposed the potential beneficial role of IF-DR regimen initiated in young and old age rats in attenuating reactive astrogliosis and neuronal plasticity (Sharma and Kaur 2008; Kaur et al. 2008). Young adult rats on IF-DR regimen for 12 weeks demonstrated that kainic acid (KA) excitotoxicity-induced reactive astrogliosis was suppressed and neuronal plasticity was enhanced in response as evident from reduced immunoreactivity of GFAP and enhanced expression of neuronal plasticity markers, PSA-NCAM and NCAM (Sharma and Kaur 2008). The data suggested that IF-DR regimen modulated reactive astrogliosis and prevented age-associated neuronal dysfunction.
Hippocampal neurons play a pivotal role in learning and memory and are vulnerable to neurodegeneration and dysfunction with advancing age. Fasting stimulates the production of BDNF as a result of increased activity of these neurons. BDNF promotes and maintains the growth of dendrites and synapses and also enhances the neurogenesis (Wrann et al. 2013; Longo and Mattson 2014). The newly synthesized cells then integrate into the existing network of neuronal circuits, thus strengthening the synapse and prompting plasticity. After activation, BDNF binds to its high-affinity receptor tyrosine kinase TrkB, resulting in the activation of PI3K/Akt/mitogen-activated protein kinase (MAPK) signaling cascade (Marosi and Mattson 2014). Recent findings have suggested that peripheral signals such as muscle-derived factors can enter the brain and contribute to neuroplasticity and stress resistance (Mattson 2015). For instance, muscle-derived protein FNDC5 is cleaved and secreted as irisin can cross the blood-brain barrier and stimulate BDNF production in the brain which is associated with improved cognitive function following exercise (Wrann et al. 2013).
Apart from the significant contribution of BDNF signaling in maintaining the optimal cognitive functioning and neuronal bioenergetics in the brain in response to IF-DR, glutamate, insulin, and glucagon-like peptide 1 (GLP-1) also contribute to the adaptive responses of the brain to protect it from neurodegeneration (Longo and Mattson 2014). Glutamate activates AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and NMDA (N-methyl-D-aspartate) receptors resulting in the calcium influx followed by activation of protein kinase (CaM) and phosphatases (CaN) which are calcium-dependent regulators of learning and memory (Longo and Mattson 2014). Their activation in turn leads to activation of transcription factors cyclic AMP response element-binding protein (CREB) and NF-κB (nuclear factor kappa B). Insulin binds to its receptor leading to activation of mTOR signaling pathway implicated in protein synthesis and cell growth. Finally, GLP-1 activates its receptor, followed by cyclic AMP production in a coupled manner, CREB activation, and BDNF production. All these signaling cascades mediate neuroprotective effects in response to fasting by regulating neuronal bioenergetics.
Both late-onset and early-onset short-term IF-DR regimens have shown beneficial effects in terms of synaptic plasticity. Synaptophysin is a presynaptic marker used as an index of synaptic number and density, and fall in its expression reflects a decrease in neurotransmission affecting spatial memory (Liu et al. 2005). The stabilization in synaptophysin levels in response to short-term IF-DR in discrete brain regions indicates the prevention of decline in synaptic function in both middle and old age Wistar rats (Singh et al. 2012, 2015). Further the studies have shown that hippocampal synaptic potentiation by signaling molecules like CaMKII and CaN was strengthened by IF-DR regimen. Both of these proteins are enriched in postsynaptic density and are involved in calcium signaling and homeostasis, neural transmission, learning, and memory.
Although IF-DR regimen showed neuroprotective role in middle and old age rats, adverse effects were seen on reproductive functions of young adult female rats via estrous cycle disruption and altered levels of estradiol, testosterone, and luteinizing hormone in both male and female rats (Kumar and Kaur 2013). Further, the decrease in gonadotropin-releasing hormone (GnRH) and PSA-NCAM was observed in median eminence region of the hypothalamus. The study suggested that neuroendocrine energy regulators such as leptin, NPY, and kisspeptin target the GnRH neurons on hypothalamus-hypophysial-gonadal axis (HPA) and disrupt the reproductive functions and cause nutritional infertility in the face of energy status of the rats. The findings of this study may suggest that although IF-DR regimen is beneficial in all age groups, but, females in reproductive age may have adverse effects on their reproductive functions.
13.3 IF-DR and Oxidative Stress
A steady and notable observation from different model systems reported in various studies is that DR promotes healthy aging by reducing oxidative stress and associated damage (Walsh et al. 2014). In addition to oxidative stress induced within the body due to imbalanced homeostasis, IF-DR has been also reported effective against age-related and LPS-induced oxidative stress in rat hippocampus. LPS-induced increase was observed in lipid peroxidation indicated by TBARS levels, nitric oxide (NO), and protein nitrosylation similar to age-related changes. IF-DR for 30 days (every alternative day feeding) was found to prevent LPS-induced changes in these parameters (Vasconcelos et al. 2015).
Further, a clinical study reported by Johnson evidenced that IF-DR regimen reduced expression of oxidative and inflammatory markers (Johnson et al. 2007). Protein carbonyls are well-reported markers of oxidative stress because of their relative formation in early phase and stability (Dalle-Donne 2003). In 8-week IF-DR study, protein carbonyls and other markers of oxidative stress such as nitrotyrosine, 8-isoprostane, histidine, and lysine-4-hydroxy nonenal adducts were significantly decreased. Uric acid, the major scavenger of peroxynitrite and hydroxyl radical concentrations, was significantly enhanced in urine of ADCR subjects (Johnson et al. 2007). Age-associated increase in protein carbonyl content was also reported by our lab (Singh et al. 2015). The brain regions of MAL (middle-aged ad libitum fed) rats were found to have higher protein carbonyl content which was significantly reduced in MRD (middle-aged dietary restriction) rats. Reduced protein carbonyl content indicates reduced oxidative stress by IF-DR intervention which may be due to early initiation of repair and maintenance (Singh et al. 2015).
HNE (4-hydroxynonenal), a major aldehydic product from peroxidation-induced breakdown of membrane phospholipids, was found to be progressively increased with age in heart tissue of rat along with protein carbonyl content. Both HNE and protein carbonyl content were found significantly reduced in hearts of alternate day fed (ADF) animals (Castello et al. 2010). In addition to lipid and protein oxidation, ADF dietary regime also improved the reduced glutathione (GSH) concentrations and significantly decreased GSH/GSSG (oxidized glutathione) ratio, thus improving the antioxidant levels within the body.
Plasma membrane redox system (PMRS) enzymes which are important for antioxidant recycling and antioxidant levels such as coenzyme Q and α-tocopherol were also upregulated by calorie restriction (Hyun et al. 2006). Further age-related increase in protein carbonyls, PM lipid peroxidation, and nitrotyrosine were significantly attenuated by CR in cultured neuronal cells (Hyun et al. 2006).
13.3.1 Dietary Restriction, Oxidative Stress, and Neurodegenerative Diseases
Dietary restriction is well documented to prolong life span and to render the nervous system resistant to age-associated neurodegenerative diseases (Prolla and Mattson 2001). Dietary restriction is beneficial to vulnerable neurons in PD brain as it is reported to bolster brain and peripheral biogenesis (Longo and Mattson 2014). DR has also been reported to confer resistance to dopaminergic neurons against MPTP-induced Parkinsonism and motor deficit amelioration (Duan and Mattson 1999). Two- to 4-month DR regime was reported to be effective against kainic acid-induced Alzheimer’s disease, ameliorating degeneration of pyramidal hippocampal neurons, learning, and memory deficits (Bruce-Keller et al. 1999). Further, DR increased resistance against excitotoxicity and oxidative stress in presenillin-1 and APP-1 mutant mice as compared to ad libitum fed animals (Zhu et al. 1999; Mattson et al. 2001). DR was found beneficial against excitotoxic insults caused by 3-nitropropionic acid (NP) to induce Huntington’s disease. Striatal neurons in rats maintained on 3-month DR regime developed resistance against 3-NP along with improved motor functioning (Bruce-Keller et al. 1999). Interestingly, DR was also observed to confer protection and extend life span in Drosophila model of AD (Kerr et al. 2011). In addition to these conditions, dietary restriction has been reported beneficial in models of ischemic stroke (Yu and Mattson 1999; Fann et al. 2017).
13.3.2 Cellular Effectors of Dietary Restriction
Mitochondria is the main focus of aging research since decades. Increased oxidative stress and loss of mitochondrial bioenergetic efficiency are considered as characteristic features of senescence, neurodegeneration, and aging. Dietary restriction imparts reduced workload and wear and tear of mitochondria by promoting mitochondrial biogenesis and induction of autophagy.
13.3.2.1 Mitochondrial Biogenesis
Activation of distinct genetic programs by nuclear and mitochondrial transcription factors allows synthesis of new mitochondria in response to damaged organelles or increased oxidative stress, known as mitochondrial biogenesis. Nuclear respiratory factor (NRF) and mitochondrial transcription factor A (TFAM) are reported downstream transcription factors which coordinate nuclear and mitochondrial gene transcription responsible for mitochondrial bioenergetics (Wu et al. 1999; St-Pierre et al. 2003). PPARγ is an upstream regulator of TFAM and NRF, which acts as nutrient and energy sensor, signals mitochondrial biogenesis, and further transfers utilization of substrate for cellular energy from carbohydrates to fats (Lopez-Lluch et al. 2006). Sirt1 directly deacylates PPARγ, regulates biogenesis and bioenergetics, and prolongs the healthy life span (Rodgers et al. 2005; Nemoto et al. 2005). In a clinical study on human population, an increase in muscular mitochondrial DNA was observed in response to calorie restriction along with reduced oxidative stress and DNA damage, which suggests that calorie restriction exerts positive effects on mitochondrial functioning in young nonobese individuals (Civitarese et al. 2007). Lopez and group reported CR-induced mitochondrial biogenesis and bioenergetics regulation both in vitro and in vivo by studying mitochondrial membrane potential, a bioenergetic parameter (Lopez-Lluch et al. 2006). Calorie restriction is proposed to carry out efficient electron transfer in respiratory chain which meets equivalent ATP production even under reduced oxygen consumption and reduced ROS production. Attenuation of cellular and molecular damage due to oxidative stress and reduced rate of aging is attributed to this change in mitochondrial efficiency in different organisms (Lopez-Lluch et al. 2006).
13.3.2.2 Autophagy
Autophagy is a strictly regulated process of recycling of damaged organelles and damaged and aggregated cellular proteins into biosynthetic and bioenergetic products in order to maintain cellular homeostasis (Morselli et al. 2010; Wohlgemuth et al. 2010; Yang et al. 2014; Ntsapi and Loos 2016; Pani 2015). This cellular mechanism is potentially induced by CR and plays an important role in CR exerted antiaging effects (Ntsapi and Loos 2016). Several studies have revealed that CR-induced autophagy is controlled by Sirtuin 1 expression in in vitro human cells and in C. elegans in vivo, whereas knockout of Sirtuin 1 abolished the autophagy induction by nutrient deprivation in cultured human cells as well as autophagy induced in C. elegans by dietary restriction (Morselli et al. 2010). Mammalian target of rapamycin (mTOR) pathway negatively regulates autophagy, and its inhibition by CR is also one of the reported underlying mechanisms of CR-induced autophagy (Yang et al. 2014). Thorough literature studies revealed that dysfunctional autophagy in the brain, muscle, liver, and other organs leads to degeneration and aging, and dietary restriction mends the dysfunctional autophagy and delays aging by regulating different pathways (Kume et al. 2010; Morselli et al. 2010; Wohlgemuth et al. 2010; Yang et al. 2014; Ntsapi and Loos 2016; Pani 2015).
13.3.3 Molecular Effectors of Dietary Restriction
Dietary restriction targets the pathways and molecules responsible for energy metabolism, maintaining homeostasis and synthesis.
13.3.3.1 Sirtuins
Sirtuins are family of NAD+-dependent mitochondrial deacetylases, which monitor oxidative and energy metabolism along with mitochondrial dynamics within mitochondrial matrix and maintain cellular homeostasis (Tang et al. 2017; Su et al. 2017). Dietary restriction has been reported to enhance SIRT1 expression and mitochondrial biogenesis through SIRT-1-mediated deacetylation of PGC-1α, a major regulator of biogenesis (Amigo and Kowaltowski 2014; Cohen et al. 2004; Nemoto et al. 2005). SIRT1 orchestrates oxidation of fatty acids in the muscle and liver and mobilization of lipid in adipose tissue, thus suggesting that its activation in dietary restriction may induce metabolic reprogramming (Rodgers et al. 2005; Fiskum et al. 2008; Amigo and Kowaltowski 2014). In yeast, protein Sir2, a gene product of SIR2, catalyzes histone deacetylation and NAD+ cleavage. Since NAD+ and NADH are metabolic cofactors in various key reactions, Sir2 may be used as metabolic sensor which could regulate gene expression according to cellular metabolic state (Guarente 2000; Tanner et al. 2000). Based on this hypothesis, several studies proposed that Sir2/SIR2 mediate cytoprotective effects of dietary restriction in yeast (Canto and Auwerx 2009). Beneficial effects of CR in mammals have been attributed to sirtuins and their interconnections with other cell circuitries such as AMPK1, CREB, and PGC1, which are reported to be activated by fasting (Schulz et al. 2007; Canto and Auwerx 2009; Price et al. 2012; Pani 2015). In several cellular and animal studies, Sirt1 activity has been linked to neuronal plasticity, rendering protection against misfolded protein excitotoxicity in Parkinson’s, Alzheimer’s, and Huntington disease (Parker et al. 2005; Gao et al. 2011; Donmez et al. 2012). Clinically the neuroprotective effects of Sirt1/SIRT1 can be mimicked and enhanced experimentally by resveratrol which is known to mimic effects of dietary restriction (Amigo and Kowaltowski 2014; Pani 2015).
13.3.3.2 AMPK and Cross Talk with mTOR, SIRT1, and FOXO Encoded Proteins
Adenosine 5′ monophosphate-activated protein kinase (AMPK) pathway has been reported to play an important role in preventing aging and senescence (Ido et al. 2015). It is regulated by intracellular ATP/AMP ratio and serves as cellular nutrient and energy sensor with ability to modulate whole body metabolism (Xu et al. 2012). AMPK might arbitrate the beneficial effects of dietary restriction via regulating mitochondrial metabolism and biogenesis. AMPK has been reported to prevent oxidative stress-mediated senescence and aging by inducing autophagy via suppression of mTOR pathway (Canto and Auwerx 2011). Mammalian target of rapamycin (mTOR) is another important pathway to regulate energy balance which responds to hormonal and nutritional cues (Powell et al. 2012; Xu et al. 2012). The role of mTOR as important longevity pathway suggests that inhibition of mTOR complex 1 (mTORC1) activity is sufficient to increase life span. In mammals calorie restriction has been shown to reduce mTOR signaling (Stanfel et al. 2009). So it is hypothesized that in need of energy/fasting/calorie restriction, ATP demand increases which suppresses mTOR pathway, thus leading to autophagic destruction of damaged cells resulting in longevity and increased life span.
In a recent review by Pani (2015), it has been proposed that neurodegenerative diseases, mTOR promotes tau and amyloid β aggregation by promoting protein synthesis and inhibiting onset of autophagy. CR inhibits mTOR thus inducing autophagy, protects from cognitive decline, and ameliorates disease-related pathology in AD (Pani 2015). Further, AMPK phosphoregulates PGC-1α and enhances NAD+ levels which acts as rate-limiting step in SIRT1 deacetylation (Canto and Auwerx 2009). Thus AMPK allows specific and higher activity of SIRT1 and promotes neuroprotective effects of sirtuins (Canto and Auwerx 2011). The other family of transcription factors, FOXO, provides another evidence of CR and AMPK and increased life span correlation. Genetic evidences suggest that FOXO proteins have the ability to enhance longevity by providing resistance to oxidative stress, protein structure protection, promotion of autophagy, and lipid metabolism (Fontana et al. 2010; Gross et al. 2008). AMPK directly phosphorylates different members of FOXO, which act as mediators of AMPK-induced autophagy (Nakashima and Yakabe 2007).
13.3.3.3 CREB and CREB-Sirt1 Cross Talk
Neurotrophins, the neurotrophic factors, promote neuronal heath by modulating genetic factors majorly via cAMP-responsive element-binding (CREB) factor (Riccio et al. 1999; Finkbeiner 2000). The beneficial neuroprotective effects of calorie restriction in the forebrain of mice lacking CREB were reported to be abolished, thus suggesting the important role of CREB in CR-mediated neuroprotection (Fusco et al. 2012). Further, reports have evidenced significantly reduced levels of CREB expression in aged and neurodegenerative disease associated brains. The CREB-mediated neuroprotection is also dependent on CREB-sirtuin cross talk (Cui et al. 2006; Caccamo et al. 2010). Increased neurotrophins in response to CR and DR increase the CREB expression which further induce Sirt1 expression and its mediated pathway. Sirt1 expression is reported to be highly reduced in absence of CREB, and nutrient availability regulates expression of CREB and CREB-related genes in the brain (Fusco et al. 2012). CREB has been also reported to transactivate neurotrophin BDNF and TrkB encoding gene expression in the brain (Deogracias et al. 2004). Furthermore, deletion or mutation of CREB leads to neurodegeneration and neuronal damage induced by huntingtin mutant (Cui et al. 2006). In C. elegans CREB has been reported to be essential for memory, and differential regulation of CREB is one important factor underlying age-related decline in memory. In mammalian brains also CREB is referred to as memory regulator, and overexpression of CREB in the hippocampus enhanced the performance of aged animals in long-term memory experiments (Kauffman et al. 2010).
13.4 IF-DR and Neuroinflammation
CR is known to inhibit immunosenescence (Koubova and Guarente 2003) which refers to the age-associated decline of immune functions (Solana et al. 2006). Food restriction inhibits the pro-inflammatory pathways and enhances anti-inflammatory pathways in various tissues including the brain. In the hypothalamus, increased mRNA expression of anti-inflammatory signaling molecules including suppressor of cytokine signaling 3 (SOCS3), interleukin-10 (IL-10), and neuropeptide-Y (NPY) was observed in CR animals (MacDonald et al. 2011). CR suppressed LPS-induced release of IL-1β, IL-6, and TNF-α and enhanced anti-inflammatory corticosterone (MacDonald et al. 2014). Increase in glucocorticoids (GCs) after CR is one of the possible mechanisms by which caloric restriction exerts its anti-inflammatory effect (Levay et al. 2010). GCs show dual effects on regulation of inflammation under stressful conditions. Mild stress results in anti-inflammatory effects of GCs as they reduce pro-inflammatory cytokine production and increase the expression of anti-inflammatory proteins. On the other hand, pathological stressful stimuli lead to chronically elevated GCs and also promote pro-inflammatory cytokines and microglia activation (Vasconcelos et al. 2016). CR suppresses the activation of microglial cells, which are primary immune cells in the brain (Jochen Gehrmann et al. 1995). Microglial cells possess receptors for hormones such as leptin and ghrelin, which are known to be altered by CR. Leptin is an appetite hormone and also has pro-inflammatory effects (Luheshi et al. 1999) which is reduced by CR (Govic et al. 2008).
CR reduces the ionized calcium-binding adapter molecule-1(Iba1) expression in LPS-induced animals. LPS induction causes upregulation of Iba1, protein specifically expressed by microglia, in activated microglia (Imai and Kohsaka 2002). In AL fed animals, LPS increases the mean intensity of Iba1, but this increase in Iba1 expression was not observed in animals exposed to CR which may suggest that CR inhibits microglial activation. Significant decline of Iba1 expression was also observed in both hippocampus and piriform cortex (PC) in animals put on IF-DR with herbal supplementation which indicates that CR is effective to prevent inflammation during aging (Singh et al. 2017). CR also attenuated LPS-stimulated microglial activation in hypothalamus arcuate nucleus (ARC) (Radler et al. 2014) and inhibited NF-κB activation and NF-κB-driven inflammatory gene expression in aged rats (Grosjean et al. 2006). NF-κB is considered as a master regulator of innate immunity, and NF-κB in cytosol fraction is in inactive state, complexed with inhibitory IKβα protein. The activation of NF-κB occurs by phosphorylation of IKβα at serine residue 32 and 36 by IKKα complex (Grosjean et al. 2006). The phosphorylation of IKβα causes degradation of inhibitory IKβα, thus allowing translocation of NF-κB to the nucleus, and permits the binding of NF-κB to regulatory elements in DNA promoters subsequent to genes involved in inflammatory response. NF-κB regulates expression of pro-inflammatory molecules such as tumor necrosis factors (TNF-α and TNF-β), interleukins (IL-1β, IL-2, and IL-6), chemokines (IL-8 and CCL5), adhesion molecules (ICAM-1, VCAM, and E-selectin), and enzymes like iNOS and COX2 (Chung et al. 2002).
Hunger is an adaptive response to fasting that involves neuroendocrine signals in addition to sensory and cognitive changes that activate food seeking behavior. Several studies have reported that hunger-related neuropeptides and hormones play a pivotal role in mediating the beneficial effects of IF-DR on aging and its associated diseases. A recent study from our lab has reported that NPY which is a “hunger peptide” and energy regulator was activated in the hypothalamus of middle-aged male Wistar rats in response to IF-DR regimen as compared to their AL counterparts (Singh et al. 2015). The study further showed that rats maintained on IF-DR exhibited reduced expression of leptin receptor in the hypothalamus. NPY expression is influenced by peripherally produced hormone signals, including appetite suppressor leptin and appetite stimulant ghrelin. Leptin regulates NPY release through leptin-NPY-GnRH pathway (Fernandez-Fernandez et al. 2006). In addition, leptin also regulates energy homeostasis via central activation of the nervous system through its receptor, Ob-Rb. Low leptin levels are known to induce orexigenic signals in hypothalamus and suppressing energy expenditure (Valassi et al. 2008). A study by Shi et al. (2012) showed that caloric restriction was unable to elevate the levels of circulating adiponectin in NPY deficient mice thus suggesting the central role of this neuropeptide in peripheral adaptation to energy restriction. In addition, NPY activation is linked to anti-inflammatory response following CR by suppression of microglial activation (Sonti et al. 1996; Sousa-Ferreira et al. 2011). Several studies have shown that CR exerts anti-inflammatory role by altering the level of circulating leptin and ghrelin resulting in enhanced production of NPY (Felies et al. 2004; Sousa-Ferreira et al.. 2011; Radler et al. 2015).
Recently, our lab has reported the effect of IF-DR along with supplementation of herbal extracts Withania somnifera and Tinospora cordifolia. This regimen suppressed inflammation induced due to aging in middle-aged female rats by reducing and normalizing the expression of inflammatory molecules such as NF-κB, Iba1, TNFα, IL-1β, and IL-6 in both hippocampus and PC regions of the brain. This was observed in both IF-DR and IF-DR + Herbal (IFDRH) supplementation groups (Singh et al. 2017). This study also demonstrated that IFDRH regimen reduced anxiety-like behavior in middle-aged rats, which was mediated by anti-inflammatory effect posed by this dietary intervention.
13.5 Conclusion
As discussed in the preceding sections, IF-DR regimen provides neuroprotection by various mechanisms (Fig. 13.1), thereby raising the possibility of extended health span and delayed onset of age-related disorders. The effects of IF-DR regimen have shown evolutionary conservation ranging from simple organisms such as Saccharomyces cerevisiae (yeast), Caenorhabditis elegans (nematode), and Drosophila melanogaster (fruit fly) to mammals (Longo and Mattson 2014). The lifestyle in present-day society, which has seen technological advances in food processing, agriculture, as well as transportation, has given rise to sedentary work culture in many fields. Besides consuming energy dense food, people are also less exposed to vigorous exercise schedules. This has resulted in impaired physical and mental health and early onset of diseases. Further, medical practitioners and pharmaceutical industry also aim to treat diseases with chemically synthesized drugs and/or surgery; instead the focus should be on the prevention of diseases. This can be achieved by proper education of children as well as parents regarding the importance of intermittent challenges to the brain for sustaining optimal brain health. IF-DR regimen can prove to be a boon in this scenario. Different cultures and religious groups have been practicing fasting since times immemorial. These include Hindus, Buddhists, Christians, Jews, as well as Muslims, who fast during the month of Ramadan. IF-DR regimen, thus, provides a scientific validation to the traditional practice of fasting. Though the scientific literature provides immense evidence for the potential beneficial effects of IF-DR, yet its translation to human subjects on a regular basis is still a challenge.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADCR:
-
Alternate day caloric restriction
- ADF:
-
Alternate day fed
- AL:
-
Ad libitum
- AMP:
-
Adenosine monophosphate
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ARC:
-
Arcuate nucleus
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- CA:
-
Cornu ammonis
- CaM:
-
Calmodulin
- CaN:
-
Calcineurin
- COX2:
-
Cyclooxygenase-2
- CR:
-
Caloric restriction
- CREB:
-
Cyclic AMP response element-binding protein
- DNA:
-
Deoxyribonucleic acid
- DR:
-
Dietary restriction
- FOXO:
-
Forkhead box O3
- GCs:
-
Glucocorticoids
- GFAP:
-
Glial fibrillary acidic protein
- GLP-1:
-
Glucagon-like peptide 1
- GluR2:
-
Glutamate receptor subunit
- GnRH:
-
Gonadotropin-releasing hormone
- GSH:
-
Glutathione
- HNE:
-
4-Hydroxynonenal
- HPA:
-
Hypothalamic-pituitary-adrenal axis
- Iba1:
-
Ionized calcium-binding adapter molecule-1
- ICAM:
-
Intracellular adhesion molecules
- IF-DR:
-
Intermittent fasting-dietary restriction
- IFDRH:
-
Intermittent fasting-dietary restriction plus herbal supplementation
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- KA:
-
Kainic acid
- LPS:
-
Lipopolysaccharide
- MAL:
-
Middle-aged ad libitum fed
- MAPK:
-
Mitogen-activated protein kinase
- MDR:
-
Middle-aged dietary restriction
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (neurotoxin)
- mRNA:
-
Messenger ribonucleic acid
- mTOR:
-
Mammalian target of rapamycin
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADH:
-
Nicotinamide adenine dinucleotide plus hydrogen
- NCAM:
-
Neural cell adhesion molecule
- NF-кB:
-
Nuclear factor kappa B
- NMDA:
-
N-methyl-D-aspartate
- NO:
-
Nitric oxide
- NP:
-
3-Nitropropionic acid
- NPY:
-
Neuropeptide-Y
- NRF:
-
Nuclear respiratory factor
- NT-3:
-
Neurotrophic factor-3
- PC:
-
Piriform cortex
- PD:
-
Parkinson’s disease
- PGC-1α:
-
Proliferator-activated receptor gamma coactivator 1-alpha
- PI3K:
-
Phosphatidylinositol 3-kinase
- PM:
-
Plasma membrane
- PMRS:
-
Plasma membrane redox system
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PSA-NCAM:
-
Polysialylated neural cell adhesion molecule
- PSD95:
-
Postsynaptic density protein 95 kDa
- ROS:
-
Reactive oxygen species
- SIRT-1:
-
Sirtuin
- SOCS3:
-
Suppressor of cytokine signaling 3
- TBARS:
-
Thiobarbituric acid reactive substances
- TFAM:
-
Mitochondrial transcription factor A
- Trk:
-
Tyrosine kinase
- VCAM:
-
Vascular cell adhesion molecule
References
Amigo I, Kowaltowski AJ (2014) Dietary restriction in cerebral bioenergetics and redox state. Redox Biol 2:296–304
Anton S, Leeuwenburgh C (2013) Fasting or caloric restriction for healthy aging. Exp Gerontol 48:1003–1005
Bruce-Keller AJ, Umberger G, McFall R et al (1999) Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol 45:8–15
Caccamo A, Maldonado MA, Bokov AF et al (2010) CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 107:22687–22692
Canto C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20(2):98
Canto C, Auwerx J (2011) Calorie restriction: is AMPK a key sensor and effector? Physiology 26(4):214–224
Castello L, Froio T, Maina M et al (2010) Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med 48:47–54
Catenacci VA, Pan Z, Ostendorf D et al (2016) A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 24:1874–1883
Chung HY, Kim HJ, Kim KW et al (2002) Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech 59:264–272
Civitarese AE, Carling S, Heilbronn LK et al (2007) Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4(3):e76
Cohen HY, Miller C, Bitterman KJ et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472
Cui L, Jeong H, Borovecki F et al (2006) Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69
Dalle-Donne I, Rossi R, Giustarini D et al (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1):23–38
Deogracias R, Espliguero G, Iglesias T et al (2004) Expression of the neurotrophin receptor trkB is regulated by the cAMP/CREB pathway in neurons. Mol Cell Neurosci 26(3):470–480
Donmez G, Arun A, Chung CY et al (2012) SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J Neurosci 32(1):124–132
Duan W, Mattson MP (1999) Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res 57(2):195–206
Duan W, Guo Z, Mattson MP (2001) Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem 76:619–626
Duan W, Guo Z, Jiang H et al (2003) Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 144:2446–2453
Egan MF, Kojima M, Callicott JH et al (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269
Fann DYW, Ng GYQ, Poh L et al (2017) Positive effects of intermittent fasting in ischemic stroke. Exp Gerontol 89:93–102
Felies M, Von Hörsten S, Pabst R et al (2004) Neuropeptide Y stabilizes body temperature and prevents hypotension in endotoxaemic rats. J Physiol 561:245–252
Fernandez-Fernandez R, Martini AC, Navarro VM et al (2006) Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol 25:127–132
Finkbeiner S (2000) CREB couples neurotrophin signals to survival messages. Neuron 25:11–14
Fiskum G, Danilov CA, Mehrabian Z et al (2008) Post ischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci 1147:129–138
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328(5976):321–326
Fusco S, Ripoli C, Podda MV et al (2012) A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci U S A 109(2):621–626
Gao Z, Zhang J, Kheterpal I et al (2011) Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem 286(25):22227–22234
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev 3:269–287
Govic A, Levay EA, Hazi A et al (2008) Alterations in male sexual behaviour, attractiveness and testosterone levels induced by an adult-onset calorie restriction regimen. Behav Brain Res 190:140–146
Greenberg ME, Xu B, Lu B et al (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 29:12764–12767
Grosjean J, Kiriakidis S, Reilly K et al (2006) Vascular endothelial growth factor signalling in endothelial cell survival: a role for NFκB. Biochem Biophys Res Commun 340:984–994
Gross DN, Van Den Heuvel APJ, Birnbaum MJ (2008) The role of FoxO in the regulation of metabolism. Oncogene 27(16):2320–2336
Guarente L (2000) Sir2 links chromatin silencing, metabolism, and aging. Genes Dev 14:1021–1026
Hamilton ML, Van Remmen H, Drake JA et al (2001) Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A 98:10469–10474
Hariri AR, Goldberg TE, Mattay VS et al (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23:6690–6694
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Horne BD, Muhlestein JB, Anderson JL (2015) Health effects of intermittent fasting: hormesis or harm? A systematic review. Am J Clin Nutr 102:464–470
Hyun DH, Emerson SS, Jo DG et al (2006) Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A 103(52):19908–19912
Ido Y, Duranton A, Lan F et al (2015) Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One 10(2):e0115341
Idrobo F, Nandy K, Mostofsky DI et al (1987) Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriatr 6:355–362
Imai Y, Kohsaka S (2002) Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia 40:164–174
Johnson JB, Summer W, Cutler RG et al (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 42(5):665–674
Kauffman AL, Ashraf JM, Corces-Zimmerman MR et al (2010) Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol 8(5):e1000372
Kaur G, Lakhman SS (2012) Dietary restriction as a potential intervention to retard age-associated impairment of brain functions. In: Thakur MK, Rattan SIS (eds) Brain aging and therapeutic interventions, 1st edn. Springer, Netherlands, pp 147–157
Kaur M, Sharma S, Kaur G (2008) Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 9:441–454
Kerr F, Augustin H, Piper MD et al (2011) Dietary restriction delays aging, but not neuronal dysfunction, in Drosophila models of Alzheimer’s disease. Neurobiol Aging 32(11):1977–1989
Komatsu T, Chiba T, Yamaza H et al (2008) Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol 43:339–346
Koubova J, Guarente L (2003) How does calorie restriction work? Genes Dev 17:313–321
Kuipers SD, Bramham CR (2006) Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel 9:580–586
Kumar S, Kaur G (2013) Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS One 8:e52416
Kumar S, Parkash J, Kataria H et al (2009) Interactive effect of excitotoxic injury and dietary restriction on neurogenesis and neurotrophic factors in adult male rat brain. Neurosci Res 65:367–374
Kume S, Uzu T, Horiike K et al (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120(4):1043–1055
Lara-Padilla E, Godínez-Victoria M, Drago-Serrano ME et al (2015) Intermittent fasting modulates IgA levels in the small intestine under intense stress: a mouse model. J Neuroimmunol 285:22–30
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhance-ment of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375
Levay EA, Tammer AH, Penman J et al (2010) Calorie restriction at increasing levels leads to augmented concentrations of corticosterone and decreasing concentrations of testosterone in rats. Nutr Res 30:366–373
Li L, Wang Z, Zuo Z (2013) Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One 8:e66069
Liu HX, Zhang JJ, Zhen P et al (2005) Altered expression of MAP-2, GAP-43 and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Mol Brain Res 139:169–177
Loeb LA, Wallace DC, Martin GM (2005) The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci U S A 102(52):18769–18770
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19:181–192
Lopez-Lluch G, Hunt N, Jones B et al (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 103(6):1768–1773
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217
Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323
Luheshi GN, Gardner JD, Rushforth DA et al (1999) Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci U S A 96:7047–7052
MacDonald L, Radler M, Paolini AG et al (2011) Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. Am J Physiol Regul Integr Comp Physiol 301:R172–R184
MacDonald L, Hazi A, Paolini AG et al (2014) Calorie restriction dose-dependently abates lipopolysaccharide-induced fever, sickness behavior, and circulating interleukin-6 while increasing corticosterone. Brain Behav Immun 40:18–26
Mattson MP (2009) Mitochondria in Neuroplasticity, Neurologic Disease and Aging. Blood 114:SCI-2
Marosi K, Mattson MP (2014) BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 25:89–98
Martin B, Mattson MP, Maudsley S (2006) Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 5(3):332–353
Mattson MP (2003) Gene–diet interactions in brain aging and neurodegenerative disorders. Ann Intern Med 139:441–444
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7
Mattson MP (2015) Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev 20:37–45
Mattson MP, Duan W, Pedersen WA et al (2001) Neurodegenerative disorders and ischemic brain diseases. Apoptosis 6(1–2):69–81
Mattson MP, Duan W, Guo Z (2003) Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem 84:417–431
Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39:46–58
Morselli E, Maiuri MC, Markaki M et al (2010) Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1(1):e10
Munch G, Lüth HJ, Wong A et al (2000) Crosslinking of α-synuclein by advanced glycation endproducts—an early pathophysiological step in Lewy body formation? J Chem Neuroanat 20(3):253–257
Nakashima K, Yakabe Y (2007) AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem 71(7):1650–1656
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280:16456–16460
Ntsapi C, Loos B (2016) Caloric restriction and the precision-control of autophagy: a strategy for delaying neurodegenerative disease progression. Exp Gerontol 83:97–111
Pani G (2015) Neuroprotective effects of dietary restriction: evidence and mechanisms. Semin Cell Dev Biol 40:106–114
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Parker JA, Arango M, Abderrahmane S et al (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37(4):349–350
Powell JD, Pollizzi KN, Heikamp EB et al (2012) Regulation of immune responses by mTOR. Annu Rev Immunol 30:39–68
Price NL, Gomes AP, Ling AJ et al (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15(5):675–690
Prolla TA, Mattson MP (2001) Molecular mechanisms of brain aging and neurodegenerative disorders: lessons from dietary restriction. Trends Neurosci 24:21–31
Qiu G, Spangler EL, Wan R, Miller M, Mattson MP, So K, de Cabo R, Zou S, Ingram DK (2012) Neuroprotection provided by dietary restriction in rats is further enhanced by reducing glucocortocoids. Neurobiol Aging 33(10):2398–2410
Radler ME, Hale MW, Kent S (2014) Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun 38:13–24
Radler ME, Wright BJ, Walker FR et al (2015) Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience 285:236–247
Rattan SIS (2017) Hormetins as drugs for healthy aging. In: Vaiserman AM (ed) Anti-aging drugs: from basic research to clinical practice, 1st edn. Royal Society of Chemistry, London, pp 170–180
Riccio A, Ahn S, Davenport CM et al (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286:2358–2361
Robinet C, Pellerin L (2011) Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience 192:155–163
Rodgers JT, Lerin C, Haas W et al (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118
Sarkar D, Fisher PB (2006) Molecular mechanisms of aging-associated inflammation. Cancer Lett 236:13–23
Schulz TJ, Zarse K, Voigt A et al (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6(4):280–293
Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3:431–443
Sharma S, Kaur G (2008) Dietary restriction enhances kainate-induced increase in NCAM while blocking the glial activation in adult rat brain. Neurochem Res 33:1178–1188
Shi Y, Felley-Bosco E, Marti TM et al (2012) Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer 12:571
Singh R, Lakhanpal D, Kumar S et al (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 34:917–933
Singh R, Manchanda S, Kaur T et al (2015) Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 16:775–788
Singh H, Kaur T, Manchanda S et al (2017) Intermittent fasting combined with supplementation with Ayurvedic herbs reduces anxiety in middle aged female rats by anti-inflammatory pathways. Biogerontology 18(4):601–614
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273(5271):59–63
Solana R, Pawelec G, Tarazona R (2006) Aging and innate immunity. Immunity 24:491–494
Sonti G, Ilyin SE, Plata-Salamán CR (1996) Neuropeptide Y blocks and reverses interleukin-1β-induced anorexia in rats. Peptides 17:517–520
Sousa-Ferreira L, Garrido M, Nascimento-Ferreira I et al (2011) Moderate long-term modulation of neuropeptide Y in hypothalamic arcuate nucleus induces energy balance alterations in adult rats. PLoS One 6:e22333
Stanfel MN, Shamieh LS, Kaeberlein M et al (2009) The TOR pathway comes of age. Biochim Biophys Acta 1790(10):1067–1074
St-Pierre J, Lin J, Krauss S et al (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278:26597–26603
Stranahan AM, Lee K, Martin B et al (2009) Voluntary exercise and caloric restriction enhance hippocampal den-dritic spine density and BDNF levels in diabetic mice. Hippocampus 19:951–961
Su J, Liu J, Yan XY et al (2017) Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia–reperfusion injury. Int J Mol Sci 18(7):E1599
Tang X, Chen XF, Chen HZ et al (2017) Mitochondrial Sirtuins in cardiometabolic diseases. Clin Sci 131(16):2063–2078
Tanner KG, Landry J, Sternglanz R et al (2000) Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A 97:14178–14182
Tinsley GM, La Bounty PM (2015) Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev 73:661–674
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168
Varady KA (2011) Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev 12:e593–e601
Vasconcelos AR, Kinoshita PF, Yshii LM et al (2015) Effects of intermittent fasting on age-related changes on Na, K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol Aging 36:1914–1923
Vasconcelos AR, Cabral-Costa JV, Mazucanti CH et al (2016) The role of steroid hormones in the modulation of neuroinflammation by dietary interventions. Front Endocrinol (Lausanne) 7(9)
Walsh ME, Shi Y, Van Remmen H (2014) The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med 66:88–99
Wohlgemuth SE, Seo AY, Marzetti E et al (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45(2):138–148
Wrann CD, White JP, Salogiannnis J et al (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659
Wu Z, Puigserver P, Andersson U et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Xu J, Ji J, Yan XH (2012) Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr 52(5):373–381
Yang F, Chu X, Yin M et al (2014) mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res 264:82–90
Yu ZF, Mattson MP (1999) Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res 57(6):830–839
Zhu H, Guo Q, Mattson MP (1999) Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res 842(1):224–229
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kaur, G. et al. (2018). Intermittent Fasting-Dietary Restriction as a Geroprotector. In: Rizvi, S., Çakatay, U. (eds) Molecular Basis and Emerging Strategies for Anti-aging Interventions. Springer, Singapore. https://doi.org/10.1007/978-981-13-1699-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-1699-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1698-2
Online ISBN: 978-981-13-1699-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)