Abstract
Intermittent fasting (IF) is widely practiced for health benefits among people of various societies by adopting regimens which vary in terms of dietary patterns and duration of fast. Also, sustained periods of caloric restriction (CR) without malnutrition have been shown to be a potent modulator of lifespan resulting in lower incidence of metabolic disorders like type 2 diabetes, cardiovascular diseases, cancer, and neurological disorders. IF regimens such as alternate day fasting, time restricted feeding, protein restriction etc. have recently emerged as potential alternate approaches to CR which do not involve any major changes in quality and quantity of nutritional intake. This chapter reviews the different regimens of IF and CR used in model organisms and in humans to ascertain their efficacy for metabolic fitness, resistance to age-related diseases and longevity as well as their underlying molecular and cellular mechanisms. Moreover, promoting health-oriented and disease preventive approaches are more viable options for healthy aging and longevity than continuing with disease-oriented research and therapeutic strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Caloric restriction
- Dietary restriction
- Time restricted fasting
- Metabolic syndrome
- Circadian rhythms
- Alternate day fasting
1 Introduction
Biological aging and its underlying molecular and cellular processes cannot be considered as a disease, and therefore, reorienting the focus of aging research to health-oriented and disease-preventive strategies is required (Rattan 2014). Amongst such approaches, dietary restriction (DR) has emerged to be of prime importance in maintaining and improving mental as well as physical health status in older adults (Zupo et al. 2020; Currenti et al. 2021). Caloric restriction (CR) refers to a dietary intervention which recommends an overall 20–40% reduction in daily caloric intake, whereas, DR is based on a broader scope of dietary interventions that involves restrictions CR without compromising on the quality of nutrition is an effective non-pharmacological intervention which is reported to promote health span in numerous non-human species (Weindruch 1996; Ingram et al. 2007; Mattison et al. 2012) as well as in humans (Fontana et al. 2004, 2010; Fontana and Partridge 2015).
Several clinical trials conducted in the last decade have reported the benefits of short and prolonged bouts of CR in weight reduction and improvement in several physiological markers of health (Most et al. 2017, 2018; Redman and Ravussin 2011). However, long term implementation of daily traditional CR has lower success rate due to poor compliance by individuals (Barte et al. 2010; Scheen 2008). Moreover, ample availability of energy-rich food and beverages in the present-day societies baffles the individual’s ability to continue with traditional CR regimen (Swinburn and Egger 2002). Keeping in view the rapid rate of population aging, some innovative and easy to implement strategies are needed to improve healthspan (Dzau et al. 2019). Intermittent fasting-dietary restriction (IF-DR) regimen based on feeding/fasting timings manipulation is emerging as an alternative and innovative intervention to promote healthy aging.
2 Efficacy of CR Intervention in Aging
Aging-associated changes in physiological functions affect the nutrient requirements of individuals by directly altering their appetite and body weight. Moreover sensory changes such as loss of taste and smell also reduce caloric intake in the aged people. Similarly loss of protein and lean body mass (sarcopenia) in old persons is associated with reduction in energy requirements. Although recent studies suggest that reducing calorie consumption and maintaining below-average body weight throughout life does reduce chronic disease load and increases life span, there is scant data available to confirm the potential beneficial effect of CR, specifically in the older populations. Also, it will not be appropriate to suggest at this juncture that applying lifelong CR is the only way to achieve beneficial long term health benefits such as reducing inflammatory markers, and improving metabolic functions. Therefore, additional studies as well as policy development in this direction are urgently required to establish appropriate nutritional requirements and CR regimens for older adults before making firm recommendations for this population.
After the initial report by McCay showing that CR intervention can extend lifespan in rats, several studies have reported robust potential of CR in delaying age-related impairments and lifespan extension in humans (McDonald and Ramsey 2010; Anderson and Weindruch, 2012). The applicability and efficacy of CR as an aging intervention and lifespan extension have also been studied in detail in non-human primates. A significant improvement in morbidity and mortality was reported with 25% daily CR in adult monkeys (Colman et al. 2009). The efficacy of CR in primates has been found to be dependent on the age of CR onset as in the young onset animals, CR failed to show any survival advantage. Moderate CR onset in adult primates delayed the onset of age-associated pathologies and significant lifespan extension. Moreover, timing of onset of CR also requires consideration of its negative impact on reproductive health in young animals in addition to the advantage of adult-onset CR in lifespan extension (Mattison et al. 2012).
Several recent studies on animals and humans have reported beneficial effects of CR on different health markers, thus providing new approaches for prevention of lifestyle diseases and healthy aging. Some well-known examples are population of Okinawa Island (Willcox and Willcox 2014), Calorie Restriction in Biosphere 2 (Walford et al. 2002), and members enrolled in a clinical trial by Calorie Restriction Society International, who self-impose CR and believed that it will enhance their lifespan (Fontana et al. 2004; Holloszy and Fontana 2007; Kraus et al. 2019). CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) study in humans resulted in improved insulin sensitivity and cardiovascular health (Das et al. 2007; Redman et al. 2011). Initial reports from short-term CR studies of 6- or 12-months part of CALERIE-I in overweight individuals showed reduction in body weight, improved glucose regulation and cardiovascular health (Most et al. 2017, 2018). CALERIE-II conducted in lean individuals with 25% CR at 12 and 24 months showed lower resting metabolism, energy expenditure and sustained metabolic fitness (Ravussinet al. 2015; Kraus et al. 2019). Although both CALERIE studies were unable to completely match many physiological effects of CR in rodents, these results are consistent with some beneficial effects of CR earlier reported in monkeys (Edwards et al. 1998) showing promise of CR as a practical tool for healthy lifespan in humans. There is not adequate evidence available in literature regarding nutritional requirements of old people and their healthy body weight.
3 Cellular and Molecular Basis of Potential Beneficial Effects of CR
Over the years extensive research in a wide variety of species has uncovered several pathways for the beneficial effect of CR including lifespan extension. This section deals with cellular and molecular effectors of the proposed mechanisms of CR. The lifespan extension effect of CR has been attributed to multiple neural, systemic, tissue-specific, and cell autonomous mechanisms (Fontana and Partridge 2015; Fontana 2017). At cellular level, the lifespan extension effect of CR involves increased stress resistance (Hine et al. 2015a; b), autophagy (Singh and Cuervo 2012) and chromatin remodeling (Dang et al. 2014). CR targets molecular effectors involved in energy sensing and utilization to improve cellular energetics and metabolic homeostasis. CR also promotes anti-inflammatory intestinal microbiota, and reduces obesity and metabolic dysfunctions (Tilg and Kaser 2011).
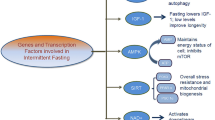
Molecular effectors of CR-related lifespan extension include a variety of kinases, deacetylase enzymes, transcription factors and co-activators involved in cellular energetics pathways. FOXO, a member of forkhead family of transcription factors, in mammals and its invertebrate homologue DAF-16, both have been implicated in increased lifespan (Seo et al. 2015). FOXO has a very interesting role in both stress resistance and apoptosis under variety of environmental conditions and its activity therefore may influence target gene expression relevant for energy homeostasis, glucose metabolism, ROS, oxidative stress, stress resistance, autophagy and cell cycle (Webb and Brunet 2014; Wang et al. 2014). AMP-activated protein kinase (AMPK) is involved in the adaptive response to cellular energy deficit or changes in cellular energetic demand. Reduction in AMPK activation has been reported with aging, whereas, activation of AMPK pathways in multiple tissues is reported with CR (Reznick et al. 2007; Canto and Auwerx 2011). The lifespan extension effect of CR is at least in part dependent on mTOR signaling to regulate metabolism, insulin sensitivity, autophagy, immunity and stress response (Kapahi et al. 2010; Kennedy and Lamming 2016). Metabolic sensors like SIRT1 and AMPK directly regulate PGC1a (peroxisome proliferator activated receptor gamma coactivator 1alpha, a family of nuclear receptor transcription factors) activity through deacetylation and phosphorylation, respectively, and improve metabolic fitness. The mechanistic Target of Rapamycin (mTOR) is a protein kinase implicated in nutrient and energy sensing pathways and mTOR is negatively regulated by AMPK. Overexpression of sirtuins including SIRT1 involved in histone deacetylation improves cellular energetics and metabolic homeostasis in addition to reducing NF-kB signaling (Guarente 2013). All of these studies suggest that regulation of nutrient and fuel sensitive pathways by CR is a shared mechanism to increase metabolic health and lifespan extension. Future studies using genomic, proteomic, and metabolomic approaches may help to understand the tissue-specific effects of CR in both animals and humans, and to elucidate the complex underlying biological processes involved in the anti-aging and life-prolonging effects of CR.
4 IF-DR: Novel Strategies to Improve Metabolic Health and Longevity
Pioneer research to explore the potential of CR-stimulated longevity was spearheaded by Mark Mattson (Mattson 2005; Mattson and Wan 2005). However, recent advances in this area of research have provided much deeper insights into the impact of novel dietary restriction approaches on longevity and healthspan in animal models as well as in humans (Harvie et al. 2011, 2013; Mattson et al. 2017; Anton et al. 2018). Several alternative approaches to traditional CR have acclaimed prominence as novel dietary regimens which may be more efficient to stimulate positive adaptive processes without energy restriction and weight loss (Dorling et al. 2020). IF is the most acclaimed of these novel approaches that requires either adjustment of timings for nutrient intake or the frequency of eating to enforce periodic bouts of fasting i.e. 100% energy restriction, generally recommended for ≥12 h (Anton et al. 2018; Patterson and Sears 2017). IF based approaches are hypothesized to enhance physiological functions and slow down disease progression attributed to prolonged gaps of daily energy restriction (Anton et al. 2018). The potential benefits of IF observed in animal studies have challenged the dogmatic viewpoint that CR is a prerequisite of longevity-promoting diets, and have encouraged scientists in the aging field to test the efficacy of these newer dietary strategies in humans (Anton et al. 2018; Fontana and Partridge 2015). Some emerging IF strategies to improve health such as alternate day fasting (ADF), alternate-day modified fasting (ADMF), 5:2IF, time restricted fasting/feeding (TRF), and protein restriction (PR) in diet have been reported to improve markers of aging in both pre-clinical and clinical set up. Therefore, this area of aging research is gaining momentum to explore whether these novel strategies offer superiority compared to the traditional CR to stimulate improvements in health and longevity (Dorling et al. 2020; Hoddy et al. 2020).
4.1 Alternate-Day Fasting
Alternate-day fasting (ADF) is one of the widely studied IF regimens in animals that involves food withdrawal for 24 h on every other day with ad libitum access to water (Varady and Hellerstein 2007). The lifespan extension efficacy of the ADF regimen in rodents varies with species and age of onset (Goodrick et al. 1990; Arum et al. 2009). Several studies performed with rodents, including those from our lab, have shown promising effects of alternate day IF regimen on stress response, neural and synaptic plasticity and cognition (Duanet al. 2001, 2003; Lee et al. 2002a, b; Sharma and Kaur 2005, 2007, 2008; Kumar et al. 2009). Interestingly, we observed that the beneficial effects of early onset of IF in rats negatively influenced hypothalamo-hypophysial-gonadal axis and compromised their reproductive health (Kumar and Kaur 2013). On the other hand, IF regimen started either in middle-age (Singh et al. 2015, 2017) or in late-age in rats have shown health promoting effects in reversal of age-related impairments in stress, neuronal plasticity, inflammation and cognition (Kaur et al. 2008; Sharma et al. 2010; Singh et al. 2012).
Keeping in view the human’s limitation to constantly maintain a certain level of CR, Stekovic et al. (2019) carried out a clinical trial of ADF in 30 healthy non-obese and 60 controls on conventional western diet, and observed striking reduction in overall calorie intake for a period of more than 6 months. Moreover, ADF regimen was more easily tolerated than chronicCR, and showed similar beneficial effects on the cardiovascular health and fat mass. Further proteome and metabolome of subjects categorized as long-term adopters of ADF showed a significant increase in circulating levels of lipids and a decrease in amino acids like methionine on fasting days. Low systemic levels of methionine and other amino acids have been reported in model organisms to extend lifespan by reducing mTOR pathway activity and corresponding upregulation in cell autophagy (deCabo and Mattson 2019). Safety and tolerability of ADF was evaluated in another study by Catenacci et al. (2016), which reported that alterations in body weight and composition, lipids, and insulin sensitivity index were comparable with moderate daily CR regimen. Similarly, another recent study observed comparable effects of ADF to CR on health parameters over eight weeks in women with obesity and reported that higher energy intake on feeding days could offset hunger pangs on calorie restriction days and assisted in compliance to ADF regimen (Hutchison et al. 2019). Apart from potential negative consequence on reproductive health related to early initiated CR, practical implications in terms of continuously practicing ADF in daily life is another limitation for human population.
4.2 Alternate-Day Modified Fasting
Keeping in view the difficulties of compliance due to 100% CR during ADF regimen on the fasting day, its modified approach (ADMF) has examined IF strategies that permits ≤25% consumption of habitual daily calories intake during fasting days and ad libitum feeding on alternate days (Johnson et al. 2007). Subsequent study by Wegman et al. (2015) reported that ADMF was well tolerated and decreased plasma insulin of healthy and lean subjects. Similar studies in obese but healthy individuals reported that 2–3 months of ADMF lowered their adiposity and improved CVDs as well as inflammatory markers (Bhutani et al. 2013; Varady et al. 2013; Hoddy et al. 2014), irrespective of macronutrientcomposition (Klempel et al. 2013) and meal timings (Hoddy et al. 2014) on fasting days. Moreover, the health benefits of ADMF, and traditional CR in terms of weight loss, weight maintenance, or cardioprotection were found comparable over 12 months in 100 obese participants in the age group of 18–65 years (Trepanowski et al. 2017, 2018; Gabel et al. 2019). Although no foolproof evidence is available till date to demonstrate that ADMF offers significant advantage to markers of aging as compared to traditional CR, but relatively easy compliance to ADMF regimen as compared to traditional CR certainly suggests its superiority for implementation of ADMF as an effective lifestyle intervention in the current obesogenic environment.
4.3 Intermittent Fasting Regimen 5:2
Another novel dietary approach which has been tested recently (Anton et al. 2018) is categorized as 5:2IF, which allows ad libitum normal diet eating for 5 days in a week and severe/complete energy restriction on 2 days per week. Fasting is recommended on either consecutive or non-consecutive days. The advantage of 5:2IF regimen is its flexibility of fasting bouts which makes it easy to adopt by individuals having inconsistent work schedules and social commitments. In a pilot study, Harvie et al. (2011) selected 107 premenopausal overweight or obese women for either six months of traditional CR or 5:2IF and restricted the overall energy intake of subjects by 25% from baseline energy requirements. The main benefit of 5:2IF over CR was greater improvements in insulin resistance and fasting insulin levels, whereas no difference was observed in markers of energy metabolism, inflammation, and quality of life between these two regimens. The data from such studies may help to assess the importance of fasting independent of energy balance between different regimens. Similar findings have been reported by some other recent studies assessing 5:2IF in relation to traditional CR with no between-group changes in glycemic control, weight loss, quality of life and attrition (Carter et al. 2016; Conley et al. 2018; Headland et al. 2018). Taken together, the current evidence does not clearly explain the superiority of 5:2IF over traditional CR in improving markers of aging and longevity and demands future long-term studies in different population groups.
4.4 Time Restricted Feeding/Fasting as an Emerging IF Strategy
Eating behaviors are mostly evaluated based on nutritional quality and quantity of food, but little attention is paid to the temporal patterns of eating and their role in the etiology of diseases. Food overeating behaviors as well as excessive consumption of processed foods with high salt, sugars and fats are the major factors in the development of chronic lifestyle-associated pathologies (Zollner 1990; Zarrinpar et al. 2016; Mozaffarian 2016; Micha et al. 2017). Period between start of first meal to the end of the last meal of a day is considered as the daily feeding time window. Recently, Kant (2018) collected the data of daily feeding time from an American cohort of 15,000 adults and reported that for most of the individuals the estimated feeding time was 12 h, which even reached 15 h for more than half of them. Similarly, another study from India found that erratic eating pattern and prolonging daily feeding time may be a risk factor in the development of metabolic disorders (Gupta et al. 2017). These recent studies suggest that the onset of non-communicable diseases may be prevented/slowed down by time-restricted feeding (TRF), a regimen of IF in which everyday’s nutrient intake is restricted to few hours (usually to 12 h during the day), without any consideration given to alter nutrient quality or calories intake. TRF regimen suggests that the daily food consumption be limited within a period of 4–12 h, which introduces a fasting window of 12–20 h per day (Chaix et al. 2014). The major difference between IF and TRF regimens is that although caloric restriction is not required during feeding time in TRF, but a daily eating window must be consistently maintained (Moon et al. 2020). Studies on experimental animals have reported that TRF dietary regimen attenuates the onset/progression of metabolic diseases against pre-existing obesity, T2D, hyperinsulinemia, hepatic steatosis, inflammation (Hatori et al. 2012; Rothschild et al. 2014).
The concept of TRF was developed keeping in view its relevance to circadian rhythms, which are daily 24 h rhythms of body in physiology, metabolism and behavior sustained under constant light or dark conditions (Xie et al. 2019). Several recent pilot studies on humans have reported potential beneficial effects of TRF regimen on metabolic health parameters (Jamshed et al. 2019; Sutton et al. 2018; Tinsley et al. 2017). Jamshed et al (2019) studied the effects of early TRF (skipping dinner) on eleven overweight adults and observed that only 4 days of early TRF altered the expression of 6 circadian clock genes as well as upregulated the expression of both SIRT1 and LC3A which play important role in autophagy.
Autophagy is well reported to play a key role in protecting the body against multiple chronic diseases like diabetes, CVDs, cancers, and neurodegenerative diseases, by recycling used and damaged proteins and organelles. Sutton et al. (2018) reported that 8 h of early time restricted feeding without reducing the food intake for 5 weeks although did not cause weight loss but enhanced insulin sensitivity in pre-diabetic men. Similarly, a recent study carried out on subjects undergoing orthodox religious fasting reported that time restricted eating may be beneficial to provide better metabolic and glycemic profile (Karras et al. 2021). Due to variations in protocols used and nature of study samples, TRF studies have so far produced mixed data on the superiority of this regimen compared to other IF paradigms. However, Sutton et al. (2018) hypothesized that TRF implemented during earlier periods of the waking phase may elicit more noticeable benefits due to added advantage of utilizing circadian rhythms. Specifically, they postulated that the food consumed during the early waking hours may give greater insulin sensitivity and thermic effect thus providing a more suitable time window for food consumption to enhance metabolic endpoints. Their study on pre-diabetic men reported that early hours TRF regimen improved secondary outcome measures such as insulin sensitivity, hypertension, and oxidative stress in these subjects but without any weight loss (Sutton et al. 2018).
5 Cellular and Molecular Basis of Potential Beneficial Effects of IF-DR and Its Modified Versions
Fasting involving meal skipping on designated days of the week or certain times during a calendar year is a feature of traditional rituals among many religious groups including Hindus, Muslims, Christians, Jews, Buddhists and others. In economically rich societies, there is ample supply and access to food for most of the people; but during evolution both humans and animals had intermittent access to the food, and this is still true for wild animals which face extended bouts of fasting in nature. Organisms tend to adapt their physiology and adjust cellular energetics to achieve metabolic homeostasis while facing this fundamental challenge of uncertain and extended fasting bouts to enhance their survival under such adverse conditions. Quiescence is an example of one such adaptive survival mechanisms evolved in response to ecological constraints like prolonged fasting. It is not surprising that quiescence-related genes are also important in the control of lifespan (Baugh 2013). Food deprivation has been shown to extend lifespan in a variety of organisms including E. coli (Gonidakis et al. 2010), yeast (Longo et al. 2012), nematodes (Kaeberlein et al. 2006; Lee et al. 2006) and flies (Partridge et al. 2005). Mammals respond to acute fasting bouts lasting 12–14 h by lowering blood glucose by more than 20% and increasing levels of fat-derived ketone bodies, free fatty acids and gluconeogenesis. Interestingly a similar metabolic adaptation resulting in acetic acid accumulation in response to food deprivation was reported in both bacteria and yeast (Gonidakis et al. 2010; Longo et al. 2012). These metabolic adaptations involving switching to alternate fuel may have originated first in microorganism and later evolved and appeared in mammals which can respond to glucose deprivation by switching to fatty acids and ketone bodies as an alternate fuel (Cahill 2006).
Fasting increases metabolic efficiency at the mitochondrial level, increases levels of chaperones, inducesautophagy and genomic stability (Mattson et al. 2014). Several studies have reported that in alternate day feeding regimen, IF-DR improves sensory, motor, and cognitive functions including learning and memory in rodents (Singh et al. 2012; Fontan-Lozano et al. 2007) attributed to increased neurogenesis from neural stem cells and synaptic plasticity (Lee et al. 2002a; b). These results can be explained with evolutionary perspective where hunger can engage sensory and cognitive functions accompanied with neuroendocrine changes leading to increased motivation that enables foraging and food seeking behaviors. Additionally, where hungry animals are hypervigilant and active during foraging and on the contrary food consumption and satiety supports a relatively sedentary state. Fasting and aerobic exercise share many similarities in the physiological responses involved in glucose metabolism, cardiovascular and autonomic regulation (Anson et al. 2003). Brain derived neurotrophic factor (BDNF) signaling has been shown to be sensitive to physical activity and exercise. Several studies have shown increased BDNF signaling in response to IF-DR in rodents (Duan et al. 2001, 2003). Thus, BDNF signaling seems to be a shared mechanism explaining common responses to both fasting and activity involving appetite, metabolism, cardiovascular and autonomic regulation (Rothman et al. 2012).
From evolutionary perspective, individuals whose bodily functions were maintained under the fasted state were more successful in acquiring food as well as had better fertility rates (Mattson et al. 2018). IF in combination with physical activity results in depletion of liver glycogen stores on one hand and simultaneously enhances ketone bodies production from adipose-cell-derived fatty acids. Mild stressful conditions such as IF and exercise that initially have a physiological burden but bring about potentially beneficial effects are collectively called ‘hormetins’ (Rattan 2008). Pioneer work by Mattson’s team reports that intermittent metabolic switching, i.e. repeating cycles of fasting and feeding on daily basis may optimize brain functions and provide resilience throughout the lifespan and further explain that the phenomenon of hormesis represents a key concept for such beneficial effects of IF (Mattson 2008). Santoro et al. (2020) propose that aging may be the integrated result of the adaptive responses such as flexibility of energy metabolism, inflammaging and stress response, and consider IF has hormetic effects to promote healthy aging.
6 Cross Talk Between Circadian Rhythms, and Time-Restricted Feeding for Healthy Aging
The role of orexigenic and anorexigenic factors controlling short term homeostatic feeding is well studied. Daily feeding patterns are controlled by circadian clocks, including the resetting of master clock in the suprachiasmatic nuclei by ambient light and other brain clocks by feeding time, via hormonal, nutrient and visceral cues (Challet 2019).The plasticity of the circadian system to accommodate change in ambient light or food availability although is an advantage in nature to adapt to different seasons, but such plasticity may also become a liability in modern societal setup where light and food are both available ad libitum round the clock. As a result, almost all human beings in modern society are voluntarily overriding this natural cycle of diurnal circadian rhythm by self-selecting sleep–wake timings as per their convenience and suitability to work schedule. Night-shift workers have been observed to experience such chronodisruption with hormonal imbalance, metabolic disorders, and increased incidence of cancer (Davis and Mirick 2006). Furthermore, there is bidirectional link between feeding and sleep patterns. In the modern human lifestyle, extended wakefulness allows food ingestion behavior to continue late into the night, which significantly contributes to increased caloric intake that often correlates with modern human lifestyle. Moreover, eating at a sub-optimal time of the 24 h circadian rhythm promotes excessive energy storage instead of energy expenditure and often results in overweight/obesity. Chronic disruptions of diurnal rhythms of feeding, fasting and sleep-wake cycles i.e. chrono-disruption, can lead to circadian desynchronization with deleterious health consequences (Manoogian and Panda 2017). Transgenic knockout mouse models of circadian rhythm genes like CLOCK, BMAL1 and PER 1,2 have also been shown to result in premature aging, increased age-related health impairments and reduced lifespan (Dubrovsky et al. 2010; Kondratov et al. 2006; Lee 2005). Mice subjected to long-term daytime restricted feeding showed increased amplitude of CLOCK gene expression, increased expression of catabolic factors and reduced levels of disease markers (Sherman et al. 2011). Daytime TRF does not entrain SCN, whereas, on the contrary CR is shown to entrain SCN, indicating that energy reduction can affect central oscillator (Froy 2018). Interestingly, the timing of food presentation in IF determines its effect on circadian rhythms such that when food is introduced in the light period, mice exhibited arrhythmicity in clock gene expression and in contrast, night-time feeding yielded rhythms like those generated during ad libitum feeding (Froy et al. 2009). Based on these findings, it may be suggested that adherence to timed feeding schedules especially restricting feeding to daytime may help to prevent circadian desynchronization and improve energy homeostasis and metabolic fitness during aging.
7 Conclusion
CR and different regimens of IF when integrated into the standard medical care may hold great potential for the prevention and treatment of aging-associated chronic metabolic diseases. The regimens such as ADF, 5:2IF, TRF, and protein restriction have recently emerged as potential alternate approaches to CR, which do not involve any major changes in quality and quantity of nutritional intake. These novel approaches have been shown in several pilot studies to achieve strong effects on several disease markers which constitute the underlying basis of metabolic syndrome, cardiovascular diseases, cancer, and neurodegenerative diseases. Although the mechanisms of action of these regimens is still poorly understood, their major impact appears to promote coordinated beneficial effects on the process of aging as well as aging-associated diseases, unlike conventional pharmacological therapies which target inhibition of specific molecules/enzymes. Moreover, TRF regimen which recommends restricting the daily feeding time window in alignment with circadian rhythms may prove to be more beneficial in prevention and/or slow down of aging process and promote healthspan. Taking lessons from Okinawa island people, balanced diet consumed in moderate quantities during time-restricted window may be the mantra to achieve wholesome nutrition as it does not require much conscious effort and is also easy to compliant for lifelong. These practices, which are already gaining popularity as lifestyle interventions may eventually accommodate modern healthcare in various settings. Further future interventional studies on basic and translational research are warranted on a large number of human subjects to elucidate the impact of TRF on different parameters of physical and mental health.
References
Anderson RM, Weindruch R (2012) The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol 24:101–106
Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 100:6216–6220
Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG III, Leeuwenburgh C, Mattson MP (2018) Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (silver Spring) 26:254–268
Arum O, Bonkowski MS, Rocha JS, Bartke A (2009) The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell 8:756–760
Barte JCM, Ter Bogt NCW, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJE (2010) Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 11:899–906
Baugh LR (2013) To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194:539–555
Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA (2013) Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 21:1370–1379
Cahill GF Jr (2006) Fuel metabolism in starvation. Annu Rev Nutr 26:1–22
Canto C, Auwerx J (2011) Calorie restriction: is AMPK a key sensor and effector? Physiology 26:214–224
Carter S, Clifton PM, Keogh JB (2016) Intermittent energy restriction in type 2 diabetes: a short discussion of medication management. World J Diabetes 7:627–630
Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Troy Donahoo W (2016) A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 24:1874–1883
Chaix A, Zarrinpar A, Miu P, Panda S (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20:991–1005
Challet E (2019) The circadian regulation of food intake. Nat Rev Endocrinol. 15:393–405
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204
Conley M, LeFevre L, Haywood C, Proietto J (2018) Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? Randomised Pilot Study. Nutr Diet. 75:65–72
Currenti, W., Godos, J., Castellana, S., Caruso, G., Ferri, R., Caraci, F., Grosso, G., Galvano, F.: Association between time restricted feeding and cognitive status in older Italian adults. Nutrients 13:191 (2021)
Dang W, Sutphin GL, Dorsey JA, Otte GL, Cao K, Perry RM, Wanat JJ, Saviolaki D, Murakami CJ, Tsuchiyama S, Robison B (2014) Inactivation of yeast Isw2 chromatin remodeling enzyme mimics longevity effect of calorie restriction via induction of genotoxic stress response. Cell Metab 19:952–966
Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE (2007) Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85:1023–1030
Davis S, Mirick DK (2006) Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control 17:539–545
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. Nengl J Med 381(26):2541–2551
Dorling JL, Martin CK, Redman LM (2020) Calorie restriction for enhanced longevity: the role of novel dietary strategies in the present obesogenic environment. Aging Res Rev 64:101038
Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP (2003) Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A 100:2911–2916
Duan W, Lee J, Guo Z, Mattson MP (2001) Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J MolNeurosci 16:1–12
Dubrovsky YV, Samsa WE, Kondratov RV (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2:936–944
Dzau VJ, Inouye SK, Rowe JW, Finkelman E, Yamada T (2019) Enabling healthful aging for all-the national academy of medicine grand challenge in healthy longevity. N Engl J Med 381:1699–1701
Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Cefalu WT (1998) Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci 53:443–448
Fernandes L, Paúl C (2017) Editorial: aging and mental health. Front Aging Neurosci 1–3
Flatt T (2012) A new definition of aging? Front Genet 3:148
Fontana L, Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161:106–118
Fontana L (2017) The science of nutritional modulation of aging. Aging Res Rev 39:1–2
Fontana L, Meyer TE, Klein S, Holloszy JO (2004) Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. PNAS 101:6659–6663
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328:321–326
Fontán-Lozano Á, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión ÁM (2007) Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci 27:10185–10195
Froy O (2018) Circadian rhythms, nutrition and implications for longevity in urban environments. Proc Nutr Soc 77:216–222
Froy O, Chapnik N, Miskin R (2009) Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech Aging Dev 130:154–160
Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, Varady KA (2019) Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity 27:1443–1450
Gonidakis S, Finkel SE, Longo VD (2010) Genome-wide screen identifies Escherichia coli TCA-cycle-related mutants with extended chronological lifespan dependent on acetate metabolism and the hypoxia-inducible transcription factor ArcA. Aging Cell 9:868–881
Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N (1990) Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev 55:69–87
Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27:2072–2085
Gupta NJ, Kumar V, Panda S (2017) A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 12:0172852
Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S (2013) The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 110:1534–1547
Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (lond) 35:714–727
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–860
Headland ML, Clifton PM, Keogh JB (2018) Effect of intermittent energy restriction on flow mediated dilatation, a measure of endothelial function: a short report. Int J Environ Res Public Health 15:1166
Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Treviño-Villarreal JH, Mejia P, Ozaki CK, Wang R (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160:132–144
Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Treviño-Villarreal JH, Mejia P, Ozaki CK, Wang R (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160(1–2):132–144
Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA (2014) Meal timing during alternate day fasting: impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 22(12):2524–2531
Hoddy KK, Marlatt KL, Çetinkaya H, Ravussin E (2020) Intermittent fasting and metabolic health: from religious fast to time‐restricted feeding. Obesity 28:S29–S37
Holloszy JO, Fontana L (2007) Caloric restriction in humans. Exp Gerontol 42:709–712
Hutchison AT, Liu B, Wood RE, Vincent AD, Thompson CH, O’Callaghan NJ, Wittert GA, Heilbronn LK (2019) Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity 27:50–58
Ingram DK, Young J, Mattison JA (2007) Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience 145:1359–1364
Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM (2019) Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11:1234
Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S, Carlson O (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biol Med 42:665–674
Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M (2006) Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5:487–494
Kant AK (2018) Eating patterns of US adults: meals, snacks, and time of eating. Physiol Behav 193:270–278
Kapahi P, Chen D, Rogers AN, Katewa SD, Li PWL, Thomas EL, Kockel L (2010) With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 11:453–465
Karras SN, Koufakis T, Adamidou L, Antonopoulou V, Karalazou P, Thisiadou K, Mitrofanova E, Mulrooney H, Petróczi A, Zebekakis P, Makedou K (2021) Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int J Food Sci Nutr 72:82–92
Kaur M, Sharma S, Kaur G (2008) Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 9:441–454
Kennedy BK, Lamming DW (2016) The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab 23:990–1003
Klempel MC, Kroeger CM, Varady KA (2013) Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism 62:137–143
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20:1868–1873
Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Das SK, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E, Holloszy JO (2019) 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 7:673–683
Kumar S, Kaur G (2013) Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS ONE 8, e52416
Kumar S, Parkash J, Kataria H, Kaur G (2009) Interactive effect of excitotoxic injury and dietary restriction on neurogenesis and neurotrophic factors in adult male rat brain. Neurosci Res 65:367–374
Lee CC (2005) The circadian clock and tumor suppression by mammalian period genes. Methods Enzymol 393:852–861
Lee GD, Wilson MA, Zhu M, Wolkow CA, De Cabo R, Ingram DK, Zou S (2006) Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5:515–524
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375
Lee J, Seroogy KB, Mattson MP (2002) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19:181–192
Longo VD, Shadel GS, Kaeberlein M, Kennedy B (2012) Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab 16:18–31
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Manoogian EN, Panda S (2017) Circadian rhythms, time-restricted feeding, and healthy aging. Aging Res Rev 39:59–67
Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489:318–321
Mattson MP, Wan R (2005) Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J NutrBiochem 16:129–137
Mattson MP (2005) Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr 25:237–260
Mattson MP (2008) Dietary factors, hormesis and health. Aging Res Rev 7:43–48
Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN (2014) Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 111:16647–16653
Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Aging Res Rev 39:46–58
Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A (2018) Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci 19:63–80
McDonald RB, Ramsey JJ (2010) Honoring Clive McCay and 75 years of calorie restriction research. J Nutr 140:1205–1210
Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D (2017) Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 317:912–924
Moon S, Kang J, Kim SH, Chung HS, Kim YJ, Yu JM, Cho ST, Oh CM, Kim T (2020) Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients 12:1267
Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM (2018) Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol-Endocrinol Metab 314:E396–E405
Most J, Tosti V, Redman LM, Fontana L (2017) Calorie restriction in humans: an update. Aging Res Rev 39:36–45
Mozaffarian D (2016) Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133:187–225
Partridge L, Piper MD, Mair W (2005) Dietary restriction in Drosophila. Mech Ageing Dev 126:938–950
Patterson RE, Sears DD (2017) Metabolic effects of intermittent fasting. Annu Rev Nutr 37:371–393
Rattan SI (2008) Hormesis in aging. Aging Res Rev 7:63–78
Rattan SI (2014) Aging is not a disease: implications for intervention. Aging Dis. 5:196–202
Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR (2015) A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 70:1097–1104
Redman LM, Ravussin E (2011) Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal 14:275–287
Redman LM, Huffman KM, Landerman LR, Pieper CF, Bain JR, Muehlbauer MJ, Stevens RD, Wenner BR, Kraus VB, Newgard CB, Kraus WE (2011) Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab 96(2):E312–E321
Reznick RM, Zong H, Li J, Morino K, Moore IK, Hannah JY, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D (2007) Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156
Rothman SM, Griffioen KJ, Wan R, Mattson MP (2012) Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci 1264:49–63
Rothschild J, Hoddy KK, Jambazian P, Varady KA (2014) Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev 72:308–318
Rowe GC, Arany Z (2014) Genetic models of PGC-1 and glucose metabolism and homeostasis. Rev Endocr Metab Disord 15:21–29
Santoro A, Zhao J, Wu L, Carru C, Biagi E, Franceschi C (2020) Microbiomes other than the gut: inflammaging and age-related diseases. Semin Immunopathol 42(5):589–605
Scheen AJ (2008) CB1 receptor blockade and its impact on cardiometabolic risk factors: overview of the RIO programme with rimonabant. J Neuroendocrinol 20:139–146
Seo M, Seo K, Hwang W, Koo HE, Hahm J, Yang J, Han SK, Hwang D, Kim S, Jang SK, Lee Y, Nam HG, Lee SV (2015) RNA helicase HEL-1 promotes longevity by specifically activating DAF-16/FOXO transcription factor signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA 112(31):E4246–4255
Sharma S, Kaur G (2005) Neuroprotective potential of dietary restriction against kainate-induced excitotoxicity in adult male Wistar rats. Brain Res Bull 67:482–491
Sharma S, Kaur G (2007) Intermittent dietary restriction as a practical intervention in aging. Ann N Y Acad Sci 1114:419–427
Sharma S, Kaur G (2008) Dietary restriction enhances kainate-induced increase in NCAM while blocking the glial activation in adult rat brain. Neurochem Res 33:1178–1188
Sharma S, Singh R, Kaur M, Kaur G (2010) Late-onset dietary restriction compensates for age-related increase in oxidative stress and alterations of HSP 70 and synapsin 1 protein levels in male Wistar rats. Biogerontology 11:197–209
Sherman H, Frumin I, Gutman R, Chapnik N, Lorentz A, Meylan J, le Coutre J, Froy O (2011) Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J Cell Mol Med 15:2745–2759
Singh R, Cuervom AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:2820–2041
Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (dordr) 34:917–933
Singh R, Manchanda S, Kaur T, Kumar S, Lakhanpal D, Lakhman SS, Kaur G (2015) Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 16:775–788
Singh H, Kaur T, Manchanda S, Kaur G (2017) Intermittent fasting combined with supplementation with Ayurvedic herbs reduces anxiety in middle aged female rats by anti-inflammatory pathways. Biogerontology 18:601–614
Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S (2019) Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab 30:462–476
Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27:1212–1221
Swinburn B, Egger G (2002) Preventive strategies against weight gain and obesity. Obes Rev 3:289–301
Tilg H, Kaser A (2011) Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121:2126–2132
Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW (2017) Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci 17:200–207
Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, Varady KA (2018) Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr 37:1871–1878
Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, Ravussin E (2017) Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Internal Med 177:930–938
Varady KA, Hellerstein MK (2007) Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr 86:7–13
Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y (2013) Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 12:1–8
Walford RL, Mock D, Verdery R, MacCallum T (2002) Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 57:B211–B224
Wang L, Karpac J, Jasper H (2014) Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol 217:109–118
Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, Xu J, Williams TA, Lu X, Hsu SI, Anton SD (2015) Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res 18:162–172
Weindruch R (1996) The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol 24:742–745
Webb A, E., Brunet, A., (2014) FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci 39:159–169
Willcox B, J., Willcox, D, C., (2014) Caloric restriction, CR mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care 17:51–58
Xie Y, Tang Q, Chen G, Xie M, Yu S, Zhao J, Chen L (2019) New insights into the circadian rhythm and its related diseases. Front Physiol 10:682–701
Zarrinpar A, Chaix A, Panda S (2016) Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab 27:69–83
Zöllner N (1990) The relevance of diet for civilization diseases, especially atherosclerosis. Wien Med Wochenschr Supplement 106, suppl-11
Zupo R, Castellana F, Bortone I, Griseta C, Sardone R, Lampignano L, Lozupone M, Solfrizzi V, Castellana M, Giannelli G, De Pergola G (2020) Nutritional domains in frailty tools: working towards an operational definition of nutritional frailty. Aging Res Rev 101148
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
All authors declare they have no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharma, S., Kaur, G. (2021). Fasting and Caloric Restriction for Healthy Aging and Longevity. In: Rattan, S.I.S., Kaur, G. (eds) Nutrition, Food and Diet in Ageing and Longevity. Healthy Ageing and Longevity, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-030-83017-5_24
Download citation
DOI: https://doi.org/10.1007/978-3-030-83017-5_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83016-8
Online ISBN: 978-3-030-83017-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)