Abstract
Plant genetic variations provide opportunity to develop new and improved cultivars with desired characteristics, hence gaining major attention from the scientists and breeders all over the world. Harnessing genetic variability is the key factor in the adaptation of plants to ever-rising temperature. Nowadays, such characteristic traits among the population can be used to develop various heat-resilient crop varieties and have a profound effect on restoring the balance between climate change and agriculture. Genetic variations in physiological and molecular traits proved to be the major components for breeding programs to augment the gene pool. With genetic variations, it is possible to identify the phenotypic variations governed either by a single gene or by many genes that will be helpful for mapping associated quantitative trait loci. Genetic variations can also be traced by examining various physiological traits of a crop plant like growth traits (biomass, plant height, and root growth), leaf traits (stomatal conductance, chlorophyll content, chlorophyll fluorescence, photosynthetic rate, membrane stability, sucrose content, and canopy temperature depression), and floral traits (mainly associated with male gametophyte). Yield traits can also display enormous variation, making it highly useful/reliable for screening purposes. Further, genetic variation at the biochemical level can be assessed by measuring the expression of enzymes (related to oxidative stress and antioxidants) and metabolites (both primary and secondary). Evaluating how genetic variation influences phenotype is the ultimate objective of genetics, and using omics approaches can improve the understanding of heat tolerance-governing mechanisms. Further, collecting molecular data at different levels of plant growth and development will help to accelerate our understanding of the mechanisms linking genotype to phenotype.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Genetic variations
- Physiological and molecular traits
- Metabolites
- Phenotype
- Heat tolerance
- Omics approaches
2.1 Introduction
The Earth’s rising average surface temperature, possibly due to global warming, poses a significant threat to the production potential of plants (Bita and Gerats 2013). Temperature is one of the main factors affecting plant phenology and plays a significant role in plant species distribution around the globe (Li et al. 2018). All plant species have a threshold temperature for growth to reach their yield potential; temperatures beyond the threshold are stressful at all plant growth stages, affecting overall performance (Wahid et al. 2007). Heat stress is supraoptimal temperatures that cause irreversible damage to plants (Hasanuzzaman et al. 2013). The impact of heat stress depends on species, specific growth stage, and intensity and duration of the stress (Farooq et al. 2017; Li et al. 2018).
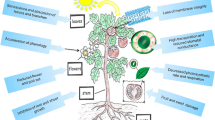
Heat stress affects all stages of plant growth, viz., (1) seed germination (decreases seed germination rate and seedling root and shoot lengths), (2) vegetative growth (decreases plant height, biomass production, and root growth), (3) leaf structure and function (damages membrane structure, increases canopy temperature, decreases stomatal conductance, chlorophyll fluorescence, photosynthetic rate, and sucrose metabolism), (4) reproductive traits (mainly male gametophyte), (5) cellular homeostasis (elevated reactive oxygen species production), and (6) yield (reduced seed number, seed weight, and seed-filling rate). The reproductive stage is much more sensitive to heat stress than the vegetative stages, leading to lower seed weights and thus yield (Farooq et al. 2017). Plants are sessile organisms that can develop various adaptive mechanisms to endure heat waves, such as antioxidant production, synthesis of low-molecular-weight secondary metabolites, increasing heat-shock proteins (HSPs), and upregulating various transcription factors (Fig. 2.1). These endurance mechanisms vary between crop species, growth stage, and growth traits (Bita and Gerats 2013; Prasad et al. 2017).
Impacts, defense mechanisms against heat stress, and possible screening traits used for selecting temperature-resilient crops. High temperature adversely affects plant growth, causes tissue damage, and impairs vital processes such as photosynthesis, respiration, and reproduction. The injuries caused by heat stress lead to oxidative stress due to the production of reactive oxygen species, reducing crop yields. Plants implement various mechanisms to cope with heat stress, including antioxidant and metabolite production, accumulation and adjustment of compatible solutes, and most importantly chaperone (heat-stress proteins, HSPs) signaling and transcriptional activation. These mechanisms, regulated at the molecular level, enable plants to thrive under heat stress. Various growth traits [e.g., plant biomass, plant height, root system architecture (RSA)], leaf traits [e.g., cell membrane thermostability (CMT), canopy temperature depression (CTD), stay-green trait (SGT), stomatal conductance, chlorophyll fluorescence, photosynthetic rate], reproductive traits (e.g., pollen viability, pollen germination), biochemical traits [e.g., reactive oxygen species (ROS) detoxification, various metabolites, HSP levels], and yield traits have been explored as heat-tolerance indicators for screening and breeding for heat tolerance
2.2 Heat Stress and Legumes
Food legumes are an indispensable part of the human diet in developing countries. The major food legumes consumed worldwide are pea (Pisum sativum L.), chickpea (Cicer arietinum L.), common bean (Phaseolus vulgaris L.), lentil (Lens culinaris Medik.), mung bean/green gram (Vigna radiata L.), urdbean/black gram (Vigna mungo L.), and cowpea [Vigna unguiculata (L.) Walp.], and the major oilseed legumes include peanut (Arachis hypogaea L.) and soybean (Glycine max L.) (Maphosa and Jideani 2017). Due to their high nutritional value, legumes are ranked second after cereals. They are rich in protein (20–45%), carbohydrates (60%), dietary fiber (5–37%), and mineral matter (calcium, iron, potassium, phosphorus, copper, and zinc) with no cholesterol and low fat (Iqbal et al. 2006). Environmental factors, mainly rising temperatures, are major constraints on the growth and yield of food legumes. Heat stress adversely affects physiological and reproductive stages, resulting in poor seed yield and quality (Sita et al. 2017). Table 2.1 shows the threshold temperatures for commonly grown legumes in different regions of the world. Various studies have reported the impact of heat stress on seed germination, including poor emergence, germination percentage and radicle and plumule growth, and abnormal seedling vigor. For instance, chickpea germinated well at temperatures from 15 to 35 °C but poorly at temperatures above 40 °C (Kumari et al. 2018). Temperature beyond the threshold range showed lethal effects on the chickpea seedlings (Kumari et al. 2018). Similarly, a 50 °C heat treatment for 30 min significantly reduced seed germination, seed vigor, and seedling growth of dry black gram (Piramila et al. 2012).
Heat stress affects early vegetative growth, decreasing biomass accumulation and root growth and stunting plant height (Huang and Xu 2008; Kaushal et al. 2013). Various studies have reported that heat stress inhibits physiological processes and cellular response activation, including decreased cellular membrane thermostability (Xu et al. 2006). Heat stress dramatically affects the photosynthetic process by disrupting chloroplast structures (thylakoid leakiness and grana stacking) and damaging the D1 protein of PSII due to the accumulation of reactive oxygen species (ROS) (Allakhverdiev et al. 2008; Sharkey 2005). Deactivation of the RuBisCo enzyme even at moderate–high temperatures further hampers photosynthesis (Allakhverdiev et al. 2008).
High temperatures significantly affect the reproductive phase, as reported in various food legumes, including mung bean (Kaur et al. 2015), chickpea (Kaushal et al. 2013), lentil (Bhandari et al. 2016; Sita et al. 2017), and peanut (Prasad et al. 1999). The main reproductive events affected by heat stress are male gametophyte development (meiosis in microspore mother cell, tapetum development in viable pollen, reduced pollen germination, reduced pollen tube growth), female gametophyte development (meiosis in the megaspore mother cell, tapetum development in viable eggs, altered stigmatic and style positions, reduced stigma receptivity), and fertilization (double fertilization and triple fusion) (Farooq et al. 2017; Prasad et al. 2017). Heat stress accelerates seed filling, inhibiting the accumulation of reserves in developing seeds, resulting in poor-quality seeds (Calderini et al. 2006) and reduced seed yields in food legumes such as chickpea (Awasthi et al. 2014) and lentil (Sehgal et al. 2018).
Understanding the impact of heat stress and the related mechanisms will help improve crop genotypes under heat stress. Therefore, identifying traits through extensive screening experiments related to heat tolerance is important for selecting better performing heat-tolerant genotypes of food legumes. This chapter identifies various traits in genotypes of various food legumes with different heat sensitivity/tolerance levels (Fig. 2.1) and offers insight into the overall traits and mechanisms used to select heat-tolerant genotypes.
2.3 Growth-Based Studies
High temperature adversely affects the growth and development of various legumes, restricting the growth cycle from emergence to seed set (Sehgal et al. 2018). Seed germination and seedling establishment, including root and shoot lengths and seedling vigor, are highly sensitive to high temperature. For instance, mung bean seedlings exposed to 45/35 °C had reduced growth (Kumar et al. 2011), and chickpea seedlings exposed to 40 °C for 96 h died (Kumari et al. 2018). Heat stress accelerates early vegetative growth, decreasing leaf number and dry matter accumulation (Tahir et al. 2008). Even moderate heat stress leads to rapid growth and development, resulting in shorter crop duration and less carbon assimilation over the plant’s life cycle (Driedonks et al. 2016; Hatfield and Prueger 2015). Many studies have shown that disturbances in fundamental physiological processes, such as photosynthesis, respiration, water status, membrane stability, primary and secondary metabolites, and ROS generation, due to metabolic disparity resulted in fewer and malformed plant parts (Wahid et al. 2007). Reduced vegetative growth also results from various anatomical and structural changes in cellular organelles, leading to necrosis, chlorosis, sunburn, senescence, and abscission of leaves, twigs, branches, and stems. Further, heat stress negatively affects plant architecture, including branching pattern, leaf area, internode elongation, and leaf/branch angles (Sabagh et al. 2020). The above studies indicate that several processes and molecules are involved in heat stress, reducing plant growth. Many studies have reported reduced vegetative growth in legumes, suggesting an interaction between potential yield and vegetative growth traits, for instance, in chickpea (Awasthi et al. 2014), common bean (Soltani et al. 2019; Yoldas and Esiyok 2009), faba bean (Siddiqui et al. 2015), lentil (Sita et al. 2017), mung bean (Kumar et al. 2011; Sharma et al. 2016), and soybean (Sabagh et al. 2020). Thus, the impact of heat stress on plant growth can be evaluated by assessing traits such as plant height, biomass, and root system architecture. Studies on contrasting genotypes revealed genetic variation in these traits in response to heat stress, which will help identify the mechanisms associated with heat tolerance in legumes.
2.3.1 Biomass
Biomass is an indicator of dry matter accumulation during plant growth, which is adversely affected by heat stress in various legumes (Sabagh et al. 2020). Several studies have revealed genetic variations in biomass accumulation in legumes under high temperatures. Thus, chickpea under heat stress (˃32/20 °C) in a greenhouse had 22–30% less biomass than control plants (Kaushal et al. 2013). High temperature decreased biomass more in heat-sensitive chickpea genotypes (ICC5912, ICC10685) than heat-tolerant genotypes (ICC15614 and ICCV92944) (Kaushal et al. 2013). In another greenhouse study, heat stress (38/35 °C) decreased alfalfa (Medicago sativa) biomass, more so in heat-sensitive Wl712 than heat-tolerant Bara310SC, compared to the control (25 °C) (Wassie et al. 2019). In the field, heat stress (˃32/20 °C) significantly decreased lentil biomass (Sita et al. 2017). Genotypes IG3263, IG2507, IG3297, IG3312, IGG3327, IG3330, IG3546, IG3745, IG4258, and FLIP2009 retained the most biomass and were considered heat tolerant, while genotypes IG2519, IG2802, IG2506, IG2849, IG2821, IG2878, IG3326, IG3290, IG3973, IG3964, IG4242, DPL15, DP315, IG4221, and IG3568 were considered heat sensitive. High temperature (˃40/28 °C) in the field significantly reduced (76%) plant biomass in 45 mung bean accessions from the World Vegetable Center, compared to control conditions (34/16 °C)—genotypes EC693357, EC693358, EC693369, Harsha, and ML 1299 retained the most biomass under heat stress and were considered heat tolerant, while genotypes EC693363, EC693361, KPS1, EC693370, and IPM02-3 retained the least biomass and were considered heat sensitive (Sharma et al. 2016).
2.3.2 Plant Height
Heat stress suppresses the overall vegetative growth of plants by affecting various growth-related mechanisms involving hormones and enzymes (Siddiqui et al. 2015). Plant height at different growth stages is a vital indicator of plant growth under stress situations and has been correlated with heat stress sensitivity (Prasad et al. 2008). A field study was undertaken to screen 12 Kabuli chickpea lines through delayed sowing for heat exposure (39.4 °C) (Mishra and Babbar 2014). Four chickpea lines—KAK2, JGK2, ICCV07311, and ICCV06301—were selected as heat tolerant based on plant height and other yield traits, with positive correlations between phenological traits (days to flowering, days to 50% flowering, maturity days, number of secondary branches, plant height) and yield traits (Mishra and Babbar 2014). Soybean genotypes (64) exposed to heat stress (40/32 °C; seedling stage for 20 days) varied in plant height—IREANE, CZ4898RY, CZ5242LL, CZ5375, ELLIS, 5N393R2, CZ4181, and 45A46 were categorized as heat tolerant, and 5115LL, S47-K5, S45-W9, 483C, 38R10, R01-416F, JTN-5110, S48RS53, and DG4825RR2/STS as heat sensitive, with the remainder categorized as moderately heat tolerant or moderately heat sensitive (Alsajri et al. 2019). Similarly, high temperature imposed on four common bean genotypes (Gima, Volare, Amboto, Nassan) by delaying normal sowing (late-sown) significantly reduced yields, relative to normal-sown plants, due to a shorter vegetative cycle, and genotypes Gima and Volare maintained taller plants than Amboto and Nassan (Yoldas and Esiyok 2009). In a greenhouse study, ten faba bean genotypes raised under high temperatures (HT1: 31 °C and HT2: 37 °C) had markedly reduced plant height compared to the control plants. Genotype C5 produced the tallest plants (heat tolerant), while Espan produced the shortest plants (heat sensitive) (Siddiqui et al. 2015).
2.3.3 Root System Architecture
Root system architecture (RSA) is the structure and spatial and temporal configuration of plant root systems (de Dorlodot et al. 2007). On a macroscale, RSA can determine the organization of the primary and secondary roots (Smith and De Smet 2012). On a microscale, RSA can determine root microstructures, such as fine root hairs and root tips and their interactions with soil and soil microorganisms responsible for water and mineral uptake (Wu et al. 2018). The spatial and temporal distribution of roots determines the crop’s ability to exploit heterogeneously distributed soil resources (Brussaard et al. 2007). Heat stress directly affects plant roots by restricting carbohydrate transport from shoots to roots (Huang and Xu 2008). A comprehensive understanding of RSA helps us understand the effect of environmental conditions and management practices on crops, decreasing the deviation between potential and actual average yields (Garnett et al. 2009; Judd et al. 2015; Ryan et al. 2016). RSA plays an important role in plant–soil–microbe interactions and resolving the cross talk with beneficial soil microbes in the rhizosphere (Ryan et al. 2016).
Root architecture adapts to fluctuating environments. Therefore, we can improve crop performance by increasing root traits, such as root development allocation, and morphological, anatomical, or developmental plasticity (Sultan 2000). Thus, understanding the genetic and molecular mechanisms determining root phenotypic plasticity is necessary for effective selection and crop breeding efforts. Direct relationships between individual root architectural plasticity and yield have been reported across changing environments in various species (Niones et al. 2013; Sadras 2009). Root branching is important for improving soil anchorage and root surface area, enabling plants to reach more distant water reserves. In plants, high- and low-temperature stress generally reduces primary root length, lateral root density (number of lateral roots per unit primary root length), and emergence angle of lateral roots from the primary root, but does not affect the average lateral root length (McMichael and Quisenberry 1993; Nagel et al. 2009). Heat stress affects nutrient uptake due to a decline in root biomass and root hair surface area. In mung bean, high temperatures of 40/30 °C and 45/35 °C inhibited root growth by 13% and 23%, respectively (Kumar et al. 2011).
Root growth has lower optimal growth temperatures and is more sensitive to high temperatures than shoot growth (Huang and Gao 2000; Xu and Huang 2000). Some plant roots synthesize heat-shock proteins (HSPs) by ameliorating their working efficiency (Nieto-Sotelo et al. 2002). Root phenotyping of 577 common bean genotypes in variable heat environments revealed significant relationships between seed yield and seedling basal root number, seedling adventitious root abundance, and seedling taproot length (Strock et al. 2019). The Mesoamerican genotypes yielded higher than the Andean genotypes under heat stress (Strock et al. 2019). In another study, five chickpea genotypes were assessed for thermotolerance at 30, 35, and 40 °C using root length and root branching as criteria, which identified CSJD 884 and RSG 895 as heat tolerant and C 235 as heat sensitive (Kumari et al. 2018). The 40 °C treatment for 96 h negatively affected root branching in chickpea (Kumari et al. 2018).
Similarly, screening 48 lentil genotypes in a growth chamber at 34 °C using root length as one of the selection criteria identified Ranjan, Moitree, 14-4-1, IC 201710, and IC 208329 as heat tolerant (Choudhury et al. 2012). In another lentil study, heat-tolerant genotypes (IG2507, IG3263, IG3745, IG4258, and FLIP2009) had 1.8–22-fold more root nodulation than heat-sensitive genotypes (IG2821, IG2849, IG4242, IG3973, IG3964) under heat stress (>32/20 °C) (Sita et al. 2017).
2.4 Yield-Based Traits
Heat stress negatively impacts reproductive efficiencies and seed development stages, reducing crop yield and quality (Sehgal et al. 2018). Various studies have shown that the relative performance of plants in terms of yield under heat stress is useful for selecting genotypes for crop improvement programs (detailed below). Heat stress severely affects seed development and seed filling in many crop species, resulting in abnormal and shriveled seeds (Egli 1998). The direct effect of heat stress on the sink potential of maturing seeds (Commuri and Jones 1999) disrupts cell division in the endosperm, decreases the number of starch granules, and reduces starch accumulation. Many screening studies under heat stress have included yield traits, such as seed number, seed weight, seed-filling rate, and duration (Farooq et al. 2017).
2.4.1 Seed Number
Heat stress disrupts pollination and fertilization events that directly curtail seed number. For instance, high temperature (45/32 °C) reduced seed number in mung bean genotypes relative to the control (34/16 °C), more so in heat-sensitive genotypes (EC693363, EC693361, KPS1, EC693370, and IPM02-3) than heat-tolerant genotypes (EC693357, EC693358, EC693369, Harsha, and ML 1299) (Sharma et al. 2016). Similarly, in a greenhouse study, the 33/30 °C treatment reduced pod number and seed number per pod the most in 24 common bean genotypes exposed to varying temperatures (24/21 °C, 27/24 °C, 30/27 °C, 33/30 °C), more so in heat-sensitive genotypes (−66%; A55, Labrador, Majestic, IJR) than heat-tolerant genotypes (−31%; Brio, Carson, G122, HB1880, HT38, Venture) (Rainey and Griffiths 2005). In another study, heat stress (36/27 °C) reduced seed number per pod in all but two cowpea lines (heat-tolerant B89-600 and TN88-63) evaluated for heat tolerance in a greenhouse (Ehlers and Hall 1998). In another greenhouse study, high temperature (38 °C) during the reproductive stage of 211 pea genotypes revealed HUDP-25, IPF-400, HFP-4, and DDR-56 as heat tolerant and VL-40, KPMR-615, DDR-61, and KPMR-557 as heat susceptible based on yield parameters; for example, heat-tolerant genotypes had more seeds per plant (35–197) than heat-sensitive genotypes (1–58) (Mohapatra et al. 2020).
2.4.2 Seed Weight
Seed weight is one of the major traits governing crop yield and is thus used as a screening trait in many studies to select heat-tolerant varieties. For example, chickpea exposed to different temperatures (35/25 °C, 40/30 °C, and 45/35 °C) in a growth chamber decreased seed weight at 40/30 °C by 37–45% in sensitive genotypes (ICC14183, ICC5912) relative to tolerant genotypes (ICCV07110, ICCV92944). However, higher temperature (45/35 °C) had a more severe effect, with fewer seeds in tolerant genotypes and no pod set in sensitive genotypes (Kumar et al. 2013). Similar findings were recorded in mung bean when high temperatures (45/32 °C) coincided with reproductive growth; seed weights declined by 48.3% in the sensitive genotype (SML668) and 35.1% in the tolerant genotype (SML832), relative to the control (Kaur et al. 2015). Likewise, seed weight of lentil plants exposed to high temperature (˃32/20 °C) in the field declined, relative to control plants (Bhandari et al. 2016), more so in the heat-sensitive genotypes (−50%; LL699 and LL1122) than the heat-tolerant genotype (−33%; LL931).
In common bean, a high temperature of 33/30 °C was adequate for selecting heat-tolerant (Carson, G122, Brio, HB1880, HT38, Venture) and heat-sensitive genotypes (Labrador, A55, Majestic, IJR), based on seed weight trait in the field; seed weights decreased by 88% in heat-sensitive genotypes compared with 35% in heat-tolerant genotypes (Rainey and Griffiths 2005). Different location-based yield trials—Coachella (USA; 41/25 °C) and Riverside (USA; 36/17 °C)—were used to screen three groups of cowpea genotypes differing in heat sensitivity (Ismail and Hall 1999). Yield parameters, mainly seed weight, and seeds/pod, decreased significantly as the temperature increased. Tolerant genotypes (H36, H8-9, DLS99) retained more seed weight (193 mg/seed) at higher temperature (41/25 °C) than heat-sensitive genotypes (168 mg/seed; CB5, CB3, DLS127). Mohapatra et al. (2020) reported that heat stress reduced 25-seed weight in pea in heat-susceptible genotypes (VL-40, KPMR-615, DDR-61, KPMR-557) to a mean value of 4.13 g, while heat-tolerant genotypes (HUDP-25, IPF-400, HFP-4, DDR-56) had higher seed weights (4.60 g).
Heat stress accelerates the seed-filling rate but decreases the seed-filling duration. In cowpea, increasing the temperature from 15.5 to 26.6 °C increased the seed-filling duration by 14–21 days (Nielsen and Hall 1985). During seed development, heat stress (˃32/20 °C) increased the seed-filling rate in six chickpea genotypes relative to the optimum temperature, and shortened the seed-filling duration, more so in heat-sensitive (ICC4567) than heat-tolerant (ICC1356, ICC15614) genotypes (Awasthi et al. 2014). Thus, reduced seed weight due to heat stress could be related to a decline in seed-filling processes (Sehgal et al. 2017).
2.5 Pollen Grain Traits
Pollen grains are sensitive to extreme temperatures from early pollen development to fertilization, including meiosis I and meiosis II of the microspore mother cell, early dissolution of the tapetum layer, anther dehiscence, pollen shedding, pollen viability, pollen germination, pollen tube growth, and fertilization (Barnabas et al. 2008; Hedhly 2011; Kumar et al. 2013). Observations on heat stress-induced arrest of male gametophyte development revealed the importance of starch accumulation during pollen development because it gives rise to carbohydrates at maturity (Raja et al. 2019). Heat stress prevents starch accumulation during pollen development, which possibly contributes to reduced pollen viability (Pressman et al. 2002). High temperature during anthesis leads to yield losses due to poor pollen traits such as pollen viability, pollen production, and pollen tube length in crop plants, including chickpea (Devasirvatham et al. 2012; Kaushal et al. 2013), common bean (Suzuki et al. 2001), mung bean (Kaur et al. 2015), lentil (Kumar et al. 2016; Sita et al. 2017), and soybean (Salem et al. 2007). Heat-tolerant and heat-sensitive common bean genotypes were identified based on pollen stainability—exposure to high temperature (˃28 °C) for 8–11 days before anthesis decreased pollen stainability and increased flower abortion, reducing pod yield (Suzuki et al. 2001). Heat-sensitive genotypes (Kentucky Wonder, Oregon, and Okinawa Local) had ˂20% pollen stainability, while the heat-tolerant genotype (Haibushi) had 60% pollen stainability under heat-stress conditions. Heat stress (43/30 °C and 45/32 °C) in mung bean adversely affected reproductive components, reducing pollen viability, pollen germination, and pollen tube length (Kaur et al. 2015), compared to the controls (˃40/25 °C). Moreover, high temperature during microsporogenesis reduced pollen number and produced shriveled pollen grains, more so in the heat-sensitive genotype than the heat-tolerant genotype. Another field study exposed 45 mung bean genotypes to high temperature (42 °C) during the flowering stage (Sharma et al. 2016).
An in vitro pollen study revealed that heat-tolerant mung bean genotypes (C693357, EC693358, EC693369, Harsha, ML1299) had better pollen viability and pollen germination than sensitive genotypes (KPS1, EC693361, EC693363, EC693370, IPM02-3) (Sharma et al. 2016). Other pollen traits (pollen germination and pollen load) were used to screen chickpea, identifying heat-tolerant (ICC15614, ICCV92944) and -sensitive (ICC10685, ICC5912) genotypes (Kaushal et al. 2013). Another study identified tolerant and sensitive chickpea genotypes using pollen traits (Devasirvatham et al. 2013) under heat stress (≥35 °C); pollen grains were more sensitive to high temperature than stigmas, with genotype ICC1205 identified as heat tolerant and ICC4567 as heat sensitive. Kumar et al. (2016) screened 334 lentil accessions for heat tolerance under field conditions (˃35/25 °C) and selected heat-tolerant genotypes (FLIP2009-55L, IG2507, and IG4258) based on pollen traits. Sita et al. (2017) revealed that high temperature (˃32/20 °C) in the field reduced pollen viability to a greater extent than control (<32/20 °C), with higher pollen germination in heat-tolerant genotypes (48–50%; IG2507, IG3263, IG3745, IG4258, and FLIP2009) than heat-sensitive genotypes (28–33%).
Sixteen pea accessions were screened for heat tolerance by exposing plants to 45 °C for 2 h; the Ran1 line was selected as heat tolerant and R–Af-1, C–Af-2, and Cs–Af–3 as heat sensitive based on pollen traits (pollen viability, pollen germination, pollen tube growth) (Petkova et al. 2009). In another study, two pea cultivars were tested for their differential sensitivity to high temperature (27/18 °C, 30/18 °C, 33/18 °C, and 36/18 °C) based on in vitro pollen germination, pollen tube length, pollen surface morphology, and pollen wall structure; as a result, CDC Sage was classified as tolerant and CDC Golden as sensitive genotype based on its higher pollen germination and stable lipid composition in pollen than the heat-sensitive genotype at 36 °C (Jiang et al. 2015).
Pollen-based traits were also used to screen 44 soybean genotypes for heat tolerance at 38/30 °C (Salem et al. 2007). The total stress response index based on reproductive traits such as pollen germination and pollen tube length was used to categorize the genotypes. Three of these genotypes, heat tolerant (DG 5630RR), heat intermediate (PI 471938), and heat sensitive (Stewart III), were selected for pollen grain morphology; the heat-sensitive genotype had deformed pollen with reduced aperture. Based on the studies mentioned above, pollen grain structure and function could be used as a screening tool for heat tolerance in soybean (Salem et al. 2007).
2.6 Leaf-Based Parameters
2.6.1 Stomatal Conductance
Stomatal conductance is a measure of stomatal opening or the rate of passage of CO2 entering and water vapor releasing through leaf stomata. Stomatal conductance is affected by many environmental factors, including high temperature. Stomatal conductance increases with increasing temperature to increase photosynthesis, which can help plants endure short heat waves (Urban et al. 2017). Moreover, plants acclimatize to high temperatures by evaporating more water, keeping their canopies cool despite the presence of fewer stomata (Crawford et al. 2012). Therefore, regulating stomatal conductance under high temperatures is a useful trait for screening contrasting genotypes. Stomatal conductance can be recorded with a leaf porometer and expressed in mmol m−2 s−1 (Priya et al. 2018). Heat-tolerant chickpea genotypes (ICC15614, ICCV92944) had higher stomatal conductance (265–271 mmol H2O m−2 s−1) than heat-sensitive genotypes (ICC5912, ICC10685; 187–210 mmol H2O m−2 s−1) under high temperatures (˃32/20 °C) imposed by late sowing (Kaushal et al. 2013). Similarly, for late-sown mung bean genotypes, the heat-tolerant genotype (SML 868) had higher stomatal conductance (99 mmol m−2 s−1) than the heat-sensitive genotype (SML 668; 90 mmol m−2 s−1) (Kaur et al. 2015). In another study, five common bean genotypes (SB761, SB776, SB781, Jaguar, TB1) were screened in the greenhouse at three temperature regimes (35/30 °C, 40/35 °C, 45/40 °C); stomatal conductance in all genotypes increased with increasing temperature until 40/35 °C but declined at 45/40 °C except in genotype TB1, which was identified as heat tolerant (Traub et al. 2018). Similarly, Sita et al. (2017) identified heat-tolerant (IG2507, IG3263, IG3745, IG4258, FLIP2009) and heat-sensitive (IG2821, IG2849, IG4242, IG3973, IG3964) lentil genotypes based on stomatal conductance—the heat-tolerant genotypes had higher stomatal conductance values (390–497 mmol m−2 s−1) than heat-sensitive genotype (205–313 mmol m−2 s−1) in a late-sown environment.
2.6.2 Stay-Green Trait
Heat stress negatively affects photosynthesis by decreasing leaf pigment content and damaging leaf ultrastructure in heat-sensitive genotypes. Chloroplasts play a vital role in photosynthesis as one of the most heat-sensitive organelles (Abdelmageed and Gruda 2009; Krause and Santarius 1975). Decreased total chlorophyll content and changes in the chlorophyll a/b ratio have been correlated with reduced photosynthesis during heat stress due to reduced “antenna (pigment unit)” size that reduces light harvesting (Blum 1986; Harding et al. 1990; Shanmugam et al. 2013). Chlorophyll retention (chlorophyll content) is an integrative trait and is considered a good criterion for screening heat-stress tolerance in legume crops. For example, high-temperature (38/28 °C) stress for 14 days at the flowering stage in a growth chamber caused anatomical and structural changes, including damaged plasma membrane, chloroplast membrane, and thylakoid membranes and reduced leaf photosynthetic rate, in the leaves of soybean genotype K 03-2897. Plant chlorophyll maintenance, also known as the stay-green (SGR) trait, is affected by high temperature. Understanding the physiological and molecular mechanisms of the stay-green trait is important for controlling photosynthetic ability (Abdelrahman et al. 2017). The SGR trait, or delayed leaf senescence (DLS), allows plants to retain leaves in an active photosynthetic state under high temperatures to maintain assimilation and increase crop yield (Gregersen et al. 2013; Kumari et al. 2013). Stay-green genotypes can carry out photosynthesis for longer than senescent types, often with yield benefits (Borrell et al. 2014). The development of contrasting F6 and F7 recombinant-inbred lines of cowpea for the DLS trait under heat stress revealed that the DLS trait increased plant survival and seed size under heat stress (Ismail et al. 2000). Of ten common bean genotypes, only BRS Expedito, FT-Taruma, and BAF071 had the stay-green trait, with higher initial chlorophyll a contents, less chlorophyll degradation, and higher grain yields under heat stress than the other genotypes (Schmit et al. 2019).
A field experiment screening 58 chickpea genotypes for high-temperature tolerance (25–40 °C) during the reproductive phase identified eight genotypes—Pusa 1103, Pusa 1003, KWR 108, BGM 408, BG 240, PG 95333, JG 14, and BG 1077—as heat tolerant, with higher chlorophyll contents than the heat-sensitive genotypes (ICC1882, PUSA 332, PUSA 112, RSG 803) (Kumar et al. 2017). Two heat-tolerant chickpea genotypes (ICC1356, ICC15614) maintained higher chlorophyll contents under heat stress (>32 °C/20 °C) in the field than two heat-sensitive genotypes (ICC4567, ICC5912) (Awasthi et al. 2017). In another study, chickpea genotypes were grown in the greenhouse to flowering (42 and 46 DAS) and then in a growth chamber under increasing temperatures (by 2 °C per day from 27/18 °C to 42/25 °C; day/night) for 8 days (anthesis), which revealed that genotype JG14 (heat tolerant) had higher total leaf chlorophyll content than genotype ICC16374 (heat sensitive) (Parankusam et al. 2017). Similarly, heat-tolerant chickpea genotypes Pusa-1103 and BGD-72 had significantly higher chlorophyll contents than heat-sensitive genotypes Pusa-256 and RSG-991 under high temperatures (25/35 °C) in wooden polyethylene chambers (Singh et al. 2018). Likewise, Kaushal et al. (2013) identified two heat-tolerant (ICC15614, ICCV92944) and two heat-sensitive (ICC10685, ICC5912) chickpea genotypes based on the chlorophyll content, after exposure to heat stress (>32/20 °C) in the field during reproductive development. A field study on lentils measured the stay-green trait as the loss of total chlorophyll (Chl) in leaves under high temperature (>32/20 °C) during the reproductive phase; heat-stressed plants had lower total chlorophyll concentrations than the control plants, and the heat-tolerant genotype (IG3263) retained more Chl than the heat-sensitive genotype (IG4242) (Sita et al. 2017). Similarly, lentil genotypes LL699 and LL931 (heat tolerant) retained more chlorophyll than genotype LL1122 (heat sensitive) in outdoor conditions (>32/23 °C), which was confirmed in a controlled environment with plants subjected to 33/15 °C or 35/20 °C during reproductive growth (Bhandari et al. 2016). Heat stress in the field (>30/20 °C) during reproductive growth and seed filling revealed two lentil heat-tolerant genotypes (1G 2507 and 1G 4258) with high leaf chlorophyll concentrations and two heat-sensitive genotypes (1G 3973 and 1G 3964) with lower chlorophyll concentrations (Sehgal et al. 2017). In another study, common bean genotypes exposed to 32/25 °C at the V4 developmental stage identified two genotypes (Sacramento and NY-105) with high chlorophyll contents, indicating their high thermotolerance, relative to the thermosensitive genotype Redhawk with low chlorophyll content (Soltani et al. 2019). Likewise, in a heat-sensitive mung bean genotype (SML668), chlorophyll content declined, relative to the heat-tolerant genotype (SML832), grown under heat stress (43/30 °C and 45/32 °C) in outdoor late-sown conditions, contributing to an increase in leaf temperature (Kaur et al. 2015). Mung bean genotypes EC693357, EC693358, EC693369, Harsha, and ML 1299 produced more chlorophyll content under heat stress than genotypes EC693363, EC693361, KPS1, EC693370, and IPM02-3 (Sharma et al. 2016). Screening of ten faba bean genotypes for heat-stress tolerance (37 °C) revealed that genotype C5 tolerated high temperature by retaining more chlorophyll, while genotype Espan had less chlorophyll and was relatively more sensitive to heat stress (Siddiqui et al. 2015). In a recent study, 4-week-old seedlings of 15 alfalfa cultivars were exposed to heat treatment (38/35 °C) for 7 days in a growth incubator; genotypes Gibraltar, WL354HQ, Golden Queen, Siriver, WL712, and Sanditi had significantly lower Chl contents (heat sensitive) than genotypes Bara310SC, WL363HQ, WL656HQ, and Magna995 (heat tolerant) (Wassie et al. 2019).
2.6.3 Chlorophyll Fluorescence
Chlorophyll (Chl) fluorescence (Fv/Fm ratio) is used as an indicator of functional changes in photosynthetic apparatus under abiotic or biotic stress (Yamada et al. 1996). The relationships between essential photosynthetic responses and chlorophyll fluorescence are pivotal as they provide information on the plant’s photosynthetic ability and acclimation limit under stress conditions (Kalaji et al. 2018; Lichtenthaler 1987). Chlorophyll fluorescence is a fast, nondestructive, and effective common tool for determining heat-stress responses as it can reveal damage before visible stress symptoms appear (Baker 2008; Méthy et al. 1994; Wilson and Greaves 1990). Of the photosynthetic apparatus, photosystem II (PSII) is the most heat-labile cell structure (Vacha et al. 2007). Since damage to PSII is often the first response of plants subjected to thermal stress (Mathur et al. 2011), measuring chlorophyll a fluorescence is an effective and noninvasive technique for identifying damage to PSII efficiency (Baker 2008; Baker and Rosenqvist 2004). The ratio between variable fluorescence (Fv) and maximum fluorescence (Fm), or Fv/Fm, reflects the maximum quantum efficiency of PSII (Butler 1978). When plants are exposed to abiotic stress, including thermal stress, Fv/Fm often declines (Molina-Bravo et al. 2011; Sharma et al. 2012; Willits and Peet 2001). Screening methodologies have used chlorophyll fluorescence to detect and quantify damage in PSII and thylakoid membranes in several legume crops under heat stress, including chickpea, groundnut, pigeon pea, and soybean (Herzog and Chai-Arree 2012; Srinivasan et al. 1996). Recent study assessed the response of four chickpea genotypes to a natural temperature gradient during the reproductive stage in the field and a climate chamber using chlorophyll fluorescence. Field experiments were conducted over two winter seasons; two genotypes (Acc#RR-3, Acc#7) showed tolerance (Fv/Fm 0.83–0.85) and two (Acc#2, Acc#8) showed sensitivity (Fv/Fm 0.78–0.80) to heat stress. The field results were validated in the climate chamber experiment, where Fv/Fm declined more in the heat-sensitive (0.74–0.75 at 35/30 °C) than heat-tolerant (0.78–0.81 at 35/30 °C) genotypes when exposed to short-term heat treatments (30/25 °C and 35/30 °C) (Makonya et al. 2019). In another chickpea study, heat stress (>30 °C) in the field during the reproductive stage reduced Fv/Fm more in two heat-sensitive genotypes ICC10685 and ICC5912 (0.48, 0.41) than in two heat-tolerant genotypes ICC15614 and ICCV92944 (0.64, 0.60) (Awasthi et al. 2014; Kaushal et al. 2013). A similar study, where four contrasting chickpea genotypes—two heat tolerant (ICC1356, ICC15614) and two heat sensitive (ICC4567, ICC5912)—were analyzed in the field, revealed that the tolerant genotypes maintained higher chlorophyll fluorescence (Fv/Fm 0.60) on exposure to heat stress (>32/20 °C) than the sensitive genotypes (Fv/Fm 0.50) (Awasthi et al. 2017). In lentils, photosynthetic efficiency was measured as PSII function (Fv/Fm ratio) in the field by exposing plants to heat stress (>32/20 °C) during the reproductive stage. Heat-tolerant genotypes—IG2507, IG3263, IG3297, IG3312, IG3327, IG3546, IG3330, IG3745, IG4258, and FLIP2009—maintained higher chlorophyll fluorescence (Fv/Fm 0.71) under stress than heat-sensitive genotypes IG2821, IG2849, IG4242, IG3973, and IG3964 (Fv/Fm 0.58) (Sita et al. 2017). Similarly, two heat-tolerant lentil genotypes (1G 2507 and 1G 4258) exposed to heat stress (>25 °C) during reproductive growth and seed filling in the field had higher chlorophyll fluorescence (Fv/Fm 0.67) than two heat-sensitive genotypes (1G 3973 and 1G 3964; Fv/Fm 0.57) (Sehgal et al. 2017). Likewise, the screening of 41 mung bean lines grown outdoors and exposed to high temperatures (>40/28 °C) during the reproductive stage revealed several promising heat-tolerant lines (EC693358, EC693357, EC693369, Harsha, ML1299) with high Fv/Fm ratios (0.73–0.75) compared to sensitive lines (0.61–0.67), which could serve as useful donor/s for breeding programs and as a suitable base plant source to gain insight into heat stress-induced effects in cell metabolism (Sharma et al. 2016). Nine common bean lines were evaluated for changes in chlorophyll fluorescence under heat stress during flowering (45 °C for 2 h) in a greenhouse; thermotolerant lines 83201007 and RRR46 had higher Fv/Fm values under heat stress than the heat-sensitive line Secuntsa (Petkova et al. 2009). In another study, 12 varieties and lines of common bean were exposed to 42 °C in the field during the reproductive period; two genotypes (Ranit and Nerine) maintained their Fv/Fm values at 42 °C, relative to the controls at 26 °C, and were considered heat tolerant. These two genotypes also showed good productivity and quality and can be used as parental lines in bean breeding programs (Petkova et al. 2007). Screening of 15 alfalfa genotypes by exposing seedlings to 38/35 °C day/night for 7 days in a growth chamber identified Bara310SC (Fv/Fm 0.79) and WL712 (Fv/Fm <0.79) as heat-tolerant and heat-sensitive cultivars, respectively (Wassie et al. 2019), showing that Fv/Fm is an effective tool for phenotyping contrasting genotypes for heat tolerance.
2.6.4 Photosynthetic Rate
Heat stress affects the stay-green trait, chlorophyll content, and chlorophyll fluorescence, which affects RuBisCo activation, decreasing the photosynthetic rate (Salvucci Michael and Crafts-Brandner 2004; Sharkey 2005). Hence, photosynthetic rate can be used as a screening parameter for selecting heat-tolerant genotypes. Variation in photosynthetic rate among plant species in response to heat stress has been well documented. For example, the response of four chickpea genotypes to a natural temperature gradient in the field at the flowering stage identified two heat-tolerant genotypes (Acc#RR-3, Acc#7) with high Pn and two heat-sensitive genotypes (Acc#2, Acc#8) with lower Pn; these results were validated in a climate chamber experiment set at 30/25 °C and 35/30 °C (Makonya et al. 2019). In another study, 56 chickpea genotypes were exposed to high temperatures in the field from flowering to crop maturity (maximum temperatures 25–40 °C)—the tolerant genotypes (PUSA1103, PUSA1003, KWR108, BGM408, BG240, PG95333, JG14, BG) had higher Pn than the sensitive genotypes (ICC1882, PUSA372, PUSA2024) (Kumar et al. 2017). In a similar study in lentil, two heat-tolerant (1G 2507 and 1G 4258) genotypes had higher photosynthetic rate (Pn) than two heat-sensitive (1G 3973 and 1G 3964) genotypes exposed to heat stress (>25 °C) in the field during reproductive growth and seed filling (Sehgal et al. 2017).
Soybean cultivars IA3023 and KS4694 and PI lines PI393540 and PI588026A expressed heat tolerance and susceptibility with high and low Pn, respectively (Djanaguiraman et al. 2019). The two cultivars had less thylakoid membrane damage than the PI lines. In an earlier study on soybean, genotype K 03-2897, exposed to high temperature (38/28 °C) in a growth chamber for 14 days at the flowering stage, significantly decreased Pn due to anatomical and structural changes (increased thickness of palisade and spongy layers and lower epidermis) in cells and cell organelles, particularly damage to chloroplasts and mitochondria (Djanaguiraman and Prasad 2010).
2.6.5 Sucrose
Leaf photosynthates are transported to sink organs primarily as sucrose, and sucrose synthase (SS) is a key enzyme for sucrose to enter various metabolic pathways (Calderini et al. 2006). Downregulation of SS indirectly inhibits carbohydrate production, eventually reducing yield and quality. Maintaining sucrose levels is vital during stressed conditions, which depend on its synthesis and hydrolysis. Heat-stressed plants had significantly lower activities of key enzymes—sucrose phosphate synthase (SPS) and SS—involved in sucrose synthesis than non-stressed plants. Sucrose availability to reproductive organs is crucial for sustaining their function (Kaushal et al. 2013). Heat-tolerant genotypes can stabilize the photosynthetic process better than heat-sensitive genotypes. Heat stress disturbs sucrose production in leaves and impairs its transportation to reproductive organs (Kaushal et al. 2013; Li et al. 2012). Limitations in sucrose supply to reproductive organs, particularly under thermal stress, restrict flower development and function and pod and seed filling, reducing crop yield (Kaushal et al. 2013; Li et al. 2012). Measuring sucrose concentrations reveals the photosynthetic status of plants under heat stress (Awasthi et al. 2014). Sucrose synthase is strongly associated with heat tolerance in chickpea; heat-sensitive genotypes produced far less leaf sucrose than heat-tolerant genotypes, which impaired its supply to developing reproductive organs (flowers, pods, and seeds) in chickpea (Kaushal et al. 2013). Screening a large core collection of chickpea genotypes for heat tolerance (32/20 °C) in field condition identified two heat-tolerant (ICC15614, ICCV92944) and two heat-sensitive (ICC10685, ICC5912) genotypes. The heat-sensitive genotypes had significantly greater inhibition of RuBisCo (carbon-fixing enzyme), SPS, and SS than the heat-tolerant genotypes and thus produced less sucrose than the tolerant genotypes (Kaushal et al. 2013). Heat-sensitive (ICC16374) and heat-tolerant (JG14) chickpea genotypes exposed to gradually increasing temperatures (2 °C per day from 27/18 °C to 42/25 °C; day/night) for 8 days at anthesis in a growth chamber revealed greater sucrose synthase expression in JG14 than ICC16374 (Parankusam et al. 2017). Two tolerant chickpea genotypes (Acc#7 and Acc#RR-3) had higher starch contents and were relatively unaffected by heat-stress exposure compared to two heat-sensitive genotypes (Acc#2, Acc#8) at high temperature (35/30 °C) in a control chamber (Makonya et al. 2019). Therefore, an increased abundance of sucrose synthase in the tolerant genotype reasserted its potential role during heat-stress tolerance; this may ensure successful fertilization due to sustained pollen viability under heat stress, enhancing pod set and yield, as reported earlier for the tolerant genotype (ICC15614) (Krishnamurthy et al. 2011).
In lentil, sucrose production is vital for leaf and anther function and has been correlated with SPS activity in natural high-temperature environments (>32/20 °C). Heat-tolerant lentil genotypes (IG2507, IG3263, IG3297, IG3312, IG3327, IG3546, IG3330, IG3745, IG4258, FLIP2009) produced more sucrose in leaves (65–73%) and anthers (35–78%) than heat-sensitive genotypes (IG2821, IG2849, IG4242, IG3973, IG3964), which was associated with superior reproductive function and nodulation in tolerant genotypes (Sita et al. 2017). Limitations in sucrose supply may disrupt the development and function of reproductive organs (Prasad and Djanaguiraman 2011; Snider et al. 2011). In a similar study, two heat-tolerant (1G 2507 and 1G 4258) lentil genotypes exposed to heat stress (>25 °C) in the field had higher SS activity and thus higher sucrose contents in leaves and seeds than two heat-sensitive (1G 3973 and 1G 3964) genotypes (Sehgal et al. 2017). Thus, sucrose synthase in seeds and leaves is strongly correlated with seed yield; therefore, reductions in seed size and weight are attributed mainly to reductions in sucrose content.
Mung bean genotypes tested under heat stress (>40/25 °C day/night) during flowering and podding outdoors and in a controlled environment showed that two heat-tolerant genotypes (SML832 and SML668) had more sucrose than the heat-susceptible genotype (SML832). Thus, sucrose concentrations in leaves and anthers and SS and SPS activities declined significantly in sensitive genotypes under heat stress (Kaur et al. 2015). Exposure of common bean genotypes at the V4 developmental stage to heat treatment (32/25 °C) in a growth chamber significantly reduced leaf sucrose concentration in genotype Redhawk (most heat-sensitive genotype) and increased sugar contents in Sacramento (58%) and NY-105 (most heat tolerant) (Soltani et al. 2019).
2.6.6 Cell Membrane Thermostability
Under heat stress, protein denaturation, lipid liquefaction, and loss of membrane integrity are some of the chief physiological, biochemical, and molecular changes in plant metabolism (Gulen and Eris 2004). Most of the changes that appear during acclimation to heat stress are reversible, but death can occur if the stress is too intense (Saelim and Zwiazek 2000). Cell membranes are the principal target of environmental stresses, including heat stress (Chen et al. 2014; Sita et al. 2017). Protein denaturation and increased membrane fluidity, enzyme inactivation, decreased protein synthesis, protein degradation, and alterations in membrane integrity are documented injuries under heat stress (Howarth 2005). By accelerating the kinetic energy and movement of molecules across membranes, heat stress releases chemical bonds within the molecules of biological membranes, resulting in membrane fluidity by protein denaturation or increased unsaturated fatty acids (Savchenko et al. 2002). Decreased cell membrane thermostability or increased ionic leakage caused by the alteration of membrane protein structure is an important indicator of heat stress. The increased membrane fluidity caused by protein denaturation and increased unsaturated fatty acids in the membrane under high temperatures affect membrane structure and function (Wahid et al. 2007), causing symptoms, such as photooxidation of chlorophyll pigments, impaired electron flow, inhibited carbon fixation, and water loss from leaves (Prasad et al. 2017; Sharifi et al. 2012; Sita et al. 2017). The relationship between cell membrane thermostability (CMT) and crop yield changes from plant to plant under high temperatures. Ion leakage from plant tissues has been used as a membrane damage indicator in plants exposed to heat stress. Thus, CMT is an indirect indicator of heat-stress tolerance in legumes, such as soybean (Martineau et al. 1979), lentil (Sita et al. 2017), chickpea (Kaushal et al. 2013), and mung bean (Sharma et al. 2016). Membrane damage occurs under heat and cold stress, more so under heat stress, as reported for Medicago (Mo et al. 2011). Cell membrane thermostability (CMT) tends to decline during the late developmental phase of plants (Ahmad and Prasad 2011).
In addition to conventional breeding techniques, noticeable variations in membrane thermostability among genotypes, combined with biochemical and physiological screening methods, could be used to improve the selection for breeding objectives (Hemantaranjan et al. 2014). Membrane thermostability has been used to assess thermotolerance in many food crops worldwide. Depending on the growing season, electrolyte leakage in plants varies among tissues, organs, and growth stages and is affected by plant/tissue age, sampling organ, developmental stage, growing season, degree of hardening, and plant species. A significant positive relationship between CMT and yield was reported in sorghum (Sullivan and Ross 1979). In crop plants such as barley (Hordeum vulgare L.), cotton (Gossypium spp.), sorghum, and cowpea, increased electrolyte leakage decreased membrane thermostability (Wahid et al. 2007; Wahid and Shabbir 2005). In leguminous crops, electrolyte leakage has been used to assess thermotolerance. For example, heat stress at 34 °C in lentil revealed genotypes Ranjan, Moitree, 14-4-1, IC201710, and IC208329 as heat tolerant and genotypes ICC201655, ICC201661, ICC201662, ICC201670, ICC201675, ICC201681, ICC201698, ICC201743, ICC201794, ICC248959, Asha, Sagardeep Local, and UP local as heat sensitive, based on cell membrane stability in field and growth chamber studies (Choudhury et al. 2012). In another study, lentil genotypes exposed to high temperature (45 °C) at the flowering stage revealed Qazvin and B4400 as heat-tolerant and -sensitive genotypes, with 98.13% and 33.19% CMT, respectively (Barghi et al. 2013). At 38/28 °C and 40/30 °C in a controlled environment, heat-tolerant lentil genotypes IG2507, IG3263, IG3745, IG4258, and FLIP2009 had less membrane damage (<20% electrolyte leakage) than heat-sensitive genotypes IG2821, IG2849, IG4242, IG3973, and IG3964 (>30%) (Sita et al. 2017).
Among various legumes (pigeon pea, peanut, chickpeas, and soybean), chickpea was the most sensitive to high temperature based on CMT (Devasirvatham et al. 2012). Heat-tolerant chickpea genotypes ICCV07110 and ICCV92944 had less membrane damage (22.6% and 20.6%) than heat-sensitive genotypes ICC14183 and ICC5912 (30.4% and 33.3%) under high temperatures of 40/30 °C and 45/35 °C (Kumar et al. 2013). In another study, high temperature (>32/20 °C) during the reproductive stage caused the most membrane damage in heat-sensitive chickpea genotypes ICC10685 (28.3%) and ICC5912 (26.3%) and the least membrane damage in heat-tolerant genotypes ICC15614 (17.3%) and ICCV 92944 (19.6%) (Kaushal et al. 2013). A gradual rise in temperature (42/25 °C) at anthesis for 8 days increased electrolyte leakage (EL) by 20–25% greater in heat-sensitive chickpea genotype ICC16374 compared to heat-tolerant genotype ICCV92944 (Parankusam et al. 2017). At 37/27 °C, electrolyte leakage increased by a maximum of 16–25% in chickpea genotypes (Pareek et al. 2019), with ICC1205 identified as heat tolerant (13–14%). Similarly, Dua et al. (2001) reported ICCV88, ICC512, and ICC513 as heat-tolerant chickpea genotypes under heat stress. Another study on six chickpea genotypes revealed DG36 (EL: 36.7%) and Pusa 372 (EL: 50.7%) as heat-tolerant and heat-sensitive genotypes, respectively, when exposed to high temperature (>38 °C) under field conditions, based on EL (Singh et al. 2004). Of 115 chickpea genotypes screened at high temperature (36.5 °C) in the field, GNG 663 and Pusa 244 were selected as heat tolerant and heat sensitive, with electrolyte leakage values of 23% and 50%, respectively (Kumar et al. 2012). Among 30 chickpea genotypes screened for heat tolerance (>30 °C), Pusa 240 and GG2 genotypes were identified as heat-tolerant and -sensitive genotypes, respectively, with minimum (45%) and maximum (69%) cell membrane injury (Kumar et al. 2013).
Screening of nine cowpea genotypes exposed to heat stress (33/20 °C) during flowering and pod revealed less leaf electrolyte leakage in heat-tolerant genotypes H36, H8-9, and DLS99 (35.8–36.7%) than heat-susceptible genotypes CB5, CB3, and DLS127 (66.2–79.0%) (Ismail and Hall 1999). In another study at high temperature (38/30 °C), cell membrane injury was negatively corelated with yield in heat-tolerant (CB 27, Prima, UCR 193) and heat-sensitive genotypes (CB 5, CB 46) (Singh et al. 2010), with less membrane damage in heat-tolerant genotypes.
Screening of 15 Medicago cultivars at high temperature (38/35 °C) using membrane damage revealed “Bara310SC” and “WL712” as heat-tolerant and heat-sensitive genotypes with 24.07% and 53.2% electrolyte leakage, respectively (Wassie et al. 2019). Similarly, screening studies on 116 green gram genotypes at high temperature (45/25 °C) identified EC 3398889 and LGG460 as heat tolerant and heat sensitive, with minimum and maximum cell membrane damage, respectively (Basu et al. 2019). Gradual exposure to high temperature (35–50 °C) of 4-week-old three common bean genotype seedlings in a growth chamber revealed “local genotype” and “Ferasetsiz” as heat-sensitive genotypes, while “Balkız” was a relatively heat-sensitive genotype (Tokyol and Turhan 2019). Gross and Kigel (1994) used electrolyte leakage as a criterion for assessing heat tolerance at 32/28 °C during the reproductive stage and reported PI 271998 and BBL 47 as heat-tolerant and heat-sensitive genotypes in common bean, respectively. High-temperature studies (>40/28 °C) at the reproductive stage in mung bean showed high electrolyte leakage (21.8–23.6%) in heat-sensitive lines (EC 693363, EC 693361, EC 693370, KPS1, IPM02-3) compared to heat-tolerant lines (16.8–20.4%; EC693357, EC693358, EC693369, Harsha, ML1299) (Sharma et al. 2016). Another study on mung bean at high temperature (>35 °C) identified genotype MH 421 as heat tolerant and Basanti as heat sensitive, with low (34.88%) and high (41.34%) electrolyte leakage, respectively (Jha et al. 2015). Screening of ten faba bean genotypes exposed to heat stress (37 °C) 60 days after sowing revealed C5 as heat tolerant and Espan as heat sensitive, based on low (57.67%) and high (76%) membrane damage, respectively (Siddiqui et al. 2015).
2.6.7 Canopy Temperature Depression
Canopy temperature depression (CTD) is the plant canopy temperature deviation from the ambient temperature (Balota et al. 2007). At the whole-crop level, leaf temperature decreases below air temperature when water evaporates. CTD acts as an indirect measure of transpiration (Reynolds et al. 2001) and plant water status (Araus et al. 2003) and indicates the relative metabolic fitness of genotypes in a given environment (Reynolds 1997). CTD is a key trait for assessing the response of genotypes to low water usage, high temperature, and other stresses (Balota et al. 2007). At high temperatures, transpiration increases for some time, with plants using more water during growth due to more open stomata and lower CTD. A positive CTD value [i.e., difference between air temperature (Ta) and canopy temperature (Tc)] occurs when the canopy is cooler than the air (CTD = Ta − Tc) (Balota et al. 2008).
Canopy temperature depression is heritable and can be measured on cloudless days using an infrared thermometer (Reynolds et al. 1997). To maintain canopy temperature at a metabolically comfortable range, plants transpire through open stomata. Plants close stomata during stress acclimation, increasing the canopy temperature (Kashiwagi et al. 2008). Canopy temperature can be affected by biological and environmental factors, such as soil water status, wind, evapotranspiration, cloudiness, conduction systems, plant metabolism, air temperature, relative humidity, and continuous radiations (Reynolds et al. 2001). Canopy temperature is an indicator of plant water status or the equilibrium between root water uptake and shoot transpiration (Berger et al. 2010). CTD can act as a desirable criterion for selecting heat-tolerant genotypes based on phenotypic variation (Mason and Singh 2014). It can be used to determine yield potential and metabolic fitness of crop plants under specific environmental conditions (Kumari et al. 2013). It acts as a mechanism of heat escape and is strongly correlated with yield (Reynolds et al. 2001); affected by many physiological factors, it is a strong trait for determining genotype fitness.
Epicuticular leaf wax QTL and CTD are strongly interlinked, with wax load affecting plant canopy temperature (Awika et al. 2017). Stay-green genotypes have high CTD values and thus low canopy temperature due to transpirational cooling under heat stress (Fischer et al. 1998; Reynolds et al. 1994). In chickpea, CTD is negatively correlated with water potential, osmotic pressure, relative leaf water content, and seed yield (Sharma et al. 2015). Differences in canopy temperature are not detectable in high-humidity environments because the effect of evaporative leaf cooling is negligible (de Souza et al. 2012). CTD has been successfully used to select for heat tolerance in various crop species, including legumes. For example, heat-tolerant chickpea genotypes ICCVs 95311, 98902, 07109, and 92944 had higher CTD values than sensitive genotypes ICCVs 07116, 07117, and 14592, which had negative CTD values (Devasirvatham et al. 2015). Another study screened 30 chickpea genotypes exposed to temperature >30 °C to reveal Pusa 240 as a heat-tolerant genotype due to its cooler canopy than other genotypes (Kumar et al. 2013). Similarly, screening chickpea genotypes subjected to 36.5 °C identified GNG 663 and Vaibhavaas as heat tolerant and heat sensitive, respectively, with CTD values of 4.8 °C (maximum) and 1.8 °C (minimum) (Kumar et al. 2012). In a screening study of 56 chickpea genotypes for heat tolerance (40 °C), CTD values ranged from 5.0 to 7.5 °C; eight genotypes (Pusa 1103, Pusa 1003, KWR 108, BGM 408, BG 240, PG 95333, JG 14, BG 1077) were identified as heat tolerant, with maximum CTD values compared to other genotypes (Kumar et al. 2017). In mung bean, seed yield positively correlated with CTD, while canopy temperature negatively correlated with root traits, such as the number of lateral branches and dry root weight (Raina et al. 2019). In another study, mung bean genotype MH 421 (CTD 5.78 °C) was selected as heat tolerant compared to Basanti (CTD 4.37 °C) when tested at high temperature (>35 °C) (Jha et al. 2015). In pea, CTD is affected by canopy structure, and increased pod number and pod-to-node ratio associated with CTD (Tafesse et al. 2019).
2.7 Biochemical Traits
2.7.1 Oxidative Stress and Antioxidants
Heat stress is a major environmental factor affecting vital metabolic processes in plants, hampering proper growth and development. Disturbances in these metabolic processes lead to ROS generation, such as hydrogen peroxide, hydroxyl radicals, and superoxides (Chakraborty and Pradhan 2011). ROS production damages cellular activity by inactivating enzymes, denaturing proteins, and damaging membranes and DNA. Plants shield such injuries by activating cascades of enzymatic activities, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR), and nonenzymatic activities, such as glutathione (GSH) and ascorbic acid (ASC) (Suzuki et al. 2012). The selection of contrasting genotypes based on the expression level of these antioxidants is effective in leguminous plants (Kumar et al. 2013). For example, chickpea genotypes raised under natural conditions until 50% flowering and then in a growth chamber for heat treatment (30/20 °C, 35/25 °C, 40/30 °C, and 45/35 °C) revealed that heat-tolerant genotypes (ICCV92944, ICCV07110) had lower H2O2 and MDA concentrations than sensitive genotypes (ICC5912, ICC14183). Tolerant genotypes face fewer injuries due to greater expression of antioxidants, such as APX and GR (Kumar et al. 2013). Similarly, 41 mung bean genotypes were screened, and contrasting genotypes were selected based on oxidative stress damage and antioxidant activity. Heat-tolerant genotypes (EC693357, EC693358, EC693369, Harsha, ML1299) experienced less oxidative damage (1.52–2.0-fold increase in MDA; 1.59–1.96-fold increase in H2O2) than sensitive genotypes (2.2–2.4-fold increase in MDA; 2.21–2.93-fold increase in H2O2) (Sharma et al. 2016). Moreover, heat-tolerant genotypes increased APX activity (by 1.48–1.77-fold) more than sensitive genotypes (1.27–1.37-fold). Likewise, of 38 lentil genotypes screened for heat tolerance (˃35/20 °C) during the reproductive phase, heat-tolerant genotypes (IG2507, IG3263, IG3745, IG4258, FLIP2009) had less oxidative damage (MDA and H2O2 contents increased) and higher SOD, CAT, APX, and GR activities than heat-sensitive genotypes (IG2821, IG2849, IG4242, IG3973, IG3964) (Sita et al. 2017). In another study on lentil exposed to heat stress (30, 35, 40, 45, and 50 °C for 4 h) in plant growth chambers, SOD, CAT, and APOX activities initially increased in four heat-tolerant lentil varieties (IPL 81, IPL 406, Asha, Subrata) at 35, 40, and 45 °C but decreased at 50 °C, and decreased in heat-sensitive genotypes (Sehore and Lv) at all temperatures, except 30 °C (Chakraborty and Pradhan 2011). Further accumulation of carotenoids and ascorbate followed a similar trend, indicating the association of heat sensitivity with antioxidant expression.
2.7.2 Metabolites
Metabolite detection and quantification are an effective and powerful tool for selecting genotypes in response to environmental stresses (Bueno and Lopes 2020). Metabolites include low-molecular-weight compounds, including precursors and intermediate metabolic pathways, which are an indispensable part of plant metabolism, regulating vital biological processes and involved in stress tolerance (Wahid et al. 2007). The primary metabolites upregulated during abiotic stress are amino acids (proline), carbohydrates (sucrose, hexoses, polyhydric alcohols), polyamines (spermidine, spermine, putrescine), and glycine betaine. Correspondingly, secondary metabolites include terpenoids (saponins, tocopherols), phenolic compounds (flavonoids, isoflavonoids, anthocyanins), and nitrogen-containing metabolites (alkaloids and glucosinolates) (Rodziewicz et al. 2014). About one million specific metabolites varying in chemical structures, polarity, and physiochemical properties are present in the plant kingdom and can be analyzed through metabolomics profiling and metabolic fingerprinting. Due to heat stress, plants reshuffle their metabolites to sustain plant growth (Serrano et al. 2019). Metabolite production is regulated by genes; thus, the activation of heat-shock factors, mainly HSFA2 and HSFA3, increases metabolite content, such as galactinol (Song et al. 2016). Knowledge on metabolite production is important for developing metabolite markers to select heat-tolerant varieties.
Chebrolu et al. (2016) raised heat-tolerant (04025-1-1-4-1-1) and heat-sensitive (DT97-4290) soybean genotypes in a growth chamber, which were maintained under control conditions (28/22 °C) until flowering. Heat stress [moderate (36/24 °C) and severe (42/26 °C)] was imposed from flowering to maturity, with metabolite profiling undertaken on harvested seeds. The seeds of genotypes collected at 42/26 °C were highly abnormal and small and had high nitrogen levels compared with the sensitive genotype. Two hundred and seventy-five metabolites were traced and compared for 36/24 °C and 28/22 °C; 83 metabolites (48 downregulated and 35 upregulated were differentially altered in tolerant than sensitive genotypes) significantly differed between genotypes at 36/24 °C, compared to 61 metabolites (−30 and +31 in tolerant than sensitive genotypes) at 28/22 °C. Most traced compounds were antioxidants belonging to tocopherol, terpenoid, and flavonoid precursors. The tolerant genotype had more gulono-1,4-lactones (precursor for ascorbic acid) than the sensitive genotype, which was attributed to its higher tolerance to heat stress and positively correlated with seed vigor, seed germination, seed weight, and oil content.
Proline is a multifunctional amino acid involved in plant growth and development that acts as a compatible osmolyte and ROS scavenger to regulate plant function in stressed environments (Szepesi and Szőllősi 2018). Under stress, proline has diverse roles, such as stabilizing membranes, proteins, subcellular structures, and energy sources, thus maintaining cellular homeostasis. Therefore, an increase in compatible solutes such as proline under stressful conditions is valuable for plants (Kaur and Asthir 2015). Leaf proline concentrations were measured in four chickpea genotypes varying in their sensitivity to high temperature (4.5 °C higher than the ambient temperature for 15 days); heat-treated genotypes had significant higher proline concentrations than the control, more so in Pusa 1103 and BGD-72 (tolerant genotypes) than Pusa 256 and Pusa 261 (sensitive genotypes) (Arunkumar et al. 2012). Similarly, a high-temperature treatment (45 °C for 8 h) on 6-day-old common bean seedlings increased proline content compared to control plants (25 °C) (Babu and Devaraj 2008).
2.7.3 Heat-Shock Proteins
Heat-shock proteins are specific proteins accumulated during rapid heat stress. Heat-shock genes are upregulated for plant survival under heat stress and responsible for encoding HSPs (Chang et al. 2007). A sudden change in temperature increases HSP production (Wahid et al. 2007). In all organisms, HSP expression is a general response to high temperature (Vierling 1991). HSP90, HSP70, and low-molecular-weight proteins are three classes of proteins according to molecular weight. Under stress conditions, HSPs perform chaperone-like functions in protein synthesis, maturation, targeting, renaturation, and membrane stabilization (Reddy et al. 2010, 2016). HSPs also play a role in protein translation and translocation, perform proteolysis and protein folding, and reactivate denatured proteins (Zhang et al. 2005). Under heat stress, the expression of HSPs protects the machinery of protein biosynthesis (Miroshnichenko et al. 2005). Membrane lipid composition, membrane integrity osmoprotectants, and HSPs play important roles in heat tolerance (Blum 2018). HSPs are located mainly in the cytoplasm, nucleus, mitochondria, chloroplast, and endoplasmic reticulum (Waters et al. 1996). In plant species such as potato, maize, soybean, and barley, specific HSPs have been identified in mitochondria in response to high temperature (Neumann et al. 1994). HSPs maintain membrane stability and protect PSII from oxidative stress (Barua et al. 2003). In Medicago truncatula, the role of HSPs was determined by cloning and characterization (Li et al. 2016). The roots of some plants also synthesize HSPs to cope with heat stress (Nieto-Sotelo et al. 2002). The expression profiles of HSPs have been compared in plant species/genotypes contrasting in heat sensitivity. In a comparative study on cowpea and eight common bean varieties at 40 °C, cowpea showed more HSP expression than common bean and was thus more tolerant to high temperature. IPA 7 had the highest HSP expression of the eight common bean genotypes (Simões-Araújo et al. 2003).
In chickpea exposed to high temperature (42/25 °C) at anthesis, the levels of HSPs increased in genotype JG14 compared to ICC16374 (Parankusam et al. 2017). In another study, five chickpea genotypes were assessed for thermotolerance at 30, 35, and 40 °C, with CSJD 884 and RSG 895 identified as heat tolerant and C 235 as heat sensitive (Kumari et al. 2018). In peanut genotypes exposed to 50 °C for 30 min, ICGS 76, COC038, COC050, COC041, and COC068 were identified as heat tolerant and COC812, COC166, COC115, COC277, COC227, Tamrun OL 02, and Spanco as heat sensitive (Selvaraj et al. 2011). Heat-tolerant peanut genotype ICGS 44 had higher HSP expression than heat-sensitive genotypes AK 159 and DRG 1 under heat stress (45 °C) (Chakraborty et al. 2018). The level of thermotolerance positively correlated with HSP accumulation. Thirty varieties of pea seedlings exposed to high temperature (46–49 °C) in growth chambers for different time intervals (1–3 h) identified Acc#623 and Acc#476 as heat-tolerant and heat-sensitive varieties, respectively, with Acc#623 having higher levels of HSP70, HSP90, and HSP104 than Acc#476 (Srikanthbabu et al. 2002). In soybean under 38/30 °C, cultivar PI 471938 had higher HSP expression (especially HSP70), conferring heat tolerance, than R95-1705 (Katam et al. 2020).
2.8 Genes for Heat Tolerance
Diverse genes have been identified using omics analyses (transcriptomics, genomics, and proteomics) in various plant species for heat resilience mechanisms; these genes are essential for developing stable cultivars (Singh et al. 2019). A lentil population was developed by crossing heat-tolerant (PDL-1 and PDL-2) and heat-sensitive (JL-3 and E-153) genotypes for molecular mapping and genetics studies (Singh et al. 2017). For this purpose, simple sequence repeat (SSR) marker analysis and QTL analysis were performed, using 495 SSR markers, which detected seven SSR markers and two QTLs—qHt_ss and qHt_ps were closely linked with SSR markers (PBA_LC_1507, PLC_105, PBA_LC_1288, LC_03, PBA_LC_1684, PBA_LC_1752, PBA_LC_1480). Further, SSR marker PBA_LC_1507 was closely linked to pod set and seedling survival trait. Another lentil study revealed genetic diversity for heat tolerance among 119 genotypes using SSR markers (Zhang et al. 2005). High-temperature stress was applied at the seedling (35/33 °C) and anthesis (35/20 °C) stages to study the effects on morphophysiological and reproductive traits of non-stressed and stressed plants in the field. A set of 209 alleles were identified using 35 SSR markers. Genotypes were clustered into nine groups based on SSR markers. Clusters 1 and 6 had significant variation, which could help produce better segregants for heat tolerance. The genotypes in clusters 2, 3, 4, 5, 7, 8, and 9 were moderately tolerant or moderately sensitive to heat stress. Significant differences among clusters were observed for seedling survivability, heat tolerance scores, membrane stability index, pollen viability, pollen germination, pod and seed set, and seed yield. The finding suggests that identifying the genetic distances between clusters will maximize their use for breeding heat-tolerant lentils. Results from the RT-PCR confirmed differential gene expression in heat-sensitive fescue genotype PI283316 and heat-tolerant genotype PI297901 (Zhang et al. 2005).
Similarly, in chickpea, phenotyping of RILs developed from a cross between ICC4567 (heat-sensitive) and ICC156614 (heat-tolerant) genotypes exhibited two genomic regions (CaLG05 and CaLG06) with four QTLs for the number of filled pods, seed number, grain yield, and pod set. Further, 25 genes responsible for heat tolerance were reported in these two genomic regions—five encoding HSPs and heat-shock transcription factors, three responsible for detoxifying ROS, five encoding proteins like farnesylated protein 6 and ethylene-responsive transcription factors, and all these genes collectively upregulating other genes like MYB4, AKH3, and RAN1 that are involved in the mitigation of heat stress in chickpea (Paul et al. 2018). Molecular characterization in mung bean genotype VC1973A revealed 24 VrHsf genes responsible for the synthesis of heat-shock transcription factors that mediate plant responses under heat stress, suggesting their potential role in investigating mechanisms related to heat tolerance (Liu et al. 2019). Similarly, in a soybean study, 26 GmHsf genes coded for heat-shock transcription factors, with GmHsf12, GmHsf28, GmHsf34, GmHsf35, and GmHsf47, highly upregulated during heat stress (Chung et al. 2013).
2.9 Scope of Harnessing Germplasm for Designing Heat Tolerance
Harnessing crop germplasm variability is one of the cheapest and most environmentally friendly approaches for developing abiotic stress, including heat stress tolerance (Jha et al. 2014). Like other crops, substantial genetic variation has been harnessed to develop grain legumes that tolerate heat stress (Craufurd et al. 2003; Jha et al. 2017; Krishnamurthy et al. 2011). Several breeder-friendly techniques, such as field-based screening of grain legumes in targeted heat-stress environments, enabled the selection of potential heat-tolerant grain legumes in chickpea, soybean, common bean, pea, lentil, and cowpea. Based on the early phenology, an important heat stress, some important chickpea genotypes, viz., ICC 14346, ACC 316, and ACC 317, showing heat stress escape mechanisms have been reported (Canci and Toker 2009; Upadhyaya et al. 2011). Selection relying on yield and yield-related traits, such as high pod and seed set, low grain yield reduction, and maintaining high biomass, has been used to directly identify heat-tolerant lines, including ICC1205, ICC15614, BG256, and Vaibhav in chickpea (Devasirvatham et al. 2013; Gaur et al. 2012; Jha et al. 2015; Jumrani et al. 2018); G122, PI 163120, PI 271998, G122, A55, and Cornell 503 in common bean (Miklas et al. 2000; Rainey and Griffiths 2005; Shonnard and Gepts 1994); TN88-63, Tvu 4552, and Prima in cowpea (Nielsen and Hall 1985; Warrag and Hall 1983); 55-437, 796, 796, 55-437, ICG 1236, ICGV 86021, ICGV 87281, and ICGV 92121 in groundnut (Craufurd et al. 2003; Ntare et al. 2001); 72578, 70548, 71457, and 73838 in lentil (Delahunty et al. 2015); Dieng, IA3023, and KS4694 in soybean (Djanaguiraman et al. 2019; Puteh et al. 2013); C.52/1/1/1 and C.42 in faba bean (Abdelmula and Abuanja 2007); and JP-625, IARI-2877, PMR-38 II, EC-318760, EC-328758, and IARI-2904 in pea (Mohapatra et al. 2020). Similar studies based on various physiological parameters, including cell membrane stability, identified heat-tolerant ILC 482, Annegiri, and ICCV 10 in chickpea (Srinivasan et al. 1996), PI 271998 in common bean (Marsh et al. 1985), and SPT 06-07 in groundnut (Singh et al. 2016), and studies based on pollen germination and fertilization under heat stress identified heat-tolerant ICC 15614, ICCV 92944, and ICC1205 in chickpea (Devasirvatham et al. 2010; Kaushal et al. 2013), 55-437, ICG 1236, TMV 2, and ICGS 11 in groundnut (Kakani et al. 2002), DG 5630RR, NRC 7, and EC 538828 in soybean (Jumrani et al. 2018; Salem et al. 2007), and Haibushi in common bean (Tsukaguchi et al. 2003). In addition, studies based on superior yield performance and genotype × genotype × environment biplot analysis identified heat-tolerant ICC 4958, RVG 203, RVG 202, JAKI 9218, and JG 130 in chickpea (Jha et al. 2018, 2019), and studies based on several heat-stress tolerance indices identified heat-tolerant lines in soybean (Sapra and Anaele 1991), chickpea (Jha et al. 2018), and common bean (Porch 2006). Harnessing existing genetic variability in crop wild relatives and landraces should be considered to broaden the genetic base of grain legumes for higher heat tolerance in the future.
2.10 Genetics of Heat Tolerance
Classical genetics and quantitative genetics approaches, such as generation mean analysis and diallel analysis, provided preliminary information on heat-stress tolerance in chickpea (Jha et al. 2019), cowpea (Marfo and Hall 1992; Patel and Hall 1988), and common bean (Miklas et al. 2000; Rainey and Griffiths 2005) based on yield and yield-related traits under heat stress. However, this genetic information does not provide a complete picture of heat tolerance in these grain legumes, as this trait is governed by multigenes and highly influenced by G × E interactions (Upadhyaya et al. 2011).
2.11 Genomic Resources for Heat Tolerance
Unprecedented advances in genomic resource development have enabled the precise mapping of various traits of breeding importance, including heat-stress tolerance in various grain legume crops (Jha et al. 2021; Paul et al. 2018; Pottorff et al. 2014; Varshney et al. 2019). In parallel, the availability of reference genome sequences for major grain legumes has enriched the genomics resources in legume crops. Using a biparental mapping approach, several QTLs controlling heat-stress tolerance have been elucidated in chickpea (Jha et al. 2019; Paul et al. 2018), cowpea (Lucas et al. 2013; Pottorff et al. 2014), lentil (Singh et al. 2017), and pea (Huang et al. 2017). In chickpea, four important QTLs related to yield traits were identified on CaLG05 and CaLG06 from an ICC15614 × ICC4567 RIL population under heat stress (Paul et al. 2018). Jha et al. (2021) reported that 37 major QTLs related to heat tolerance in chickpea were discovered. Five QTLs were elucidated in cowpea under heat stress (Lucas et al. 2013). Similarly, an evaluation of IT93K-503-1 × CB46 and IT84S-2246 × TVu14676 RIL populations identified three QTLs (Hbs-1, Hbs-2, and Hbs-3) contributing to heat tolerance in cowpea (Pottorff et al. 2014). Many QTLs contribute to phenological traits, such as days to flowering, with yield-related QTLs reported in pea under heat stress (Huang et al. 2017).
The availability of high-throughput SNP markers elucidated genomic regions controlling heat tolerance across the whole genome in a large set of chickpea germplasm using a genome-wide association mapping approach (Tafesse et al. 2020; Varshney et al. 2019). In this context, several marker-trait associations (MTAs) for various heat-stress traits have been deciphered in chickpea (Thudi et al. 2014; Varshney et al. 2019), pea (Tafesse et al. 2020), and common bean (López-Hernández and Cortés 2019). In whole genome resequencing derived SNP markers based GWAS analysis involving a large panel of chickpea germplasm, several significant MTAs for various physiological and yield traits were unveiled under heat stress (Varshney et al., 2019). Likewise, Tafesse et al. (2020) identified several significant MTAs for chlorophyll content, photochemical reflectance index, canopy temperature, and pod number in pea under heat stress. In common bean, GWAS in 78 “geo-referenced” wild common bean accessions revealed several candidate genes (e.g., MED23, MED25, HSFB1, HSP40, HSP20, phospholipase C, MBD9, PAP) related to heat-stress tolerance (López-Hernández and Cortés 2019). These MTAs could be important in marker-assisted breeding for developing heat-tolerant grain legumes.
2.12 Transcriptomics for Unfolding Candidate Genes for Heat Tolerance
In the past decade, technical interventions in functional genomics, especially next-generation sequencing-based RNA-seq facility, have offered great insights into gaining function of candidate gene(s) controlling various complex traits, including heat stress in various grain legumes (Agarwal et al. 2016; Singh et al. 2019; Wang et al. 2018). Using the RNA-seq technique, Ca_25811, Ca_23016, Ca_09743, Ca_17680, and Ca_25602 candidate genes were deciphered from heat-treated reproductive tissues of heat-tolerant and heat-sensitive chickpea genotypes (Agarwal et al. 2016). In soybean, RNA-seq analysis of contrasting genotypes treated with combined drought and heat stress revealed several differentially expressed genes, primarily involved in the defense response, photosynthesis, and metabolic processes (Wang et al. 2018). RNA-seq analysis of heat-treated soybean leaf tissue at the reproductive stage revealed a plethora of up- and down-regulatory differentially expressed genes and unearthed genes involved in flowering, oxidative stress, osmoregulation, HSPs, and ethylene biosynthesis (Xu et al. 2020). Transcriptional analysis of heat-treated soybean root tissue revealed numerous differentially expressed genes involved in regulating the heat-stress response (Valdés-López et al. 2016). In lentil, transcriptome analysis of contrasting heat-tolerant and heat-sensitive genotypes (PDL-2 and JL-3) revealed several genes encoding a WRKY transcription factor, DnaJ homolog subfamily B member 13, and 17.1 kDa class II heat-shock protein and cell wall (Singh et al. 2019). However, higher expression of NAC and WRKY transcription factor genes conferred heat tolerance in the PDL-2 genotype.
2.13 Proteomics and Metabolomics Resolving Gene Networks for Heat Tolerance in Grain Legumes
A proteomics approach could endow us with the whole landscape of proteins responding to various biotic and abiotic stresses (Ramalingam et al. 2015). A series of proteins contributing to switching on various complex signal transduction mechanisms and intricate gene networks associated with adapting the plant response to heat stress have been investigated (Rathi et al. 2016). However, the role of proteomics in mediating heat-stress tolerance remains limited in grain legumes. Various types of HSPs, such as ClpB/HSP100 and VfHsp17.9-CII (Kumar et al. 2015), EF-Tu protein (Das et al. 2016), tissue-specific proteins (Ahsan et al. 2010), and early response to dehydration (ERD)-related proteins (ERD10 and ERD14) (Kovacs et al. 2008), act as chaperones, protecting cells from heat stress-related injuries. Similarly, heat stress increased HSP expression in chickpea genotype JG14 (Parankusam et al. 2017) and groundnut genotype ICGS 44 (Chakraborty et al. 2018). Further, Das et al. (2016) reported 25 proteins contributing to various cellular metabolic activities under heat stress in soybean. Furthermore, the participatory role of dehydrin-like proteins recovered from mitochondria and their plausible role in safeguarding mitochondrial membrane in yellow lupin under heat stress are worth noting (Rurek 2010). Valdés-López et al. (2016) reported 30 commonly up- and downregulated heat stress-responsive proteins involved in cell wall formation, amino acid and lipid biosynthesis, and ROS reduction in soybean.
Like proteomics, metabolomics is a robust approach for enriching our understanding of various primary and secondary metabolites produced in response to abiotic stresses, including heat stress (Janni et al. 2020; Ramalingam et al. 2015). Among the various metabolites, tocopherol and its isoforms, ascorbate, flavonoids, phenolic compounds, proline, polyamines, and glycine betaine help plants adjust to heat stress (Chebrolu et al. 2016; Kaplan et al. 2004). For example, a heat-tolerant soybean genotype had a higher abundance of flavonoids and tocopherols acting as antioxidants than a heat-sensitive genotype (Chebrolu et al. 2016). Further technical innovations and bioinformatic analysis of metabolomics-derived data could shed light on the complex gene network of heat-stress adaptation in grain legumes.
2.14 Conclusions
Increasing episodes of heat stress are becoming a serious issue worldwide, challenging the yield potential of various crops, including grain legumes. Harnessing genetic resources could be an important approach for sustaining legumes under rising temperatures. In addition to yield traits, incorporating various physiological traits could enable plants to adapt and sustain grain yield under heat stress (Reynolds and Langridge 2016).
As crop wild relatives are the reservoir of novel gene(s)/QTLs for various stress tolerance including heat-stress tolerance, introgression of heat-tolerance genomic region into elite legume cultivars using a pre-breeding approach could sustain legume yields under rising global temperatures (Chaudhary et al. 2020). Likewise, capitalizing on the various adaptive traits conferring heat tolerance from legume landraces could assist in developing grain legumes that tolerate heat stress. Furthermore, advances in grain legume genomics, especially molecular markers, and availability of grain legume genome assemblies have helped pinpoint heat-tolerance genomic regions in various legumes. Whole-genome resequencing efforts have also enabled the discovery of novel haplotypes controlling heat tolerance (Varshney et al. 2019). In parallel, progress in functional genomics, including RNA-seq-based transcriptomics, has enabled the discovery of underlying candidate gene(s) involved in heat tolerance and putative functions (Agarwal et al. 2016; Singh et al. 2019; Wang et al. 2018). Additionally, advances in proteomics and metabolomics have uncovered various participatory proteins, especially HSPs and heat stress-responsive metabolites, and various novel signaling molecules in legumes (Chebrolu et al. 2016; Parankusam et al. 2017). Therefore, leveraging various breeding, physiological, and “omics” approaches combined with emerging “speed breeding,” genomic selection, and genome editing technology could help develop climate-resilient grain legumes to meet the increasing demand for plant-based dietary protein.
References
Abdelmageed AHA, Gruda N (2009) Influence of high temperatures on gas exchange rate and growth of eight tomato cultivars under controlled heat stress conditions. Eur J Hortic Sci 74:152–159
Abdelmula AA, Abuanja IK (2007) Genotypic responses, yield stability, and association between characters among some of Sudanese faba bean (Vicia faba L.) genotypes under heat stress. In: Conference on international agricultural research for development, University of Kassel-Witzenhausen and University of Göttingen, Germany, 9–11 October 2007
Abdelrahman M, El-Sayed M, Jogaiah S, Burritt DJ, Tran LSP (2017) The “STAY-GREEN” trait and phytohormone signalling networks in plants under heat stress. Plant Cell Rep 36:1009–1025. https://doi.org/10.1007/s00299-017-2119-y
Agarwal G, Garg V, Kudapa H, Doddamani D, Pazhamala LT, Khan AW, Thudi M, Lee S, Varshney RK (2016) Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol J 14:1563–1577. https://doi.org/10.1111/pbi.12520
Ahmad P, Prasad MNV (2011) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York
Ahsan N, Donnart T, Nouri MZ, Komatsu S (2010) Tissue-specific defense and thermo-adaptive mechanisms of soybean seedlings under heat stress revealed by proteomic approach. J Proteome Res 9:4189–4204. https://doi.org/10.1021/pr100504j
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550. https://doi.org/10.1007/s11120-008-9331-0
Alsajri FA, Singh B, Wijewardana C, Irby J, Gao W, Reddy KR (2019) Evaluating soybean cultivars for low- and high-temperature tolerance during the seedling growth stage. Agronomy 9:1–20. https://doi.org/10.3390/agronomy9010013
Anitha Y, Vanaja M, Kumar VG (2016) Identification of attributes contributing to high temperature tolerance in blackgram (Vigna mungo L. Hepper) genotypes. Int J Sci Res 5:1021–1025
Araus JL, Bort J, Steduto P, Villegas D, Royo C (2003) Breeding cereals for Mediterranean conditions: ecophysiological clues for biotechnology application. Ann Appl Biol 142:129–141. https://doi.org/10.1111/j.1744-7348.2003.tb00238.x
Arunkumar R, Sairam RK, Deshmukh PS, Pal M, Khetarpal S, Pandey SK, Kushwaha SR, Singh TP (2012) High temperature stress and accumulation of compatible solutes in chickpea (Cicer arietinum L.). Indian J Plant Physiol 17:145–150
Awasthi R, Kaushal N, Vadez V, Turner NC, Berger J, Siddique KH, Nayyar H (2014) Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct Plant Biol 41:1148–1167. https://doi.org/10.1071/FP13340
Awasthi R, Gaur P, Turner NC, Vadez V, Siddique KHM, Nayyar H (2017) Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop Pasture Sci 68:823–841. https://doi.org/10.1071/CP17028
Awika HO, Hays DB, Mullet JE, Rooney WL, Weers BD (2017) QTL mapping and loci dissection for leaf epicuticular wax load and canopy temperature depression and their association with QTL for staygreen in Sorghum bicolor under stress. Euphytica 213:1–22. https://doi.org/10.1007/s10681-017-1990-5
Babu NR, Devaraj VR (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust J Crop Sci 2:40–48
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Balota M, Payne WA, Evett SR, Lazar MD (2007) Canopy temperature depression sampling to assess grain yield and genotypic differentiation in winter wheat. Crop Sci 47:1518–1529. https://doi.org/10.2135/cropsci2006.06.0383
Balota M, Payne WA, Evett SR, Peters TR (2008) Morphological and physiological traits associated with canopy temperature depression in three closely related wheat lines. Crop Sci 48:1897–1910. https://doi.org/10.2135/cropsci2007.06.0317
Barghi SS, Mostafaii H, Peighami F, Zakaria RA, Nejhad RF (2013) Response of in vitro pollen germination and cell membrane thermostability of lentil genotypes to high temperature. Int J Agric Res Rev 3:13–20
Barnabas B, Jager K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38. https://doi.org/10.1111/j.1365-3040.2007.01727.x
Barua D, Downs CA, Heckathorn SA (2003) Variation in chloroplast small heat-shock protein function is a major determinant of variation in thermotolerance of photosynthetic electron transport among ecotypes of Chenopodium album. Funct Plant Biol 30:1071–1079. https://doi.org/10.1071/FP03106
Basu PS, Pratap A, Gupta S, Sharma K, Tomar R, Singh NP (2019) Physiological traits for shortening crop duration and improving productivity of greengram (Vigna radiata L. Wilczek) under high temperature. Front Plant Sci 10:1–18. https://doi.org/10.3389/fpls.2019.01508
Berger B, Parent B, Tester M (2010) High-throughput shoot imaging to study drought responses. J Exp Bot 61:3519–3528. https://doi.org/10.1093/jxb/erq201
Bhandari K, Siddique KHM, Turner NC, Kaur J, Singh S, Agrawal SK, Nayyar H (2016) Heat stress at reproductive stage disrupts leaf carbohydrate metabolism, impairs reproductive function, and severely reduces seed yield in lentil. J Crop Improv 30:118–151. https://doi.org/10.1080/15427528.2015.1134744
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:1–18. https://doi.org/10.3389/fpls.2013.00273
Blum A (1986) The effect of heat stress on wheat leaf and ear photosynthesis. J Exp Bot 37:111–118. https://doi.org/10.1093/jxb/37.1.111
Blum A (2018) Plant breeding for stress environments. CRC Press, Boca Raton, FL
Borrell AK, van Oosterom EJ, Mullet JE, George-Jaeggli B, Jordan DR, Klein PE, Hammer GL (2014) Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol 203:817–830. https://doi.org/10.1111/nph.12869
Brussaard L, De Ruiter PC, Brown GG (2007) Soil biodiversity for agricultural sustainability. Agric Ecosyst Environ 121:233–244. https://doi.org/10.1016/j.agee.2006.12.013
Bueno PC, Lopes NP (2020) Metabolomics to characterize adaptive and signaling responses in legume crops under abiotic stresses. ACS Omega 5:1752–1763. https://doi.org/10.1021/acsomega.9b03668
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29:345–378. https://doi.org/10.1146/annurev.pp.29.060178.002021
Calderini DF, Reynolds MP, Slafer GA (2006) Source–sink effects on grain weight of bread wheat, durum wheat, and triticale at different locations. Aust J Agric Res 57:227–233. https://doi.org/10.1071/AR05107
Canci H, Toker C (2009) Evaluation of yield criteria for drought and heat resistance in chickpea (Cicer arietinum L.). J Agron Crop Sci 195:47–54. https://doi.org/10.1111/j.1439-037X.2008.00345.x
Chakraborty U, Pradhan D (2011) High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J Plant Interact 6:43–52. https://doi.org/10.1111/jac.12260
Chakraborty K, Bishi SK, Singh AL, Zala PV, Mahatma MK, Kalariya KA, Jat RA (2018) Rapid induction of small heat shock proteins improves physiological adaptation to high temperature stress in peanut. J Agron Crop Sci 204:285–297. https://doi.org/10.1111/jac.12260
Chang PFL, Jinn TL, Huang WK, Chen Y, Chang HM, Wang CW (2007) Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. Plant Sci 172:64–75. https://doi.org/10.1016/j.plantsci.2006.07.017
Chaudhary S, Devi P, Bhardwaj A, Jha UC, Sharma KD, Prasad PVV, HanumanthaRao B, Kumar S, Nayyar H (2020) Identification and characterization of contrasting genotypes/cultivars for developing heat tolerance in agricultural crops: current status and prospects. Front Plant Sci 11:1–34. https://doi.org/10.3389/fpls.2020.587264
Chebrolu KK, Fritschi FB, Ye S, Krishnan HB, Smith JR, Gillman JD (2016) Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 12:1–14. https://doi.org/10.1007/s11306-015-0941-1
Chen X, Lin S, Liu Q, Huang J, Zhang W, Lin J, Wang Y, Ke Y, He H (2014) Expression and interaction of small heat shock proteins (sHsps) in rice in response to heat stress. Biochim Biophys Acta 1844:818–828. https://doi.org/10.1016/j.bbapap.2014.02.010
Choudhury DR, Tarafdar S, Das M, Kundagrami S (2012) Screening lentil (Lens culinaris Medik.) germplasms for heat tolerance. Trends Biosci 5:143–146
Chung E, Kim KM, Lee JH (2013) Genome-wide analysis and molecular characterization of heat shock transcription factor family in Glycine max. J Genet Genomics 40:127–135. https://doi.org/10.1016/j.jgg.2012.12.002
Commuri PD, Jones RJ (1999) Ultrastructural characterization of maize (Zea mays L.) kernels exposed to high temperature during endosperm cell division. Plant Cell Environ 22:375–385. https://doi.org/10.1046/j.1365-3040.1999.00424.x
Craufurd PQ, Bojang M, Wheeler TR, Summerfield RJ (1998) Heat tolerance in cowpea: effect of timing and duration of heat stress. Ann Appl Biol 133:257–267. https://doi.org/10.1111/j.1744-7348.1998.tb05826.x
Craufurd PQ, Prasad PV, Kakani VG, Wheeler TR, Nigam SN (2003) Heat tolerance in groundnut. Field Crops Res 80:63–77. https://doi.org/10.1016/S0378-4290(02)001557
Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA (2012) High temperature exposure increases plant cooling capacity. Curr Biol 22:R396–R397. https://doi.org/10.1016/j.cub.2012.03.044
Das A, Eldakak M, Paudel B, Kim DW, Hemmati H, Basu C, Rohila JS (2016) Leaf proteome analysis reveals prospective drought and heat stress response mechanisms in soybean. Biomed Res Int 2016:1–23. https://doi.org/10.1155/2016/6021047
de Dorlodot S, Forster B, Pages L, Price A, Tuberosa R, Draye X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12:474–481. https://doi.org/10.1016/j.tplants.2007.08.012
de Souza MA, Pimentel AJB, Ribeiro G (2012) Breeding for heat-stress tolerance. In: Fritsche-Neto R, Borem A (eds) (1) Plant breeding for abiotic stress tolerance. Springer, Berlin, pp 137–156
Delahunty A, Nuttall J, Nicolas M, Brand J (2015) Genotypic heat tolerance in lentil. Paper presented in the Proceedings of the 17th ASA Conference, Hobart, Australia, 20–24 September 2018
Devasirvatham V, Tan DKY, Trethowan RM, Gaur PM, Mallikarjuna N (2010) Impact of high temperature on the reproductive stage of chickpea. Paper presented at the Food security from sustainable agriculture proceedings of the 15th Australian Society of Agronomy Conference, New Zealand, Lincoln, 15–18 November 2010
Devasirvatham V, Tan DKY, Gaur PM, Raju TN, Trethowan RM (2012) High temperature tolerance in chickpea and its implications for plant improvement. Crop Pasture Sci 63:419–428. https://doi.org/10.1071/CP11218
Devasirvatham V, Gaur PM, Mallikarjuna N, Raju TN, Trethowan RM, Tan DKY (2013) Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. Field Crops Res 142:9–19. https://doi.org/10.1016/j.fcr.2012.11.011
Devasirvatham V, Gaur PM, Raju TN, Trethowan RM, Tan DKY (2015) Field response of chickpea (Cicer arietinum L.) to high temperature. Field Crops Res 172:59–71. https://doi.org/10.1016/j.fcr.2014.11.017
Djanaguiraman M, Prasad PVV (2010) Ethylene production under high temperature stress causes premature leaf senescence in soybean. Funct Plant Biol 37:1071–1084. https://doi.org/10.1071/FP10089
Djanaguiraman M, Schapaugh W, Fritschi F, Nguyen H, Prasad PV (2019) Reproductive success of soybean (Glycine max L. Merrill) cultivars and exotic lines under high daytime temperature. Plant Cell Environ 42:321–336. https://doi.org/10.1111/pce.13421
Driedonks N, Rieu I, Vriezen WH (2016) Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod 29:67–79. https://doi.org/10.1007/s00497-016-0275-9
Dua RP, Chaturvedi SK, Sewak S (2001) Reference varieties of chickpea for IPR regime. Indian Institute of Pulses Research, Kanpur duration genotypes in chickpea (Cicer arietinum L.). Legume Res 21:121–124
Egli DB (1998) Seed biology and the yield of grain crops. CAB International, Oxford, UK, p 178
Ehlers JD, Hall AE (1998) Heat tolerance of contrasting cowpea lines in short and long days. Field Crops Res 55:11–21. https://doi.org/10.1016/S0378-4290(97)00055-5
Farooq M, Nadeem F, Gogoi N, Ullah A, Alghamdi SS, Nayyar H, Siddique KHM (2017) Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pasture Sci 68:985–1005. https://doi.org/10.1071/CP17012
Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38:1467–1475. https://doi.org/10.2135/cropsci1998.0011183X003800060011x
Garnett T, Conn V, Kaiser BN (2009) Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ 32:1272–1283. https://doi.org/10.1111/j.1365-3040.2009.02011.x
Gaur PM, Jukanti AK, Varshney RK (2012) Impact of genomic technologies on chickpea breeding strategies. Agron J 2:199–221. https://doi.org/10.3390/agronomy2030199
Gregersen PL, Culetic A, Boschian L, Krupinska K (2013) Plant senescence and crop productivity. Plant Mol Biol 82:603–622. https://doi.org/10.1007/s11103-013-0013-8
Gross Y, Kigel J (1994) Differential sensitivity to high temperature of stages in the reproductive development of common bean (Phaseolus vulgaris L.). Field Crops Res 36:201–212. https://doi.org/10.1016/0378-4290(94)90112-0
Gulen H, Eris A (2004) Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci 166:739–744. https://doi.org/10.1016/j.plantsci.2003.11.014
Harding SA, Guikema JA, Paulsen GM (1990) Photosynthetic decline from high temperature stress during maturation of wheat: I. Interaction with senescence processes. Plant Physiol 92:648–653. https://doi.org/10.1104/pp.92.3.648
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extremes 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
Hedhly A (2011) Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ Exp Bot 74:9–16. https://doi.org/10.1016/j.envexpbot.2011.03.016
Hemantaranjan A, Bhanu AN, Singh MN, Yadav DK, Patel PK, Singh R, Katiyar D (2014) Heat stress responses and thermotolerance. Adv Plants Agric Res 1:1–10
Herzog H, Chai-Arree W (2012) Gas exchange of five warm-season grain legumes and their susceptibility to heat stress. J Agron Crop Sci 198:466–474. https://doi.org/10.1111/j.1439-037X.2012.00517.x
Howarth CJ (2005) Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris P (eds) Abiotic stresses: plant resistance through breeding and molecular approaches, 1st edn. CRC Press, Boca Raton, FL, pp 277–300
Huang B, Gao H (2000) Growth and carbohydrate metabolism of creeping bentgrass cultivars in response to increasing temperatures. Crop Sci 40:1115–1120. https://doi.org/10.2135/cropsci2000.4041115x
Huang B, Xu C (2008) Identification and characterization of proteins associated with plant tolerance to heat stress. J Integr Plant Biol 50:1230–1237. https://doi.org/10.1111/j.1744-7909.2008.00735.x
Huang S, Gali KK, Tar’an B, Warkentin TD, Bueckert RA (2017) Pea phenology: crop potential in a warming environment. Crop Sci 57:1540–1551. https://doi.org/10.2135/cropsci2016.12.0974
Iqbal A, Khalil IA, Ateeq N, Khan MS (2006) Nutritional quality of important food legumes. Food Chem 97:331–335. https://doi.org/10.1016/j.foodchem.2005.05.011
Ismail AM, Hall AE (1999) Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Sci 39:1762–1768. https://doi.org/10.2135/cropsci1999.3961762x
Ismail AM, Hall AE, Ehlers JD (2000) Delayed-leaf-senescence and heat-tolerance traits mainly are independently expressed in cowpea. Crop Sci 40:1049–1055. https://doi.org/10.2135/cropsci2000.4041049x
Janni M, Gullì M, Maestri E, Marmiroli M, Valliyodan B, Nguyen HT, Marmiroli N (2020) Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J Exp Bot 71:3780–3802. https://doi.org/10.1093/jxb/eraa034
Jha UC, Bohra A, Singh NP (2014) Heat stress in crop plants: its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed 133:679–701. https://doi.org/10.1111/pbr.12217
Jha UC, Basu P, Singh D (2015) Genetic variation and diversity analysis of chickpea genotypes based on quantitative traits under high temperature stress. Int J Stress Manag 6:700–706. https://doi.org/10.5958/0976-4038.2015.00108.6
Jha UC, Bohra A, Jha R, Parida S (2017) Integrated ‘omics’ approaches to sustain major global grain legume productivity under heat stress. Plant Breed 136:437–459. https://doi.org/10.1111/pbr.12489
Jha UC, Jha R, Singh NP, Shil S, Kole PC (2018) Heat tolerance indices and their role in selection of heat stress tolerant chickpea (Cicer arietinum) genotypes. Indian J Agric Sci 88:260–267
Jha UC, Kole PC, Singh NP, Shil S, Kumar H (2019) GGE bi-plot analysis for grain yield in chickpea (Cicer arietinum) under normal and heat stress conditions. Indian J Agric Sci 89:721–725
Jha UC, Palakurthi R, Nayyar H, Jha R, Valluri VK, Bajaj P, Chitikineni A, Singh NP, Varshney RK, Thudi M (2021) Major QTLs and potential candidate genes for heat stress tolerance identified in chickpea (Cicer arietinum L.). Front Plant Sci 12:1–16. https://doi.org/10.3389/fpls.2021.655103
Jiang Y, Lahlali R, Karunakaran C, Kumar S, Davis AR, Bueckert RA (2015) Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ 38:2387–2397. https://doi.org/10.1111/pce.12589
Judd LA, Jackson BE, Fonteno WC (2015) Advancements in root growth measurement technologies and observation capabilities for container-grown plants. Plants 4:369–392. https://doi.org/10.3390/plants4030369
Jumrani K, Bhatia VS, Pandey GP (2018) Screening soybean genotypes for high temperature tolerance by in vitro pollen germination, pollen tube length, reproductive efficiency and seed yield. Indian J Plant Physiol 23:77–90. https://doi.org/10.1007/s40502-018-0360-1
Kakani VG, Prasad PVV, Craufurd PQ, Wheeler TR (2002) Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotypes to temperature. Plant Cell Environ 25:1651–1661. https://doi.org/10.1046/j.1365-3040.2002.00943.x
Kalaji HM, Rastogi A, Živčák M, Brestic M, Daszkowska-Golec A, Sitko K, Cetner MD (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:953–961. https://doi.org/10.1007/s11099-018-0766-z
Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Guy CL (2004) Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 136:4159–4168. https://doi.org/10.1104/pp.104.052142
Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Gaur PM (2008) Rapid screening technique for canopy temperature status and its relevance to drought tolerance improvement in chickpea. J Agric Res 6:105–104
Katam R, Shokri S, Murthy N, Singh SK, Suravajhala P, Khan MN, Reddy KR (2020) Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS One 15:1–29. https://doi.org/10.1371/journal.pone.0233905
Kaur G, Asthir BJBP (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619. https://doi.org/10.1007/s10535-015-0549-3
Kaur R, Bains TS, Bindumadhava H, Nayyar H (2015) Responses of mungbean (Vigna radiata L.) genotypes to heat stress: effects on reproductive biology, leaf function and yield traits. Sci Hortic 197:527–541. https://doi.org/10.1016/j.scienta.2015.10.015
Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique KHM, Nayyar H (2013) Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct Plant Biol 40:1334–1349. https://doi.org/10.1071/FP13082
Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol 147:381–390. https://doi.org/10.1104/pp.108.118208
Krause GH, Santarius KA (1975) Relative thermostability of the chloroplast envelope. Planta 127:285–299. https://doi.org/10.1007/BF00380726
Krishnamurthy L, Gaur PM, Basu PS, Chaturvedi SK, Tripathi S, Vadez V (2011) Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet Resour 9:59–69. https://doi.org/10.1017/S1479262110000
Kumar S, Kaur R, Kaur N, Bhandhari K, Kaushal N, Gupta K, Nayyar H (2011) Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol Plant 33:2091–2101. https://doi.org/10.1007/s11738-011-0748-2
Kumar S, Gupta D, Nayyar H (2012) Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Physiol Plant 34:75–86. https://doi.org/10.1007/s11738-011-0806-9
Kumar S, Thakur P, Kaushal N, Malik JA, Gaur P, Nayyar H (2013) Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch Agron Soil Sci 59:823–843. https://doi.org/10.1080/03650340.2012.683424
Kumar R, Lavania D, Singh AK, Negi M, Siddiqui MH, Al-Whaibi MH, Grover A (2015) Identification and characterization of a small heat shock protein 17.9-CII gene from faba bean (Vicia faba L.). Acta Physiol Plant 37:1–13. https://doi.org/10.1007/s11738-015-1943-3
Kumar J, Kant R, Kumar S, Basu PS, Sarker A, Singh NP (2016) Heat tolerance in lentil under field conditions. Legume Genomics Genet 7:1–11. https://doi.org/10.5376/lgg.2016.07.0001
Kumar P, Shah D, Singh MP (2017) Evaluation of chickpea (Cicer arietinum L.) genotypes for heat tolerance: a physiological assessment. Indian J Plant Physiol 22:164–177. https://doi.org/10.1007/s40502-017-0301-4
Kumari M, Pudake RN, Singh VP, Joshi AK (2013) Association of stay green trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica 190:87–97. https://doi.org/10.1007/s10681-012-0780-3
Kumari P, Singh S, Yadav S (2018) Analysis of thermotolerance behaviour of five chickpea genotypes at early growth stages. Rev Bras Bot 41:551–565. https://doi.org/10.1007/s40415-018-0484-6
Lavania D, Siddiqui MH, Al-Whaibi MH, Singh AK, Kumar R, Grover A (2015) Genetic approaches for breeding heat stress tolerance in faba bean (Vicia faba L.). Acta Physiol Plant 37:1–9. https://doi.org/10.1007/s11738-014-1737-z
Li Z, Palmer WM, Martin AP, Wang R, Rainsford F, Jin Y (2012) High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J Exp Bot 63:1155–1166. https://doi.org/10.1093/jxb/err329
Li Y, Chen X, Chen Z, Cai R, Zhang H, Xiang Y (2016) Identification and expression analysis of BURP domain-containing genes in Medicago truncatula. Front Plant Sci 7:1–16. https://doi.org/10.3389/fpls.2016.00485
Li B, Gao K, Ren H, Tang W (2018) Molecular mechanisms governing plant responses to high temperatures. J Integr Plant Biol 60:757–779. https://doi.org/10.1111/jipb.12701
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Liu Y, Li J, Zhu Y, Jones A, Rose RJ, Song Y (2019) Heat stress in legume seed setting: effects, causes, and future prospects. Front Plant Sci 10:1–12. https://doi.org/10.3389/fpls.2019.00938
López-Hernández F, Cortés AJ (2019) Last-generation genome–environment associations reveal the genetic basis of heat tolerance in common bean (Phaseolus vulgaris L.). Front Genet 10:1–22. https://doi.org/10.3389/fgene.2019.00954
Lucas MR, Ehlers JD, Huynh BL, Diop NN, Roberts PA, Close TJ (2013) Markers for breeding heat-tolerant cowpea. Mol Breed 31:529–536. https://doi.org/10.1007/s11032-012-9810-z
Makonya GM, Ogola JB, Muasya AM, Crespo O, Maseko S, Valentine AJ, Chimphango SB (2019) Chlorophyll fluorescence and carbohydrate concentration as field selection traits for heat tolerant chickpea genotypes. Plant Physiol Biochem 141:172–182. https://doi.org/10.1016/j.plaphy.2019.05.031
Maphosa Y, Jideani VA (2017) The role of legumes in human nutrition. In: Hueda MC (ed) Functional food-improve health through adequate food. Intech Open, Rijeka, p 1, 13. https://doi.org/10.5772/intechopen.69127
Marfo KO, Hall AE (1992) Inheritance of heat tolerance during pod set in cowpea. Crop Sci 32:912–918. https://doi.org/10.2135/cropsci1992.0011183X003200040015x
Marsh LE, Davis DW, Li PH (1985) Selection and inheritance of heat tolerance in the common bean by use of conductivity. J Am Soc Hortic Sci 110:680–683
Martineau JR, Specht JE, Williams JH, Sullivan CY (1979) Temperature tolerance in soybeans. I. Evaluation of a technique for assessing cellular membrane thermostability. Crop Sci 19:75–78. https://doi.org/10.2135/cropsci1979.0011183X001900010017x
Mason RE, Singh RP (2014) Considerations when deploying canopy temperature to select high yielding wheat breeding lines under drought and heat stress. Agronomy 4:191–201. https://doi.org/10.3390/agronomy4020191
Mathur S, Jajoo A, Mehta P, Bharti S (2011) Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol 13:1–6. https://doi.org/10.1111/j.1438-8677.2009.00319.x
McMichael BL, Quisenberry JE (1993) The impact of the soil environment on the growth of root systems. Environ Exp Bot 33:53–61. https://doi.org/10.1016/0098-8472(93)90055-K
Méthy M, Olioso A, Trabaud L (1994) Chlorophyll fluorescence as a tool for management of plant resources. Remote Sens Environ 47:2–9. https://doi.org/10.1016/0034-4257(94)90121-X
Miklas PN, Hannan R, Smith JR, Beaver JS, Riley R, Antonius S (2000) Transferring heat tolerance and indeterminancy from Indeterminate Jamaica Red (PI 163122) to kidney bean. Annu Rep Bean Improv Crop 43:68–69
Miroshnichenko S, Tripp J, Nieden UZ, Neumann D, Conrad U, Manteuffel R (2005) Immunomodulation of function of small heat shock proteins prevents their assembly into heat stress granules and results in cell death at sublethal temperatures. Plant J 41:269–281. https://doi.org/10.1111/j.1365-313X.2004.02290.x
Mishra S, Babbar A (2014) Selection strategies to assess the promising kabuli chickpea promising lines under normal and heat stress environments. Electron J Plant Breed 5:260–267
Mo Y, Liang G, Shi W, Xie J (2011) Metabolic responses of alfalfa (Medicago sativa L.) leaves to low and high temperature induced stresses. Afr J Biotechnol 10:1117–1124. https://doi.org/10.5897/AJB10.1433
Mohapatra C, Chand R, Tiwari JK, Singh AK (2020) Effect of heat stress during flowering and pod formation in pea (Pisum sativum L.). Physiol Mol Biol Plant 26:1119–1125. https://doi.org/10.1007/s12298-020-00803-4
Molina-Bravo R, Arellano C, Sosinski BR, Fernandez GE (2011) A protocol to assess heat tolerance in a segregating population of raspberry using chlorophyll fluorescence. Sci Hortic 130:524–530. https://doi.org/10.1016/j.scienta.2011.07.022
Nagel KA, Kastenholz B, Jahnke S, Van Dusschoten D, Aach T, Mühlich M, Schurr U (2009) Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Funct Plant Biol 36:947–959. https://doi.org/10.1071/FP09184
Nahar K, Hasanuzzaman M, Fujita M (2016) Heat stress responses and thermotolerance in soybean. In: Miransari M (ed) Abiotic and biotic stresses in soybean production, 1. Academic, San Diego, pp 261–284. https://doi.org/10.1016/B978-0-12-801536-0.00012-8
Neumann D, Lichtenberger O, Günther D, Tschiersch K, Nover L (1994) Heat-shock proteins induce heavy-metal tolerance in higher plants. Planta 194:360–367. https://doi.org/10.1007/BF00197536
Nielsen CL, Hall AE (1985) Responses of cowpea (Vigna unguiculata (L.) Walp.) in the field to high night air temperature during flowering. I. Thermal regimes of production regions and field experimental system. Field Crops Res 10:167–179. https://doi.org/10.1016/0378-4290(85)90024-3
Nieto-Sotelo J, Martínez LM, Ponce G, Cassab GI, Alagón A, Meeley RB, Yang R (2002) Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 14:1621–1633. https://doi.org/10.1105/tpc.010487
Niones JM, Suralta RR, Inukai Y, Yamauchi A (2013) Roles of root aerenchyma development and its associated QTL in dry matter production under transient moisture stress in rice. Plant Prod Sci 16:205–216. https://doi.org/10.1626/pps.16.205
Ntare BR, Williams JH, Fatondji D (2001) Evaluation of groundnut genotypes for heat tolerance under field conditions in a Sahelian environment using a simple physiological model for yield. J Agric Sci 136:81–88. https://doi.org/10.1017/S0021859600008583
Parankusam S, Bhatnagar-Mathur P, Sharma KK (2017) Heat responsive proteome changes reveal molecular mechanisms underlying heat tolerance in chickpea. Environ Exp Bot 141:132–144. https://doi.org/10.1016/j.envexpbot.2017.07.007
Pareek A, Rathi D, Mishra D, Chakraborty S, Chakraborty N (2019) Physiological plasticity to high temperature stress in chickpea: adaptive responses and variable tolerance. Plant Sci 289:110258. https://doi.org/10.1016/j.plantsci.2019.110258
Patel PN, Hall AE (1988) Inheritance of heat-induced brown discoloration in seed coats of cowpea. Crop Sci 28:929–932. https://doi.org/10.2135/cropsci1988.0011183X002800060011x
Paul PJ, Samineni S, Thudi M, Sajja SB, Rathore A, Das RR, Gaur PM (2018) Molecular mapping of QTLs for heat tolerance in chickpea. Int J Mol Sci 19:1–20. https://doi.org/10.3390/ijms19082166
Petkova V, Denev ID, Cholakov D, Porjazov I (2007) Field screening for heat tolerant common bean cultivars (Phaseolus vulgaris L.) by measuring of chlorophyll fluorescence induction parameters. Sci Hortic 111:101–106. https://doi.org/10.1016/j.scienta.2006.10.005
Petkova V, Denev I, Stefanov D (2009) Resistance to high temperature stress of various bean (Phaseolus vulgaris L.) cultivars and lines. Gen Appl Plant Physiol 35:117–121
Piramila BHM, Prabha AL, Nandagopalan V, Stanley AL (2012) Effect of heat treatment on germination, seedling growth and some biochemical parameters of dry seeds of black gram. Int J Pharm Phytopharmacol Res 1:194–202
Porch TG (2006) Application of stress indices for heat tolerance screening of common bean. J Agron Crop Sci 192:390–394. https://doi.org/10.1111/j.1439-037X.2006.00229.x
Pottorff M, Roberts PA, Close TJ, Lonardi S, Wanamaker S, Ehlers JD (2014) Identification of candidate genes and molecular markers for heat-induced brown discoloration of seed coats in cowpea [Vigna unguiculata (L.) Walp]. BMC Genomics 15:1–11. https://doi.org/10.1186/1471-2164-15-328
Prasad PV, Djanaguiraman M (2011) High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Funct Plant Biol 38:993–1003. https://doi.org/10.1071/FP11035
Prasad PV, Craufurd PQ, Summerfield RJ (1999) Sensitivity of peanut to timing of heat stress during reproductive development. Crop Sci 39:1352–1357. https://doi.org/10.2135/cropsci1999.3951352x
Prasad PVV, Staggenborg SA, Ristic Z (2008) Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In: Ahuja LR, Reddy VR, Saseendran SA, Yu Q (eds) Response of crops to limited water: understanding and modeling water stress effects on plant growth processes. ASA-CSSA-SSSA, Baltimore, MD, pp 301–356
Prasad PV, Bheemanahalli R, Jagadish SK (2017) Field crops and the fear of heat stress—opportunities, challenges and future directions. Field Crop Res 200:114–121. https://doi.org/10.1016/j.fcr.2016.09.024
Pressman E, Peet MM, Pharr DM (2002) The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann Bot 90:631–636. https://doi.org/10.1093/aob/mcf240
Priya M, Siddique KHM, Dhankhar OP, Prasad PV, Rao BH, Nair RM, Nayyar H (2018) Molecular breeding approaches involving physiological and reproductive traits for heat tolerance in food crops. Indian J Plant Physiol 23:697–720. https://doi.org/10.1007/s40502-018-0427-z
Puteh AB, Thu Zar M, Mondal MMA, Abdullah APB, Halim MRA (2013) Soybean [Glycine max (L.) Merrill] seed yield response to high temperature stress during reproductive growth stages. Aust J Crop Sci 7:1472–1479. https://doi.org/10.3316/informit.618691733672149
Raina SK, Rane J, Raskar N, Singh AK, Govindasamy V, Kumar M, Minhas PS (2019) Physiological traits reveal potential for identification of drought tolerant mungbean [Vigna radiata (L.) Wilczek] genotypes under moderate soil-moisture deficit. Indian J Genet 79:427–437. https://doi.org/10.31742/IJGPB.79.2.6
Rainey KM, Griffiths PD (2005) Differential response of common bean genotypes to high temperature. J Am Soc Hortic Sci 130:18–23. https://doi.org/10.21273/JASHS.130.1.18
Raja MM, Vijayalakshmi G, Naik ML, Basha PO, Sergeant K, Hausman JF, Khan PSSV (2019) Pollen development and function under heat stress: from effects to responses. Acta Physiol Plant 41:1–20. https://doi.org/10.1007/s11738-019-2835-8
Ramalingam A, Kudapa H, Pazhamala LT, Weckwerth W, Varsh-ney RK (2015) Proteomics and metabolomics: two emerging areas for legume improvement. Front Plant Sci 6:1–21. https://doi.org/10.3389/fpls.2015.01116
Rathi D, Gayen D, Gayali S, Chakraborty S, Chakraborty N (2016) Legume proteomics: progress, prospects, and challenges. Proteomics 16:310–327. https://doi.org/10.1002/pmic.201500257
Reddy PS, Mallikarjuna G, Kaul T, Chakradhar T, Mishra RN, Sopory SK, Reddy MK (2010) Molecular cloning and characterization of gene encoding for cytoplasmic Hsc70 from Pennisetum glaucum may play a protective role against abiotic stresses. Mol Genet Genomics 283:243–254. https://doi.org/10.1007/s00438-010-0518-7
Reddy PS, Chakradhar T, Reddy RA, Nitnavare RB, Mahanty S, Reddy MK (2016) Role of heat shock proteins in improving heat stress tolerance in crop plants. In: Asea AAA, Kaur P, Calderwood SK (eds) Heat shock proteins and plants. Springer, Cham, Switzerland p, pp 283–307
Reynolds MP (1997) Using canopy temperature depression to select for yield potential of wheat in heat-stressed environments, vol No. 42. CIMMYT, Mexico
Reynolds M, Langridge P (2016) Physiological breeding. Curr Opin Plant Biol 31:162–171. https://doi.org/10.1016/j.pbi.2016.04.005
Reynolds MP, Balota M, Delgado MIB, Amani I, Fischer RA (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Funct Plant Biol 21:717–730. https://doi.org/10.1071/PP9940717
Reynolds MP, Nagarajan S, Razzaque MA, Ageeb OAA (2001) Heat tolerance. In Reynolds MP, Ortiz-Monasterio JI, McNab A (eds) Application of physiology in wheat breeding CIMMYT, Mexico, p 124–135
Reynolds MP, Nagarayan S, Razzaue MA, Ageeb OAA (1997) Using canopy temperature depression to select for yield potential of wheat in heat-stressed environments. Wheat Special Rep. No. 42. CIMMYT, Mexico
Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M (2014) Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol Plant 36:1–19. https://doi.org/10.1007/s11738-013-1402-y
Rurek M (2010) Diverse accumulation of several dehydrin-like proteins in cauliflower (Brassica oleracea var. botrytis), Arabidopsis thaliana and yellow lupin (Lupinus luteus) mitochondria under cold and heat stress. BMC Plant Biol 10:1–17. https://doi.org/10.1186/1471-2229-10-181
Ryan PR, Delhaize E, Watt M, Richardson AE (2016) Plant roots: understanding structure and function in an ocean of complexity. Ann Bot 118:555–559. https://doi.org/10.1093/aob/mcw192
Sabagh AE, Hossain A, Islam MS, Iqbal MA, Fahad S, Ratnasekera D et al (2020) Consequences and mitigation strategies of heat stress for sustainability of soybean (Glycine max L. Merr.) production under the changing climate. In: Plant stress physiology. Intech Open, London, UK. https://doi.org/10.5772/intechopen.92098
Sadras VO (2009) Does partial root-zone drying improve irrigation water productivity in the field? A meta-analysis. Irrig Sci 27:183–190. https://doi.org/10.1007/s00271-008-0141-0
Saelim S, Zwiazek JJ (2000) Preservation of thermal stability of cell membranes and gas exchange in high temperature acclimated Xylia xylocarpa seedlings. J Plant Physiol 156:380–385. https://doi.org/10.1016/S0176-1617(00)80077-2
Salem MA, Kakani VG, Koti S, Reddy KR (2007) Pollen-based screening of soybean genotypes for high temperatures. Crop Sci 47:219–231. https://doi.org/10.2135/cropsci2006.07.0443
Salvucci Michael E, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470. https://doi.org/10.1104/pp.103.038323
Sapra VT, Anaele AO (1991) Screening soybean genotypes for drought and heat tolerance. J Agron Crop Sci 167:96–102. https://doi.org/10.1111/j.1439-037X.1991.tb00939.x
Savchenko GE, Klyuchareva EA, Abramchik LM, Serdyuchenko EV (2002) Effect of periodic heat shock on the inner membrane system of etioplasts. Russ J Plant Physiol 49:349–359. https://doi.org/10.1023/A:1015592902659
Schmit R, de Melo RC, Trevisani N, Guidolin AF, Coimbra JLM (2019) Screening and agronomic benefits of the stay-green trait in common bean genotypes. J Biosci 35:869–877. https://doi.org/10.14393/BJ-v35n3a2019-42022
Sehgal A, Sita K, Kumar J, Kumar S, Singh S, Siddique KH, Nayyar H (2017) Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front Plant Sci 8:1–19. https://doi.org/10.3389/fpls.2017.01776
Sehgal A, Sita K, Siddique KHM, Kumar R, Bhogireddy S, Varshney RK et al (2018) Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front Plant Sci 871:1–19. https://doi.org/10.3389/fpls.2018.01705
Selvaraj MG, Burow G, Burke JJ, Belamkar V, Puppala N, Burow MD (2011) Heat stress screening of peanut (Arachis hypogaea L.) seedlings for acquired thermotolerance. Plant Growth Regul 65:83–91. https://doi.org/10.1007/s10725-011-9577-y
Serrano N, Ling Y, Bahieldin A, Mahfouz MM (2019) Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-018-36484-z
Shanmugam S, Kjær KH, Ottosen CO, Rosenqvist E, Kumari Sharma D, Wollen Weber B (2013) The alleviating effect of elevated CO2 on heat stress susceptibility of two wheat (Triticum aestivum L.) cultivars. J Agron Crop Sci 199:340–350. https://doi.org/10.1111/jac.12023
Sharifi P, Amirnia R, Majidi E, Hadi H, Nakhoda B, Alipoor HM, Moradi F (2012) Relationship between drought stress and some antioxidant enzymes with cell membrane and chlorophyll stability in wheat lines. Afr J Microbiol Res 6:617–623. https://doi.org/10.5897/AJMR
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28:269–277. https://doi.org/10.1111/j.1365-3040.2005.01324.x
Sharma DK, Andersen SB, Ottosen CO, Rosenqvist E (2012) Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Funct Plant Biol 39:936–947. https://doi.org/10.1071/FP12100
Sharma DK, Andersen SB, Ottosen CO, Rosenqvist E (2015) Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant 153:284–298. https://doi.org/10.1111/ppl.12245
Sharma L, Priya M, Bindumadhava H, Nair RM, Nayyar H (2016) Influence of high temperature stress on growth, phenology and yield performance of mungbean [Vigna radiata (L.) Wilczek] under managed growth conditions. Sci Hortic 213:379–391. https://doi.org/10.1016/j.scienta.2016.10.033
Shonnard GC, Gepts P (1994) Genetics of heat tolerance during reproductive development in common bean. Crop Sci 34:1168–1175. https://doi.org/10.2135/cropsci1994.0011183X003400050005x
Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS (2015) Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J Biol Sci 22:656–663. https://doi.org/10.1016/j.sjbs.2015.06.002
Simões-Araújo JL, Rumjanek NG, Margis-Pinheiro M (2003) Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Braz J Plant Physiol 15:33–41. https://doi.org/10.1590/S1677-04202003000100005
Singh N, Sandhu KS, Kaur M (2004) Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J Food Eng 63:441–449. https://doi.org/10.1016/j.jfoodeng.2003.09.003
Singh SK, Kakani VG, Surabhi GK, Reddy KR (2010) Cowpea (Vigna unguiculata [L.] Walp.) genotypes response to multiple abiotic stresses. J Photochem Photobiol B 100:135–146. https://doi.org/10.1016/j.jphotobiol.2010.05.013
Singh D, Singh CK, Tomar RSS, Chaturvedi AK, Shah D, Kumar A, Pal M (2016) Exploring genetic diversity for heat tolerance among lentil (Lens culinaris Medik.) genotypes of variant habitats by simple sequence repeat markers. Plant Breed 135:215–223. https://doi.org/10.1111/pbr.12341
Singh D, Singh CK, Singh Tomar RS, Pal M (2017) Genetics and molecular mapping of heat tolerance for seedling survival and pod set in lentil. Crop Sci 57:3059–3067. https://doi.org/10.2135/cropsci2017.05.0284
Singh AK, Ganapathysubramanian B, Sarkar S, Singh A (2018) Deep learning for plant stress phenotyping: trends and future perspectives. Trends Plant Sci 23:883–898. https://doi.org/10.1016/j.tplants.2018.07.004
Singh D, Singh CK, Taunk J, Jadon V, Pal M, Gaikwad K (2019) Genome wide transcriptome analysis reveals vital role of heat responsive genes in regulatory mechanisms of lentil (Lens culinaris Medikus). Sci Rep 9:1–19. https://doi.org/10.1038/s41598-019-49496-0
Sita K, Sehgal A, Kumar J, Kumar S, Singh S, Siddique KHM, Nayyar H (2017) Identification of high-temperature tolerant lentil (Lens culinaris Medik.) genotypes through leaf and pollen traits. Front Plant Sci 8:744. https://doi.org/10.3389/fpls.2017.00744
Smith S, De Smet I (2012) Root system architecture: insights from Arabidopsis and cereal crops. Phil Trans R Soc B 367:1441–1452. https://doi.org/10.1098/rstb.2011.0234
Snider JL, Oosterhuis DM, Loka DA, Kawakami EM (2011) High temperature limits in vivo pollen tube growth rates by altering diurnal carbohydrate balance in field-grown Gossypium hirsutum pistils. J Plant Physiol 168:1168–1175. https://doi.org/10.1016/j.jplph.2010.12.011
Soltani A, Weraduwage SM, Sharkey TD, Lowry DB (2019) Elevated temperatures cause loss of seed set in common bean (Phaseolus vulgaris L.) potentially through the disruption of source-sink relationships. BMC Genomics 20:1–18. https://doi.org/10.1186/s12864-019-5669-2
Song C, Chung WS, Lim CO (2016) Overexpression of heat shock factor gene HsfA3 increases galactinol levels and oxidative stress tolerance in Arabidopsis. Mol Cells 39:477–483. https://doi.org/10.14348/molcells.2016.0027
Srikanthbabu V, Krishnaprasad BT, Gopalakrishna R, Savitha M, Udayakumar M (2002) Identification of pea genotypes with enhanced thermotolerance using temperature induction response technique (TIR). J Plant Physiol 159:535–545. https://doi.org/10.1078/0176-1617-00650
Srinivasan A, Takeda H, Senboku T (1996) Heat tolerance in food legumes as evaluated by cell membrane thermostability and chlorophyll fluorescence techniques. Euphytica 88:35–45. https://doi.org/10.1007/BF00029263
Strock CF, Burridge J, Massas AS, Beaver J, Beebe S, Camilo SA, Fourie D, Jochua C, Miguel M, Miklas PN, Mndolwa E, Nchimbi-Msolla S, Polania J, Porch TG, Rosas JC, Trapp JJ, Lynch JP (2019) Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res 237:53–64. https://doi.org/10.1016/j.fcr.2019.04.012
Sullivan CY, Ross WM (1979) Selecting for drought and heat resistance in grain sorghum. In: Stress physiology in crop plants. Wiley, New York, pp 263–281
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542. https://doi.org/10.1016/S1360-1385(00)01797-0
Suzuki K, Tsukaguchi T, Takeda H, Egawa Y (2001) Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. J Am Soc Hortic Sci 126:571–574. https://doi.org/10.21273/jashs.126.5.571
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270. https://doi.org/10.1111/j.1365-3040.2011.02336.x
Szepesi Á, Szőllősi R (2018) Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In: Plant metabolites and regulation under environmental stress. Academic, San Diego, pp 337–353. https://doi.org/10.1016/B978-0-12-812689-9.00017-0
Tafesse EG, Warkentin TD, Bueckert RA (2019) Canopy architecture and leaf type as traits of heat resistance in pea. Field Crops Res 241:1–11. https://doi.org/10.1016/j.fcr.2019.107561
Tafesse EG, Gali KK, Lachagari VB, Bueckert R, Warkentin TD (2020) Genome-wide association mapping for heat stress responsive traits in field pea. Int J Mol Sci 21:1–26. https://doi.org/10.3390/ijms21062043
Tahir ISA, Nakata N, Yamaguchi T, Nakano J, Ali AM (2008) Influence of high shoot and root-zone temperatures on growth of three wheat genotypes during early vegetative stages. J Agron Crop Sci 194:141–151. https://doi.org/10.1111/j.1439-037X.2008.00298.x
Thudi M, Upadhyaya HD, Rathore A, Gaur PM, Krishnamurthy L, Roorkiwal M et al (2014) Genetic dissection of drought and heat tolerance in chickpea through genome-wide and candidate gene-based association mapping approaches. PLoS One 9:1–12. https://doi.org/10.1371/journal.pone.0096758
Tokyol A, Turhan E (2019) Heat stress tolerance of some green bean (Phaseolus vulgaris L.) genotypes. Agron Ser Sci Res 61:472–479
Traub J, Porch T, Naeem M, Urrea CA, Austic G, Kelly JD, Loescher W (2018) Screening for heat tolerance in Phaseolus spp. using multiple methods. Crop Sci 58:2459–2469. https://doi.org/10.2135/cropsci2018.04.0275
Tsukaguchi T, Kawamitsu Y, Takeda H, Suzuki K, Egawa Y (2003) Water status of flower buds and leaves as affected by high temperature in heat-tolerant and heat-sensitive cultivars of snap bean (Phaseolus vulgaris L.). Plant Prod Sci 6:24–27. https://doi.org/10.1626/pps.6.24
Upadhyaya HD, Dronavalli N, Gowda CLL, Singh S (2011) Identification and evaluation of chickpea germplasm for tolerance to heat stress. Crop Sci 51:2079–2094. https://doi.org/10.2135/cropsci2011.01.0018
Urban J, Ingwers M, McGuire MA, Teskey RO (2017) Stomatal conductance increases with rising temperature. Plant Signal Behav 12:1–3. https://doi.org/10.1080/15592324.2017.1356534
Vacha F, Adamec F, Valenta J, Vacha M (2007) Spatial location of photosystem pigment–protein complexes in thylakoid membranes of chloroplasts of Pisum sativum studied by chlorophyll fluorescence. J Luminesc 122–123:301–303. https://doi.org/10.1016/j.jlumin.2006.01.148
Valdés-López O, Batek J, Gomez-Hernandez N, Nguyen CT, Isidra-Arellano MC, Zhang N et al (2016) Soybean roots grown under heat stress show global changes in their transcriptional and proteomic profiles. Front Plant Sci 7:1–12. https://doi.org/10.3389/fpls.2016.00517
Varshney RK, Thudi M, Roorkiwal M, He W, Upadhyaya HD, Yang W, Bajaj P, Cubry P, Rathore A, Jian J et al (2019) Resequencing of 429 chickpea accessions from 45 countries provides insights into genome diversity, domestication and agronomic traits. Nat Genet 51:857–864. https://doi.org/10.1038/s41588-019-0401-3
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Biol 42:579–620. https://doi.org/10.1146/annurev.pp.42.060191.003051
Wahid A, Shabbir A (2005) Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul 46:133–141. https://doi.org/10.1007/s10725-005-8379-5
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang L, Liu L, Ma Y, Li S, Dong S, Zu W (2018) Transcriptome profiling analysis characterized the gene expression patterns responded to combined drought and heat stresses in soybean. Comput Biol Chem 77:413–429. https://doi.org/10.1016/j.compbiolchem.2018.09.012
Warrag MOA, Hall AE (1983) Reproductive responses of cowpea to heat stress: genotypic differences in tolerance to heat at flowering. Crop Sci 23:1088–1092. https://doi.org/10.2135/cropsci1983.0011183X002300060016x
Wassie M, Zhang W, Zhang Q, Ji K, Chen L (2019) Effect of heat stress on growth and physiological traits of alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 9:1–20. https://doi.org/10.3390/agronomy9100597
Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 47:325–338. https://doi.org/10.1093/jxb/47.3.325
Willits DH, Peet MM (2001) Measurement of chlorophyll fluorescence as a heat stress indicator in tomato: laboratory and greenhouse comparisons. J Am Soc Hortic Sci 126:188–194. https://doi.org/10.21273/JASHS.126.2.188
Wilson JM, Greaves JA (1990) Assessment of chilling sensitivity by chlorophyll fluorescence analysis. In: Wang YC (ed) Chilling injury of horticultural crops. CRC Press, Boca Raton, FL, pp 129–141
Wu W, Ma BL, Whalen JK (2018) Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: perspectives for stress adaptation from root system architecture. Adv Agron 151:87–157. https://doi.org/10.1016/bs.agron.2018.05.002
Xu Q, Huang B (2000) Effects of differential air and soil temperature on carbohydrate metabolism in creeping bentgrass. Crop Sci 40:1368–1374. https://doi.org/10.2135/cropsci2000.4051368x
Xu P, Zhao PX, Cai XT, Mao JL, Miao ZQ, Xiang CB (2020) Integration of jasmonic acid and ethylene into auxin signaling in root development. Front Plant Sci 11:271. https://doi.org/10.3389/fpls.2020.00271
Xu S, Li J, Zhang X, Wei H, Cui L (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285. https://doi.org/10.1016/j.envexpbot.2005.03.002
Yamada M, Hidaka T, Fukamachi H (1996) Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci Hortic 67:39–48. https://doi.org/10.1016/S0304-4238(96)00931-4
Yoldas F, Esiyok D (2009) The influence of temperature on growth and yield of green beans for processing. Int J Agric Res 4:123–130
Zhang J, Huang W, Pan Q, Liu Y (2005) Improvement of chilling tolerance and accumulation of heat shock proteins in grape berries (Vitis vinifera cv. Jingxiu) by heat pretreatment. Postharvest Biol Technol 38:80–90. https://doi.org/10.1016/j.postharvbio.2005.05.008
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Devi, P. et al. (2023). Harnessing Genetic Variation in Physiological and Molecular Traits to Improve Heat Tolerance in Food Legumes. In: Muthu Arjuna Samy, P., Ramasamy, A., Chinnusamy, V., Sunil Kumar, B. (eds) Legumes: Physiology and Molecular Biology of Abiotic Stress Tolerance. Springer, Singapore. https://doi.org/10.1007/978-981-19-5817-5_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-5817-5_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5816-8
Online ISBN: 978-981-19-5817-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)