Abstract
Seed development is a temperature-sensitive process that is much more vulnerable than vegetative tissues to abiotic stresses. High temperatures during soybean (Glycine max) seed development frequently results in seed with poor germination, increased incidence of pathogen infection, and decreased economic value. Climate change is expected to increase the incidence and severity of summer heatwaves, and the impact of heat stress on seed development will become more widespread during the course of the twenty-first century. Global metabolite profiles were contrasted between seed from heat-tolerant and heat-susceptible genotypes produced under 28/22 °C (control), 36/24 °C, and 42/26 °C day/night temperatures. Germination of seeds from the heat-susceptible genotype was strongly reduced (50 %) for the 36/24 °C treatment and completely inhibited for the 42/26 °C. In contrast, germination was unaffected for the heat-tolerant genotype for the 36/24 °C, and while strongly inhibited, some (25 %) seed from the heat-tolerant genotype were able to germinate even for the 42/26 °C treatment, and in general were less impacted by elevated temperatures as compared to a commonly grown high-yielding, but heat-sensitive genotype. A total of 275 seed metabolites were analyzed by three metabolite profiling methods, and genotype-specific differences and temperature specific differences were identified. A diverse set of antioxidant metabolites, including tocopherols, flavonoids, phenylpropanoids, and ascorbate precursors were found to be enriched in seed of the heat tolerant genotype. The generally greater abundance of these metabolites at both temperatures in the tolerant genotype indicates that these compounds are likely responsible, at least in part, for the greater tolerance to high temperatures during seed development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soybean (Glycine max L. Merr) is a major food and feed crop whose principal economic value is derived from the high quality oil and protein present in seed. Breeding efforts in the United States have resulted in considerable seed yield gains over the last century, ~23.4 kg ha−1 year−1 from 1924 to 2011 (Suhre et al. 2014). A substantial portion of these yield gains can be directly attributed to genetic factors and focused breeding (Rowntree et al. 2013; Specht et al. 1999; Suhre et al. 2014). While impressive, these gains have been made with an extremely limited amount of genetic diversity; modern high yielding North American cultivars trace back to less than 15 original founding lines (Gizlice et al. 1993, 1994, 1996). The common breeding practice of crossing “elite by elite” materials has only further reduced soybean genetic diversity (Hyten et al. 2006).
Soybean is generally considered to be relatively heat-tolerant in comparison to other crop plants (e.g. Zea maize), with a vegetative optimum temperature of ~30 °C (Hesketh et al. 1973). However, pollination and seed development are much more sensitive than vegetative tissues to increased temperature; the optimal reproductive temperature is a relatively low 22–24 °C (Hatfield et al. 2011). The overwhelming consensus of the scientific community is that the average temperature of the planet is increasing, resulting in ongoing climate change (Cook et al. 2013). Water availability has long been well known as one of the most limiting factors in soybean productivity (Specht et al. 1999). Predicting future changes in precipitation patterns in response to climate change has proven to be quite problematic (Redden et al. 2014). Nevertheless, some predictions indicate that summer precipitation patterns may be altered, which could increase the risk and/or severity of drought in the future [(Thornton et al. 2014) http://www.epa.gov/climatechange/impacts-adaptation/midwest.html, verified 8 August 2015]. Moreover, heat stress during key reproductive events, including pollination and seed development, has been demonstrated to be a major factor limiting soybean seed yield (Redden et al. 2014; Siebers et al. 2015). Significant, complex interactions of temperature, drought, and increased carbon dioxide are expected for future climatic conditions, but predictions on the impact and severity of these stresses on soybean yield are inconsistent (Hatfield et al. 2011; Redden et al. 2014).
In the Midsouth region of the United States, consistent late season drought has historically reduced seed yield and economic return for farmers (Heatherly 1999; Heatherly and Spurlock 1999). Irrigation can partially ameliorate the reduction in seed yield (Heatherly 1996), and a large proportion of Midsouth/Mississippi soybeans are irrigated (~44 %, (Agriculture 2015)). However, irrigation remains an expensive and uncommon practice overall; less than 10 % of total United States soybean acreage is reported as irrigated (Agriculture 2015). Traditional soybean practices in the Midsouth featured maturity group (MG) V, VI and VII soybeans planted in May/June and harvested in October and November. An alternative production method, the Early Soybean Production System (ESPS) has shifted planting and harvest times to minimize late season drought stress (Heatherly 1999). Hence, cultivars that flower and mature faster (MG III–V) are planted as early in spring as possible (early to mid-April). Seed are then harvested earlier, typically in mid-August to mid-September. The ESPS has been demonstrated to consistently increase seed yield and economic return on investment (Heatherly 1996; Heatherly and Spurlock 1999) under both irrigated and non-irrigated conditions in the Midsouth (Heatherly and Spurlock 1999). Consequently, seed that develop under the conditions of the ESPS are frequently exposed to much higher day and nighttime temperatures, leading to reduced seed weight, seed quality, germination and vigor, increased seed wrinkling, impermeable seed coat, and higher incidence of pathogen infection (Heatherly 1996, 1999; Heatherly and Spurlock 1999; Smith et al. 2008). Although ESPS has increased seed yield, seed quality has been negatively impacted.
Unadapted landraces and/or wild relatives are an excellent genetic source for disease-resistance and stress-tolerance genes which do not exist in elite germplasm. An example of this approach was a successful screen for genotypes which resist the high temperatures associated with the ESPS in the Midsouth region (Smith et al. 2008). One exotic landrace, PI 587982A (National Genetic Resources Program-Germplasm Resources Information Network, http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=PI+587982A verified 9 August 2015), was identified as having the most consistent and robust heat tolerance (near absence of Phomopsis longicolla infection and seed wrinkling, and >90 % germination). This genotype has been used in traditional hybridization to generate experimental genotypes with improved seed yield and agronomics (as compared to the PI line) which also bear the heat tolerance trait.
Several recent studies have highlighted the utility of metabolomic analysis to probe physiology and diversity in soybean. Selected examples include: A study of root hairs in response to Bradyrhizobium japonicum infection (Brechenmacher et al. 2010), the response of roots to flooding (Komatsu et al. 2014), a comparison of transgenic soybean seed with seed of conventional cultivars (Clarke et al. 2013; García-Villalba et al. 2008), and a recent characterization of soybean seed metabolomic profiles among 29 different germplasm entries (Lin et al. 2014).
In this study, we compared mature seed of an unreleased experimental line which is tolerant to heat-induced seed degradation with a released soybean genotype which is high-yielding but which has typical heat-sensitivity (Paris et al. 2006). During seed development and maturation, soybean plants were exposed to optimum, moderate, or high temperatures. A total of 275 seed metabolites were identified and their relative abundances were compared. Seed metabolome was very responsive to elevated temperature, whereas genotypic-specific differences were more attenuated. A diverse set of antioxidant compounds were enriched in seed of the heat tolerant genotype, as compared to seed from the heat sensitive genotype. These results reveal the impact of elevated temperatures on soybean seed metabolite profiles and provide insights into mechanisms underlying tolerance to high temperatures during seed development.
2 Methods and materials
2.1 Plant material and experimental design
A heat tolerant genotype, ‘04025-1-1-4-1-1’ (an F5-derived line from DT98-9102 × PI 587982A and hereafter referred to as TG) and a high-yielding, but heat-sensitive line ‘DT97-4290’, (hereafter referred to as SG) were sown in 6.2 L pots filled with a 1:1 ratio of course sand and Mexico silt loam (fine, smectitic, mesic Aeric Vertic Epiaqualf) soil from Bradford experimental research farm in Columbia, MO. Inoculant was applied prior to planting (N-Dure, INTX Microbials, LLC, NC, USA). Pots were placed in growth chambers operated at 28 °C/22 °C day/night temperatures, 14 h photoperiod, and maintained at 50 %/70 % day/night relative humidity. Plants were grown at these optimal temperatures until flower initiation, and immediately after onset of flowering, a subset of plants from both genotypes were transferred to moderate (36 °C/24 °C day/night) or severe (42 °C/26 °C day/night) heat conditions, with the remainder maintained at optimal temperature conditions (28 °C/22 °C day/night (S Fig. 1). Seed from five plants per genotype/temperature treatment were harvested as soon as they reached harvest maturity as judged by the color of individual pods. Harvested seed intended for metabolite analysis were immediately flash frozen and stored at −80 °C. The remaining seed were stored at 4 °C and ~35 % relative humidity until used for seed composition or germination analysis as described below.
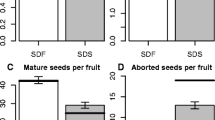
Influence of heat during seed development on seed quality parameters. Germination percentage at 25 °C after 72 h (a), radicle length (b), and mature seed weight (c) in two soybean genotypes: TG (heat tolerant) and SG (heat sensitive). Results of ANOVA and t test statistical analyses (α = 0.05) are indicated by letters above columns; overlapping letters indicate insignificant differences among means. Bars indicate one standard error
2.2 Soybean seed germination, and oil and nitrogen analyses
Seed weight, seed oil and seed protein concentration were measured on five replicates (five different plants) per genotype and temperature treatment. Total oil concentration was determined using an NMR equipped with MQC Oilseeds Analyzer with a 26 mm probe, according to manufacturer’s recommendations (Oxford Instruments America, Concord, MA, USA). To determine nitrogen concentration, five replicates of five seed per genotype and temperature treatment were ground to a fine powder and nitrogen concentration was determined for a 200 mg subsample using the Dumas method and a LECO truSpec model FP-428 nitrogen analyzer according to manufacturer’s recommendations (LECO, St. Joseph, MI). Protein concentration was inferred using a nitrogen-to-protein conversion factor of 6.25.
Due to the limited seed for certain temperature treatments only three replicates, consisting of ten seed of each genotype/treatment combination, were examined for germination. Seed were first surface-sterilized by soaking in 5 % sodium hypochlorite for 30 s, soaking in 95 % ethanol for 15 s and rinsing twice in sterilized distilled water for 30 s. Two layers of blue seed germination paper (Hoffman Mfg., Jefferson, OR) were placed in clean seed aging boxes and pre-wetted with 20 mL of nano-pure water, closed to ensure adequate humidity and placed into a darkened growth chamber at 25 °C. Germination was scored if a radicle of ≥1 cm was present. Germination status and radicle length were recorded at 72 h after the start of imbibition. Extraction of soybean seed proteins and its separation by SDS-PAGE was carried out as described earlier (Kim et al. 2013).
2.3 Extraction of metabolites and analysis on LCMS and GCMS platforms
Three seed from one plant (comprising one biological replicate) were freeze-dried and ground together using a mortar and pestle with liquid nitrogen. A total of 30 samples (2 genotypes × 3 temperature treatments × 5 biological replications) were analyzed for 275 biochemicals at Metabolon Inc., Research Triangle Park, North Carolina, USA. Extraction and metabolite analysis were performed essentially as described in previous publications (Evans et al. 2009; Ohta et al. 2009). A subsample of 20 mg of each sample was thawed on ice and extracted in 400 mL of methanol containing recovery standards using an automated MicroLab STAR system (Hamilton Company), and was then split into three aliquots for analysis by: (1) UPLC/MS/MS2 performed using a Waters Acquity UPLC (Waters Corporation) coupled to an LTQ mass spectrometer (Thermo Fisher Scientific Inc.) equipped with an electrospray ionization source optimized for acidic species; (2) The same UP LC/MS/MS2 instrumentation but with methods optimized for basic species, and (3) GC/MS using Bis-trimethyl-silyl-triflouroacetamide derivatized samples that were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole MS operated at unit mass resolving power (Ohta et al. 2009). For UPLC methods, chromatographic separation was performed and followed by full scan mass spectra to record retention time, molecular weight (m/z) and MS/MS2 of ions (Evans et al. 2009). Metabolites in seed were identified by automated analysis of ion features in comparison with reference libraries as previously described (DeHaven et al. 2010, 2012).
2.4 Data imputation, statistical analysis and principle component analysis
Missing values for metabolites were imputed with the observed minimum detected value, based upon the assumption that results were below the instrument limit of detection. Statistical analyses were performed using JMP Version 11 software (SAS Institute Inc., Cary, NC, USA) and “R” (http://cran.r-project.org/), essentially as previously described (Evans et al. 2009; Ohta et al. 2009). Principle component analysis, ANOVA and t-tests were performed in JMP software using the normalized data obtained from the LCMS and GCMS platforms. All statistical analyses were performed using a confidence threshold of α = 0.05.
3 Results
Two soybean genotypes: “SG”, a conventional, high-yielding released line (DT97-429); and “TG”, an experimental line (04025-1-4-1-1) which has tolerance to heat-induced seed degradation and was derived from plant introduction line PI 587982A (Smith et al. 2008), were grown in growth chambers at near-ideal temperatures (28 °C/22 °C day/night). At the onset of flowering, one of three temperature regimes was imposed: (1) 28 °C/22 °C day/night; (2) 36 °C/24 °C day/night; or (3) 42 °C/26 °C day/night (SI1).
3.1 Seed weight, germination and radical growth
Analysis of variance of germination efficiency revealed robust temperature (P < 0.0001) and genotype (P = 0.0010) effects as well as significant genotype × temperature (P = 0.0153) interactions. All seed produced at 28 °C exhibited normal (>90 %) germination (Fig. 1a). Although germination percent at 28 °C was not different between TG and SG, early radicle emergence in TG resulted in an overall ~30 % longer radicle as compared to SG seed (Fig. 1b). Seed maturation at temperatures consistent with the ESPS (36 °C), did not alter seed weight (Fig. 1c), but resulted in a dramatic reduction of germination potential for the SG seed (~50 % decrease) and a 4-fold decrease in radicle length (Fig. 1a, b) compared to the 28 °C treatment. In contrast, germination percentage of TG seed produced at 36 °C remained at 100 % and radicle length was maintained (Fig. 1a, b). Seed of both genotypes produced at 42 °C, a temperature above the reported lethal temperature for soybean seed (Boote et al. 2005), were noticeably shriveled and very atypical in appearance (SI2). None of the SG seed that developed at 42 °C were able to germinate. In contrast, a modest proportion of TG seed (~25 %) were able to germinate, although they exhibited reduced radicle growth (Fig. 1a, b). These germination results are consistent with previous heat stress studies (Dornbos and Mullen 1991; Egli and Wardlaw 1980; Smith et al. 2008).
3.2 Metabolomic profile and principle component analysis of metabolic phenotypes
In order to gain insight into the physiological mechanisms that underlay tolerance to heat-induced seed degradation, we utilized a combination of metabolite profiling platforms to probe mature soybean seed. A total of 275 metabolites were identified and their relative abundances were compared (Supplementary File 1, Supplementary Table 1, Supplementary Fig. 3). This includes 76 amino acids or amino acid derivatives (28 %), 55 lipids (20 %), 50 carbohydrates (18 %), 27 peptides (10 %), 21 secondary metabolites (7 %), 21 cofactors (7 %), 21 nucleotides (7 %), 2 hormones (1 %) and 2 xenobiotic chemicals (1 %). These compounds were subjected to principal component analysis (PCA) to examine genotype and temperature treatments effects on their seed metabolic profiles (Fig. 2). PCA revealed six clusters corresponding to the six genotype-by-temperature treatments examined in this study. A total of 67 % of the variation among treatments was explained by principal component 1 (PC1, 56.1 %) and principal component 2 (PC2, 10.9 %). The small amount of variation between genotypes at 28 °C is reflected by the close proximity of the clusters. The data points representing the 36 °C treatments were less tightly grouped than those of the 28 °C treatment, and there was more separation between the genotype clusters. TG and SG clusters for metabolite profiles of seed that developed at 42 °C were clearly separated on both PC1 and PC2. In addition, the two genotypes were represented by clusters that were less compact than those representing seed grown at 28 or 36 °C. Seed produced by SG at 42 °C clustered towards the farthest positive extreme on PC1 and failed to germinate. In contrast, seed from the treatments that fell into the upper left quadrat of Fig. 2 (PC1, 0 to −20; PC2, 0–20) were fully viable. Limited germination was observed for seeds from treatments that fell into the bottom half of Fig. 2 (PC1, −20 to 30; PC2, 0 to −20).
3.3 Impact of seed development at 42 °C on seed metabolites
Seed development and maturation at 42 °C resulted in dramatically reduced seed size, abnormal seed appearance and severely reduced germination (Fig. 1a and SI2). Moreover, the concentration of the majority (>200/275) of seed metabolites were altered in seed from the 42 °C treatment compared to all other treatments in both genotypes (Supplementary Table 1).
Seed of SG accumulated extraordinarily higher levels of nitrogen at 42 °C (Fig. 3a, Supplemental File 1); however the-fold changes were much greater in SG as opposed to TG (Supplemental File 1). One-dimensional gel electrophoresis revealed significantly lower protein (particular from the β-subunit of 7S globlin) accumulation on a dry weight basis in all seed which developed at 42 °C (Fig. 3b). Interestingly, certain seed protein bands were not differential; suggesting that protein translation per se was not blocked. One possible explanation is that certain proteins are more sensitive to heat-induced protein degradation, leading to a futile cycle of protein synthesis and degradation during seed fill/maturation.
Post heat stress influence on percentage seed oil and protein concentration in two soybean genotypes: TG (heat tolerant) and SG (heat sensitive). a Percentage seed oil concentration; b Percentage seed nitrogen concentration. Bars indicate standard error, and results of T-test (α = 0.05) are indicated by letters above columns; overlapping letters indicate insignificant differences among means. c One-dimensional denaturing protein gel electrophoresis of protein from equal amounts of seed powder. Ladder indicates size standards in kilodaltons. The approximate position of major seed storage proteins is indicated by arrows
3.4 Genotypic differences in seed metabolites at 28 and 36 °C
Out of 275 metabolites analyzed (Supplementary Table 1), 61 were significantly different (P < 0.05) between the two genotypes at 28 °C (31 higher and 30 lower in TG seed as compared to SG seed). At 36 °C, levels of 83 metabolites were significantly differential (35 higher and 48 lower in TG seed as compared to SG seed). Interestingly, the top twelve compounds that showed higher levels in TG seed are all well-known antioxidant compounds (Fig. 4, e.g. flavonoids, phenylpropanoids, ascorbate precursors, tocopherols, etc.)
3.5 Differential flavonoid/phenylpropanoid metabolites between genotypes at 28 or 36 °C
Remarkable differences were apparent in the flavonoid levels between the two genotypes at 28 °C (Figs. 4, 5). Five out of thirteen flavonoids detected were higher in TG seed but none were found to be higher in SG. For example, apigenin, glycitin, genistin, genistein and naringenin-7-O-glucoside were 4-fold, 2.7-fold, 2.1-fold, 2.1-fold and 3-fold higher in abundance, respectively, in TG seed (Figs. 4, 5). Daidzin and glycitein, while elevated, were more variable and differences were not statistically significant. Among other members in the phenylpropanoid pathway, ferulic acid, which is an important structural component of the plant cell wall and an antioxidant compound present at relatively high levels in plant tissues (Ou and Kwok 2004.), was also found to be higher (9-fold) in TG seed as compared to SG seed at 28 °C. However, at 36 and 42 °C, ferulic acid levels were not different between genotypes.
Abundance of prominent antioxidants from shikimate, homogenistate and ascorbate pathways in seed of two soybean genotypes produced at a 28 °C or b 36 °C. Metabolites in red boxes showed significant (P < 0.05) over-abundance in TG over SG, metabolites in white boxes did not significantly change while metabolites in grey boxes are not analyzed in this study
At 36 °C, 10 out of the 13 analyzed flavonoids were present at higher levels in TG as compared to SG seed. In addition to those flavonoids elevated in TG seed at 28 °C, daidzein, daidzin, glycitein, kaempferol 3-O-beta-glucoside and naringenin-7-O-glucoside were also significantly higher (2.9-fold, 2.9-fold, 2.3-fold, 2-fold and 2.9-fold respectively, Fig. 4).
Metabolites significantly elevated at only 28 °C were syringic acid (2.2-fold), ferulate (8.7-fold). Metabolites significantly elevated at only 36 °C included kaempferol-3.6-O-beta-glucoside (2.0-fold), naringenin (1.8-fold), daidzein (2.9-fold), daidzin (2.9-fold), and glycitein (2.3-fold).
3.6 Differential terpenoid, tocopherol, and ascorbate precursors between genotypes at 28 or 36 °C
The two terpenoids analyzed differed between genotypes at either 28 or 36 °C. Soyasaponin I levels were 1.2-fold higher at 28 °C and soyasaponin II was 1.5-fold more abundant in TG than SG seed at 36 °C.
Tocopherol metabolism was significantly elevated in TG compared to SG seed at 28 °C. At this temperature, β-, γ-, and δ-tocopherol were 3.8-fold, 4.0-fold and 9.3-fold higher in TG compared to SG seed (Figs. 4, 5, 6). At 36 °C, the differences between the genotypes were not as pronounced and only δ-(2.4-fold) and γ-tocopherols (2.3-fold) were significantly different.
Ascorbic acid levels are negligible (<10 ppm) in mature dry seed (Bates and Matthews 1975); therefore it was not detected during this analysis. However, differences in other compounds revealed differences in ascorbate metabolism between the two genotypes. The levels of gulono-1,4-lactone, the immediate precursor of ascorbic acid, was 12-fold higher in TG seed as compared to SG seed at 28 °C (Figs. 4, 5, 6) and 13-fold higher at 36 °C (Figs. 4, 5, 6). Threonate, a metabolite that occurs downstream from ascorbate synthesis, was present at 1.5-fold higher concentrations in TG compared to SG seed at both temperatures.
3.7 Differential amino acids between genotypes at 28 or 36 °C
Out of 20 proteogenic amino acids analyzed, only valine and leucine were significantly different between the two genotypes at both temperatures (Fig. 4). Concentrations of valine were 0.7-fold lower and those of leucine were 0.8-fold lower in TG than in SG seed. Asparagine was significantly higher (2.9-fold) in TG compared to SG seed at 36 °C.
A number of other amino acids and amino acid derivatives (non-proteogenic amino acids, di- and tri-peptides, etc.) were found to be different between genotypes (Supplemental File 1). At both temperatures, gamma-glutamylglutamine concentration was lower in TG seed (28 °C, 0.6-fold; 36 °C, 0.7-fold) than in SG seed. In contrast, gamma-glutamyltyrosine (28 °C, 1.5-fold; 36 °C, 1.5-fold), N-methyl-4-aminobutyric acid (28 °C, 1.5-fold; 36 °C, 2-fold), and dimethylarginine (28 °C, 2.7-fold; 36 °C 1.8-fold) levels were more abundant in TG seed at both temperatures. Methionine sulfoxide was lower at 28 °C (0.6-fold) and higher at 36 °C (1.4-fold) in TG than SG seed. A number of amino acid derivatives were more abundant in TG than in SG seed at 28 °C (cadaverine, 4-methyl-2-oxopentanoate, gamma-glutamylhistidine, gamma-glutamyltryptophan, and N-monomethylarginine) whereas only one amino acid derivative (4-methyl-2-oxopentanoate) was less abundant in TG seed. In contrast, levels of numerous amino acid-derived compounds (6-oxopiperidine-2-carboxylic acid, S-adenosylhomocysteine, 2-aminoadipate, pipecolate, pyroglutamine, gamma-aminobutyrate, N-delta-acetylornithine) were lower in TG than SG seed at 36 °C and only one amino acid derived compound (gamma-glutamylphenylalanine) was found at higher levels in TG seed at this temperature.
3.8 Significant genotypic differences in fatty acid or lipid-associated species at 28 or 36 °C
Only a small number of lipid associated metabolites differed between genotypes (Fig. 4, Supplemental File 1). Four metabolites were less abundant in TG than in SG seed at both 28 and 36 °C: glycerol 3-phosphate (0.6-fold/0.6-fold the levels in SG seed), glycerophosphorylcholine (0.3-fold/0.3-fold the levels in SG seed), azelate (0.6-fold/0.4-fold the levels in SG seed), and pantothenate (0.8-fold/0.8-fold the levels in SG seed). Compounds that differed between genotypes only at 28 °C included 1-palmitoylglycerophosphoglycerol, caproate, and malonate for which concentrations in TG were 0.6-, 0.5-, and 1.4-fold those in SG, respectively. Two metabolites were lower in abundance in TG than in SG at 36 °C: oleic-ethanolamide (0.4-fold the levels in SG seed) and traumatic acid (0.4-fold the levels in SG seed).
3.9 Differential carbohydrate metabolites between genotypes at 28 and/or 36 °C
Two seed carbohydrates, glucose (28 °C, 1.7-fold; 36 °C, 2-fold) and fructose (28 °C, 1.6-fold; 36 °C, 2-fold), were consistently higher in TG than SG seed at both temperatures (Fig. 4). In contrast, two minor seed carbohydrates fucitol (28 °C, 0.6-fold; 36 °C, 0.7-fold) and mannitol (28 °C, 0.7-fold; 36 °C, 0.7-fold) were consistently lower in TG than SG seed at both temperatures. At 28 °C, succinate (1.5-fold) and threitol (1.4-fold) were more abundant in TG than SG seed while 3-deoxyoctulosonate (0.7-fold), citrate (0.828-fold), and cis-aconitate (0.9-fold) were less abundant in TG than SG seed.
A modest number of carbohydrate derivatives were significantly higher in TG seed, but only at 36 °C: glycolate (2.0-fold), ribose (2.2-fold), 1,3-dihydroxyacetone (3.4-fold). Another four carbohydrate derivatives were lower in TG seed as compared to SG seed at 36 °C: arabitol (0.7-fold), erythritol (0.5-fold), pinitol (0.6-fold), and chiro-inositol (0.7-fold).
While not different at 28 °C, phytic acid biosynthesis was influenced by genotype at 36 °C. Both myo-inositol pentakisphosphate and myo-inositol hexakisphosphate levels in TG seed were ~0.6-fold those in SG seed. Another minor inositol derivative, scyllo-inositol, was 1.8 fold higher in TG than SG seed at both temperatures.
3.10 Impact of seed development at 36 °C on seed oil, protein and associated metabolite concentrations
A large number of metabolites were impacted by seed development at 36 °C (Supplementary Table 1, Fig. 4, Supplemental File 1); in SG seed 76 compounds were significantly elevated and 21 were significantly lower at 36 °C compared to seed produced at 28 °C. Comparison of TG seed produced at 36 °C revealed 58 were elevated and 14 were at lower abundance, in comparison to TG seed produced at 28 °C (Supplementary Table 1).
Seed oil concentration for both genotypes was significantly elevated at 36 °C (Fig. 3c).Consistent with these results, many phospholipids and free fatty acids were more abundant in seed produced at 36 °C as compared 28 °C (Supplemental File 1). All three of the fatty acid amides and sugar derivatives detected were elevated for SG seed at 36 °C (compared with only one of the three elevated for TG seed at 36 °C). Free fatty acids were strongly elevated in abundance due to growth at 36 °C, with six of nine detected fatty acids elevated in SG seed at 36 °C. In contrast, free fatty acids in TG seed were less affected by the increased temperature: one of nine detected free fatty acids was more abundant and two of nine were less abundant due to elevated temperature. Phospholipids were strongly increased by growth at 36 °C, with six of eight more abundant in SG seed, and seven of eight more abundant in TG seed. Another interesting free fatty acid, traumatic acid, a plant wound hormone (Zimmerman and Coudron 1979), was 1.5-fold higher in SG lines while it decreased 2-fold in TG seed at 36 °C, as compared to 28 °C.
Seed nitrogen concentration, an indicator for protein abundance, did not differ between 28, 36 and 42 °C in TG seed. In contrast, a slight increase in apparent protein concentration occurs at 36 °C (Fig. 3a), and a dramatic increase at 42 °C was observed in SG lines. Of the 76 amino acid related metabolites analyzed 22 amino acids or amino acid derivatives were significantly altered in SG line while only 16 changed in TG line due to growth at 36 °C as compared to 28 °C (Supplemental File 1). Perhaps the most striking difference among treatments was found for methionine sulfoxide, which was less abunt in TG (0.6-fold) than SG seed at 28 °C. In contrast, methionine sulfoxide was more abundant (1.7-fold) in TG than SG seed at 36 °C. Among proteogenic amino acids, aspartate levels decreased but leucine levels increased in both genotypes when seed developed at 36 °C as opposed to 28 °C. Similar to leucine, proline levels were also higher (1.4–1.5 fold increase) in both genotypes at 36 °C relative to 28 °C. In addition, several other amino acids were more abundant at 36 °C than 28 °C in TG but not SG seed: methionine (1.4-fold), glutamine (1.5-fold) and isoleucine (1.2-fold). A prominent amino acid derivative, cadaverine, was significantly less abundant (2.5-fold) at 36 °C than 28 °C in TG seed, yet was unaltered in SG seed. Gamma-aminobutyrate (GABA) was 1.5-fold more abundant in SG at 36 °C relative to 28 °C, but did not change in TG seed in response to increased temperature.
The influence of elevated temperature on seed carbohydrates was more pronounced in SG than TG seed (Fig. 4, Supplemental File 1). A large number (16/25) of minor and major seed carbohydrates were at significantly higher levels at 36 °C (1.3–2.1 fold) in SG seed as compared to SG seed at 28 °C. This includes myo-inositol, verbascose, several sugar alcohols (e.g. galactinol, mannitol, arabitol), and phytic acid biosynthesis intermediates (Fig. 4, Supplemental File 1).
Of the 12 carbohydrates that were different at 36 °C compared to 28 °C in TG seed, 11 were more abundant and only one was less abundant at the elevated temperature (Fig. 4, full details in Supplemental File 1). Sucrose was the only carbohydrate that was lower (0.8-fold) in TG seed at 36 °C than 28 °C. The remaining 11 which were elevated are relatively minor carbohydrates, sugar alcohols, and phytic acid intermediates (Supplemental File 1).
4 Discussion
Soybean seed maturation is optimal at 22–24 °C (Boote et al. 2005), and even moderately elevated temperatures (≥30 °C) can lead to developmental irregularities and cause detrimental effects on seed viability (Dornbos and Mullen 1991; Heatherly 1996; Ren et al. 2009; Smith et al. 2008). Commercially grown high yielding soybean seed are derived from a very narrow genetic basis of less than 20 ancestors (Gizlice et al. 1993, 1994, 1996; Hyten et al. 2006), which do not have a high degree of heat tolerance (Smith et al. 2008). The SG line evaluated here, derived from cultivars A5979 and DP3478, is typical of the genetic base of soybean cultivars for the Midsouth region (Paris et al. 2006). It has high seed yield and excellent agronomic qualities, but suffers from heat-induced seed degradation (Smith et al. 2008). The recently identified soybean accession, PI 587982A (Smith et al. 2008), has been shown to bear remarkable tolerance to heat-induced seed degradation, giving hope to soybean breeders and farmers for developing new heat tolerant soybean varieties suited to growth in areas with endemic heat stress. Mature soybean seed obtained from the TG line, which derives heat-tolerance from PI 587982A, displayed 100 % seed viability at both 28 and 36 °C, and possible enhancement of seedling vigor as measured by radicle length (Fig. 1a, b). Seed maturation at the extremely high 42 °C resulted in severe seed wrinkling (Supplemental Fig. 1) and dramatically impaired germination (Fig. 1a, b), although a modest proportion of seed from the TG line were able to germinate. These seed weight/germination results are consistent with previous heat stress studies (Dornbos and Mullen 1991; Egli and Wardlaw 1980; Smith et al. 2008). The overabundance of >200 compounds, and a diverse range of amino acids, is likely a result of the dramatically reduced seed size and overall reduced seed protein (Supplementary Table 1, Supplemental File 1). Seed from TG plants, while still featuring reduced protein accumulation, are less impacted as compared to the sensitive SG seed (Fig. 3a, b). We conclude that the extreme stress associated with seed development at 42 °C has generated substantial artifacts in our 42 °C dataset. Although seed germination is impaired in TG seed produced under this extreme stress, it is remarkable that a small proportion of seed are still able to germinate (Fig. 1a, b).
In order to further investigate this tolerance, we utilized global seed metabolomic analysis to identify the impact of high temperatures on seed specific metabolites, which may directly or indirectly be responsible for enhanced seedling viability and vigor. There was a significant increase in oil concentration and apparent amino acids/nitrogen concentration (but not seed protein per se) in the SG line due to elevated temperature. Curiously only seed oil concentration, but not seed protein, significantly increased in TG seed. In this study, a dramatic majority of lipids analyzed accumulated to a greater degree at 36 °C, as compared to 28 °C in both genotypes (Fig. 4, Supplemental File 1). These results are consistent with previous studies that show a positive correlation of soybean seed oil concentration with increased temperature (Bellaloui et al. 2015; Gibson and Mullen 1996; Piper and Boote 1999; Ren et al. 2009).
We identified 25 metabolites which were significantly different between the two genotypes at both 28 and 36 °C (Fig. 6). This includes two amino acids, several minor constituent chemical and amino acid derivatives, ascorbate precursors/derivatives, numerous flavonoids/phenylpropanoids, and several tocopherol isoforms.
Flavonoids have been hypothesized to at least partially mitigate the negative effects of heat stress in plants (Coberly and Rausher 2003; Gill and Tuteja 2010). In soybean seed, flavonoids principally occur in the form of isoflavones. The genotypic differences in flavonoid concentrations were more pronounced at 36 °C than at 28 °C (Figs. 4, 5, Supplemental File 1). At 28 °C, only five flavonoids (apigenin, genistein, genistin, glycitin, and naringenin-7-O-glucoside) were more abundant in TG than SG. But at 36 °C, concentrations of ten flavonoids (apigenin, daidzein, daidzin, genistein, genistin, glycitein, glycitin, kaempferol 3-O-beta-glucoside, naringenin and naringenin-7-O-glucoside) were more abundant in TG compared to SG seed.
Tocopherols are potent lipophilic antioxidant molecules, which exist in 4 distinct isoforms in soybean: α, β, γ and δ (Packer et al. 2001). A recent study showed a strong positive correlation between tocopherol levels and soybean seed viability and concluded that tocopherols are the major antioxidants in soybean seed. Tocopherols are known to interact strongly with membrane lipids and help in stabilization of the plasma membranes under heat stress (Havaux 1998). They are also implicated in scavenging ROS and preventing membrane damage and lipid peroxidation (DellaPenna and Pogson 2006). The vte2-1 Arabidopsis mutant, which is nearly devoid of tocopherols, exhibits loss of seed viability and showed severe growth defects due to lipid peroxidation (Sattler et al. 2004). Seed of TG showed much higher levels of almost all tocopherol species at both 28 °C (β, δ, and γ-tocopherol are 3.8-fold, 4.0-fold and 9.3-fold higher) and 36 °C (δ, and γ-tocopherol are 2.4-fold and 2.3-fold higher).
Gulono-1,4-lactone is one of the primary precursors for l-ascorbic acid biosynthesis in animals, and a significant portion of this pathway is conserved in plants (Valpuesta and Botella 2004). In Arabidopsis, overexpression of gulono-1,4,-lactone oxidase rescued vitamin C deficient mutants, improving both overall plant growth and restoring vitamin C content of leaves (Radzio et al. 2003). In our study, gulono-1,4-lactone was substantially higher in TG seed as compared to SG seed from both 28 °C (11-fold) and 36 °C treatments (13-fold higher, Figs. 4, 5, 6). Further, gulono-1,4-lactone was responsive to heat stress in both genotypes, with increased amounts observed in response to elevation of temperature from 28 to 36 °C (2.2-fold for SG seed and 2.5-fold for TG seed). In addition to its’ well-known role in controlling reactive oxygen species, ascorbic acid has been suggested to have important roles in root and shoot development, and biomass accumulation by modulating cell cycle and cell enlargement (Lisko et al. 2013). The very high levels of gulono-1,4-lactone (11–13-fold higher) in TG seed may contribute to control of peroxidation damage during maturation, storage, and imbibition leading to successful seed germination in TG seed after exposure to heat stress.
In a recent study, overexpression of key enzymes for the synthesis of myo-inositol and gulono-1,4-lactone resulted in increased plant abiotic stress tolerance (Lisko et al. 2013). The role of ascorbate/ascorbate peroxidases in heat tolerance is well known (Caverzan et al. 2012). In previous studies, peroxidase activity was highly correlated to high heat stress (Chaitanya et al. 2002; Gulen and Eris 2004; Mazorra et al. 2002). Moreover, certain peroxidases utilize plant phenolics for cell growth and development (Coberly and Rausher 2003; Fini et al. 2011; Pérez et al. 2002; Teklemariam and Blake 2003).
5 Concluding remarks
We compared levels of 275 soybean seed metabolites between two soybean genotype that differ in their tolerance to heat-induced seed degradation. In seed of a heat tolerant genotype, while impacted by an increase in temperature during seed development, differences were significantly attenuated as compared to seed of a commonly grown, high yielding but typically heat-sensitive genotype. The concentration of 61 compounds differed between the two genotypes at control conditions (28 °C). Importantly, the top 12 out of the 31 metabolites more abundant in TG seed (in the order of their-fold change between genotypes) are well known antioxidants. At 36 °C, 83 metabolites were different between the genotypes, and 11 of the top 12 metabolites (in the order of their-fold changes between genotypes) also were antioxidant compounds and were present in higher concentrations in TG seed than SG seed. The higher concentrations of flavonoids, ascorbate precursors and tocopherols in the heat tolerant genotype likely act by attenuating the deleterious repercussions of heat-induced ROS damage occurring during seed maturity at elevated temperature.
References
Bates, R. P., & Matthews, R. F. (1975). Ascorbic acid and β-carotene in soybeans as influenced by maturity, sprouting, processing and storage. Proceedings of Florida State Horticulture Society, 88, 266–271.
Bellaloui, N., Bruns, H. A., Abbas, H. K., Mengistu, A., Fisher, D. K., & Reddy, K. N. (2015). Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Frontiers in Plant Science, 6, 31.
Boote, K. J., Allen, L. H., Prasad, P. V. V., et al. (2005). Elevated temperature and CO2 impacts on pollination, reproductive growth, and yield of several globally important crops. Journal of Agricultural Meteorology, 60, 469–474.
Brechenmacher, L., Lei, Z., Libault, M., et al. (2010). Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiology, 153, 1808–1822.
Caverzan, A., Passaia, G., Rosa, S. B., Ribeiro, C. W., Lazzarotto, F., & Margis-Pinheiro, M. (2012). Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genetics and Molecular Biology, 35, 1011–1019.
Chaitanya, K. V., Sundar, D., Masilamani, S., & Ramachandra Reddy, A. (2002). Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regulation, 36, 175–180.
Clarke, J. D., Alexander, D. C., Ward, D. P., et al. (2013). Assessment of genetically modified soybean in relation to natural variation in the soybean seed metabolome. Scientific Reports, 3.
Coberly, L. C., & Rausher, M. D. (2003). Analysis of a chalcone synthase mutant in Ipomoea purpurea reveals a novel function for flavonoids: Amelioration of heat stress. Molecular Ecology, 12, 1113–1124.
Cook, J., Nuccitelli, D., Green, S. A., et al. (2013). Quantifying the consensus on anthropogenic global warming in the scientific literature. Environmental Research Letters, 8, 024024.
DeHaven, C., Evans, A., Dai, H., & Lawton, K. (2010). Organization of GC/MS and LC/MS metabolomics data into chemical libraries. Journal of Cheminformatics, 2, 9.
DeHaven, C. D., Evans, A. M., Dai, H., & Lawton, K. A. (2012). Software techniques for enabling high-throughput analysis of metabolomic datasets. In D. U. Roessner (Ed.), Metabolomics. Rijeka: InTech.
DellaPenna, D., & Pogson, B. J. (2006). Vitamin synthesis in plants: Tocopherols and carotenoids. Annual Review of Plant Biology, 57, 711–738.
Dornbos, D. L, Jr, & Mullen, R. E. (1991). Influence of stress during soybean seed fill on seed weight, germination, and seedling growth rate. Canadian Journal of Plant Science, 71, 373–383.
Egli, D. B., & Wardlaw, I. F. (1980). Temperature response of seed growth characteristics of soybeans. Agronomy Journal, 72, 560–564.
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M., & Milgram, E. (2009). Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry, 81, 6656–6667.
Fini, A., Brunetti, C., Di Ferdinando, M., Ferrini, F., & Tattini, M. (2011). Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behaviour, 6, 709–711.
García-Villalba, R., León, C., Dinelli, G., et al. (2008). Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis–time-of-flight mass spectrometry. Journal of Chromatography A, 1195, 164–173.
Gibson, L. R., & Mullen, R. E. (1996). Soybean seed composition under high day and night growth temperatures. Journal of the American Oil Chemists’ Society, 73, 733–737.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Gizlice, Z., Carter, T. E., & Burton, J. W. (1993). Genetic diversity in north american soybean: I. Multivariate analysis of founding stock and relation to coefficient of parentage. Crop Science, 33, 614–620.
Gizlice, Z., Carter, T. E., & Burton, J. W. (1994). Genetic base for north american public soybean cultivars released between 1947 and 1988. Crop Science, 34, 1143–1151.
Gizlice, Z., Carter, T. E., Gerig, T. M., & Burton, J. W. (1996). Genetic diversity patterns in north american public soybean cultivars based on coefficient of parentage. Crop Science, 36, 753–765.
Gulen, H., & Eris, A. (2004). Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Science, 166, 739–744.
Hatfield, J. L., Boote, K. J., Kimball, B. A., et al. (2011). Climate impacts on agriculture: Implications for crop production. Agronomy Journal, 103, 351–370.
Havaux, M. (1998). Carotenoids as membrane stabilizers in chloroplasts. Trends in Plant Science, 3, 147–151.
Heatherly, L. G. (1996). Yield and germinability of seed from irrigated and nonirrigated early- and late-planted MG IV and V soybean. Crop Science, 36, 1000–1006.
Heatherly, L. G. (1999). Early soybean production system (ESPS). In L. G. Heatherly & H. F. Hodges (Eds.), Soybean production system in the Midsouth (pp. 103–115). Boca Raton: CRC.
Heatherly, L. G., & Spurlock, S. R. (1999). Yield and economics of traditional and early soybean production system (ESPS) seedings in the midsouthern United States. Field Crops Research, 63, 35–45.
Hesketh, J. D., Myhre, D. L., & Willey, C. R. (1973). Temperature control of time intervals between vegetative and reproductive events in soybeans. Crop Science, 13, 250–254.
Hyten, D. L., Song, Q., Zhu, Y., et al. (2006). Impacts of genetic bottlenecks on soybean genome diversity. Proceedings of the National Acadamy of Sciences, 103, 16666–16671.
Kim, W.-S., Gillman, J., & Krishnan, H. (2013). Identification of a plant introduction soybean line with genetic lesions affecting two distinct glycinin subunits and evaluation of impacts on protein content and composition. Molecular Breeding, 32, 291–298.
Komatsu, S., Nakamura, T., Sugimoto, Y., & Sakamoto, K. (2014). Proteomic and metabolomic analyses of soybean root tips under flooding stress. Protein and Peptide Letters, 21, 865–884.
Lin, H., Rao, J., Shi, J., et al. (2014). Seed metabolomic study reveals significant metabolite variations and correlations among different soybean cultivars. Journal of Integrative Plant Biology, 56, 826–836.
Lisko, K. A., Torres, R., Harris, R. S., et al. (2013). Elevating vitamin C content via overexpression of myo-inositol oxygenase and l-gulono-1,4-lactone oxidase in Arabidopsis leads to enhanced biomass and tolerance to abiotic stresses. In-Vitro Cellular Development and Biology—Plant, 49, 643–655.
Mazorra, L. M., Núñez, M., Hechavarria, M., Coll, F., & Sánchez-Blanco, M. J. (2002). Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biologia Plantarum, 45, 593–596.
Ohta, T., Masutomi, N., Tsutsui, N., et al. (2009). Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in fischer 344 male rats. Toxicologic Pathology, 37, 521–535.
Ou, S., & Kwok, K.-C. (2004). Ferulic acid: Pharmaceutical functions, preparation and applications in foods. Journal of Science Food and Agriculture, 84, 1261–1269.
Packer, L., Weber, S. U., & Rimbach, G. (2001). Molecular aspects of α-tocotrienol antioxidant action and cell signalling. Journal of Nutrition, 131, 369–373.
Paris, R. L., Mengistu, A., Tyler, J. M., & Smith, J. R. (2006). Registration of soybean germplasm line DT97–4290 with moderate resistance to charcoal rot registration by CSSA. Crop Science, 46, 2324–2325.
Pérez, F. J., Villegas, D., & Mejia, N. (2002). Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry, 60, 573–580.
Piper, E., & Boote, K. (1999). Temperature and cultivar effects on soybean seed oil and protein concentrations. Journal of the American Oil Chemists’ Society, 76, 1233–1241.
Radzio, J., Lorence, A., Chevone, B., & Nessler, C. (2003). l-Gulono-1,4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Molecular Biology, 53, 837–844.
Redden, R. J., Hatfield, J. L., Vara Prasad, P. V., Ebert, A. W., Yadav, S. S., & O’Leary, G. J. (2014). Temperature, climate change, and global food security (pp. 181–202)., Temperature and Plant Development New York: Wiley.
Ren, C., Bilyeu, K. D., & Beuselinck, P. R. (2009). Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Science, 49, 1010–1022.
Rowntree, S. C., Suhre, J. J., Weidenbenner, N. H., et al. (2013). Genetic gain × management interactions in soybean: I. Planting date. Crop Science, 53, 1128–1138.
Sattler, S. E., Gilliland, L. U., Magallanes-Lundback, M., Pollard, M., & DellaPenna, D. (2004). Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell, 16, 1419–1432.
Siebers, M. H., Yendrek, C. R., Drag, D., et al. (2015). Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Global Change Biology, 21, 3114–3125.
Smith, J. R., Mengistu, A., Nelson, R. L., & Paris, R. L. (2008). Identification of soybean accessions with high germinability in high-temperature environments. Crop Science, 48, 2279–2288.
Specht, J. E., Hume, D. J., & Kumudini, S. V. (1999). Soybean yield potential—A genetic and physiological perspective. Crop Science, 39, 1560–1570.
Suhre, J. J., Weidenbenner, N. H., Rowntree, S. C., et al. (2014). Soybean yield partitioning changes revealed by genetic gain and seeding rate interactions. Agronomy Journal, 106, 1631–1642.
Teklemariam, T., & Blake, T. J. (2003). Effects of UVB preconditioning on heat tolerance of cucumber (Cucumis sativus L.). Environmental and Experimental Botany, 50, 169–182.
Thornton, P. K., Ericksen, P. J., Herrero, M., & Challinor, A. J. (2014). Climate variability and vulnerability to climate change: A review. Global Change Biology, 20, 3313–3328.
USDA. (2015). Census of agriculture—United States—Summary and state data.
Valpuesta, V., & Botella, M. A. (2004). Biosynthesis of l-ascorbic acid in plants: New pathways for an old antioxidant. Trends in Plant Science, 9, 573–577.
Zimmerman, D. C., & Coudron, C. A. (1979). Identification of traumatin, a wound hormone, as 12-oxo-trans-10-dodecenoic acid. Plant Physiology, 63, 536–541.
Acknowledgments
The authors would like to acknowledge the superb technical assistance of USDA-ARS technicians Alexandria Berghaus, and Jeremy Mullis. This work was partially supported by two grants to F. B. Fritschi, J.D. Gillman and J.R. Smith from United Soybean Board (Project No. 1420-532-5613), and by project funds from the USDA-Agricultural Research Service. Mention of a trademark, vendor, or proprietary product does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products or vendors that may also be suitable. The US Department of Agriculture, Agricultural Research Service, Midwest Area, is an equal opportunity, affirmative action employer and all agency services are available without discrimination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no pertinent conflict of interest.
Research involving animal and human rights
This article does not contain any studies with animals or humans, performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11306_2015_941_MOESM2_ESM.pptx
Influence of temperature on seed quality in sensitive genotype; SG (DT97-4290) and tolerant genotype; TG (04025-1-1-4-1-1). Supplementary Fig. 2 (PPTX 756 kb)
11306_2015_941_MOESM3_ESM.pptx
Characterization of 275 metabolites studied in two soybean genotypes; SG (heat sensitive) and TG (heat tolerant). Supplementary Fig. 3 (PPTX 307 kb)
Rights and permissions
About this article
Cite this article
Chebrolu, K.K., Fritschi, F.B., Ye, S. et al. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 12, 28 (2016). https://doi.org/10.1007/s11306-015-0941-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-015-0941-1