Abstract
Optic pathway gliomas (OPGs) are low-grade neoplasms arising from the optic nerve, chiasm, tracts, or radiations. Although they can arise sporadically, they are commonly associated with neurofibromatosis type 1 (NF-1). The clinical course of OPGs is variable and unpredictable, which has limited the development of standardized treatment protocols. This chapter reviews the clinical presentation of OPGs, screening recommendations, diagnostic tools, current treatment options, and future directions for management.
Aparna Ramasubramanian, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
13.1 Introduction
Optic pathway gliomas (OPGs) are low-grade neoplasms arising from the optic nerve, chiasm, tracts, or radiations. Although they can arise sporadically, they are commonly associated with neurofibromatosis type 1 (NF-1). The clinical course of OPGs is variable and unpredictable, which has limited the development of standardized treatment protocols. This chapter reviews the clinical presentation of OPGs, screening recommendations, diagnostic tools, current treatment options, and future directions for management.
13.2 Defining Optic Pathway Gliomas
OPGs are low-grade tumors that can arise anywhere along the optic pathway, including the optic nerve, optic chiasm, optic tracts, and optic radiations [1,2,3], and most commonly present in the first decade of life [4]. OPGs account for 2–5% of pediatric central nervous system tumors [1, 4,5,6]. While OPGs can arise sporadically, there is a strong association with NF-1, a genetic neuro-cutaneous disorder that affects 1:3000 persons annually (ref). Approximately 15–20% of patients with NF-1 will develop an OPG [1, 7,8,9,10], most commonly along the optic nerve or chiasm [7]. Because of the frequency of OPGs in NF-1, one of the seven potential diagnostic qualifications for NF-1, as defined by the NIH, is the presence of an optic glioma [6, 11]. Histologically, OPGs are most commonly classified as pilocytic astrocytoma (WHO grade I) [1, 2, 12, 13] but are less commonly classified as other, sometimes more aggressive LGG-subtypes, including pilomyxoid astrocytoma, pleomorphic xanthoastroctyoma, or diffuse fibrillary astrocytoma [1].
13.2.1 Symptoms
-
Impaired color vision [15].
-
Visual field defects—bitemporal hemianopia from chiasm involvement [10, 14].
-
Central retinal vein occlusion [14].
-
Dissociated vertical nystagmus [14].
13.2.2 Prognostic Factors
Better prognosis | Worse prognosis |
|---|---|
Tumor limited to optic nerve [9] | Tumor involving optic chiasm, posterior to the optic chiasm, or involving the hypothalamus [16] |
NF1 association | |
Female gender: Higher rate of requiring therapy and 5–10× more likely than males to lose vision [10] | |
Sporadic (non-NF1 associated) OPG [12, 17]: More aggressive course [12, 17], higher chance of causing increased intracranial pressure [8] |
13.2.3 Pathophysiology of NF1-Associated OPGs
OPGs that arise in the context of NF1 are driven by biallelic silencing of the NF1 gene silencing by mutation, methylation, or deletion [10]. The gene product normally produced by NF1, the protein neurofibromin, is analogous to GTPase activating proteins and plays a role in inhibiting the proto-oncogenic effects of RAS [10]. When neurofibromin is not functional, RAS activates AKT and MEK, allowing dysregulated cell growth through the mTOR complex and ERK [10]. Absent neurofibromin and unchecked RAS also decrease levels of cAMP, which leads to apoptosis of the retinal ganglion cell (RGC) layer [10]. Therefore, inhibiting RAS or increasing cAMP levels can decrease RGC loss and preserve vision for patients with NF1-OPGs. Additionally, microglia are implicated in tumor growth through the production of inflammatory cytokines like IL-1ß and IL-6 that act as neurotoxins to the RGCs and retinal nerve fiber layer (RNFL) [18]. Estradiol potentiates this effect; this may partly explain why vision loss is more severe in females [2]. Sporadic OPGs often arise from alterations to BRAF (most commonly BRAFV600E mutation or BRAF-KIAA1549 fusion) that activate the mitogen-activated protein kinase (MAPK) pathway [19].
13.3 Diagnosis
13.3.1 Dodge Classification
In 1958, Dodge and colleagues published a series of 46 patients with OPG divided into categories meant to distinguish management based on tumor location [20]. Type A tumors involved the optic nerve, Type B tumors involved the optic chiasm, and Type C “tractal” tumors were more diffuse [20]. Criteria derived from this series have been adapted into the Modified Dodge Classification System, also known as the PLAN score after the four centers that contributed to its development (Padua, Leeds, Augsburg, and Nottingham) [21]:
-
Dodge 1—optic nerve only.
-
1a: Single optic nerve
-
1b: Bilateral optic nerves
-
1c: Cisternal segment optic nerve
-
-
Dodge 2—optic chiasm ± optic nerve.
-
2a: Central chiasmatic
-
2b: Asymmetric chiasmatic
-
-
Dodge 3—anterior optic tracts.
-
3b: Asymmetric optic tracts
-
-
Dodge 4—posterior optic tracts ± hypothalamus.
-
4b: Asymmetric posterior tracts
-
H±: Hypothalamic involvement.
-
LM±: Leptomeningeal dissemination.
-
NF1±: Neurofibromatosis type 1.
-
13.3.2 Screening
Progression of OPGs can have devastating consequences on vision emphasizing the importance of early detection. In 1997, the OPG Task Force set forth a set of recommendations for ophthalmologic screening and surveillance of NF1-OPGs, which are still in practice today (Table 13.1) [8]. A study of 51 patients with NF1-OPGs found no tumor progression beyond age 12, which supports more frequent surveillance in younger patients and advocates for the extension of annual screening up to age 12 from the current recommendation of 8 years [23]. Table 13.2 outlines tools utilized to assess visual acuity during ophthalmologic exams based on the patient’s age and literacy.
13.3.3 Additional Recommendations
-
For patients with suspected OPG on ophthalmic exam, magnetic resonance imaging (MRI) brain and orbits should be obtained.
-
Patients with NF1-OPG should undergo ophthalmologic evaluation every 3 months for 1–2 years after diagnosis after which the frequency should be defined by their treating ophthalmologist. If no vision exam is stable by age 18, surveillance may be discontinued.
-
Routine neuroimaging is not indicated as part of a screening protocol unless a sufficient ophthalmic exam cannot be attained.
-
Routine Visual Evoked Potentials are not indicated.
-
Formal visual field testing is difficult for pediatric patients to complete and is prone to many fixation errors; therefore, confrontation visual fields as part of an ophthalmic exam is sufficient.
-
Annual physical examination should include screening for precocious puberty.
13.3.4 Imaging and Diagnostics
13.3.4.1 MRI
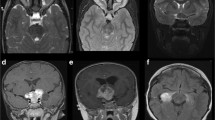
MRI is the gold standard imaging modality for identifying and monitoring OPGs in pediatric patients. The recommended imaging protocol includes coronal and axial thin-section T1-weighted and fat-saturated T2-weighted images [14], plus T1 sequences with gadolinium contrast and fluid-attenuated inversion recovery [11]. T1-weighted images are best for characterizing the size of the tumor, which will appear hypo- to isodense compared to the optic pathway (Fig. 13.2a) [4, 14]. T2-weighted images are useful for assessing anatomical involvement, and the tumor will appear mildly hyperdense compared to surrounding structures (Fig. 13.2b) [4, 14]. Volumetric MRI is gaining popularity as an adjunctive tool for measuring tumor size since traditional measurements of these complex tumors from T1-enhanced images are limited by the two-dimensional view [4, 9]. With volumetric MRI, the complex anatomy of a tumor can be more accurately estimated [9], though it is currently limited in assessing tumors of the posterior tract [9].
13.3.4.2 DTI
Diffusion tensor imaging (DTI), another MR technique, tracks the diffusion of water along axons and can map white matter tracts like the optic tract [9]. Areas with decreased fractional anisotropy (FA) do not have coherent diffusion, representing white matter damage [9]. Decreased FA has been correlated with decreased visual acuity in patients with NF1-OPGs [9]. Interestingly, increased diffusion on DTI has also been observed as a harbinger for future tumor progression and subsequent vision loss [9]. Larger scale studies need to be conducted to determine if DTI can be consistently used as a prognostic predictor of impending vision loss.
DTI has limitations for evaluating optic nerves due to artifacts from adjacent bone, fat, and airspaces [9]. Readout-segmented multi-shot echo planar imaging (rsDTI) was found to be superior to single-shot echo planar imaging (ssDTI) for providing better resolution with less artifact of the optic nerve and optic chiasm [24].
13.3.4.3 CT
Because of the radiation exposure from computed tomography (CT) and the increased propensity for patients with NF-1 to develop cancer, CT is less commonly utilized to characterize OPGs, and MRI is the preferred method for diagnosis and serially monitoring [8].
13.3.4.4 PET
Although positron emission tomography (PET) also carries a radiation burden, it may have a role in tumor surveillance and differentiation between benign and malignant neoplasms. One study utilized fluorodeoxyglucose (FDG) as their marker of metabolic activity and found that high-grade tumor elements could be detected, which aided in risk stratification for tumor advancement given the otherwise unpredictable growth patterns of OPGs [25]. Additionally, FDG-PET was able to detect residual tumor after surgical intervention as well as tumor progression [22, 25]. Therefore, PET may have an adjunctive role in monitoring patients with OPGs, especially in postsurgical patients. Further research is needed to discern if PET is a useful prognostic tool for predicting tumor progression.
13.3.4.5 OCT
Optical coherence tomography (OCT) measures the thickness of the RNFL and inner plexiform layer/ganglion cell layer (IPL-GCL) [18]. Its use in surveillance of glaucoma demonstrated a correlation between visual field defects and peripapillary RNFL thinning [26] which represents the underlying principle of using OCT to evaluate patients with OPG. In one study, a correlation between RNFL thickness and visual field defects was found such that patients with abnormal vision tended to have a RNFL <80 μm on Stratus OCT [27]. Another study utilized spectral-domain handheld OCT and found that RNFL was thinner in patients with abnormal visual acuity or visual field defects [26]. In this second study, the average RNFL thickness for normally seeing eyes was 125.1 μm, while patients with abnormal vision had an average RNFL thickness of 75.8 μm [26]. The disadvantage of OCT in this study was that patients had to be sedated in order to obtain quality images. The correlation between vision and RNFL thickness indicates that OCT can be a useful correlate for vision loss in patients who are unable to perform acuity or visual field screening. Cooperative children who can fixate long enough to obtain a quality scan can avoid the need for sedation, and OCT may provide additional data for analysis of disease progression and evaluation for possible treatment (Fig. 13.3).
13.3.4.6 VEPs
Flash and/or pattern visual evoked potentials (VEPs), which are an electrophysiologic measure of the integrity of the visual pathway, are not recommended in screening for OPGs due to high variability in results and difficulty in making meaningful conclusions from changes on serial examinations [8]. Additionally, the test can take up to 30 min, which restricts its utility in a pediatric population with a limited attention span [4]. One study advocated for use of VEPs as a “pre-symptomatic screening tool” because it is an objective measure of visual function that may be able to demonstrate visual changes prior to neuroradiological changes [28]. However, most authors conclude that the results from VEPs are too inconsistent to warrant routine use [4]. Therefore, VEPs are not recommended as part of a routine screening protocol for NF1-OPGs [8]. VEPs may be utilized in select patients on an individualized basis, though the value they add to the diagnostic evaluation is limited.
13.3.4.7 Biopsy
Tumor biopsy is not routinely performed for pediatric patients with OPG given the risk for vision deterioration from this procedure when for most tumors, histopathology will not change the management plan [11]. However, biopsy may be informative in cases with unique tumor characteristics on MRI or ambiguous clinical presentation [4, 8]. One case study documented the increased likelihood of utilizing biopsy to confirm diagnosis in patients with sporadic OPGs compared to patients with NF1-OPGs [17]. However, for most patients, a biopsy is redundant and merely increases the risk of a negative outcome from the procedure.
13.4 Treatment
OPGs tend to be slow growing and have a low potential for malignant transformation [22]. They may even spontaneously regress or may remain stable over a long period of time with no apparent impact on vision [14]. However, OPGs can still create space-occupying symptoms, permanent vision damage [22], and rarely death [9]. Because the clinical course and progression of symptoms in OPGs are unpredictable, development of a standardized surveillance regimen has been unsuccessful thus far [13]. Some trends in treatment have emerged in the literature, while more definitive guidelines are still forthcoming.
13.4.1 Observation
Observation is a satisfactory course of action for many patients with OPG, especially those with NF1 or isolated optic nerve glioma, since spontaneous regression may occur [4, 13]. On routine screening examination, several findings that warrant increased surveillance but not necessarily treatment include change in color vision, disc pallor, optic nerve swelling, afferent pupillary defect, strabismus, and nystagmus [9]. Association with precocious puberty is also not an indication to initiate treatment for an OPG since it can be medically managed with a GnRH antagonist [9].
13.4.2 Indications for Treatment
Patients with OPG benefit from care by a multidisciplinary team consisting of an ophthalmologist, neurosurgeon, and neuro-oncologist [17]. Patients with sporadic OPG tend to have more aggressive disease and are more likely to require therapy [4]. Adolescents with OPG often present with symptomatic disease necessitating treatment [17]. To balance the risk-benefit ratio, treatment should only be considered for patients with symptomatic tumors [22], significant growth [22], and/or visual decline [14, 22]. Criteria for defining “progression” of visual deficits include the following [5]:
-
Two-line change in visual acuity as measured by Snellen, HOTV, or Lea visual acuity tests, as compared to previous exams.
-
Two-octave decline in Teller visual acuity.
-
OR, 0.2 logMAR or greater change in visual acuity as compared to an age-matched control [9].
Although imaging may be helpful for monitoring tumor progression, it does not predict growth patterns [1] and may not correlate with actual visual acuity loss [4] or threatened visual loss [9]. Therefore, tumor growth on neuroimaging should rarely be the sole qualifier for advancing to treatment except in instances whereby a patient is unable to cooperate with vision testing and is otherwise limited in metrics other than imaging for monitoring disease progression [9]. Changes in visual fields are also an unreliable surrogate for disease progression, as patients may have worsening visual field defects without any associated decline in visual acuity [29].
Predicting timing between OPG diagnosis and onset of treatment is not well defined. Patients with NF1-OPGs tend to have a milder course and can tolerate a longer duration of observation without harmful disease progression, with suggested follow up every 3 months for the first 2 years after diagnosis [23]. Sporadic OPGs advance more rapidly and, in most cases, require treatment soon after diagnosis [23].
Though the goal of treatment is to preserve vision, a large cohort study in France found that approximately 25% of pediatric patients who underwent treatment for OPGs went on to have visual disability in adulthood [29]. Rather, treatment may halt further progression in visual decline, but evidence suggests that reversal of vision damage is unlikely.
13.4.3 Chemotherapy
Due to the unresectable nature of OPGs and the untoward effects of radiation therapy, chemotherapy is widely recognized as the current first-line treatment for OPGs [5, 10, 13, 22]. Packer and colleagues were among the first to investigate chemotherapeutic regimens for OPGs (low-grade gliomas; LGGs) with their work, published in 1993, on vincristine and carboplatin that includes a 10-week induction cycle, followed by eight 6-week maintenance cycles [30]. The overall response rate for 37 patients with newly diagnosed disease was 62%. Another commonly used chemotherapy regimen for LGG includes 6-thioguanine (6-TG), procarbazine, lomustine (CCNU), and vincristine (TPCV). This regimen was compared head-to-head with carboplatin/vincristine through the Children’s Oncology Group for children with newly diagnosed LGG (including OPG); objective response rates were similar between regimens, and 5-year event-free survival was 39% and 52% for carboplatin/vincristine and TPCV, respectively [31]. Weekly vinblastine, another commonly used chemotherapeutic agent for LGG, has yielded similar rates of disease control for both newly diagnosed and relapsed/refractory low-grade gliomas [32, 33]. Dosing regimens for common chemotherapy used for OPG are outlined in Table 13.3. Of note, subjects with NF1 generally fare more favorably, both in terms of response and duration of response to chemotherapy, compared to patients with non-NF-associated LGG, including OPG.
Chemotherapy is often successful in halting tumor progression or even inducing regression [10]. Despite good tumor response, full recovery of vision is rare [1, 10, 22, 33], and visual outcome is dependent on the pretreatment visual acuity [11]. The most common outcome after chemotherapy is stable vision [16]. A review study of visual outcomes found that less than 15% of patients treated with chemotherapy had improved vision and 40% had a decline in vision [33]. The authors suggest that visual changes in disease progression are irreversible regardless of the tumor’s chemosensitivity [33]. Additional chemotherapy cycles are associated with either no improvement or worse visual prognosis [16, 34]. Improved visual acuity after chemotherapy was independent of any radiologic improvement in tumor size [3, 33]. Preliminary data using bevacizumab (VEGF monoclonal antibody) suggests some potential for visual improvement, though more robust longitudinal studies are needed [45, 46].
13.4.4 Radiation Therapy
Despite the efficacy of RT against OPG [1, 4, 17], the adverse effects of RT, including neurocognitive and pituitary deficits as well as vascular injury and risk of secondary malignancy, have dramatically reduced utilization of this modality for OPG. Patients with NF1 are especially susceptible to malignant transformation of low-grade glioma and development of RT-induced malignancy, such that RT should be avoided except in extreme circumstances [1, 4, 10, 22]. Patients with NF1 are also at an increased risk of the complication of moyamoya vasculopathy in which neovascularization occurs about 40 months after treatment due to ischemia of the internal carotid or cerebral arteries during radiation [5]. It is believed that the proximity of OPGs to the Circle of Willis contributes to the increased prevalence of moyamoya syndrome in this patient population [4, 10].
Because of the heavy burden of complications, radiation is typically used as salvage therapy for patients initially treated with chemotherapy [14], or as a “last resort” treatment for refractory disease or older patients [1]. It is contraindicated in patients younger than 5-years old unless disease progression occurs while undergoing chemotherapy [14]. Radiation used as salvage therapy tends to be less effective for visual outcomes than primary radiation [47].
13.4.5 Surgical Resection and Debulking
When Packer and colleagues published their work on the vincristine + carboplatin regimen for OPGs in 1993, they noted “a relative consensus that for surgically excisable lesions, gross total resection is the treatment of choice” [30]. With newer vision-preserving treatment options for OPGs, such as chemotherapy, surgery is less readily performed due to the high risk of permanent blindness and even binocular vision loss [5]. The new consensus is that surgery should only be performed on eyes with no visual potential [4, 11, 22]. In conjunction with severe visual impairment, additional indications for surgery include:
-
Evidence of tumor growth toward chiasm [5].
Standard surgical approach is a lateral orbitotomy, with entrance to the orbital rim at the angle of the lateral canthus [1]. Resection of the entire length of the optic nerve requires a transcranial approach, which carries a higher risk of endocrinological and cerebrovascular damage [1]. New technology in computer-assisted surgery has improved surgical navigation by using a patient’s neuroimaging to aid in surgical planning, simulation, and intraoperative safety near critical structures [48]. One example reported in the literature is a Medtronic Stealthstation S7® Surgical Navigation System, which utilizes an electromagnet near the surgical site to calibrate a tracker based on the patient’s preoperative imaging [48]. The mirroring technique involves the projection of a patient’s anatomy from the normal side onto the surgical site to allow intraoperative visualization of normal anatomy, which is another tool for minimizing damage to vital structures [48]. Although these surgical advances are streamlining the operative process for patients needing debulking or resection, other alternative treatments such as chemotherapy are still preferred.
13.4.6 Novel Treatments and Future Directions
13.4.6.1 Murine Models
Research on molecular targets for pharmaceuticals requires an animal model that can closely simulate human disease progression. The genetically engineered mouse (GEM) for NF1-OPGs has proven to be a successful model for the evaluation of the pathogenesis of OPG development and subsequent visual loss [7]. In particular, the Nf1 GEM strain (Nf1flox/mut; GFAP-Cre, FMC mice) develops low-grade pre-chiasmatic and chiasmatic optic gliomas analogous to the human variants [7].
13.4.6.2 Molecular Targeting
The mitogen-activated protein kinase (MAPK) pathway plays a role in the pathogenesis of OPGs, and more recently available MAPK inhibitors are being increasingly integrated into the treatment plan for refractory or progressive OPGs (particularly if associated with NF1) [1, 19]. One such agent, selumetinib, specifically acts by inhibiting MEK-1/2 and has shown early promising results for pediatric LGG [19]. Banerjee et al. reported outcomes from the pediatric phase I clinical trial of selumetinib in children with refractory or recurrent LGG [19]. Dose levels ranged from 25 to 43 mg/m2/dose BID, and the most common dose-limiting toxicities were elevated amylase/lipase, headache, mucositis, and rash. The recommended phase 2 dose was defined as 25 mg/m2/dose BID, which resulted in a 2-year PFS of 69 ± SE 9.8%. Fangusaro et al. have since reported outcomes from the phase II study on which 13 patients with NF1-associated OPG and 25 with sporadic, non-NF1-associated hypothalamic/optic pathway LGG experienced 2-year PFS of 96% and 78%, respectively [35, 49]. Other clinical trials evaluating other MEK1/2 inhibitors and other targeted therapies, such as RAF, FGFR, and NTRK inhibitors, are ongoing.
Sorafenib, a multi-kinase inhibitor, was discontinued in a phase II trial due to concern for the acceleration of tumor growth [10] possibly due to resistance to BRAF inhibition causing paradoxical MAPK activation [19].
Lovastatin is a nonspecific RAS inhibitor that decreases RAS/mTOR activity implicated in tumor growth while increasing cAMP production that is protective to the RGC [7]. Lovastatin is currently utilized as a treatment for learning disabilities in children with NF-1 [7]. In an FMC mouse model, administration of lovastatin decreased microglia infiltration, increased RGC survival, and decreased RNFL thinning compared to controls [7]. This effect persisted for 2 months after cessation of treatment, at which point tumor activity was stable. This study corroborates the efficacy of targeting the MAPK pathway, and it highlights the opportunity to intervene early in disease progression in order to prevent further irreversible damage to the RGCs and RNFL.
13.5 Differences Between Pediatric and Adult Patients
Briefly, OPGs are rare in adults but tend to be malignant when they do occur. Since 1973, approximately 70 cases of malignant optic pathway gliomas in adults have been reported, with a mean age of onset in the sixth decade [50]. While the pediatric population has a strong association with NF-1, adult OPGs arise sporadically [50]. Tissue biopsy is often necessary for diagnosis, with pathology most commonly demonstrating anaplastic astrocytoma (WHO grade III) or glioblastoma multiforme (WHO grade IV) [50]. For these histologies, treatment involves surgical resection, RT (54–60 Gy), and often chemotherapy [50]. Temozolomide, an alkylating agent, has been shown to be effective in targeting malignant gliomas [50]. Unlike pediatric patients, survival rates are poor and most patients die within a year of disease onset [50].
Key Points
-
1.
OPGs arise sporadically or in conjunction with NF-1. Although they are benign and tend to be slow-growing, the clinical course is variable and may result in significant and debilitating vision loss.
-
2.
Yearly screening with a thorough ophthalmologic exam is recommended for pediatric patients with NF-1 under 8 years of age.
-
3.
Although consensus on a treatment regimen for OPGs is lacking, there is strong evidence to support the use of chemotherapy as a first-line approach for patients with symptomatic OPGs (carboplatin and vincristine).
-
4.
Radiation can be highly effective but use is highly discouraged given acceptable efficacy of numerous chemotherapy regimens and availability of newer targeted agents. Surgery should be reserved for management of painful proptosis in an eye with no meaningful vision.
-
5.
Molecularly targeted therapy is a promising future direction for the treatment of OPGS. MEK inhibitors have shown early success, though long-term studies are needed.
References
Farazdaghi MK, Katowitz WR, Avery RA. Current treatment of optic nerve gliomas. Curr Opin Ophthalmol. 2019;30(5):356–63.
Freret ME, Gutmann DH. Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1. J Neurosci Res. 2019;97(1):45–56.
Shofty B, Ben-Sira L, Kesler A, Jallo G, Groves ML, Iyer RR, et al. Isolated optic nerve gliomas: a multicenter historical cohort study. J Neurosurg Pediatr. 2017;20(6):549–55.
Fried I, Tabori U, Tihan T, Reginald A, Bouffet E. Optic pathway gliomas: a review. CNS Oncol. 2013;2(2):143–59.
Shapey J, Danesh-Meyer HV, Kaye AH. Diagnosis and management of optic nerve glioma. J Clin Neurosci. 2011;18(12):1585–91.
Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18(7):471–8.
Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro-Oncology. 2017;19(6):808–19.
Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–98.
de Blank PMK, Fisher MJ, Liu GT, Gutmann DH, Listernick R, Ferner RE, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37(Suppl 1):S23–32.
Campen CJ, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1. J Child Neurol. 2018;33(1):73–81.
Karaconji T, Whist E, Jamieson RV, Flaherty MP, Grigg JRB. Neurofibromatosis type 1: review and update on emerging therapies. Asia-Pacific J Ophthalmol (Philadelphia, Pa). 2019;8(1):62–72.
Opocher E, Kremer LC, Da DL, van de Wetering MD, Viscardi E, Caron HN, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42(12):1807–16.
Hamideh D, Hoehn ME, Harreld JH, Klimo PD, Gajjar A, Qaddoumi I. Isolated optic nerve glioma in children with and without neurofibromatosis: retrospective characterization and analysis of outcomes. J Child Neurol. 2018;33(6):375–82.
Nair AG, Pathak RS, Iyer VR, Gandhi RA. Optic nerve glioma: an update. Int Ophthalmol. 2014;34(4):999–1005.
Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–6.
Dodgshun AJ, Elder JE, Hansford JR, Sullivan MJ. Long-term visual outcome after chemotherapy for optic pathway glioma in children: site and age are strongly predictive. Cancer. 2015;121(23):4190–6.
Chong AL, Pole JD, Scheinemann K, Hukin J, Tabori U, Huang A, et al. Optic pathway gliomas in adolescence—time to challenge treatment choices? Neuro-Oncology. 2013;15(3):391–400.
Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017;214(1):17–25.
Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Poussaint TY, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a pediatric brain tumor consortium (PBTC) study. Neuro-Oncology. 2017;19(8):1135–44.
Dodge HW Jr, Love JG, Craig WM, Dockerty MB, Kearns TP, Holman CB, et al. Gliomas of the optic nerves. AMA Arch Neurol Psychiatry. 1958;79(6):607–21.
Taylor T, Jaspan T, Milano G, Gregson R, Parker T, Ritzmann T, et al. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol. 2008;81(970):761–6.
Bergqvist C, Servy A, Valeyrie-Allanore L, Ferkal S, Combemale P, Wolkenstein P, et al. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):33–7.
Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single Centre. Eur J Pediatr. 2000;159(9):692–6.
Ho CY, Deardorff R, Kralik SF, West JD, Wu YC, Shih CS. Comparison of multi-shot and single shot echo-planar diffusion tensor techniques for the optic pathway in patients with neurofibromatosis type 1. Neuroradiology. 2019;61(4):431–41.
Kruer MC, Kaplan AM Jr, Etzl MM Jr, Carpentieri DF, Dickman PS, Chen K, et al. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neuro-Oncol. 2009;95(2):239–45.
Avery RA, Hwang EI, Ishikawa H, Acosta MT, Hutcheson KA, Santos D, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132(3):265–71.
Avery RA, Liu GT, Fisher MJ, Quinn GE, Belasco JB, Phillips PC, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–9.e2.
Moradi P, Robson AG, Rose GE, Holder GE. Electrophysiological monitoring in a patient with an optic nerve glioma. Doc Ophthalmol Ophthalmol. 2008;117(2):171–4.
Heidary G, Fisher MJ, Liu GT, Ferner RE, Gutmann DH, Listernick RH, et al. Visual field outcomes in children treated for neurofibromatosis type 1-associated optic pathway gliomas: a multicenter retrospective study. J AAPOS. 2020;24(6):349.e1–5.
Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–6.
Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the children’s oncology group. J Clin Oncol. 2012;30(21):2641–7.
Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, Carret AS, Lafay-Cousin L, Larouche V, Hawkins CE, Pond GR, Poskitt K, Keene D, Johnston DL, Eisenstat DD, Krishnatry R, Mistry M, Arnoldo A, Ramaswamy V, Huang A, Bartels U, Tabori U, Bouffet E. Phase II weekly vinblastine for chemotherapy-Naïve children with progressive low-grade Glioma: a Canadian pediatric brain tumor consortium study. J Clin Oncol. 2016;34(29):3537–43.
Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–63.
Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a pediatric brain tumor consortium (PBTC) study. Neuro-Oncology. 2017;19(8):1135–44.
Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, Pollack IF, Qaddoumi I, Jakacki RI, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Jones DTW, Pfister SM, Vezina G, Stern JS, Panigrahy A, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–22.
Ronsley R, Hounjet CD, Cheng S, Rassekh SR, Duncan WJ, Dunham C, Gardiner J, Ghag A, Ludemann JP, Wensley D, Rehmus W, Sargent MA, Hukin J. Trametinib therapy for children with neurofibromatosis type 1 and life-threatening plexiform neurofibroma or treatment-refractory low-grade glioma. Cancer Med. 2021;10(11):3556–64.
Awada G, Serruys D, Schwarze JK, Van De Voorde L, Duerinck J, Neyns B. Durable complete response of a recurrent mesencephalic glioblastoma treated with trametinib and low-dose dabrafenib in a patient with neurofibromatosis type 1. Case Rep Oncol. 2020;13(2):1031–6.
Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, Pajtler KW, Sahm F, Bahr A, Simon M, Jones DTW, Well L, Mautner VF, Capper D, Hernáiz Driever P, Gnekow A, Pfister SM, Witt O, Milde T. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neuro-Oncol. 2020;149(3):499–510.
Manoharan N, Choi J, Chordas C, Zimmerman MA, Scully J, Clymer J, Filbin M, Ullrich NJ, Bandopadhayay P, Chi SN, Yeo KK. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neuro-Oncol. 2020;149(2):253–62.
Gururangan S, Cavazos CM, Ashley D, Herndon JE 2nd, Bruggers CS, Moghrabi A, Scarcella DL, Watral M, Tourt-Uhlig S, Reardon D, Friedman HS. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20(13):2951–8.
Wright KD, Yao X, London WB, Kao PC, Gore L, Hunger S, Geyer R, Cohen KJ, Allen JC, Katzenstein HM, Smith A, Boklan J, Nazemi K, Trippett T, Karajannis M, Herzog C, Destefano J, Direnzo J, Pietrantonio J, Greenspan L, Cassidy D, Schissel D, Perentesis J, Basu M, Mizuno T, Vinks AA, Prabhu SP, Chi SN, Kieran MW. A POETIC phase II study of continuous oral everolimus in recurrent, radiographically progressive pediatric low-grade glioma. Pediatr Blood Cancer. 2021;68(2):e28787.
Cappellano AM, Petrilli AS, da Silva NS, Silva FA, Paiva PM, Cavalheiro S, Bouffet E. Single agent vinorelbine in pediatric patients with progressive optic pathway glioma. J Neuro-Oncol. 2015;121(2):405–12.
Khaw SL, Coleman LT, Downie PA, Heath JA, Ashley DM. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49(6):808–11.
Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Fahey F, Vajapeyam S, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Fouladi M, Kun LE. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric brain tumor consortium study. Neuro-Oncology. 2014;16(2):310–7.
Yamasaki F, Takano M, Yonezawa U, Taguchi A, Kolakshyapati M, Okumichi H, Kiuchi Y, Kurisu K. Bevacizumab for optic pathway glioma with worsening visual field in absence of imaging progression: 2 case reports and literature review. Childs Nerv Syst. 2020;36(3):635–9.
Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–4.
Awdeh RM, Kiehna EN, Drewry RD, Kerr NC, Haik BG, Wu S, et al. Visual outcomes in pediatric optic pathway glioma after conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(1):46–51.
Hussain A, Wan M, DeAngelis D. Progressive optic nerve glioma: orbital biopsy technique using a surgical navigation system. Can J Ophthalmol. 2018;53(1):e18–22.
Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, Campagne O, Banerjee A, Gururangan S, Kilburn L, Goldman S, Qaddoumi I, Baxter P, Vezina G, Bregman C, Patay Z, Jones JY, Stewart CF, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. A phase 2 trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a pediatric brain tumor consortium study. Neuro Oncol. 2021:noab047. https://doi.org/10.1093/neuonc/noab047. Epub ahead of print
Alabiad CR, Shah VS, Eatz TA, Sternau LL, Lam BL. Malignant optic nerve glioma in a young woman with 7 year follow up without recurrence. Am J Ophthalmol case reports. 2020;19:100862.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tukan, A., Hoffman, L.M., Ramasubramanian, A. (2022). Current Management of Optic Pathway Glioma. In: Ramasubramanian, A. (eds) Pediatric Ophthalmology. Current Practices in Ophthalmology. Springer, Singapore. https://doi.org/10.1007/978-981-19-4963-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-19-4963-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4962-3
Online ISBN: 978-981-19-4963-0
eBook Packages: MedicineMedicine (R0)