Abstract
The management of progressive unresectable low-grade glioma remains controversial. Treatment options have included radiotherapy, and more recently chemotherapy, usually following an initial period of observation. Within this context, we evaluated vinorelbine, a semi-synthetic vinca alkaloid that has shown evidence of activity against glioma. From July 2007 an institutional protocol with vinorelbine (30 mg/m2 days 0, 8, 22) for a total of 18 cycles, has been conducted at IOP/GRAACC/UNIFESP for children with optic pathway glioma (OPG). The main objectives were clinical and radiological response, as well as toxicity profile. Twenty-three patients with progressive OPG with a mean age of 69 months (4–179) were enrolled. Three patients had a diagnosis of neurofibromatosis type 1. Twenty-two patients were assessable for response with an overall objective response rate of 63 %, with eight patients showing stable disease. The most important toxicity was hematologic (grade III/IV neutropenia) observed in four patients. Gastrointestinal toxicity (grade I/II vomiting) was observed in seven patients and only 1 patient showed grade I peripheral neuropathy. The median progression-free survival (PFS) was 33 months (6.9–69) with a 3 and 5 year PFS of 64 ± 19 and 37 ± 20 %, respectively, for an overall 3 and 5 year-survival of 95 ± 10 %. This study suggests that vinorelbine may be an interesting option for pediatric low-grade gliomas, showing low toxicity profile and providing a good quality of life for patients with such chronic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-grade gliomas (LGGs) represent 30–40 % of all childhood brain tumors [1]. Optimal treatment, when possible, is surgical resection [2–4]. However, for patients with midline or recurrent tumors, appropriate management remains unclear. Although radiotherapy afford some disease control, it may promote long term adverse effects, especially in younger children and patients with neurofibromatosis type1 (NF1) [5, 6]. Therefore, chemotherapy has become the mainstay treatment for children with progressive unresectable LGG. A variety of regimens have showed efficacy, inducing mostly objective tumor response [7–18]. However, given the chronicity of these conditions, administration of repeated lines of chemotherapy can lead to undesirable side effects [8–12, 14, 15]. In this context, and based on evidence of activity of single agent vinca alkaloids, the authors evaluated vinorelbine; this semi-synthetic vinca alkaloid preferentially binds to the mitotic spindle instead of axonal neurons, promoting less neurotoxicity than other compounds in this class [17–19]. Despite evidence of activity on solid tumors, there are few reports on vinorelbine in brain tumors [20–24]. We conducted this study in children and adolescent with optic pathway gliomas (OPG).
Materials and methods
This phase II study was approved by the local Institutional Research Ethics Board and opened to accrual in July 2007 at IOP/GRAACC/Federal University of São Paulo. Data were collected and analysed in June 2013.
Eligibility criteria
The following criteria were required for enrolment in the study: (1) Patients with newly diagnosed OPG that required immediate treatment due to progressive symptoms or patients with indolent OPG that showed progression on consecutive imaging studies and/or visual deterioration. (2) Patients with recurrent/refractory tumors, recurrence defined as progression following completion of previous treatment and refractoriness as progression during chemotherapy; (3) age ≤ 21 years old when originally diagnosed; (3) Histologic confirmation at diagnosis was recommended. However, histology was not mandatory for patients with intrinsic chiasmatic tumors and OPG associated with NF1; (4) Patients with evidence of dissemination were eligible for the study; (5) Recurrent/refractory patients must have evidence of radiographic progression, (i.e. >25 % enlargement on magnetic resonance imaging (MRI), and/or clinical deterioration such as impairment of visual acuity; (6) interval of at least 4 weeks from previous treatment; (7) Corticosteroids were allowed to control progressive symptoms, if necessary. Patients must be on a stable or decreasing dose for at least 1 week prior to enrolment; (8) adequate hematologic, renal and hepatic functions; (8) written informed consent as approved by local institutional board. Cytologic examination of the cerebrospinal fluid and MRI scan of the neuroaxis was recommended but not mandatory.
Treatment
The treatment plan consisted of the administration of intravenous vinorelbine at a dose of 30 mg/m2 on days 0, 8 and 22, during eighteen 4 weekly cycles for a total of 54 injections. For children weighting <10 kg, doses were calculated by body weight (1 mg/kg/dose). Before each cycle the absolute neutrophil count was to be ≥500/mm3, platelet count >100.000/mm3, creatinine level <1.5 mg/dL and transaminases <1.5× the institutional normal level. Therapy was delayed if the patient did not meet the criteria, and in case of fever and neutropenia until recovery, with decrease by 25 % of the dose, depending on individual situations. In case of grade III/IV vinorelbine-related neurotoxicity, treatment was withheld until evidence of improvement and the dose was reduced by 25 % during the following cycle.
Evaluation of response
Initial staging consisted of a brain MRI without/with contrast administration ± neuroaxis if clinically indicated. Patients underwent a detailed clinical examination at study entry. Whenever possible, visual assessment was performed at the time of inclusion, during and after treatment. Assessment was obtained using the Snellen linear chart method and single field analyses. A 2-line decrease in visual acuity compared with the pre-chemotherapy examination was defined as worsening. Similarly, improvement was defined as a 2-line increase in acuity. For visual fields, deterioration was defined as a loss of 25 % or more of the field. In children who were not able to proceed with this method, visual acuity was assessed using visual-evoked potentials.
MRI assessments were performed after the 4th, 8th, 12th and 18th cycles and every 4 months after the treatment. Tumor measurements were assessed by two physicians (FAS and EB) blinded from clinical information and calculated on bi-or tri-dimensional measures, depending on the shape of the lesion and in the non-enhanced FLAIR and enhanced T1-weighted images. Complete response (CR) was defined as no radiological evidence of tumor. Partial response (PR) as ≥50 % reduction in the product of the two greatest tumor diameters (≥65 % for 3D measurement). Minor Response (MR) as 25–50 % reduction (40–65 % for 3D measurement). Stable disease (SD) < 25 % decrease (40 % for 3D measurement) and Progressive disease (PD) as >25 % increase (40 % for 3D measurement) in the tumor size. Because previous studies have shown some early progression followed by stabilization or response, progression had to be confirmed by a second scan at 4–8 weeks showing further increase in tumor size. In this trial, objective response (OR) was defined as CR, PR or MR with stable or improved clinical findings. In addition and independently of radiological changes, children who showed visual deterioration on two consecutive visual assessments were deemed to have PD.
Statistical considerations
The primary endpoint of the trial was response rate to single agent vinorelbine and secondary endpoints were the 3 and 5-year progression free and overall survival, safety and duration of response. The best response rates reported in the literature using single agents are 36 % with vinblastine and 29 % with carboplatin [11, 18]. A two-stage design was used for patient accrual, based on the occurrence of OR. Initially, ten patients were to be accrued. If ≤2 patients responded to vinorelbine, the study would be discontinued due to lack of efficacy. If ≥3 patients had responded, ten additional patients would be enrolled and treated (Simon’s optimal two stage design). With adjustment for potential incomplete data, the required sample size would be 23 patients. Response was evaluated clinically and with MRI scan overall survival (OS) was defined as the time in months to death from any cause from date of entrance into the study. Progression-free survival (PFS) was defined as the time in months to first disease progression, disease recurrence or disease related-death from date of entrance into the study. Survival times (OS and PFS) were calculated according to the Kaplan–Meier methods.
Results
Patient characteristics
A total of 23 patients with a diagnosis of OPG were enrolled in the study. Eleven patients had biopsy proven low grade glioma and 12 patients—including three with NF1—had lesions that were considered typical on radiology, therefore were not confirmed histologically. There were 15 males. Three patients had NF1, three presented with features of diencephalic syndrome and one had disseminated disease at diagnosis. The mean age at diagnosis was 69 months (range 4–179 months). The mean time between the initial symptoms and diagnosis was 14 months (range 1–48 months). Eighteen patients were newly diagnosed and chemotherapy naïve, whereas five patients had previously been treated with ≥1 line of chemotherapy (range 1–4). Eligibility criteria were met for all patients: nine patients on observation were included due to visual deterioration, including two with concomitant radiographic progression; 14 patients had radiographic progression or large tumors that were considered for immediate treatment including three with diencephalic syndrome. Patients’ characteristics are listed in Table 1. Eleven patients underwent surgery, including six partial resections and five biopsies. On histology, ten tumors were consistent with the diagnosis of pilocytic astrocytoma and one was described as grade two astrocytic tumors. Two patients were on steroids (dexamethasone, 0.10–0.15 mg/kg/dose BID) at the time of treatment initiation.
At the time of last follow-up analysis in June 2013, all OPG patients had completed therapy or had discontinued treatment because of progression, toxicity or death. The median follow-up for the population was 45.8 months (range 6.8–69.4 months).
Toxicity
One patient developed atypical pneumonia after 8 cycles of chemotherapy. This patient was receiving steroids for symptom control with SD on MRI scan. He rapidly deteriorated, required intubation and died despite mechanical ventilation. Outside this complication, treatment was mostly well tolerated. Overall 1,124 doses of vinorelbine were administered, and 48 adverse events that were likely related to the treatment were reported in 14 patients. The most common toxicity observed was hematologic with eight patients experiencing 13 episodes of grade I/II anemia. Grade I/II neutropenia was observed in three patients (4 episodes) and grade III/IV in four patients (7 episodes). Two patients under the age of 3, experienced severe neutropenia. The first developed febrile neutropenia and sepsis after two doses of vinorelbine and was taken off study. The second patient developed 2 episodes of febrile neutropenia, treated with IV antibiotics in an outpatient setting, with temporarily reduction in 25 % of vinorelbine’s dose. No patient required platelet or red cell transfusion.
Seven patients experienced gastrointestinal toxicity (12 episodes) with grade I/II vomiting. Neurotoxicity was observed in four patients and included grade I abdominal pain (6 episodes) and grade I peripheral neuropathy in one patient.
Response
All patients were assessable for response except for one patient who discontinued treatment after two doses of vinorelbine due to sepsis. Responses to vinorelbine are listed in Fig. 1. The best response observed for the 22 evaluable patients was 1CR, 9PR, and 4MR, for an overall OR rate (CR + PR + MR) of 63 % (95 % CI 43–81 %) and a mean of 8 cycles to achieve the best response (Fig. 2). The response rate including only CR + PR was 45 % (95 % CI 27–65 %). The remaining patients (n = 8, 36 %) had SD.
The three NF1 patients initiated treatment after a period of observation with evidence of tumor progression. One patient with a 3 cm chiasmatic glioma extending to the right optic nerve initiated treatment due to radiological progression on consecutive scans. No surgery was performed. He achieved a CR after 12 cycles, with disappearance of contrast enhancement and normalisation of chiasmatic and optic nerve enlargement. The two other NF1 patients showed MR and SD, respectively. All three children with diencephalic syndrome demonstrated clinical response, two with radiological improvement (1MR and 1PR).
Of the nine patients who had baseline visual assessment, four were stable and five showed improvement, one of them with recovery of color vision. Other patients were considered to be too young to have their vision reliably assessed at the time of initiation of vinorelbine.
During treatment, two patients experienced radiological progression, with previous SD and MR, 11 and 12 months after starting treatment, respectively; seven other patients experienced progression 4 months to 2 years and 10 months after completion of treatment. In four patients, progression was diagnosed on imaging, while in the other three was based on deterioration of visual field with SD on MRI. Among the five patients who received vinorelbine after failure of previous chemotherapy regimens, two showed SD, 2MR and 1PR by the end of treatment. Three of them experienced further progression after completion of treatment. One of these three patients had previously experienced early progression with previous chemotherapy regimens (carboplatin/vincristine, vinblastine, cisplatin/etoposide and temozolomide). He showed sustained stabilization with vinorelbine until progression occurred, based on visual impairment while the tumor remained stable on imaging.
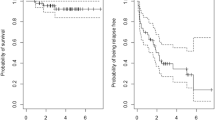
Median PFS for the 22 evaluable patients was 33 months (range 6.9–69 months), with a 3-year PFS and OS of 64 % (95 % CI 45–83 %) and 95 % (95 % CI 85–100 %), respectively; and an estimated 5-year PFS of 37 % (95 CI 17–57 %) and OS of 95 (95 % CI 85–100 %) (Fig. 3).
Discussion
The results of this phase II study support the use of single agent vinorelbine in the management of pediatric patients with LGG of the optic pathway. These tumors account for 5 % of all childhood brain tumors and represent a relatively homogeneous group of progressive unresectable LGG. Most OPG arise in children younger than 5 years of age although they may also be seen in teenagers. The behavior of these tumors is highly unpredictable [25]. The main threat associated with OPG is visual impairment and ultimately blindness [26]. Still, some OPG can also show aggressive behavior and may lead to death, particularly in the younger population [25]. Treatment with surgery is usually associated with a risk of serious morbidity, including visual loss and endocrine impairment [27]. The role of radiotherapy remains controversial and is reserved to older children and patients who have failed chemotherapy [28]. While there is certainly some role for observation in the context of indolent OPG, increasingly, physicians are using chemotherapy as a standard first line treatment for progressive unresectable LGG. Over the last 30 years, various protocols of chemotherapy have been investigated. Several agents and combinations have demonstrated activity, with a response rate up to 36 % for single agents and 78 % for combinations (see Table 2). Currently, the combination of vincristine and carboplatin is the most used first line option, although this combination did not show significant advantage over the TPCV regimen (thioguanine, procarbazine, lomustine and vincristine) in a large randomised study conducted by the Children’s Oncology Group (COG) [8, 9, 29].
Promising results were reported in a phase II study using the single agent vinblastine, a vinca alkaloid that has traditionally be used in the management of some solid tumors as in Hodgkin’s disease [18]. Vinorelbine being a semi-synthetic vinca alkaloid, it was logical to test its activity against pediatric LGG. This agent has a broad-spectrum activity with low level of toxicity. It has demonstrated efficacy as a single agent and in combination therapy against some adult tumors and more recently against pediatric sarcomas, with a response rate between 36–58 % in phase II studies conducted by COG and the Istituto Nazionale Tumori [20, 30]. The COG study enrolled 22 patients with CNS tumors: with two responses, one recurrent medulloblastoma patient with PR sustained over a period of 80 weeks and one astrocytoma patient with PR that lasted for 24 weeks. In addition, three CNS tumor patients had SD (diagnosis not provided). Reports of SD in CNS tumor patients were also noted in a COG phase I study, with tumor control in three patients-astrocytoma, meningioma and ependymoma, respectively [22]. A sustained response was also described by Biassoni et al. in pediatric patient with recurrent glioblastoma [31]. Outside these early clinical trials and anecdotal reports, vinorelbine has not been studied against a large spectrum of CNS tumors. In particularly, there has been no trial of vinorelbine in pediatric LGG. Matthew et al. evaluated the activity of vinorelbine against xenografts derived from adult and pediatric CNS malignancies. In this experiment, vinorelbine demonstrated activity against several human glioma xenografts [22].
Our experience confirms previous reports from Rosenstock and Bouffet et al., suggesting that vinca alkaloids have an activity against paediatric LGG [18, 19]. The response rate observed in the current study is excellent, with 63 % of the patients showing OR. The projected 5-year PFS is in keeping with the results of other chemotherapy studies, summarized in Table 2. These results are interesting as there is increasing evidence that progressive unresectable LGG often have a growth potential that requires several lines of treatment [32]. The efficacy/toxicity profile of vinorelbine is without any doubt another advantage, as the repeated use of different chemotherapy agents exposes patients to a risk of cumulative toxicity, including hearing loss and bone marrow failure [9, 12]. There is no recognized long term or cumulative toxicity associated with prolonged use of vinorelbine. This makes this agent an appealing option in the armamentum of drugs to treat paediatric LGG. However, in our experience, two infants developed severe neutropenia. Increased haematological toxicity in infants has not been reported in previous studies of vinorelbine. Future trials should pay attention to this group of patients in order to provide more information on the potential relationship between age and toxicity. Whether vinorelbine acts as a cell cycle-dependent antimitotic agent or through an anti-angiogenic mechanism is unknown. In the absence of available animal models of LGG, the understanding of the activity of antineoplastic agents, in particular vinca alkaloids, in this tumor is only speculative. The results of this study confirm the important role of vinca alkaloids in the management of pediatric LGGs, as both vinblastine and vinorelbine have shown promising activity and a low toxicity profile. Although the response rate observed with vinorelbine appears superior compared to that observed in the recent phase II of vinblastine (63 vs. 35 %), the 5-year progression free survival in the two studies is comparable. At this time no conclusions concerning the superiority of either of these therapeutic options can be made. However, since vinorelbine has an oral formulation, this offers the prospect to consider an oral agent for the management of this condition, with inherent benefits in terms of quality of life [23].
The limitation of this trial is the small size sample of the patient population. However, they represent an unselected group of consecutive patients seen in a large single institution. The lack of correlative biology study is another limitation. The recent description of the BRAF-KIAA 1549 fusion and its potential role in predicting the behavior of paediatric LGG may help in the treatment decision and may contribute to an interpretation of the chemotherapy trials [33]. However, in the present trial, the shortage histology material did not allow such correlative study. Finally, the young age of many participants in this trial precluded a systematic and thorough assessment of visual changes during the period of treatment. Such limitation is not unique to our trial, as none of the paediatric OPG clinical trials conducted lately has used a standardized visual acuity testing protocol to measure the visual benefit of chemotherapy in children with OPG [34–36]. There is an urgent need to develop international studies with clear ophthalmologic endpoints to overcome the lack of knowledge on visual outcome in OPG patients.
In conclusion, this phase II study showed interesting efficacy of another vinca alkaloid as single agent for the treatment of progressive unresectable LGG and particularly OPG, with low toxicity and excellent quality of life. Future studies should consider oral vinorelbine as a potential option for children with LGG.
References
CBTRUS (2000–2004) Statistical report: primary brain tumors in the United States. 2000–2004, the central brain tumor. Registry of the United States, Hinsdale
Medlock MD, Scott RM (1997) Optic chiasm astrocytomas of childhood. 2. Surgical management. Pediatr Neurosurg 27(3):129–136
Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y et al (1997) Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol 15(8):2792–2799
Watson GA, Kadota RP, Wisoff JH (2001) Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol 11(2):152–162
Grabenbauer GG, Schuchardt U, Buchfelder M, Rödel CM, Gusek G, Marx M et al (2000) Radiation therapy of optico-hypothalamic gliomas (OHG)–radiographic response, vision and late toxicity. Radiother Oncol 54(3):239–245
Grill J, Couanet D, Cappelli C, Habrand JL, Rodriguez D, Sainte-Rose C, Kalifa C (1999) Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 45(3):393–396
Perilongo G (2005) Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol 75(3):301–307
Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R et al (1993) Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol 11(5):850–856
Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS et al (1997) Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 86(5):747–754
Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA et al (1997) Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol 32(3):235–241
Gururangan S, Cavazos CM, Ashley D, Herndon JE 2nd, Bruggers CS, Moghrabi A et al (2002) Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol 20(13):2951–2958
Massimino M, Spreafico F, Cefalo G, Riccardi R, Tesoro-Tess JD, Gandola L et al (2002) High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol 20(20):4209–4216
Fouladi M, Hunt DL, Pollack IF, Dueckers G, Burger PC, Becker LE et al (2003) Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer 98(6):1243–1252
Massimino M, Spreafico F, Riva D, Biassoni V, Poggi G, Solero C et al (2010) A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol 100(1):65–71
Gururangan S, Fisher MJ, Allen JC, Herndon JE 2nd, Quinn JA, Reardon DA et al (2007) Temozolomide in children with progressive low-grade glioma. Neuro Oncol 9(2):161–168
Packer RJ, Jakacki R, Horn M, Rood B, Vezina G, MacDonald T et al (2009) Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 52(7):791–795
Lafay-Cousin L, Holm S, Qaddoumi I, Nicolin G, Bartels U, Tabori U et al (2005) Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer 103(12):2636–2642
Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M et al (2012) Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 30(12):1358–1363
Rosenstock JG, Evans AE, Schut L (1976) Response to vincristine of recurrent brain tumors in children. J Neurosurg 45(2):135–140
Casanova M, Ferrari A, Spreafico F, Terenziani M, Massimino M, Luksch R et al (2002) Vinorelbine in previously treated advanced childhood sarcomas: evidence of activity in rhabdomyosarcoma. Cancer 94(12):3263–3268
Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C et al (2004) Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer 101(7):1664–1671
Hanley ML, Elion GB, Colvin OM, Modrich PL, Keir S, Adams DJ et al (1998) Therapeutic efficacy of vinorelbine against pediatric and adult central nervous system tumors. Cancer Chemother Pharmacol 42(6):479–482
Johansen M, Kuttesch J, Bleyer WA, Krailo M, Ames M, Madden T et al (2006) Phase I evaluation of oral and intravenous vinorelbine in pediatric cancer patients: a report from the Children’s Oncology Group. Clin Cancer Res 12(2):516–522
Cappellano AM, Bouffet E, Cavalheiro S, Seixas MT, da Silva NS et al (2011) Diffuse intrinsic brainstem tumor in an infant: a case of therapeutic efficacy with vinorelbine. J Pediatr Hematol Oncol 33(2):116–118
Qaddoumi I, Sultan I, Broniscer A (2009) Pediatric low-grade gliomas and the need for new options for therapy: why and how? Cancer Biol Ther 8(1):4–10
Avery RA, Fisher MJ, Liu GT (2011) Optic pathway gliomas. J Neuroophthalmol 31(3):269–278
Steinbok P, Hentschel S, Almqvist P, Cochrane DD, Poskitt K et al (2002) Management of optic chiasmatic/hypothalamic astrocytomas in children. Can J Neurol Sci 29(2):132–138
Nicolin G, Parkin P, Mabbott D, Hargrave D, Bartels U, Tabori U et al (2009) Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer 53(7):1231–1237
Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR et al (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30(21):2641–2647
Kuttesch JF Jr, Krailo MD, Madden T, Johansen M, Bleyer A, Children’s Oncology Group (2009) Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: a Children’s Oncology Group study. Pediatr Blood Cancer 53(4):590–593
Biassoni V, Casanova M, Spreafico F, Gandola L, Massimino M et al (2006) A case of relapsing glioblastoma multiforme responding to vinorelbine. J Neurooncol 80(2):195–201
Scheinemann K, Bartels U, Tsangaris E, Hawkins C, Huang A, Dirks P et al (2011) Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer 57(1):84–88
Rodriguez FJ, Lim KS, Bowers D, Eberhart CG (2013) Pathological and molecular advances in pediatric low-grade astrocytoma. Annu Rev Pathol 8:361–379
Avery RA, Bouffet E, Packer RJ, Reginald A (2013) Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci 54(2):1034–1038
Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ et al (2012) Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol 14(6):790–797
Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S (2010) Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer 46(12):2253–2259
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cappellano, A.M., Petrilli, A.S., da Silva, N.S. et al. Single agent vinorelbine in pediatric patients with progressive optic pathway glioma. J Neurooncol 121, 405–412 (2015). https://doi.org/10.1007/s11060-014-1652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1652-6