Abstract
The plant rhizosphere hosts a vast array of microbes including bacteria, fungi, and others that provide nutrient absorption and plant protection among other crucial functions. Recent research shows that the plant defense system through the influence of secondary metabolites in root exudates and defense hormones shapes the rhizosphere and endosphere microbiome, promoting certain taxa while removing others. The root-associated microbiota deploys their repertoire of secondary metabolites to antagonize pathogens even before they get to the plant, acting as the true first line of defense while also priming systemic plant defense. Attempts to promote plant protection through the use of one or more such beneficial microbes have not yielded consistent results in field settings. Disease-protective soils that confer strong plant protection have spurred interest in the use of the microbiome to bolster plant protection. The consistent theme arising in recent research has been that healthy resilient microbiomes corresponding to better plant protection are characterized by a higher diversity of microbes, likely nurtured by richer host root exudates. Relatively higher microbial diversity is detected in wild relatives of crops, organic farms, and disease-suppressive soils as opposed to domesticated crops with inorganic fertilizer farming, which also display reduced symbiotic interactions. These observations suggest that a good investment in sustainable farming would be to harness diverse beneficial microbial communities for agriculture and to engineer crop plants to recruit and retain the same, akin to their wild relatives. Microbiome-based agriculture, free from toxic and polluting pesticide and fertilizer use, is, therefore, an exciting advance towards sustainability.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
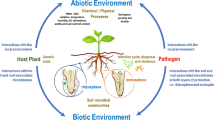
All eukaryotes display complex associations with microbial communities (Lareen et al. 2016). The rhizosphere microbiome refers to the teeming diversity of microbes including bacteria, fungi, oomycetes, archaea, viruses, and protists inside, on, or around plant roots in the soil forming a complex ecosystem (Compant et al. 2019); specifically, the term microbiome indicates the genetic information that identifies these microbes. Rhizosphere microbes compete with each other and the plant for soil nutrients and organic compounds and often assist the plant in accessing the trove of nutrients from the soil. Some of these microbes are free-living, and some colonize the root surface (rhizoplane), while others can live inside the roots and are referred to as endophytes. The best-characterized endophytes include the nitrogen-fixing Rhizobium and arbuscular mycorrhizal fungi. Fungi are also vital members of the rhizosphere microbiome and an estimated 80% of angiosperm species are supposed to associate with mycorrhizal fungi (Wang and Qiu 2006).
The microbiome of an organism serves as an extension of its genome (Turner et al. 2013), conferring new genomic and biochemical functional capabilities. The rhizosphere microbiome bestows on the plants a vastly extended capability of nutrient absorption, disease resistance, immune regulation, and stress tolerance and is an important determinant of growth and productivity (Berendsen et al. 2012; Perez-Jaramillo et al. 2016). The rhizosphere microbiome of each plant is influenced by many factors—primarily the soil microbial diversity which is used to seed the microbiome; the nature of the soil, including water, nutrient, mineral content, and pH; plant genotype; and other environmental conditions. Microbes are attracted to root exudates and other organic material secreted by the roots which contain nutrients and signals to attract microbes for colonization through a process referred to as rhizodeposition, which alters the chemical nature of the rhizosphere environment. Up to 40% of photosynthetically fixed carbon and 20% of plant nitrogen may be released into the soil environment (Odelade and Babalola 2019; Whipps 1990). This highlights the significant investment made by the plant to nurture its microbiome. The quality of the root exudates is dependent on the host genotype and its products of primary and secondary metabolism and is also influenced by the environment; hence, the rhizosphere microbiome composition is a function of the genotype-environment interactions with soil being the major seeding factor.
From a plant defense standpoint, the microbiome functions as an additional layer of protection against pathogens. The existence of certain microbes in the rhizosphere can reduce or avert plant disease (Newitt and Prudence 2019). Rhizosphere microbes add to the repertoire of defense proteins that plants produce such as chitinases and proteases to suppress pathogens (Pinski and Betekhtin 2019). The microbes can synthesize novel antimicrobials that the plant cannot make (Rout 2014), restricting the growth of certain microbes, including potential pathogens. Rhizosphere colonization of bacteria can also induce systemic defense in a process referred to as induced systemic resistance (ISR), wherein plants are primed for a faster and stronger response for defense against infections.
The plant immune system also plays a significant role in selecting microbes from the soil environment (Leach et al. 2017). Plants respond to microbes in the rhizosphere through a process is known as MAMP-triggered immunity (MTI), which senses microbial structures and secretions and limits microbial access to the root environment. In addition, plants utilize a large diversity of secondary metabolites to selectively retain certain microbes while targeting others. For example, the plant stress hormone salicylic acid gates the plant endosphere and limits access to certain microbes, thus shaping the microbiome composition (Lebeis et al. 2015). The plant commensals and symbionts have evolved to tolerate or dampen plant immunity to survive in the rhizosphere and endosphere. The beneficial survivors in the rhizosphere not only stimulate plant growth but also protect them from stress in return for organic carbon and other nutrients. Thus, the plant immune system and the selected rhizosphere microbiota mutually benefit each other.
Crop disease accounts for major losses in agriculture and disease resistance can be bred into crops, but evolving pathogens can overcome the resistance in field settings (Wille et al. 2019). Modern agriculture has been based heavily on chemical application and the effect of pesticides has adverse effects on the environment (Gomez Exposito et al. 2017). For generations, humans have unwittingly as well as knowingly manipulated the rhizosphere microbiome to optimize plant growth. Soil amendments ranging from manure to compost involving microbe-driven fermentation processes constitute an important part of organic farming and enrich the root microbiome. In recent decades, farmers have used one or more beneficial plant growth-promoting rhizobacteria (PGPR) such as Pseudomonas and Bacillus species to enhance plant growth and protection through biological control of pests and pathogens (Rosier et al. 2016; Kloepper et al. 1980). Appreciating that microbes are crucial drivers of agricultural productivity (Qiu et al. 2019), recently the focus has shifted to utilizing the soil microbiome to sustainably improve crop production without the use of polluting fertilizers and harmful pesticides (Philippot et al. 2013). To realize this, it is important to approach plants that need protection as holobionts that are intimately and inseparably tied to their microbiome and maximize the positive effects of the microbiome (Wille et al. 2019). The one plant-one pathogen model is now giving way to the pathobiome concept, which considers that the effects of the pathogen are moderated by the action of the commensals and symbionts such as those in the rhizosphere microbiome as well as the environment (Bass et al. 2019).
Only a fraction of the microbiome can be cultivated in artificial media and early estimates ranged from only 1% to 10% (Conn et al. 1918) as it is a challenge to reproduce the conditions required to sustain many species; recent research shows that these predictions are an underestimate. Culture-independent identification of bacteria through DNA sequencing has enabled the identification of bacteria recalcitrant to culture. The development of high-throughput next-generation sequencing has facilitated shotgun metagenome sequencing and made possible the identification of millions of sequences per sample and dramatically improved the resolution of identification to include even rare species (Turner et al. 2013). Most importantly, this has led to the identification of microbes that are recalcitrant to culture and broadening of our understanding of three-way plant-rhizosphere microbiome-pathogen interactions in an unprecedented fashion (Wille et al. 2019). The study of the metaphenome, which encompasses not only the metagenome and metatranscriptome but also the metaproteome and metametabolome can help appreciate the full functional potential of the rhizosphere microbiome on a global scale (Jansson and Hofmockel 2018).
The improved ability to culture bacteria has also enabled the development of synthetic communities (SynComs) of bacteria that have enabled a deeper understanding of microbial community functions, their interactions with the plant, and plant responses to them. This information can facilitate the development of new strategies including improving plants to adopt better microbiomes, applying optimal microbial communities, plant probiotics, and microbe-derived products for better plant growth and biological control of pests and pathogens (Levy et al. 2018; Rosier et al. 2016). The discovery that plant genotype influences microbiome composition has also important connotations to improve agriculture (Leach et al. 2017).
Sustainable agriculture is a priority in serving the burgeoning human population, which has increased sevenfold since the beginning of the nineteenth century. It will be an important strategy to combat the rising challenge to grow food and fodder in less than ideal conditions including dwindling arable land and more hostile climate conditions triggered by climate change (Tilman et al. 2002; McNear 2013). Harnessing the rhizosphere microbiome could improve crop productivity, decrease losses from plant disease, and reduce the use of pesticides (Turner et al. 2013). In this chapter, we discuss the rhizosphere microbiome in the context of agriculture and how the understanding of plant immunity-microbiome interactions can be utilized for sustainable agriculture.
9.2 Understanding Rhizosphere Microbiome Interactions with Plant Defense
9.2.1 Rhizosphere Colonization
Plant-microbe association in the rhizosphere is largely driven by mutual metabolic needs. Competing or cooperating microbes influence each other’s survival and abundance, while the plant recruits and selects microbes from the pool in the soil environment. The plant genotype, as well as the environment, can affect the morphology of the root as well as the chemical composition of the root exudates and other plant material. The amount of organic compounds like sugars and amino acids and inorganic nutrients can dictate the composition and abundance of microbial species in the rhizosphere (Fierer 2017; Rout 2014). The colonization of the rhizosphere by microbes proceeds through several steps: recruitment and motility, root surface colonization, and in some cases biofilm formation (Pinski and Betekhtin 2019). Additionally, endophytic microbes also invade the host tissue for colonization.
9.2.1.1 Recruitment
The recruitment of specific microbes by plant roots to form the rhizosphere microbiome is an active process involving rhizodeposition (Quiza et al. 2015). Rhizodeposition involves the secretion or release of root exudates, gases, macromolecules, sloughed-off cells, and intact root border cells enriched in organic compounds into the rhizosphere environment (Jones et al. 2009). Root exudates are predominated by sugars, organic acids (as in tomato) (de Weert et al. 2002), and amino acids (as in rice) (Bacilio-Jiménez et al. 2004) and also include metabolites such as fatty acids, sterols, vitamins, secondary metabolites like phenolic compounds and putrescine, volatile compounds as well as macromolecules such as proteins, and complex carbohydrates such as cellulose and mucilage (Badri and Vivanco 2009; Bertin et al. 2003; Quiza et al. 2015; Mendes et al. 2013). The molecules in root exudates, released mainly from root cap cells, can attract microbes in the surrounding soil, which can utilize them as carbon and nitrogen sources or as signals that trigger chemotaxis (Reinhold-Hurek et al. 2015). Only microbes that survive host defenses and competition among each other and sense these molecules as preferred substrates venture into the rhizosphere for successful colonization (Zhalnina et al. 2018). Many beneficial bacteria like rhizobia and Bacillus and Pseudomonas spp. migrate to the plant through chemotaxis and can colonize on or inside the plant. Thus, root exudates are critical determinants of the root and rhizosphere microbiome composition (Rout 2014; Turner et al. 2013).
Certain metabolites in root exudates help recruit beneficial bacteria. For instance, the release of the organic acid malic acid in exudates triggered by foliar infection with Pseudomonas syringae enlists the beneficial bacterium Bacillus subtilis (Rudrappa et al. 2008). Likewise, citric acid and malic acid released by tomato, watermelon, and cucumber roots promoted positive chemotaxis of beneficial Pseudomonas fluorescens WCS365, Paenibacillus polymyxa, and Bacillus amyloliquefaciens SQR9, respectively (de Weert et al. 2002; Ling et al. 2011; Zhang et al. 2014). Thus, the attraction of beneficial bacteria by exuding organic acids is a common phenomenon in the rhizosphere.
9.2.1.2 Surface Colonization and Biofilm Formation
Root exudates attract a variety of bacteria, but only those that can make contact with the root can colonize the root surface and access the interior (Pinski and Betekhtin 2019). Protein-based fimbriae, pili, adhesins, and curli fibers can facilitate the physical attachment of bacteria to surfaces (Mohan et al. 2018). Bacteria can then autoaggregate and form microcolonies. Bacteria communicate through a process known as quorum sensing (QS), which is fundamental to the colonization of plants by bacteria. Through this process, they sense or estimate the density of their population or that of other bacteria by monitoring levels of certain secreted signaling molecules called autoinducers and regulate gene expression accordingly. Autoinducers include N-acyl homoserine lactones (AHLs) (e.g., Pseudomonas), lipid-based diffusible signal factors (DSF) (e.g., Xanthomonas, Stenotrophomonas), and oligopeptides (e.g., Bacillus) (Eberl 1999) (reviewed in Mohan et al. 2018). Different bacterial species may share the same signal and display interspecies cooperativity, or interfere with quorum sensing in other bacteria in a process known as quorum quenching. QS communication is critical for the coordination of various population density-driven processes such as motility, adhesion, biofilm formation (Lareen et al. 2016), and virulence functions in pathogens. Once bacteria adhere to root surfaces, they can form microcolonies and in some cases proceed to develop a biofilm (Rout 2014).
Microcolonies can grow into biofilms where bacteria aggregate in several layers ensheathed in a matrix. Biofilm-forming bacteria may shed their flagella and secrete a glutinous substance called exopolysaccharide (EPS) among others to aid the formation of a biofilm (Meneses et al. 2011; Żur et al. 2016). The secretion of these substances requires cooperation between bacteria of the same or different species, coordinated through QS (Hassani et al. 2018). Root exudates, particularly amino acids, have an important role in the dynamics of biofilm formation and disassembly (Kolodkin-Gal et al. 2010). Bacteria within a biofilm can also communicate to coordinate the density-dependent discharge of plant growth-promoting compounds (Rudrappa et al. 2008). Biofilms not only serve to shield the component bacteria from other bacteria and host immunity (Van Acker et al. 2014) but also occupy niches to deny phytopathogens access to space, thus physically protecting the root surface.
9.2.1.3 Invasion
Bacteria, particularly endophytes, may enter into roots passively through cracks or may actively produce cell wall- and middle lamella-degrading enzymes (Turner et al. 2013; Viaene et al. 2016) to disrupt the barriers and gain entry into the root. The production of these enzymes (frequently hydrolases) may be triggered by root exudate components and amplified by QS (Levy et al. 2018). In sum, the bacteria that establish in the rhizosphere survive a competitive environment and go through several steps to establish contact with roots in the rhizosphere. The colonized microbes confer numerous benefits to the plant as illustrated in Fig. 9.1.
Various benefits of the rhizosphere microbiota—belowground and aboveground. Epiphytes colonize root surfaces (purple), while endophytes colonize root interiors (red). Roots release exudates containing primary and, more selective, secondary metabolites and microbes (bacteria shown as blue) respond to the exudates; rhizosphere microbes facilitate nutrient absorption, mineral scavenging, and nitrogen fixation; they also recruit other microbes to the root; microbe-derived signals stimulate various systemic responses in the aerial parts of the plant as shown
9.2.2 Selection of the Rhizosphere Microbiome by Plant Immunity
Microbial diversity decreases from the surrounding soil to the rhizosphere and is least in the endosphere, indicating that the rhizosphere and root interiors are strong selective environments (Rodriguez et al. 2019). At the same time, the abundance of microbes of each type is enriched within the rhizosphere implying that the selected microbes experience a supportive environment. Recent evidence strongly suggests that plant immunity plays a major role in selecting the microbes in the rhizosphere.
Plants appear to have a strong capacity to influence the composition of the rhizosphere through the secretion of secondary metabolites and phytohormones (Bulgarelli et al. 2015). Exudation of nutrients and antimicrobial metabolites and proteins encourages certain microbes while deterring others (Quiza et al. 2015). It appears that the competitive shield of rhizosphere microbes operates as the very first layer in plant protection, while additional layers of plant immunity exist. The first line of plant defense is the basal resistance conferred by preexisting physical and chemical defenses. Then comes the molecular machinery of induced defense that is activated when the plant perceives potential intruders by detecting microbial structures or contents. Finally, induced defense involves signaling that culminates in transcriptional and posttranslational activation of protein-based defenses in addition to refortification of physical structures and recharging of chemical defenses.
9.2.2.1 Basal Immunity
9.2.2.1.1 Physical Defenses
The waxy cuticle of the root serves as the primary physical barrier to microbial ingress (Martin 1964). The root cap and the border cells that constitute the distal part of the cap are also important defensive structures in the root. While the root cap protects the growing root tip, the root border cells are sloughed off periodically and participate in the physical and chemical defense against potentially pathogenic microbes (Gunawardena and Hawes 2002). The sloughed-off cells and root border cells serve a protective function for the plant by acting as bait to distract phytopathogens while attracting beneficial bacteria (Hawes et al. 2000).
9.2.2.1.2 Basal Chemical Defenses
Root exudates, in addition to primary metabolites like sugars, amino acids, and organic acids, are also enriched in secondary metabolites relevant to plant immunity and thus begin to target specific microbes even before they have come into contact with the plant. Several defense-related metabolites differentially influence (attract, deter, or kill) different sets of microbes, and the resultant microbial community is a consequence of the collective selective pressure exerted by the plant metabolites and proteins in combination with those released by microbes. Some defense metabolites are produced before the onset of stress and are coined phytoanticipins (VanEtten et al. 1994). Phytoanticipins include benzoxazinoids, cyanogenic glycosides, glucosinolates, and saponins (Pedras and Yaya 2015).
9.2.2.1.2.1 Phenolic Compounds
Application of a mixture of root exudate-based phytochemicals followed by 16S rRNA profiling in Arabidopsis revealed that phenolic compounds in root exudates had a stronger impact than other metabolites on the root microbiome composition through suppression of certain members while promoting the growth of others (Badri et al. 2013). Moreover, plant phenolic compounds induced the expression of the antifungal compounds 2,4-diacetylphloroglucinol (DAPG) and pyoluteorin (PLT) in the beneficial P. fluorescens CHA0 (de Werra et al. 2011). Phenolics may serve as substrates or as signals to certain bacteria and are positively correlated with the enrichment of certain beneficial bacteria such as Streptomyces (Newitt and Prudence 2019). Alteration in phenolic compound profile in poplar cinnamyl-Co reductase (CCR) mutant resulted in shifts in the root microbiota composition (Beckers et al. 2016), illustrating the importance of phenolics in microbiome homeostasis.
Phenolics may be simple phenols like coumarins or complex phenols like flavonoids. Coumarins are secondary metabolites that protect plants from pathogenic fungi. The release of coumarins by roots is triggered by beneficial bacteria during iron starvation and is dependent on a root-specific transcription factor, MYB72 (Fig. 9.2) (Stringlis et al. 2018). One such coumarin, scopoletin, not only mobilizes iron but also exhibits antimicrobial activity against pathogenic fungi, while not affecting some beneficial bacteria. Recent evidence suggests coumarins also inhibit biofilm formation in bacteria (Reen et al. 2018), thus potentially affecting the ability to compete for bacteria to establish themselves in rhizosphere niches. Thus, beneficial bacteria can restructure the microbiome by triggering the release of selective metabolites like coumarin from plants. Flavonoids are plant-specific polyphenols that are critical determinants of the root microbiome (Weston and Mathesius 2013), particularly enriched in the maize and Arabidopsis rhizospheres (Pétriacq et al. 2017). The role of flavonoids in plant-microbe interactions was underscored when they were identified as plant signals exuded by legume hosts to recruit modulating Rhizobium species (Cooper 2007).
Local and systemic protection conferred by rhizosphere microbes. Beneficial bacteria such as Bacillus sp. and Pseudomonas sp. can antagonize other microbes, including potential pathogens, through antibiosis, for instance, by producing antimicrobial compounds. Bacillus sp. can suppress plant defense (MTI/PTI, MAMP/PAMP-triggered immunity) using effector proteins; this allows them to colonize. Iron deficiency can signal through the ethylene pathway (EIN3/EIL), which promotes iron import through transporters. Both ethylene signaling and Pseudomonas simiae (fluorescens) can activate the transcription factor MYB72 which can trigger the production of the secondary metabolite coumarin and also induce ISR (induced systemic resistance). Coumarin secretion helps with the iron acquisition as well as serves as an antimicrobial to reshape the microbiome. Pathogen attack can stimulate malate release which triggers biofilm formation in Bacillus. The physical occupation by a biofilm protects the plants from pathogens. ISR stimulates the priming of defense in systemic tissues. While the defense hormones salicylic acid, jasmonic acid, and ethylene can also stimulate structural defenses, they can also activate primed defense gene expression (PR, MYC, PDF) against both biotrophic and necrotrophic pathogens
9.2.2.1.2.2 Other Secondary Metabolites
Benzoxazinoids are an important group of secondary metabolites functioning in defense against pathogens and pests and are derived from indole (Zhou et al. 2018). They are abundant in maize and other Poaceae members. Benzoxazinoids have been shown to affect rhizosphere microbiome composition in maize by specifically affecting certain groups of bacteria (Hu et al. 2018). Additionally, a type of benzoxazinoid termed DIMBOA also helps recruit and colonize beneficial microbes like Pseudomonas putida (Neal et al. 2012). Saponins are constitutive phytoanticipin antimicrobial metabolites with defense functions, derived from the fusion of triterpenoid or steroid groups with sugar groups (Pedras and Yaya 2015). Well-known examples are saponins avenacin A-1 and avenacoside B that influence microbiota in oats and confer resistance to fungal pathogens (Papadopoulou et al. 1999). Strigolactones are often released by roots during nitrogen or phosphate starvation and help recruit beneficial microbes (Yoneyama et al. 2012). Like flavonoids, strigolactones also serve as signals for a symbiosis of plants with mycorrhizal fungi and parasitic plants (Perez-Jaramillo et al. 2016). Some secondary metabolites mimic bacterial AHLs and manipulate bacterial quorum sensing. For example, plants like sweet basil release rosmarinic acid (RA) in root exudates in response to infection with Pseudomonas aeruginosa. RA directly binds to a QS response regulator and triggers premature QS signaling to suppress microbial growth (Corral-Lugo et al. 2016). Thus, plants have a versatile array of secondary metabolites that exert a strong effect on the rhizosphere and endosphere to sculpt the root microbiome.
9.2.2.2 Induced Immunity
9.2.2.2.1 MAMP-Triggered Immunity (MTI)
An important challenge that plants face when encountering a myriad of microbes in the rhizosphere is distinguishing between pathogenic and nonpathogenic species. In some cases, plant pathogens and nonpathogens are physically not very different, and the functional differences may arise simply by the gain or loss of a few pathogenicity islands in some cases (Melnyk et al. 2019b); this complicates the distinction between pathogens and nonpathogens for the plant. Induced plant defense responses may be triggered by recognition of conserved bacterial structures (microbe-associated molecular patterns or MAMPs) on bacteria in a process known as MAMP-triggered immunity (MTI). Plant cell surface pattern recognition receptors (PRRs) recognize MAMPs such as lipopolysaccharide (LPS), EF-Tu, and flagellin through cognate PRRs (e.g., FLS2, a leucine-rich repeat receptor-like kinase or LRR-RLK) to trigger an immune response. A typical MTI defense response includes the generation of reactive oxygen species (ROS), proton influx, calcium level spike, MAP kinase signaling, and transcription of antimicrobial pathogenesis-related (PR) genes, and collectively, these processes serve to limit pathogens (Trdá et al. 2014). MTI is important to limit microbial growth (Dangl et al. 2013) and is expected to be an important factor in gating the root microbiome.
For symbiotic bacteria and fungi, microbially produced signals are recognized by the plant to enable colonization (Pinski and Betekhtin 2019). For example, Rhizobium, an endosymbiont establishes symbiosis with legume hosts through a lipochitooligosaccharide NOD factor signal, while mycorrhizal fungi use chitooligosaccharides that are recognized by host roots (Leach et al. 2017). These signals are structurally similar to the bacterial MAMP peptidoglycan and the fungal MAMP chitin, respectively (Liang et al. 2014) and recognized by receptor-like kinases (RLKs) in plants to initiate symbiosis (Zipfel and Oldroyd 2017). Although MTI is an important defense response in the roots, profiling the PRR FLS2 expression in roots suggests that MTI may be more actively induced in the inner layers of the root (e.g., pericycle in stele) and in areas most susceptible to infection—the entry sites (Beck et al. 2014; Chuberre et al. 2018; Wyrsch et al. 2015). The abundance of MAMPs in the soil may prompt desensitization of the MTI response in the outer layers. Recently, mounting evidence indicates that beneficial microbes actively suppress or evade host immunity to engage in symbiosis (Yu et al. 2019).
9.2.2.2.2 Induced Chemical Defenses
In contrast to phytoanticipins that are constitutively produced, phytoalexins are secondary metabolites that are produced in response to pathogen infection. Phytoalexins are produced in both root and shoot infections (Duan et al. 2014) and can impact rhizospheric and endophytic bacteria composition (Pinski and Betekhtin 2019). A variety of phytoalexins are produced by plants, many in a genotype-specific manner; for example, camalexin in Brassicaceae members, capsidiol in capsicum, gossypol in cotton, and pisatin in pea (Preisig et al. 1990). Such defense metabolites play significant roles in defining the characteristic microbiomes of various plant species.
9.2.2.3 Plant Defense Hormones
That phytohormones are important in the regulation of microbial community composition is evident with the observation that treatment with hormones as well as defense hormone signaling mutants altered root exudate and microbial profiles (Leach et al. 2017). Three major plant defense hormones are salicylic acid (SA), jasmonic acid (JA), and ethylene. Salicylic acid mediates defense against biotrophic pathogens and is important for systemic acquired resistance (SAR), a resistance mechanism that is triggered in the shoot (Glazebrook 2005). On the other hand, JA and ethylene function in resistance to necrotrophic pathogens in the shoot, but are also required for induced systemic resistance, a resistance pathway initiated in roots upon interaction with beneficial microbes. These three hormone pathways can function in defense signaling with additive and synergistic effects, and the loss of all three hormonal pathways results in aberrant rhizosphere microbiome composition or dysbiosis that may be linked with reduced field survival (Lebeis et al. 2015). Each of these hormones play an active role in shaping the rhizosphere and/or endosphere microbiome.
9.2.2.3.1 Salicylic Acid
Salicylic acid (SA) has been detected in root exudates of plants (Khorassani et al. 2011; Ling et al. 2013) and is among the preferred nutritional substrates for certain rhizosphere bacteria, alongside other organic acids, as observed in the oat, Avena barbata (Zhalnina et al. 2018). Besides serving as a nutrient, SA could also serve as a signaling molecule for some bacteria (Lebeis et al. 2015). The biosynthesis of SA in plants is suppressed by beneficial microbes; for example, an effector protein produced by the beneficial fungus Piriformospora indica suppressed the expression of the plant SA biosynthetic transcription factor CBP60g presumably to suppress SA-mediated defense and to facilitate its own colonization (Akum et al. 2015). SA also has a marked influence on the rhizosphere microbiome composition and can inhibit mycorrhizal and root nodule symbioses (Rodriguez et al. 2019). A defect in SA-mediated defense leads to increased colonization of certain bacterial species including Salmonella enterica and the nitrogen-fixing Gluconacetobacter diazotrophicus (consistent with the inhibition of nitrogen-fixing bacteria by SA), but not other bacteria such as Klebsiella pneumoniae (Pinski and Betekhtin 2019). Arabidopsis mutants exhibiting altered SA synthesis and signaling, but not JA and ethylene mutants, showed distinct core root microbiomes at the family level (Lebeis et al. 2015), while previous studies showed little effect of SA on the microbiome (Bodenhausen et al. 2014; Carvalhais et al. 2014; Doornbos et al. 2011). SA appeared to limit the growth of several families of bacteria as they were enriched in SA defense-deficient mutants in root interiors, suggesting that SA plays an important role in restricting the growth of certain taxa in wild-type plants while allowing the growth of others. The disruption of SA-mediated defense also reduced leaf endophytic diversity (Kniskern et al. 2007). Thus, it is clear that SA is a strong component of plant defense in gating rhizosphere microbes and regulating the microbiota composition. Consistently, beneficial bacteria such as Pseudomonas putida appear to modify the microbial community by activating SA signaling in Arabidopsis (Sheoran et al. 2016).
9.2.2.3.2 Jasmonic Acid
The effect of JA on symbiosis varies with plant genotype and conditions (Reverchon et al. 2019). Certain microbes not only suppress SA defenses, but some like the mycorrhizal fungus Laccaria bicolor also inhibit JA signaling to enable colonization; an L. bicolor effector prevents the degradation of the JA repressor JAZ to keep early JA-mediated defense inhibited to allow colonization (Plett et al. 2014). Other beneficial fungi, P. indica, and the beneficial bacteria Bacillus subtilis suppress early PTI in Arabidopsis using the JA pathway as the defense suppression is lost in JA signaling mutants, jar1 and jin1 (Jacobs et al. 2011; Lakshmanan et al. 2012). While the loss of SA defense reduced endophytic diversity in Arabidopsis roots, on the contrary, activation of JA signaling through exogenous JA application reduced root endophytic diversity in wheat (Liu et al. 2017). The shift in microbiome composition following JA application is attributed to changes in root exudate composition (Yu et al. 2019).
9.2.2.3.3 Ethylene
Colonization of plants such as Medicago truncatula (Iniguez et al. 2005) and sugarcane (Cavalcante et al. 2007) with beneficial microbes triggered ethylene signaling and gene expression early on and an ethylene-insensitive mutant of M. truncatula was observed to be over-colonized by the endophyte K. pneumoniae (Iniguez et al. 2005), indicating that ethylene plays a restrictive role in microbial colonization consistent with its role in plant defense. Ethylene also inhibits root nodule symbiosis as well as the association with mycorrhizal fungi (Rodriguez et al. 2019). It is, therefore, not surprising that some bacterial species including Bacillus and Pseudomonas produce the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which reduces root ethylene biosynthesis by degrading the ethylene precursor, ACC (Compant et al. 2019; Glick 2014), and this was shown to enhance plant stress tolerance and root development and possibly improved general microbial colonization. Suppression of ethylene production can be beneficial to the plant as ethylene is a stress hormone that can be detrimental to plant growth at higher levels (Vaseva et al. 2018). Unexpectedly, ablation of ethylene biosynthesis and signaling in Nicotiana attenuata mutants reduced endophytic microbial diversity, suggesting that ethylene affects microbial homeostasis within the plant and certain bacteria may require plant ethylene signaling for invasive colonization in roots (Long et al. 2010). In contrast, in Arabidopsis, root microbial diversity was not affected, but rhizosphere bacterial abundance was reduced in ethylene mutant ein2. Thus, ethylene, like SA and JA, functions inflict both positive and negative effects on root microbiota. SA and JA/ethylene pathways generally function antagonistically as they confer resistance to different kinds of pathogens, but in the roots, they modulate microbial homeostasis as they all appear to generally prevent microbial ingress and overgrowth of certain bacteria while in some cases promoting endophytic diversity. The activation of these pathways during stress may be further instrumental in reshaping the microbiome.
9.2.3 Modulation of Plant Immunity by the Rhizosphere Microbiome
While the root microbiome is, in large part, selected by the plant immune system, they also have a reciprocal effect on plant immunity. It is now well established that the root microbiome expands plant immunity and functions as an additional layer of defense against pathogenic microorganisms, providing unique opportunities to develop novel tools in crop protection and enhance crop productivity sustainably. Two of the ways the root microbiota participates in plant disease resistance are direct disease suppression (DDS) and induced systemic resistance (ISR) (Fig. 9.2).
9.2.3.1 Local Disease Suppression
DDS takes place either in the rhizosphere or the root interior and is commonly based on competition for nutrients and niches, parasitism, antibiosis, or combinations of the abovementioned mechanisms. DDS has ideally exemplified in disease-suppressive soils, soils in which a soilborne pathogen cannot cause disease because of the presence and/or increased abundance of potent antagonistic microbes. The mechanisms involved in direct pathogen suppression include mainly competition for carbon and siderophore-mediated competition for iron, the production of cell-wall-degrading enzymes such as chitinases, and the production of various antibiotics including the well-studied antibiotic compounds 2,4-diacetylphloroglucinol (DAPG) and phenazines (PHZ) (Rout 2014). More recently, volatile molecules have been proposed to contribute to DDS in suppressive soils. These functions are further elaborated below in the context of disease-suppressive soils.
9.2.3.2 Induced Systemic Resistance
ISR is initiated in the roots upon microbial colonization and confers broad-spectrum systemic resistance to aboveground plant tissues against pathogens and even insects (Pieterse et al. 2014). ISR was first described in studies of the early 1990s focusing on the ability of Pseudomonas sp. rhizobacteria to trigger systemic resistance in carnation, wheat, and common beans. Since then, the phenomenon has been shown to occur in numerous dicotyledonous and monocotyledonous plant species, suggesting that ISR represents a conserved function of the root microbiome. Interestingly, novel findings in Arabidopsis suggest that plants experiencing pathogen attack in the aboveground tissues modify the composition of the exudates they excrete in the root vicinity to recruit a potent consortium of ISR-inducing rhizobacteria (Melnyk et al. 2019a). Such microbiota-dependent legacy that plants generate in the soil under stress conditions has been shown to enhance the defense capacity of future generations against pathogens thereby promoting offspring survival in hostile environments. The catalog of ISR-eliciting microorganisms is long and includes both individual strains and microbial consortia. Epiphytic or endophytic soilborne bacteria belonging to the genera Pseudomonas, Bacillus, Serratia, and Streptomyces represent typical examples of ISR-eliciting microbes. Symbiotic rhizofungi such as Trichoderma spp., mycorrhizal fungi like Rhizophagus irregularis (syn. Glomus intraradices), the mycorrhizal-like endosymbiotic fungus Piriformospora indica, and nonpathogenic Fusarium species are also capable of eliciting ISR. Interestingly, several of the same strains involved in LDS have been shown to be potent ISR inducers.
Epiphytic ISR-inducing bacteria capable of colonizing the root system of host plants form biofilms in the root epidermis, whereas endophytic ISR-inducing bacteria enter the root interior by either actively penetrating the external root layers or entering passively through wounds and discontinuing in the epidermis such as those formed during lateral root emergence (Pieterse et al. 2014). Although ISR-inducing rhizobacteria are not enveloped in symbiotic organs, such as the root nodules in the Rhizobium symbiosis, they commonly induce significant alterations in the root system architecture. Such alterations contribute to plant growth promotion but also enhance the exudation of energy-rich compounds taking into consideration that most of the root exudation takes place in the elongation zone of young roots. Yet plant growth promotion and ISR are mediated by distinct signaling pathways in the host tissues. Evidence is also accumulating that rhizobacteria of the root microbiome, including ISR-inducing bacteria, suppress plant defense responses at the early stages of the interaction to efficiently colonize plant tissues. Yet plants have evolved immunity-based genetic networks to control the population of epiphytic and endophytic communities of microbes. In Arabidopsis, disruption of such networks has been recently shown to result in a form of dysbiosis.
Several microbial determinants have been proposed to function as ISR elicitors, among them, molecules with well-established immune-stimulatory properties such the MAMPs flagellin and LPS, but also iron-regulated siderophores, the antibiotics DAPG and pyocyanin, N-acyl homoserine lactones, and biosurfactants such as cyclic lipopeptides (Rout 2014). These elicitors are likely to act redundantly during the elicitation of ISR. More recently, volatiles emitted by ISR-inducing strains have been shown to trigger the expression of the essential for ISR establishment MYB72 transcription factor (Fig. 9.2). Despite the extended list of ISR-eliciting molecules, with few exceptions such as the volatiles mentioned above, little is known on the hierarchy that those molecules function during the initiation of ISR and the exact contribution of each determinant to the phenomenon.
The molecular mechanisms underpinning rhizobacteria-mediated ISR are well-studied in Arabidopsis (Pieterse et al. 2014). In Arabidopsis, ISR triggered upon root colonization by the model strains Pseudomonas simiae WCS417 depends on an intact jasmonic acid (JA) and ethylene (ET) signaling pathway and further requires the transcriptional regulators MYC2 and NPR1. In contrast to the costly plant defenses activated by pathogens or insects, the establishment of ISR is not correlated with substantial reprogramming of the host’s transcriptome. Instead, upon pathogen attack, immunized plants display a boosted immune reaction resulting in enhanced resistance to the attacker encountered. This phenomenon is called priming and shares striking similarities with the potentiation of cellular defense responses in primed monocytes and macrophages in mammals. In roots, initiation of ISR is regulated by the root-specific transcription factor MYB72, a member of the R2R3 family of MYB transcription factors, and components of the ET signaling pathway that locally act in the generation or translocation of a thus-far unidentified systemic signal. Importantly, MYB72 is also required for ISR triggered upon root colonization by the beneficial fungus Trichoderma asperellum strain T34, suggesting that this transcription factor is a node of convergence in signaling pathways induced by diverse types of beneficial soilborne microbes. MYB72 regulates the secretion of plant-derived coumarins, suggesting that these molecules are essential components of the ISR signaling pathway. Thus, root microbes play a vital role in stimulating local and systemic plant defenses for enhanced disease resistance, which, in turn, can reshape the rhizosphere microbiome through altered root exudation.
9.3 Microbiome and Modern Agriculture
9.3.1 Impact of Modern Agricultural Practices on the Rhizosphere Microbiome
9.3.1.1 Plant Domestication
Generations of modern agricultural practices have markedly altered rhizosphere microbes. Plant protection in agriculture has long involved breeding for resistance and, more recently, genetic modification for enhanced resistance, but the development of broad-spectrum resistant crops is time-consuming and subject to stringent regulation and public approval (Syed et al. 2018). Moreover, resistance in crops can break down over the years, as observed for grapevine mildew, wheat rust, and rice blast. One of the reasons behind the resistance breakdown is that pathogens can evolve rapidly (Peressotti et al. 2010) and recently there has been an alarming rise in new fungal phytopathogens (Fisher et al. 2012). To counter this, modern agriculture has witnessed a massive surge in the use of biocides, including toxic pesticides and herbicides and yield-promoting fertilizers that can have a telling nontarget effect on the rhizosphere microbial community either directly or indirectly through their impact on the plants (Turrini et al. 2015).
9.3.1.1.1 Changes in the Rhizosphere Microbiome
Plant domestication through agriculture appears to have resulted in a reduction in both plant and microbial genetic diversity through the loss of plant traits and wild microbial species that were originally adapted for the plants (Perez-Jaramillo et al. 2016; Compant et al. 2019). These changes in the microbiome may be small in some cases but significant, as observed in wild and cultivated barley, beans, and sugarbeet (Bulgarelli et al. 2015; Zachow et al. 2014; Perez-Jaramillo et al. 2017). In general the bacterial phylum Bacteroidetes was comparatively less abundant in the rhizospheres of cultivated crop plants such as beans and other plant species compared to their wild counterparts, which are colonized more abundantly by Proteobacteria and Actinobacteria (Perez-Jaramillo et al. 2017; Pérez-Jaramillo et al. 2018). Members of Bacteroidetes, also an abundant phylum in the human gut, are known for their propensity to metabolize complex carbohydrates, a component that may have become more limited in agricultural crop rhizospheres. Thus, changes in root microbiota composition could be associated with simplification of plant exudates.
Several studies have suggested that microbial community changes during domestication likely resulted from changes in root architecture, root exudate composition, plant physiological changes, and alteration of the chemical environment (Perez-Jaramillo et al. 2016). These changes appear to have hampered beneficial associations with mycorrhizae and nitrogen-fixing rhizobia. Indeed, wild ancestors in maize, wheat, and breadfruit showed a greater disposition to mycorrhizal associations compared to modern varieties (Kapulnik and Kushnir 1991; Xing et al. 2012; Zhu et al. 2001). The comparison of wild and domesticated legumes grown in natural soil also revealed that the ability to attract and colonize a diverse microbial community was reduced in cultivated crops, suggesting the loss of microbial recruitment skills upon domestication (Mutch and Young 2004). The lower microbial diversity in agricultural soils may also be attributed to the reduced diversity of available microbes in agricultural soils compared to natural soils since the selection of microbes by the plant is limited by what is available in the soil. This is well exemplified in the study showing that the transformation of Amazon forest areas into agricultural land resulted in shrinkage of microbial diversity (Rodrigues et al. 2013).
The loss of rhizosphere microbial diversity has consequences to plant health. Generally, diversity in a microbial community ensures that competition for niches and resources keeps pathogens at bay. Additionally, more diverse communities are also more resilient to environmental stresses such as drought as the stress-induced loss of important microbial species (often temporary) is compensated for by the presence of new taxa that spring into action and help the plant withstand stress (Xu et al. 2018). Thus, the reduction in microbial diversity in modern agricultural soils could offer pathogenic species an opening to invade the rhizosphere and cause disease and could also render the plants less resilient to stress.
9.3.1.1.2 Changes in Plant Morphology
Soil surface watering and fertilization in agricultural plants appear to have led to the evolution of shallower root systems, as the nutrients are easily accessible at the surface negating the need for deep rooting (Jackson 1995). This change in root architecture can alter root surface niches as well as oxygen exposure near the surface and consequently affect the microbiome, as has been suggested (Micallef et al. 2009). The shallowing of roots or loss of deep rooting in domesticated plants compared to wild plants has been witnessed in many plant species including lettuce. Evolutionarily, a less deep root system may have contributed to the deselection of anaerobic root microbiota such as some members in the Bacteroidetes phylum (Pérez-Jaramillo et al. 2018).
9.3.1.1.3 Changes in Plant Physiology
Agricultural domestication of plants has resulted in an erosion of genetic diversity as witnessed in multiple plant species including rice, wheat, and bean (Perez-Jaramillo et al. 2016). A general reduction of plant genetic diversity through agriculture may be linked with a reduced ability to recruit and select rhizosphere microbial communities (Wissuwa et al. 2008). The genetic component of rhizosphere microbiome selection is evident from the analysis of maize recombinant inbred lines that revealed the significant genetic contribution to microbial selection and diversity (Peiffer et al. 2013). Specifically, plant domestication progressively selected out secondary metabolites and volatile compounds to render plants more palatable or less toxic to humans and livestock (Meyer et al. 2012) and this has rendered modern crops more susceptible to insect pest herbivory, for instance (Chen et al. 2015). Many of these metabolites are defense compounds against pathogens and insect pests, including phenols, flavonoids, terpenes, and glucosinolates, which almost always carry a strong taste such as bitterness, acridity, or astringence (Drewnowski and Gomez-Carneros 2000). Such metabolic changes may have impacted the ability of modern crops to recruit microbiota as these secondary metabolites also play a key role in the selection and shaping of the rhizosphere microbiome as discussed above. The root exudates of crops may also be less complex than wild counterparts as modern wheat showed severalfold higher exudation of simple sugars such as glucose and fructose (Shaposhnikov et al. 2016). The impact of plant domestication on rhizosphere microbes is illustrated in Fig. 9.3.
Impact of plant domestication and agriculture on rhizosphere microbes. Left panel, monoculture in agriculture has resulted in the rise of pathogens, but also nurtured the development of disease-suppressive soils which have had a protective effect in limiting pathogens; right panel, plant domestication through agriculture had led to the loss of secondary metabolites that are key selective agents in root exudates against microbes. The regular provision of water and nutrients has led to the evolution of shallow root systems, which can alter microbial niches in the rhizosphere. The root exudate composition in domesticated plants is also simpler and correlates with reduced microbial diversity and interaction with symbiotic microbes like nitrogen-fixing rhizobacteria and mycorrhizal fungi. In comparison, the undomesticated wild counterparts have more secondary metabolites, deeper root systems, more complex components in root exudates, and higher microbial diversity
9.3.1.2 Inorganic Fertilizers
Modern farming is largely inorganic farming and inorganic fertilizer treatment of soil undoubtedly enhances plant growth, but only about 60% of the nitrogen supplements are absorbed by the plant, and the rest leach into and contaminate groundwater and end up in water bodies causing environmental pollution such as eutrophication (Schmer et al. 2014). Furthermore, the treatment of plants with nitrogen-based fertilizers for a long time resulted in the displacement of mutualists by less mutualistic root bacteria, negating microbe-mediated benefits to the host (Weese et al. 2015). Similar to the enrichment of certain members by eutrophication in water bodies, fertilizer treatment promoted the growth of copiotrophic bacterial taxa like Actinobacteria and Firmicutes with a reduction in oligotrophic species in Acidobacteria and Verrucomicrobia (Ramirez et al. 2012). Phosphorus is another major macronutrient for plants, but only about 5% of soil phosphorus is accessible for uptake by the plant. To sidestep this problem, farm soil is amended with phosphate fertilizers. Fertilizers do augment the biological activity in the soil (Quiza et al. 2015), but appear to restructure the microbiome with the apparent cost of microbial diversity loss.
9.3.1.3 Pesticides
Without question, pesticides can boost crop yield through protection from pests and plant growth promotion (Syed et al. 2018). Products like fungicides carry both financial and environmental costs, in addition to the development of fungicide resistance by pathogens and the need to keep developing new products (Ma and Michailides 2005). Fungicides and other agrochemicals can also inadvertently target the microbiomes and weaken beneficial interactions of the plant with rhizobacteria and mycorrhizae (Berg 2009). For instance, products like Oryzalin and glyphosate have been shown to suppress plant-associating mycorrhizae and nitrogen-fixing bacteria, respectively (Kelley and South 2017; Santos and Flores 1995).
Taken together, many modern agricultural practices appear to have collectively caused a shift in rhizosphere microbiomes with reduced interactions with beneficial microbes and diminished microbial diversity compared to their undomesticated counterparts. Soil organic matter is the driving force for rhizosphere microbiome colonization as a source of colonization signals and sustaining nutrients. Modern farming practices reduce soil organic matter content, compromising soil microbial diversity (Lareen et al. 2016). Indeed, low-input farming is correlated with higher microbial diversity characteristics of a healthy rhizosphere microbiome (Postma-Blaauw et al. 2010).
9.3.2 Contemporary, Alternative Farming Practices
9.3.2.1 Organic Farming
Organic farming is a more sustainable alternative to modern agriculture, as it aims to replace hazardous and polluting pesticides, fungicides, herbicides, and fertilizers with the more eco-friendly options—organic matter (Quiza et al. 2015). Organic farming enriches soil organic matter content and biological activity and plants cultivated in organic soil showed greater microbial diversity and species richness than those grown in conventional mineral soil in winter wheat, clover, and other species (Hartmann et al. 2015; Long et al. 2010; Lupatini et al. 2016). The increased microbial species richness may be owed to the fact that organic matter contains complex organic substrates that may nurture a distinct and more diverse set of bacteria. Microbial 16S rRNA profiling revealed that Proteobacteria members were elevated in the organic soils compared to conventional soils which mainly contained Actinobacteria (Li et al. 2012). The enrichment of Proteobacteria is not surprising because they are among the most abundant phyla in animal feces (Shanks et al. 2011) that are often used as soil amendments and may also indicate an enrichment by the plant.
Organic farming practices emphasize soil amendments including compost, animal manure, and treated sewage sludge, rich in organic matter. Compost includes chitinous material such as crab shells, fish emulsion, and fruit pulp (Gómez Expósito et al. 2017). Often the compost possesses biocontrol activity and affords disease protection; for example, compost including chitosan, crab shell (chitin), and citrus pulp protected bell pepper from Phytophthora root and crown rot (Kim et al. 1997). In some cases, organic mulches have been supplemented with beneficial fungi to improve disease resistance, as observed for root rot resistance to the oomycete pathogen Phytophthora cinnamomi in avocado (Costa et al. 2000). Green manure, consisting of cover crop plant material left to decompose on the field, not only enriches organic matter but also acts as a mulch to retain soil moisture and suppress weed growth (Muimba-Kankolongo 2018). The application of green manure increased bacterial richness and soil microbial heterogeneity while also increasing the levels of microbes that promote nutrient cycling (Ingels et al. 2005). Thus, organic farming practices generally supported a higher microbial diversity than inorganic farming with protective effects.
9.3.2.2 Crop Rotation
Crop rotation has been utilized as an important tool to restructure the rhizosphere microbiota to benefit crop plants and is a mainstay in organic farming (Mazzola 2007), although it could also be practiced with modern inorganic farming. The alternating growth of complementary plants in crop rotation—particularly with legumes—not only increased nutrient cycling and improved soil properties but also increased disease resistance (Ingels et al. 2005). For instance, the nitrogen-fixing legume chickpea was found to recruit microbiome—including the plant-protective Penicillium sp. that benefited the subsequent wheat crops (Ellouze et al. 2013). Similarly, another legume red clover developed rhizobacterial communities that were beneficial to potato growth (Sturz et al. 2003). Thus, legumes make good partner crops for rotation with other crops. Oats produce terpenoid avenacin that confers resistance to the highly destructive fungal disease take-all (Begley et al. 1986). The growth of oat as a break crop before growing wheat transferred the resistance benefits to wheat as the protective effects persisted in the soil (Huang and Osbourn 2019). Thus, rotation or alternation of crops can result in complementary microbiomes that are tolerated by both crops, with additive or synergistic benefits from the mixed microbiome (Quiza et al. 2015). The mixed community has greater microbial diversity and resilience to pathogen invasion, contributing to a disease-suppressive effect. Furthermore, the alternation with incompatible hosts also discourages plant pathogen survival.
Although organic farming is ecologically friendly, drawbacks include the undefined nature of the amendments that limit the reproducibility of benefits (Quiza et al. 2015). Moreover, the salinity in some of the treatments and heavy metals and therapeutic agents in biosolids and other soil amendments may be toxic to the native soil microbiota. Nevertheless, organic farming is a more sustainable alternative to modern inorganic farming. The effect of organic and inorganic farming on rhizosphere microbes is compared in Fig. 9.4.
9.3.2.3 Tillage
Tilling and turning over of soil can aerate the soil, but disrupt the soil structure and microbial community organization and expose the soil to potential erosion and runoff from precipitation. No-till farming preserves the microbial communities for the next crop season and the residual plant material can sustain microbial growth. In one study comparing the microbiomes of tilled and non-tilled farms, the bacterial communities were not observed to be significantly different (Yin et al. 2017). It was suggested that the tillage may affect fungal populations more as fungal enzymes may play a more significant role in the digestion of lignocellulosic material (Baker et al. 2019).
9.3.3 Monoinoculant Biocontrol
As an alternative to inorganic and organic fertilizers, microbes such as Azospirillum can be introduced in the field as biofertilizers that can promote plant growth, generally by solubilizing nutrients and promoting absorption (Maeder et al. 2002; Namvar and Khandan 2015; Qiu et al. 2019). Plant growth-promoting rhizobacteria (PGPR) go a step further by not only improving plant growth but also enhancing protection from diseases (Compant et al. 2019). Some PGPR produce plant growth-promoting phytohormones including auxins, gibberellins, and cytokinins or modulate endogenous levels of them within the host (Compant et al. 2019; Hardoim et al. 2008). Several PGPR species including Pseudomonas, Bacillus, and Streptomyces have been employed in agricultural soils to enhance crop growth, yield, and survival (Sanchis and Bourguet 2008). Several Bacillus spp. have shown promising results in conferring plant growth promotion and disease resistance under field conditions (Syed et al. 2018). Beneficial fungal species such as Trichoderma have been employed for a similar purpose and function (Harman et al. 2004).
Plant protection by PGPR species involves pathogen antagonism as many of them grow aggressively and compete fiercely and these bacteria are also referred to as biological control or biocontrol bacteria. For example, Pseudomonas and Streptomyces can protect host plants through the function of antimicrobial/antibiotic/antifungal compounds such as phenazine derivatives and DAPG and antimicrobial lytic enzymes such as proteases (Newitt and Prudence 2019). Similarly, Bacillus spp. produce antibiotics such as iturin A and surfactants well as lipoproteins that have an antimicrobial function (Lareen et al. 2016; Turner et al. 2013). PGPR also sequester critical nutrients such as iron using iron-scavenging siderophore proteins, thus depriving their competitors and potential pathogens (Hassani et al. 2018). For instance, Pseudomonas spp. suppress fungal pathogens and disease through the use of siderophores (Mercado-Blanco and Bakker 2007). PGPR also prime the plant immune system to trigger a rapid defense to a wide range of pathogens through various mechanisms. One such process is induced systemic resistance (ISR), where rhizosphere colonization triggered systemic resistance in plants. For example, field trials showed that root colonization of Bacillus spp. enhanced resistance to the cucumber mosaic virus (CMV) in tomatoes and cucurbit wilt disease (Zehnder et al. 2000). Similar benefits of ISR have been observed in several crop species (Choudhary et al. 2007).
PGPR microbial inoculants help slash the usage of polluting biocides and fertilizers (Qiu et al. 2019), but the overall promise of biocontrol bacteria is curtailed by their limited success and unpredictability in field settings even though they were promising in laboratory and greenhouse experiments (Schlaeppi and Bulgarelli 2015). For instance, although Pseudomonas spp. exhibit promising biocontrol activity against take-all disease in wheat, these strains are sensitive to desiccation and only survive the early stages of growth on wheat in field settings and are subsequently outcompeted (Coombs et al. 2004; Schlatter et al. 2017). Moreover, plant protection is even more imperative in the context of climate change, which is expected to be hostile to monoinoculant PGPRs—where all eggs lie in one basket. These observations suggest that overreliance on single PGPR inoculants for agricultural plant protection is untenable.
9.3.4 Microbial Mixtures
Instead of single-strain PGPRs, a combination of strains holds more promise in agriculture (Nguyen et al. 2017), particularly when the strains exhibit synergistic or additive effects in conferring plant protection (Orozco-Mosqueda et al. 2018), as was shown with Bacillus spp. in field trials (Zehnder et al. 2000). Similarly, a group of six endophytes promoted resistance to tobacco wilt disease (Santhanam et al. 2015). A diverse Pseudomonas consortium led to greater pathogen suppression and disease protection in tomatoes, likely with the increased survival of the Pseudomonas strains (Hu et al. 2016). Strain mixtures including Bacillus and Cutibacetrium spp. improved growth and biocontrol of pathogens in cucumber (Raupach and Kloepper 1998). In some cases, benefits to the plant were only discernable when two Pseudomonas strains were used together resulting in synergistic interactions on chickpea (Meena et al. 2010). Various studies in grapevine (Rolli et al. 2015), maize (Molina-Romero et al. 2017), potato (De Vrieze et al. 2018), and tomato (Berg and Koskella 2018) have demonstrated that multistrain inoculations have the potential to increase plant growth-promoting effects as compared to mono-inoculations. In some cases, bacterial mixtures also improved tolerance to stresses such as drought, as was shown for a cocktail of Pseudomonas, Sphingomonas sp., Azospirillum, and Acinetobacter in maize (Molina-Romero et al. 2017).
A diverse set of microbes in a complex inoculum have the potential to occupy different niches in the rhizosphere, expanding plant protection and boosting growth promotion (Finkel et al. 2017). Furthermore, they may confer additive or synergistic benefits, especially when their benefits are afforded through different mechanisms (Timm et al. 2016). While microbial consortia often show greater potential than single strains, sometimes they may be worse than single strains as seen in the case of growth of grapevines during drought (Rolli et al. 2015). In another case, multiple strains of Pseudomonas affected community stability and did not improve plant protection (Becker et al. 2012). Other studies also witnessed multistrain inoculations being less beneficial to the plant than single inoculants (De Vrieze et al. 2018; Herrera Paredes et al. 2018). Furthermore, co-inoculation may produce a competitive process that may be subjected to environmental changes, with unpredictable outcomes. Thus, future endeavors with microbial consortia should be driven by knowledge and evidence-based selection of complementary microbial strains.
9.3.5 Disease-Suppressive Soils
With the limitations of current single and multistrain PGPR inoculants, disease-suppressive soils have proved not only to be a treasure trove to identify novel individual PGPR strains but also as sources of beneficial microbiomes in agriculture. Disease-suppressive soils are a great example of microbiome-mediated plant protection from pathogens in the soil (Gomez Exposito et al. 2017). Continual monoculture on agricultural soils can build selective pressures against pathogens to produce disease-suppressive soils enriched in beneficial microbes and microbial and plant-derived antimicrobial metabolites that mediate disease suppression (Durán et al. 2018; Santhanam et al. 2015), although this can take several years to build (Coque et al. 2020). In disease-suppressive soils, plants can continue to be healthy even in the presence of pathogens (Teixeira et al. 2019) and this partly results from higher microbial diversities than in conventional soils (Garbeva et al. 2006) that can have a protective effect against pathogens. In some cases, disease suppressiveness may also result from changes in the relative abundance and functions of specific bacterial groups rather than their presence or absence (Mendes et al. 2011; Chapelle et al. 2016). Although soil suppressiveness is a complex phenomenon, the ability of a specific plant genotype to gather in the rhizosphere disease-suppressive communities is critical for the transition of the soil from the conductive to the suppressive state.
Within disease-suppressive soils, specific microbes or groups of microbes confer disease protection to plants largely through competition, pathogen antagonism, and the production of antimicrobial compounds (Mendes et al. 2011). For example, Pseudomonas spp. obtained from Fusarium wilt-suppressive soil conferred resistance to flax (Mazurier et al. 2009). The development of disease suppressiveness involves the selective recruitment of beneficial microbes by the plant roots. For instance, foliar infection with the oomycete pathogen Hyaloperonospora arabidopsidis summoned multiple beneficial strains in the soil that functioned synergistically to promote disease suppressiveness and this effect persisted in the following generations (Berendsen et al. 2018). Thus, the development of disease suppression is accomplished through changes in the microbial community and function in the soil. Since the first report by Atkinson of a cotton-grown soil suppressive to Fusarium wilt, several bacterial and fungal species conferring DDS have been reported. Typical examples are individual bacterial strains belonging to the genera Pseudomonas, Bacillus, Paenibacillus, Enterobacter, Alcaligenes, and Pantoea; fungal strains of the genera Trichoderma, Penicillium, and Clonostachys/Gliocladium; nonpathogenic Fusarium species; and the fungal species Verticillium biguttatum and Pochonia chlamydosporia. Besides the commonly studied Bacillus, Pseudomonas, and Streptomyces, many other bacterial genera including Burkholderia, Paraburkholderia, Enterobacter, and Pantoea show pathogen antagonism (Compant et al. 2019) and are expected to play important roles in the development of disease suppression. Depending on the case, these beneficiaries have been shown to target pathogenic soilborne fungi and oomycetes but also pathogenic bacteria, protists, and parasitic root-knot and cyst nematodes (Gomez Exposito et al. 2017).
In addition to protective strains, disease-suppressive soils also contain microbe- and plant-derived protective compounds that suppress soilborne pathogen growth. This is best exemplified in the case of the wheat take-all disease caused by the fungal root pathogen, Gaeumannomyces graminis, which has the potential to wipe out wheat fields (James Cook 2003). The presence of Pseudomonas-derived antimicrobial DAPG and oat-derived avenacin in the soil corresponded with the suppression of take-all disease in wheat (Mendes et al. 2011; Huang and Osbourn 2019; Raaijmakers et al. 2009). Thus, in take-all decline, the severity of disease was reduced with every generation of wheat, consistent with the development of disease-suppressive soil (Turner et al. 2013). Compounds like DAPG and phenazines can also prime the plant immune system, further enhancing disease resistance. Streptomyces spp. have also been frequently isolated from disease-suppressive soils and their disease suppressiveness was linked with the production of antifungal volatile organic compounds and thiopeptides (Cordovez et al. 2015; Cha et al. 2016; Newitt and Prudence 2019). The disease suppressiveness of Paraburkholderia graminis PHS1 was attributed to the production of sulfur-containing volatile compounds (Carrión et al. 2018). Antimicrobials like DAPG, phenazines, and iturin A can persist in the rhizosphere soil. Therefore, the disease’s suppressive nature in soils can persist for generations, particularly if the plant- and microbe-derived compounds are not volatile. Breeding crops for traits related to the recruitment of disease-suppressive microbial communities could be an alternative breeding strategy towards durable disease resistance.
Microbiome studies have broadened our understanding of disease-suppressive soils and revealed that communities constituted by distinct taxonomic groups operate to confer disease suppression. For instance, bacterial species from Proteobacteria (including Pseudomonas producing antifungal compounds), Firmicutes, and Actinobacteria were implicated in the development of resistance to Rhizoctonia root rot through pathogen antagonism (Mendes et al. 2011). Another report revealed identified Acidobacteria, Actinobacteria, and Firmicutes as keystone groups for resistance to Fusarium wilt (Trivedi et al. 2017). In general, a diversity of microbial taxa become more abundant in disease-suppressive soils (reviewed in Gomez Exposito et al. 2017). Collectively, these studies reveal shifts in community composition with the development of disease suppression and the concomitant microbial enrichment may prevent pathogen invasion (Turner et al. 2013). Pathogen- or plant-derived compounds can promote recruitment or growth of new microbial groups; for example, fungal pathogen-derived oxalic acid or plant metabolites encouraged the growth of bacteria from specific families, including Oxalobacteraceae and Burkholderiaceae that likely served an antagonistic function (Chapelle et al. 2016; Mendes et al. 2011). Many microbial strains have been isolated from rhizospheres and developed as PGPRs for crop protection (Gopal et al. 2013). Disease-suppressive soils can thus be invaluable sources of novel bioactive strains of microbes as well as antimicrobial compounds (Weller et al. 2002). Indeed, the PGPR Streptomyces was originally isolated from disease-suppressive soils (Cha et al. 2016). A study of the rhizosphere community in take-all disease revealed Enterobacter and Serratia as promising candidates for disease suppression (Durán et al. 2018). The complexity of community interactions in disease-suppressive soils, the underlying mechanisms, and the impact of environmental factors remain to be elucidated for many disease-suppressive soils.
Disease suppressiveness can be transferred to new soils by mixing a small portion (1–10% w/w), thus seeding the new soil with a consortium of beneficial microbes (Mendes et al. 2011; Raaijmakers and Mazzola 2016; van der Voort et al. 2016). Similarly, supplementing the soil with siderophore-producing Pseudomonas or their siderophores, both isolated from suppressive soil, could suppress disease in wheat and barley (Gomez Exposito et al. 2017). The organic soil amendments employed in organic farming can also promote disease suppressiveness by increasing soil microbial activity and promoting the recruitment of beneficial microbes. However, the development of disease suppression involves continual monoculture, and crop rotation can accelerate this development of disease suppressiveness (Coque et al. 2020), although in some cases, crop rotation could break disease suppressiveness (Newitt and Prudence 2019), possibly by releasing the selective pressure on the pathogens in the soil. Understanding the mechanisms of disease suppressiveness will be a big step forward in the deployment of plant-protective microbiomes in agriculture.
9.4 Harnessing Microbes for Plant Protection in Sustainable Agriculture
9.4.1 Harnessing Beneficial Microbes for Plant Protection
9.4.1.1 Identification and Selection of Candidate Microbes
While candidate plant-protective microbes can be isolated by screening assays in laboratories, they tend to be laborious. Amplicon-based sequencing methods such as 16S ribosomal RNA offer a relatively cost-effective approach to profile and identify microbial communities, but do not provide information about whether the microbes are beneficial or their relative importance in the community (Levy et al. 2018). Metagenomic sequencing (shotgun metagenomics) can be used to sequence the genomes of the entire rhizosphere community and offer insights into their functional potential and their relative roles. Metagenome sequencing can reveal what genes and functions are enriched in various niches of the rhizosphere (endosphere vs. rhizosphere) as well as dynamic spatiotemporal changes in microbial populations. While elucidation of community structure is a good starting point, the next important step is the functional characterization of promising candidates in the community.
9.4.1.2 Isolation and Functional Characterization of Candidate Microbes
From community profiling, microbial species that are preferentially recruited and/or enriched by the plant may be identified for further characterization. It is estimated that only a small portion of the rhizosphere microbiota is culturable, but recent studies are proving that such estimates are underestimates and more microbes are amenable to culture than previously thought. The ability to grow candidate microbes and explore their functions through plant-microbe experiments is fundamental to the understanding of the plant microbiome and to exploit its full potential. Microbial culture can be employed to test if a plant recruits a microbe or microbial community of interest and can also be used to analyze the underlying mechanisms. Network analysis has been increasingly useful in guiding the selection of representative microbes and identification of hub microbes that are critical to the assembly and function of the microbiome (Gómez Expósito et al. 2017).
If microbial isolates were identified by rRNA profiling, their genomes can be sequenced to further understand their potential. Using the genome, one may explain the organism’s observed behavior or trait of interest, examine additional plant growth-promoting traits, and look for genes or gene clusters corresponding to the synthesis of bioactive compounds (e.g., hormones, antimicrobials) and other genes that indicate novel capabilities. For example, genome sequencing of Streptomyces S4-7 revealed 35 gene clusters implicated in the biosynthesis of antimicrobial compounds, following which a novel thiopeptide was isolated and showed antimicrobial activity (Cha et al. 2016). Similarly, the genome of Pseudomonas sp. contained biosynthetic clusters that allowed the identification of novel antibiotics (Helfrich et al. 2018). Microbes in such cases may be evaluated for antagonistic functions against other microbes or pathogens, although it may be noted that strains that do not show strong bioactivity against phytopathogens in vitro may do so in situ in the presence of root signals (Newitt and Prudence 2019). Good-quality genomes can also serve as reference sequences for the comparison of metagenomics data (Levy et al. 2018). Genome information is not informative of what genes are expressed or functioning in the rhizosphere. This may be accomplished through transcriptomic, proteomic, or metabolomics analysis of the microbe in the rhizosphere. Microbial genes important for plant interaction may be identified through mutational analysis. Recently, transposon sequencing (TnSeq) has turned out to be a facile strategy to create genome-wide mutants of a microbe and systematically test all mutants for a trait of interest (Levy et al. 2018). Such approaches will not only allow the identification of genes important for plant-microbe interaction, but also interactions in the microbiome. Other approaches such as stable isotope probing to assess microbial substrate preferences and metabolic potential are critical to understand the metabolic basis of the plant-microbe interaction (Radajewski et al. 2000).
9.4.1.3 Assembling Synthetic Communities of Candidate Microbes
The representative microbes identified by network analysis can be grown to constitute synthetic communities or SynComs (Gómez Expósito et al. 2017). As microbes function in concert in the microbiome, SynCom scan is employed to study their complex interactions with and impact on gnotobiotic plants in sterile culture (the plant equivalent of germ-free mice). Traditionally, microbial culture in vitro has been a limitation, but recent studies are demonstrating that it is possible to culture as much as 50% of the major members of the microbiome (Bai et al. 2015). SynCom experiments can demonstrate how each species contributes to community assembly and function and how they influence plant fitness (Rodriguez et al. 2019). SynComs also make excellent tools to assess how hub microbiota, which displays a high degree of interaction with other members in the community function as focal points in the community (Hassani et al. 2018). One study showed that the removal of one strain caused five others to disappear, indicating the disproportionately important role of specific members of the community (Niu et al. 2017). Many SynCom studies focus on small communities containing representative strains, but larger synthetic communities involving hundreds of members have also been shown to colonize the rhizosphere reproducibly, making this a powerful approach (Finkel et al. 2017). Additionally, SynComs are valuable in understanding fundamental aspects of plant-microbial community interactions, for instance, SynCom experiments confirmed the importance of the plant defense hormone, salicylic acid (SA) in gating the endosphere and limiting colonization by certain taxa in Arabidopsis (Lebeis et al. 2015).
SynCom experiments have revealed that higher diversity in the synthetic community correlates with better disease suppression (Hassani et al. 2018). More complex Pseudomonas consortia afford better protection against Ralstonia solanacearum, a root pathogen in tomato, through greater competition and pathogen antagonism (Hu et al. 2016). A simplified SynCom consisting of seven species representative of various taxa from the microbiome was collectively required for resistance to Fusarium verticilloides blight in maize (Niu et al. 2017). Generation of SynComs with complementary microbial species with different functions or mechanisms of action may issue additive and synergistic effects, resulting in a resilient microbiome (Gomez Exposito et al. 2017). Some sets of bacterial strains may interact through cohabitation in the same biofilm (Berendsen et al. 2018). The greater plant protection from higher strain diversity has been correlated with a greater diversity of secondary metabolites that can protect the plant through varying mechanisms (Hu et al. 2016). These studies collectively indicate that synthetic communities containing diverse strains, complementary and synergistic with each other, but competitive and antagonistic to other microbes such as potential pathogens, and which can stimulate plant defenses are good candidates for use in sustainable plant protection.
9.4.2 Enabling Plants to Harness Beneficial Microbes for Plant Protection
While soil is the basic source of microbial pool available for plant colonization, host plant genotype also plays an important role in selecting and sustaining the rhizosphere microbiome (Badri et al. 2013; Bulgarelli et al. 2015, 2012; Lebeis et al. 2015; Peiffer et al. 2013). Each plant species, and even different genotypes within the same species, enriches a distinct and selected set of microbes in the rhizosphere and endosphere that are generally beneficial (Perez-Jaramillo et al. 2016), although in some studies the varietal differences were more subtle (Bulgarelli et al. 2015; Peiffer et al. 2013). This selection is primarily dictated by the root exudate composition which also includes selective secondary metabolites, both of which not only serve to cull out certain species can also act as nutrients. A study of Arabidopsis accessions found qualitative differences between root exudates that corresponded to differences in rhizosphere microbiota (Micallef et al. 2009). Thus, the differences in microbial communities may be owed to differences in root exudates.
Plant breeding has traditionally focused on traits like yield and disease resistance, but the outburst of microbiome studies in the past decade has prompted consideration that plants may additionally be bred for their ability to recruit preferred partners and PGPR to build optimal microbiomes and disease-suppressive soils (Quiza et al. 2015; Ryan et al. 2009). For example, wheat varieties were selected for their ability to recruit Pseudomonas populations for resistance to Rhizoctonia solani (Mazzola 2002). Some Arabidopsis mutants with altered root exudate composition were also found to recruit beneficial bacteria. Since root exudate composition is critical for microbial recruitment and selection, many studies have focused on modifying exudate composition (reviewed in Quiza et al. 2015) and transferring these traits to crop plants through traditional breeding and genetic engineering, potentially through the CRISPR/Cas9 system (Schaeffer and Nakata 2015) to augment plant protection; however, a detailed understanding of the mechanistic basis of microbial recruitment is the priority.
Identification of plant loci involved in recruiting or supporting the growth of specific bacterial taxa in the roots may be accomplished through quantitative trait loci (QTL) mapping (Collard et al. 2005) and genome-wide association studies (GWAS) with crops and their wild relatives. One study identified several plant QTLs regulating the colonization of Bacillus cereus UW85 and the accompanied disease-suppressive effect (Smith and Goodman 1999). Wild relatives of cultivated plants are more effective recruiters of a higher diversity of rhizosphere microbes likely due to a richer root exudate and having coevolved with microbiota that enhances their fitness (Perez-Jaramillo et al. 2016). Plant breeding for improved traits over generations has been successful in improving cultivated plant traits, but often with loss of genes from their wild ancestors (Gopal and Gupta 2016). Some of these genes may have contributed to the synthesis of secondary metabolites, which presumably made the plants more palatable both to humans and, inadvertently, also to insect pests. Revisiting wild varieties to identify genes that promote microbial recruitment is a promising approach to design a fitter rhizosphere microbiome and holobiont (Perez-Jaramillo et al. 2016). A plant engineered to produce a diverse root exudate may be expected to support microbiome diversity in the rhizosphere. Desirable rhizosphere traits in plants could be incorporated into elite breeding programs to enhance crop varieties. Thus, modulation of a plant’s ability to attract and retain beneficial microbes is a promising approach to introducing beneficial bacteria in the field. However, it is important to ensure that the soil is equipped with the preferred partners of the plant and supplementing the soil with SynComs could augment the recruitment of the microbiome. Bacterial strains may also be modified for higher responsiveness to plant signals to promote colonization (Cole et al. 2017).
One other way plants can modulate rhizosphere bacterial communities is by targeting quorum sensing (QS), a signaling system used by bacterial species to monitor their population density or those of other species and activate specific coordinated functions (Mohan et al. 2018; Quiza et al. 2015). Plants engineered to produce QS signals or enzymes such as lactonases that can degrade QS signals in the rhizosphere may be able to selectively target certain bacterial groups while retaining others. In addition to improving microbes and plants for better colonization, plant or microbial metabolites could be identified that enhance recruitment by the root. Metabolite profiling and modeling can help identify candidate metabolites that affect community structure and dynamics (Botero et al. 2018). Such metabolites could be used as elicitors to enhance the colonization and retention of preferred beneficial microbes. Thus, a variety of complementary approaches are feasible to enhance the recruitment and enrichment of crop microbiome for enhanced protection; these are summarized in Fig. 9.5.
Harnessing microbes for plant protection in agriculture. Can be accomplished via three approaches: identifying microbes ideal for plant colonization and protection (left), identifying metabolites that promote microbial colonization (elicitors), and enhancing the ability of plants to recruit and retain protective microbes
9.5 Future Considerations for Sustainable Microbiome-Based Agriculture
Rhizosphere microbiomes have coevolved with plants, the local environment, and fluctuating stress conditions serving as a shaping force. The understanding of the microbiome and its dynamic interactions with plants, currently in its infancy, can be potentially applied for sustainable agriculture, particularly in resource-limited environments. An exciting array of opportunities that could transform agriculture await exploration.
9.5.1 Plant Probiotics
While the use of plant bacteria as pure inocula or microbial mixtures is not a new concept to promote plant disease resistance and even though such inoculants showed promising results in laboratory or greenhouse experiments, they fell short in field settings (Glick 2012). With more recent knowledge of the microbiome, thoughtfully selected microbial preparations produced with thorough testing using SynComs are key to success. Many attributes are ideally desirable in these consortia; these include the ability of the strains to compete and survive in the rhizosphere, protect the plant from pathogens by antagonism, tolerate the plant immune system, and stimulate both local and systemic defenses. The inclusion of hub microbes that are capable of recruiting other microbes to assemble a plant-preferred microbiome in agriculture would be beneficial. However, certain hub microbes such as Enterobacter cloacae (Niu et al. 2017) are potential human pathogens and their enrichment in agricultural fields may be considered carefully. The assembling members of the community should preferably show metabolic and functional complementarity with different mechanisms of pathogen antagonism and host defense stimulation so that they combine to afford additive or synergistic protection to the host. The starter community should be representative of the host microbiome, be inherently diverse, or be able to build a diverse microbiome, as diverse microbiomes tend to be resilient; some functional redundancy among the microbes is desirable in this aspect, especially in dynamic environments. Ideally, consortia should include indigenous stress-tolerant microbes that are adapted to the local environment (Mueller and Sachs 2015; Qiu et al. 2019) and capable of assisting plants to withstand fluctuating environmental stresses. Some of these desirable traits could be engineered in the bacteria through recombinant strain production (Quiza et al. 2015), but the risks associated with recombinant strain release and potential gene transfer should be evaluated first.
One challenge in synthesizing ideal consortia is the present limitation in being able to freely grow all microbes in culture. This is particularly true for obligate biotrophs that can only grow on a living host and some of the keystone hub species identified are obligate biotrophs. Bacterial consortia administered as probiotics may be coated onto seeds before sowing (Santhanam et al. 2015), so they can establish the microbial community early on. However, to accomplish this, they need to be competitive to overcome the indigenous microbes already present in the soil. Although fungicide or antibiotic treatments have been recommended to disrupt the existing microbiome in the soil (Quiza et al. 2015), a more sustainable option would be tilling the soil to achieve the same. To ensure invasion of the inocula in the rhizosphere, higher doses may be required, but this may promote undesirable pervasive growth of the microbes in the aerial parts of the plant; for instance, treatment of Arabidopsis roots with high doses of Pseudomonas simiae (P. fluorescens) resulted in the strain spreading to the aerial parts of the plant (Zamioudis and Mohan, unpublished observations). Even if the inoculants establish in the rhizosphere, they may not persist, as in some cases, inocula in the field have been outcompeted by indigenous microbes (van Veen et al. 1997), as has been observed for Azospirillum (Ryan et al. 2009; Herschkovitz et al. 2005). To ensure persistence, periodic soil amendments with the inocula may be necessary (Syed et al. 2018).
9.5.2 Mixed Microbiomes
The exclusive focus on bacterial microbiomes in the rhizosphere comes with the cost of inherent bias as the other kingdoms of microbes including fungi and oomycetes can in many cases play a substantial role in community dynamics, particularly in the context of plant protection. PGPRs Bacillus and Pseudomonas teamed up with mycorrhizal fungi for synergistic suppression of root-knot nematode in chickpea (Akhtar and Siddiqui 2008). Disease suppressiveness in soils is contributed not only by bacteria, but also by fungal genera such as Aspergillus, Fusarium, and Eurotium (Adam et al. 2014; Giné et al. 2016; Song et al. 2016). Certain rhizospheres such as that of pea are enriched in fungal species in addition to bacterial taxa (Turner et al. 2013). Bacteria and fungi can physically associate as some bacterial biofilms such as that of Pseudomonas sp. are formed on the hyphae of fungi like Laccaria in the soil (Guennoc et al. 2017; Hassani et al. 2018). Bacteria and fungi could be metabolically interdependent. For instance, fungal enzymes may initiate the breakdown of complex plant-derived substrates such as lignocellulosic material (Baker et al. 2019) and the breakdown products could serve as substrates for bacterial groups. Bacteria, fungi (e.g., Albugo), and oomycete species (e.g., Udeniomyces and Dioszegia) may coordinate to serve as hub microbes that are highly interactive with other microbes in the rhizosphere (Agler et al. 2016). Interkingdom molecular dialogue between bacteria and fungi is possible through quorum sensing (Jarosz et al. 2011). The inclusion of fungi in the bacterial consortium not only diversifies the inoculum but also promotes niche filling and competitive suppression of pathogens (Quiza et al. 2015). However, these interactions have to be evaluated and optimized using SynCom experiments in planta.
9.5.3 Engineered Plants
In addition to better probiotics, plants may also be better equipped to get the best support out of their microbiomes, since plants and their microbiomes function in unison as a holobiont. This is particularly relevant in the context of stress as microbiomes can respond dynamically to confer stress protection. Plant-mediated selection of microbiomes can alter traits such as flowering in Arabidopsis and Brassica spp. (Panke-Buisse et al. 2017). Genetically engineering plants to be able to modulate their microbiome is one approach as relevant genes could be transferred to crop plants (Qiu et al. 2019). Genes regulating the production of metabolites that attract beneficial microbes can be integrated into or enhanced in a plant. For example, plants releasing volatile organic compounds could attract beneficial bacteria from a distance in the soil (Schulz-Bohm et al. 2018). However, the consequences of change in plant metabolite profiles, their impact on crop quality, and the non-target effects of the metabolites on other organisms have to be carefully evaluated. Comparative genomics of domesticated crops and their wild relatives in combination with metabolite analysis and microbiome profiles can help narrow down to genes that can enrich crop microbiomes, in what is referred to as the “back to the roots” approach.
9.5.4 Disease-Suppressive Soils
Disease-suppressive soils are gold mines of beneficial microbes and can also be used to inoculate agricultural soils to transplant disease resistance to new soils even if the latter contains pathogens. Such soils can retain the suppressive effect for generations of crops and disease resistance may progress with generations due to enrichment of the microbiome and optimizing selection by the host plants. Undoubtedly, the early studies on suppressive soils focusing on single community members provide valuable insights into the mechanisms involved in disease suppression, yet they neglect the complex interactions among microbial communities as these occur in the root vicinity and within the root interior. Several seminal studies based on metagenomics and metatranscriptomics support that microbial consortia rather than individual strains function synergistically to confer solid protection against pathogens. Thus, multi-omics technologies provide opportunities to dissect disease suppressiveness to an exceptional level of detail and, in this context, may assist in the design of robust synthetic communities of microbes with enhanced disease-suppressive potential. Understanding the mechanisms of how disease suppression evolves in soils can be invaluable in engineering the plants and the soil microbiome to enhance disease suppressiveness. Presently, microbiome engineering is being pursued through artificially selecting a protective microbiome through repeated colonization over multiple generations to achieve an optimal plant-preferred community with protective functions (Mueller and Sachs 2015). This, in effect, creates a disease-suppressive soil. Such microbiomes may, in the future, be mass-cultured and cryopreserved for field application.
9.5.5 Microbiome-Mediated Organic Farming
Cultural practices in organic farming must have a pronounced impact on agricultural microbiomes. The progressive ease of sequencing and microbial community characterization affords the power to characterize the complex and diverse microbiomes that must operate in organic farms. Manure that has been traditionally used to fertilize agricultural fields is enriched in the fecal microbiomes of animals. The substrates used in organic soil amendments are degraded or fermented with microbial action which results in the enrichment of various microbial species. Recently, the microbial composition of traditional organic preparations in rural agriculture is receiving renewed attention; for instance, the compost fermentates named jeevamrutha and beejamrutha, which are made through the fermentation of organic substrates in jaggery and pulse crop flour by microbiota from cow dung, are routinely used as soil amendments in agricultural fields for sustainable crop production (Pattanaik et al. 2020). Microbial profiling of these preparations revealed that they are enriched in bacteria such as actinomycetes and fungi. Organic farming in itself promotes microbial diversity in the rhizosphere, and organic practices that support the enrichment of beneficial microbes may be explored to promote sustainability in agriculture, especially with increasing ease of microbiome profiling.
9.5.6 Considering Environmental Impacts of and on Microbiome-Based Agriculture
The introduction of a new microbial species into an ecosystem often comes with consequences that may be difficult to quantify (Delgado-Baquerizo et al. 2016). Microbial consortia have to be tested for the effects of their metabolites on nontarget organisms before application in agriculture and whether the metabolic changes in the crop could also affect human health. For instance, the inoculated rhizosphere microbes could enrich antifungal compounds such as polyene macrolide antibiotics, which have the potential to affect human cholesterol metabolism (Zotchev 2003).
Stresses such as drought are expected to aggravate plant disease and herbivory while substantially impacting yield (Bebber et al. 2014; Lobell and Field 2007). Environmental stress, particularly at high temperatures, can modulate the expression of defense genes, increase the transfer of pathogen effector proteins into host cells, reduce pathogen perception, and suppress host defense (Teixeira et al. 2019). Unfortunately, the benefits conferred by the microbiomes on hosts are also threatened by the effects of global climate change (Maclean and Wilson 2011). Microbiomes in agriculture could also be influenced by the environment, particularly climate change, including a rise in carbon dioxide levels, global warming, and altered rainfall patterns (Blankinship et al. 2011). Increased carbon dioxide levels, one of the key components of climate change, can influence rhizosphere structure through the altered root exudation patterns (Drigo et al. 2013). The activity of hub microbes has also been shown to be sensitive to environmental changes (Santoyo et al. 2017; Vacher et al. 2016). Fortunately, plant adaptations to stresses are not only accompanied by rapid compensatory changes in the rhizosphere, typically associated with changes in root exudation profiles, but also with genetic changes in microbes that are beneficial to the host (Rodriguez et al. 2008). Microbial communities have been observed to evolve and adapt faster to environmental changes than the plant itself, helping the plant overcome stress (Lau and Lennon 2012). Understanding the mechanisms of plant-microbiome dynamics during stress may help us design better strategies to harness microbiomes that can rescue plants from biotic and abiotic stresses in a changing environment. Finally, testing microbiome-based agriculture in multiple field trials across distinct locations and over multiple years is critical to overcome limitations in performance under field settings.
In conclusion, steps towards sustainable agriculture are critical to increasing global food security. The application of rhizosphere microbiomes as a sustainable alternative to chemical-based agriculture is gaining ground, thanks to recent advances in non-culture-based characterization of the microbiome and insights into the mechanisms of their interactions with the plant. This may be accomplished through a combination of microbiome treatments and enhanced recruitment and retention of healthy microbiomes by the plant to create disease-suppressive soils for durable plant protection.
References
Adam M, Westphal A, Hallmann J, Heuer H (2014) Specific microbial attachment to root knot nematodes in suppressive soil. Appl Environ Microbiol 80:2679–2686
Agler MT, Ruhe J, Kroll S, Morhenn C, Kim S-T, Weigel D, Kemen EM (2016) Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14:e1002352
Akhtar MS, Siddiqui ZA (2008) Glomusintraradices, Pseudomonas alcaligenes, and Bacillus pumilus: effective agents for the control of root-rot disease complex of chickpea (Cicer arietinum L.). J Gen Plant Pathol 74:53–60
Akum FN, Steinbrenner J, Biedenkopf D, Imani J, Kogel K-H (2015) The Piriformospora indica effector PIIN_08944 promotes the mutualistic Sebacinalean symbiosis. Front Plant Sci 6:906
Bacilio-Jiménez M, Aguilar-Flores S, Ventura-Zapata E, Pérez-Campos E, Bouquelet S, Zenteno E (2004) Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 249:271–277
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Badri DV, Chaparro JM, Zhang R, Shen Q, Vivanco JM (2013) Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem 288:4502–4512
Bai Y, Müller DB, Srinivas G, Garrido-Oter R, Potthoff E, Rott M, Dombrowski N, Münch PC, Spaepen S, Remus-Emsermann M, Hüttel B, McHardy AC, Vorholt JA, Schulze-Lefert P (2015) Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528:364–369
Baker P, Tiroumalechetty A, Mohan R (2019) Fungal enzymes for bioremediation of xenobiotic compounds. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi: volume 3: perspective for sustainable environments. Springer International Publishing, Cham, pp 463–489
Bass D, Stentiford GD, Wang HC, Koskella B, Tyler CR (2019) The pathobiome in animal and plant diseases. Trends Ecol Evol 34:996–1008
Bebber DP, Holmes T, Gurr SJ (2014) The global spread of crop pests and pathogens. Glob Ecol Biogeogr 23:1398–1407
Beck M, Wyrsch I, Strutt J, Wimalasekera R, Webb A, Boller T, Robatzek S (2014) Expression patterns of flagellin sensing 2 map to bacterial entry sites in plant shoots and roots. J Exp Bot 65:6487–6498
Becker J, Eisenhauer N, Scheu S, Jousset A (2012) Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol Lett 15:468–474
Beckers B, Op De Beeck M, Weyens N, Van Acker R, Van Montagu M, Boerjan W, Vangronsveld J (2016) Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc Natl Acad Sci U S A 113:2312–2317
Begley MJ, Crombie L, Crombie WML, Whiting DA (1986) The isolation of avenacins A-1, A-2, B-1, and B-2, chemical defences against cereal ‘take-all’ disease. Structure of their ‘aglycones’, the avenestergenins, and their anhydro dimers. J Chem Soc Perkin Trans 1:1905–1915
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Berendsen RL, Vismans G, Yu K, Song Y, de Jonge R, Burgman WP, Burmølle M, Herschend J, Bakker P, Pieterse CMJ (2018) Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J 12:1496–1507
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
Berg M, Koskella B (2018) Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr Biol 28:2487–2492.e2483
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Blankinship JC, Niklaus PA, Hungate BA (2011) A meta-analysis of responses of soil biota to global change. Oecologia 165:553–565
Bodenhausen N, Bortfeld-Miller M, Ackermann M, Vorholt JA (2014) A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet 10:e1004283
Botero D, Alvarado C, Bernal A, Danies G, Restrepo S (2018) Network analyses in plant pathogens. Front Microbiol 9:35
Bulgarelli D, Rott M, Schlaeppi K, Loren V, van Themaat E, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95
Bulgarelli D, Garrido-Oter R, Münch PC, Weiman A, Dröge J, Pan Y, McHardy AC, Schulze-Lefert P (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403
Carrión VJ, Cordovez V, Tyc O, Etalo DW, de Bruijn I, de Jager VCL, Medema MH, Eberl L, Raaijmakers JM (2018) Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J 12:2307–2321
Carvalhais LC, Dennis PG, Schenk PM (2014) Plant defence inducers rapidly influence the diversity of bacterial communities in a potting mix. Appl Soil Ecol 84:1–5
Cavalcante JJ, Vargas C, Nogueira EM, Vinagre F, Schwarcz K, Baldani JI, Ferreira PC, Hemerly AS (2007) Members of the ethylene signalling pathway are regulated in sugarcane during the association with nitrogen-fixing endophytic bacteria. J Exp Bot 58:673–686
Cha J-Y, Han S, Hong H-J, Cho H, Kim D, Kwon Y, Kwon S-K, Crüsemann M, Bok Lee Y, Kim JF, Giaever G, Nislow C, Moore BS, Thomashow LS, Weller DM, Kwak Y-S (2016) Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J 10:119–129
Chapelle E, Mendes R, Bakker PA, Raaijmakers JM (2016) Fungal invasion of the rhizosphere microbiome. ISME J 10:265–268
Chen YH, Gols R, Benrey B (2015) Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol 60:35–58
Choudhary DK, Prakash A, Johri BN (2007) Induced systemic resistance (ISR) in plants: mechanism of action. Indian J Microbiol 47:289–297
Chuberre C, Plancot B, Driouich A, Moore JP, Bardor M, Gügi B, Vicré M (2018) Plant immunity is compartmentalized and specialized in roots. Front Plant Sci 9:1692
Cole BJ, Feltcher ME, Waters RJ, Wetmore KM, Mucyn TS, Ryan EM, Wang G, Ul-Hasan S, McDonald M, Yoshikuni Y, Malmstrom RR, Deutschbauer AM, Dangl JL, Visel A (2017) Genome-wide identification of bacterial plant colonization genes. PLoS Biol 15:e2002860
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142:169–196
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37
Conn HJ, Harding HA, Kligler IJ, Frost WD, Prucha MJ, Atkins KN (1918) Methods of pure culture study preliminary report of the committee on the chart for identification of bacterial species. J Bacteriol Res 3(2):115–128
Coombs JT, Michelsen PP, Franco CMM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Cooper JE (2007) Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol 103:1355–1365
Coque JJR, Álvarez-Pérez JM, Cobos R, González-García S, Ibáñez AM, Diez Galán A, Calvo-Peña C (2020) Chapter Four - Advances in the control of phytopathogenic fungi that infect crops through their root system. In: Gadd GM, Sariaslani S (eds) Advances in applied microbiology. Academic Press, London, pp 123–170
Cordovez V, Carrion VJ, Etalo DW, Mumm R, Zhu H, van Wezel GP, Raaijmakers JM (2015) Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front Microbiol 6:1081
Corral-Lugo A, Daddaoua A, Ortega A, Espinosa-Urgel M, Krell T (2016) Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci Signal 9:ra1
Costa JLS, Menge JA, Casale WL (2000) Biological control of Phytophthora root rot of avocato with microorganisms grown in organic mulches. Braz J Microbiol 31:239–246
Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science (New York, NY) 341:746–751
De Vrieze M, Germanier F, Vuille N, Weisskopf L (2018) Combining different potato-associated pseudomonas strains for improved biocontrol of phytophthora infestans. Front Microbiol 9:2573
Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, Berdugo M, Campbell CD, Singh BK (2016) Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat Commun 7:10541
Doornbos RF, Geraats BP, Kuramae EE, Van Loon LC, Bakker PA (2011) Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. Mol Plant-Microbe Interact 24:395–407
Drewnowski A, Gomez-Carneros C (2000) Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72:1424–1435
Drigo B, Kowalchuk GA, Knapp BA, Pijl AS, Boschker HTS, van Veen JA (2013) Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Glob Chang Biol 19:621–636
Duan L, Liu H, Li X, Xiao J, Wang S (2014) Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol Plant 152:486–500
Durán P, Tortella G, Viscardi S, Barra PJ, Carrión VJ, Mora ML, Pozo MJ (2018) Microbial Community Composition in Take-All Suppressive Soils. Front Microbiol 9:2198
Eberl L (1999) N-acyl homoserinelactone-mediated gene regulation in gram-negative bacteria. Syst Appl Microbiol 22:493–506
Ellouze W, Hamel C, Vujanovic V, Gan Y, Bouzid S, St-Arnaud M (2013) Chickpea genotypes shape the soil microbiome and affect the establishment of the subsequent durum wheat crop in the semiarid North American Great Plains. Soil Biol Biochem 63:129–141
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590
Finkel OM, Castrillo G, Herrera Paredes S, Salas González I, Dangl JL (2017) Understanding and exploiting plant beneficial microbes. Curr Opin Plant Biol 38:155–163
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Garbeva P, Postma J, van Veen JA, van Elsas JD (2006) Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ Microbiol 8:233–246
Giné A, Carrasquilla M, Martínez-Alonso M, Gaju N, Sorribas FJ (2016) Characterization of soil suppressiveness to root-knot nematodes in organic horticulture in plastic greenhouse. Front Plant Sci 7:164–164
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:963401
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Gomez Exposito R, de Bruijn I, Postma J, Raaijmakers JM (2017) Current Insights into the role of rhizosphere bacteria in disease suppressive soils. Front Microbiol 8:2529
Gómez Expósito R, de Bruijn I, Postma J, Raaijmakers JM (2017) Current Insights into the role of rhizosphere bacteria in disease suppressive soils. Front Microbiol 8:2529–2529
Gopal M, Gupta A (2016) Microbiome selection could spur next-generation plant breeding strategies. Front Microbiol 7:1971
Gopal M, Gupta A, Thomas GV (2013) Bespoke microbiome therapy to manage plant diseases. Front Microbiol 4:355
Guennoc CM, Rose C, Labbé J, Deveau A (2017) Bacterial biofilm formation on soil fungi: a widespread ability under controls. bioRxiv:130740
Gunawardena U, Hawes MC (2002) Tissue specific localization of root infection by fungal pathogens: role of root border cells. Mol Plant-Microbe Interact 15:1128–1136
Hardoim PR, van Overbeek LS, Elsas JDv (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species--opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194
Hassani MA, Duran P, Hacquard S (2018) Microbial interactions within the plant holobiont. Microbiome 6:58
Hawes MC, Gunawardena U, Miyasaka S, Zhao X (2000) The role of root border cells in plant defense. Trends Plant Sci 5:128–133
Helfrich EJN, Vogel CM, Ueoka R, Schäfer M, Ryffel F, Müller DB, Probst S, Kreuzer M, Piel J, Vorholt JA (2018) Bipartite interactions, antibiotic production and biosynthetic potential of the Arabidopsis leaf microbiome. Nat Microbiol 3:909–919
Herrera Paredes S, Gao T, Law TF, Finkel OM, Mucyn T, Teixeira PJPL, Salas González I, Feltcher ME, Powers MJ, Shank EA, Jones CD, Jojic V, Dangl JL, Castrillo G (2018) Design of synthetic bacterial communities for predictable plant phenotypes. PLoS Biol 16:e2003962
Herschkovitz Y, Lerner A, Davidov Y, Rothballer M, Hartmann A, Okon Y, Jurkevitch E (2005) Inoculation with the plant-growth-promoting rhizobacterium Azospirillum brasilense causes little disturbance in the rhizosphere and rhizoplane of maize (Zea mays). Microb Ecol 50:277–288
Hu J, Wei Z, Friman V-P, Gu S-h, Wang X-f, Eisenhauer N, Yang T-j, Ma J, Shen Q-r, Xu Y-c, Jousset A (2016) Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBio 7:e01790–e01716
Hu L, Robert CAM, Cadot S, Zhang X, Ye M, Li B, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M (2018) Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738
Huang AC, Osbourn A (2019) Plant terpenes that mediate below-ground interactions: prospects for bioengineering terpenoids for plant protection. Pest Manag Sci 75:2368–2377
Ingels CA, Scow KM, Whisson DA, Drenovsky RE (2005) Effects of cover crops on grapevines, yield, juice composition, soil microbial ecology, and gopher activity. Am J Enol Vitic 56:19–29
Iniguez AL, Dong Y, Carter HD, Ahmer BM, Stone JM, Triplett EW (2005) Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant-Microbe Interact 18:169–178
Jackson LE (1995) Root architecture in cultivated and wild lettuce (Lactuca spp.). Plant Cell Environ 18:885–894
Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, Kogel KH, Schäfer P (2011) Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol 156:726–740
James Cook R (2003) Take-all of wheat. Physiol Mol Plant Pathol 62:73–86
Jansson JK, Hofmockel KS (2018) The soil microbiome—from metagenomics to metaphenomics. Curr Opin Microbiol 43:162–168
Jarosz LM, Ovchinnikova ES, Meijler MM, Krom BP (2011) Microbial spy games and host response: roles of a Pseudomonas aeruginosa small molecule in communication with other species. PLoS Pathog 7:e1002312–e1002312
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Kapulnik Y, Kushnir U (1991) Growth dependency of wild, primitive and modern cultivated wheat lines on vesicular-arbuscular mycorrhiza fungi. Euphytica 56:27–36
Kelley WD, South DB (2017) Effects of herbicides on in vitro growth of mycorrhizae of pine (Pinus spp.). Weed Sci 28:599–602
Khorassani R, Hettwer U, Ratzinger A, Steingrobe B, Karlovsky P, Claassen N (2011) Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC Plant Biol 11:121–121
Kim KD, Nemec S, Musson G (1997) Control of Phytophthora root and crown rot of bell pepper with composts and soil amendments in the greenhouse. Appl Soil Ecol 5:169–179
Kloepper JW, Schroth MN, Miller TD (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70:1078
Kniskern JM, Traw MB, Bergelson J (2007) Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol Plant-Microbe Interact 20:1512–1522
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-amino acids trigger biofilm disassembly. Science (New York, NY) 328:627–629
Lakshmanan V, Kitto SL, Caplan JL, Hsueh Y-H, Kearns DB, Wu Y-S, Bais HP (2012) Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol 160:1642–1661
Lareen A, Burton F, Schafer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90:575–587
Lau JA, Lennon JT (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci 109(35):14058–14062
Leach JE, Triplett LR, Argueso CT, Trivedi P (2017) Communication in the phytobiome. Cell 169:587–596
Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, Malfatti S, Glavina del Rio T, Jones CD, Tringe SG, Dangl JL (2015) Plant microbiome. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349:860–864
Levy A, Conway JM, Dangl JL, Woyke T (2018) Elucidating bacterial gene functions in the plant microbiome. Cell Host Microbe 24:475–485
Li R, Khafipour E, Krause DO, Entz MH, de Kievit TR, Fernando WGD (2012) Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS One 7:e51897–e51897
Liang Y, Tóth K, Cao Y, Tanaka K, Espinoza C, Stacey G (2014) Lipochitooligosaccharide recognition: an ancient story. New Phytol 204:289–296
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47:374–379
Ling N, Zhang W, Wang D, Mao J, Huang Q, Guo S, Shen Q (2013) Root exudates from grafted-root watermelon showed a certain contribution in inhibiting Fusarium oxysporum f. sp. niveum. PLoS One 8:e63383
Liu H, Carvalhais LC, Schenk PM, Dennis PG (2017) Effects of jasmonic acid signalling on the wheat microbiome differ between body sites. Sci Rep 7:41766
Lobell DB, Field CB (2007) Global scale climate–crop yield relationships and the impacts of recent warming. Environ Res Lett 2:014002
Long HH, Sonntag DG, Schmidt DD, Baldwin IT (2010) The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol 185:554–567
Lupatini M, Korthals GW, de Hollander M, Janssens TKS, Kuramae EE (2016) Soil microbiome is more heterogeneous in organic than in conventional farming system. Front Microbiol 7:2064
Ma Z, Michailides TJ (2005) Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot 24:853–863
Maclean IMD, Wilson RJ (2011) Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci 108:12337–12342
Maeder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Martin JT (1964) Role of cuticle in the defense against plant disease. Annu Rev Phytopathol 2:81–100
Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM (2009) Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J 3:977–991
Mazzola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie Van Leeuwenhoek 81:557–564
Mazzola M (2007) Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39:213–220
McNear DH Jr (2013) The rhizosphereroots, soil and everything in between. Nat Educ Knowl 4(3):1
Meena KK, Mesapogu S, Kumar M, Yandigeri MS, Singh G, Saxena AK (2010) Co-inoculation of the endophytic fungus Piriformospora indica with the phosphate-solubilising bacterium Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol Fertil Soils 46:169–174
Melnyk RA, Beskrovnaya P, Liu Z, Song Y, Haney CH (2019a) Bacterially produced spermidine induces plant systemic susceptibility to pathogens. bioRxiv:517870
Melnyk RA, Hossain SS, Haney CH (2019b) Convergent gain and loss of genomic islands drive lifestyle changes in plant-associated Pseudomonas. ISME J 13:1575–1588
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Meneses CH, Rouws LF, Simoes-Araujo JL, Vidal MS, Baldani JI (2011) Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol Plant-Microbe Interact 24:1448–1458
Mercado-Blanco J, Bakker PA (2007) Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. Antonie Van Leeuwenhoek 92:367–389
Meyer RS, DuVal AE, Jensen HR (2012) Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytol 196:29–48
Micallef SA, Shiaris MP, Colón-Carmona A (2009) Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J Exp Bot 60:1729–1742
Mohan R, Benton M, Dangelmaier E, Fu Z, Chandra Sekhar A (2018) Quorum sensing and biofilm formation in pathogenic and mutualistic plant-bacterial interactions. In: Veera P (ed) Implication of quorum sensing system in biofilm formation and virulence, B. Springer Singapore, Singapore, pp 133–160
Molina-Romero D, Baez A, Quintero-Hernandez V, Castaneda-Lucio M, Fuentes-Ramirez LE, Bustillos-Cristales MDR, Rodriguez-Andrade O, Morales-Garcia YE, Munive A, Munoz-Rojas J (2017) Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS One 12:e0187913
Mueller UG, Sachs JL (2015) Engineering microbiomes to improve plant and animal health. Trends Microbiol 23:606–617
Muimba-Kankolongo A (2018) Chapter 7 Pre- and postharvest field operations. In: Muimba-Kankolongo A (ed) Food crop production by smallholder farmers in Southern Africa. Academic Press, London, pp 59–71
Mutch LA, Young JP (2004) Diversity and specificity of Rhizobium leguminosarum biovar viciae on wild and cultivated legumes. Mol Ecol 13:2435–2444
Namvar A, Khandan (2015) Inoculation of rapeseed under different rates of inorganic nitrogen and sulfur fertilizer: impact on water relations, cell membrane stability, chlorophyll content and yield. Arch Agron Soil Sci 61:1137
Neal AL, Ahmad S, Gordon-Weeks R, Ton J (2012) Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One 7:e35498
Newitt JT, Prudence SMM (2019) Biocontrol of cereal crop diseases using streptomycetes. Pathogens 8:78
Nguyen TH, Phan TC, Choudhury ATMA, Rose MT, Deaker RJ, Kennedy IR (2017) BioGro: a plant growth-promoting biofertilizer validated by 15 years’ research from laboratory selection to rice farmer’s fields of the mekong delta. In: Singh JS, Seneviratne G (eds) Agro-environmental sustainability: volume 1: managing crop health. Springer International Publishing, Cham, pp 237–254
Niu B, Paulson JN, Zheng X, Kolter R (2017) Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci U S A 114:E2450–E2459
Odelade KA, Babalola OO (2019) Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int J Environ Res Public Health 16:3873
Orozco-Mosqueda MDC, Rocha-Granados MDC, Glick BR, Santoyo G (2018) Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol Res 208:25–31
Panke-Buisse K, Lee S, Kao-Kniffin J (2017) Cultivated sub-populations of soil microbiomes retain early flowering plant trait. Microb Ecol 73:394–403
Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE (1999) Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci 96:12923–12928
Pattanaik L, Duraivadivel P, Hariprasad P, Naik SN (2020) Utilization and re-use of solid and liquid waste generated from the natural indigo dye production process - a zero waste approach. Bioresour Technol 301:122721
Pedras MSC, Yaya EE (2015) Plant chemical defenses: are all constitutive antimicrobial metabolites phytoanticipins? Nat Prod Commun 10:1934578X1501000142
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553
Peressotti E, Wiedemann-Merdinoglu S, Delmotte F, Bellin D, Di Gaspero G, Testolin R, Merdinoglu D, Mestre P (2010) Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol 10:147
Perez-Jaramillo JE, Mendes R, Raaijmakers JM (2016) Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol 90:635–644
Perez-Jaramillo JE, Carrion VJ, Bosse M, Ferrao LFV, de Hollander M, Garcia AAF, Ramirez CA, Mendes R, Raaijmakers JM (2017) Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME J 11:2244–2257
Pérez-Jaramillo JE, Carrión VJ, de Hollander M, Raaijmakers JM (2018) The wild side of plant microbiomes. Microbiome 6:143
Pétriacq P, Williams A, Cotton A, McFarlane AE, Rolfe SA, Ton J (2017) Metabolite profiling of non-sterile rhizosphere soil. Plant J 92:147–162
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375
Pinski A, Betekhtin A (2019) Defining the genetic basis of plant-endophytic bacteria interactions. Int J Mol Sci 20:1947
Plett JM, Daguerre Y, Wittulsky S, Vayssières A, Deveau A, Melton SJ, Kohler A, Morrell-Falvey JL, Brun A, Veneault-Fourrey C, Martin F (2014) Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc Natl Acad Sci U S A 111:8299–8304
Postma-Blaauw MB, de Goede RGM, Bloem J, Faber JH, Brussaard L (2010) Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 91:460–473
Preisig CL, Bell JN, Sun Y, Hrazdina G, Matthews DE, Vanetten HD (1990) Biosynthesis of the phytoalexin pisatin: isoflavone reduction and further metabolism of the product sophorol by extracts of Pisum sativum. Plant Physiol 94:1444–1448
Qiu Z, Egidi E, Liu H, Kaur S, Singh BK (2019) New frontiers in agriculture productivity: optimised microbial inoculants and in situ microbiome engineering. Biotechnol Adv 37:107371
Quiza L, St-Arnaud M, Yergeau E (2015) Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Front Plant Sci 6:507
Raaijmakers JM, Mazzola M (2016) ECOLOGY. Soil immune responses. Science 352:1392–1393
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Radajewski S, Ineson P, Parekh NR, Murrell JC (2000) Stable-isotope probing as a tool in microbial ecology. Nature 403:646–649
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927
Raupach GS, Kloepper JW (1998) Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88:1158–1164
Reen FJ, Gutiérrez-Barranquero JA, Parages ML, Gara FO (2018) Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition. Appl Microbiol Biotechnol 102:2063–2073
Reinhold-Hurek B, Bunger W, Burbano CS, Sabale M, Hurek T (2015) Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53:403–424
Reverchon F, Garcia-Quiroz W, Guevara-Avendano E, Solis-Garcia IA, Ferrera-Rodriguez O, Lorea-Hernandez F (2019) Antifungal potential of Lauraceae rhizobacteria from a tropical montane cloud forest against Fusarium spp. Brazilian journal of microbiology. Publ Braz Soc Microbiol 50:583–592
Rodrigues JL, Pellizari VH, Mueller R, Baek K, Jesus Eda C, Paula FS, Mirza B, Hamaoui GS Jr, Tsai SM, Feigl B, Tiedje JM, Bohannan BJ, Nüsslein K (2013) Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc Natl Acad Sci U S A 110:988–993
Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2:404–416
Rodriguez PA, Rothballer M, Chowdhury SP, Nussbaumer T, Gutjahr C, Falter-Braun P (2019) Systems biology of plant-microbiome interactions. Mol Plant 12:804–821
Rolli E, Marasco R, Vigani G, Ettoumi B, Mapelli F, Deangelis ML, Gandolfi C, Casati E, Previtali F, Gerbino R, Pierotti Cei F, Borin S, Sorlini C, Zocchi G, Daffonchio D (2015) Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol 17:316–331
Rosier A, Bishnoi U, Lakshmanan V, Sherrier DJ, Bais HP (2016) A perspective on inter-kingdom signaling in plant-beneficial microbe interactions. Plant Mol Biol 90:537–548
Rout ME (2014) Chapter Eleven - The plant microbiome. In: Paterson AH (ed) Advances in botanical research. (Academic Press), London, pp 279–309
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547
Ryan PR, Dessaux Y, Thomashow LS, Weller DM (2009) Rhizosphere engineering and management for sustainable agriculture. Plant Soil 321:363–383
Sanchis V, Bourguet D (2008) Bacillus thuringiensis: applications in agriculture and insect resistance management. A review. Agron Sustain Dev 28:11–20
Santhanam R, Luu VT, Weinhold A, Goldberg J, Oh Y, Baldwin IT (2015) Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc Natl Acad Sci U S A 112:E5013–E5020
Santos A, Flores M (1995) Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria. Lett Appl Microbiol 20:349–352
Santoyo G, Hernández-Pacheco CE, Hernández-Salmerón JE, Hernández-León R (2017) The role of abiotic factors modulating the plant-microbe-soil interactions: toward sustainable agriculture. A review. Span J Agric Res 15:13
Schaeffer SM, Nakata PA (2015) CRISPR/Cas9-mediated genome editing and gene replacement in plants: transitioning from lab to field. Plant Sci 240:130–142
Schlaeppi K, Bulgarelli D (2015) The plant microbiome at work. Mol Plant-Microbe Interact 28:212–217
Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T (2017) Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107:1284–1297
Schmer MR, Vogel KP, Varvel GE, Follett RF, Mitchell RB, Jin VL (2014) Energy potential and greenhouse gas emissions from bioenergy cropping systems on marginally productive cropland. PLoS One 9:e89501
Schulz-Bohm K, Gerards S, Hundscheid M, Melenhorst J, de Boer W, Garbeva P (2018) Calling from distance: attraction of soil bacteria by plant root volatiles. ISME J 12:1252–1262
Shanks OC, Kelty CA, Archibeque S, Jenkins M, Newton RJ, McLellan SL, Huse SM, Sogin ML (2011) Community structures of fecal bacteria in cattle from different animal feeding operations. Appl Environ Microbiol 77:2992–3001
Shaposhnikov A, Morgounov A, Akin B, Makarova N, Belimov A, Tikhonovich I (2016) Comparative characteristics of root systems and root exudation of synthetic, landrace and modern wheat varieties. Agric Biol 51:68–78
Sheoran N, Kumar A, Munjal V, Nadakkakath AV, Eapen SJ (2016) Pseudomonas putida BP25 alters root phenotype and triggers salicylic acid signaling as a feedback loop in regulating endophytic colonization in Arabidopsis thaliana. Physiol Mol Plant Pathol 93:99–111
Smith KP, Goodman RM (1999) Host variation for interactions with beneficial plant-associated microbes. Annu Rev Phytopathol 37:473–491
Song J, Li S, Xu Y, Wei W, Yao Q, Pan F (2016) Diversity of parasitic fungi from soybean cyst nematode associated with long-term continuous cropping of soybean in black soil. Acta Agric Scand Sect B Soil Plant Sci 66:432–442
Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker P, Feussner I, Pieterse CMJ (2018) MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci U S A 115:E5213–E5222
Sturz AV, Arsenault W, Christie B (2003) Red clover–potato cultivar combinations for improved potato yield. Agron J 95:1089
Syed A, Rahman SF, Singh E, Pieterse CMJ, Schenk PM (2018) Emerging microbial biocontrol strategies for plant pathogens. Plant Sci 267:102–111
Teixeira PJPL, Colaianni NR, Fitzpatrick CR, Dangl JL (2019) Beyond pathogens: microbiota interactions with the plant immune system. Curr Opin Microbiol 49:7–17
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677
Timm CM, Pelletier DA, Jawdy SS, Gunter LE, Henning JA, Engle N, Aufrecht J, Gee E, Nookaew I, Yang Z, Lu TY, Tschaplinski TJ, Doktycz MJ, Tuskan GA, Weston DJ (2016) Two poplar-associated bacterial isolates induce additive favorable responses in a constructed plant-microbiome system. Front Plant Sci 7:497
Trdá L, Fernandez O, Boutrot F, Héloir MC, Kelloniemi J, Daire X, Adrian M, Clément C, Zipfel C, Dorey S, Poinssot B (2014) The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol 201:1371–1384
Trivedi P, Delgado-Baquerizo M, Trivedi C, Hamonts K, Anderson IC, Singh BK (2017) Keystone microbial taxa regulate the invasion of a fungal pathogen in agro-ecosystems. Soil Biol Biochem 111:10–14
Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol 14:209
Turrini A, Sbrana C, Giovannetti M (2015) Belowground environmental effects of transgenic crops: a soil microbial perspective. Res Microbiol 166:121–131
Vacher C, Hampe A, Porté AJ, Sauer U, Compant S, Morris CE (2016) The phyllosphere: microbial jungle at the plant–climate interface. Annu Rev Ecol Evol Syst 47:1–24
Van Acker H, Van Dijck P, Coenye T (2014) Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol 22:326–333
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus “Phytoanticipins”. Plant Cell 6:1191
Vaseva II, Qudeimat E, Potuschak T, Du Y, Genschik P, Vandenbussche F, Van Der Straeten D (2018) The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc Natl Acad Sci 115:E4130–E4139
van Veen JA, van Overbeek LS, van Elsas JD (1997) Fate and activity of microorganisms introduced into soil. Microbiol Mol Biol Rev 61:121–135
Viaene T, Langendries S, Beirinckx S, Maes M, Goormachtig S (2016) Streptomyces as a plant’s best friend? FEMS Microbiol Ecol 92:fiw119
van der Voort M, Kempenaar M, van Driel M, Raaijmakers JM, Mendes R (2016) Impact of soil heat on reassembly of bacterial communities in the rhizosphere microbiome and plant disease suppression. Ecol Lett 19:375–382
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
de Weert S, Vermeiren H, Mulders IH, Kuiper I, Hendrickx N, Bloemberg GV, Vanderleyden J, De Mot R, Lugtenberg BJ (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant-Microbe Interact 15:1173–1180
Weese DJ, Heath KD, Dentinger BT, Lau JA (2015) Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 69:631–642
Weller DM, Raaijmakers JM, Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
de Werra P, Huser A, Tabacchi R, Keel C, Maurhofer M (2011) Plant- and microbe-derived compounds affect the expression of genes encoding antifungal compounds in a pseudomonad with biocontrol activity. Appl Environ Microbiol 77:2807–2812
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol 39:283–297
Whipps JM (1990) Carbon economy. John Wiley and Sons Ltd., Chichester, pp 59–97
Wille L, Messmer MM, Studer B, Hohmann P (2019) Insights to plant-microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ 42:20–40
Wissuwa M, Mazzola M, Picard C (2008) Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 321:409
Wyrsch I, Domínguez-Ferreras A, Geldner N, Boller T (2015) Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol 206:774–784
Xing X, Koch AM, Jones AM, Ragone D, Murch S, Hart MM (2012) Mutualism breakdown in breadfruit domestication. Proc Biol Sci 279:1122–1130
Xu L, Naylor D, Dong Z, Simmons T, Pierroz G, Hixson KK, Kim Y-M, Zink EM, Engbrecht KM, Wang Y, Gao C, DeGraaf S, Madera MA, Sievert JA, Hollingsworth J, Birdseye D, Scheller HV, Hutmacher R, Dahlberg J, Jansson C, Taylor JW, Lemaux PG, Coleman-Derr D (2018) Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc Natl Acad Sci 115:E4284–E4293
Yin C, Mueth N, Hulbert S, Schlatter D, Paulitz TC, Schroeder K, Prescott A, Dhingra A (2017) Bacterial communities on wheat grown under long-term conventional tillage and no-till in the pacific northwest of the United States. Phytobiomes J 1:83–90
Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235:1197–1207
Yu K, Pieterse CMJ, Bakker PAHM, Berendsen RL (2019) Beneficial microbes going underground of root immunity. Plant Cell Environ 42:2860–2870
Zachow C, Muller H, Tilcher R, Berg G (2014) Differences between the rhizosphere microbiome of Beta vulgaris ssp. maritima-ancestor of all beet crops-and modern sugar beets. Front Microbiol 5:415
Zehnder GW, Yao C, Murphy JF, Sikora ER, Kloepper JW (2000) Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. BioControl 45:127–137
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loque D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480
Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R (2014) Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 374:689–700
Zhou S, Richter A, Jander G (2018) Beyond defense: multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol 59:1528–1537
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Zipfel C, Oldroyd GED (2017) Plant signalling in symbiosis and immunity. Nature 543:328–336
Zotchev SB (2003) Polyene macrolide antibiotics and their applications in human therapy. Curr Med Chem 10:211–223
Żur J, Wojcieszyńska D, Guzik U (2016) Metabolic Responses of Bacterial Cells to Immobilization. Molecules 21:958
Acknowledgments
This endeavor was supported by the Erie Community Foundation-Helping Today grant. We thank Grant Ruback at Colgate University and Nathan Wise at Mercyhurst University for their intellectual contribution.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mohan, R. et al. (2022). Microbiome-Based Sustainable Agriculture Targeting Plant Protection. In: Veera Bramhachari, P. (eds) Understanding the Microbiome Interactions in Agriculture and the Environment. Springer, Singapore. https://doi.org/10.1007/978-981-19-3696-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-3696-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3695-1
Online ISBN: 978-981-19-3696-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)