Abstract
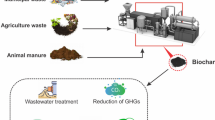
Biochar is carbon rich, porous substance produced under limited or no supply of oxygen. Pristine biochar is considered as a sustainable, beneficial, and low-cost product employed for soil conditioning, agricultural production, and pollutant removal. It is a promising product having a wide array of applications including catalytic reaction, carbon sequestration, pollution mitigation, and sustainable agriculture. The production of biochar is a sustainable practice to treat and valorize solid waste. Without any activation or modification, pristine biochar has lower surface area, porosity, and surface functional groups. To enhance the physicochemical and functional properties of pristine biochar, modification of biochar is done via physical, chemical, and biological methods. This chapter provides an overview of pristine biochar, including its production, modification, differences between pristine and engineered/modified biochar and multi-dimensional applications. Additionally this chapter covers knowledge gaps and perspectives in the domain of biochar technology and application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pristine biochar (PBC) is a carbonaceous material prepared by pyrolysis. Pyrolysis is the thermochemical conversion of raw materials at high temperature in oxygen-limited conditions. The raw materials needed to make biochar are a wide range of biomass-based wastes such as forest and crop residues, wood chips, sludge, municipal solid waste, sunflower husk, pelletized grape wine pressings, etc. (Lehmann et al. 2006; Colatoni et al. 2016).
The properties of biochar including its recalcitrant nature, surface area, porosity, varied functional groups, and the mineral content make it a suitable product for multi-dimensional applications such as pollutants removal, carbon sequestration, soil rehabilitation, wastewater purification, catalysis, reduction of greenhouse gases, geo-engineering, enhanced soil fertility, crop productivity, and sustainable energy production (Wang et al. 2017; Wani et al. 2021; Ramola et al. 2013). This product is well-liked in the wastewater treatment sector due to its significant adsorption properties for nitrogen, phosphorus, heavy metals, and other emerging contaminants (Chen et al. 2011 and Han et al. 2016). However, due to small particle size and low density, pristine biochar is hard to get separated from water (Tan et al. 2016). There is also a problem of low regeneration and potential adverse effects of spent pristine biochar disposal in the ambient environment. Characteristic properties of pristine biochar vary according to the type of raw materials used and the pyrolysis conditions operated during its preparation. Increase in textural properties such as pore volume and surface area occur when the pyrolysis temperature is optimum. These properties decline beyond the optimum temperature due to collapse of pores and structural deformation (Ramola et al. 2020a, 2021). Large surface area helps in the adsorption of heavy metals and other emerging contaminants. The surface area of pine needles pyrolyzed at 100 ºC was 0.65 m2g−1, which increased to 112.4 m2 g−1 at 400 ºC (Chen et al. 2008). Ramola et al. (2020a) also reported increased surface area of biochar-bentonite and biochar-calcite composites at 500 ºC, i.e., 53.08 m2g−1 and 32.29 m2g−1, respectively, that was maximum in comparison to the surface area of both biochar composites at 300 and 700 ºC. Surface area of some pristine biochar at different pyrolysis temperature is given in Table 1.
2 Production of Pristine Biochar
There are different methods for the production of biochar. The most common methods are pyrolysis, hydrothermal carbonization (HTC), and microwave carbonization (Table 2). These methods influence physical and chemical properties of biochar like yield, cation exchange capacity, specific surface area, functional groups, and ash content.
Pyrolysis is classified as either fast or slow pyrolysis based on the pyrolysis conditions. The temperature of 500 ºC, residence period of 2 s, and heat transfer rate of 300 ºC/min are the operational conditions for fast pyrolysis. The raw material decomposes very rapidly in fast pyrolysis because the temperature is high. Bio-oil (75 wt%), char (12 wt%), and gas (13 wt%) are the products of fast pyrolysis. Slow pyrolysis is operated at temperatures of 300–500 ºC, residence period of 5–30 min, and heat transfer rate of 5–20 ºC/min. Bio-oil (30–50 wt%), char (25–30 wt%), and gas (35 wt%) are produced via slow pyrolysis (Ramola et al. 2020b).
HTC system uses water medium for reaction under high pressure and temperature (Regmi et al. 2012; Zhang et al. 2013a, b). HTC provides significant advantages in conversion of biomass as it has high conversion efficiency, relatively low operational temperature, and no need of extensive energy for drying process (Sabio et al. 2016; Liu and Balasubramanian 2012b, a). The biochar produced by HTC is known as hydrochar. The fuel quality of hydrochar is similar to that of lignite (Liu et al. 2012) with high energy density.
Microwave pyrolysis is a comparatively modern pyrolysis technique that has several advantages over conventional techniques, including the ability to regulate the process along with power and cost reductions (Masek et al. 2013; Morgan et al. 2017). It tends to eliminate the drawbacks of traditional pyrolysis technologies, like the need to shred raw material into smaller parts. Microwave modification is more adequate than traditional pyrolysis because it is fast and efficient, and it allows for homogeneous interior temperature distribution. As a result, it produces biochar with more surface area and functional groups than by traditional pyrolysis (Wang et al. 2009). It produces efficient biochar at a low temperature (300 ºC) while also enhancing soil water holding capacity (WHC) and cation exchange capacity (CEC) (Mohamed et al. 2016a, b).
3 Modified Biochar
Biochar engineering contributes to achieve enhanced properties of biochar for specific applications. This helps to modify the desired properties of biochar with improved efficiency. The physicochemical properties like specific area, pore structure, and surface functional groups can be enhanced by modification of biochar. Biochar engineering or modification is done via different physical, chemical, and biological methods for preparing activated or modified biochar (Ok et al. 2015; Mohamed et al. 2016a, b; Rajapaksha et al. 2016; Yao et al. 2013). Figure 1 depicts different biochar modification methods.
3.1 Physical Modification
The most common methods for physical modification are ball milling, gas and steam activation, microwave modification, and magnetic modification. Physical modification contributes to improvement in pore structure and addition of oxygen containing functional groups. This modification also enhances micropores and mesopores in biochar with an improved surface area that provides enough space to adsorb organic pollutants, nutrient elements, and heavy metals (Wang et al. 2017).
3.1.1 Ball Milling Modification
The grinding of biochar particles through ball mill can improve the particle size and specific surface area that leads to more adsorption of organic and inorganic ions (Cai et al. 2016). Peterson et al. (2012) reported that using ball mill process, the surface area of corn- stover based biochar was improved 3.2 times and reached 194 m2. The improvement of the functional groups in biochar can be achieved by adding chemicals during ball milling. Ball milling of biochar can also produce nano-sized biochar particles.
3.1.2 Gas/Steam Activation
Gas/steam such as water vapor, CO2, and air can be employed for physical modification (Guo et al. 2009; Shim et al. 2015). This method improves the porosity formation in biochar by activating its carbon which leads to better surface area and surface reactivity, while removing incomplete combustible components. However, the gas/steam activation method decreases the availability of some functional groups on the biochar surface like COOH (carboxylic acid) that gets degraded in steam-based biochar activation process. COOH is considered as good adsorbent for heavy metals (Uchimiya et al. 2012). Due to its depletion during activation, the metal adsorption capacity of gas/steam engineered biochar is negatively impacted.
3.1.3 Microwave Modification
Microwave radiation with a frequency of 0.03–300 GHz may be utilized in a controlled environment to pyrolyze biochar at 200–300 ºC, yielding about 60% biochar (Tabatabaei et al. 2019). The microwave-modified biochar eliminates the need for biomass drying and shredding (Bhaskar and Pandey 2015). The biochar produced via microwave modification possess additional functional groups and greater surface area than pristine biochar (Wang et al. 2017).
3.1.4 Magnetic Biochar Modification
The small particles size of biochar with low density makes its separation difficult from water and creates hurdle in the purification of water. It can also contribute in generation of secondary pollutants (Nguyen et al. 2013). Impregnation and co-precipitation pyrolysis are two common methods for magnetic modification (Yi et al. 2020). In impregnation method, biochar is impregnated with transitional metal salts. A magnetic biochar was made using Fe 3+/Fe 2+ solution having 1.4%–80.6% of iron content (Mohan et al. 2014). This magnetized biochar has shown higher Pb2+ and Cd2+ removal capacities. The modified magnetic biochar has large number of carboxyl functional group on the surface which improves the adsorption capacity for heavy metal ions. Magnetic biochar can be derived through mixing of Fe2+/Fe3+ ions into a solution of ammonia (Yu et al. 2013) while in case of co-precipitation ammonia and NaOH solution are used with constant stirring (Yi et al.2020).
3.2 Chemical Modification
Biochar can be modified using acids and bases to increase surface area, micropores, cation exchange capacity, and functional group availability. Acid (H2O2, H3PO4, HNO3, and HCl) and other chemical agents increase carboxyl groups and mineral composition of biochar. Various alkaline agents stimulate the functional groups and promote electrostatic interaction at biochar surface that enhance the adsorption capacity for heavy metals (Mahmoud et al. 2012; Jingchun et al. 2013). Organic compounds, clays metal oxides can also be impregnated on biochar for chemical modification. Modified corncob biochar coated with magnesium salts resulted in positive surface charge of biochar that further enhanced the adsorption of phosphate ion with maximum adsorption capacity of 319.63 mg/g (Sizmur et al. 2017).
3.2.1 Chemical Coating
The surface area can be improved by coating nanoparticles or metal oxides on the surface of biochar during pyrolysis to produce biochar-based nano composite. The chemical impregnated biochar results in improved surface area, pore size, functional group, catalytic efficiency, and more active sites for binding (Kazemi et al. 2020).
−OH and −CH are very important surface functional groups that help in adsorption of heavy metals. When the pristine biochar is modified by HNO3 or NH3, a new amino functional group gets attached to the biochar surface and the modified biochar becomes more efficient to remove heavy metals. Biochar derived from cactus fibers and modified through HNO3 had more adsorption sites and surface carboxylic group (Qian et al. 2013).
Song et al. (2014) used KMnO4 for pyrolysis of corn straw at 600° C. This modified biochar showed increased O/C ratio and oxygen in Mn-OH and Mn-O structure which was effective for Cu2+ adsorption in comparison to pristine biochar. A corn straw-based biomass was dipped into FeCl3.6H2O solution before slow pyrolysis at 600 ºC for 1 h. The resultant modified biochar had more functional groups, higher stability, larger surface area (He et al. 2018) and improved As (V) adsorption capacity up to 400 times.
Similar approach of biochar activation was adopted by Shen et al. (2019), where corncob biomass was treated with MgCl2 before pyrolysis to get MgO plated biochar. This modified biochar had 380 time more surface area as compared to pristine biochar. Biochar derived from spruced wood when impregnated with AlCl3/FeCl3 solution and pyrolyzed at 650 °C for 1 h, removed fluoride ion with adsorption capacity of 13.6 mg/g (Tchomgui et al. 2010).
3.3 Biochar-Based Composite
3.3.1 Clay Coating
Clay has well-defined surface chemistry, large particle size, and surface area (Yao et al. 2014), which can efficiently adsorb inorganic and organic pollutants like dyes, heavy metals, etc. Bentonite, montmorillonite, and kaolinite are used as adsorbents for pollutant removal. Yao et al. (2014) pretreated the biomass with kaolinite and montmorillonite and the mixture was pyrolyzed at 600 ºC, for 60 min. The adsorption capacity of modified biochar increased by five times for methylene dye compared to unmodified biochar due to more ion exchange and electrostatic attraction. Takaya et al. (2016) also used clay-coated biochar for the recovery of phosphate from wastewater. The oak wood-based biochar was impregnated with magnesium by treating with MgCl2.6H2O before pyrolysis at 600 ºC for 60 min. The modified biochar efficiently removed phosphate in comparison to pristine biochar. Ramola et al. (2020a) prepared different biochar clay mineral composites with spent waste, bentonite, and calcite as raw materials. They observed an increased surface area, porosity, and adsorption capacity of biochar composites for Pb in comparison to pristine biochar. Similarly, a novel rice husk biochar-calcite composite (BRH-C) was prepared for efficient removal of phosphate at low concentration. The optimum pyrolysis conditions for preparation of BRH-C were 700 °C (temperature), 2.3 h (time), and rice husk-calcite ratio of 4.2:1 (w/w). BRH-C removed better phosphate than rice husk biochar or calcite alone, suggesting synergistic effects of both rice husk biochar and calcite as a composite. Calcite also altered the yield and textural properties of BRH-C and added characteristics calcite functional groups and minerals (Ramola et al. 2021).
3.3.2 Biological Impregnation
The small size of microorganisms makes them penetrable in minute pores of biochar, where these organisms develop a biofilm. The rigid pore structure of biochar prevents microorganisms to be washed out. The presence of these microorganisms inside the inert biochar modifies it through biofilm generation and colonization. It has been well known that microorganisms are excellent in the degradation of organic matter and adsorption of heavy metal (Hamedi et al. 2015). Biochar activated with microbial film sourced from the mining field, reported naphthenic acid degradation and removal of heavy metal at the same time from wastewater (Frankel et al. 2016). Another study by Dalahmeh et al. (2018) investigated that biofilm activated biochar, degraded pharmaceutically active compounds like caffein, carbamazepine, and ranitidine from sewage. It was observed that activated biochar degraded >90% carbamazepine in comparison to 7% in sand active biofilm during 154 days.
3.3.3 Biomass Composite Formation with Metal Oxide
Nano metal oxide and hydroxide-biochar can be produced through enrichment of targeted element by bioaccumulation, post pyrolysis nanoparticle impregnation, and biomass treatment by metal salt. The biomass activation through this way increases the active adsorption site for inorganic and organic contaminants and surface area (Tan et al. 2016). Post pyrolysis metal oxide impregnation of nanoparticle could be achieved through conventional impregnation, evaporative, and heat treatment method.
4 Applications of Biochar
The properties of biochar make it a dynamic substance for its multi-dimensional use (Fig. 2). Although pristine biochar and engineered biochar both are efficient in removing the contaminants, still the improved performance of engineered biomass have been reported in the various study.
4.1 Biochar for Soil Conditioning and Carbon Sequestration
Biochar is resistant to decay so it can stay in the soil for longer time. Biochar application in soil enhances the soil stability to act as carbon sink. The co-dopped activated biochar is also reported to enhance the carbon sequestration (Mašek et al. 2019). Biochar also reduces nitrous oxide, carbon dioxide, and methane from agricultural land. Different concentrations of biochar ranging from 2 to 60% (w/w) can reduce the emission of nitrous oxide higher than 20% (w/w) (Rawat et al. 2019).
It also increases the water holding capacity, cation exchange capacity, total nitrogen, and phosphorous (Lehmann et al. 2003). Plant response toward the uptake of nutrients, and the availability of nutrients like zinc, phosphorous, and potassium increased with the use of biochar in soil. In comparison to pristine biochar, biochar dopped with nutrients like Ca, Mg, and K on its surface promotes more soil fertility by immobilizing and releasing the nutrients simultaneously (Igalavithana et al. 2017). Acidic and alkaline biochar are also used for the treatment of soil.
Biochar provides medium for the growth of mycorrhiza that provides the suitable conditions for colonization of plant roots. Biochar can affect the growth of fungi by changing the properties of soil. It has also been reported that biochar improves the nitrogen fixation in Phaseolus vulgaris (Rondon et al. 2007). Biochar also protects fungal hyphae and beneficial microbes from attacks of mites, protozoans, and nematodes (Rawat et al. 2019). Biochar amended soil can suppress the growth of pathogen through its antagonist effect and with indirect interaction through induction of systemic resistance in plants. Biochar triggers the induction of system plant defense only for leaves diseases such as powdery mildew in strawberry, gray mold in tomato, and foliar blight in soybean (Jaiswal et al. 2020). There are many studies that report use of biochar to control the pathogen in soil. It can resist the growth of soil borne as well as air borne pathogen that causes powdery mildew (Bananomi et al. 2015). The biochar prepared from citrus wood can control the gray mold (Botrytis cinerea) on tomato and pepper (Rawat et al. 2019). Similarly, biochar obtained from the grounded hardwood prevents the root lesion (causal organism Fusarium oxysporum) in asparagus (Elmer et al. 2011).
4.2 Biochar for Remediation of Pollutants from Aqueous Medium
Biochar has a good adsorption capacity for different pollutants (Table 2). Biochar derived from the switchgrass was used for the removal of metribuzin herbicide as well as antibiotics like tetracycline and sulfonamide. Electron donor–acceptor interactions and specific functional groups at the surface of biochar enhanced the removal of pollutants (Peiris et al. 2017). Sulfamethoxazole (SMX) is a sulfonamide antibiotic used for animals and humans. This antibiotic was adsorbed by the digested bagasse biochar and adsorption process was governed by π-π interaction. The biochar made up of rapeseed straw, peanut at 350 °C showed maximum adsorption capacity toward the removal of methyl violet, i.e., 123.5–195.4 mg/g. Electrostatic interaction was reported as the main mechanism behind this removal. Several studies reported that the performance of biochar for pollutants removal depends on the polarity index, the quantity of functional groups, and aromaticity index (Braghiroli et al. 2018; Cha et al. 2016).
Ca-Mg loaded biochar enriched with nano- CaO and MgO particles had more organic functional groups and increased surface area as compared to pristine biochar that resulted in more adsorption of phosphate ions with adsorption capacity of 294.22, 315.33, and 326.63 mg/g respectively (Fang et al. 2015).
Inyang et al. (2012) revealed that biochar made from anaerobic digestion of sugar beetroot at 600 °C can remove Ni (II), Pb (II), Cd (II), and Cu (II) effectively up to 97%. Wheat straw-based biochar when pyrolyzed at 200 °C, can remove Cr (VI) with a maximum adsorption capacity of 35.78 mg/g. There are four mechanisms that work behind the adsorption of heavy metals: the electrostatic attraction between biochar surface and heavy metals, complexation with a surface functional group or π electron-rich domain, exchange of ion between the surface of biochar and heavy metals, and process of co-precipitation to form insoluble compounds (Wang et al. 2020; Ramola et al. 2020b).
Pristine biochar is very effective in adsorbing the inorganic ions. The exhausted nitrogen and phosphorus laden biochar can further be recycled and reapplied for soil conditioning. A study on bamboo biochar reveals that biochar has adsorption capacity of 6.4 mM/g for NH4+ ion from wastewater. At high pH (pH > pH PZC) biochar easily adsorbs the NH4+ ions through electrostatic force (Viglasova et al. 2018).
For removal of phosphate ions from the wastewater, the sludge derived, and walnut derived biochar was used, and it was found that there was an electrostatic attraction between phosphate and biochar surface (Ajmal et al. 2020). Rice husk is an abundantly available agricultural waste with a global annual production of approximately 1.2 Giga tonne (Costa and Paranhos 2018). Optimized, ecofriendly, and cost-effective rice husk biochar-calcite composite was used to remove phosphate from the aqueous solution. Results demonstrate efficient removal of phosphate (87.3%) in comparison to pristine biochar (Ramola et al. 2021).
A biochar from maize straw at 300, 500, and 700 °C was studied for the removal of thiacloprid. The process of adsorption occurred through pore filling hydrophobic interaction as well as π-π interaction. Similar study was done with pyrolyzed swine manure biochar for understanding the adsorption mechanism for imidacloprid (Wang et al. 2020).
Antibiotics are sometimes difficult to degrade in environment. Biochar can be an effective adsorbent that can reduce the toxicity of antibiotics. Sulfonamides (SAs) and Tetracyclines (TCs) are the most commonly used antibiotics in agricultural fields. Their excessive amount is harmful for the environment. Adsorption mechanism of pristine biochar for the removal of SAs has been done by Peris et al. (2017). They found that biochar prepared at high temperature makes a strong π-π electron donor-acceptor bond between the arene ring on SAs and surface of biochar.
4.3 Biochar Application for Removal of the Pathogen
Urban storm water is highly contaminated with pathogens. There is a need to decontaminate this water before its confluence into surface water. The mechanism which works behind the removal of pathogens is filtration of large size pathogen and adsorption of negatively charged cell of bacteria. Biochar can act as filtration layer for microorganism. The size of biochar particles is crucial to act as a filtration layer. The minimum particle size of 1.4 mm has the capacity to remove atleast 1 log 10 CFU of microorganism from the wastewater. Studies show that biochar can filter >1 log 10 CFU of Saccharomyces cerevisiae from a diluted wastewater (Perez-Mercado et al. 2019).
5 Factors Affecting Adsorption by Biochar
Biochar has stacked layers of graphene and aromatic structure that are interconnected to one another (Conte et al. 2021). This makes biochar armored with rich pore structure and large surface area. Different mechanisms involved in the removal of pollutants from aqueous solution by biochar may work solely or side by side with one another depending upon the properties of biochar and pollutants. The main mechanisms involved in the adsorption process are cation exchange, electrostatic interactions, precipitation, complexation, and chemical reduction (Ramola et al. 2020b). Some of the factors that affect the removal mechanisms are discussed as follow.
5.1 pH
The pH of solution affects the charge present at the surface of biochar. The value of pH, where the net charge at the surface of biochar is zero is called as zeta potential (pHPZC). When the value of pH is less than pHPZC, biochar surface is positively charged and it can bind anionic form of metals like HAsO42− and HCrO42−. When the value of pH is greater than pHPZC the biochar becomes negatively charged and binds with cations like Hg2+, Cd 2+, and Pb2+ (Li et al. 2017).
5.2 Functional Groups Present in Biochar
The carboxylic (COOH) and phenolic (OH) group of biochar gravitate the sorption of methyl violet on biochar (Xu et al. 2011). It has been reported that the graphitization degree of biochar and electron donor–acceptor (EDA) interaction between electron acceptors and electron donor of graphite surface magnifies the sorption rate of sulfonamides (SAs) on different surfaces of biochar (Xie et al. 2014). Phenolic group (−OH) and methine group (−CH) also play vital role in adsorption of heavy metals.
5.3 Minerals in Biochar
Different minerals at the surface of biochar increase their adsorption efficiency. It has been reported that minerals like CO3− (carbonate), HCO3−(bicarbonate), and H2PO4− (dihydrogen phosphate ion) adsorb Pb (II) at the surface of digested cow dung biochar through surface precipitation. When Pb (II) reacts with CO3−, HCO3−, and H2PO4−, it forms PbCO3 (lead carbonate), Pb5(PO4)3X (X can be ions of fluoride, chloride, bromine, etc.) and Pb (CO3)2 (Lead II carbonate) (Inyang et al. 2012). Similarly, the addition of bentonite (montmorillonite and quartz) and calcite in a cigarette waste biochar composite was found to increase the removal of Pb from aqueous solution by acting as a catalyst and altering the physicochemical properties, textural properties, and functional properties of the biochar composite (Ramola et al. 2020a). Addition of calcite in rice husk biochar demonstrated higher phosphate removal in comparison to pristine biochar without any calcite addition. Calcite changed the physicochemical and functional properties of biochar and removed phosphate via pore filling, electrostatic interaction, and precipitation working simultaneously side by side (Ramola et al. 2021).
6 Conclusion and Recommendation for Future Direction
Biochar is a renewable, cost-effective, ecofriendly, and sustainable product. Pristine biochar is easy to prepare, but its wide application is still in the testing stage. Although a broad range of raw materials are available for biochar preparation, but a thorough comprehension of the production and modifications are required for its specific applications. Biochar is gaining popularity as an adsorbent for removing pollutants from air, water, and soil. Organic pollutants are removed by hydrophobic, electrostatic attraction/repulsion via—electron donor–acceptor and partitioning between biochar and pollutant, whereas inorganic pollutants are removed via ion exchange, surface-complexation, precipitation, and ionic interactions. Despite the fact that modified biochar is more efficient than pristine biochar, appropriate biochar activation and modification methods are still not fully understood. Since the engineered biochar production is more expensive than pristine biochar, more research is required to explore the engineered biochar manufacturing methods and its cost-effectiveness. Currently, wide range of research is being conducted on pristine and engineered biochar, with most of the work being done in the laboratory only. There are limited reports on the effectiveness of modified biochar in a wide range of applications. To determine its fate and contribution for a sustainable environment, a thorough examination of the raw materials, engineering process, pyrolysis temperature, collection, and recycling of modified biochar is required.
References
Ajmal Z, Muhmood A, Dong R, Wu S (2020) Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J Environ Manag 253:109730 https://doi.org/10.1016/j.jenvman.2019.109730
Bhaskar T, Pandey A, (2015) Advances in thermochemical conversion of biomass introduction. In: Pandey A, Bhaskar T, Stocker M, Sukumaran RK € (eds) Recent advances in thermo-chemical conversion of biomass. Elsevier, pp 3–30
Bonanomi G, Ippolito F, Scala F (2015) “Black” future for plant pathology? biochar as a new soil amendment for controlling plant diseases. J Plant Patho 97(2):223–234
Braghiroli F, Bouafif H, Neculita C, Koubaa A (2018) Activated biochar as an effective sorbent for organic and inorganic contaminants in water. Water, Air and Soil Pollut 229:230. https://doi.org/10.1007/s11270-018-3889-8
Cai H, Xu L, Chen G et al (2016) Removal of fluoride from drinking water using modified ultrafine tea powder processed using a ball-mill. Appl Surf Sci 375:74–84
Cha J, Park S, Jung S et al (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15. https://doi.org/10.1016/j.jiec.2016.06.002
Chen BL, Zhou DD, Zhu LZ (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143. https://doi.org/10.1016/j.jbiosc.2013.05.035
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102(2):716–723
Colantoni N, Evic R, Lord S et al (2016) Characterization of biochar’s produced from pyrolysis of pelletized agricultural residues. Renew Sust Energ Rev 64:187–194. https://doi.org/10.1016/j.rser.2016.06.003
Conte P, Bertani R, Sgarbossa P et al (2021) Recent developments in understanding biochar’s physical-chemistry. J Agron 11(4):615. https://doi.org/10.3390/agronomy11040615
Costa JAS, Paranhos CM (2018) Systematic evaluation of amorphous silica production from rice husk ashes. J Clean Prod 192, 688e697. https://doi.org/10.1016/j.jclepro.2018.05.028
Dalahmeh S, Ahrens L, Gros M et al (2018) Potential of biochar filters for onsite sewage treatment: adsorption and biological degradation of pharmaceuticals in laboratory filters with active, inactive, and no biofilm. Sci Total Environ 612:192–201
Devi P, Saroha AK (2015) Simultaneous adsorption and dechlorination of pentachlorophenol from effluent by Ni–ZVI magnetic biochar composites synthesized from paper mill sludge. Chem Eng J 271:195–203
Elmer WH, Pignatello JJ (2011) Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis 95:960–966
Fang C, Zhang T, Li P (2015) Phosphorus recovery from biogas fermentation liquid by Ca– Mg loaded biochar. J Environ Sci 29:106–114
Frankel ML, Bhuiyan TI, Veksha A (2016) Removal and biodegradation of naphthenic acids by biochar and attached environmental biofilms in the presence of co-contaminating metals. Bioresour Technol 216:352–361
Guo S, Peng J, Li W (2009) Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl Surf Sci 255:8443–8449. https://doi.org/10.1016/j.apsusc.2009.05.150
Hamedi J, Dehhaghi M, Mohammdipanah F (2015) Isolation of extremely heavy metal resistant strains of rare actinomycetes from high metal content soils in Iran. Int J Environ Res 9(2):475–480
Han Y, Cao X, Ouyang X et al (2016) Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: effects of production conditions and particle size. Chemosphere 145:336–341
He R, Peng Z, Lyu HH et al (2018) Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci Total Environ 612:1177–1186
Igalavithana AD, Lee S-E, Lee YH et al (2017) Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 174:593–603. https://doi.org/10.1016/j.chemosphere.2017.01.148
Inyang M, Gao B, Yao Y et al (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour Technol 110:50–56
Jaiswal AK, Alkan N, Elad Y et al (2020) Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci Rep 10:13934. https://doi.org/10.1038/s41598-020-70882-6
Jingchun T, Wenying Z, Rai K, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116(6):653–659
Kazemi H, Dehhaghi M, Ok YS et al (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270:122462. https://doi.org/10.1016/j.jclepro.2020
Kong HL, He J, Gao YZ et al (2011) Cosorption of phen-anthrene and mercury from aqueous solution by soybean stalk-based biochar. J Agric Food Chem 59:12116–12123
Lehmann J, da Silva JP Jr, Steiner C et al (2003) Nutrient availability and leaching in an archaeological anthrosol and a ferrasol of the Central Amazon basin: fertilizer, manure, and charcoal amendments. Plant Soil. 249:343–357
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adap Strateg Glob Chang. 11(2):395–419
Li H, Dong X, Da Silva EB (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Liu Z, Balasubramanian R (2012a) Hydrothermal carbonization of waste biomass … production of solid biochar fuel from waste biomass by hydrothermal carbonization. Appl Energ 115:394–404. https://doi.org/10.1016/j.apenergy.2013.11.036
Liu Z, Balasubramanian R (2012b) Greenpeat: an innovative sustainable material recovered from waste. Proc Environ Sci Eng Manag 16:159–166
Liu Z, Quek A, Balasubramanian R (2012) Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour Technol 16:159–166. https://doi.org/10.1016/j.biortech.2012.06.063
Mahmoud DK, Salleh MAM, Karim WAWA et al (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181:449–457
Manariotis ID, Fotopoulou KN, Karapanagioti HK (2015) Preparation and characterization of biochar sorbents produced from malt spent rootlets. Ind Eng Chem Res 54:9577–9584. https://doi.org/10.1021/acs.iecr.5b02698
Mašek O, Buss W, Brownsort P et al (2019) Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-41953-0
Mašek,O, Brownsort, P, Cross,A, Sohi,Saran (2013) Influence of production conditions on the yield and environmental stability of biochar. Fuel 103:151–155, ISSN 0016-2361. https://doi.org/10.1016/j.fuel.2011.08.044
Mohamed BA, Ellis N, Kim CS et al (2016a) Engineered biochar from microwave-assisted catalytic pyrolysis of switchgrass for increasing water-holding capacity and fertility of sandy soil. Sci Total Environ 566:387–397
Mohamed BA, Ellis N, Kim CS, Bi X, Emam AE (2016b) Engineered biochar from microwave-assisted catalytic pyrolysis of switchgrass for increasing water-holding capacity and fertility of sandy soil. Sci Total Environ 566–567:387–397. https://doi.org/10.1016/j.scitotenv.2016.04.16
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman CU (2014) Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem Eng J 236:513–528. https://doi.org/10.1016/j.cej.2013.09.057
Morgan HM Jr, Bu QL et al (2017) A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour Technol 230:112–121
Nguyen BT, Lehmann J, Kinyangi J (2009) Long-term black carbon dynamics in cultivated soil. Biogeochemistry 92:163–176
Nguyen BT, Lehmann J, Hockaday WC et al (2011) Temperature sensitivity of black carbon decomposition and oxidation. Environ Sci Technol 44:3324–3331
Nguyen T, Ngo H, Guo W et al (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Ok YS, Uchimiya SM, Chang SX, Bolan N (2015) Biochar—production, characterization and applications. CRC Press, Taylor and Francis, London
Peiris C, Gunatilake SR, Mlsna TE et al (2017a) Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour Technol 246:150–159
Perez-Mercado LF, Lalander C, Joel A (2019) Biochar filters as an on-farm treatment to reduce pathogens when irrigating with wastewater-polluted sources. J Environ Manage 248:109295. https://doi.org/10.1016/j.jenvman.2019.109295
Peterson SC, Jackson MA, Kim S, Palmquist DE (2012) Increasing biochar surface area: optimization of ball milling parameters. Powder Technol 228:115–120
Qian K, Kumar A, Patil K et al (2013) Effects of biomass feedstocks and gasification conditions on the physiochemical properties of char. Energies 6:3972–3986. https://doi.org/10.3390/en6083972
Rajapaksha AU, Chen SS, Tsang DC, Zhang M, Vithanage M, Mandal S, ... Ok YS (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Ramola S, Srivastava RK, Vasudevan P (2013) Effect of biochar application in combination with domestic wastewater on biomass yield of bioenergy plantations. Int J Energy Sect Manag 7(3):355–363
Ramola S, Belwal T, Srivastava RK (2020b) Thermochemical conversion of biomass waste-based biochar for environment remediation. In: Kharissova O, Martínez L, Kharisov B (eds) Handbook of nanomaterials and nanocomposites for energy and environmental applications. Springer, Cham, pp p1-16
Ramola S, Belwal T, Li CJ et al (2020a) Improved lead removal from aqueous solution using novel porous bentonite and calcite-biochar composite. Sci Total Enviro 709:136171. https://doi.org/10.1016/j.scitotenv.2019.136171
Ramola S, Belwal T, Li CJ et al (2021) Preparation and application of novel rice husk biochar-calcite composites for phosphate removal from aqueous medium. J Clean Prod 299:126802. https://doi.org/10.1016/j.jclepro.2021.126802
Rawat J, Saxena J, Sanwal P (2019) Biochar—an imperative amendment for soil and the environment. Biochar: a sustainable approach for improving plant growth and soil properties. https://doi.org/10.5772/intechopen.82151
Regmi P, Moscoso JLG, Kumar S (2012) Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J Environ Manage 109(17):61–69
Rondon M, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708
Sabio E, Álvarez-Murillo A, Román S, Ledesma B (2016) Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag 47:122–132
Shen Z, Zhang J, Hou D et al (2019) Synthesis of MgO coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ Int 122:357–362
Shim T, Yoo J, Ryu C, Park Y, Jung J (2015) Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresour Technol 197:85–90. https://doi.org/10.1016/j.biortech.2015.08.055
Sizmur T, Fresno T, Akgül G et al (2017) Biochar modification to enhance sorption of inorganics from water. Bioresour Technol 246:34–47. https://doi.org/10.1016/j.biortech.2017.07.082
Song Z, Lian F, Yu Z et al (2014) Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem Eng J 242:36–42. https://doi.org/10.1016/j.cej.2013.12.061
Tabatabaei M, Aghbashlo M, Dehhaghi M et al (2019) Reactor technologies for biodiesel production and processing: a review. Prog Energy Combust Sci 74:239e303
Takaya C, Fletcher L, Singh S et al (2016) Recovery of phosphate with chemically modified biochar’s. J Environ Chem Eng 4:1156–1165
Tan X-F, Liu Yg, Gu Y-L, et al (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333
Tang J, Zhu W, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116(6):653–659
Tchomgui-Kamga E, Alonzo V, Nanseu-Njiki CP et al (2010) Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon 48:333–343. https://doi.org/10.1016/j.carbon.2009.09.034
Uchimiya M, Bannon DI, Wartelle LH (2012) Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J Agric Food Chem 60:1798–1809
Viglašová E, Galamboš M, Danková Z (2018) Production, characterization, and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manage 79:385–394. https://doi.org/10.1016/j.wasman.2018.08.005
Wang XH, Chen HP, Ding XJ, Yang HP, Zhang SH, Shen YQ (2009) Properties of gas and char from microwave pyrolysis of pine sawdust. BioResources 4(3):946–959
Wang Y, Lu J, Wu J et al (2015) Adsorptive removal of fluoroquinolone antibiotics using bamboo biochar. Sustainability 7(9):12947–12957
Wang B, Gao B, Fang J (2017) Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 47:2158–2207
Wang X, Guo Z, Hu Z, Zhang J (2020) Recent advances in biochar application for water and wastewater treatment: a review. Peer J 8:e9164. https://doi.org/10.7717/peerj.9164
Wani I, Ramola S, Garg A, Kushvaha V (2021) Critical review of biochar applications in geo-engineering infrastructure: Moving beyond agricultural and environmental perspectives. Biomass Convers. Biorefin. https://doi.org/10.1007/s13399-021-01346-8
Xie M, Chen W, Xu Z et al (2014) Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ Pollut 186:187–194. https://doi.org/10.1016/j.envpol.2013.11.022
Xu R, Xiao S, Yuan J, Zhao A (2011) Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour Technol 102:10293–10298. https://doi.org/10.1016/j.biortech.2011.08.089
Yao Y, Gao B, Chen J et al (2013) Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: characterization and phosphate removal potential. Bioresour Technol 138:8–13
Yao Y, Gao B, Fang J et al (2014) Characterization and environmental applications of clay biochar composites. Chem Eng J 242:136–143
Yi Y, Huang Z, Lu B et al (2020) Magnetic biochar for environmental remediation: a review. Bioresour Technol 298:122468. https://doi.org/10.1016/j.biortech.2019.122468
Yu J, Wang L, Chi R et al (2013) Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl Surf Sci 268:163–170. https://doi.org/10.1016/j.apsusc.2012.12.047
Zhang W, Mao S, Chen H et al (2013a) Pb (II) and Cr (VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour Technol 147:545–555
Zhang ZB, Cao XH, Liang P (2013b) Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonization. J Radioanal Nucl Chem 295(2):1201–1208
Zhou Y, Gao B, Zimmerman AR (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518. https://doi.org/10.1016/j.cej.2013.07.036
Zhou F, Wang H, Zhang W et al (2015) Pb (II), Cr (VI) and atrazine sorption behavior on sludge-derived biochar: role of humic acids. Environ Sci Pollut Res 22(20):16031–16039
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chhimwal, M., Pandey, D., Srivastava, R.K. (2022). Pristine Biochar and Engineered Biochar: Differences and Application. In: Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T. (eds) Engineered Biochar. Springer, Singapore. https://doi.org/10.1007/978-981-19-2488-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-2488-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2487-3
Online ISBN: 978-981-19-2488-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)