Abstract
Biochar, produced from biodegradable waste through advanced thermochemical processes like pyrolysis, offers substantial environmental benefits due to its carbon-rich composition and versatile applications. This review delves into the transformative potential of biochar derived from diverse agricultural and municipal solid wastes. With its unique properties-including a high surface area, porosity, and functional groups such as –COOH, –OH, and –NH2 biochar stands out as a highly effective adsorbent for heavy metals and organic pollutants. Factors like feedstock type, pyrolysis temperature, and residence time critically shape biochar’s characteristics and yield. Moreover, pretreatment methods and activation techniques further amplify its adsorption capacity. Biochar’s environmental applications are vast, spanning soil remediation, water purification, carbon sequestration, and waste management, all promoting ecological sustainability. Its remarkable stability and efficacy in pollutant removal highlight its potential as a catalyst and a vital component in improving soil health. Despite its many advantages, a thorough assessment of biochar’s environmental impact and long-term stability is crucial for its sustainable use. This review highlights recent biochar production, characterization, and application advances, emphasizing the need to balance economic growth with environmental development. By strategically integrating biochar into various sectors, we can pave the way for a greener, more sustainable future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
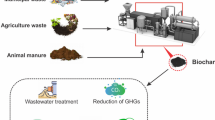
Biodegradable waste has a remarkable ability to produce biochar and is the key constituent of feedstock. Several studies have established that biomass is a rich and powerful source for obtaining renewable energy and mineral resources [1]. Various forms of waste material, such as algal biomass, agricultural waste, animal waste, urban activated sludge, agri-residue are to be considered as primary resources of biomass. Different validated methodologies such as, thermochemical, physical as well as biochemical may be implemented to convert biomass into products with high-values [2, 3]. Biochar can be obtained with the help of thermochemical conversions of biomass with carbon base at high range of temperature (300–900 °C) in the absence or limited oxygen, various methodologies such as gasification, pyrolysis, torrefaction and hydrothermal carbonization [4]. Biochar posses multiple physical, chemical and thermochemical properties and these properties may fluctuate according to the application of raw material and method administered [5, 6].
Application of Biochar is widespread in the arena of waste handling, originating from various sources such as plant waste, animal waste and agri waste [7]. The production of biochar from different waste material are low cost and highly efficient, on the other hand, the administration of biochar must be taken care in the condition where char has been obtained from sewage, urban waste and with heavy metal content [2]. Many authors mentioned that conversion of biomass into biochar is one of the finest and most effective methods for carbon sequestering to control the environmental toxicity due to different heavy metals and emerging toxicants [8]. Further, several authors emphasized about the benefits of biochar production through the process of pyrolysis and obtain bioenergy, which may be utilized as a substitute of fossil fuel with the added advantage of lower carbon do oxide emission [9]. Multiple methodologies have been emerged to enhance the functional capacity of Biochar to mitigate the toxicants and emerging toxicants [4].
Various methodologies have been approved by different research attempts for the production of biochar from different sources of biomass, these methods such as gasification is based on the vaporization mechanism, whereas pyrolysis works on transmutation of biomass, torrefaction method is based on drying or roasting of waste and hydrothermal carbonization works on aqueous carbonization at high pressure and temperature for conversion [10]. The most often used methodology for the production of biochar is pyrolysis, i.e., the heating of a biomass. The organic compounds (molecules that contain carbon in biomass) degrade at a temperature range of 300–900 °C [4] in an anaerobic environment. The factors that affect pyrolysis products include heating, thermal efficiency, biomass quality and residence time [11]. The basic factor influencing the properties/possession of biochar is temperature and the functional groups attached at the surface of biochar molecules [12]. Consequently, in many sectors, the roles of potency and biochar rely on organic matter to produce it [1].
Various waste sources and Agricultural waste materials may be converted into different chemical resources through thermochemical process. Furthermore, pyrolysis is a thermochemical method to convert the Plant and animal waste into bio-oil, biochar and syn-gas at the temperature range of 300–900 °C [4] (Fig. 1).In the process of pyrolysis, the content proportion of biochar, bio-oil and syn-gas depends upon the reaction parameters such as temperature, presence of oxygen and residual time [13].
Biochar derived from different waste sources possess various functional groups at the surface to bind with toxicants, heavy metals and other contaminants through the process of adsorption [5]. Some of the unique mechanical and elemental characteristics, such as functional sets (–COOH, –OH, and –NH4), superficial area, high porosity and cation exchange capacity (CEC), are absolutely linked to the adsorptive efficiency of biochar [14]. To modify the surface area, biochar is treated with acid, and for this purpose, nitric acid and sulfuric acid are most frequently used. To increase the understanding of different aspects of biochar potency, the assessment and specification of comprehensive information on biochar characteristic features and approaches would be easy. Because of its many advantages and environmentally safe nature, including pollutant absorption, composting, soil remediation, catalysis, decreased greenhouse gas emissions, and energy production, a pyrogenic black carbon is successfully used to resolve diverse ecological issues. The affinities for nonpolar groups and high surface-to-volume ratios are the basis for the adsorption potential of biochar for carbon-rich and metallic contaminants [15, 16].
Hemi-cellulose, cellulose and lignin, a class of complex organic polymers, are significant components of the biological waste used for biochar production. During the thermal decomposition process (pyrolysis), these elements are thermally degraded at different temperatures, and the mechanism of degradation (thermolysis) has been thoroughly investigated. The following reports concentrate on a complete analysis and evaluation of precarious contaminant elimination, the advantages as well as the consequences of the process parameters, remarkable pressure, heating rate, temperature, residence time, type of biomass, etc. [17]. The synthesis of biochar is accomplished through different thermochemical processes, including pyrolysis, flash carbonization, hydrothermal gasification and torrefaction. Most essentially, the current advances in biochar stability and comprehensibility predominantly/significantly illuminate the use of biochar in several applications, including the immobilization of organic and inorganic pollutants as a catalyst and carbon sequestration. Correspondingly, this subject also includes discussion regarding advancing/developing economic sustainability to create a balance between economic growth and environmental development [18, 19].
1.1 Different biochar production methods
The conversion of organic matter to biochar was the outcome of the high demand for organic matter. The most important method to produce biochar is thermochemical conversion. Some precedents of these reactions include pyrolysis, HTC, gasification and torrefaction. To obtain the maximum output of biochar because of these reactions, the approach used should be combined with the type of organic matter being used.
1.2 Pyrolysis
In chemistry, the term pyrolysis refers to the type of chemical reaction in which heat-based decomposition of carbon-based compounds occurs under anaerobic conditions at temperatures ranging between 482° and 520 °F [20]. It is a distinctive method to obtain syngas and bio-oil along with biochar from biomass (Fig. 2). At the temperature range of 300–450 °C [21] lignocellulosic substances, specifically cellulose, hemi-cellulose and lignin, undergo various reaction pathways comprising depolymerization, fragmentation and linkage related to a new variety of product phases, such as solid, liquid and gas phases. The aeriform/vapor phase outcomes include (CO + H2) syngas or synthesis gas (C1–C2 hydrocarbons), carbon dioxide (CO2), carbon monoxide (CO), and bio-oil in the aqueous state, while biochar is in solid phase [22]. A variety of bioreactors can be used for biochar production, such as agitated sand spinning boilers, paddle furnaces, bubbling fluidized bed reactors and wagon reactors. The type and composition of the raw material used, pyrolysis environment and temperature determine the amount of biochar obtained as a product during pyrolysis. The quality of the product is dependent on the temperature that is kept during the process. An increase in temperature during this process increases the yield of syngas, decreasing biochar production [23].
On the basis of the operating conditions (heating rate, residence time and pressure) in the reactor, pyrolysis can be categorized into three categories:
The process of fast pyrolysis is a rapid, efficient thermochemical pathway for deliquescing carbon-based compounds to an immense amount of energy, yielding aqueous bio-oil. It is adjusted by the following factors: (i) average pyrolysis temperature 300–900 °C [4], (ii) instantaneous heating of biomass particles (below 200 °C/min) [24] and (iii) small heating duration of the configuration along with pyrolysis vapors (1/2–2 s) [25], at increased degrees. Reducing the fume residence duration in the heated zone to extract standard bio-oil is a crucial differentiating characteristic of fast pyrolysis [26]. For the records, fast pyrolysis produces a greater quantity of bio-oil. This could be achieved by confirming that the gas swiftly doused or condensed. In contrast to fast pyrolysis, slow pyrolysis occurs at a comparatively low heating rate, ~ 410–440 °F [27, 28]. The residence time is more than 60 min. The amount of char produced during slow pyrolysis is greater than that produced during other pyrolysis or carbonization reactions. Most of the raw materials used in the process consist of cellulose, hemi-cellulose and lignin. Different reaction parameters are used along with different reaction pathways to convert these organic molecules into biochar.
1.3 Hydrothermal carbonization (HTC)
Because biochar is directed at temperatures varying/extending from 180 °C to 250 °C, HTC is a cost-effective practice for manufacturing biochar [29]. The outcome manufactured by arid techniques such as gasification and pyrolysis are the byproduct generated by hydrothermal synthesis for differentiation and is referred to as hydrochar. During the formation of biochar, biomass and organic matter are liquefied in H2O and sediment inside a sealed furnace. A moderate increase in temperature leads to sustained solidity. The products produced by HTC at distinct temperatures/degrees could be similar: below 250 °C, biochar is produced; when the temperature ranges between 250 °C and 400 °C, and the product formed by hydrothermal liquefaction is bio-oil; and when the reaction temperature exceeds 400 °C, gases such as carbon monoxide, carbon dioxide, methane, and methylene are formed by hydrothermal gasification [30].
The resulting hydrochar was obtained via chemical processes (polymerization), intramolecular dehydration and liquefaction/condensation [31]. The mechanism is intricate because of the high molecular mass and composite structure of lignin. The breakdown of lignin begins with chemical reactions such as alkylation and base hydrolysis of esters that generate carbolic acids such assyringols and phenols, along with catechol. Char is produced by the breakdown of polymers and interstrand crosslinks (ICLs). During pyrolysis, complex organic polymers (lignin) that are unblended under liquefiable conditions are transferred to hydrochar [24].
1.4 Gasification
Gasification is a technological process in which organic or fossil-based carbon-rich raw materials are thermochemically converted into vaporized compounds, such as the synthesis gases carbon monoxide, carbon dioxide, methane, and hydrogen, at high temperature and traces of other hydrocarbons in the presence of gasification agents such as air, oxygen or stream to increase the heating value of the producer gas. Notably, the reaction temperature (700 °C to 1300 °C) is a necessary factor affecting the production of syngas (CO + H2). The formation of the syngas components CO and H2increased with increasing temperature, while the formation of other components, such as carbon dioxide, hydrocarbons and methane, decreased. The immediate outcome of the operation is syngas, while the solid product called char is regarded as a resultant product with a lower yield [32] (Fig. 3).
The multiple steps involved in the whole gasification process are as follows:
1.4.1 Drying
By vaporization, the amount of water in the biological residue is eliminated at the time of drying, and the solid fuel is dried without energy recovery. Depending on environmental factors and other parameters, the amount of moisture varies among different biomass materials. The process of drying is carried out independently of gasification and is considered complete when a biomass temperature of 150 °C is achieved [33].
1.4.2 Oxidation/combustion
For the mechanism of gasification, the oxidation/burning actions of suitable agents constitute the principal energy source. These suitable gasification media collaborate with gasifier particles capable of burning to yield CO2, CO and water [34].
1.5 Torrefaction and flash carbonization
For charcoal formation, torrefaction is a comparatively modern approach. The methodology is referred to as median pyrolysis since it is conducted at a lagging heating rate. Under anaerobic conditions at a heating rate < 122 °F/min, a residence time of less than half an hour and a temperature increase from 300 °C to 500 °C [31], torrefaction is a form of fast pyrolysis. The running mechanism of torrefaction is divided into several steps: heating, dehydrating, torrefy and condensing [35] (Fig. 4).
The method of torrefaction can be conducted as follows:
(i) Steam torrefaction: This approach involves vapor utilization for raw material conversion with a residence duration of ~ 600 s and a temperature exceeding the upper limit of 260 °C [36].
(ii) Wet torrefaction: A temperature range between 180 and 260 °C is set up for residence durations between 5 and 240 min, and the organic matter is kept exposed to water. Wet torrefaction is also called hydrothermal carbonization (HTC). In this technique, thermal energy is liberated during combustion by the application of gaseous oxidants. This energy is used to increase the temperature to a certain extent [31].
Under anaerobic conditions at a heating rate < 122°F/min, a residence time of less than half an hour and a temperature increase from 200 °C to 300 °C, torrefaction is a form of incompetent/deficient pyrolysis. Heating, dehydrating, torrefaction and cooling are the phases into which dry torrefaction is split. Furthermore, this process can be classified into two stages: pre-drying and post drying [37].
1.6 Factors affecting biochar properties
To produce biochar from biomass by thermochemical conversion pyrolysis, certain associated criteria need to be met. The raw material (feedstock), particle proportions, reaction temperature and heating rate significantly control and determine the characteristic features of the biochar produced [38]. Additionally, the caliber of biochar produced is directly dependent on these parameters. Not only the quality but also the quantity of biochar produced is also under the control of these factors. To use biochar in a suitable way, it is necessary to have comprehensive knowledge of its properties. The type of raw material needed for biochar production is not limited to one source but rather to any organic waste, such as ecological waste, farming waste, timber, and solid remnants. However, in comparison to biomass from trees, agricultural land, etc., solid junk and animal excreta produce greater amounts of biochar [39].
1.7 Feedstock
Biomass derived from natural and synthetic components is a complex substance that consists of biotic, animate, or inanimate elements. Raw matter has two forms, either ligneous or non-ligneous. Ligneous biomass refers to the form derived from tree and woodland waste. It has an elevated heating value (the amount of heat released during burning), a decreased thickness, a low content of decaying matter, a decreased volume and a low water content (Table 1). On the other hand, non-ligneous materials include animal excreta, farmland and factory-based organic residue [40].
1.8 Temperature
Temperature is the most fundamental property that significantly influences other parameters. A thermochemical decomposition process that produces biochar from organic and inorganic biomass is known as pyrolysis. The physicochemical qualities and structures of biochar, including its functional groups, surface area, pore structure, and elemental composition, are strongly influenced by its pyrolysis temperature [41].The elimination of different particles (C, H and O) in the form of fumes and evaporates consequently reduces the H/C and O/C atomic fractions and accordingly increases the aromatic property and amount of carbon, which ultimately boosts the solidity of the biochar. With increasing reaction temperature, this feature is enhanced [42]. The highly acquiescent nature of biochar for biocontamination eradication is a consequence of its enhanced water repelling nature, surface area and micropore capacity due to an increase in pyrolysis temperature. Although a lower reaction temperature (below 500 °C) subsequently yielded biochar with reduced pore dimensions, a lower superficial dimension and more O2-rich functional sets were concluded to be more competent in the removal of inorganic/metallic pollutants. An increase in pyrolysis temperature also elevates the pH of the yield due to the presence of ashes [43].
1.9 Retention time
The duration of the HTL reaction at a certain temperature, excluding the time spent heating and cooling, is known as the retention time [44]. The length of retention may have an impact on the production of bio-oil since a shorter period leads in partial degradation, while a longer period causes the polymerization reaction to reach an intermediate stage, which eventually reduces the yield of bio-crude oil, for example. Furthermore, a later increase in retention time has a detrimental influence on the yield of bio-oil due to breaking and polymerization. Reaction time changed the amount of residue (solid) produced; in the HTL, biochar was reported to drop from 16.2% after 40 min to 14.5% at 70 min [45]. Additionally, the output of gas increased as the residence duration increased (28.4% for 130 min). These results showed that the bio-crude oil output nearly reached its maximum level at different reaction temperatures and time intervals [46].
1.10 Pretreatment
The properties of biochar are influenced by the pretreatment of the raw supply before pyrolysis. The process of biochar formation from biomass is the most common step. Overall, these prior operation techniques can be corporal, actinic or physiological. Reducing the proportions of constituents and dissolving/submerging noncomplex elements in liquids are common pathways for conducting pretreatment [47]. The yield of biochar significantly increased as the amount of biomass molecules decreased. The organic mass of the pine timber was soaked in a nonconcentrated acidic mixture, for instance, as a pretreatment. The manufacturing and maintenance of biochar are affected by nitrogen and metal doping. The fundamental formation and properties of biochar are likely impacted by pretreatment approaches such as steaming and submergence. To produce orchestrated biochar with a distinct orifice anatomy, enhanced functional groups on the exterior of the particles, increased surface area, etc., for pretreatment, caustic chemicals such as acid, alkali and oxidizing agents have also been used.
1.11 Biochar properties and characterization
1.11.1 Functional groups
The important functional groups that are found to attach superficially to biochar to enhance the adsorptive tendency of its particles are organic acid (COOH), hydroxyl (OH), amine (NH4), amide (CONH4) and lactone groups. The temperature of the thermochemical conversion and the type of raw material used determine the functional groups on the surface of biochar. Furthermore, these attached groups could be lessened due to increases in parameters such as permeability, surface area and pH [48]. Pretreatment of the raw material before conversion and posttreatment of the product could significantly influence the characteristics of the biochar [49].
1.11.2 Surface area and porosity
Ordinarily, the ability of biochar to adsorb is directly proportional to the surface area and permeability, i.e., the greater both given parameters are, the greater the adsorption [50]. As the process of pyrolysis progresses, during the drying step, continuous loss of moisture from the biomass results in increased porosity of the surface. The pores of biochar are classified into three groups depending on their presize: microspores (2 µm), mesopores (2–5 0 µm), or macrospores (> 50 µm) [41]. Regardless of their polarity or charge, biochar particles with reduced pore sizes are inefficient for pesticide adsorption [51]. The pore size of biochar particles might increase with increasing temperature in biochar tests [52]. Moreover, the production of extremely eligible sweet scents in biochar therefore of an increase in the crystallinity of the mineral portion with increasing pyrolysis temperature is also possible.
Since the sorption and ion exchange properties of biochar are directly related to its surface area and bulk, the surface area of biochar particles is a necessary factor for pollutant eradication in the bulk land and water ecosphere. In two different approaches, the surface area is characterized by (i) the intrinsic and extrinsic surface areas and (ii) the dimension and approachability of the pores. The lateral surface area of biochar is characterized by prominent, deep cracks along with pores (macropores and mesopores). In contrast, the internal surface area consists entirely of a surface of intellectual depth and few unenclosed cracks and space/pores/cavities (microspores). Considering that the contributions of macropores and mesopores were noticeably less than average to the entire permeable structure of black carbon (biochar), mainly with respect to its adsorption capability, such as its ability to act as a groove for adsorbable substances to achieve micropores), the inner surface dimensions of black carbon [41] are well documented.
Although unrefined specimens are set side by side to the alternative form of biochar, a substantial increase in the Brunauer‒Emmett‒Teller (BET) surface area occurs during subsequent pyrolysis. During pyrolysis, char was formed with new nanopores, as organic biomasses are deficient in very fine pores (physical micropores). The development of a high permeability along with a countless pore composition and a low volume of biochar is caused by the excretion of numerous volatile matter [53]. The surface area of the raw material that was used in the experiment might be distinguishable from that of the material that was not used in the experiment. The functional properties of economically obtainable biochar include its prominent surface area. In the absence of activation, biochar has a restricted surface area with few pores. Consequently, biochar activation is utilized throughout the synthesis of biochar to increase its surface area and increase its porosity. Both chemical and physical activation are needed during biochar treatment [54].
1.11.3 Biochar stability
Several investigations to determine biochar stability have been performed [27, 30]. Techniques for investigating the stability of biochar can be divided into three groups: (a) straight or devious calibration (b) quantitative and qualitative determination and (c) determination of biochar growth [55]. The latter approach is a biological method for evaluating biochar stability and serves as the foundation of the other two techniques. The yield of the first two methods may be collated to estimate the indirect stability produced utilizing gestation and portrayal techniques [56].
1.11.4 Biochar and environment
To reduce any undesirable outcomes, despite its various uses, the effects of biochar on nature should be studied. Stability is the principal element that should be considered before use. Biochar consists of carbon arrangements. Therefore, it could be concluded that the durability of carbon structures determines biochar stability. The foremost indices of carbon arrangement are the aromaticity and aromatic condensation of biochar. The effective levels of solidity, flexibility and aromaticity are affected by the fused organic matter of biochar. The carbon content of water increases when industry-based liquid waste is treated with biochar due to the elements liberated by the biochar. The biochar produced from waste material rich in heavy metals might be collected from bioremediation systems, causing heavy metal pollution [57].
As a result, the stability of biochar continuously decreases because it is frequently utilized as a reaction impetus (catalyst). The conformational degeneration of biochar particles causes a negative change (decline) in stability. Hence, it could be concluded that with regard to nature’s interest, the solidity of biochar is crucial. In addition, the toxicity of biochar to land dwellers must be investigated before its utilization. Furthermore, meticulous determination of the perilous effect of biochar on the circumambient environment is needed because its physical and chemical behavior changes with the type of raw material used. For instance, bacteria, algae or fish can be utilized to perform certain toxicity tests. The heat-based chemical decomposition of natural formations under anaerobic conditions and their comprehensive use in farmlands to assist in alleviating ecological issues are crucial. The evolution of biochar was reported to enhance the loam porosity, water amount, acidity, and variable carbon and nitrogen reserve proportions, affecting CO2 discharge from the land. The advancement of biochar could be an applicable tool for alleviating ecological fluctuations accompanied by reduced CO2 discharge and escalated drought problems.
1.11.5 Application of biochar
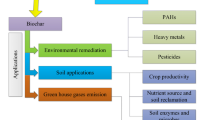
Due to its environmentally safe nature, ample reserves, easy availability of media and uncomplicated process of formation from a variety of raw materials with the assistance of heat-based chemical conversion, biochar is a popular subject of exploration and experimentation because of its ability to perform a broad range of ecological functions Table 2.
Based on the kind of raw material used and the temperature of pyrolysis, biochar has a significant role in eliminating adulterants and impurities from polluted water. The potential of organic biochar produced at increased temperatures by pyrolysis to remove bio pollutants from water is attributed to its amplified characteristic features, such as pore size, surface area, raw material used, acidity, decreased fused carbon amount and water repellent nature. Similarly, for inorganic pollutant elimination, biochar produced at decreased temperatures has oxygen as a functional set, low permeability and a greater amount of fused carbon. The degree of adsorption is also dependent on supplemental factors such as potential hydrogen (pH) and residence duration. In countering these ecological concerns, biochar has extensive capability and could be used as a stimulus to achieve sustainable development goals (SDGs) for ecological legitimacy. Overall, biochar can be used as a driving force for polluted water remediation, carbon absorption, composting, soil modification and energy production [58].
1.12 Water and wastewater decontamination
Biochar, a byproduct of pyrolysis, can have extensive application in the removal of different pollutants from wastewater. The bioactive components of pollutants and the type of biochar used are the parameters that determine the ability of biochar to adsorb organic contaminants and heavy metals present in greater concentrations inside water. For example, biochar extracted from wood dust may exterminate 20.3 mg/L of sulfamethoxazole by using a 20 mg/L absorbent dose; on the other hand, the elimination capacity of sulfamethoxazole is significantly lower, 20 to 30%, by biochar generated from wood at room temperature (37 °C). Sulfamethoxazole sorption (< 6%) is extremely inadequate for biochar produced from sustainable agriculture and natural farms [50]. Any change in pyrolysis temperature resulted in a change in the tetracycline removal efficiency of the biochar generated from rice hulls (i.e., the protective covering of grain).When the inceptive amount of tetracycline was 0.2 g/L and the pyrolysis temperature (thermal decomposition temperature) was ~ 1472°F, the sorption capacity of tetracycline was ~ 20–60% [59]. For tetracycline, when the initial concentration was 5 mg/L and the pyrolysis temperature was 932°F, the removal efficiency was ~ 90%, as demonstrated by additional investigations [55]. Consequently, biochar's adsorption capability is significantly influenced by the pyrolysis temperature. Parameters other than pyrolysis temperature, such as pyrolysis time, can remarkably affect the physical and chemical properties of biochar, therefore influencing its adsorptive efficiency.
Quick attention is required for heavy metal pollutants that cause significant environmental threats. Adsorption is a remarkably efficient methodology for the extraction of hazardous heavy metals from aquatic ecosystems. The kinds of heavy metals and adsorptive substances utilized are basic characteristics required for the exclusion of hazardous compounds by biochar in the case of organic pollutants [46]. For Cd2+ and As5+, biochar derived from biomass has more inadequate removal potential than biochar derived from other heavy metals, such as Pb2+. Adsorption capability is influenced mainly by the temperature at which thermal decomposition (pyrolysis) of biochar occurs. For instance, biochar generated from corn residue presented a variable adsorption capability for Cu2+ [57]. To remove 1 mM Cu2+, 1 g/L biochar was added at a pyrolysis temperature of 1472 °F. At a pyrolytic temperature of 752 °F, 20 g/L biochar was needed for the elimination of 2 mg/L Cu2+. It is often found that biochar generated from Eichhornia crassipes (water hyacinths) has a variety of removal potentials for Cd2+ and Pd2+ depending upon the heavy metal targeted, and the elimination rate of biochar differs accordingly [60]. It should be emphasized that when functional groups alter biochar, the adsorption energy is strongly impacted by the functional groups on the biochar surface. For example, the amino-modified surface of biochar has an enhanced cupric ion adsorption capability due to vigorous chelation.
Together with organic and heavy metals, an evaluative analysis inferred that biochar extracted from sludge is efficient at entirely extracting ammonium through monolayer chemisorption, a form of adsorption in which the adsorbed material is held together by chemical bonds. These results suggest that extremely competitive biosorption occurs quickly when organic pollutants and heavy metals are eliminated in the presence of ammonium, where biochar is utilized as an adsorbent.
Biochar has many important roles other than adsorption, and biochar can promote microbial growth, so accelerating the rate of biodegradable waste elimination. Biochar derived from fruitwood has a remarkable fraction of Archaebacteria, fruitwood-derived biochar reduces acid and ammonia stress on flourishing biological microbes, thereby increasing microbial activity [61]. In addition to the redox-active moieties, the authorization of tetrabromobisphenol A is enhanced by the incorporation of biochar, which speeds up the conversion of adsorbed tetrabromobisphenol A. It should be considered and emphasized that biochar is utilized for the treatment of environmental pollutants, refining and, furthermore, reuse. Biochar can be recycled due to magnetic modification [62]. A remarkable increase in the magnetic properties of biochar derived from corn stalks was seen upon the addition of a mixture of FeCl2 and ZnCl2 [63]. Following ongoing research analysis, to eliminate specific pollutants, massive evaluations are being performed on biochar. However, in reality, numerous pollutants reside in drinking water after treatment, making it unfit for consumption. However, in certain cases, competitive adsorption may occur, which leads to findings that vary from those observed in the laboratory. In addition, the real flow state may increase the ability of biochar to adsorb impurities. Thus, to simulate real-world conditions, supplemental investigations need to be conducted to determine the potential of biochar for eliminating contaminants.
1.13 Catalyst and catalyst support
In the execution of different types of reactions, in domains such as farming, environment and energy, biochar acts as a reaction catalyst. The properties of biochar make it a catalyst with desirable capabilities. For the stimulant applications of biochar, its broad surface area is crucial since it accommodates a variety of functional sets. In particular, the carboxyl and hydroxyl groups are optimal for the adsorption of ammonium ions, and for norfloxacin sorption, the OAH group is optimal. More importantly, the triggered and operationalized biochar can attain greater surface area and plentiful functional sets, hence showing a notable role as a catalyst or catalyst for different chemical conversions and surface assimilation/adsorption and absorption/enhancement of less contaminant water flows. Biochar can be utilized in biorefineries for the manufacturing of upgraded commodities in addition to being used as a stimulant or stimulant for the decomposition of biowaste. The functions of biochar include organic fuel (biodiesel) production, pollutant reduction, energy production, tar elimination, organic fuel gas production, terminal fuel cells and actinic formations [64].
1.14 Waste management
A wide variety of synthetic organic substances that are manufactured in research centers strongly protect against biodegradation and are not responsive to biological treatments. These artificially produced products could cause cancer in humans, microbes, plants and higher animals. With the anticipated technique of the catalytic ozonation process (COP), these bio-resistant chemicals could be degraded. To eliminate a resistant organic compound, a reactive colorant red 198 dye, COP is used, and this reaction is catalyzed by a cost-friendly biochar produced from a biomass with a permeable conformation and phenol (C6H5OH) and hydroxyl (OH) functional groups [65].
1.15 Control of air pollutants
In addition to low-temperature catalysts, selective catalytic reduction of biochar has been highly studied. Experiments on organic matter such as rice chaff and biosolids were conducted to generate biochar and temperature catalysts in combination with ammonia as a useful solvent. The fear of being switched on biochemically or physically along with the effectiveness of the treatment, accompanied by unfastening, was regulated. In comparison with physical activation, physical activation was also more efficacious at separating pollutants. This shows that chemical attributes, such as adsorption sites and functional groups, play a remarkable role in regulating removal capability. Charcoal catalysis was applied for sulfate and free radical transfer. The oxygen-rich surface of biochar accommodates complex catalytic activity via diverse processes. As a result of biochar, amalgamation enhanced the catalytic activity of the stimulus [66].
1.16 Biochar: an ideal approach for regenerative economic sustainability
The approach liable for manufacturing biochar from organic matter is through the use of thermochemical materials, largely in the hinterland, to assist in flourishing a certain region along with small and medium-sized enterprises to fabricate adequate power to enhance agriculturalist conclusions for horticulture waste minimization. This grant considers the relation of minor-scale construction techniques to wide-reaching techniques, therefore setting up a hermetic system replica in which waste from a particular citation can be utilized as an insert for more, following companionability, which is profitable and has an environmental impact on restoring economic security. Comparable interconnections in the middle of diverse biochar manufacturing and waste recycling innovation are essential for the expansion of new plans. By using waste, particularly for agro-refining factories, to illuminate toxic waste matter and embody the consequent sustainable economic growth, a soil application method has been developed, allowing for the latest products and process growth and the emergence of the latest collection. The strategy which affects stability amid the ease of energy saving, operation and awkward discharge, conceivably amalgamated into the regional network to authorize the attainable creation of biochar, considering both mechanical and commercial deliberation, along with heat emission and recovery of the biochar (black carbon).
2 Conclusion
These days, the presence of toxicants in the environment due to human activity has steadily grown to be a serious worldwide environmental problem that not only threatens human health but also the health of ecosystems and agricultural output. Biochar posses large surface area with attached functional groups and higher dimensions to ensnare organic contaminants and toxicants are described as promising contrivances. Biochar may be used through a variety of processes, including adsorption and other physicochemical reactions, to reduce the bioavailability of toxins. The characteristics of biochar, such as the kind of feedstock materials, pyrolysis temperature, and retention period, have a significant impact on its efficiency. With recent studies revealing new avenues for its application, the scientific community has begun to administer the biochar with serious consideration. Thus, by employing multipurpose biochar components, the utility of biochar efforts may be increased.
Data availability
No datasets were generated or analysed during the current study.
References
Sadh PK, Duhan S, Duha JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess. 2018;5(1):1–15.
Nadarajah K, Asharp T, Jeganathan Y. Biochar from waste biomass, its fundamentals, engineering aspects, and potential applications: an overview. Water Sci Technol. 2024;89(5):1211–39.
Enaime G, Baçaoui A, Yaacoubi A, Lübken M. Biochar for wastewater treatment-conversion technologies and applications. Appl Sci (Switzerland). 2020;10(10):3492.
Nidheesh PV, Gopinath A, Ranjith N, Praveen Akre A, Sreedharan V, Suresh Kumar M. Potential role of biochar in advanced oxidation processes: a sustainable approach. Chem Eng J. 2021;405:126582.
Amalina F, Farah A, Razak Y, Krishna S, Sulaiman H, Zularisam AW, Nasrullah M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—a review. J Hazardous Mater Adv. 2022;7: 100134.
Ge S, Nai P, Yek Y, Wang Y, Xia C, Adibah W, et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: a batch to continuous approach. Renewable Sustain Energy Rev. 2021;135:110148.
Kwapinski W, Byne C, Kryachko E, Wolfram P, Adley C, Leahy JJ, Novotny EH, Hayes MHB. Biochar from biomass and waste. J Waste Biomass Volarization. 2012;2(1):123–34.
Yang F, Wang B, Shi Z, Li L, Li Y, Mao Z, Liao L, Zhang H, Wu Y. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Env Pollut Bioavail. 2021;33:55–65.
Mingjing H, Zibo X, Deyi H, Bin G, Xinde C, Yong Sik O, Rinklebe J, Bolan N, Tsang DCW. Waste-derived biochar for water pollution control and sustainable development. Nat Rev Earth Environ. 2022;3(4):253–61.
Kumar A, Bhattacharya T, Akram Shaikh W, Roy A, Chakraborty S, Vithanage M, Kumar Biswas J. Multifaceted applications of biocharvin environmental management: a bibliometric. Biochar. 2023;5:11.
Manyà JJ, García-Morcate D, González B. Adsorption performance of physically activated biochars for postcombustion Co2 capture from dry and humid flue gas. Appl Sci (Switzerland). 2020;10(1):1–17.
Qin X, Luo J, Liu Z, Fu Y. Preparation and characterization of mgo-modified rice straw biochars. Molecules. 2020;25:1–39.
Mishra A, Kumar M, Bolan NS, Kapley A, Kumar R, Singh L. Multidimensional approaches of biogas production and upgradation : opportunities and challenges. Bioresour Technol. 2021;338:125514.
Sakhiya AK, Anand A, Kaushal P. Production, activation, and applications of biochar in recent times. Biochar. 2020;2(3):253–85.
Liu Z, Wang Z, Chen H, Cai T, Liu Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: a critical review. Environ Pollut. 2020;268:115910.
Nyoo J, Ju Y, Edi F. Biosorption of dyes. In: Green chemistry and water remediation: research and applications. Elsevier, Amsterdam. 2021.
Rahman MA, Hasegawa H. Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere. 2011;83(5):633–46.
Pan S-Y, Dong C-D, Su J-F, Wang P-Y, Chen C-W, Chang J-S, Kim H, Huang C-P, Hung C-M. The role of biochar in regulating the carbon, phosphorus, and nitrogen cycles exemplified by soil systems. Sustainability (Switzerland). 2021;13(10):5612.
Shokry H, Elkady M, Salama E. Eco-friendly magnetic activated carbon nanohybrid for facile oil spills separation. Sci Rep. 2020;10(1):1–18.
Singh JK, Chaurasia B, Dubey A, Manuel A, Noguera F, Gupta A, et al. Biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eicchornia crassipes) biomass hydrolysis. Sustainability. 2021;13:245.
Zhou R, Zhang M, Li J, Zhao W. Optimization of preparation conditions for biochar derived from water hyacinth by using response surface methodology (RSM) and its application in Pb2+ removal. J Environ Chem Eng. 2020;8(5): 104198.
Xiang W, Zhang X, Chen J, Zou W, He F, Hu X, Tsang DCW, Ok YS, Gao B. Biochar technology in wastewater treatment: a critical review. Chemosphere. 2020;252: 126539.
Sonu K, Sogani M, Syed Z, Dongre A, Sharma G. Enhanced decolorization and treatment of textile dye wastewater through adsorption on acid modified corncob derived biochar. ChemistrySelect. 2020;5(39):12287–97.
Ambaye TG, Rene ER, DuPont C, Wongrod S, van Hullebusch ED. Anaerobic digestion of fruit waste mixed with sewage sludge digestate biochar: influence on biomethane production. Front Energy Res. 2020;8:1–14.
Bhattacharjee C, Dutta S, Saxena VK. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ Adv. 2020;2:100007.
Srivatsav P, Bhargav BS, Shanmugasundaram V. Biochar as an ecofriendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: a review. Water. 2020;12:3561.
Senthil C, Lee CW. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew Sustain Energy Rev. 2021;137: 110464.
Barnossi AE, Moussaid F, Housseini AI. Tangerine, banana and pomegranate peels valorization for sustainable environment: a review. Biotechnol Rep. 2020;29:e00574.
Brown AE, Adams JMM, Grasham OR, Camargo-Valero MA, Ross AB. An assessment of different integration strategies of hydrothermal carbonization and anaerobic digestion of water hyacinth. Energies. 2020;13(22):5983.
Yaashikaa PR, Kumar PS, Varjani S, Saravanan A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol Rep. 2020;28: e00570.
Mitter EK, Tosi M, Obregón D, Dunfield KE, Germida JJ. Rethinking crop nutrition in times of modern microbiology: innovative biofertilizer technologies: innovative biofertilizer technologies. Front Sustain Food Syst. 2021;5:606815.
Gale M, Nguyen TU, Moreno M, Gilliard-Abdul Aziz KL. Physiochemical properties of biochar and activated carbon from biomass residue: influence of process conditions to adsorbent properties. ACS Omega. 2021;6(15):10224–33.
Han L, Sun KE, Yang Y, Xia X, Li F, Yang Z, Xing B. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma. 2020;364:114184.
Lee DJ, Lu JS, Chang JS. Pyrolysis synergy of municipal solid waste (MSW): a review. Bioresour Technol. 2020;318:123912.
Kazemi H, Panahi S, Dehhaghi M, Sik Y, Nizami A, Khoshnevisan B, Shiung S. A comprehensive review of engineered biochar : production, characteristics, and environmental applications. J Cleaner Prod. 2020;270:122462.
Saha A, Basak BB. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind Crops Prod. 2020;145:111979.
Sun Y, Xiong X, He M, Xu Z, Hou D, Zhang W, Sik Y. Roles of biochar derived from different raw materials. Environ Process. 2021;2(1):314–45.
Hu Q, Jung J, Chen D, Leong K, Song S, Li F, Mohan BC, Yao Z, Prabhakar AK, Lin XH, Lim EY, Zhang L, Souradeep G, Ok YS, Kua HW, Li SFY, Tan HTW, Dai Y, Tong YW, Peng Y, Joseph S, Wang C-H. Biochar industry to circular economy. Sci Total Environ. 2021;757: 143820.
Reza MS, Yun CS, Afroze S, Radenahmad N, Bakar MSA, Saidur R, Taweekun J, Azad AK. Preparation of activated carbon from biomass and its’ applications in water and gas purification: a review. Arab J Basic Appl Sci. 2020;27(1):208–38.
Tomczyk A, Sokołowska Z, Boguta P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol. 2020;19(1):191–215.
Labiadh L, Kamali AR. Textural, structural and morphological evolution of mesoporous 3D graphene saturated with methyl orange dye during thermal regeneration. Diam Relat Mater. 2020;103: 107698.
Zoroufchi K, MotalebiDamuchali A, Soltan J, McPhedran KN. Treatment of aqueous arsenic—a review of biochar modi fi cation methods. Sci Total Environ. 2020;739: 139750.
Arrebola J, Rodriguez-Fernandez N, Caballero A. Decontamination of wastewater using activated biochar from agricultural waste: a practical experiment for environmental sciences students. J Chem Educ. 2020;97(11):4137–44.
Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W, Jiang S, Li H, Huang H. An overview on engineering the surface area and porosity of biochar. Sci Total Environ. 2021;763: 144204.
Rajput, V., Kumar, V., Vlaskin, M.S., Nanda, M., Verma, M (2023). Hydrothermal liquefaction of waste agricultural biomass for biofuel and biochar. In: Agriculture waste management and bioresource: the circular economy perspective. Wiley, New York.
Bolan N, Hoang SA, Beiyuan J, Gupta S, Hou D, Karakoti A, Sherif A. Multifunctional applications of biochar beyond carbon storage. Int Mater Rev. 2021;1(2):1–51.
Feng Q, Wang B, Chen M, Wu P, Lee X, Xing Y. Resources, conservation & recycling invasive plants as potential sustainable feedstocks for biochar production and multiple applications : a review. Resour Conserv Recycl. 2021;164: 105204.
Samsami S, Mohamadizaniani M, Sarrafzadeh M-H, Rene ER, Firoozbahr M. Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process Saf Environ Prot. 2020;143:138–63.
Jesudoss NR, Kumar JS, Kamyab H, Sujana JAJ, Al-Khashman OA, Kuslu Y, Ene A, Suresh Kumar B. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector—a comprehensive review. J Cleaner Prod. 2020;272: 122636.
Shukla SK, Al Mushaiqri NRS, Al Subhi HM, Yoo K, Al Sadeq H. Low-cost activated carbon production from organic waste and its utilization for wastewater treatment. Appl Water Sci. 2020;10(2):1–9.
Kumar V, Nagappan S, Bhosale RR, Lay C. Bioresource technology review on sustainable production of biochar through hydrothermal liquefaction: physicochemical properties and applications. Bioresour Technol. 2022;310:121414.
You X, Jiang H, Zhao M, Suo F, Zhang C, Zheng H, Sun K, Zhang G, Li F, Li Y. Biochar reduced Chinese chive (Allium tuberosum) uptake and dissipation of thiamethoxam in an agricultural soil. J Hazard Mater. 2020;390: 121749.
Li Y, Xing B, Ding Y, Han X, Wang S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour Technol. 2020;312:123614.
Li Z, Sun Y, Yang Y, Han Y, Wang T, Chen J. Biochar supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J Hazard Mater. 2020;383: 121240.
Zhang H, Xu F, Xue J, Chen S, Wang J, Yang Y. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: behavior and mechanism. Sci Rep. 2020;10(1):1–14.
Esteves BM, Morales-Torres S, Maldonado-Hódar FJ, Madeira LM. Fitting biochars and activated carbons from residues of the olive oil industry as supports of fe-catalysts for the heterogeneous fenton-like treatment of simulated olive mill wastewater. Nanomaterials. 2020;10(5):876.
Jeffrey B, Novak M. Biochar from Biomass and Waste. Willy Publications. ISBN: 9780128117309. 2018.
Ha JH, Lee I-G. Study of a method to effectively remove char byproduct generated from fast pyrolysis of lignocellulosic biomass in a bubbling fluidized bed reactor. Processes. 2020;8(11):1–14.
Naghdi M, Taheran M, Brar SK, Kermanshahi-Pour A, Verma M, Surampalli RY. Fabrication of nanobiocatalyst using encapsulated laccase onto chitosan-nanobiochar composite. Int J Biol Macromol. 2019;124:530–6. https://doi.org/10.1016/j.ijbiomac.2018.11.234.
Zhang X, Sun P, Wei K, Huang X, Zhang X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem Eng J. 2020;385: 123921.
Rashid MI, Shah GA, Sadiq M, Amin NU, Ali AM, Ondrasek G. Nanobiochar and copper oxide nanoparticles mixture synergistically increases soil nutrient availability and improves wheat production. Plan Theory. 2023;12:1312. https://doi.org/10.3390/plants12061312.
Kumar S, Kumar G, Avasthe R. Applications of biomass derived biochar in modern science and technology. Environ Technol Innovation. 2021;21: 101306.
Alkharabsheh HM, Seleiman MF, Battaglia ML, Shami A, Jalal RS, Alhammad BA, Almutairi KF, Al-Saif AM. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: a review. Agronomy. 2021;11(5):993.
Zhang P, Min L, Tang J, Rafq MK, Sun H. Sorption and degradation of imidacloprid and clothianidin in Chinese paddy soil and red soil amended with biochars. Biochar. 2020;2(3):329–41.
Gopinath A, Divyapriya G, Srivastava V, Laiju AR, Nidheesh PV, Kumar MS. Conversion of sewage sludge into biochar: a potential resource in water and wastewater treatment. Environ Res. 2021;194: 110656.
Pan H, Yang X, Chen H, Sarkar B, Bolan N, Shaheen SM, Wu F, Che L, Ma Y, Rinklebe J, Wang H. Pristine and iron-engineered animal- and plant-derived biochars enhanced bacterial abundance and immobilized arsenic and lead in a contaminated soil. Sci Total Environ. 2021;763: 144218.
Benkhaya S, Souad M, Harfi AE. A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun. 2020;115:107891.
Jiang C, Bo J, Xiao X, Zhang S, Wang Z, Yan G. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manage. 2020;102:732–42. https://doi.org/10.1016/j.wasman.2019.11.019.
Al M, Wonik S, Sarker A, Ardie S, Das K, Deen MD, Iqbal MA, Abu R, Islam T, Malafaia G. A critical review of sustainable application of biochar for green remediation: research uncertainty and future directions. Sci Total Environ. 2023;1(4):239.
Khanh T, Kim N, Jyh H. ScienceDirect The production of hydrogen gas from modified water hyacinth (Eichhornia Crassipes) biomass through pyrolysis process. Int J Hydrogen Energy. 2020;46:13976.
Talaiekhozani A, Rezania S, Kim K-H, Sanaye R, Amani AM. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J Cleaner Prod. 2021;278: 123895.
Ferlazzo A, Bressi V, Espro C, Iannazzo D, Piperopoulos E, Neri G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J Electroana Chem. 2023;928: 117071. https://doi.org/10.1016/j.jelechem.2022.117071.
Khan HA, Naqvi SR, Mehran MT, Khoja AH, Khan Niazi MB, Juchelková D. A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture. Chemosphere. 2021;285: 131382. https://doi.org/10.1016/j.chemosphere.2021.131382.
Ramanayaka S, Tsang DCW, Hou D, Ok YS, Vithanage M. Green synthesis of graphitic nanobiochar for the removal of emerging contaminants in aqueous media. Sci Total Environ. 2020;706: 135725. https://doi.org/10.1016/j.scitotenv.2019.135725.
Silvani L, Hjartardottir S, Bielska L, Skulcova L, Cornelissen G, Nizzetto L, Hale SE. Can polyethylene passive samplers predict polychlorinated biphenyls (PCBs) uptake by earthworms and turnips in a biochar amended soil? Sci Total Environ. 2019;662:873–80.
Gu A. Removal of dyes and pigments from industrial effluents. 2021. https://doi.org/10.1016/B978-0-12-817742-6.00005-0.
Jorge L, Mar A, Ana Á, Tarr J, Naya S. The complexity of lignin thermal degradation in the isothermal context. Processes. 2021;9:1154.
Nath BK, Chaliha C, Kalita E. Iron oxide permeated mesoporous rice-husk nanobiochar (IPMN) mediatedremoval of dissolved arsenic (as): chemometric modelling and adsorption dynamics. J Environ Manag. 2019;246:397–409. https://doi.org/10.1016/j.jenvman.2019.06.008.
Jenie SNA, Kristiani A, Khaerudini DS, Takeishi K. Sulfonated magnetic nanobiochar as heterogeneous acid catalyst for esterification reaction. J Environ Chem Eng. 2020;8: 103912. https://doi.org/10.1016/j.jece.2020.103912.
Zhang Y, Piao M, He L, Yao L, Piao T, Liu Z. Immobilization of laccase on magnetically separable biochar for highly efficient removal of bisphenol a in water. RSC Adv. 2020;10:4795–804. https://doi.org/10.1039/C9RA08800H.
Chiappero M, Norouzi O, Hu M, Demichelis F, Berruti F, Di Maria F, Mašek O, Fiore S. Review of biochar role as additive in anaerobic digestion processes. Renewable Sustain Energy Rev. 2020;131: 110037.
Korpe S, Rao PV. Application of advanced oxidation processes and cavitation techniques for treatment of tannery wastewater—a review. J Environ Chem Eng. 2021;9(3): 105234.
Acknowledgements
None.
Funding
No funding was received for this project.
Author information
Authors and Affiliations
Contributions
Vishal Rajput: investigation, software, validation, writing—original draft preparation; Bindu Naik: Conceptualization, investigation, analysis, writing—original draft preparation;Vijay Kumar: conceptualization, methodology, formal analysis, writing—review and editing; Isha Saini:Original draft writing, Reviewing and Editing; Simran Parmar: Original draft writing, Reviewing and Editing; Vedansh Pundir: Original draft writing, Reviewing and Editing,Vivek Kumar; Original draft writing, Reviewing and Editin:Sarvesh Rustagi: Original draft writing, Reviewing and Editing
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The conducted research was not related to either human or animal use.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajput, V., Saini, I., Parmar, S. et al. Biochar production methods and their transformative potential for environmental remediation. Discov Appl Sci 6, 408 (2024). https://doi.org/10.1007/s42452-024-06125-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06125-4