Abstract
Core–shell nanoparticles have been the centre of attention of the researchers in various fields as the transition from bulk/micro particles to nanoparticles to core shell nanoparticles was seen to lead to enormous changes in the physical and chemical properties of the materials like increased surface to volume ratio, dominance of surface atoms over those in the core etc. Core–shell nanohybrid structures are nanocomposites which incorporates the advantages of both core–shell nanoparticles and other component of the hybrid material like polymer, ceramic, oxide structures. In recent times core–shell nanohybrid structures have gained wide attention in different energy and environment applications including sorption of pollutants from aquatic medium. Drinking water pollution is one of the major problems the world is facing today. Various technologies have been developed for removal of various contaminants from aquatic streams. Core–shell hybrid nanostructures can be tailored by chemical modification or by incorporation in the polymeric matrix. This not only makes them specific to some metal ions, radionuclides even nanoparticles but also enhances their sorption capacity. These materials can be synthesised by dispersion of building blocks in polymeric network, in situ polymerisation, sol–gel process, self-assembly of unit building blocks through layered structures or interpenetrating networks. In this book chapter, a series of core shell nanohybrid structures having application in water decontamination have been reviewed and discussedextensively. Synthesis strategies, sorptive properties of these core shell nanohybrid structures are summarised with emphasis on decontamination of conventional pollutants, radionuclides, dyes and organic pollutants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanoparticle can be defined as any particle with size in the range of 1–100 nm. The properties of materials change drastically as one enters into nano world due to increase in surface area to volume ratio and onset of quantum confinement effects [1]. Due to their distinguished features, they find applications in various sectors. In healthcare area, nanoparticles are being synthesised to aid in the migration of chemotherapy drugs, straightway to the site of cancerous growth. Also, Au nanoparticles are commercially used as probe in order to detect the sequence of nucleic acids. Cosmetic sector makes use of TiO2 nanoparticles in the sunscreens, which works by reflecting the visible light and absorbing the UV light, thus acting as a transparent barrier which shields the skin from harmful ultraviolet rays. Apart from this, nano TiO2 also finds use in coatings which are applied to make self-cleaning surfaces [2]. Use of carbon nanotubes in baseball bats has made it lighter, thereby improving the performance. Transistors, which make all the modern computing possible have become smaller and smaller with the advent of nanotechnology [3]. Ultra-High Definition (UHD) displays are available that makes use of quantum dots to generate much vibrant colors and at the same time being more energy efficient [4]. Nanotechnology can also assist in meeting the requirements of clean drinking water by developing novel materials that could help in easy and affordable detection of harmful pollutants in water followed by their facile eradication [5]. In food industry, nanotechnology can bring improvement in food processing and packaging, food safety and in extending the shelf life of food products. Thus, it is clear that there is hardly any area which is not benefitted by the arrival of nanotechnology. Coming to the classification, Siegel classified the nanomaterials based on their dimensionality as 0D, 1D, 2D and 3D (Fig. 1).
-
(i)
0-dimensional nanomaterials: In 0-D nanomaterials, all the dimensions are in the nanorange. It comprises of nanospheres and nanoclusters.
-
(ii)
1-dimensional nanomaterials: In 1-D nanomaterials, one dimension is not in the nanorange. This category includes nanorods, nanowires, nanofibers etc.
-
(iii)
2-dimensional nanomaterials: In 2-D nanomaterials, two dimensions are not in the nanorange. Nanolayers and nanofilms come under this class of materials.
-
(iv)
3-dimensional nanomaterials: In 3-D nanomaterials, three dimensions are not in the nanorange. They are made up of multiple nanosize crystals arranged in different directions.
Core shell nanoparticles (CSNP) are concentric multilayer particles where dimension of each layer is in nanometre range [6]. Core–shell nanoparticles have been the centre of attention of the researchers in the field as the transition from bulk/micro particles to nanoparticles to core shell nanoparticles was seen to lead to enormous changes in the physical and chemical properties of the materials [7]. The properties of core shell nanohybrids are not only dependent on size but also on shape. Different physical and chemical features such as melting point, catalytic activity, coercivity, selectivity, optical properties are affected by shape [7,8,9]. In addition to these, the sensitivity towards Surface Enhanced Raman Scattering (SERS) is influenced by morphology of core–shell nanohybrids. PengzhenGuo et al. reported the synthesis of Au@Agnanohybrid and used it for the detection of pesticide thiram [10]. The detection limit was found to be different for nanocubes and nanocuboids. The schematic showing the different shapes of core–shell nanohybrid material is shown in Fig. 2 [11].
Different core/shell nanoparticles: a spherical; b multicore spherical; c movable core in hollow shell; d multiple small cores coated by single shell; e Single core coated by multiple small particles as shell; f hexagonal (Reprinted with permission from Ref. [11])
The core shell nanohybrids are superior over conventional nanomaterials as their properties can be easily tailored either by varying the constituents or by changing the core to shell ratio. The shell coating provides multiple benefits such as an increase in functionality, dispersibility, stability as well as the ability to release the core in a regulated manner [12]. Apart from this, they are of immense significance from economic frame of reference. A valuable material can be coated onto a cheap material in order to decrease the consumption of expensive material rather than using the costly pure material of the same size [13]. The synthesis of core shell nanomaterial results in incorporation of two functions in one single structure. By adding more shells or by increasing the number of constituents, the versatility of the hybrid material can be increased, leading to an extensive range of applications in various fields [14, 15]. The schematic illustrating the significance of core–shell nanohybrids in diverse areas is shown in Fig. 3. Here in, we have mainly focused on the usage of core–shell nanohybrid for the decontamination of water.
The quality of water is degrading continuously making it more and more toxic for humans and environment. Rapid industrialisation, improper planning and urbanisation has resulted in the worldwide contamination of water resources [15]. Heavy metals, pharmaceuticals, pesticides, dyes, radionuclides are some of the important categories of pollutants which are deteriorating the water quality parameters. Even with the arrival and advancements of new technologies, the heavy metal concentration in potable water is still beyond the prescribed limits set be regulatory bodies in various parts of the globe [16, 17]. The exposure to the heavy metals results in increased risk towards cardiovascular disorders, renal injuries, immune system dysfunction, gastrointestinal and neuronal problems, genomic instability and many more [18, 19]. Apart from heavy metals, the textile industry discharges huge quantities of dyes into water bodies resulting in an enhancement in the chemical and biochemical oxygen demand, inhibition in the growth of plant, bioaccumulation and an increased likelihood of mutagenicity and carcinogenicity. The indiscriminate usage of antibiotics on a large scale and their subsequent presence in water bodies has given birth to antibiotic resistance. Other pollutants like pesticides enter into water bodies through surface run off and are well known to adversely affect the health of aquatic species and humans. In view of the increasing load of pollutants, and the crisis for clean drinking water supply across globe, it is necessary to find suitable means to deal with the problem. Various methodologies have been adopted for the treatment of water such as membrane separation, advanced oxidation processes, photochemical degradation, sedimentation, flocculation, adsorption, ozonation etc. [20].
Among all these methods, adsorption is preferred widely because of its cost effectiveness, eco-friendly nature, easy handling and availability of large number of adsorbents. Various adsorbents such as graphene oxide, reduced graphene oxide, boron nitride, cellulose, chitosan, activated carbon, alumina, ferrites, iron oxide NPs etc. have been used by researchers for the removal of different kinds of pollutants [21,22,23,24,25]. Core–Shell Nanohybrids have proven to be a better adsorbent because of the high surface area and the ability to tailor the properties of core using suitable type of shell and its thickness [26, 27]. In view of the immense significance of core shell nanohybrids, here in we have discussed in detail their classification, synthetic strategies, general characterisation and finally their role in the removal of different kinds of pollutants.
3 Inorganic/Inorganic CSNP
It is the most significant class among all the types as it finds applications in different fields ranging from information storage, biological labelling, optical bioimaging, catalysis, optoelectronics and many more. These CSNP can be further divided into following types.
3.1 Silica-Based Shell
The reasons for which silica is chosen by researchers for encapsulation of core nanomaterials are its extra ordinary colloidal stability especially in aquatic media, easy and controllable synthesis, modifiable porosity, chemical inertness and optical transparency [28,29,30]. Apart from this, due to the presence of silica shell, the core nanomaterial becomes biocompatible and the rich silica chemistry allows conjugation with functional groups easy which has resulted in widespread use of silica coated nanomaterials for various diagnostic and therapeutic purposes. Due to these distinguished properties, silica coatings have been made on different inorganic materials such as metals, metal oxides and metal salts. Silica coating is usually done by employing the classic Stober method involving hydrophobic silanes such as tetraethylorthosilicate (TEOS), tetramethylorthosilicate (TMOS) as silica precursor [31, 32]. Recently, water soluble silanes such as MPTES (3-(mercaptopropyl-triethoxysilane), MPTMS (3-(mercaptopropyl)-trimethoxysilane), MTMS (3-(methyltrimethoxysilane) have also been utilised [33].
3.2 Non Silica-Based Shell
Instead of Silica, various metals such as Au, Ni, Co, Pd, Pt, Cu, metal oxides, semiconductors can also be used as a shell material. Metal nanoshells consist of a dielectric core having a nano range metallic layer (usually of Au) over it. By changing the relative core and shell thickness, a broad variation can be seen in the color of nanoshells making it useful for biomedical applications [34]. Likewise, Fe2O3 coating on CaO and MgO nanoparticles can lead to an increase in the adsorption capacity of toxic materials compared to pure CaO and MgO [35].
3.3 Semiconductor CSNP
Here, either the core or the shell or both are made up of semiconducting material. They can be further classified as Nonsemiconductor/Semiconductor or/Semiconductor/Non-semiconductor Core Shell Nanoparticles. Among various types of nonsemiconductor/semiconductor materials, the ones consisting of a magnetic core having a semiconducting shell around them are very much versatile. The photocatalytic properties of magnetic core semiconductor shell is higher than pure semiconductor [36]. Researchers investigated the photocatalytic activity of TiO2 and Fe2O3/TiO2 core shell hybrid in malignant tumour therapy and they found out that survival of tumour cells is lesser for core/shell nanohybrid compared to pure semiconductor [37].
3.4 Semiconductor/Semiconductor CSNP
Both the core and shell are made up of semiconductor. The major advantage of these particles is that the outer coating of second semiconducting material over first leads to an increase in optical activity and stability towards photooxidation. Depending upon the band gap three subdivisions can be made: type-I, inverse type-1 and type-II [38]. In type I, the shell bandgap is larger than core and hence both hole (h) and electrons (e) are restricted within the core. In inverse type, shell bandgap is smaller than core; the h and e may be partially or wholly restricted to shell depending upon its thickness. In the last type, the edge of valence or conduction edge lies within the core bandgap. Hence, one carrier is chiefly restricted to the core and other to the shell.
4 Inorganic/Organic CSNP
These are made up of metal, metal oxide, metallic compound, or silica core having a polymeric shell or a shell build-up of any organic material of high density. Coating of organic material around metal decreases the susceptibility of metal towards surface oxidation and also makes it biocompatible for applications in biological field [39]. In many cases, the core particles are coated to enhance their stability in suspension media. On the basis of material properties, they can be further divided into following two groups.
4.1 Magnetic/Organic CSNP
Magnetic nanoparticles are coated with organic materials in order to reduce the agglomeration. Polysaccharides and hydrophilic polymers are commonly employed as coating materials. This class of materials find application in magnetic sealing, MRI, magnetic recording, electromagnetic shielding, magnetic shell separation and drug targeting [40,41,42].
4.2 Nonmagnetic/Organic CSNP
This class of materials can be further categorised on the basis of material constituting the nonmagnetic core- metal, metal or metalloid oxide, and metal salts. Among metals, silver and gold have been the centre of attraction because of their distinct optical properties. Au surrounded by polyaniline acts as biosensor for glucose [43]. In metal oxides, polystyrene, poly(methylmethacrylate), polyvinyl chloride are widely used for coating on silica core and the polymer coated silica nanoparticles find application in material additives, sensors, optical and electrical devices. For metal salts, coating is usually done with conducting polymers such as polyaniline, polythiophene, polypyrole etc. [44]. They usually find application in light emitting devices, chemical sensors and electronic devices.
5 Organic/Inorganic CSNP
The core is made up of polymeric material such as polystyrene, polyurethane, poly(ethylene oxide), poly(vinylpyrrolidone), poly(vinyl benzyl chloride), surfactants, dextrose, styrene-methyl methacrylate, poly(styrene-acrylic acid) etc. while the shell is composed of metals, metal chalcogenides, metal oxides or silica [45, 46]. The coating of an inorganic material, mainly that of metal oxide over an organic material has several benefits such as increment in the strength of whole material, better colloidal and thermal stability and resistance towards oxidation and abrasion [47, 48]. Further, they could be used as a template for the synthesis of hollow inorganic material via dissolution of the core using an appropriate solvent or by calcination. The preparation of silica microspheres via formation of polystyrene@silica core shell nanohybrid is shown in Fig. 5 [48, 49].
6 Organic/Organic CSNP
Both the core and shell are composed of polymeric or some organic material. The profits gained by developing one polymer coating onto another is the modification in the physical properties of whole material, such as its toughness and glass transition temperature. Since, the glass transition temperature plays an important role in deciding whether a synthesised material is most suited for rigid or flexible application; tuning this property could help in getting desired properties. For instance, it has been reported that a high Tg core material improves the mechanical stability while a low Tg shell aids in the improvement of film formation capability. Apart from this, this class of materials also find application in controlled release of drugs. For instance, Dina M. Silva etal synthesised polymeric core shell nanohybrid and utilised it for the co-delivery of two drugs [50]. The curcumin drug is trapped in the core and ciprofloxacin was incorporated in the shell layer. The synthesis of nanohybrid material is shown in Fig. 6.
Schematic representation of the processing steps of the core–shell particles. (Reprinted with permission from Ref. [50]).
7 Core/Multishell Nanoparticles
Bimetallics form an important part of this category of core shell NPs. Benito Rodrı́guez-González synthesised Au–Ag multishell nanoparticles [51]. The optical properties were found to change as second and third shells are placed. The color of pure gold was deep red, when silver shell is placed over it, the color changes to yellow, upon placement of second gold shell it changed to blue hue. Finally, when second silver shell is deposited over it, orange color was observed. When silica shell is placed onto CdSe/ZnS core–shell quantum dots, an enhancement in stability in biological buffers was observed apart from an increase in biocompatibility in case of fluorescence imaging [52].
8 Movable Core/Hollow Shell Nanoparticles
Template assist route is usually followed for the synthesis of movable core/shell NPs. In this methodology, first core with a double shell is prepared and then removal of double shell is done by using an appropriate technique such as calcination or dissolution. The synthetic scheme for the preparation of movable Au core inside a carbon shell following template assisted route is shown in Fig. 7 [53].
One-step synthesis of Au–SiO2–RF polymer CSS nanocomposite and its conversion to Au@C yolk–shell nanostructure. (Reprinted with permission from Ref. [53])
9 Synthesis Strategies for Core–Shell Nanohybrid Materials
For carrying out the synthesis of these nanomaterials two approaches are generally followed (a) Top-down approach (b) Bottom up approach. In the top down approach, the bulk material is broken down to produce nanosized particles. The techniques included in this approach are ball milling, electrospinning, lithographic technique, laser ablation, sputtering and arc discharge method. The main problem associated with this approach is the creation of imperfections on the surface structure and difficult to maintain uniformity in the core and shell size and dimensions [54]. Contrary to this, the bottom up approach involves the building up of a nanomaterial from bottom, that is either atom by atom or molecule by molecule (Fig. 8). Techniques like chemical vapor deposition, solvothermal methods, sol–gel method, reverse micelle methods, soft and hard templating methods, laser pyrolysis etc. come under the category of bottom up approach methodology [55, 56]. This approach is advantageous in the sense that it can give rise to smaller size nanoparticles as well as creation of less defects. As far as synthesis of core shell nanohybrid is concerned, bottom up approach is found to be more convenient as it gives better control in achieving uniform coating. The two approaches can also be used in combination, for example the core particles can be synthesised via top down approach and then coating can be carried out by bottom up approach so as to maintain more regular shell thickness [57].
A two-step process is followed for the synthesis of core/shell nanohybrids. The synthesis strategy can be categorised into two types depending on core particles availability: (i) In first method, synthesis of core particles is done separately followed by washing and drying. Subsequently, appropriate surface modification of the core is carried out in order to coat it with the shell material. (ii) In the second method, core particles are synthesised in situ and afterwards coating with shell is done. The main advantage offered by first method is that the core particles obtained are in the pure form and hence chances of impurities on its surface would be less. While, in the in situ approach, probability of having some impurities lying between core and shell increases [58, 59].
The two things which are of utmost concern in the synthesis of core/shell nanohybrids is to develop a uniform coating of shell around the core and to have a control over its thickness. The major problems encountered by researchers in achieving these goals is primarily because of the following reasons: (i) core particles get agglomerated in reaction media, (ii) separate formation of shell particles instead of onto the core, (iii) core surface is impartially covered, (iv) managing the rate of reaction. Various routes such as microemulsion, precipitation, polymerization, sol–gel condensation, layer by layer adsorption have been adopted for the synthesis of core shell nanohybrids [6, 60,61,62]. Considering the material properties only, synthetic methods utilised for the preparation of inorganic and organic materials irrespective of whether they are acting as core or shell are briefly described below:
9.1 Synthesis of Inorganic CSNP
Inorganic core–shell nano materials can be divided into three classes: (i) metal, (ii) oxides of metal or metalloid, (iii) metal salts and metal chalcogenides.
9.1.1 Synthesis of Metallic CSNP
Following strategies are adopted for the synthesis of cores or shells made up of metal.
9.1.1.1 Reduction
It involves the reduction of a metal salt in the presence of a stabilizer. Various reducing agents have been used such as sodium borohydride, hydrazine, lithium aluminium hydride, tannic acid, sodium citrate; the former two being the most common [6, 63, 64]. Apart from the chemical reduction, greener reducing agents such as plant extract, bacteria and fungi have also been tried in order to reduce the use of harmful chemicals (Fig. 9). The main advantages associated with chemical reduction are its simplicity, cost-effectiveness and ability to control the size of particles by regulating various parameters such as nature of reducing agent, type of stabilizer, amount of reducing agent relative to salt precursor and the molar ratio of salt precursor and stabilizer [65, 66]. The choice of a reducing agent is a significant factor because size, shape as well as particle size distribution are strongly affected by it. For the reduction of metal salts, the reactivity of reducing agent needs to be adjusted appropriately. This is because if during the synthesis the rate of reaction is too fast, large number of nuclei will be formed rapidly resulting in generation of smaller size nanoparticles. Whereas if rate of reaction is very small, agglomeration is more likely to take place giving rise to bigger size nanoparticles.
9.1.1.2 Reduction Transmetallation
This technique is mainly used for the formation of bimetallic type of core–shell nanoparticles. Compared to common reduction methods, redox transmetallation offers numerous advantages for the synthesis of core/shell nanoparticles: (i) Additional reducing agent is not required (ii) Occurrence of spontaneous deposition of shell layer onto the surface of core (iii) Self Nucleation of shell is prevented. The major demerit associated with this method is that only those bimetallic core shell nanoparticles can be synthesised where reduction potential of shell is more than that of the core. Ni/Au, Ni/Ag, Ag/Au, Co/Pt, Co/Cu, Co/Pd, Co/Au are some examples of bimetallic core–shell structures prepared by this technique [67,68,69,70,71]. In this method, first the synthesis of core is carried out. Thereafter addition of metal salt to the solution is done. When these salts come in contact with the surface of core, they get reduced by core atoms present on the surface and thus are deposited on it. Consequently, a part of the metallic core is oxidised to the salt which then diffuses through shell into the solution. Woo-ram Lee reported the synthesis of Co/Pd, Co/Pt, and Co/Cu using redox tranmetallation route [67].
9.1.1.3 Thermal Decomposition
Metal nanoparticles especially that of core can also be synthesised by thermally decomposing the organometallic compounds at high temperature. The surfactants are generally employed for the stabilisation of nanoparticles. A very common example is thermal decomposition of Co2(CO)8. As metal is already in zero-valent state it is possible to dissociate M-CO bond in organic solvent through thermal activation in the presence of a stabilizer [72]. Hess et al. reported the synthesis of Co nanoparticles through decomposition of Co2(CO)8 at 110 °C in toluene [73]. Likewise, Fe NPs have been prepared by decomposition of Fe(CO)5. Thereafter nanoparticles are precipitated and then stored in hexane so as to prevent the oxidation of surface. As the metal carbonyls are expensive and toxic, therefore other organometallic precursors such as [bis(salicylidene) cobalt(II)] oleylamine complex have also been tried for the synthesis of cobalt NPs [74]. Apart from organometallic compounds, metal nanoparticles are also made through thermal decomposition of metal complexes. MasoudSalavati-Niasari reported the synthesis of copper NPs by thermal decomposition of copper oxalate [75]. Similarly, synthesis of Ni and Au nanoparticles from thermal decomposition of their corresponding acetates have been reported [76, 77]. Once the metal is separated, it is washed with anhydrous alcohol for removing the stabilizer from the surface and then coating with shell is carried out. This technique is more helpful in the preparation of core shell nanohybrids of metal/metal oxide type. This is because after the synthesis of core, the only thing needed to be done is the removal of stabilizer from the surface of the core. After this surface atoms will get oxidised by atmospheric oxidation to metal oxide.
9.1.1.4 Wire Electrical Explosion (WEE)
It involves the application of a high voltage across a thin wire under inert conditions in a closed chamber having high pressure. This leads to the generation of an intense pulse with large current density. When such huge current passes through the wire, it becomes overheated causing it to evaporate rapidly. As a result, metal nanoparticles of different size are formed inside chamber. WEE is not commonly used in industry as it is highly expensive and secondly cannot be used for all metals. It can be employed for only those metals which have high electrical conductivity and can be easily obtained in the form of thin wire. Nanoparticles of metals such as Cu, Al, Ni and core shell alloys such as Cu/Zn, Ti/Ni have been synthesised via WEE [78, 79]. The schematic diagram for the synthesis of Nickel nanoparticle using wire electrical explosion followed by coating with silica shell is shown in Fig. 10.
9.1.2 Synthesis of Oxide Nanoparticles
They form an important class of materials with interesting applications. The widely used methods for their synthesis are described below.
9.1.2.1 Sol–Gel Method
It comes under wet chemical techniques and is widely employed for the the synthesis of metal oxide nanoparticles. Hydrolysis followed by polycondensation are the two key steps leading to the generation of a solid phase network gradually. Metal alkoxides and metal salts are commonly used as precursors in solgel method. By varying various parameters, a wide range of materials such as fine powder, aerogel, xerogel, thin films and monoliths can be prepared [80, 81]. The overall process can be written as:
-
(1)
Hydrolysis of precursors (usually alkoxides or chlorides)
$$M - OR + H_{2} O \to M - OH + R - OH$$Here, M = metal, R = alkyl
-
(2)
Polycondensation: This step involves the formation of metal oxide linkages with elimination of water or alcohol. The polymeric network gradually grows and ultimately reaches colloidal dimensions. The size of colloidal factors depends mainly on the pH of medium and ratio of precursor to water.
$$M - OX + M - OH \to H - OX + M - O - M$$Here, X = alkyl or H.
-
(3)
Aging: During this process the polycondensation reaction continues to take place.
-
(4)
Drying: There are different ways in which drying can be done: Supercritical, thermal, and freeze drying leading to formation of aerogel, xerogel and cryogel respectively.
The schematic for the process is shown in Fig. 11 [82, 83].
9.1.2.2 Coprecipitation
Precipitation reaction involves the reaction of two or more than two water soluble salts to generate at least one insoluble salt which precipitates out of the media. The solubility product is the key parameter for precipitation reaction. This is because initially the particles formed will be in liquid phase, and once their concentration exceeds solubility product only then particle formation will begin. This technique has been widely used for the synthesis of iron oxide nanoparticles and various ferrites. K. Petcharoen reported the synthesis of magnetite nanoparticles by the coprecipitation of FeCl2.4H2O and FeCl3 in the presence of ammonium hydroxide which is acting as precipitating agent [83]. Both hexanoic and oleic acid were used as coating agents for the stabilisation of formed nanoparticles. Finally, the formed coated nanoparticles were filtered and subsequently washed. First washing is carried out with water in order to remove Cl− ions and then with ethanol so as to get rid of excess coating agent. Similarly, Yeong Il Kim prepared CoFe2O4 nanoparticles by the coprecipitation of CoCl2.6H2O and FeCl3.6H2O using sodium hydroxide as the precipitating agent [84]. They also found that particle size can be tuned by properly regulating the temperature during coprecipitation. Apart from ferrites, other metal oxide nanoparticles such as ZnO have been prepared following this route. The extension of this route for the synthesis of core/shell nanohybrids is simple. For instance, after the synthesis of Fe3O4 core by co-precipitation, urea and titanium sulfate are added in the same solution. Decomposition of urea generate ammonia which undergoes reaction with Ti(SO4)2 to form TiO2, thus ultimately forming Fe3O4/TiO2 core shell structure [85].
9.1.2.3 Thermal Decomposition
Metal complexes can be decomposed by heat treatment to generate metal oxide, a process also known as thermolysis. This methodology is mainly adopted for the synthesis of core particles and is quite simple. Various transition metal oxides have been synthesised following this route. Teresa Palacios-Hernández synthesised Co3O4 and CuO nanoparticles by performing calcination of tartarate complexes of Co and Cu complexes respectively for 5 h at a temperature of 500 °C [86]. Similarly, Sun et al. prepared Fe3O4 nanoparticles by thermal decomposition of Fe(acac)3. Oleic acid and oleyl amine were added in the reaction mixture as stabilizer [87]. They also found that particle size can be tuned by changing the reaction conditions or by following seed mediated growth. Likewise, ZnO nanoparticles are prepared by thermally decomposing [bis(acetylacetonato)zinc(II)]–oleylamine complex. Oleyl amine and triphenylphosphine were used for restricting the particle size. The synthetic scheme is shown in Fig. 12 [88].
Synthesis of ZnO NPs by the thermal decomposition of Zn-Oleylamine (Reprinted with permission from Ref. [88])
9.1.3 Synthesis of Metal Salt and Metal Chalcogenide Nanoparticles
Metal chalcogenides, especially sulfides of transition elements are employed as semiconducting materials in electronic industry and metal salts of rare earth metals are utilized for bioimaging. The synthesis of this class of materials is usually carried out by the use of precipitation reaction. Various semiconducting core shell nanohybrids which have been prepared via precipitation are CdS/ZnS, CdS/PbS, CdS/CdSe, ZnS, CdSe etc. [89,90,91,92].
9.2 Synthesis of Organic Nanoparticles
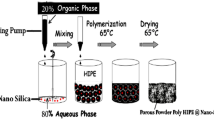
Most of the organic nanoparticles come under the class of polymers. Therefore, the technique most commonly used for the synthesis of organic core or shell is the addition or condensation polymerisation. There are various techniques by which polymerisation can be done- bulk, solution, suspension and emulsion polymerization. Generally, the synthesis of organic core is carried out by emulsion polymerisation and then it is used as a template for the preparation of core shell nanostructure [93, 94]. The development of polymeric shell on either the organic or inorganic material is usually done insitu. For the enhancement of shell coating the surface of core is commonly modified with a polyelectrolye in case of an organic core or via a surfactant for the inorganic core. After surface modification is achieved, polymerisation proceeds on the surface of the core by the technique of solution or bulk polymerisation [95]. A variety of nanohybrids have been synthesised where polymeric nanoparticle is playing the role of either core or shell or both. For instance, Chunlei Wang et al. prepared Fe3O4@CaCO3@PMMA core shell nanohybrid by insitu emulsion polymerisation on the surface of oleic acid altered Fe3O4@CaCO3 nanoparticles. The synthetic scheme is shown in Fig. 13 [96]. The advantage gained by having a polymeric coating of hydrophilic nature around an inorganic core is an enhancement in the biocompatibility of the core.
Fe3O4@CaCO3@PMMA core shell nanohybrid prepared by insitu emulsion polymerisation on the surface of oleic acid altered Fe3O4@CaCO3 nanoparticles [OA: Oleic Acid, SDBS: Sodium dodecylbenzene sulfonate, MMA: Methyl methacrylate, PMMA: Poly Methyl methacrylate] (Reprinted with permission from Ref. [97])
9.3 General Characterisation of Core Shell Nanohybrid
After the successful synthesis of core shell nanohybrid, the next task is to analyse it with respect to size, shape, particle size distribution, morphology and structural composition. Dynamic light scattering (DLS) which is also known by the name of photon correlation spectroscopy or quasi-elastic light scattering is used for the measurement of size of particles. The technique works by measuring the Brownian motion and correlate it with the size of particle. The bigger the size of particle, the slower will be its Brownian motion. The diameter that is obtained by DLS is actually hydrodynamic diameter because it comes from the way in which a particle diffuses in a fluid. Also, the diameter acquired refers to the diameter of a sphere having identical translational diffusion coefficient to that of the particle. Ionic strength of the medium, alternation in surface structure and degree of non-sphericity in particle have a significant influence on the result of DLS measurements. Further, temperature should be kept stable, otherwise convection currents will result in having a non-random movement in fluid leading to erroneous results. For the determination of shape as well as surface morphology, scanning electron microscopy (SEM) is employed. It is usually done in conjunction with energy dispersive spectrometry (EDS) in order to have a better picture of structural composition. Whereas if one wants to know information about inner structure, transmission electron microscopy (TEM) proves to be very useful. Atomic force microscopy (AFM) is beneficial for checking the uniformity of shell coating onto the core. In order to study the surface functionalisation, Fourier transform infrared (FTIR) spectroscopy in different modes- transmittance, reflectance and attenuated total reflectance (ATR) as well as Raman spectroscopy is widely used. For the identification of crystal structure, X-Ray diffractomers are utilized. If the core shell nanohybrid is to be used for water decontamination or for any other application relying on surface, determination of surface area becomes very much crucial. Brunauer–Emmett–Teller (BET) isotherm is usually used by researchers for finding the surface area of a nanosorbent. Zeta potential is an important parameter which determines the stability of nanohybrids and its variation with pH aids in the mechanistic understanding of sorption. The measurements are done with the help of a zetasizer. For characterising the composition, numerous techniques such as FTIR, X-Ray fluorescence, inductively coupled plasma atomic emission spectrometry, inductively coupled plasma mass spectrometry, flame and graphite furnace atomic absorption spectrometry are available.
9.4 Application of Core–Shell Nanohybrids for Water Treatment
Water contains numerous kinds of pollutants ranging from organic dyes, heavy elements, radionuclides, nanoparticles, pharmaceuticals, pesticides etc. Here, we have discussed the role of nanohybrids in the treatment of water.
9.5 Removal of Conventional Pollutants
Heavy metals are naturally present in earth but anthropogenic activities have caused tremendous increase in their concentration thus posing a major risk on the health of plants, animals and humans. Typical conventional pollutants like As, F, Cr, Cu, Hg, Ni etc. can enter the human body through drinking water causing serious health effects [97, 98]. Several regions of the world are already facing higher concentrations of arsenic and fluoride in groundwater. Various core shell nanohybrids have been reported for the removal of conventional pollutants. Abdel Halim A. Saad et al. fabricated ZnO/Chitosan core shell nanocomposite and used it for the removal of Cu2+, Cd2+ and Pb2+ [99]. The reusability of the material was found to be high. Meng Zhang prepared Ni/Mg(OH)2 and used it for the adsorption of Zn2+, Cu2+ and Cd2+ ions. Magnesium hydroxide is an ideal sorbent for heavy metal ions and has been already used for the removal of nickel, lead, cadmium, zinc, copper and cobalt [11, 100, 101]. But the problem associated with it is its poor recyclability. In view of this, the researchers synthesised Ni/Mg(OH)2 core shell nanostructure as it can be easily removed from the media by application of external magnetic field [102]. Apart from this the shell acts as a protective layer and aids in preventing the oxidation of Ni to NiO, thereby enabling it to retain its magnetism. Further, removal efficiency of all the metals was found to be close to 100% and remained 95% even after 5 cycles. The recycling strategy is shown as a schematic in Fig. 14.
The recycling strategy of Ni@Mg(OH)2 water treatment agent (Reprinted with permission from Ref. [103])
Another example of a nanohybrid where the shell leads to enhancement in the adsorptive capacity is a core double shell structure prepared by KairuZhe where in the core is made up of magnetic Fe3O4 nanoparticles having an inner polydopamine shell and an outer shell made up of zeoliticidazolate frameworks-8 (MP@ZIF-8). The adsorption capacity of MP@ZIF-8 was found to be 136.56 mg g−1 for Cr(VI) which is greater as compared to MP (92.27 mg g−1). Further from XPS studies it was found that Cr(VI) was partially reduced to Cr(III) during the adsorption process. The authors attributed this to the presence of nitrogen atom groups on polydopamine and ZIF8 which are acting as reducing agents [103]. In another study, core shell silica coated Fe3O4 functionalised with diglycolic acid (FGA-1) was found to be a better adsorbent for the removal of Pb(II) and Cr(VI) ions [104]. The magnetic core has the advantage of ease of isolation by the application of external magnetic field. Further, the presence of different functional groups around the core protects it against agglomeration and also enhances its selectivity. Different sort of chemical modification of core can be done in order to make it selective for the capture of desired metal ions. As the prepared nanosorbent (FGA-1) bears terminal acidic moieties and since it is known that Pb(II) and Cr(VI) ions exhibit excellent binding towards acidic functionalities, they are chosen for carrying out further uptake measurements. From the zeta potential studies, it was found that the surface of nanosorbent is negatively charged at neutral pH, which was attributed to the ionisation of carboxylic acid. As a result, adsorption of Pb(II) is preferred over Cr(VI) ions. However, at lower pH the uptake of Cr(VI) was found to be higher, which the authors thought might be due to the protonation of sorbent. The adsorption capacity for both the ions was found to be high under optimised conditions with reasonably good recyclability upto 5 cycles.
Numerous core–shell nanohybrids have been reported in the literature highlighting their immense significance in the adsorption of a wide range of ions (Zn(II), Cd(II), Hg(II), Pb(II), Cu((II), Cr(VI), Cr(III), Ni(II), Co(II), Co(III), As(VI), As(III) etc.), a comprehensive list of which is outlined in Table 1 [99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141]. Though actinides are too heavy elements, they are dealt in a separate subsection which is exclusively dedicated to role of core shell nanohybrids in radionuclide removal. The table also enlists the sorption capacities of different sorbents for the sake of comparison. But it should be noted that the maximum adsorption capacity reported necessarily need not be at room temperature and the working pH is different for different nanohybrids.
9.6 Radionuclide Removal
Water resources can get contaminated by radioactive materials. It may occur due to nuclear weapon testing, nuclear accidents, leakage from nuclear power and reprocessing plants. The devastating earthquake and tsunami that struck Japan has caused drastic damage to the Fukushima Nuclear Power Plant resulting in the release of tons of water contaminated with radionuclides into the ocean in March, 2011. Other than this, various steps involved in oil extraction also poses threat to the radioactive contamination of water. As hospitals also use numerous isotopes for diagnostic and therapeutic purposes; improper disposal of hospital waste also contributes to the generation of radioactive waste into water. The challenge to meet the energy demand is rising throughout the globe and nuclear power industry is more likely to grow in the coming years, with the risk of generating more and more radioactive waste into water bodies. In view of this, researchers have synthesised various core–shell nanohybrids for the removal of radionuclides [144].
For instance, Yan Liu et al. prepared FeS@Fe3O4 core shell nanohybrid and utilised it for the adsorption of uranyl ions [148]. The synthesis was carried out via ultrasonic assisted strategy. The nanohybrid proved to be a better adsorbent as compared to FeS and Fe3O4 alone. This is supported by their adsorption capacities. For FeS@Fe3O4, the adsorption capacity came out to be 229.03 mg g−1 which is considerably higher than FeS (211.0 mg g−1) and Fe3O4 (154.0 mg g−1). This happens because bare FeS can get easily aggregated, leading to reduction in its sorption capacity. Once it gets encapsulated by Fe3O4 layer, it becomes more stable and dispersible; thereby enhancing its adsorption capacity. The results from surface area measurement are also in agreement with this. The surface area of hybrid material is 82.47 m2g−1 which is significantly larger than that of FeS. Another example highlighting the immense importance of core shell nanohybrid material is the synthesis of silica coated Fe3O4 functionalised with amidoxime. The magnetic core was selected for facilitating the separation of nano-sorbent [150]. But since, the magnetic nanoparticles are susceptible towards oxidation and agglomeration, surface modifications are carried out in order to overcome these limitations. In view of this, the researchers coated the surface of magnetite nanoparticles with silica because of its high stability and ability to functionalise it with different kinds of groups due to the presence of hydroxyl groups on the surface. Finally the core shell material, Fe3O4@SiO2 was functionalised with amidoxime to prepare Fe3O4@SiO2-AO and various studies pertaining to adsorption of uranyl ions were conducted. From the speciation diagram and pH studies, it was proposed that oxime groups and amino nitrogen are responsible for binding with uranyl ion. A consolidated list of various core–shell materials developed for radionuclides removal from water have been listed in Table 2 [143,144,145,146,147,148,149,150,151,152,153,154,155].
9.7 Removal of Dyes
Water pollution because of dyeing industry is a matter of grave concern since a huge amount of it is released into water bodies every year. As the solubility of dyes in water is high, it becomes really difficult to eradicate them by conventional means. The presence of dyes in water leads to a reduction in the penetration of light into water bodies, thereby causing a decrease in the photosynthesis rate and hence lowering the dissolved oxygen levels. All this adversely affects the life of entire aquatic organisms. The dyes are also reported to have mutagenic and carcinogenic effects and once they enter the food chain can readily undergo biomagnification and thus species at higher trophic level are more likely to get affected. Thus, it becomes very necessary to find measures to deal with dye pollution. Among various ways, adsorption of dyes by nanomaterials can prove to be a promising approach. In particular, core shell naonybrids have emerged as recent adsorbents for tackling dye pollution. Various core–shell nanohybrids have been used for the adsorption of diverse kinds of dyes ranging from acidic to basic. Zikang Xiong et al. synthesised Fe3O4@Carbon@ZIF-8 core shell nanomaterial and used it for the adsorption of Congo red dye [133]. The adsorption capacity of the prepared nanosorbent was found to be very high (806.45 mg g-1). For the easy removal of adsorbents, magnetic cores are commonly preferred and are protected using a shell. The researchers chose amorphous carbon as the inner shell as it is easier to synthesise and more environmentally benign as compared to silica or other polymeric shells. Apart from this, it also plays the role of linker as well as stabilizer between magnetic core and outer shell layer. Metal Organic frameworks [Zeolitic Imidazolate Framework-8 (ZIF-8)] were selected as the outer shell material because of its high surface area, availability of numerous unsaturation sites, tuneable pore size and outstanding chemical stability. Further ZIF-8 was particularly chosen because of its high selectivity and superior water stability. To gain better insight into mechanistic aspects, FTIR and XPS studies are carried out after and before adsorption. It was concluded that electrostatic interaction, pi-pi bonding and hydrogen bonding are the driving forces behind the adsorption of congo red dye. Finally selectivity of the adsorbent was examined in the presence of other anionic and cationic dyes and it was revealed that Fe3O4@Carbon@ZIF-8 can be successfully used for the selective adsorption of Congo Red in complex wastewater system. Similarly, Fe3O4@lignosulfonate/phenolic core–shell microspheres has been used for the adsorption of methylene blue [164]. The nanohybrid material can be separated easily on account of the presence of magnetic core and functional groups of lignosulfonate (shell) are responsible for high adsorption capacity [165]. A list of different core shell nanohybrid materials those have been used for the adsorption of different dyes with their sorption capacities is summarized in Table 3 [165,166,167,168,169,170,171,172,173,174,175,176,177].
9.8 Removal of Organic Pollutants
Organic pollutants comprise of pharmaceuticals, pesticides, insecticides, organic solvents and are harmful for human beings because of the concentration in which they are found in aquatic medium. Pharmaceutical pollution also knows as drug pollution occurs when drugs or their metabolites reach the water bodies. This leads to various adverse consequences. To mention a few, it has been revealed from numerous studies that estrogen and chemicals which behave similar to it result in a feminizing effect on fishes thus causing an increase in the population of intersex and female fishes. Apart from this, various commonly used antidepressants were found to be accumulated in the tissues of brain in fishes. Further, as antibiotics are largely used for the treatment of variety of infections, their presence in sewage treatment systems can lead to the inhibition of sewage bacteria, thereby deteriorating the decomposition of organic matter. Some studies also indicate that presence of antibiotics in water has given rise to antibiotic resistance. Besides this, pesticides also poses threats to environment due to their presence in drinking water in significant concentration. Organic solvents are widely used in almost all industries and are too a major contributor in water pollution. A variety of methods are available for the removal of organic pollutants, the particular method being chosen depends upon the nature of pollutant [178,179,180]. Core–Shell nanohybrids also provide a means of getting rid of these organic pollutants from water bodies. Most of the core shell nanohybrids either employ adsorption or photocatalytic degradation for removing the pollutant a list of which is given in Table 4 [178,179,180,181,182,183,184,185,186,187,188] and. As already discussed, many times, most of the core–shell nanosorbents synthesised use magnetite as a core for its facile recovery. The shell material and its further functionalisation is carried out on the basis of nature of pollutant to be removed.
9.9 Conclusion
In this book chapter, various core shell nanohybrid structureshaving application in water decontamination have been discussed. Core shell nanohybrid structuresare obtained through self-assembling techniques, sol–gel method, using polymers for dispersion of building blocks. While major percentage of these nanohybrid structuresare reported for conventional pollutants removal (mainly inorganic, organic pollutants);nanohybrid structures are comparatively less for removal of radionuclides, nanoparticles. Many core shell nanohybrid structures discussed here are potential alternate to conventional water treatment processes due to their advance properties, environment friendliness and cost effectiveness. Most nanostructures, however, are in nascent stage for industrial and large-scale applications due to technical challenges in scaling up, reusability and sometimes owing to lack of stability under ambient environment.
References
Roduner E (2006) Size matters: why nanomaterials are different. Chem Soc Rev 35(7):583–592
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl Catal B 176:396–428
Kim J, Lee HC, Kim KH, Hwang MS, Park JS, Lee JM ... Park HG (2017) Photon-triggered nanowire transistors. Nat Nanotechnol 12(10):963–968
Liu Z, Lin CH, Hyun BR, Sher CW, Lv Z, Luo B ... He JH (2020) Micro-light-emitting diodes with quantum dots in display technology. Light: Sci Appl 9(1):1–23
Jassby D, Cath TY, Buisson H (2018) The role of nanotechnology in industrial water treatment. Nat Nanotechnol 13(8):670–672
Gawande MB, Goswami A, Asefa T, Guo H, Biradar AV, Peng DL ... Varma RS (2015) Core–shell nanoparticles: synthesis and applications in catalysis and electrocatalysis. Chem Soc Rev 44(21):7540–7590
Ghosh Chaudhuri R, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112(4):2373–2433
Kwizera EA, Chaffin E, Shen X, Chen J, Zou Q, Wu Z ... Huang X (2016) Size-and shape-controlled synthesis and properties of magnetic–plasmonic core–shell nanoparticles. J Phys Chem C 120(19):10530–10546
Radi A, Pradhan D, Sohn Y, Leung KT (2010) Nanoscale shape and size control of cubic, cuboctahedral, and octahedral Cu− Cu2O core− shell nanoparticles on Si (100) by one-step, templateless, capping-agent-free electrodeposition. ACS Nano 4(3):1553–1560
Guo P, Sikdar D, Huang X, Si KJ, Xiong W, Gong S, ... Cheng W (2015) Plasmonic core–shell nanoparticles for SERS detection of the pesticide thiram: size-and shape-dependent Raman enhancement. Nanoscale 7(7):2862–2868
Basu H, Saha S, Kailasa SK, Singhal RK (2020) Present status of hybrid materials for potable water decontamination: a review. Environ Sci: Water Res Technol 6(12):3214–3248
Singh R, Bhateria R (2021) Core–shell nanostructures: A simplest two-component system with enhanced properties and multiple applications. Environ Geochem Health 43(7):2459–2482
Leng Z, Li L, Zhang D, Li G (2018) Tunable green/red dual-mode luminescence via energy management in core-multishell nanoparticles. Mater Des 152:119–128
Lei L, Dai X, Cheng Y, Wang Y, Xiao Z, Xu S (2019) Dual-mode color tuning based on upconversion core/triple-shell nanostructure. J Mater Chem C 7(11):3342–3350
Dhanalakshimi T, Devi MS (2020) Water pollution. A primer on earth pollution: pollution types and disposal 97
WHO (2006) Guidelines for drinking-water quality [Electronic Resource]: Incorporating First Addendum (vol. I) Recommendations, pp 375–377, 448–1456
WHO (1993) Guidelines for drinking water quality recommendations. Geneva
Yakamercan E, Ari A, Aygün A (2021) Land application of municipal sewage sludge: human health risk assessment of heavy metals. J Clean Prod 319:128568
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning in the modern world-new tricks for an old dog 10
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17(1):145–155
Rathi BS, Kumar PS (2021) Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ Pollut 280:116995
Suárez-Iglesias O, Collado S, Oulego P, Díaz M (2017) Graphene-family nanomaterials in wastewater treatment plants. Chem Eng J 313:121–135
Peng B, Yao Z, Wang X, Crombeen M, Sweeney DG, Tam KC (2020) Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ 5(1):37–49
Yu S, Wang X, Pang H, Zhang R, Song W, Fu D ... Wang X (2018) Boron nitride-based materials for the removal of pollutants from aqueous solutions: a review. Chem Eng J 333, 343–360
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375
Wan X, Zhan Y, Long Z, Zeng G, He Y (2017) Core@ double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem Eng J 330:491–504
Alijani H, Shariatinia Z (2018) Synthesis of high growth rate SWCNTs and their magnetite cobalt sulfidenanohybrid as super-adsorbent for mercury removal. Chem Eng Res Des 129:132–149
Meaney SP, Follink B, Tabor RF (2018) Synthesis, characterization, and applications of polymer-silica core–shell microparticle capsules. ACS Appl Mater Interf 10(49):43068–43079
Guo X, Liu X, Xu B, Dou T (2009) Synthesis and characterization of carbon sphere-silica core–shell structure and hollow silica spheres. Colloids Surf, A 345(1–3):141–146
Tang F, Liu L, Alva G, Jia Y, Fang G (2017) Synthesis and properties of microencapsulated octadecane with silica shell as shape–stabilized thermal energy storage materials. Sol Energy Mater Sol Cells 160:1–6
Rao KS, El-Hami K, Kodaki T, Matsushige K, Makino K (2005) A novel method for synthesis of silica nanoparticles. J Colloid Interf Sci 289(1):125–131
Khanal A, Inoue Y, Yada M, Nakashima K (2007) Synthesis of silica hollow nanoparticles templated by polymeric micelle with core− shell− corona structure. J Am Chem Soc 129(6):1534–1535
Deng TS, Zhang QF, Zhang JY, Shen X, Zhu KT, Wu JL (2009) One-step synthesis of highly monodisperse hybrid silica spheres in aqueous solution. J Colloid Interf Sci 329(2):292–299
Basu H, Saha S, Mahadevan IA, Pimple MV, Singhal RK (2019) Humic acid coated cellulose derived from rice husk: a novel biosorbent for the removal of Ni and Cr. J Water Process Eng 32:100892
Decker S, Klabunde KJ (1996) Enhancing effect of Fe2O3 on the ability of nanocrystalline calcium oxide to adsorb SO2. J Am Chem Soc 118(49):12465–12466
Basu H, Saha S, Pimple MV, Singhal RK (2019) Novel hybrid material humic acid impregnated magnetic chitosan nano particles for decontamination of uranium from aquatic environment. J Environ Chem Eng 7(3):103110
Basu H, Pimple MV, Saha S, Patel A, Dansena C, Singhal RK (2020) TiO2 microsphere impregnated alginate: a novel hybrid sorbent for uranium removal from aquatic bodies. New J Chem 44(10):3950–3960
Nayak MK, Singh J, Singh B, Soni S, Pandey VS, Tyagi S (2017) Introduction to semiconductor nanomaterial and its optical and electronics properties. In Metal semiconductor core-shell nanostructures for energy and environmental applications, pp 1–33. Elsevier
Chiozzi V, Rossi F (2020) Inorganic–organic core/shell nanoparticles: progress and applications. Nanoscale Adv 2(11):5090–5105
Zhou Z, Liu R (2017) Fe3O4@ polydopamine and derived Fe3O4@ carbon core–shell nanoparticles: comparison in adsorption for cationic and anionic dyes. Colloids Surf, A 522:260–265
Gutierrez AM, Bhandari R, Weng J, Stromberg A, Dziubla TD, Hilt JZ (2019) Novel magnetic core-shell nanoparticles for the removal of polychlorinated biphenyls from contaminated water sources. Mater Chem Phys 223:68–74
Karsten S, Nan A, Turcu R, Liebscher J (2012) A new access to polypyrrole-based functionalized magnetic core-shell nanoparticles. J Polym Sci, Part A: Polym Chem 50(19):3986–3995
Xian Y, Hu Y, Liu F, Xian Y, Wang H, Jin L (2006) Glucose biosensor based on Au nanoparticles–conductive polyaniline nanocomposite. Biosens Bioelectron 21(10):1996–2000
Yan W, Feng X, Chen X, Li X, Zhu JJ (2008) A selective dopamine biosensor based on AgCl@ polyaniline core–shell nanocomposites. Bioelectrochemistry 72(1):21–27
Nakate UT, Ahmad R, Patil P, Bhat KS, Wang Y, Mahmoudi T, … Hahn YB (2019) High response and low concentration hydrogen gas sensing properties using hollow ZnO particles transformed from polystyrene@ ZnO core-shell structures. Int J Hydrogen Energy 44(29):15677–15688
Zhao Y, Chen Z, Zhu X, Möller M (2016) A facile one-step approach toward polymer@ SiO2 core–shell nanoparticles via a surfactant-free miniemulsion polymerization technique. Macromolecules 49(5):1552–1562
Basu H, Singhal RK, Pimple MV (2016) Highly efficient removal of TiO2 nanoparticles from aquatic bodies by silica microsphere impregnated Ca-alginate. New J Chem 40:3177–3186
Basu H, Singhal RK, Pimple MV, Reddy AVR (2015) Synthesis and characterization of silica microsphere and their application in removal of Uranium and Thorium from water. Int J Environ Sci Technol 12(6):1899–1906
Basu H, Singhal RK, Pimple MV, Saha S (2018) Graphene-prussian blue nanocomposite impregnated in alginate for efficient removal of cesium from aquatic environment. J Environ Chem Eng 6(4):4399-4407
Silva DM, Liu R, Gonçalves AF, da Costa A, Gomes AC, Machado R ... Sencadas V (2021) Design of polymeric core-shell carriers for combination therapies. J Colloid Interf Sci 587:499–509
Rodríguez-González B, Burrows A, Watanabe M, Kiely CJ, Marzán LML (2005) Multishell bimetallic AuAg nanoparticles: synthesis, structure and optical properties. J Mater Chem 15(17):1755–1759
Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP (2001) Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J Phys Chem B 105(37):8861–8871
Liu R, Qu F, Guo Y, Yao N, Priestley RD (2014) Au@ carbon yolk–shell nanostructures via one-step core–shell–shell template. Chem Commun 50(4):478–480
Arole VM, Munde SV (2014) Fabrication of nanomaterials by top-down and bottom-up approaches-an overview. J Mater Sci 1:89–93
Indiarto R, Indriana LPA, Andoyo R, Subroto E, Nurhadi B (2021) Bottom–up nanoparticle synthesis: a review of techniques, polyphenol-based core materials, and their properties. Eur Food Res Technol 1–24
Ealia SAM, Saravanakumar MP (2017, November). A review on the classification, characterisation, synthesis of nanoparticles and their application. In IOP conference series: materials science and engineering, Vol 263, No 3, p 032019. IOP Publishing
Mehta VN, Desai ML, Basu H, Singhal RK, Kailasa SK (2021) Recent developments on fluorescent hybrid nanomaterials for metal ions sensing and bioimaging applications: a review. J Mol Liq 333:115950
Basu H, Singhal RK, Pimple MV, Saha S (2018) Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J Environ Chem Eng 6(2):1625–1633
Basu H, Singhal RK, Pimple MV, Kumar A, Reddy AVR (2014) Association and migration of uranium and thorium with silica colloidal particles in saturated subsurface zone. J Radio Anal Nucl Chem 303:2283–2290
Malik MA, O’Brien P, Revaprasadu N (2002) A simple route to the synthesis of core/shell nanoparticles of chalcogenides. Chem Mater 14(5):2004–2010
Ban Z, Barnakov YA, Li F, Golub VO, O’Connor CJ (2005) The synthesis of core–shell iron@ gold nanoparticles and their characterization. J Mater Chem 15(43):4660–4662
Khatami M, Alijani HQ, Nejad MS, Varma RS (2018) Core@ shell nanoparticles: greener synthesis using natural plant products. Appl Sci 8(3):411
Khan A, Rashid A, Younas R, Chong R (2016) A chemical reduction approach to the synthesis of copper nanoparticles. Int Nano Lett 6(1):21–26
Guzmán MG, Dille J, Godet S (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int J Chem Biomol Eng 2(3):104–111
Jana NR, Gearheart L, Murphy CJ (2001) Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mater 13(7):2313–2322
Kailasa SK, Koduru JR, Desai ML, Park TJ, Singhal RK, Basu H (2018) Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological sample., Trends Anal Chem 105:106–120
Basu H, Singhal RK, Pimple MV, Saha S (2017) Association and migration behavior of trace metals with humus colloidal particles in aquatic subsurface medium. J Radio Anal Nucl Chem 311(1): 503–551
Lee WR, Kim MG, Choi JR, Park JI, Ko SJ, Oh SJ, Cheon J (2005) Redox− transmetalation process as a generalized synthetic strategy for core− shell magnetic nanoparticles. J Am Chem Soc 127(46):16090–16097
Chen D, Liu S, Li J, Zhao N, Shi C, Du X, Sheng J (2009) Nanometre Ni and core/shell Ni/Au nanoparticles with controllable dimensions synthesized in reverse microemulsion. J Alloy Compd 475(1–2):494–500
Park JI, Cheon J (2001) Synthesis of “solid solution” and “core-shell” type cobalt− platinum magnetic nanoparticles via transmetalation reactions. J Am Chem Soc 123(24):5743–5746
Ji Y, Yang S, Guo S, Song X, Ding B, Yang Z (2010) Bimetallic Ag/Au nanoparticles: a low temperature ripening strategy in aqueous solution. Colloids Surf, A 372(1–3):204–209
Lee CC, Chen DH (2006) Large-scale synthesis of Ni–Ag core–shell nanoparticles with magnetic, optical and anti-oxidation properties. Nanotechnology 17(13):3094
Singhal RK, Gangadhar B, Basu H, Venkatesh M, Naidu GRK, Reddy AVR (2012) Remediation of malathion contaminated soil using zero valent iron nano-particles. Am J Anal Chem 3:76–82
Hess PH, Parker PH Jr (1966) Polymers for stabilization of colloidal cobalt particles. J Appl Polym Sci 10(12):1915–1927
Salavati-Niasari M, Davar F, Mazaheri M, Shaterian M (2008) Preparation of cobalt nanoparticles from [bis (salicylidene) cobalt (II)]–oleylamine complex by thermal decomposition. J Magn Magn Mater 320(3–4):575–578
Salavati-Niasari M, Davar F, Mir N (2008) Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron 27(17):3514–3518
Wang L, Wang L, Luo J, Fan Q, Suzuki M, Suzuki IS ...Zhong CJ (2005) Monodispersed core− shell Fe3O4@ Au nanoparticles. J Phys Chem B 109(46):21593–21601
De Jesus JC, González I, Quevedo A, Puerta T (2005) Thermal decomposition of nickel acetate tetrahydrate: an integrated study by TGA, QMS and XPS techniques. J Mol Catal A: Chem 228(1–2):283–291
Fu Y, Shearwood C (2004) Characterization of nanocrystallineTiNi powder. Scripta Mater 50(3):319–323
Wang Q, Yang H, Shi J, Zou G (2001) Preparation and characterization of nanocrystalline powders of Cu–Zn alloy by wire electrical explosion method. Mater Sci Eng, A 307(1–2):190–194
Basu H, Singh S, Venkatesh M, Pimple MV, Singhal RK (2021) Graphene oxide-MnO2-goethite microsphere impregnated alginate: a novel hybrid nanosorbent for As (III) and As (V) removal from groundwater. J Water Process Eng 42:102129
Basu H, Singhal RK, Pimple MV, Manisha V, Bassan MKT, Reddy AVR, Mukherjee T (2011) Development of naturally occurring siliceous material for the preferential removal of thorium from U-Th from aquatic environment. J Radio Anal Nucl Chem 289:231–237
Parashar M, Shukla VK, Singh R (2020) Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J Mater Sci: Mater Electron 31(5):3729–3749
Petcharoen K, Sirivat A (2012) Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater Sci Eng, B 177(5):421–427
Kim YI, Kim D, Lee CS (2003) Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Physica B 337(1–4):42–51
He Q, Zhang Z, Xiong J, Xiong Y, Xiao H (2008) A novel biomaterial—Fe3O4: TiO2 core-shell nano particle with magnetic performance and high visible light photocatalytic activity. Opt Mater 31(2):380–384
Palacios-Hernández T, Hirata-Flores GA, Contreras-López OE, Mendoza-Sánchez ME, Valeriano-Arreola I, González-Vergara E, Méndez-Rojas MA (2012) Synthesis of Cu and Co metal oxide nanoparticles from thermal decomposition of tartrate complexes. Inorganica Chimica Acta 392:277–282
Sun S, Zeng H (2002) Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc 124(28):8204–8205
Salavati-Niasari M, Davar F, Mazaheri M (2008) Preparation of ZnO nanoparticles from [bis (acetylacetonato) zinc (II)]–oleylamine complex by thermal decomposition. Mater Lett 62(12–13):1890–1892
Tian Y, Newton T, Kotov NA, Guldi DM, Fendler JH (1996) Coupled composite CdS− CdSe and core− shell types of (CdS) CdSe and (CdSe) CdS nanoparticles. J Phys Chem 100(21):8927–8939
Korgel BA, Monbouquette HG (2000) Controlled synthesis of mixed core and layered (Zn, Cd) S and (Hg, Cd) S nanocrystals within phosphatidylcholine vesicles. Langmuir 16(8):3588–3594
Zhou HS, Honma I, Komiyama H, Haus JW (1993) Coated semiconductor nanoparticles; the cadmium sulfide/lead sulfide system’s synthesis and properties. J Phys Chem 97(4):895–901
Kortan AR, Hull R, Opila RL, Bawendi MG, Steigerwald ML, Carroll PJ, Brus LE (1990) Nucleation and growth of CdSe on ZnS quantum crystallite seeds, and vice versa, in inverse micelle media. J Am Chem Soc 112(4):1327–1332
Xie XL, Li RKY, Liu QX, Mai YW (2004) Structure-property relationships of in-situ PMMA modified nano-sized antimony trioxide filled poly (vinyl chloride) nanocomposites. Polymer 45(8):2793–2802
Ballauff M, Lu Y (2007) “Smart” nanoparticles: preparation, characterization and applications. Polymer 48(7):1815–1823
Singh S, Basu H, Bassan MKT, Singhal RK (2022) Thiol functionalised silica microsphere loaded polymeric hydrogel: development of a novel hybrid sorbent for removal of lead and cadmium. Chemosphere 286:131659
Wang C, Yan J, Cui X, Cong D, Wang H (2010) Preparation and characterization of magnetic hollow PMMA nanospheres via in situ emulsion polymerization. Colloids Surf, A 363(1–3):71–77
Mohod CV, Dhote J (2013) Review of heavy metals in drinking water and their effect on human health. Int J Innov Res Sci, Eng Technol 2(7):2992–2996
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 30(3):167–176
Saad AHA, Azzam AM, El-Wakeel ST, Mostafa BB, Abd El-latif MB (2018) Removal of toxic metal ions from wastewater using ZnO@ Chitosan core-shell nanocomposite. Environ Nanotechnol Monitor Manag 9:67–75
Mehta VN, Desai ML, Basu H, Singhal RK, Kailasa SK (2021) Recent developments on fluorescent hybrid nanomaterials for metal ions sensing and bioimaging applications: a review. J Mol Liq 333:115950
Basu H, Singhal RK, Pimple MV, Reddy AVR (2015) Arsenic removal from ground water by goethite impregnated calcium alginate beads. Water, Air, Soil Pollut 226:22
Zhang M, Song W, Chen Q, Miao B, He W (2015) One-pot synthesis of magnetic Ni@ Mg (OH) 2 core–shell nanocomposites as a recyclable removal agent for heavy metals. ACS Appl Mater Interf 7(3):1533–1540
Zhu K, Chen C, Xu H, Gao Y, Tan X, Alsaedi A, Hayat T (2017) Cr (VI) reduction and immobilization by core-double-shell structured magnetic polydopamine@ zeoliticidazolate frameworks-8 microspheres. ACS Sustain Chem Eng 5(8):6795–6802
Nawaz T, Zulfiqar S, Sarwar MI, Iqbal M (2020) Synthesis of diglycolic acid functionalized core-shell silica coated Fe 3 O 4 nanomaterials for magnetic extraction of Pb (II) and Cr (VI) ions. Sci Rep 10(1):1–13
Liu L, Liu S, Zhao L, Su G, Liu X, Peng H, ... Tang A (2020) Fabrication of novel magnetic core-shell chelating adsorbent for rapid and highly efficient adsorption of heavy metal ions from aqueous solution. J Mol Liq 313:113593
Ma Z, Zhao D, Chang Y, Xing S, Wu Y, Gao Y (2013) Synthesis of MnFe2O4@ Mn–Co oxide core–shell nanoparticles and their excellent performance for heavy metal removal. Dalton Trans 42(39):14261–14267
Amiri O, Hosseinpour-Mashkani SM, Rad MM, Abdvali F (2014) Sonochemical synthesis and characterization of CdS/ZnS core–shell nanoparticles and application in removal of heavy metals from aqueous solution. Superlattices Microstruct 66:67–75
Yang W, Wang Y, Wang Q, Wu J, Duan G, Xu W, Jian S (2021) Magnetically separable and recyclable Fe3O4@ PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 189:110229
Jahanbakhsh Z, Hosseinzadeh H, Masoumi B (2021) Synthesis of carboxymethyl β-cyclodextrin bonded Fe3O4@ SiO2–NH2 core-shell magnetic nanocomposite adsorbent for effective removal of Pb (II) from wastewater. J Sol-Gel Sci Technol 1–13
Jiang X, Su S, Rao J, Li S, Lei T, Bai H, ... Yang X (2021) Magnetic metal-organic framework (Fe3O4@ ZIF-8) core-shell composite for the efficient removal of Pb (II) and Cu (II) from water. J Environ Chem Eng 9(5):105959
Bhardwaj S, Sarkar T (2020) Core–shell type magnetic Ni/NiO nanoparticles as recyclable adsorbent for Pb (II) and Cd (II) ions: one-pot synthesis, adsorption performance, and mechanism. J Taiwan Inst Chem Eng 113:223–230
Patiño-Ruiz D, Rehmann L, Mehrvar M, Quiñones-Bolaños E, Herrera A (2020) Synthesis of FeO@ SiO 2–DNA core–shell engineered nanostructures for rapid adsorption of heavy metals in aqueous solutions. RSC Adv 10(64):39284–39294
Zhao Z, Zhang X, Zhou H, Liu G, Kong M, Wang G (2017) Microwave-assisted synthesis of magnetic Fe3O4-mesoporous magnesium silicate core-shell composites for the removal of heavy metal ions. Microporous Mesoporous Mater 242:50–58
Ji S, Miao C, Liu H, Feng L, Yang X, Guo H (2018) A hydrothermal synthesis of Fe 3 O 4@ C hybrid nanoparticle and magnetic adsorptive performance to remove heavy metal ions in aqueous solution. Nanoscale Res Lett 13(1):1–10
Chen J, Liu K, Jiang M, Han J, Liu M, Wang C, Li C (2019) Controllable preparation of porous hollow carbon sphere@ ZIF-8: Novel core-shell nanomaterial for Pb2+ adsorption. Colloids Surf, A 568:461–469
Fathy M, Zayed MA, Moustafa YM (2019) Synthesis and applications of CaCO3/HPC core–shell composite subject to heavy metals adsorption processes. Heliyon 5(8):e02215
Venkateswarlu S, Yoon M (2015) Core–shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@ DAPF) for fast removal of Pb (II) from aqueous solutions. ACS Appl Mater Interf 7(45):25362–25372
Luo H, Zhang S, Li X, Liu X, Xu Q, Liu J, Wang Z (2017) Tannic acid modified Fe3O4 core–shell nanoparticles for adsorption of Pb2+ and Hg2+. J Taiwan Inst Chem Eng 72:163–170
Ren C, Ding X, Fu H, Li W, Wu H, Yang H (2017) Core–shell superparamagnetic monodisperse nanospheres based on amino-functionalized CoFe2O4@ SiO2 for removal of heavy metals from aqueous solutions. RSC Adv 7(12):6911–6921
Ashrafi A, Rahbar-Kelishami A, Shayesteh H (2017) Highly efficient simultaneous ultrasonic assisted adsorption of Pb (II) by Fe3O4@ MnO2 core-shell magnetic nanoparticles: synthesis and characterization, kinetic, equilibrium, and thermodynamic studies. J Mol Struct 1147:40–47
Gui CX, Li QJ, Lv LL, Qu J, Wang QQ, Hao SM, Yu ZZ (2015) Core–shell structured MgO@ mesoporous silica spheres for enhanced adsorption of methylene blue and lead ions. RSC Adv 5(26):20440–20445
Xiong T, Yuan X, Cao X, Wang H, Jiang L, Wu Z, Liu Y (2020) Mechanistic insights into heavy metals affinity in magnetic MnO2@ Fe3O4/poly (m-phenylenediamine) core− shell adsorbent. Ecotoxicol Environ Saf 192:110326
Wang S, Wang K, Dai C, Shi H, Li J (2015) Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem Eng J 262:897–903
Wan S, Yu C, Li Y, Lu Z, Wang Y, Wang Y, He F (2021) Highly selective and ultrafast removal of cadmium and copper from water by magnetic core-shell microsphere. Chem Eng J 405:126576
Abolhasani J, Khanmiri RH, Ghorbani-Kalhor E, Hassanpour A, Asgharinezhad AA, Shekari N, Fathi A (2015) An Fe3O4@ SiO2@ polypyrrole magnetic nanocomposite for the extraction and preconcentration of Cd (II) and Ni (II). Anal Methods 7(1):313–320
Sun L, Li Y, Sun M, Wang H, Xu S, Zhang C, Yang Q (2011) Porphyrin-functionalized Fe3O4@ SiO2 core/shell magnetic colorimetric material for detection, adsorption and removal of Hg2+ in aqueous solution. New J Chem 35(11):2697–2704
Zhu H, Shen Y, Wang Q, Chen K, Wang X, Zhang G ... Bai R (2017) Highly promoted removal of Hg (II) with magnetic CoFe2O4@ SiO2 core–shell nanoparticles modified by thiol groups. RSC Adv 7(62):39204–39215
Xia K, Guo Y, Shao Q, Zan Q, Bai R (2019) Removal of mercury (II) by EDTA-functionalized magnetic CoFe2O4@ SiO2 nanomaterial with core-shell structure. Nanomaterials 9(11):1532
Removal of Hg(II) by poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic nanoparticles Water Res 69C:252–260 (2014) https://doi.org/10.1016/j.watres.2014.11.030
Afraz A, Hajian A, Niknam Z, Mosayebi E, Yusefi A, Sillanpää M (2017) Amin-functionalized magnetic-silica core-shell nanoparticles for removal of Hg2+ from aqueous solution. J Dispers Sci Technol 38(5):750–756
Wu A, Jia J, Luan S (2011) Amphiphilic PMMA/PEI core–shell nanoparticles as polymeric adsorbents to remove heavy metal pollutants. Colloids Surf, A 384(1–3):180–185
Chang L, Pu Y, Jing P, Cui Y, Zhang G, Xu S ... Qiao C (2021). Magnetic core-shell MnFe2O4@ TiO2 nanoparticles decorated on reduced graphene oxide as a novel adsorbent for the removal of ciprofloxacin and Cu (II) from water. Appl Surf Sci 541:148400
Xiong Z, Zheng H, Hu Y, Hu X, Ding W, Ma J, Li Y (2021) Selective adsorption of Congo red and Cu (II) from complex wastewater by core-shell structured magnetic carbon@ zeoliticimidazolate frameworks‑8 nanocomposites. Separ Purif Technol 119053
Zuo B, Deng Q, Shao H, Cao B, Fan Y, Li W, Huang M (2021) Fe3O4@ Mesoporous-SiO2@ Chitosan@ polyaniline core-shell nanoparticles as recyclable adsorbents and reductants for hexavalent chromium. ACS Appl Nano Mater 4(2):1831–1840
Ekka B, Dhar G, Sahu S, Mishra M, Dash P, Patel RK (2021) Removal of Cr (VI) by silica-titania core-shell nanocomposites: in vivo toxicity assessment of the adsorbent by Drosophila melanogaster. Ceram Int 47(13):19079–19089
Zhang Y, Xu S, Zhou H, Qi H, Wang H (2021) Adsorption of organic pollutants and heavy metal by Co-doped core-shell MoO2/Mo2C adsorbent. J Solid State Chem 293:121801
Yang Q, Wang H, Li F, Dang Z, Zhang L (2021) Rapid and efficient removal of Cr (vi) by a core–shell magnetic mesoporous polydopamine nanocomposite: roles of the mesoporous structure and redox-active functional groups. J Mater Chem A 9(22):13306–13319
Wang T, Zhang L, Li C, Yang W, Song T, Tang C ... Luo J (2015) Synthesis of core–shell magnetic Fe3O4@ poly (m-phenylenediamine) particles for chromium reduction and adsorption. Environ Sci Technol 49(9):5654–5662
Facile synthesis of layered core-shell structure Fe3O4 magnetic composites and its application for the Co2+ removal. J Mol Liq 325:114517 (2020). https://doi.org/10.1016/j.molliq.2020.114517
Zhang X, Wang Y, Yang S (2014) Simultaneous removal of Co (II) and 1-naphthol by core–shell structured Fe3O4@ cyclodextrin magnetic nanoparticles. Carbohyd Polym 114:521–529
Huo JB, Xu L, Yang JCE, Cui HJ, Yuan B, Fu ML (2018) Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As (III) from water. Colloids Surf, A 539:59–68
Wu J, Zhu H, Liu G, Tan L, Hu X, Chen C ... Tan X (2017) Fabrication of core–shell CMNP@ PmPD nanocomposite for efficient As (V) adsorption and reduction. ACS Sustain Chem Eng 5(5):4399–4407
Singhal RK, Basu H, Manisha V, Reddy AVR, Mukherjee T (2011) Removal of low level americium-241 from potable water originated from different geochemical environments by calcium alginate. Desalination 280:313–318
Wang Z, Wang J, Zhu L, He Y, Duan T (2020) Scalable Fe@ FeO core-shell nanoparticle-embedded porous wood for high-efficiency uranium (VI) adsorption. Appl Surf Sci 508:144709
Dai Z, Zhang H, Sui Y, Ding D, Hu N, Li L, Wang Y (2018) Synthesis and characterization of a novel core–shell magnetic nanocomposite via surface-initiated RAFT polymerization for highly efficient and selective adsorption of uranium (VI). J Radio Anal Nucl Chem 316(1):369–382
Tan L, Zhang X, Liu Q, Wang J, Sun Y, Jing X ... Liu L (2015) Preparation of magnetic core–shell iron oxide@ silica@ nickel-ethylene glycol microspheres for highly efficient sorption of uranium (VI). Dalton Trans 44(15):6909–6917
Liu Q, Li W, Zhao W, Tan L, Jing X, Liu J ... Wang J (2016) Synthesis of ketoxime-functionalized Fe 3 O 4@ C core–shell magnetic microspheres for enhanced uranium (vi) removal. RSC Adv 6(26):22179–22186
Yang Y, Wang J, Wu F, Ye G, Yi R, Lu Y, Chen J (2016) Surface-initiated SET-LRP mediated by mussel-inspired polydopamine chemistry for controlled building of novel core–shell magnetic nanoparticles for highly-efficient uranium enrichment. Polym Chem 7(13):2427–2435
Liu Y, Yang P, Li Q, Liu Y, Yin J (2019) Preparation of FeS@ Fe3O4 core–shell magnetic nanoparticles and their application in uranyl ions removal from aqueous solution. J Radio Anal Nucl Chem 321(2):499–510
Li P, Wang J, Wang X, He B, Pan D, Liang J ... Fan Q (2018) Arsenazo-functionalized magnetic carbon composite for uranium (VI) removal from aqueous solution. J Mol Liq 269:441–449
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@ SiO2 core–shell magnetic microspheres for highly efficient sorption of U (VI). Chem Eng J 235:275–283
Hu S, Lin X, Zhang Y, Huang R, Qu Y, Luo X, Zhou J (2017) Preparation and application of alginate-Ca/attapulgite clay core/shell particle for the removal of uranium from aqueous solution. J Radio Anal Nucl Chem 314(1):307–319
Feng J, Cai Y, Wang X, Wang X, Zhu M, Fang M ... Tan X (2021) Designed Core–Shell Fe 3 O 4@ polydopamine for effectively removing uranium (VI) from aqueous solution. Bull Environ Contamin Toxicol 106:165–174
Li L, Lu W, Ding D, Dai Z, Cao C, Liu L, Chen T (2019) Adsorption properties of pyrene-functionalized nano-Fe3O4 mesoporous materials for uranium. J Solid State Chem 270:666–673
Zhu K, Lu S, Gao Y, Zhang R, Tan X, Chen C (2017) Fabrication of hierarchical core-shell polydopamine@ MgAl-LDHs composites for the efficient enrichment of radionuclides. Appl Surf Sci 396:1726–1735
Tan X, Fang M, Tan L, Liu H, Ye X, Hayate T, Wang X (2018) Core–shell hierarchical C@Na2Ti3O7·9H2O nanostructures for the efficient removal of radionuclides. Environ Sci: Nano 5:1140–1149
Zhao M, Cui Z, Pan D, Fan F, Tang J, Hu Y ... Wu W (2021) An efficient uranium adsorption magnetic platform based on amidoxime-functionalized flower-like Fe3O4@ TiO2 Core–Shell microspheres. ACS Appl Mater Interf 13(15):17931–17939
Xu S, Yingguo Z, Yingguo Z, Zheng F, Zheng F, Zhan Y (2016) Hollow Fe3O4@mesoporous carbon core–shell microspheres for efficient sorption of radionuclides, Springer. J Mater Sci 51(5). https://doi.org/10.1007/s10853-015-9567-y
Wu Y, Li B, Wang X, Yu S, Pang H, Liu Y ... Wang X (2019) Magnetic metal-organic frameworks (Fe3O4@ ZIF-8) composites for U (VI) and Eu (III) elimination: simultaneously achieve favorable stability and functionality. Chem Eng J 378:122105
Tan L, Zhang X, Liu Q, Jing X, Liu J, Song D ... Wang J (2015) Synthesis of Fe3O4@ TiO2 core–shell magnetic composites for highly efficient sorption of uranium (VI). Colloids Surf A: Physicochem Eng Aspects 469:279–286
Yang S, Yang S, Zong P, Zong P, Ren X, Ren X, Wang X, Wang X (2012) Rapid and highly efficient preconcentration of Eu(III) by Core–Shell structured Fe3O4@Humic acid magnetic nanoparticles, ACS Appl Mater Interf 4(12):6891–6900
Zhang Y, Lin X, Hu S, Zhang X, Luo X (2016) Core–shell zeolite@ Alg–Ca particles for removal of strontium from aqueous solutions. RSC Adv 6(78):73959–73973
Fakhri H, Mahjoub AR, Aghayan H (2019) Effective adsorption of Co2+ and Sr2+ ions by 10-tungsten-2-molybdophosphoric acid supported amine modified magnetic SBA-15. J Radio Anal Nucl Chem 321(2):449–461
Mou W, Du S, Yu Q, Li X, Wei H, Yang Y (2018) Efficient capture of radioactive strontium from water using magnetic WO3 assembled on grapheme oxide nanocomposite. Chem Select 3(24):6992–6997
Wang G, Liu Q, Chang M, Jang J, Sui W, Si C, Ni Y (2019) Novel Fe3O4@ lignosulfonate/phenolic core-shell microspheres for highly efficient removal of cationic dyes from aqueous solution. Ind Crops Prod 127:110–118
Didehban A, Zabihi M, Faghihi M, Akbarbandari F, Akhtarivand H (2021) Design and fabrication of core-shell magnetic and non-magnetic supported carbonaceous metal organic framework nanocomposites for adsorption of dye. J Phys Chem Solids 152:109930
Review on nickel-based adsorption materials for Congo red. J Hazardous Mater 403:123559 (August 2020). https://doi.org/10.1016/j.jhazmat.2020.123559
Yang S, Zong P, Ren S, Wang X (2014) Highly regenerable mussel-inspired Fe3O4@polydopamine-Ag core-shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl Mater Interf 6(11):8845–8852, Jun 11. https://doi.org/10.1021/am501632f
Huang W, Xu J, Lu D, Deng J, Shi G, Zhou T (2018) Rational design of magnetic infinite coordination polymer core-shell nanoparticles as recyclable adsorbents for selective removal of anionic dyes from colored wastewater. Appl Surf Sci 2018–12–01. https://doi.org/10.1016/j.apsusc.2018.08.122
Huo Y, Wu H, Wang Z, Wang F, Liu Y, Feng Y, Zhao Y (2018) Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the efficient adsorption of anionic dyes, Author links open overlay panel. Colloids Surf A: Physicochem Eng Aspects 549, 20 July 2018:174–183
Zhang Z, Kong J (2011) Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution, Author links open overlay panel. J Hazardous Mater 193:325–329, 15 October
Konickia W, Hełminiakb A, Arabczykb W, Mijowska E (2018) Adsorption of cationic dyes onto Fe@graphite core–shell magnetic nanocomposite: equilibrium, kinetics and thermodynamics, Author links open overlay panel. Chem Eng Res Design 129:259–270, Jan 2018
Huang B, Liu Y, Li B, Wang H, Zeng G (2019) Adsorption mechanism of polyethyleneimine modified magnetic core–shell Fe3O4@SiO2 nanoparticles for anionic dye removal. RSC Adv 9:32462–32471
Wang W, Jiao T, Zhang Q, Luo X, Hu J, Chen Y, Peng Q, Yan X, Li B (2015) Hydrothermal synthesis of hierarchical core–shell manganese oxide nanocomposites as efficient dye adsorbents for wastewater treatment. RSC Adv 5:56279–56285
Konickia W, Hełminiak A, Arabczyk W, Mijowska E (2017) Removal of anionic dyes using magnetic Fe@graphite core-shell nanocomposite as an adsorbent from aqueous solutions, Author links open overlay panel. J Colloid Interf Sci 497:155–164, 1 July
Zheng Y, Liub J, Cheng B, You W, Ho W, Tang H (2019) Hierarchical porous Al2O3@ZnO core-shell microfibres with excellent adsorption affinity for Congo red moleculeAuthor links open overlay panel. Appl Surf Sci 473:251–260, 15 April
Shaoa Y, Zhou L, Bao C, Ma J, Liu M, Wang F (2016) Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue, This paper is dedicated to the memory of Professor Yanfeng Li.Author links open overlay panel. Chem Eng J 283:1127–1136, 1 Jan
Wo R, Li Q-L, Zhu C, Zhang Y, Qiao G-f, Lei K-y, Du P, Jiang W (2019) Preparation and characterization of functionalized metal–organic frameworks with core/shell magnetic particles (Fe3O4@SiO2@MOFs) for removal of congo red and methylene blue from water solution. J Chem Eng Data 64(6):2455–2463
Kollarahithlu SC, Balakrishnan RM (2021) Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. Author links open overlay panel. J Clean Prod 294:12615, 20 April
Guo J, Yu M, Wei X, Huang L (2018) Preparation of core–shell magnetic molecularly imprinted polymer with uniform thin polymer layer for adsorption of dichlorophen. J Chem Eng Data 63(8):3068–3073
Wang Z, Tang H, Li W, Li J, Xu R, Zhang K, He G, Shearing PR, Brett DJL (2019) Core–shell TiO2@C ultralong nanotubes with enhanced adsorption of antibiotics. J Mater Chem A 7:19081–19086
Soares SF, Fernandes T, Sacramento M, Trindade T, Daniel-da-Silva AL (2019) Magnetic quaternary chitosan hybrid nanoparticles for the efficient uptake of diclofenac from waterAuthor links open overlay panel. Carbohydr Polym 203:35–44, 1 Jan
Du ZD, Cui YY, Yang CX, Yan X-P (2019) Core–Shell magnetic amino-functionalized microporous organic network nanospheres for the removal of tetrabromobisphenol A from aqueous solution. ACS Appl Nano Mater 2(3):1232–1241
Zhoua Q, Wang Y, Xiao J, Fan H (2016) Adsorption and removal of bisphenol A, α-naphthol and β-naphthol from aqueous solution by Fe3O4@polyaniline core–shell nanomaterialsAuthor links open overlay panel. Synthetic Metals 212:113–122, Feb
Zhou L, Pan S, Chen X, Zhao Y, Zou B, Jin M (2014) Kinetics and thermodynamics studies of pentachlorophenol adsorption on covalently functionalized Fe3O4@SiO2–MWCNTs core–shell magnetic microspheres. Chem Eng J 257:10–19, 1 Dec
Zhang X, Wang Y, Yang S (2014) Simultaneous removal of Co(II) and 1-naphthol by core–shell structured Fe3O4@cyclodextrin magnetic nanoparticles. Carbohydrate Polymers 114:521–529, 19 Dec
Gao M, Deng L, Kang X, Fua Q, Zhang K, Wang M, Xia Z, Gao D (2020) Core-shell structured magnetic covalent organic frameworks for magnetic solid-phase extraction of diphenylamine and its analogs, J Chromatogr A 1629:461476, 11 Oct 2020
Li Y, Zhang H, Chen Y, Huang L, Lin Z, Cai Z (2019) Core–Shell structured magnetic covalent organic framework nanocomposites for Triclosan and Triclocarban adsorption. ACS Appl Mater Interf 11(25):22492–22500
Zhou Q, Wang Y, Xiao J, Fan H (2017) Fabrication and characterisation of magnetic graphene oxide incorporated Fe3O4@polyaniline for the removal of bisphenol A, t-octyl-phenol, and α-naphthol from water. Sci Rep 7. Article number: 11316
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Basu, H., Singh, S., Kumar Kailasa, S., Kumar Singhal, R. (2022). Application of Core–Shell Nanohybrid Structures in Water Treatment. In: K. Swain, S. (eds) Nanohybrid Materials for Water Purification. Composites Science and Technology . Springer, Singapore. https://doi.org/10.1007/978-981-19-2332-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-2332-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2331-9
Online ISBN: 978-981-19-2332-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)