Abstract
FeS@Fe3O4 magnetic nanoparticles were prepared by ultrasonic-assisted method and characterized by TEM, FTIR, XRD, SEM, EDS, BET and VSM. The factors affecting the adsorption properties of uranyl ions by FeS@Fe3O4 were studied. Results show that the FeS@Fe3O4 nanoparticles have core–shell structure and superparamagnetism. Under the optimized conditions, the maximum adsorption capacity can reach 229.03 mg/g. The optimum adsorption conditions were as follows: pH = 6, temperature 80 °C, C0 = 35 mg/L, contact time 2.5 h, adsorbent dosage 10 mg. Adsorption kinetics and thermodynamic studies show that the adsorption process accords with the Freundlich isotherm adsorption model and the pseudo-second-order kinetic model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of energy is partly due to the development of nuclear technology. Uranium is an important nuclear fuel which is inevitably released into the environment in the process of exploitation and utilization [1]. Uranyl ions in aqueous solution have high radioactivity, high mobility and biotoxicity, which bring immeasurable harm to human beings and the environment [2]. Therefore, it is of great significance to find an economical and friendly method for the treatment of uranium-containing wastewater. Currently, these methods to treat uranium-containing wastewater include chemical precipitation [3,4,5], electrochemical treatment [6], solvent extraction [7,8,9,10], membrane separation [11, 12] and adsorption [13, 14] and so on. Among the above methods, the adsorption method is one of the most economical, reusable and easy-to-use methods, and has attracted more and more attention [15].
In recent years, studies have shown that nano-metal sulfides, as adsorbents, have the advantages of fast adsorption speed, strong adsorption capacity, and good adsorption properties to heavy metal ions, radioactive elements and organic compounds in water and soil substrates, and have potential research and application value [16,17,18,19,20]. Fang et al. [21] used zinc sulfide nanocrystals to remove and separate heavy metal ions in wastewater. The results showed that the adsorption capacities of zinc sulfide nanocrystals to Hg2+, Cu2+, Pb2+ and Cd2+ in wastewater were 475 mg/g, 384 mg/g, 52 mg/g and 82 mg/g, respectively. Sun et al. [22] used iron sulphide (FeS) nanoparticles to treat Hg(II)-containing wastewater, and the maximum adsorption capacity reached 1989 mg/g. However, nano-metal sulfides also have some disadvantages, for example, it is easy to aggregate in aqueous solution, and it is difficult to separate from wastewater after adsorption of heavy metal ions. These shortcomings restrict its engineering application to a certain extent. Therefore, it is necessary to modify the existing materials [23,24,25,26,27,28].
In this paper, FeS@Fe3O4 magnetic nanoparticles with core–shell structure and superparamagnetism were prepared by ultrasonic assisted method. The physicochemical properties of FeS@Fe3O4 magnetic nanoparticles were detected by TEM, FTIR, XRD, EDS, SEM, BET and VSM. And the adsorption properties of FeS@Fe3O4 magnetic nanoparticles for uranyl ions were investigated. The adsorption types were analyzed from the aspects of kinetic, isotherms, and thermodynamic.
Experimental
Materials
UO2 (NO3)2·6H2O was from Chushengwei Chemical Technology Co., Ltd. Anhydrous ferric chloride (FeCl3, > 99%) was from Sinopharm Chemical Reagent Co., Ltd (China). Iron(II) chloride tetrahydrate (FeCl2·4H2O, > 99%) came from Tianjin Fengchuan Chemical Reagent Technologies Co., Ltd. Sodium sulfide nonahydrate (Na2S·9H2O, > 98%), Ethyl alcohol absolute (CH2CH3OH, > 99%) and Ammonia solution (NH3·H2O, 25–28%) was purchased from Tianjin Hongyan Chemical Reagent Factory (China), Hunan Huihong and Guandong Guanghua, respectively. The other chemicals were purchased from other Chinese reagent companies. Deionized water was homemade. All chemical purchased were analytical grade and used as received without any pretreatment.
Analytical methods
Bruker D8 powder X-ray diffractometer (XRD) was used to identify the phases of samples. Nicolet-460 Fourier Infrared Spectrometer (FT-IR) was used to detect the structure of samples and chemical bonds in it. The morphologies of samples were observed by Tecnai G20 transmission electron microscopy (TEM) and JSM-7500F scanning electron microscopy (SEM). The components and elements of samples were determined by Oxford X-Max EDS meter (EDS). The magnetic intensity of absorbent was detected by Squid-Vibrating sample magnetometer (VSM). T6 Xinrui visible spectrophotometer was carried out measure uranium concentration in solution.
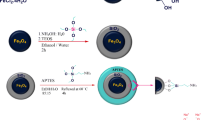
Preparation of magnetic Fe3O4 nanoparticles
1.3 g FeCl3 was dissolved in 140 mL deionized water in a 250 mL beaker. The above mixture was treated by ultrasonic treatment for 15 min and deoxidized with N2 for 30 min.
Then 1.1 g FeCl2·4H2O was added in the above solution and the mixture were stirred quickly until completely dissolved. 0.4 g sodium dodecyl benzene sulfonate (SDBS) was dissolved in 5 mL deionized water then the SDBS solution was added into the above mixture solution to prevent Fe3O4 nanoparticles from agglomerating. Afterwards, 30 mL 2 M ammonia solution was dropped into the obtained solution in the three-necked flask until the pH value was between 9 and 10. Meanwhile, under the protection of N2, the mixture solution was being stirred vigorously at a speed of 1000 rpm and kept for 30 min. After stirring, the final black solution aged for 30 min in the 60 °C water. Then the resulting product was separated from solution in an external magnetic field and washed for three times with deionized water and ethanol, respectively, until the pH of filtered solution reached 7. Finally, the black precipitate was dried in a vacuum drying chamber at 60 °C for 6 h.
Preparation of Fe3O4@FeS
0.2 g Fe3O4 was dispersed in 10 mL deionized water and transferred to 50 mL beaker. In order to improve the dispersibility of the synthesized nanocomposites, 0.9 mL 7.4% carboxyl methyl cellulose (CMC) solution was also added into the above solution and the mixture solution was ultrasonic treated for 30 min. After ultrasonic treatment, 1.4 g Na2S·9H2O was dissolved in above solution and further ultrasonic treatment was carried out for 10 min. Then 0.6 g FeCl2·4H2O was dissolved in the 10 mL deoxidized deionized water, and added dropwise to the above solution under N2 protection. At the same time, the mixture solution was stirred, and then the obtained mixture solution was ultrasonic treated for 30 min. Finally, the product was washed several times with deionized water. The product was then dried in a vacuum drying chamber at 60 °C for 6 h.
Adsorption experiments
The effects of pH, temperature, contact time, absorbent dose and initial concentration of uranyl ions on the absorption efficiency were investigate by batch experiments. 10 mg absorbents were added into a series of 80 mL 35 mg/L uranyl ions solutions in the conical flasks then the above conical flasks all were put in a thermostatic oscillator set at 80 °C then shaken for 2.5 h at constant temperature until the achievement of adsorption equilibrium. The solid phase was separated from the solution by an external magnet, and the concentration of uranyl ion in the supernatant was detected by visible light spectrophotometer, respectively. The adsorption capacity (qe) and the removal rate (A) were calculated according to the following formula [29].
where C0 (mg/L) is the initial concentration of uranyl ions. Ce (mg/L) is the remnant concentration of uranyl ions in the supernatant after absorption. V (L) is the volume of aqueous solution. M (g) is the adsorbent dose.
Desorption experiments
In order to explore reusability of the absorbent, desorption experiments were carried out. 10 mg absorbent was desorbed with 10 mL 1.5 mol/L Na2CO3 solution at a temperature of 80 °C, and the mixture was placed in a thermostatic oscillator and shaken for 2.5 h. After desorption, the absorbent was separated from the solution by magnetic separation and the concentration of uranyl ions in supernatant was measured by visible light spectrophotometer. Finally, the adsorbent was washed with distilled water until it reached a neutral state and left for the next round of adsorption–desorption experiments.
Interference experiment
The effect of competitive ions including Ni2+, Mg2+, Zn2+, Al3+ and Cu2+ on the absorption efficiency of uranyl ions were investigated. A series of different concentrations of Ni2+, Mg2+, Zn2+, Al3+, Cu2+ were mixed with 10 mL 35 mg/L uranyl ions solution, respectively, and desorbed at 80 °C for 2.5 h.
Results and discussion
Characterization
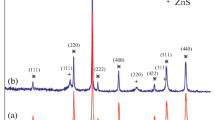
The XRD patterns (10° < 2θ < 90°) of Fe3O4, FeS@Fe3O4 and U-laden FeS@Fe3O4 were shown in Fig. 1. The six characteristic peaks of Fe3O4 at 2θ values of 30.32°, 35.40°, 43.26°, 53.80°, 57.36° and 62.96° correspond to crystal planes (220), (311), (400), (422), (511) and (440) according to JCPDS card No.19-0629. In the XRD pattern of the FeS@Fe3O4, the above six characteristic peaks did not change significantly, which means that the coating of FeS did not change the structure of Fe3O4. However, the XRD pattern of FeS@Fe3O4 exhibits two new XRD characteristic peaks at 2θ values of 18.4° and 33.3°, which correspond to the XRD characteristic peaks of FeS [30]. A broad peak of about 18.4º can be observed, which indicates that the crystal of FeS is not well crystallized and the crystal may be nano-sized [31, 32]. In the XRD pattern of U-laden FeS @Fe3O4, according to JCPDS card No.24-0749, the new characteristic XRD peak at 2θ values of 36.2°, 52.2° and 65.8° is attributed to the adsorption of uranyl ions. And in this diffraction, all peaks of Fe3O4 and FeS are weakened, which means that a large amount of uranyl ions are successfully adsorbed on the adsorbents.
The FT-IR spectra of Fe3O4, FeS@Fe3O4 and U-laden FeS@Fe3O4 are shown in Fig. 2. As shown in the figure, there are two peaks at 3400 cm−1 and 570 cm−1 in the spectrum of the three samples, which belong to the O–H stretching bond and the Fe–O bond, respectively [33]. In addition, a broad peak in the Fe3O4 spectrum at 1072 cm−1 splits into two peaks at 1094 cm−1 and 1019 cm−1 in the FeS@Fe3O4 spectrum, which may be due to the local deformation of the lattice caused by the coating of FeS [34]. In U-laden FeS@Fe3O4 spectrum, these two peaks are moved to 1094 cm−1 and 1019 cm−1, which is mainly due to the adsorption of uranyl ions by FeS@Fe3O4 [35, 36].
The magnetic properties of FeS@Fe3O4 nanoparticles were measured at room temperature by using an external magnetic field of − 20 k Oe ≤ H ≤ 20 k Oe. The results are shown in Fig. 3. The saturation magnetization of FeS@Fe3O4 can be clearly observed in the curve, about 57.18 emu/g, which is lower than the saturation magnetization of Fe3O4 reported in many literatures which is 90 emu/g [37]. This may be attributed to the encapsulation of FeS and quantum size effects. There is no coercivity and no residual magnetism nearly in the magnetic hysteresis loop and the superparamagnetism of FeS@Fe3O4 could be proved [38]. The incidental pattern in the lower right corner indicates that FeS@Fe3O4 has an excellent magnetic response to the external magnet and can be separated by an external magnet within 10 s.
EDS analysis was used to determine the main elements in the FeS@Fe3O4 and U-laden FeS@Fe3O4. As clearly exhibited in Fig. 4, the distributions of main elements of the FeS@Fe3O4 and U-laden FeS@Fe3O4 can be observed obviously. The presence of sulfur elements in FeS@Fe3O4 (Fig. 4a) attests to the successful loading of FeS on the Fe3O4 surface. In the Fig. 4b, the presence of uranium element could be obviously observed and the wt % of U reached 3.68%. It is proved that FeS@Fe3O4 has good adsorption ability for uranyl ion.
The morphology of Fe3O4 and FeS@Fe3O4 were characterized by transmission electron microscope (TEM). The morphology of U-laden FeS@Fe3O4 was characterized by scanning electron microscope (SEM). As shown in Fig. 5, the morphology of Fe3O4 is sphere-like and its size is about 10–20 nm. In Fig. 5b, the Fe3O4 nanoparticles are coated with FeS, and the core–shell structure of FeS@Fe3O4 can be proved. As shown in Fig. 5c, the white materials on the surface of U-laden FeS@Fe3O4 is the crystal of uranyl ion adsorbed on it, which proves that uranyl ion is successfully adsorbed by the FeS@Fe3O4 nanoparticles.
A comparison of adsorption capacity for uranyl ions between Fe3O4, FeS and Fe3O4@FeS
80 mL 35 mg/g uranyl ion solution was added to each of the three conical flasks, the pH of the solution was adjusted to 6, and then 10 mg Fe3O4, FeS and FeS@Fe3O4 were added, respectively, and these solutions were shaken for 2.5 h at 80 °C in a thermostatic oscillator. The supernatants of the three solutions were taken out at different adsorption time, and the uranyl ion concentration was measured to calculate the adsorption capacity and the adsorption rate. The adsorption properties of Fe3O4, FeS and FeS@Fe3O4 for uranyl ions are shown in Fig. 6. It can be seen from the figure that the adsorption capacities of Fe3O4 and FeS are 154.0 mg/g and 211.0 mg/g, respectively. The adsorption capacity of FeS@Fe3O4 nanocomposites is 229.03 mg/g, which is 48.7% higher than that of Fe3O4 nanoparticles and 8.6% higher than that of FeS nanoparticles. This is because FeS is easy to aggregate when it is present alone, which greatly reduces its adsorption capacity. When the FeS is encapsulated on the Fe3O4 nanoparticles, the composite adsorbent becomes dispersed and more stable, and therefore, the adsorbent can provide a larger surface area and facilitate adsorption. According to the specific surface area test results, the specific surface area of FeS was 29.0264 m2/g, and the specific surface area of FeS@Fe3O4 was 82.4732 m2/g. It is proved that FeS@Fe3O4 has better adsorption performance for uranyl ions than Fe3O4 and FeS.
Effect of the contact time
In order to study the effect of contact time on the adsorption of uranyl ions by FeS@Fe3O4, the contact time was from 15 to 210 min, the adsorption dosage was 10 mg, the initial uranyl ion concentration was 35 mg/L, the volume of the solution was 80 mL, the pH of solution was 6 and the temperature was 80 °C, under these conditions, a series of adsorption experiments were carried out. The results are shown in Fig. 7. As can be seen from the figure, with the increase of the contact time, the adsorption rate and the adsorption capacity increase rapidly. When the adsorption process reaches 150 min, the adsorption process reaches the adsorption equilibrium, and the adsorption rate reaches 81.8%, and remains basically unchanged in the subsequent time. This is because the adsorption of uranyl ions by FeS@Fe3O4 is firstly caused by diffusion of uranyl ions into the outer surface of the material, followed by transfer from the outer surface to the inner surface [39]. The adsorption process takes a longer time. Therefore, the adsorption rate and the adsorption capacity of uranyl ions by FeS@Fe3O4 increases with time until the adsorption equilibrium is reached. And the maximum adsorption capacity is 229.03 mg/g.
Effect of the adsorbent dosage
To investigate the effect of adsorbent dosage on the adsorption of uranyl ions by FeS@Fe3O4, the dosage of FeS@Fe3O4 was from 5 to 25 mg, the contact time was 150 min, the initial uranyl ion concentration was 35 mg/L, the volume of the solution was 80 mL, the pH of solution was 6 and the temperature was 80 °C, under these conditions, a series of adsorption experiments were carried out. The results are shown in Fig. 8. As can be seen from the figure that with the increase of initial uranyl ion concentration, the adsorption capacity of FeS@Fe3O4 to uranyl ion increases and the adsorption rate decreases. When the dosage of adsorbent is 10 mg, the adsorption rate reaches 81.8% and the adsorption capacity is 229.03 mg/L. When the dosage of adsorbent is 20 mg, the adsorption rate reaches 82.4% and the adsorption capacity is 115.29 mg/L. The main reason is that the content of uranyl ion in uranyl solution is constant. With the increase of adsorbent dosage, the number of effective adsorption active sites increases, and the removal rate of uranyl ion increases, but the amount of uranyl ion adsorbed by unit mass FeS@Fe3O4 decreases [40]. The adsorption capacity of adsorbents per unit mass was decreased. When the dosage of the adsorbent is more than 10 mg, the removal rate with the addition of the adsorbent is increased, but the adsorption capacity of the unit mass of the adsorbent is decreased rapidly. From the optimal point of view, the optimal dosage of the adsorbent is 10 mg.
Effect of pH
The pH value of uranyl ion solution was adjusted with nitric acid and sodium hydroxide solutions. In order to study the effect of pH on the adsorption of uranyl ions by FeS@Fe3O4, the pH of solution was from 2 to 8, the contact time was 150 min, the adsorption dosage was 10 mg, the initial uranyl ion concentration was 35 mg/L, the volume of the solution was 80 mL and the temperature was 80 °C, under these conditions, a series of adsorption experiments were carried out. The results are shown in Fig. 9. It is obvious that when the pH value changes from 2 to 5, the adsorption efficiency of FeS@Fe3O4 for uranyl ions increases with the increase of pH value. When the pH value is 6, the adsorption efficiency reaches the maximum value. After that, with the increase of pH (pH > 6), the adsorption efficiency begins to decrease slightly. The main reason for the above phenomenon is that the degree of protonation is different in different pH solutions [41, 42]. In the solution with lower pH, the degree of protonation is greater, and H+ in the solution is competitive with UO22+ for adsorption, which affects the adsorption performance of uranyl ion by FeS@Fe3O4, which results in the low adsorption capacity and removal rate of uranyl ion by FeS@Fe3O4. On the other hand, there are different forms of uranium in different pH. In the case of pH = 6, the uranium in solution mainly exists in the form of UO22+. In this case, the adsorption ability of FeS@Fe3O4 is the strongest. With the increase of pH, OH− and UO22+ form ions with lower adsorption affinity, including [UO2OH]+, [(UO2)3(OH)4]2+, [(UO2)2OH]3+, [(UO2)3OH]5+, [(UO2)4OH]7+, [UO2(OH)4]2−, [(UO2)3(OH)7]− [43], which reduce the adsorption amount of uranyl ion, resulting in the decrease of adsorption capacity and removal rate of uranyl ion by FeS@Fe3O4. Therefore, the solution pH = 6 is chosen as the optimal reaction condition.
Effect of temperature
Temperature was also an important parameter. A series of experiments were performed in 80 35 mg/L of the uranyl ions solutions (adjusted pH = 6) under different temperature ranging from 30 to 90 °C. 10 mg adsorbent was added into the above solution and shaken for 2.5 h at 80 °C. Figure 10 shows that when the temperature is less than 80 °C, the adsorption capacity and removal rate of uranyl ion by FeS@Fe3O4 increase with temperature increasing, and when the temperature is higher than 80 °C, The adsorption capacity and removal rate of uranyl ion by FeS@Fe3O4 decreased slightly with temperature increasing. As the temperature increases, it is advantageous for the adsorbed molecules to diffuse from the outer surface to the inner surface of the adsorbent, so that the surface of the absorbent can provide more active sites, thereby increasing the amount of adsorption. The adsorption of uranyl ions by FeS@Fe3O4 contains chemical adsorption and physical adsorption. As the temperature gradually increases, the physical adsorption efficiency decreases and the chemisorption reaches equilibrium. Therefore, when the temperature exceeds 80 °C, the adsorption capacity of FeS@Fe3O4 for uranyl ions is slightly decreased. Therefore, the optimum adsorption temperature is 80 °C.
Effect of the initial concentration of uranyl ions
In order to study the effect of initial uranyl ion concentration on the adsorption of uranyl ions by FeS@Fe3O4, the initial uranyl ion concentration was from 10 to 60 mg/L, the pH of solution was 6, the contact time was 150 min, the adsorption dosage was 10 mg, the volume of the solution was 80 mL and the temperature was 80 °C, under these conditions, a series of adsorption experiments were carried out. The results are shown in Fig. 11. It can be seen from Fig. 11 that as the initial uranyl ion concentration increases, the adsorption capacity of FeS@Fe3O4 on uranyl ions increases, and the adsorption rate decreases. This is because when the initial concentration of uranyl ions is low, the adsorption of uranyl ions by the adsorbent is far from saturation, so the removal rate is high and the adsorption amount is low. As the initial uranyl ion concentration increases, and the adsorbent gradually reaches the adsorption equilibrium, a part of the uranyl ion exists in the free state in the solution, resulting in a decrease in the removal rate but an increase in the adsorption capacity. Since the level of uranium in wastewater is around 35 mg/L, therefore, the concentration of initial uranium (VI) in the subsequent study was selected to be 35 mg/L.
Adsorption kinetic
Pseudo-first-order and pseudo-second-order kinetic models were used to simulate the adsorption of uranyl ions on FeS@Fe3O4 and the fitting curves are shown in Fig. 12. The pseudo-first-order kinetic equation and the pseudo-second kinetic equation are formulas (3) and (4) respectively [44]:
where qe and qt are the mass of solute adsorbed by the unit adsorbent in the equilibrium time and t time respectively. K1 and K2 are the rate constants of the pseudo-first-order kinetic equation and the pseudo-second-order kinetic equation, respectively.
The fitting parameters are shown in Table 1. It can be seen that the correlation coefficient of the pseudo-second-order kinetic model is 0.9994, the correlation coefficient of the pseudo-first-order kinetic model is 0.9773, and the correlation coefficient of the pseudo-second-order kinetic model is larger than correlation coefficient of the first-order kinetic model. Therefore, this adsorption behavior is more suitable to be described by pseudo-second-order kinetic model, which indicates that the adsorption behavior is dominated by chemical adsorption.
Adsorption isotherm
In order to understand the adsorption behavior of the adsorbent, the adsorption data were analyzed using the Langmuir model (formula 5) and the Freundlich model (formula 6). The two equations are as follows:
where qe (mg/g) and Ce (mg/L) are equilibrium adsorption capacity and equilibrium concentration. Qm is the maximum adsorption capacity, and KL is the equilibrium adsorption constant. KF (mL1/n μg1−1/n) and n are the Freundlich constants. The maximum adsorption capacity (Qm) can be calculated from the slope of the linear plot of Ce/qe versus Ce. The values of n and KF can be obtained from slope of linear plot of ln qe versus ln Ce [45].
The Langmuir and Freundlich isotherm parameters are shown in Table 2 and the fitting curves are shown in Fig. 13. It is clear that the correlation coefficient of the Freundlich model is 0.9991, which is higher than the correlation coefficient of the Langmuir model (R2 = 0.9641). The adsorption data agree well with the Freundlich model, indicating that the adsorption of uranyl ions by FeS@Fe3O4 is closer to that of multi-molecular layer adsorption [46].
Adsorption thermodynamics
Gibbs free energy (ΔG), enthalpy change (ΔH) and entropy change (ΔS) were calculated by the following three Eqs. (7)–(9), the results are shown in Table 3. Plotting ln K0 against 1/T gives a straight line with slope and intercept equal −H/R and S/R, respectively [47]. The fitting curve of ln K0 vs 1/T is shown in Fig. 14. The results show that: ΔH > 0, ΔS > 0, and ΔG < 0. The positive enthalpy (ΔH) indicates that the adsorption process is endothermic and the increase of temperature is beneficial to the adsorption. The entropy change (ΔS) is positive, which reflects the increase of the degree of system confusion. During the adsorption process, the exchanged hydrogen ions diffuse into the solution to form a disordered motion. The decrease in entropy caused by adsorption is less than the increase in entropy caused by disordered motion, resulting in an increase in entropy of the entire system. [48]. The Gibbs free energy (ΔG) is negative, which reflects the adsorption process is a spontaneous.
where ΔG is the Gibbs free energy (kJ/mol). ΔH is enthalpy change and ΔS is entropy change. R and T are the ideal gas constant [8.314 J/(mol K)] and absolute temperature, respectively. K0 is adsorption distribution coefficient.
Effect of interfering ions
In order to further study the selective adsorption of uranyl ions on the adsorbent, the effects of the interfering ions were investigated. Industrial wastewater contains a variety of metal ions, including Na+, K+, Ni2+, Mg2+, Zn2+, Al3+, Fe2+, Ca2+,Cu2+ and so on. So we choosed randomly the five metal ions (Ni2+, Mg2+, Zn2+, Al3+ and Cu2+) in these above metal ions as interfering ions and applied in the experiment. As shown in Fig. 15, Ni2+, Mg2+, Zn2+ and Cu2+ had certain effect on the adsorption when the concentration of these cations reached to 20 mg/L, however, when the concentration of Ni2+, Mg2+ and Zn2+ increased, the adsorption capacity remained about 227 mg/g. When the concentration of Cu2+ increased to 45 mg/L, the adsorption capacity of FeS@Fe3O4 on uranyl ions decreased from 227 to 226.6 mg/L. When the concentration of Al3+ was lower than 20 mg/L, it had no influence on the adsorption. When the concentration of Al3+ increased, the adsorption capacity of FeS@Fe3O4 on uranyl ions only decreased from 229.0 to 226.6 mg/g.
Reusability of FeS@Fe3O4
To test the reusability of FeS@Fe3O4, five successive adsorption and desorption cycles were performed. The results are shown in Fig. 16. It is obvious that the adsorption rate of FeS@Fe3O4 only decreased from 83.2 to 80.6% after 5 adsorption–desorption operations, so the reusability of FeS@Fe3O4 can be convincingly proved.
Conclusion
In this paper, core–shell magnetic nanoparticles of FeS@Fe3O4 were successfully prepared by the ultrasonic-assisted method. The average diameter of the nanoparticles is about 30 nm, and the saturation magnetic field intensity is 57.18 emu/g, and it has better superparamagnetism. The prepared adsorbents have good adsorption performance for uranyl ions. The optimum adsorption conditions were as follows: pH = 6, temperature 80 °C, initial uranium concentration 35 mg/L, contact time 2.5 h, adsorbent dosage 10 mg. Under optimal conditions, the adsorption capacity can reach 229.03 mg/g. The experimental results show that the adsorption kinetics accords with the pseudo second order kinetic model, the fitting degree of R2 is 0.9994. The adsorption isotherm is in accordance with the Freundlich isothermal adsorption model, and the fitting degree of R2 is 0.9991. The research of adsorption thermodynamics shows that the adsorption process is endothermic and spontaneous. Interference experiments and adsorption desorption cycle experiments proved that the prepared FeS@Fe3O4 had good selective adsorption and reuse performance for uranyl ions.
References
Feng ML, Sarma D, Qi X, Du KZ, Huang XY, Kanatzidis MG (2016) Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J Am Chem Soc 138(38):12578–12585
Tavengwa NT, Cukrowska E, Chimuka L (2015) Sequestration of U(VI) from aqueous solutions using precipitate ion imprinted polymers endowed with oleic acid functionalized magnetite. J Radioanal Nucl Chem 304(2):933–943
Gong Y, Tang J, Zhao D (2016) Application of iron sulfide particles for groundwater and soil remediation: a review. Water Res 89:309–320
Liu R, Yang Z, He Z, Wu L, Hu C, Wu W, Qu J (2016) Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): inhibitive effects of S(0)-enriched surfaces. Chem Eng J 304:986–992
Suzuki T, Kawasaki T, Takao K, Harada M, Nogami M, Ikeda Y (2012) A study on selective precipitation ability of cyclic urea to U(VI) for developing reprocessing system based on precipitation method. J Nucl Sci Technol 49(10):1010–1017
Tran TK, Leu HJ, Chiu KF, Lin CY (2017) Electrochemical treatment of heavy metal-containing wastewater with the removal of COD and heavy metal ions. J Chin Chem Soc 64(5):493–502
Chen L, Bai Z, Zhu L, Zhang L, Cai Y, Li Y, Liu W, Wang Y, Chen L, Diwu J (2017) Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework. ACS Appl Mater Interfaces 9(38):32446–32451
Endrizzi F, Leggett C, Rao L (2016) Scientific basis for efficient extraction of uranium from seawater, I: understanding the chemical speciation of uranium under seawater conditions. Ind Eng Chem Res 55(15):4249–4256
Wang LL, Luo F, Dang LL, Li JQ, Wu XL, Liu SJ, Luo MB (2015) Correction: ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide- and carboxyl-functionalized metal–organic framework. J Mater Chem A 3(34):17880
Zhe X, Jiangtao HU, Wang MH, Zhang WL, Shineng LI, Gao QH, Guozhong WU (2013) Properties and evaluation of amidoxime-based UHMWPE fibrous adsorbent for extraction of uranium from seawater. Sci China Chem 56(11):1504–1509
Hoyer M, Zabelt D, Steudtner R, Brendler V, Haseneder R, Repke JU (2014) Influence of speciation during membrane treatment of uranium contaminated water. Sep Purif Technol 132:413–421
Torkabad MG, Keshtkar AR, Safdari SJ (2017) Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Prog Nucl Energy 94:93–100
Ma H, Hsiao BS, Chu B (2013) Ultrafine cellulose nanofibers as efficient adsorbents for removal of UO2 2+ in water. ACS Macro Lett 1(1):213–216
Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B (2011) Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J 171(3):1185–1193
Kaynar ÜH, Ayvacıklı M, Kaynar SÇ, Hiçsönmez Ü (2014) Removal of uranium(VI) from aqueous solutions using nanoporous ZnO prepared with microwave-assisted combustion synthesis. J Radioanal Nucl Chem 299(3):1469–1477
Jing L, Lei Z, Dong F, Hudson-Edwards KA (2016) Enhancing As(V) adsorption and passivation using biologically formed nano-sized FeS coatings on limestone: implications for acid mine drainage treatment and neutralization. Chemosphere 168:529–538
Liu X, Ai L, Jiang J (2015) Interconnected porous hollow CuS microspheres derived from metal-organic frameworks for efficient adsorption and electrochemical biosensing. Powder Technol 283:539–548
Qu Z, Yan L, Li L, Xu J, Liu M, Li Z, Yan N (2014) Ultraeffective ZnS nanocrystals sorbent for mercury(II) removal based on size-dependent cation exchange. ACS Appl Mater Interfaces 6(20):18026–18032
Wolthers M, Charlet L, Linde PRvD, Rickard D, Weijden CHvD (2005) Surface chemistry of disordered mackinawite (FeS). Geochim Cosmochim Acta 69(14):3469–3481
Watson JHP, Ellwood DC, Deng Q, Mikhalovsky S, Hayter CE, Evans J (1995) Heavy metal adsorption on bacterially produced FeS. Miner Eng 8(10):1097–1108
Fang L, Li L, Qu Z, Xu H, Xu J, Yan N (2018) A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J Hazard Mater 342:617–624
Sun Y, Liu Y, Lou Z, Yang K, Lv D, Zhou J, Xu X (2018) Enhanced performance for Hg(II) removal using biomaterial (CMC/gelatin/starch) stabilized FeS nanoparticles: stabilization effects and removal mechanism. Chem Eng J 344:616–624
Dzade NY, Roldan A, Leeuw NHD (2017) Structures and properties of As(OH)3 adsorption complexes on hydrated mackinawite (FeS) surfaces: a DFT-D2 study. Environ Sci Technol 51(6):3461–3470
Shao D, Ren X, Wen J, Hu S, Xiong J, Jiang T, Wang X, Wang X (2016) Immobilization of uranium by biomaterial stabilized FeS nanoparticles: effects of stabilizer and enrichment mechanism. J Hazard Mater 302:1–9
Sun Y, Lou Z, Yu J, Zhou X, Lv D, Zhou J, Baig SA, Xu X (2017) Immobilization of mercury (II) from aqueous solution using Al2O3-supported nanoscale FeS. Chem Eng J 323:483–491
Chen L, Zhao D, Chen S, Wang X, Chen C (2016) One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal. J Colloid Interface Sci 472:99–107
Yan LG, Yang K, Shan RR, Yan T, Wei J, Yu SJ, Yu HQ, Du B (2015) Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J Colloid Interface Sci 448:508–516
Zhang D, Gao G, Ma W, Zhu J, Qiu G, Liu X (2013) Facile synthesis and properties of bifunctional magnetic-optical Fe3O4@ZnS nanocomposites with core-shell structure. Appl Mech Mater 320:92–98
Zhao D, Zhang Q, Xuan H, Chen Y, Zhang K, Feng S, Alsaedi A, Hayat T, Chen C (2017) EDTA functionalized Fe3O4/graphene oxide for efficient removal of U(VI) from aqueous solutions. J Colloid Interface Sci 506:300–307
Tavengwa NT, Cukrowska E, Chimuka L (2016) Modeling of adsorption isotherms and kinetics of uranium sorption by magnetic ion imprinted polymers. Toxicol Environ Chem Rev 98(1):1–12
Kera NH, Bhaumik M, Pillay K, Ray SS, Maity A (2017) Selective removal of toxic Cr(VI) from aqueous solution by adsorption combined with reduction at a magnetic nanocomposite surface. J Colloid Interface Sci 503:214–228
Yu Y, Yu L, Shih K, Chen JP (2018) Yttrium-doped iron oxide magnetic adsorbent for enhancement in arsenic removal and ease in separation after applications. J Colloid Interface Sci 521:252–260
Zhang S, Zhang Y, Liu J, Xu Q, Xiao H, Wang X, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226(24):30–38
Iranmanesh P, Saeednia S, Mehran M, Dafeh SR (2016) Modified structural and magnetic properties of nanocrystalline MnFe2O4 by pH in capping agent free co-precipitation method. J Magn Magn Mater 425:31–36
Xu Y, Ke G, Yin J, Lei W, Yang P (2019) Synthesis of thiol-functionalized hydrotalcite and its application for adsorption of uranium (VI). J Radioanal Nucl Chem 319(3):791–803
Wang CF, Liu ZR, Xue GR, Lei Y, Wang Y, Zhou LM (2016) Adsorptive properties of sunflower seed shells for UO2 2+ in aqueous solution. J Nucl Radiochem 38(2):107–115
Gholizadeh A, Jafari E (2017) Effects of sintering atmosphere and temperature on structural and magnetic properties of Ni-Cu-Zn ferrite nano-particles: magnetic enhancement by a reducing atmosphere. J Magn Magn Mater 422:328–336
Ma YX, Xing D, Shao WJ, Du XY, La PQ (2017) Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg(II) in aqueous solution. J Colloid Interface Sci 505:352–363
Li SL, Zhou YP, Liu JJ (2016) Physical chemistry (2009), 5th edn. Higher Education Press, Beijing, pp 594–595
Gao Y, Yuan Y, Ma D, Li L, Li Y, Xu W, Tao W (2014) Removal of aqueous uranyl ions by magnetic functionalized carboxymethylcellulose and adsorption property investigation. J Nucl Mater 453(1–3):82–90
Dolatyari L, Yaftian MR, Rostamnia S (2016) Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J Environ Manage 169:8–17
Lu BQ, Li M, Zhang XW, Huang CM, Wu XY, Fang Q (2018) Immobilization of uranium into magnetite from aqueous solution by electrodepositing approach. J Hazard Mater 343:255–265
Sepehrian H, Asadi Z (2012) Studies on the recovery of uranium from nuclear industrial effluent using nanoporous silica adsorbent. Int J Environ Sci Technol 9(4):629–636
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366(1):165–172
Qian J, Zhang S, Zhou Y, Dong P, Hua D (2014) Synthesis of surface ion-imprinted magnetic microspheres by locating polymerization for rapid and selective separation of uranium(VI). RSC Adv 5(6):4153–4161
Khani R, Sobhani S, Beyki MH (2016) Highly selective and efficient removal of lead with magnetic nano-adsorbent: multivariate optimization, isotherm and thermodynamic studies. J Colloid Interface Sci 466:198–205
Donia AM, Atia AA, El-Boraey H, Mabrouk DH (2006) Uptake studies of copper(II) on glycidyl methacrylate chelating resin containing Fe2O3 particles. Sep Purif Technol 49(1):64–70
Zhang X, Wang J, Li R, Dai Q, Gao R, Liu Q, Zhang M (2013) Preparation of Fe3O4@C@layered double hydroxide composite for magnetic separation of uranium. Ind Eng Chem Res 52(30):10152–10159
Acknowledgements
This study was financially supported by Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol Fiber Material, Huaihua University (HGY201805) and the Natural Science Foundation of Hunan Province (2017JJ2231).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Yang, P., Li, Q. et al. Preparation of FeS@Fe3O4 core–shell magnetic nanoparticles and their application in uranyl ions removal from aqueous solution. J Radioanal Nucl Chem 321, 499–510 (2019). https://doi.org/10.1007/s10967-019-06626-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06626-2