Abstract
Extracting the nanostructured molecules of plant species for measuring the corrosion inhibition ability is being researched nowadays. Due to the toxicity involved in man-made materials, a paradigm shift towards concentrating over the eco-friendly materials has been carried out. Extracts from plant species such as Musa paradisiaca have been investigated by varying parameters such as concentration and time for its corrosion inhibition efficiency for High Yield Strength Deformed (HYSD) bar under 0.25 molarity sulphuric acid medium. The investigation was done using Weight Loss Technique (WLT), and the outcomes were compared with the output of various parameters. Scanning the samples with SEM and FTIR equipment has been carried out to understand the intermolecular mechanism involved in eradicating the corrosion in the HYSD bars. Finally, the result obtained for Musa plant powder extract on the HYSD bar specimen at different concentrations and time period proved that natural products can be effectively used as corrosion inhibitors replacing the synthetic ones on planned effective use.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Many heavy metals are being slowly prohibited with the help of enormous by-laws due to their poisonous behaviour along with their disposal hurdles especially in the marine environment [1]. In order to replace inorganic compounds, nowadays extracts of plants are considered the vital source of naturally concocted one that involves simple low-cost extraction procedure [2,3,4,5,6]. These extracts have the tendency to replace synthetic organic and inorganic inhibitors with many successive literatures. Active ingredients present in the natural extract help in the treatment of many wastes and helped many researchers to formulate related theories [7,8,9,10].
Most of the industries such as cleaning industries, petrochemical related works, pickling processes and others, using corrosion inhibitors are the most critical thing in the current trends of research [12,13,14]. Inhibitors with oxygen, nitrogen and sulphur proved to be effective in the majority areas. Among various synthetic compounds showing good anticorrosive properties, they emerge as toxic to human beings and their surrounding environment. Characteristics such as natural, low cost, availability and renewable nature make the extracts of plants suitable in all scenarios. In this regard, one of the perennial trees like Musa paradisiaca (Banana) is used in many research-related activities [15]. Researches to find more compounds as corrosion inhibitors in various acidic and alkaline media are yet to be done. More works are done to find active plant compounds from various natural substances [11, 16]. Thus, in this work, the suitability of Musa extract under varying time and concentration to act as a green corrosion inhibitor over HYSD bar specimen is planned to be tested [17].
2 Experimental Work
2.1 Concoction of Leaves Powder
Musa were dried naturally and used to make the fine powder. Initially, leaves were collected from local farmers and washed thoroughly and soaked in deionized water for 5 h followed by air drying using natural sunlight. This extract of leaf powder was used as an inhibitor for HYSD bars [18].
2.2 Making of Trial Compound
Acidic compound of 0.25 molarity H2SO4 was prepared by the dilution of Analytical Grade 98% H2SO4 with deionized water to a volume of 100 ml in the existence or non-existence of various concentrations of leaves extract varying from 3 to 10 g/l.
2.3 Arrangement of Specimen
Initially, the HYSD specimen of 16 mm diameter was cleaned with various emery paper up to 300 grade and cut into 2.5 cm length and used for further process. Each specimen was thoroughly cleaned with deionized water then air dried with acetone and preserved in an air tight container.
2.4 Weight Loss Technique
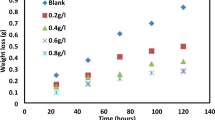
Weight loss technique (WLT) is one of the common corrosive action observing methods. It includes exposure of specimen to a specific duration, then removed for measurement of the same. It is a basic measurement where the WL happening over a particular period of exposure is given in terms of rate of corrosive action. In this work, HYSD bars in three trials were submerged in 100 ml of the trial compound of acidic nature (0.25 molarity H2SO4) in the existence or non-existence of the inhibitor at varying temperatures.
The metal samples were taken out from the trial compounds after 5 h at temperatures of 27 and 40 °C. After cleaning the specimen with Dimethyl ketone, the WL was found as the difference in weight of the samples before and after immersion using analytical balance with an accuracy of ± 0.1 mg. For the authenticity of the outcome and to report the average value of the WL, observations were performed triple time. Using this technique, the average rate of corrosive action was expressed in mg per sq cm per hr. Assuming, that uniform corrosion taking place over the sample, corrosiveness is calculated. Using the WL measurements, the rate of corrosion will be found using the following Eq. 1:
where MMPY = Milli metre per year, WL = Weight Loss (mg), d = Density (g per cubic metre), A = Area of sample (square cm), t = time (hours).
The Inhibition Efficiency (% I.E.) and Surface Coverage (θs) were found using the below Eqs. (2) and (3),
where Wt1 = Rate of Corrosion with the extract and Wt2 = Rate of Corrosion without the extract.
3 Results and Discussion
As per Fig. 1, the SEM image of Banana leaf powder represents the formation of shielding coat around the HYSD bar. Because of 5 h contact period, for a sample length of 2.5 cm, most of the surface has been covered with the particles of Musa. This shielding coat will play an important role in inhibiting the corrosion in HYSD bar specimen used in this work.
Figures 2 and 3 substantiate the effective role of Banana leaf powder as a protective sheath over the HYSD bar. Various elements present in the leaf powder help to analyze the inhibiting property of Musa extract.
Figure 4 shows the composition of various elements in the duplicate sample of Musa powder. Elements such as C, O are present in enormous amount, whereas minor elements such as Mg, Si, Mo, Cl are present in minimal amount. Carbon and Oxygen play an important role as protective cover for the high-yield strength deformed bars.
From Fig. 5, it is found that the percentage transmittance of the peaks obtained in FTIR has around a value of 100. High peaks were obtained around 455.20 per cm. Between 3956.65 and 2000 per cm, the peaks followed an uniform pattern layer of single bond, which varies from 2500 to 4000 per cm; the layer of triple bond varies from 2000 to 2500 per cm; the layer of double bond varies from 1500 to 2000 per cm; and the layer of fingerprint region varies from 600 to 1500 per cm. It contains methyl as band regions between 3650 and 3250 per cm exist, along with the presence of hydrogen bond. At above 3000 per cm, aromatic C–H stretch exists along with functional groups such as acetylenic (2140–2100), alcohol hydroxy compound (3570–3200 per cm), ether and oxy compound (2820–2810 per cm), tertiary amino( 1210–1150 per cm), carbonyl compound (2100–1800 per cm) and others.
4 Conclusion
With the help of the above points, it is proved that Musa plant extract plays a vital role in effective green inhibition against high-yield strength deformed bar specimen. It is justified that with the help of WLT, corrosion inhibition of HYSD in acidic environment can be verified. Scanning the specimen treated with or without Musa extract using SEM and FTIR equipments, shows the increment in the activation energy values of the corrosion process; and also indicates this plant extract creates a hurdle to charge and mass transfer thereby producing decrement in the rate of corrosive action over HYSD bar specimen when 0.25 molarity Sulphuric acid medium used. With the above data, it is confirmed that both physical adsorption and chemical sorption are involved while treating the specimen with Musa. Thus, the study done confirms that the corrosion inhibition due to Musa extract over HYSD specimen using 0.25 molarity sulphuric acid is due to the adsorption mechanism of the extract.
References
Nandiyanto ABD (2019) How to read and interpret FTIR spectroscope of organic material, pp 97–118
Al-Asadi AA, Abdullah AS, Khaled NI, Alkhafaja RJM (2015) Effect of an Aloe Vera as a natural inhibitor on the corrosion of mild steel in 1 wt% NaCl. Int J Eng Technol 02(06)
Singh A, Ahamad I, Quraishi MA (2016) Piper longum extract as green corrosion inhibitor for aluminium in NaOH solution. Arab J Chem 9:S1584–S1589
Khadom AA, Abd AN, Ahmed NA (2017) Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in HCl: Kinetics and mathematical studies. S Afr J Chem Eng 25:13–21
Yaro AS, Khadom AA, Wael RK (2013) Apricot Juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex Eng J 52:129–135
Nahe A, Abu-Abdoun I, Abdel- Rahman I (2010) UAE Neem Extract as a corrosion inhibitor for carbon steel in HCl solution. Int J Corros 2010. (de Souza ECCA, de Andrade Ripper B, Perrone D, D’Elia E (2016) Roasted coffee extracts as corrosion inhibitors for mild steel in HCl solution. Mater Res 19(6):1276–1285)
Jeetendra Bhaswar PK, Jain PJ (2015) Experimental and computational studies of Nicotiana tabacum leaves extract as green corrosion inhibitor for mild steel in acidic medium. Alex Eng J 54:769–775
Loto CA, Loto RT, Popoola API (2011) Effect of neem leaf (Azadiratcha indica) extract on the corrosion inhibition of mild steel in dilute acids. Int J Phys Sci 6(9):2249–2257
Chigondo M, Chigondo F (2016) Recent natural corrosion inhibitors for mild steel: an overview, J Chem
Muthukrishnan P, Jeyaprabha B, Prakash P (2017) Adsorption and corrosion inhibiting behavior of Lannea coromandelica leaf extract on mild steel corrosion. Arab J Chem S2343–S2354
Chaubey N, Singh VK, Quarishi MA (2016) Papaya peel extract as potential corrosion inhibitor for Aluminium alloy in 1M HCl: electrochemical and quantum chemical study. Ain Shams Eng J
Michael NC, Olubunmi JA (2014) The corrosion inhibition of mild steel in sulphuric acid solution by flavonoid (catechin) separated from Nypa fruticans Wurmb leaves extract. Sci J Chem 2(4):27–32
Odewunmi NA, Umoren SA, Gasem ZM (2015) Watermelon waste products as green corrosion inhihbitors for mild steel in HCl solution. J Environ Chem Eng 285–296
Mayangalambam RS, Sharma V, Singh G (2011) Musa paradisiacal extract as a green inhibitor for corrosion of mild steel in 0.5 M sulphuric acid solution. Portugaliae Elecrochimica Acta 29(6):405–417
Ramananda Singh M, Gupta P, Gupta K (2015) The litchi (Litchi Chinesis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5 M H2SO4 solution. Arab J Chem
Sangeetha M, Rajendran S, Sathiyabama J, Prabhakar P (2012) Eco friendly extract of Banana Peel as corrosion inhibitor for carbon steel in sea water. Sch Res Libr 2(5):601–610
Sharma SK, Peter A, Obot IB (2015) Potential of Azadiratcha indica as a green corrosion inhibitor against mild steel, aluminium, and tin: a review. J Anal Sci Technol 6:26
Sastri VS (2011) Green Corrosion inhibitors: theory and Practice. Wiley Publications
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Nandini, G.K.M., Gokul, N., Chandra, B., Khalid (2022). Concocting Nanostructured Plant Molecules for Inhibiting the Corrosion Activity in HYSD Bars. In: Satyanarayanan, K.S., Seo, HJ., Gopalakrishnan, N. (eds) Sustainable Construction Materials. Lecture Notes in Civil Engineering, vol 194. Springer, Singapore. https://doi.org/10.1007/978-981-16-6403-8_16
Download citation
DOI: https://doi.org/10.1007/978-981-16-6403-8_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6402-1
Online ISBN: 978-981-16-6403-8
eBook Packages: EngineeringEngineering (R0)