Abstract
The impact of environment can cause many types of degradations such as pitting corrosion, stress corrosion cracking and sulphide stress cracking of metal structures. One of the serious problems of oil extracting industry is the corrosion process. Recently, there were a number of resource failures caused by internal corrosion phenomena recorded in oil and gas industry; the reports confirmed that the failures were due to the effect of traces amounts of Hydrochloric acid. The objective of this study is to use the plant extracts as corrosion inhibitors for API 5L X52 steel. Indeed, these natural extracts contain many families of natural organic compounds “Green”, readily available and renewable. The conducted mechanics tests in this study in the presence of green inhibitors of plant origin will give very promising results on the fracture mechanics properties. The importance of this area of research is to be attributed to the fact that natural products can replace the currently used toxic organic molecules that are condemned by the world directives for using environmentally unacceptable inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pipelines play an important role in gas and oil transportation. Reduction of failure to increase a possible safe service life is one of the major challenges for oil and gas industry to prevent economic, life and ecological damages. Pipe corrosion is one of the major causes for material loss encountered in oil/gas industry and it is primarily caused by the presence of water together with acidic gas [1,2,3]. For natural gas transmission pipeline accidents due to corrosion, 36% were caused by external corrosion and 63% were caused by the internal corrosion [4,5,6,7,8,9]. Failure cases recorded in oil and gas industry, confirmed that the internal corrosion is caused by the effect of traces of H2S, HCl, or CO2 [10]. The concentration of these parameters increases as the pipes get older. This requires the development of an in situ process to stop any further corrosion from taking place. The system provides a continuous protective properties using inhibitor/coating application after the cleaning process. Inhibitors are substances or mixtures that once added in low concentration and in aggressive environment to a protection system, it inhibit, prevent or minimise the corrosion [11]. Generally the mechanism of action of the added inhibitor is one or more of the following mechanisms:
-
The inhibitor is chemically adsorbed (chemisorption) on the surface of the metal and forms a protective thin film with inhibitor effect or by combination between inhibitor ions and metallic surface.
-
The inhibitor leads a formation of a film by oxide protection of the base metal.
-
The inhibitor reacts with a potential corrosive component present in the aqueous media and the product is a complex.
Inhibitors form a layer on the corroding metal surface modifying the surface to reduce the apparent corrosion rate. They represent the largest class of inhibitive substances. Adsorption-type inhibitors are the most common barrier layer inhibitors. In general, these organic compounds are adsorbed and form a stable bond with the metal surface. The apparent corrosion rate decreases as surface adsorption is completed.
The objective of this paper is the study of efficiency of the green inhibitor to protect the pipe network for the internal corrosion phenomena which is caused by the 1 M/l of HCl acid media.
For this reason, we study the influence of the green inhibitors, on the mechanical properties of steel grade APL 5L X 52 manufactures to SONATRACH company. This research is divided into two parts, the first part investigates the influence of the 30% of synthetic and green inhibitors in HCl media on the corrosion materiel by the tensile test, for 7 days of immersion time. The second part studies the degradation of the mechanical properties of the API 5L X52 steel by different concentrations of the 1M/l of HCl acid media by using the Charpy test. The specimen preparation is passed through the following steps: The pipe is cut into shapes of bars of 15 mm width with a chain saw to the transverse direction. The bars were adjusted by a hydraulic press machine. Machining the bars so that their sections are of dimensions 10 × 10 mm by a conventional milling machine. The bars were cut in the form of lengths of 55 mm specimens by an electric saw. Correcting curvatures of specimens. Each specimen was notched in the middle V-shaped by a manual sewing machine.
Neutralising inhibitors reduce hydrogen ions in the environment. Typical neutralising inhibitors are amines, ammonia and morpholine. Scavenging inhibitors remove corrosive ions from solutions. Some well-known scavenging inhibitors are hydrazine and sodium sulphite. Cleaning and protecting process by inhibitor is used in steel pipeline diameters from 4 to 36 inches and larger diameters are possible. The protected system using corrosion inhibitor is made by injection in steel pipelines, and works with oil, gas, water, petroleum products, food and chemical products. It has the ability to cover pits and channel corrosion and will cover all lateral and girth welds. Injection of inhibitors is made according to the device described in Fig. 1.
When the pipeline is in service, it is necessary to pig the line to maintain efficiency and to control the corrosion. It is necessary to remove liquids in wet gas systems, remove accumulated water in product pipelines and paraffin in crude oil pipelines. Table 1 shows some reported results that highlight the difference in efficiency between synthetic corrosion inhibitors from a study conducted at production aerial facilities of crude gas in Hassi R’Mel field (Algeria).
The results reported in the above-mentioned table are for two used two commercial inhibitors labelled A and B. The protection methodology using these synthetic inhibitors is very expensive, toxic for humans and not friendly to the environment [12]. The use of “Green” inhibitors to replace toxic synthetic inhibitors was the subject of many recent studies [13,14,15,16,17].
Recently, various “Green” inhibitor solutions for pipe protection were obtained by extraction of the inhibitive solutions from natural plants. The developed “Green” inhibitors contain many families of natural organic compounds (flavonoids, alkaloids, tannins....), readily available and renewable.
In this paper, hydrogen embrittlement which occurs by chemical reaction of hydrochloric acid on iron is evaluated by tensile and bending properties as well as Charpy energy and dynamic fracture toughness. The prevention of hydrogen embrittlement was achieved using a green inhibitor which was obtained from the Mediterranean plant (Ruta chalepensis). A significant amelioration of mechanical properties was obtained by using this green inhibitor. The inhibition action can be attributed to the formation of a thin film of the inhibitor on the metal surface. The influence of both inhibitor concentration and immersion time was carefully addressed in this study.
2 Material
This study is related to pipe steel API 5L X52. The material is delivered as tubes which are manufactured by hot rolling. The chemical composition of steel and mechanical properties are correspondingly given in Tables 2, 3 and 4.
The microstructure of this steel was investigated by optical microscope after etching the surface of the specimen with 2% nital solution. Microstructure reveals bands of ferrite and perlite along the rolling direction (Fig. 2).
3 Green Inhibitor from R. chalepensis
In this study, the extracts of R. chalepensis plant were used as green inhibitors against steel hydrogen embrittlement. R. chalepensis is a species of flowering plant in the citrus family known by the common name “fringed rue”. It is native to Eurasia and North Africa. It has been found elsewhere as an introduced species. It is a perennial herb growing up to 80 cm tall. The leaves are compound, each divided into several segments which are subdivided into smaller leaflets. The inflorescence is a cluster of flowers, each with four or five bright yellow petals with rolled, fringed edges. The fruit is a textured capsule which is divided into pointed lobes. In traditional medicine, the plant is used as a herbal remedy for a number of ailments, such as fever and inflammation. The aerial part of the plant was harvested in May 2015 from the Sidi-Maafa Forest (Chlef western Algeria). The dried plant material was stored in the laboratory at room temperature (298 K) and in shade before extraction.
The corrosion inhibitor was prepared at the Laboratory Industrial Chemistry department of Faculty of Technology, University of Chlef Algeria, as to maintain a constant composition throughout experiments. R. chalepensis plant leaves (Fig. 3) were soaked in deionized water (500 mL) and refluxed for 5 h. The aqueous solution was filtered and concentrated to 100 mL. This concentrated solution was used to prepare solutions of different concentrations by dilution method. To obtain the mass of plant extract, drying at 100 °C under vacuum in vaporizer was made. From the weight of the vacuum-dried liquid, plant extract was found to contain 50 mg mL−1 of plant compounds.
4 Influence of Green Inhibitors on Tensile Properties of Steel API 5L X52 After Immersion in Hydrochloric Acid Solution
The stress–strain curves have been obtained from specimens made of API 5L X52 steel and immersed in different solutions of hydrochloric acid of the following compositions: 1.2 M/l HCl, 1 M HCl + 30% green inhibitor and 1M HCl + 30% synthetic inhibitor. The immersion time for the steel samples in these solutions was 7 days at 298 K. Table 5 shows the tensile properties (yield stress σy, ultimate tensile strength σul and elongation A%) after these different immersions. Results are the mean value of two test specimens and a reference value (non-immersed) is also included.
Hydrogen embrittlement for this kind of steel affected particularly the elongation at failure which was reduced by 46% after immersion in HCl. One notes that only the use of green inhibitor is able to recover the initial elongation at the failure step. The use of synthetic inhibitors induced a lower degradation of A% of 35%. Degradation of tensile properties is attributed to hydrogen content produced by chemical reaction of hydrochloric acid on iron. The green inhibitor is chemically adsorbed (chemisorption) on the surface of the metal and forms a barrier to hydrogen diffusion (Fig. 4).
5 Influence of Green Inhibitors on Bending Properties of Steel API 5L X52 After Immersion in Hydrochloric Acid Solution
Charpy V-notched specimens were used as three-point Single Edge Bending specimens (SEB) to quantify the evolution of fracture energy and toughness as a function of green inhibitor concentration. Four corrosion green inhibitor concentrations (0, 5, 20 and 30%) with 1 M hydrochloric acid were considered in the present study. Specimens were loaded by bending (with a span of 40 mm) until a fracture of 1 mm/min cross head speed. The load–midspan deflection curves were recorded until fracture (Fig. 5). The values of fracture energy were obtained from these curves by integration.
The curves in Fig. 5 exhibit three stages: the first stage describes a linear load which increases by until general yielding Pgy. The second stage is a non-linear load which increases until maximum load Pmax and for the last stage, the load decreases until failure. Load at general yielding Pgy does not affect by immersion in hydrochloric acid with or without green inhibitors. Maximum load Pmax and failure displacements affect by immersion in hydrochloric acid with or without green inhibitors. The results are reported in Table 6. The area under the curve load–displacement until failure is equal to failure energy Uc. Fracture toughness represents the resistance of fracture initiation by the form of energy to initiation Ui. This energy is the area under the curve load–displacement until initiation or critical load which occurs before maximum load. A conventional definition of critical load is given by (1):
It notes that the severe degradation of the energy to fracture initiation occurs after immersion in hydrochloric acid. With the 30% of green inhibitor sample, the fracture initiation energy had practically recovered its initial value.
6 Influence of Green Inhibitor on Charpy Energy of Steel API 5L X52 After Immersion in Hydrochloric Acid Solution
6.1 Charpy Energy After Immersion in Hydrochloric Acid
Charpy V-notch made in steel API 5L X52 was immersed in hydrochloric acid solution with the following concentrations 0.25, 0.5, 0.75 and 1 M. Immersion times before tests were: 0.25, 5, 10 and 15 days. Only the specimen notch was subjected to the action of hydrochloric acid (Fig. 6) and the rest of the specimens were coated with epoxy-based anticorrosive paint. The Charpy impact test energy was measured for different hydrochloric acid concentrations for the same exposure time (0.25 day). Results are presented in Table 7 and show a decrease in the Fracture energy (J) upon increasing the HCl concentration.
The Charpy impact test energy was measured versus immersion time for the same hydrochloric acid concentration (0.25 M). Results are presented in Table 8 and show an opposite relationship between the fracture energy and the immersion time.
6.2 Charpy Energy After Immersion in Hydrochloric Acid Solution with Green Inhibitors
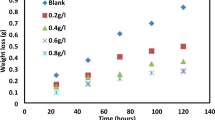
We have also studied the influence of adding a green inhibitor to the HCl acid solution (100 mL, 1 M) on the mechanical properties of immersed steel specimens in the above corrosive solution containing 5, 20 and 30% of the R. Chalepensis extract. Tests were conducted during four time durations (6 h, 5, 10 and 15 days) in the presence and absence of corrosion inhibitors.
The modification of fracture energy in the presence of inhibitors occurred in a relatively narrow band [195–180 J] and close to the reference value. Charpy energy decreased slowly with time while increased with concentration. The decrease in the Charpy energy was severe when the corrosion inhibitor was absent which confirms the beneficial effect of corrosion inhibitors on the mechanical properties. Figure 7 shows the results of the fracture energy versus the immersion time. In the absence of any added green corrosion inhibitor to the system, fracture energy decreased from 195 to 165 J after 15 days of continuous immersion in the corrosive medium.
7 Influence of Green Inhibitors on Dynamic Fracture Toughness of API 5L X52 Steel
Dynamic fracture toughness was determined from load–displacement curve obtained from instrumented Charpy impact test. A typical recorded load–displacement curve is shown in Fig. 8.
Critical load Pc was determined according to the definition of Eq. 1 which allows the determination of fracture initiation energy Ui. Fracture toughness Jc was assumed to be proportional to the specific energy for initiation \({U_{\text{i}}}/Bb,\) where B is the specimen thickness and b the ligament width [18].
where η is a coefficient of proportionality which has the value of 1.65 for a TPB specimen with a notch of relative depth a/W = 0.2 (a notch depth, W specimen width) and a notch radius ρ = 0.25 mm [19] (Figs. 9, 10).
Charpy specimens were immersed in hydrochloric acid solution having and free of green inhibitors for 7 days. The concentrations of green inhibitor in the solution were 3, 5, 10 and 30%. Results are depicted in Table 8.
Figure 11 depicts the beneficial effect of increasing the green inhibitor concentration on the static fracture toughness of steel API 5L X52 after immersion in hydrochloric acid solution containing and free of added green inhibitors.
8 Influence of Immersion Time in Hydrochloric Solution with Green Inhibitors on Dynamic Fracture Toughnesss of Steel API 5L X52
Charpy specimens were immersed in hydrochloric acid solution with and without green inhibitors for an immersion time vary from 3 to 10 days at a temperature of 80 °C. The concentration of the added green inhibitor was fixed to be 5%. Instrumented Charpy impact tests were performed on specimens after immersion. Load–displacement diagrams were recorded (Fig. 12). The critical load Pc, the fracture initiation energy Ui and dynamic fracture toughness Jc were extracted from these recorded diagrams according to the above-mentioned procedure. Results are presented in Table 9.
Table 9 indicates clearly that the beneficial effect of the added green inhibitor on the dynamic fracture toughness of API 5L X52 steel after immersion in hydrochloric acid solution free and containing 5% of the green inhibitor. Detrimental effect of immersion in hydrochloric acid solution increased upon increasing the immersion time.
9 Hydrogen Embrittlement of Steel API 5LX52
The detrimental effect of immersing steel API5L X52 samples in hydrochloric acid is attributed to the hydrogen embrittlement. Anodic dissolution of iron is done according to chemical reaction:
Recombination of adsorbed hydrogen
Absorption of Had
Hydrogen diffuses very rapidly to defect where its concentration increases locally due to the effect of the stress triaxiality [20, 21]. When the local concentration is great enough, the transition ductile to brittle fracture occurs and cleavage occurs as mentioned. Hydrogen embrittlement of API 5LX52 has been studied recently by [22,23,24,25,26]. Specimens are hydrogen charged in NS4 solution at some constant potential of polarisation, which is slightly negative than free corrosion potential for the given steels. The hydrogen-charging process is controlled by registration of the cathodic polarisation current \({I_{{\text{cath}}}}\left( \tau \right)\). The total quantity of evaluated hydrogen on metal surface can be assessed as:
Hydrogen concentration in metal has been determined on the basis of hydrogen discharging process under anodic polarisation using hydrogen electrochemical oxidation method proposed in this work. Here, the standard three-electrode electrochemical cell has been used. According to the recommendation of work (Capelle et al. [22]), the hydrogen discharging of specimen is carried out in 0.2 M NaOH (pH 12.4) solution under anodic polarisation \({E_{{\text{anodic}}}}=+{\text{168mV}}\left( {{\text{SCE}}} \right)\) during some defined time \({\tau _{{\text{dis}}}}\). The total quantity of absorbed hydrogen by metal can be defined as:
where \({I_H}\left( \tau \right)\) is an anodic polarisation current for hydrogen-charged specimen and \({I_{{\text{ref}}}}\left( \tau \right)\) is anodic polarisation current for specimen without hydrogen (reference curve). The calculation of hydrogen concentration was done according to the following formula:
where z is the number of electrons take in reaction; F is the Faraday constant; v is the effective volume of specimen: \({C_H}\left[ {{\text{mol}}/{\text{c}}{{\text{m}}^3}} \right]\);\(~Q_{H}^{{{\text{abs}}}}\left[ {A \cdot s} \right]\); \(z=1\); \(F=9.{\text{65}} \cdot {\text{1}}{{\text{0}}^4}\,{\text{C}}/{\text{mol}}\); \(v=0.{\text{256c}}{{\text{m}}^3}\).
Tensile tests were also performed and the tensile properties (yield stress σy, ultimate tensile strength σul and elongation at failure) after hydrogen embrittlement are reported in Table 10.
Hydrogen has a reduced influence on the stress–strain behaviour of API 5L X52 steel. Tensile tests indicate that the strain hardening exponent n and coefficient k are increasing of about 10% of their values obtained by test made with steel specimens without hydrogen absorption. In local fracture test, the initiation was detected by acoustic emission and energy to fracture initiation Ui deduced from load–displacement diagram (Fig. 13).
The main observation based on the results is the existence of some critical time of exposition and as a consequence of some critical hydrogen concentration \(C_{H}^{{}}\), when the essential decreasing of fracture toughness value occurs (Fig. 14) [23]. The value of \(C_{H}^{{}}\)for steel X52 is 4.3 × 10−6 mol/cm3.
Determining of critical hydrogen concentration \(C_{H}^{{}}\) in steels API X 52 [23]
10 Role of Green Inhibitors in Prevention of Hydrogen Embrittlement of Steel
The influence of corrosion inhibitors for the protection of steel against hydrogen embrittlement has been studied since a long time as Tkachenko et al. in 1968 [11]. It has been found that a concentration of the combination of propargylamine and an ethylene oxide adduct of phenylbutynol between 0.01 and 0.5% by weight of the aqueous sulphuric acid solution is an effective hydrogen embrittlement-inhibiting concentration, with a concentration between 0.01 and 0.1% being particularly advantageous even for aqueous acid systems.
The US patents US 3337469 A [12] is based on this chemical composition. The use of green inhibitors is more recent and the study of their effects on hydrogen embrittlement has started at the beginning of this century. Green corrosion inhibitors are biodegradable and do not contain heavy metals or other toxic compounds and these are the major reasons for their choice. Several plant extracts have been recognised as potential green inhibitors for steel; see Table 11.
The performance of an organic inhibitor is related to the chemical structure and physicochemical properties of the compound like functional groups, electron density at the donor atom, p-orbital character, and the electronic structure of the molecule. The inhibition could be due to:
-
(i)
Adsorption of the molecules or its ions on anodic and/or cathodic sites,
-
(ii)
Increase in cathodic and/or anodic over voltage, and the formation of a protective barrier film. Some factors that contribute to the action of inhibitors are (i) chain length, (ii) size of the molecule,
-
(iii)
Bonding, aromatic/conjugate,
-
(iv)
Strength of bonding to the substrate,
-
(v)
Cross-linking ability,
-
(vi)
Solubility in the environment.
The role of inhibitors is to form a barrier of one or several molecular layers against acid attack. This protective action is often associated with chemical and/or physical adsorption involving a variation in the charge of the adsorbed substance and transfer of charge from one phase to the other. The plastic deformation of metals is influenced by the surface. Surface effects can be modified with the chemical environment. The principal effects are on yield stress and strain hardening which appears after appreciable strain. This effect was fist mentioned by Roscoe [24] and called now “Roscoe effect”.
11 Conclusion
Immersion of API 5L X65 in hydrochloric acid induces hydrogen embrittlement. Hydrogen embrittlement is prevented through the use corrosion inhibitors. The use of synthetic inhibitors is very expensive, toxic for humans and not friendly to the environment. Therefore, it is preferable to use green inhibitors. In order to protect Algerian pipelines, a Mediterranean plant which is based on R. chalepensis has been chosen to produce a green inhibitor.
Extracts of this plant have improved their efficiency against degradation of mechanical properties such as elongation to failure, Charpy energy, energy for fracture initiation and fracture toughness. Efficiency increases with green inhibitor concentration and time of immersion. Adsorption of green inhibitor and formation of a protective layer which prevents the formation of adsorbed hydrogen is the proposed mechanism of action.
Change history
13 November 2018
In the Introduction section of the article, the reference to the study referred to in the following sentence is missing: Table 1 shows some reported results that highlight the difference in efficiency between synthetic corrosion inhibitors from a study conducted at production aerial facilities of crude gas in Hassi R’Mel field (Algeria) [1]. In addition, the photo credit for Fig. 1 is missing. The images are from a Master Thesis by Gharbi Kheira, Ouargla University, Algeria (2015).
References
Ossai CI (2012) Review article, advances in asset management techniques, an overview of corrosion mechanisms and mitigation, strategies for oil and gas Pipelines”; International Scholarly Research Network, ISRN Corrosion
Hadj-Meliani M, Bouledroua O, Ould-M’beirick M, El-Miloudi K, Neggaz D, Nateche T, El-Azzizi A, Bokort H, Houari F, Pluvinage G (2016) The two-parameter approach for fracture mechanics: some industrial applications In: Pluvinage G, Milovic L (eds) Fracture at all scales. Lecture Notes in Mechanical Engineering. Springer, New York, pp 105–134
Bouledroua O, Hadj-Meliani M, Azari A, Sorour N, Merah GP (2017) Effect of sandblasting on tensile properties, hardness and fracture resistance of a line pipe steel used in algeria for oil transport. J Fail Anal Prev 17(5):890–904
Hadj-Meliani M, Matvienko YG, Pluvinage G (2011) Corrosion defect assessment on pipes using limit analysis and notch fracture mechanics. Eng Fail Anal 18(1):271–283
Palmer-Jones R, Paisley D (2000) Repairing internal corrosion defects in pipelines-a case study, 4th International Pipeline Rehabilitation and Maintenance Conference, Prague
Nateche T, Hadj-Meliani M, Khan SMA, Matvienko YG, Merah N, Pluvinage G (2015) Residual harmfulness of a defect after repairing by a composite patch. Eng Fail Anal 48:166–173
Hadj-Meliani M, Benarous M, Azari Z, Pluvinage G (2009) Constraint parameter for a longitudinal surface notch in a pipe submitted to internal pressure. Key Eng Mater 399:3–11
Meriem-Benziane M, Abdul-Wahab SA, Zahloul H, Babaziane B, Hadj-Meliani M, Pluvinage G (2015) Finite element analysis of the integrity of an API X65 pipeline with a longitudinal crack repaired with single-and double-bonded composites. Compos B 77:431–439
CAPP (2009) Best management practices: mitigation of internal, corrosion in oil effluent pipeline systems. DocId = 155641&DT = PDF
Michael Baker Jr., Inc. ,Raymond R. Fessler, BIZTEK ,Pipeline Corrosion FINAL REPORT, U.S. Department of Transportation (2008) Department of transportation, pipeline and hazardous materials safety administration, Office of Pipeline Safety, Integrity Management Program, Under Delivery Order DTRS56-02-D-70036
Tkachenko N, Vasilenko II, Liskevich IY (1968) Inhibitors for protecting steel against hydrogen embrittlement in acid media” Soviet materials science: a transl. of Fiziko-khimicheskayamekhanikamaterialov. Acad Sci Ukrainian SSR 3(3):268–269 N
US 3337469 patent (1964) A Hydrogen-embrittlement-inhibition with propargy lbenzylamine and ethylene oxide adduct of phenylbutynol”. 15 Apr,
Lyon S (2004) A natural way to stop corrosion. Nature 427(406):407
Amitha Rani BE, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros. https://doi.org/10.1155/2012/380217
Benghalia MA, Fares C, Khadraoui A, Hadj-Meliani M, Obot IB, Sorrour A, Dmytrakh M, Azari Z (2018) Performance evaluation of a natural and synthetic compound as corrosion inhibitors of API 5 l X52 steel in hydrochloric acid media. Mor J Chem 6:51–61
Mohamed S, Hadj-Meliani M, El-Miloudi K, Fares C, Benghalia MA (2017) Corrosion effects and green scale inhibitors in the fracture mechanics properties of gas pipelines. J Struct Integrity Life 17((1):25–31
Khadraoui A, Khelifa A, Hadj-Meliani M, Mehdaoui R, Hachama K, Tidu A, Azari Z (2016) Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies. J Mol Liq 216:724–731
Pluvinage G (2003) Fracture and Fatigue emanating from stress concentrators, Editeur Kluwer, Alphen aan den Rijn
Akourri O, Louah M, Kifani A, Gilgert J, Pluvinage G (2000) The effect of notch radius on fracture toughness J1c. Eng Fract Mech 65:491–505
Hadj Meliani M, Matvienko YG, Pluvinage G (2011) Two-parameter fracture criterion (K ρ, c-T ef, c) based on notch fracture mechanics. Int J Fract 167(2):173–182
Bouledroua O, Elazzizi A, Hadj-Meliani M, Pluvinage G, Matvienko YG (2017) T-stress estimation by the two-parameter approach for a specimen with a v-shaped notch. J Appl Mech Tech Phys 58(3):546–555
Capelle J, Gilgert J, Dmytrakh I, Pluvinage G (2008) Sensitivity of pipelines with steel API X52 to hydrogen embrittlement. J Hydrog Energy 33:7060–7641
Capelle J, Dmytrakh I, Pluvinage G (2009) Hydrogen effect on local fracture emanating from notches in pipeline with steel API X52. Probl Strength 5:401
Roscoe R (1926) The plastic deformation of cadmium single crystals. Phil Magn 21:399–406
Elazzizi A, Hadj-Meliani M, Khelil A, Pluvinage G, Matvienko YG (2015) The master failure curve of pipe steels and crack paths in connection with hydrogen embrittlement. Int J Hydrog Energy 40(5):2295–2302
Hadj-Meliani M, Azari Z, Matvienko YG, Pluvinage G (2011) The effect of hydrogen on the master failure curve of APL 5L gas pipe steels. Procedia Eng 10, 942–947
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soudani, M., Hadj Meliani, M., El-Miloudi, K. et al. Efficiency of Green Inhibitors Against Hydrogen Embrittlement on Mechanical Properties of Pipe Steel API 5L X52 in Hydrochloric Acid Medium. J Bio Tribo Corros 4, 36 (2018). https://doi.org/10.1007/s40735-018-0153-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0153-0