Abstract
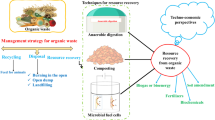
Much of the solid waste produced annually is high in organic content, and while the definition of what exactly is considered to be organic waste differs based on locale, the sheer volume of organic waste produced is shocking. Organic waste contributes to greenhouse gas (GHG) emissions and often carries costly disposal fees. Redirecting the organics from the waste into a higher value use can (1) mitigate emissions, (2) potentially reduce cost, (3) save time and effort on producing primary resources, (4) produce valuable goods and commodity chemicals. The organic fraction of municipal solid waste (OFMSW) can be recycled via biodegradation into readily used methane gas or into chemical building blocks such as acids or alcohols. Biodegradation may include anaerobic digestion, fungal transformation, and composting. This chapter will explore selected types of biodegradation, factors affecting each type, how the composition of the organic fraction affects the outcome of the biodegradation products, and the mitigation potential for recycling OFMSW via biodegradation. Upcycling, recycling, or repurposing carbon-rich wastes will enhance the carbon circular economy and reduce the burden on primary production. This chapter aims to demonstrate the utility of biodegradation to produce market-ready products from otherwise wasted resources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Agricultural residues
- Biodegradation
- Anaerobic digestion
- Lactic acid
- Food waste
- Organic fraction municipal solid waste
1 Introduction
Global waste generation and disposal has become a salient issue after the turn of the century. In an effort to shift from a linear to a more circular economy, reuse and conversion of wastes away from the landfill and into more suitable and higher value products is necessary. A potential solution is to use the carbon present in the organic fraction of municipal solid waste (OFMSW) – in the form carbohydrates (i.e. sugars) – as a resource for biochemical degradation and conversion. OFMSW is rich in both simple and complex sugars: food waste has been shown to have concentrations of up to 71.5% total sugars on a dry basis [1], and cotton waste fibers are around 95.4 wt % cellulose [2].
Sugar-rich waste is an ideal candidate for biodegradation to mitigate the ever growing stream of organic waste by diverting it from a landfill and into a process by which microorganisms consume the sugars to produce higher-value products. The aim of this chapter is to highlight recent findings and to demonstrate the current understanding of how OFMSW and other organic wastes can act as a feedstock for biodegradation.
Biodegradation of OFMSW into valuable products – specifically commodity chemicals or petro-chemical replacements – reduces demand on virgin resources and assists in the goal for national energy independence. In 2007, the United States passed the “Energy Independence and Security Act” with a goal to reduce reliance on foreign entities for their energy sources [3]. While energy independence is a significant driving force, reduction in volume of organic solid wastes annually landfilled is another. Of the 268 million tons of municipal solid waste (MSW) produced in the USA in 2017, the majority (53%) was landfilled [4]. Due to the organic nature of the OFMSW, degradation continues regardless of final resting place. When landfilled, OFMSW degrades resulting in both leachate and greenhouse gas emissions [5]. The leachate, which is the liquid excreted from landfills to the environment, levels increase with OFMSW decomposition and can reduce landfill gas collection operation [6]. Landfilling as a waste management solution for organic wastes also increases the negative impacts on human health (e.g., cardiovascular, pulmonary) by the waste treatment chemicals used [7, 8].
Background and current status of research into OFMSW and other waste organics as they pertain to biodegradation to produce either liquid or gaseous products that have a demonstrated market demand will be elucidated [3]. First, liquid-based products produced through the acetone-butanol-ethanol (ABE) fermentation schema will be discussed followed by the sequential production of lactic acid and methane gas.
The major organic wastes that will be discussed are categorized as either agricultural residues or organic fraction of municipal solid wastes. All wastes discussed are organic in composition and have either been previously studied as potential candidates for a biodegradation or have a high potential for conversion to higher value products.
1.1 Agricultural Residues
Agricultural residues are considered to be wastes derived from agricultural processing. The term ‘agricultural processing’ refers to anything from tapioca production, resulting in cassava bagasse, to rice harvesting, resulting in rice straw generation. The defining characteristic of agricultural residues, as applied to this chapter, is that these parts of the crop are generally deemed inedible and are conventionally discarded as refuse [9]. The term bagasse is used to describe the more fibrous waste of a crop [10]. The agricultural residues highlighted in this chapter are cassava bagasse, three different straws (rice, barley, and wheat), sugar beet pulp, and corn stover.
Cassava is predominately found in diets of the people living in Asian, African, and Latin American locales, making cassava the sixth most important global food crop. The major solid wastes from cassava processing are bagasse and peels, which are inedible due to their deadly concentration of cyanide [11]. For every 250–300 tons of edible tubers of cassava processed, about 280 tons of bagasse and 1.6 tons of peels are produced [9]. Cassava bagasse contains water, residual starches, and cellulose fibers, where the residual starches and cellulose fibers can be used in microbial biodegradation [3, 12]. The global production of cassava in 2019 was 304 million tons, up from 287 million tons in 2017 [13].
Rice straw is the residue remaining in the field after harvest [14, 15]. The volume is dependent upon the techniques employed for grain removal, including the cutting height, as well as the choices by the growers regarding the treatment of the straw not harvested with the grains. When these factors are considered, the global rice straw production in 2019 was between 370 and 520 million tons [13]. Of the rice straw production in 2017 the United States produced only 4.3 million dry tons and the United States Department of Energy (DOE) forecasts there to be 10.8 million dry tons by 2030 [3]. The DOE used an estimate of the moisture content of the grain to be 13.5% and a ratio of 1 dry gram of rice to 1 dry gram rice straw [16]. While grown to a lesser extent globally than rice, barely straw is also a significant agricultural residue to consider. The worldwide production of barley straw in 2019 was 159 million tons and it is estimated that between 0.33 to 0.53 kg of straw is produced per kg of barley [17].
Sugar beets are mainly grown for use as a source of crystalline sugars with 270 million tons produced annually with a dry basis of five million tons of sugar beet pulp annually, 20% of which is produced within the USA [18, 19]. The process to transform sugar beets into crystalline sugar also results in three major byproducts: tops, pulp, and molasses [20]. While sugar beet molasses has been used in fermentation to produce alcohol and methane, sugar beet pulp is the reside of focus for this chapter due to its high-solids composition [20, 21].
Corn stover is the solid agricultural waste byproduct created when growing corn; generally, corn stover is most every part of the grass that is not the ear. [22]. Barley straw and rice straw account for less than a 1:1 ratio for their product by weight, corn stover accounts for 1 kg stover per kg grain [3]. This fact, along with the fact that corn takes up over 12 times more land in the USA for growing than barley and rice combined, makes corn stover an attractive potential waste residue for biodegradation [3].
1.2 Municipal Solid Wastes (MSW)
The specific organic content of OFMSW is highly dependent on source, season, and region [17, 23, 24], though is usually abundant in food wastes (FW ), whether they be from residences, markets, or restaurants [24]. In 2015 the DOE reported that 15.1% of OFMSW was food wastes, 6.2% was wood, 13.3% from yard trimmings, and 25.9% was non-recycled paper products [25]. OFMSW tends to have higher moisture content than MSW and varied concentrations of rejected materials. Materials that may inhibit a biodegradation process and are present to varying degrees within OFMSW include plastics, cardboard, paper, metal, glass, bones, and fruit kernels [16]. Glass (4.4% of MSW), metals (9.1% of MSW), plastics (13.1% of MSW), as well as “other inorganic species found in MSW” (3.6% of MSW) are not usable as feedstock for anaerobic digestion [16, 26]. It is estimated that between 1.6 and 2.0 billion tons of MSW are produced globally per year. Of that, around 70% is landfilled, and only around 19% is recycled [27]. The production of MSW is around three times that of the combined production of agricultural residues of rice straw (520 Mt), barley straw (84 Mt), and cassava bagasse (76 Mt).
Food wastes are not the only organic-rich feedstock in MSW; textiles are another fraction of MSW and have a cellulosic makeup between 30 and 40% on average [28, 29]. In the United States, over the past two decades, the production of textile MSW by weight has increased 80%, from 9.48 million tons in 2000 to 17.03 million tons in 2018 [30]. Of the textile waste produced in these two decades, on average, 66% was landfilled, which is equivalent to 11.3 million tons in 2018 [30].
In 2019, the fraction of textile waste in MSW found in Lahore, Pakistan was 9.21%. This percentage was determined through a case study and does not define the percent of textiles found in MSW worldwide. In 2015 the fraction of MSW that can be attributed to rubber, leather, and textiles was 9.3% [26]. In the textile market globally, cotton is attributed to 30% of the market share and cellulose from cotton requires significantly more time to degrade naturally as compared to amorphous cellulose [2].
1.3 Biodegradation Processes Overview
The biodegradation processes discussed in this chapter focus on the production of either a liquid or gaseous product. The focus will be on two general schema, acetone-butanol-ethanol (ABE) and anaerobic digestion for the production of lactic acid and methane. Each will be discussed in detail in the Sects. 7.3 and 7.4. Table 7.1 is included here to highlight and summarize microorganism type and use on the substrates of interest. Table 7.1 displays strain, along with key operating parameters, sorted by process and also highlights the feedstocks pertinent to the present work.
2 Pretreatment to Overcome Substrate Challenges
To maximize yield of a desired product from organic solid waste, pretreatment may be required [36, 47,48,49,50,51,52]. Pretreatment methods that have been employed for organic wastes and agricultural residues can be categorized by one of the following groups: mechanical, physical, chemical, physicochemical, or biological. The prevalence and availability of these methods depends upon the scale of the study, the composition of the waste, and the availability of the resource. Section 7.2 aims to provide sufficient background to more acutely explain the differences in parameters and reasons for increased yield in each of the products discussed. Table 7.2 illustrates how different combinations of the pretreatment methods are related to each of the main feedstocks and how the pretreatment methods relate to the final products of interest. Note that while other pretreatment methods exist and are common for lignocellulosic materials that are not considered waste, such as switchgrass grown for energy production, they will only be discussed if they have demonstrated use in the pretreatment of organic solid wastes as well.
2.1 Physical and Mechanical Pretreatments
Physical and mechanical pretreatments employ either a physical or mechanical action or change to the substrate to increase yields of target components (e.g., cellulose, hemicellulose) [51]; examples include ultrasonication [67, 68], grinding [69, 70], rotary drum reactor [59, 63], and microwave treatment [65, 71, 72].
Grinding is a basic pretreatment that uses mechanical blades or impellers to reduce the size of the substrate [69, 70]. A hammer mill was used to grind wheat straw, barley straw, and corn stover with the goal of determining the physical properties at three particle sizes (0.8 mm, 1.6 mm, and 3.2 mm screen sizes) [70]. The bulk density achieved for the wheat straw, barely straw, and corn stover was highest for the smallest hammer mill screen sizes tested [70]. A household garbage disposal, which imparted the same type of action as a hammer mill, was compared to a bead mill to reduce FW particle and increase VFA production and methane yield [69]. A household garbage disposal was tested as a pretreatment option due to the distributed nature and volume of food waste production. It was reported that reducing FW particle size below a lower limit threshold of 0.6 mm particles resulted in VFA accumulation and decreased methane yield, as well as a reduction in FW particle solubility in the anaerobic chamber. The optimal particle size for methane production from FW was 0.62 mm [69]. Note that standard garbage disposal was only able to reduce the FW particles to 0.88 mm, whereas the bead mill achieved the optimal size of 0.62 mm and could further reduce the FW particles to 0.4 mm [69].
Ultrasonication uses targeted sound waves to sunder substrates prior to fermentation [67, 68]. Ultrasonic waves between 10 kHz and 20 MHz are emitted and weaken cell walls and break down lignin to improve accessibility of sugars to microorganisms [73]. The ultrasonic waves cause the formation of cavitation bubbles that, when disrupted, create a high pressure burst to lyse cells [73]. Ultrasonication was used on sugar beet pulp pellets that had been ground to 2 mm pieces to increase methane production 26% and methane concentration 79% when ultrasonication was paired with enzyme pretreatment, a magnetic field pretreatment, and grinding [74].
The rotary drum reactor has the same technological basis to traditional composting techniques, in that air is added to drums from the unloading side to assist in aerobic biodegradation and mechanical force degradation [59, 63]. A rotary drum reactor was able to separate biodegradable materials from MSW which resulted in a feedstock that was consistent in biogas yield to the quantity produced from FW alone [63]. A rotary drum reactor was successfully scaled-up to a 100-L capacity for use in the pretreatment of sugar cane bagasse to produce ethanol using SSF operating conditions [59].
Microwave pretreatment irradiates substrates with electromagnetic waves, between 0.3 and 300 GHz frequency, and can be used concurrently with chemical pretreatments to enhance cellulose concentrations from organic solid wastes by disrupting cells through polar and dielectric molecular interactions [65, 71, 72]. Microwave pretreatment has shown to increase glucose concentrationswhile reducing the concentration of fermentative inhibitors (e.g., 5-hydroxymethylfurfural and furfural) [71]. When microwave pretreatment was applied to a stand-in version of OFMSW, formulated based on previous characterization of Canadian kitchen waste, after size reduction pretreatment, resulted in an increased biogas production between 4 and 7%, suggesting synergy from combining pretreatments [65].
2.2 Chemical Pretreatments
Chemical pretreatments employ solvents – often acids – to reduce the long chain carbohydrates into shorter chain sugars that are more readily consumed during biodegradation. Organosolv is conventionally used for delignification of lignocellulosic biomass and it relies on an organic solvent (e.g., MgSO4, HCl, (NH4)3PO4) to degrade the lignin barrier of biomass [75]. For rice straw, an ethanol organosolv pretreatment (with a solid-to-liquid ratio of 1:8 and 75% (v/v) aqueous ethanol containing 1% (w/w) sulfuric acid catalyst) was shown to improve butanol production from 43.6 g/kg rice straw to 80.3 g/kg rice straw [56].
Alkaline pretreatment was considered as an alternative to organosolv to improve the yield of ABE from rice straw [52]. Alkaline pretreatment is often viewed as a promising path for pretreatment of agricultural residues due to its ability to simultaneously increase the internal surface area, decrease in cellulose crystallinity, and disrupt the lignin [52].
Phosphoric acid-acetone is a combination of two organic acids for enhanced organosolv pretreatment that has been shown to effectively pretreat cotton-based textile wastes to recover cellulose [2, 76]. The two-step method follows: (1) phosphoric acid (H3PO4) is used to treat solid textile cotton waste (2) acetone ((CH3)2CO) is used to recover cellulose. Step 1 dissolved 94% solids using 19 g H3PO4/g cotton compared to the initial solid mass. Step 2 recovered 97% cellulose using 41 g (CH3)2CO/g solid. Recovered cellulose here is defined by: grams cellulose precipitated per gram of dissolved cellulose [2]. Phosphoric acid-acetone pretreatment was shown to increase the glucose yield compared to untreated rice straw from 101.8 g glucose/kg rice straw [52].
2.3 Physiochemical Pretreatments
Physiochemical pretreatments take advantage of the discord between physical and chemical properties to synergistically create a force that breaks the barrier between complex substrates and fermentation. Examples of physiochemical pretreatments are hydrothermal pretreatment [50, 58], steam explosion [55, 77], and the use of supercritical CO2 (SC-CO2) [60, 78, 79].
Hydrothermal pretreatment (HTP) uses a high temperature water bath, between 160 °C and 180 °C for 30–60 min, to surround a vessel containing the substrate of interest in an effort to increase the hydrolysis rate [58]. HTP structurally changes organic solid wastes to allow more straightforward degradation and increases the soluble chemical oxygen demand by degrading insoluble organic carbohydrates and proteins [50]. The production of inhibitory compounds (i.e., melanoidins) is an inherent pitfall of HTP due to interactions between carbohydrates and amino acids during HTP, but it is possible to design the reaction vessel to counteract this by addition of acid consuming bacteria (i.e., Lactobacillus plantarum SF5.6) [50]. A two-phase anaerobic digestion of MSW was coupled with HTP and it was determined that biogas production in the two-phase anaerobic digestion was 31.5% higher if HTP was employed first as compared to the control; two-phase anaerobic digestion could reach a net energy output 97.4% higher than the one phase anaerobic digestion with HTP [50].
Steam explosion is an attractive pretreatment method for lignocellulosic biomass since it requires no chemical bar water and generates small amounts of waste [55]. Steam explosion and hydrothermal pretreatments both take advantage of high temperature water, but steam explosion uses saturated steam, whereas hydrothermal pretreatment uses the liquid phase of water [77]. The scientific basis behind steam explosion is that the high temperatures and high pressure is applied to the biomass, followed by a rapid drop in pressure, causing an explosive decompressive state that breaks down the hemicellulose [77].
Supercritical CO2 (SC-CO2) is another temperature dependent physiochemical pretreatment technique, but it uses CO2 to break down complex organic wastes [79]. When SC-CO2 was used to pretreat sugarcane bagasse resulting in an increase in fermentable sugar by 280%, compared to untreated sugarcane bagasse [60]. This pretreatment is favorable when used on biomass or organic solid waste because it does not result in the formation of harmful solvent byproducts [78].
2.4 Biological Pretreatments
Biological pretreatments use microorganisms, enzymes, or a combination thereof to reduce substrates, with similar complexity as to what is found in organic solid waste, and to maximize the fermentative production potential improving the carbon accessibility in the substrates [73, 80, 81].
Enzymatic hydrolysis may be necessary to access the organic solid waste as a carbon source in the fermentation media [73]. Selection of enzymatic hydrolysis over other pretreatment methods is based on both substrate composition and the downstream degradation process. The main goal of the enzymes used for pretreatment of organic solid wastes is to reduce carbon chain length [73, 82]. Enzymatic hydrolysis often targets hydrogen bonding within a carbon chain at designated bonding sites to reduce chain length [82]. Microorganisms can more readily consume a shortened carbon chain and thus increase the yield of a given product of interest. Cellulase, amylase, and β-glucosidase are commonly used enzymes for cellulose hydrolysis [81]. Microbial selections involving some Clostridia species, render enzymatic hydrolysis unnecessary, due to the innate enzymatic production of these microbes [83,84,85]. Section 7.3.1 will explore these features of Clostridium bacteria in more depth.
One of the microorganism-based approaches uses fungus to synthesize enzymes, with the goal of reducing operational cost increases seen from the use of industrially produced enzymes [28]. One example of such a fungus is Aspergillus niger which produces several hydrolysis enzymes such as α-amylase, β-glucosidase, xylanase, and cellulase [28].
3 Acetone-Butanol-Ethanol (ABE) Process
ABE fermentation is an anaerobic process reliant upon bacteria to produce acetone , butanol, and ethanol [86]. While early reports of butanol production using the ABE process revolved around the Clostridia genus, more recent industrial processes have used genetically modified Clostridium or Saccharomyces cerevisiae [87]. Fermentations with these specific species have controlled the genetics greatly, but use of mixed cultures has also been explored; combining Clostridium cellulovorans and Clostridium beijerinckii [88]. Mixed culture fermentation (MCF) is often employed in the fermentation of organic solids, but does not always lead to increased yields [57, 85, 89,90,91]. ABE fermentation uses a bi-phasic schema of acidogenesis, which produces butyrate, hydrogen, and CO2, followed by solventogenesis [86]. Microorganisms consume the products of the acidogenic phase and produce acetone-butanol-ethanol during a second phase called the solventogenesis phase [92].
ABE fermentation emerged in response to a growing acetone market during World War I, but production began to wane in the 1980s, as the petrochemical industry expanded its reach and portfolio [87, 93]. However, due to the rise of interest in alternative liquid fuels and renewable chemicals – namely in the form of biobutanol – research on ABE fermentation has reemerged [87, 93]. Butanol is commonly considered a drop-in ready liquid fuel and is an intermediate for the production in three disparate industries: artificial flavoring, solvents, and cosmetics [94].
Figure 7.1 depicts a generic process flow for an ABE fermentation. The unit operations shown in Fig. 7.1 include: pretreatment and enzymatic hydrolysis, fermentation, in situ separations, and a final separation to purify the product of interest from the mixture. The pretreatment and enzymatic hydrolysis steps are optional and depend on the substrate used; the fermentation contains an optional in situ separation process to assist in increasing yield as butanol is toxic to the microbes present.
The ABE production process – outlined in Fig. 7.1 – is a biphasic process where microorganisms (historically Clostridia) consume feedstock to produce acetic acid and butyric acid in the acidogenic phase (phase 1), and acetone, butanol, and ethanol in the solventogenic phase (phase 2) generally in a ratio of 3:6:1 respectively [87]. Variables that have been explored in the optimization of ABE production in terms of butanol production will be discussed. Butanol is produced in the second phase of the ABE fermentation, when microorganisms convert the organic acids produced in the solventogenic phase into acetone, butanol, and ethanol. The microorganisms that produce butanol through ABE fermentation at the industrial and lab scales are reviewed and then the organic solid substrates are presented and ranked by yield of butanol, and finally the impact of in situ separation of inhibitory compounds produced during the biodegradation mechanisms is discussed.
3.1 Microorganisms
Aerobic conditions, pH , and temperature used within a given ABE fermentation depend on microorganism selection. The genus Clostridia is often selected for this fermentation due to Clostridia’s ability to produce butanol [95]. Issues regarding use of Clostridia will be discussed and have led to increases in research into genetically modifying Escherichia coli and Saccharomyces cerevisiae [94, 96]. Of the Clostridia species, biobutanol is most prevalently produced from Clostridium acetobutylicum and Clostridium beijerinckii, however the exploration of other species with similar characteristics have been explored, but to a lesser extent [61, 97].
3.1.1 Clostridia
The Clostridia species most prevalent in the production of butanol are Clostridium acetobutylicum, Clostridium beijerinckii, Clostridium saccharoperbutylacetonicum, and Clostridium saccharobutylicum [95]. Clostridia bacteria digest sugar, starch, cellulose, and lignin – a trait that is especially advantageous when using an alternative feedstocks like agricultural residues and OFMSW [87]. These species produce butanol under the following optimal conditions: 30–40 °C, pH between 6.0 and 7.5, and anaerobic conditions [87]. The effect of pH on butanol production using a non-waste feedstock was reported; the initial pH of 6.2 yielded the highest concentration of butanol (6.28 g/L) of the pH values tested (5.0, 5.5, 6.0, 6.2, 6.5, 7.0) on the C. acetobutylicum YM1 strain [33]. Table 7.3 displays the range of pH values that have been used for ABE production along with the organic solid substrates used and their butanol yields.
Optimal temperatures used in ABE fermentation using Clostridia species are reported in Table 7.3 as well, however there has been interest in increasing the optimal temperature for this process by genetically modifying Clostridium strains to improve cellulase activity and hence butanol production. A shift towards temperatures around 42 °C yields 0.18 g/g-substrate compared to between (0.08 and 0.12) g/g-substrate in usual simultaneous saccharification and fermentation (SSF) processes using temperatures of 36 °C [32].
SSF processes allow for the reduction of unit operations due to the removal of pretreatment steps that take place in the same SSF reactor [32]. One of the major challenges of SSF was the temperature optimization conundrum due to the fact that enzymatic hydrolysis operates mostly between 45 °C and 50 °C, whereas Clostridium fermentation for ABE operates between 30 °C and 40 °C [32, 76]. Non-isothermal simultaneous saccharification and fermentation (NSSF) is a process designed to circumnavigate the temperature-based challenges of SSF, namely the differences in the optimal temperatures of enzymatic hydrolysis and fermentation, where the operation is set to the optimal enzymatic temperatures until the enzymatic hydrolysis has achieved the desired hydrolysis before shifting the temperature within the reactor to a more desirable fermentative temperature [81, 98].
One development to counteract downsides of SSF and NSSF is consolidated bioprocessing (CBP). The main aspect to CBP that sets it apart from SSF and NSSF is the use of a single organism to produce enzymes and carry out the fermentation [81]. This strategy takes advantage of the advances of recombinant DNA technology that have allowed extensive modification of microbial species to carry out these tasks [81]. A few reports on these genetic modifications on Clostridium have shown enhanced yields of up to 0.39 g/g of butanol and ethanol using CBP [81, 99].
3.1.2 Escherichia coli and Saccharomyces cerevisiae
Escherichia coli and Saccharomyces cerevisiae are model organisms due to the abundance of information and genomics surrounding their use and functionality [100]. A strong baseline knowledge of both E. coli and S. cerevisiae allow for reasonable modifications to optimize ABE fermentation processes and to improve butanol yield.
A butanol production pathway was added into the native bacterial chassis of E. coli which resulted in 33 native gene deletions and five heterologous gene introductions [101]. The strain was enhanced to produce a 34% yield with 20 g/L of butanol from a synthetic medium; a marked increase over engineered Clostridia strains which can produce 18.9 g/L with yield of 29% [101].
S. cerevisiae is another model organism that has been modified to include a butanol production pathway [102,103,104,105,106]. One goal behind implementing S. cerevisiae , co-cultured with C. acetobutylicum, was to raise the butanol toxicity threshold [107]. From this exploration, the butanol concentration was able to reach 16.3 g/L, over double the threshold of 8 g/L discussed in Sect. 7.3.3 [107].
3.2 Substrate Selection
OFMSW has been either homogenized or left as a heterogenous mixture for use as the carbon source in ABE fermentation. Table 7.3 compares the output of butanol, as it pertains to various organic solid substrates, and is sorted by highest to lowest butanol yield, while maintaining categorical separation between OFMSW and agricultural residues.
The fermentations shown in Table 7.3 were conducted with the Clostridia genus, with the majority (60%) of those being of C. acetobutylicum, followed by 30% from C. beijerinckii, while only one used C. tyrobutyricum. Microorganism selection is consistent with research trends in the past few years. It should be noted that each fermentation was performed at 36 ± 1 °C with 70% of the fermentations conducted at 37 °C. Only two studies used agitated vessels, with the final yield not among the highest yields achieved indicating that agitation may not be required. The highest yield of butanol per kg of agricultural residue was achieved from cassava bagasse at 300 g/kg [38], whereas the lowest yield from an agricultural residue was from rice straw at 112.7 g/kg [56]. Of the municipal solid waste substrates, the OFMSW from Isfahan, Iran and was pretreated with a combination of ethanol extraction, dilute acid pretreatment, and enzymatic hydrolysis, demonstrated the lowest yield of 83.9 g butanol/kg substrate [108]. The decreased yield in butanol for OFMSW as compared to agricultural residues may indicate that the sugars may not be accessible for the microorganisms and that additional pretreatment may be required to increase yield.
3.3 Separation
In situ separation processes are advantageous due to the inhibitory effect of butanol at concentrations greater than 8 g/L with regards to ABE production from Clostridium bacteria [39]. Pervaporation [110,111,112,113] and gas stripping [112, 114, 115] are conventional separation processes employed for product recovery during fermentation. Gas stripping removes the solvents produced from the media throughout the fermentation process by bubbling hydrogen or carbon dioxide through the fermentation broth, effectively stripping away ABE products as they are produced [115]. Pervaporation relies on membrane permeabilities to allow selected vapors to pass through the membrane pores with assistance of a vacuum [111]. This procedure partially vaporizes the fermentation broth by increasing the temperature of the feed broth to the heat of vaporization, then the vaporized broth passes by the membrane at which point the ABE products are pulled through the membrane via vacuum and condensed back into a liquid on the other side [111].
Gas stripping has been shown to maintain butanol concentrations below the critical inhibitory concentration of 8 g/L [112, 114]. When using a gas recycle flowrate between 0.3 and 0.6 vvm (gas volume per liquid volume per minute), 18.6 g/L of butanol was produced from industrial juices, such as sugar cane juice and sweet sorghum juice, when gas-stripping was used, compared to 10.5 g/L butanol from industrial juices without gas stripping [115]. When gas-stripping was applied to cassava bagasse hydrolysate, the butanol yield shifted from 0.22 g/g to 0.25 g/g, but the fermentation was able to produce 59.81 g/L with gas-stripping , and only 9.71 g/L without gas stripping [31], demonstrating that the more significant impact of in situ gas stripping lies in production over time, and not the yield.
Pervaporation relies on a liquid-to-vapor phase change, along with membrane technology, to separate butanol, along with other ABE products, from the fermentation media [110]. A model of a pervaporation membrane was constructed to determine efficacy in ABE production and was determined that an in situ process showed 250% higher butanol concentrations compared to control with no in situ separation [111]. A techno-economic analysis on the feasibility of pervaporation, along with gas-stripping , determined that biobutanol from MSW an economically viable process only if these two separations were incorporated. Pervaporation remains a relatively new yet technologically advanced separation technique [111, 112]; the most appropriate membranes for this separation are likely still under development.
4 Lactic Acid and Methane Process
Lactic acid and methane are generally concurrently produced – especially during anaerobic digestion. First, current approaches for optimizing production of lactic acid from organic solid wastes will be discussed followed by variables that can be modified to optimize lactic acid production and consequently achieve higher methane yields in the second phase. Lactic acid (LA) can be used in the production of various marketable products (e.g., anti-aging moisturizers, chemical cleaning agents, food preservatives, and dialysis solutions) [24]. The market size of lactic acid is estimated to increase by 254%, from $2.64 billion to $9.0 billion, between 2018 and 2025 [116]. Lactic acid produced by biodegradation is viewed as a more cost-effective and environmentally beneficial alternative to chemical synthesis because of the ability to utilize in-expensive waste streams as substrates and because chemical synthesis to produce lactic acid requires elevated temperatures and higher energy input [117].
Figure 7.2 illustrates a generic process flow for LA production via biodegradation, followed by optional methanogenesis of the volatile fatty acids (VFA). The unit operations shown in Fig. 7.2 include: pretreatment and enzymatic hydrolysis, acidogenic fermentation, optional in situ separation , separation of LA as a purified product, optional methanogenesis of remaining VFAs and media, in situ separation , and a final separation to purify the methane.
Figure 7.2 shows both the acidogenesis step and the methanogenesis step of organic solid waste fermentation. Methanogens transform organic acids into methane [118, 119]. The dotted line flowing from “Other Organic Acids” to “Methanogenic Bioreactor” represent an optional step to harvest the lactic acid and utilize the remaining broth, including other VFAs, produced during acidogenesis to produce methane. This flow is one potential route to maximize the profitability of methane production [120, 121].
The sequential production of lactic acid and methane is advantageous in anerobic digestion because it overcomes an economic hurdle of methanogenesis [24]. In the European Union (EU), LA fermentation production costs can range between 0.72 and 1.13 €/kg lactic acid with the market value at 1.36 €/kg lactic acid [24].
The microorganisms that are involved in acidogenesis include: lactic acid bacteria (LAB ) [122] and Saccharomyces cerevisiae [121], whereas methanogenesis is carried out by: Methanomicrobium mobile [123] and Methanosarcina [21]. Similar to ABE production, lactic acid production has been carried out with mixed cultures, such as the combination of LAB monocultures with various fermentative abilities that were used to produce lactic acid from sugar beet pulp [49].
4.1 Lactic Acid
Anaerobic digestion is performed as either a single or two-stage process with the transformation occurring in four phases beginning with hydrolysis, followed by acidogenesis, then acetogenesis, and finally ending with the methanogenesis [50, 124,125,126]. While anaerobic digestion can be conducted using a single reactor, or in sequential reactors; the choice between one or two reactors for the production of lactic acid and/or methane influences both microorganism selection and operating conditions [24].
4.1.1 Microorganisms
Microorganisms for lactic acid production from municipal solid wastes often include a mix of various lactic acid bacteria (LAB ) [122, 127], and S. cerevisiae [120, 121]. Refer to Table 7.1 for a summary and Table 7.4 for more specifics.
4.1.1.1 Lactic Acid Bacteria (LAB)
LAB is a classification that encompasses a wide variety of bacterium that efficaciously produce lactic acid; the two genera encompassed by LAB are Enterococci and Lactobacilli [128, 129].
The Enterococci genus includes species that operate at higher temperatures than any currently known Lactobacilli strain [128, 129]. Enterococcus faecium has been studied in a scale-up operation from 3 L to 100 L. It was concluded that E. faecium was a feasible option for industrial uses with a pilot scale production rate of 3.91 g/L-h. The lab scale production rate was 4.96 g/L-h, but the slower rate in the pilot scale was deemed a significant hurdle as the yield was not significantly different between the 3 L and 100 L scales [129].
Lactobacilli is the other main genus represented within LABs, spanning over 200 species of microorganisms [118]. With the abundance of species represented in LABs a versatility is present allowing operation anywhere from 2 °C to 53 °C as well as an ability to operate under many oxygenated states for growth [118].
4.1.1.2 Saccharomyces cerevisiae
S. cerevisiae can be engineered to produce D-lactic acid at a purity of 99.9% [46, 121]. The two enantiomeric forms of lactic acid that can be produced by fermentation are D and L forms of lactic acid. When used in the production of biologically derived plastics, L-lactic acid is more susceptible to thermal modifications at temperatures as low as 58 °C, lower than D-lactic acid [46, 128]. Furthermore, mixtures of D and L enantiomers of lactic acid can form an enhanced racemic crystal stereo-complex capable of increasing the melting temperature of the bioplastic by around 50 °C [130].
S. cerevisiae , combined with indigenous microorganism consortium within food waste, rich in Enterococcus spp., was used to determine the effectiveness of breaking down FW by measuring metabolite yield. The results showed that the use of both the indigenous consortium of bacteria and yeast together achieved metabolite yields of 81%, and the conclusion stated that this is a feasible combination of microorganisms to produce lactic acid through a more targeted fermentation than the one tested in this study [131].
4.1.2 Substrate Selection
Substrate selection for the production of lactic acid through anaerobic digestion is shown in Table 7.4. As can be seen in Table 7.4, mixed cultures are a popular choice for lactic acid production in the organic solid waste sector, with 55% of studies listed utilizing this approach. Interestingly, each of the reported pH values for this acidogenic fermentation are skewed acidic. It should also be noted that the results from the studies using specific organisms, and not mixed cultures, mostly achieved higher yields than any of the mixed culture studies reported here with the average lactic acid yield for single culture studies being 79.3 g/L and the average lactic acid yield for mixed cultures being 30.7 g/L.
4.1.3 Separations
Commonly used separation techniques for lactic acid include precipitation, solvent extraction, adsorption, distillation, electrodialysis, and nanofiltration [134,135,136]. Section 7.4.1.3 will explore solvent extraction, electrodialysis, and nanofiltration as they have been applied to lactic acid produced from the substrates of interest.
Electrodialysis (ED) is a membrane separation technology that operates based on differences in electric potential of the solutes [134]. ED poses issues with scaling on the membrane, resulting in shorter membrane life or requires costly descaling techniques. To improve upon ED challenges, the coupling of nanofiltration membranes with electrodialysis or the use of anion-exchange membranes along with electrodialysis was explored and it was determined that both combinations resulted in greater deacidification, and demineralization, but these approaches are generally more energy intensive than more traditional electrodialysis configurations [134].
Solvent extraction is a liquid-liquid extraction technique used to isolate lactic acid post fermentation. Solvent extraction takes advantage of the solubility differences between extraction solvent and lactic acid. However, this extraction method is not often employed industrially due to the weaknesses that economically feasible solvents have demonstrated when separating lactic acid. Environmental impact can be addressed when choosing solvents for this liquid-liquid extraction; the lower miscibility is proportional to reductions in environmental impacts [136].
Nanofiltration has its merits individually in the separations of lactic acid, but it operates even better coupled with the other previously discussed extraction techniques. Nanofiltration is generally less energy intensive as it uses crossflow filtration which does not require as much energy input when compared to other separation techniques [134,135,136].
4.1.4 pH Control
Lactic acid was produced using three inocula under different pH values and procedures and it was determined that for the methanogenic sludge, the highest yield of lactic acid (20.7 g/L) was at pH 5 after 72 h compared to the same sludge producing 9.7 g/L of lactic acid after 144 h with uncontrolled pH [45]. The same methods were tested on anaerobic sludge and showed similar trends. The uncontrolled pH trial with anaerobic sludge produced 11.5 g/L of lactic acid after 120 h, versus the pH 5 trial with anaerobic sludge, producing 22.6 g/L after 84 h. The pH-controlled experiments were completed in a shortened time frame as compared to the uncontrolled experiments due to the noticeably faster rate of carbohydrate degradation [133].
4.2 Methane
Lactic acid production can be coupled with methane production (see Fig. 7.2) as the acidogenesis phase is a precursor in methane production. Section 7.4.2 will explore biodegradation of organic solid feedstocks used for the production of methane gas as part of the anaerobic digestion process.
4.2.1 Microorganisms
Methanogens are a class of archaea microorganisms known for the production of methane from organic acids [119, 137]. These archaea are a part of the phylum Euryarchaeota , consisting of seven orders: Methanococcales, Methanobacteriales, Methanosarcinales, Methanomicrobiales, Methanopyrales, Methanocellasles, and Methanomassiliicoccales [119, 137].
The environments that methanogens can withstand are vast and diverse, but always anoxic [119, 137]. There have been reports of methanogens in extreme locations: hydrothermal vents and saline lakes; simple environments: rice fields and marshes; the feces of animals: cattle and horses; the human body: human feces and human dental plaques; man-made environments: landfills and biogas plants [137, 138].
Two of the more important parameters for biogas production are pH and temperature [119]. The temperature ranges that methanogens operate under are range from 4 °C to 65 °C . Values for pH in which the production of methane from methanogens is optimized is similarly large (between 5.1 and 9.5, with most methanogens exhibiting optimal production near neutral pH) [119].
4.2.2 Substrate Selection
Table 7.5 shows selected studies to produce methane from agricultural residues and OFMSW. Five aspects of the studies have been reported here: substrate used, the number of bioreactor stages used, hydraulic retention time (HRT), temperature, and yield. Note that Table 7.5 does not list microorganism information; this omission is intentional as methanogenic processes most often use mixed cultures and not specific organisms [21, 24, 50, 58, 139].
5 Technoeconomic Comparisons for Biodegradation Processes
Technoeconomic assessment (TEA ) is often employed as a tool to compare nascent or inchoate processes to probe into potential trade-offs – both in terms of cost and energy use. TEA provides insight in early stages of process design and allows for early directional shifts if it is clear that a particular unit operation will be cost prohibitive for a conversion. While TEA is a useful predition tool, it is not as widely reported as expected. Table 7.6 is included to demonstrate the dearth of TEA reports for organic wastes as feedstocks within biodegradation transformations. Of the multitude of studies published, only two were identified which included any economic information on the process. This lack of information indicates that further research is needed to demonstrate the potential utility – or lack thereof – for these types of feedstocks. A general assumption may be that since the feedstocks of interest are considered to be wastes, then the process will be profitable since material costs will be low. However, this is not necessarily the case and cursory TEA may indicate that a particular process may never be fruitful since yield or some other such variable might be too low to overcome. Food waste upgrading is best performed in a facitlity focused on producing a variety of products with more than one feedstock entering the facility [5].
6 Conclusions and Future Outlook
Use of organic wastes and agricultural residues as substrates to produce valuable chemical products is possible. While many substrates have specific challenges, these can be overcome through additional unit operations; ABE fermentation of cassava bagasse has been shown to produce upwards of 76 g/L of butanol when in situ separation techniques were applied as compared to 9.71 g/L without [31]. Not only are modifications to process configuations a possibility to for increasing production, genetic manipulations of the micrroorganisms themselves may prove most beneficial.
Increasing the tolerance to butanol within certain Clostridium bacteria for ABE fermentation or reducing required feremention time are two of the most promising directions regarding microbial manipulations [150]. A significant focus on metabolic engineering has been to increase the butanol titers from ABE fermentation by modifications to Clostridium cellulovorans [99, 151], Clostridium cellulolyticum [150, 152], as well as genetic modifications of other Clostridium species. Metabolic engineering of Clostridium cellulovorans has focused on improving butanol titers for cellulosic biomass through consolidated bioprocessing (CBP) [99, 151]. Modifications to Clostridium cellulovorans can increase butanol production at least 138 times, from 0.025 g/L to 3.47 g/L after only 84 h, when using cellulose, indicating that genetic manipulations of this organism show enough potential for additional investigation with a lower-cost feedstock [99].
The use of Clostridium cellulolyticum could be advantageous for the lignocellulosic agricultural residues, such as rice straw, due to its ability to digest the lignin [152]. However, proof-of-concept studies for Clostridium cellulolyticum show limited potential; sometimes only achieving a titer of 0.04 g/L up to 0.12 g/L of butanol [150, 152].
The combination of Thermoanaerobacterium thermosaccharolyticum and Clostridium acetobutylicum was studied to determine their ability to produce butanol from hemicellulose and with 13.28 g/L of butanol produced, the concept was promising enough to explore with food waste, namely corncob [153]. With untreated corncob, CBP produced 7.61 g/L of butanol, signifying an advance in the field of butanol production through fermentation sans pretreatment [153, 154]. However, there remains many routes for expanding the use of biodegradation of agricultural residues or OFMSW.
An engineered strain of Clostridium beijerinckii was used on corn stover hydrolysate to increase the production in the solventogenic phase of ABE fermentation showing comparable results, of 20.7 g/L total solvents, to the solventogenic production when using corn alone as feedstock [154]. The histidine kinases in C. beijerinckii were altered to to increase butanol titer and production rate, concluding that deletion of cbei2073, a histidine kinase coding region, increased production rate by 40% and increased the butanol biosynthesis by 40.8%, from 9.8 g/L to 13.8 g/L of butanol [144]. This provides evidence of the role in histidine kinase in butanol production and provides insight into specific strategies moving forward in metabolic engineering of Clostridium strains for enhanced butanol production.
When anaerobic digestion is used, the production of lactic acid and methane generally rely on the use of a consortia of microorganisms rather than a specific strain like that of ABE fermentations. For this reason, only minimal effort has been spared with respect to strain development. Though there is significant attention paid to research in mixed microbial communities [57, 88, 155]. In the coming years, significant attention on the economic feasibility of biodegradation processes of organic wastes should be given. Economic potential and profitability are seminal for commercialization of these potentially valuable feedstocks.
References
Ye Z-L, Lu M, Zheng Y, Li Y-H, Cai W-M. Lactic acid production from dining-hall food waste by Lactobacillus plantarum using response surface methodology. J Chem Technol Biotechnol. 2008;83:1541–50. https://doi.org/10.1002/jctb.1968.
Seifollahi M, Amiri H. Phosphoric acid-acetone process for cleaner production of acetone, butanol, and ethanol from waste cotton fibers. J Clean Prod. 2018;193:459–70. https://doi.org/10.1016/j.jclepro.2018.05.093.
Oak Ridge National Laboratory. 2016 billion-ton report. U.S. Department of Energy; 2016. https://doi.org/10.1089/ind.2016.29051.doe.
American Society of Civil Engineers. ASCE’s 2021 infrasturcutre report card: solid waste, 2021.
Engelberth AS. Evaluating economic potential of food waste valorization: Onward to a diverse feedstock biorefinery. Green Sustain Chem. 2020;1–6:100385. https://doi.org/10.1016/j.cogsc.2020.100385.
Xu Q, Qin J, Ko JH. Municipal solid waste landfill performance with different biogas collection practices: Biogas and leachate generations. J Clean Prod. 2019;222:446–54. https://doi.org/10.1016/j.jclepro.2019.03.083.
Laurent A, Bakas I, Clavreul J, Bernstad A, Niero M, Gentil E, Hauschild MZ, Christensen TH. Review of LCA studies of solid waste management systems—Part I: Lessons learned and perspectives. Waste Manag. 2014;34(3):573–88. https://doi.org/10.1016/j.wasman.2013.10.045.
Giusti L. A review of waste management practices and their impact on human health. Waste Manag. 2009;29(8):2227–39. https://doi.org/10.1016/j.wasman.2009.03.028.
John RP. Chapter 11: Biotechnological potentials of Cassava Bagasse. In: Pandey A, Nigam PS-N, editors. Biotechnology for agro-industrial residues utilisation. Dordrecht: Springer; 2009. p. 225–37. https://doi.org/10.1007/978-1-4020-9942-7.
Food and Agriculture Organization of the United Nations. Cassava Production Globally, 2019, from http://www.fao.org/faostat/en/#data/QC, accessed 3-5-2021.
Parmar A, Sturm B, Hensel O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Security. 2017;9(5):907–27. https://doi.org/10.1007/s12571-017-0717-8.
Teixeira EDM, Pasquini D, Curvelo AAS, Corradini E, Belgacem MN, Dufresne A. Cassava bagasse cellulose nanofibrils reinforced thermoplastic cassava starch. Carbohydr Polym. 2009;78(3):422–31. https://doi.org/10.1016/j.carbpol.2009.04.034.
van Hung N, Maguyon-Detras MC, Migo MV, Quilloy R, Balingbing C, Chivenge P, Gummert M. Rice straw overview: availability, properties, and management practices. In: Gummert M, van Hung N, Chivenge P, Douthwaite B, editors. Sustainable rice straw management. Cham: Springer International Publishing; 2020. p. 1–13. https://doi.org/10.1007/978-3-030-32373-8_1.
González-García S, Morales PC, Gullón B. Estimating the environmental impacts of a brewery waste–based biorefinery: Bio-ethanol and xylooligosaccharides joint production case study. Ind Crop Prod. 2018;123:331–40. https://doi.org/10.1016/j.indcrop.2018.07.003.
Garcia-Garcia G, Rahimifard S. Life-cycle environmental impacts of barley straw valorisation. Resources Conserv Recycl. 2019;149:1–11. https://doi.org/10.1016/j.resconrec.2019.05.026.
Office of Energy Efficiency & Renewable Energy. (2019). Waste-to-energy from municipal solids wastes, U. S. Department of Energy.
Alibardi L, Cossu R. Composition variability of the organic fraction of municipal solid waste and effects on hydrogen and methane production potentials. Waste Manag. 2015;36:147–55. https://doi.org/10.1016/j.wasman.2014.11.019.
Maitah M, Řezbová H, Smutka L, Tomšík K. European sugar production and its control in the world market. Sugar Tech. 2016;18(3):236–41. https://doi.org/10.1007/s12355-016-0439-9.
Alexandri M, Schneider R, Papapostolou H, Ladakis D, Koutinas A, Venus J. Restructuring the conventional sugar beet industry into a novel biorefinery: fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain Chem Eng. 2019;7(7):6569–79. https://doi.org/10.1021/acssuschemeng.8b04874.
Cheesman OD. Use and impacts of by-products. In: Environmental impacts of sugar production: the cultivation and processing of sugarcane and sugar beet. Wallingford, UK: CABI Publishing; 2004. p. 151–72. https://doi.org/10.1079/9780851999814.0151.
Chojnacka A, Szczęsny P, Błaszczyk MK, Zielenkiewicz U, Detman A, Salamon A, Sikora A. Noteworthy facts about a methane-producing microbial community processing acidic effluent from sugar beet molasses fermentation. PLoS One. 2 015;10(5):1–23. https://doi.org/10.1371/journal.pone.0128008.
Kim S, Dale BE, Jenkins R. Life cycle assessment of corn grain and corn stover in the United States. Int J Life Cycle Assess. 2009;14(2):160–74. https://doi.org/10.1007/s11367-008-0054-4.
López-Gómez JP, Latorre-Sánchez M, Unger P, Schneider R, Coll Lozano C, Venus J. Assessing the organic fraction of municipal solid wastes for the production of lactic acid. Biochem Eng J. 2019;150:107251. https://doi.org/10.1016/j.bej.2019.107251.
Demichelis F, Pleissner D, Fiore S, Mariano S, Navarro Gutiérrez IM, Schneider R, Venus J. Investigation of food waste valorization through sequential lactic acid fermentative production and anaerobic digestion of fermentation residues. Bioresour Technol. 2017;241:508–16. https://doi.org/10.1016/j.biortech.2017.05.174.
Gutberlet J. Cooperative urban mining in Brazil: collective practices in selective household waste collection and recycling. Waste Manag. 2015;45:22–31. https://doi.org/10.1016/j.wasman.2015.06.023.
Office of Energy Efficiency & Renewable Energy. Agricultural residues and energy crops. U.S. Department of Energy; 2016.
Azam M, Jahromy SS, Raza W, Raza N, Lee SS, Kim KH, Winter F. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ Int. 2019;134:105291. https://doi.org/10.1016/j.envint.2019.105291.
Hu Y, Du C, Pensupa N, Lin CSK. Optimisation of fungal cellulase production from textile waste using experimental design. Process Saf Environ Prot. 2018;118:133–42. https://doi.org/10.1016/j.psep.2018.06.009.
Hu Y, Du C, Leu SY, Jing H, Li X, Lin CSK. Valorisation of textile waste by fungal solid state fermentation: an example of circular waste-based biorefinery. Resources Conserv Recycl. 2018;129:27–35. https://doi.org/10.1016/j.resconrec.2017.09.024.
EPA. (n.d.). 1960–2018 Data on Textiles in MSW by Weight (in thousands of U.S. tons), from https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/textiles-material-specific-data. Accessed 1-5-2020.
Lu C, Zhao J, Yang ST, Wei D. Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour Technol. 2012;104:380–7. https://doi.org/10.1016/j.biortech.2011.10.089.
Wu Y, Wang Z, Ma X, Xue C. High temperature simultaneous saccharification and fermentation of corn stover for efficient butanol production by a thermotolerant Clostridium acetobutylicum. Process Biochem. 2021;100:20–5. https://doi.org/10.1016/j.procbio.2020.09.026.
Al-Shorgani NKN, Kalil MS, Yusoff WMW, Hamid AA. Impact of pH and butyric acid on butanol production during batch fermentation using a new local isolate of Clostridium acetobutylicum YM1. Saudi J Biol Sci. 2018;25(2):339–48. https://doi.org/10.1016/j.sjbs.2017.03.020.
Jin Q, Qureshi N, Wang H, Huang H. Acetone-butanol-ethanol (ABE) fermentation of soluble and hydrolyzed sugars in apple pomace by Clostridium beijerinckii P260. Fuel. 2019;244:536–44. https://doi.org/10.1016/J.FUEL.2019.01.177.
Mutschlechner O, Swoboda H, Gapes JR. Continuous two-stage ABE-fermentation using Clostridium beijerinckii HRRL B592 operating with a growth rate in the first stage vessel close to its maximal value. J Mol Microbiol Biotechnol. 2000;2(1):101–5.
Lépiz-Aguilar L, Rodríguez-Rodríguez CE, Arias ML, Lutz G, Ulate W. Butanol production by Clostridium beijerinckii BA101 using cassava flour as fermentation substrate: enzymatic versus chemical pretreatments. World J Microbiol Biotechnol. 2011;27(8):1933–9. https://doi.org/10.1007/s11274-010-0630-1.
Li L, Ai H, Zhang S, Li S, Liang Z, Wu ZQ, Yang ST, Wang JF. Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour Technol. 2013;143:397–404. https://doi.org/10.1016/J.BIORTECH.2013.06.023.
Huang J, Du Y, Bao T, Lin M, Wang J, Yang ST. Production of n-butanol from cassava bagasse hydrolysate by engineered Clostridium tyrobutyricum overexpressing adhE2: kinetics and cost analysis. Bioresour Technol. 2019;292:121969. https://doi.org/10.1016/j.biortech.2019.121969.
Zhang J, Zong W, Hong W, Zhang ZT, Wang Y. Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab Eng. 2018;47:49–59. https://doi.org/10.1016/J.YMBEN.2018.03.007.
Nakayama S, Kiyoshi K, Kadokura T, Nakazato A. Butanol production from crystalline cellulose by Cocultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microbiol. 2011;77(18):6470–5. https://doi.org/10.1128/AEM.00706-11.
Thang VH, Kanda K, Kobayashi G. Production of Acetone-Butanol-Ethanol (ABE) in direct fermentation of cassava by Clostridium saccharoperbutylacetonicum N1-4. Appl Biochem Biotechnol. 2010;161(1–8):157–70. https://doi.org/10.1007/s12010-009-8770-1.
Kiyoshi K, Furukawa M, Seyama T, Kadokura T, Nakazato A, Nakayama S. Butanol production from alkali-pretreated rice straw by co-culture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum. Bioresour Technol. 2015;186:325–8. https://doi.org/10.1016/j.biortech.2015.03.061.
Payot T, Chemaly Z, Fick M. Lactic acid production by Bacillus coagulans—kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme Microb Technol. 1999;24(3–4):191–9. https://doi.org/10.1016/S0141-0229(98)00098-2.
Cubas-Cano E, Venus J, González-Fernández C, Tomás-Pejó E. Assessment of different Bacillus coagulans strains for l-lactic acid production from defined media and gardening hydrolysates: Effect of lignocellulosic inhibitors. J Biotechnol. 2020;323:9–16. https://doi.org/10.1016/J.JBIOTEC.2020.07.017.
Hujanen M, Linko YY. Effect of temperature and various nitrogen sources on L (+)-lactic acid production by Lactobacillus casei. Appl Microbiol Biotechnol. 1996;45(3):307–13. https://doi.org/10.1007/s002530050688.
Wang X, Wang G, Yu X, Chen H, Sun Y, Chen G. Pretreatment of corn stover by solid acid for D-lactic acid fermentation. Bioresour Technol. 2017;239:490–5. https://doi.org/10.1016/j.biortech.2017.04.089.
Mussatto SI, Dragone GM. Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In: Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier Inc.; 2016. p. 1–22. https://doi.org/10.1016/B978-0-12-802323-5.00001-3.
Yang B, Wyman CE. Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefining. 2007;6(3):246–56. https://doi.org/10.1002/bbb.49.
Ziemiński K, Kowalska-Wentel M. Effect of different sugar beet pulp pretreatments on biogas production efficiency. Appl Biochem Biotechnol. 2017;181(3):1211–27. https://doi.org/10.1007/s12010-016-2279-1.
Li W, Guo J, Cheng H, Wang W, Dong R. Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: viability, performance and microbial community evaluation. Appl Energy. 2017;189:613–22. https://doi.org/10.1016/j.apenergy.2016.12.101.
Lomovsky O, Bychkov A, Lomovsky I. Mechanical pretreatment. In: Mussatto SI, editor. Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Amsterdam: Elsevier; 2016. p. 23–55. https://doi.org/10.1016/B978-0-12-802323-5.00002-5.
Moradi F, Amiri H, Soleimanian-Zad S, Ehsani MR, Karimi K. Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel. 2013;112:8–13. https://doi.org/10.1016/j.fuel.2013.05.011.
Yang M, Kuittinen S, Zhang J, Vepsäläinen J, Keinänen M, Pappinen A. Co-fermentation of hemicellulose and starch from barley straw and grain for efficient pentoses utilization in acetone-butanol-ethanol production. Bioresour Technol. 2015;179:128–35. https://doi.org/10.1016/j.biortech.2014.12.005.
Luo H, Zeng Q, Han S, Wang Z, Dong Q, Bi Y, Zhao Y. High-efficient n-butanol production by co-culturing Clostridium acetobutylicum and Saccharomyces cerevisiae integrated with butyrate fermentative supernatant addition. World J Microbiol Biotechnol. 2017;33(4) https://doi.org/10.1007/s11274-017-2246-1.
Cebreiros F, Risso F, Cagno M, Cabrera MN, Rochón E, Jauregui G, Boix E, Böthig S, Ferrari MD, Lareo C. Enhanced production of butanol and xylosaccharides from Eucalyptus grandis wood using steam explosion in a semi-continuous pre-pilot reactor. Fuel. 2020;290 https://doi.org/10.1016/j.fuel.2020.119818.
Amiri H, Karimi K, Zilouei H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol. 2014;152:450–6. https://doi.org/10.1016/j.biortech.2013.11.038.
Berlowska J, Cieciura W, Borowski S, Dudkiewicz M, Binczarski M, Witonska I, Otlewska A, Kregiel D. Simultaneous saccharification and fermentation of sugar beet pulp with mixed bacterial cultures for lactic acid and propylene glycol production. Molecules. 2016;21(10) https://doi.org/10.3390/molecules21101380.
Ziemiński K, Romanowska I, Kowalska-Wentel M, Cyran M. Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour Technol. 2014;166:187–93. https://doi.org/10.1016/j.biortech.2014.05.021.
Lin YS, Lee WC, Duan KJ, Lin YH. Ethanol production by simultaneous saccharification and fermentation in rotary drum reactor using thermotolerant Kluveromyces marxianus. Appl Energy. 2013;105:389–94. https://doi.org/10.1016/j.apenergy.2012.12.020.
Benazzi T, Calgaroto S, Astolfi V, Dalla Rosa C, Oliveira JV, Mazutti MA. Pretreatment of sugarcane bagasse using supercritical carbon dioxide combined with ultrasound to improve the enzymatic hydrolysis. Enzyme Microb Technol. 2013;52(4–5):247–50. https://doi.org/10.1016/J.ENZMICTEC.2013.02.001.
Qureshi N, Saha BC, Dien B, Hector RE, Cotta MA. Production of butanol (a biofuel) from agricultural residues: Part I—Use of barley straw hydrolysate. Biomass Bioenergy. 2010;34(4):559–65. https://doi.org/10.1016/j.biombioe.2009.12.024.
Chu CY, Wu SY, Tsai CY, Lin CY. Kinetics of cotton cellulose hydrolysis using concentrated acid and fermentative hydrogen production from hydrolysate. Int J Hydrogen Energy. 2011;36, Pergamon:8743–50. https://doi.org/10.1016/j.ijhydene.2010.07.072.
Gikas P, Zhu B, Batistatos NI, Zhang R. Evaluation of the rotary drum reactor process as pretreatment technology of municipal solid waste for thermophilic anaerobic digestion and biogas production. J Environ Manage. 2018;216:96–104. https://doi.org/10.1016/j.jenvman.2017.07.050.
Farmanbordar S, Amiri H, Karimi K. Simultaneous organosolv pretreatment and detoxification of municipal solid waste for efficient biobutanol production. Bioresour Technol. 2018;270:236–44. https://doi.org/10.1016/j.biortech.2018.09.017.
Shahriari H, Warith M, Hamoda M, Kennedy KJ. Anaerobic digestion of organic fraction of municipal solid waste combining two pretreatment modalities, high temperature microwave and hydrogen peroxide. Waste Manag. 2012;32(1):41–52. https://doi.org/10.1016/j.wasman.2011.08.012.
Gholamzad E, Karimi K, Masoomi M. Effective conversion of waste polyester-cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem Eng J. 2014;253:40–5. https://doi.org/10.1016/j.cej.2014.04.109.
Lee KM, Hong JY, Tey WY. Combination of ultrasonication and deep eutectic solvent in pretreatment of lignocellulosic biomass for enhanced enzymatic saccharification. Cellulose. 2021;28(3):1513–26. https://doi.org/10.1007/s10570-020-03598-5.
Elbeshbishy E, Nakhla G. Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Bioresour Technol. 2011;102(11):6449–57. https://doi.org/10.1016/j.biortech.2011.03.082.
Izumi K, Okishio YK, Nagao N, Niwa C, Yamamoto S, Toda T. Effects of particle size on anaerobic digestion of food waste. Int Biodeter Biodegr. 2010;64(7):601–8. https://doi.org/10.1016/j.ibiod.2010.06.013.
Mani S, Tabil LG, Sokhansanj S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy. 2004;27(4):339–52. https://doi.org/10.1016/j.biombioe.2004.03.007.
Mikulski D, Kłosowski G, Menka A, Koim-Puchowska B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour Technol. 2019;278:318–28. https://doi.org/10.1016/j.biortech.2019.01.068.
Mishra P, ab Wahid Z, Singh L, Zaid RM, Tabassum S, Sakinah M, Jiang X. Synergistic effect of ultrasonic and microwave pretreatment on improved biohydrogen generation from palm oil mill effluent. Biomass Convers Biorefinery. 2021; https://doi.org/10.1007/s13399-021-01285-4.
Rudakiya DM. Strategies to improve solid-state fermentation technology. In: New and future developments in microbial biotechnology and bioengineering: from cellulose to cellulase: strategies to improve biofuel production; 2019. p. 155–80. https://doi.org/10.1016/B978-0-444-64223-3.00010-2.
Pessoa M, Sobrinho MAM, Kraume M. The use of biomagnetism for biogas production from sugar beet pulp. Biochem Eng J. 2020;164:107770. https://doi.org/10.1016/j.bej.2020.107770.
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S. Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res. 2011; https://doi.org/10.4061/2011/787532.
Li H, Kim NJ, Jiang M, Kang JW, Chang HN. Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour Technol. 2009;100(13):3245–51. https://doi.org/10.1016/j.biortech.2009.01.021.
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I. Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol., Elsevier Ltd,. 2016:386–97. https://doi.org/10.1016/j.biortech.2015.09.007.
Schacht C, Zetzl C, Brunner G. From plant materials to ethanol by means of supercritical fluid technology. J Supercrit Fluids., Elsevier. 2008:299–321. https://doi.org/10.1016/j.supflu.2008.01.018.
Gu T, Held MA, Faik A. Supercritical CO2 and ionic liquids for the pretreatment of lignocellulosic biomass in bioethanol production. Environ Technol (United Kingdom). 2013;34(13–14):1735–49. https://doi.org/10.1080/09593330.2013.809777.
Ng CH, He J, Yang KL. Purification and characterization of a GH11 Xylanase from biobutanol-producing Clostridium beijerinckii G117. Appl Biochem Biotechnol. 2015;175(6):2832–44. https://doi.org/10.1007/s12010-014-1470-5.
Jouzani GS, Taherzadeh MJ. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review. Biofuel Res J. 2015;2(1):152–95. https://doi.org/10.18331/BRJ2015.2.1.4.
Singh-Nee Nigam, P.; Pandey, A.; Gupta, N. (2009). Chapter 2: Pre-treatment of agro-industrial residues. In: P. Singh-Nee Nigam, A. Pandey (Eds.), Biotechnology for agro-industrial residues utilisation utilisation of agro-residues, Dordrecht, pp. 13–33.
Paquet V, Croux C, Goma G, Soucaille P. Purification and characterization of the extracellular α-amylase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1991;57(1):212–8. https://doi.org/10.1128/aem.57.1.212-218.1991.
Szymanowska-Powałowska D, Orczyk D, Leja K. Biotechnological potential of Clostridium butyricum bacteria. Braz J Microbiol. 2014;45(3):892–901. https://doi.org/10.1590/S1517-83822014000300019.
Cai J, Wang R, Wu Q, Wang G, Deng C. Characterization of a hydrogen-producing bacterium Clostridium sp. 5A-1. Int J Green Energy. 2021; https://doi.org/10.1080/15435075.2021.1875469.
Ahmad A, Banat F, Taher H. A review on the lactic acid fermentation from low-cost renewable materials: recent developments and challenges. Environ Technol Innov. 2020;20:101138. https://doi.org/10.1016/j.eti.2020.101138.
Veza I, Muhamad Said MF, Latiff ZA. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy. 2021;144:105919. https://doi.org/10.1016/j.biombioe.2020.105919.
Valdez-Vazquez I, Sanchez A. Proposal for biorefineries based on mixed cultures for lignocellulosic biofuel production: a techno-economic analysis, Biofuels. Bioprod Biorefining. 2018;12(1):56–67. https://doi.org/10.1002/bbb.1828.
Xue C, Zhao J, Chen L, Yang ST, Bai F. Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol Adv. 2017;35(2):310–22. https://doi.org/10.1016/j.biotechadv.2017.01.007.
Uçkun Kiran E, Trzcinski AP, Liu Y. Platform chemical production from food wastes using a biorefinery concept. J Chem Technol Biotechnol. 2015;90(8):1364–79. https://doi.org/10.1002/jctb.4551.
Detman A, Chojnacka A, Mielecki D, Błaszczyk MK, Sikora A. Inhibition of hydrogen-yielding dark fermentation by ascomycetous yeasts. Int J Hydrogen Energy. 2018;43(24):10967–79. https://doi.org/10.1016/j.ijhydene.2018.05.004.
Zakaria ZA, Boopathy R, Dib JR, editors. Valorisation of agro-industrial residues – Volume I: Biological approaches. 1st ed; 2020.
Bharathiraja B, Jayamuthunagai J, Sudharsanaa T, Bharghavi A, Praveenkumar R, Chakravarthy M, Devarajan Y. Biobutanol – an impending biofuel for future: a review on upstream and downstream processing tecniques. Renew Sustain Energy Rev. 2017;68:788–807. https://doi.org/10.1016/j.rser.2016.10.017.
Swidah R, Ogunlabi O, Grant CM, Ashe MP. n-Butanol production in S. cerevisiae: co-ordinate use of endogenous and exogenous pathways. Appl Microbiol Biotechnol. 2018;102(22):9857–66. https://doi.org/10.1007/s00253-018-9305-x.
Moon HG, Jang YS, Cho C, Lee J, Binkley R, Lee SY. One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett. 2016;363(3) https://doi.org/10.1093/femsle/fnw001.
Swidah R, Wang H, Reid PJ, Ahmed HZ, Pisanelli AM, Persaud KC, Grant CM, Ashe MP. Butanol production in S. cerevisiae via a synthetic ABE pathway is enhanced by specific metabolic engineering and butanol resistance. Biotechnol Biofuels. 2015;8(1):1–9. https://doi.org/10.1186/s13068-015-0281-4.
Qureshi N, Saha BC, Hector RE, Dien B, Hughes S, Liu S, Iten L, Bowman MJ, Sarath G, Cotta MA. Production of butanol (a biofuel) from agricultural residues: Part II—Use of corn stover and switchgrass hydrolysates. Biomass Bioenergy. 2010;34(4):566–71. https://doi.org/10.1016/j.biombioe.2009.12.023.
Ibrahim MF, Ramli N, Kamal Bahrin E, Abd-Aziz S. Cellulosic biobutanol by Clostridia: challenges and improvements. Renew Sustain Energy Rev., Elsevier Ltd. 2017:1241–54. https://doi.org/10.1016/j.rser.2017.05.184.
Yang X, Xu M, Yang ST. Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab Eng. 2015;32:39–48. https://doi.org/10.1016/j.ymben.2015.09.001.
Müller B, Grossniklaus U. Model organisms: a historical perspective. J Proteomics. Elsevier. 2010:2054–63. https://doi.org/10.1016/j.jprot.2010.08.002.
Dong H, Zhao C, Zhang T, Zhu H, Lin Z, Tao W, Zhang Y, Li Y. A systematically chromosomally engineered Escherichia coli efficiently produces butanol. Metab Eng. 2017;44:284–92. https://doi.org/10.1016/j.ymben.2017.10.014.
Galazzo JL, Bailey JE. Fermentation pathway kinetics and immobilized Saccharomyces cerevisiae. Enzyme Microbial Technol. 1990;12(3):162–72.
Lin Y, Zhang W, Li C, Sakakibara K, Tanaka S, Kong H. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy. 2014;47:395–401. https://doi.org/10.1016/j.biombioe.2012.09.019.
Patnaik PR. Oscillatory metabolism of Saccharomyces cerevisiae: an overview of mechanisms and models. Biotechnol Adv. Elsevier Inc,. 2003:183–92. https://doi.org/10.1016/S0734-9750(03)00022-3.
Lau MW, Dale BE. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST). Proc Natl Acad Sci U S A. 2009;106(5):1368–73. https://doi.org/10.1073/pnas.0812364106.
Jin H, Liu R, He Y. Kinetics of batch fermentations for ethanol production with immobilized Saccharomyces cerevisiae growing on Sweet Sorghum Stalk Juice. Procedia Environ Sci. 2012;12:137–45. https://doi.org/10.1016/j.proenv.2012.01.258.
Nanda S, Dalai AK, Kozinski JA. Butanol and ethanol production from lignocellulosic feedstock: biomass pretreatment and bioconversion. Energy Sci Eng. 2014;2(3):138–48. https://doi.org/10.1002/ese3.41.
Farmanbordar S, Karimi K, Amiri H. Municipal solid waste as a suitable substrate for butanol production as an advanced biofuel. Energy Convers Manage. 2017;157:396–408. https://doi.org/10.1016/j.enconman.2017.12.020.
Jesse TW, Ezeji TC, Qureshi N, Blaschek HP. Production of butanol from starch-based waste packing peanuts and agricultural waste. J Ind Microbiol Biotechnol. 2002;29(3):117–23. https://doi.org/10.1038/sj.jim.7000285.
Shao P, Huang RYM. Polymeric membrane pervaporation. J Membr Sci. 2007;287(2):162–79. https://doi.org/10.1016/j.memsci.2006.10.043.
Azimi H, Tezel H, Thibault J. Optimization of the in situ recovery of butanol from ABE fermentation broth via membrane pervaporation. Chem Eng Res Des. 2019;150:49–64. https://doi.org/10.1016/j.cherd.2019.07.012.
Cai D, Chen H, Chen C, Hu S, Wang Y, Chang Z, Miao Q, Qin P, Wang Z, Wang J, Tan T. Gas stripping-pervaporation hybrid process for energy-saving product recovery from acetone-butanol-ethanol (ABE) fermentation broth. Chem Eng J. 2016;287:1–10. https://doi.org/10.1016/j.cej.2015.11.024.
Qureshi N, Blaschek HP. Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol Prog. 1999;15(4):594–602. https://doi.org/10.1021/bp990080e.
Lin Z, Liu H, Yan X, Zhou Y, Cheng K, Zhang J. High-efficiency acetone-butanol-ethanol production and recovery in non-strict anaerobic gas-stripping fed-batch fermentation. Appl Microbiol Biotechnol. 2017;101(21):8029–39. https://doi.org/10.1007/s00253-017-8520-1.
Rochón E, Ferrari MD, Lareo C. Integrated ABE fermentation-gas stripping process for enhanced butanol production from sugarcane-sweet sorghum juices. Biomass Bioenergy. 2017;98:153–60. https://doi.org/10.1016/j.biombioe.2017.01.011.
López-Gómez JP, Pérez-Rivero C, Venus J. Valorisation of solid biowastes: the lactic acid alternative. Process Biochem. 2020;99:222–35. https://doi.org/10.1016/J.PROCBIO.2020.08.029.
Kim MS, Na JG, Lee MK, Ryu H, Chang YK, Triolo JM, Yun YM, Kim DH. More value from food waste: lactic acid and biogas recovery. Water Res. 2016;96:208–16. https://doi.org/10.1016/j.watres.2016.03.064.
Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, Pérez-Muñoz ME, Leulier F, Gänzle M, Walter J. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. 2017;41(1):S27–48. https://doi.org/10.1093/femsre/fux030.
Enzmann F, Mayer F, Rother M, Holtmann D. Methanogens: biochemical background and biotechnological applications. AMB Express. 2018;8(1):1–22. https://doi.org/10.1186/s13568-017-0531-x.
Baek SH, Kwon EY, Bae SJ, Cho BR, Kim SY, Hahn JS. Improvement of d-lactic acid production in Saccharomyces cerevisiae under acidic conditions by evolutionary and rational metabolic engineering. Biotechnol J. 2017;12(10):1–7. https://doi.org/10.1002/biot.201700015.
Ishida N, Suzuki T, Tokuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H. d-Lactic acid production by metabolically engineered Saccharomyces cerevisiae. J Biosci Bioeng. 2006;101(2):172–7. https://doi.org/10.1263/jbb.101.172.
Hayek SA, Ibrahim SA. Current limitations and challenges with lactic acid bacteria: a review. Food Nutr Sci. 2013;4:73–87.
Dworkin M, Falkow S, Rosenberg E, Karl-Heinz Schleifer ES. Bacteria: firmicutes cyanobacteria. Prokaryotes. 2006;4
Paudel SR, Banjara SP, Choi OK, Park KY, Kim YM, Lee JW. Pretreatment of agricultural biomass for anaerobic digestion: current state and challenges. Bioresour Technol. Elsevier Ltd. 2017:1194–205. https://doi.org/10.1016/j.biortech.2017.08.182.
Zhang Q, Wang M, Ma X, Gao Q, Wang T, Shi X, Zhou J, Zuo J, Yang Y. High variations of methanogenic microorganisms drive full-scale anaerobic digestion process. Environ Int. 2019;126:543–51. https://doi.org/10.1016/j.envint.2019.03.005.
Yabu H, Sakai C, Fujiwara T, Nishio N, Nakashimada Y. Thermophilic two-stage dry anaerobic digestion of model garbage with ammonia stripping. J Biosci Bioeng. 2011;111(3):312–9. https://doi.org/10.1016/j.jbiosc.2010.10.011.
Doyle N, Mbandlwa P, Kelly WJ, Attwood G, Li Y, Ross RP, Stanton C, Leahy S. Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Front Microbiol. 2019;10 https://doi.org/10.3389/fmicb.2019.02207.
Zhang Y, Yoshida M, Vadlani P v. Biosynthesis of d-lactic acid from lignocellulosic biomass. Biotechnol Lett. 2018;40(8):1167–79. https://doi.org/10.1007/s10529-018-2588-2.
Nolasco-Hipolito C, Carvajal-Zarrabal O, Kelvin E, Tan YH, Kohei M, Nyoel SA, Shoji E, Dieng H, Bujang K. Scaling up of lactic acid fermentation using Enterococcus faecalis. IOP Conference Series: Mater Sci Eng. 2019;495(1) https://doi.org/10.1088/1757-899X/495/1/012049.
Sarasua, J. R.; Arraiza, O.; Balerdi, P.; Fundací, I. M. (n.d.). Crystallization and thermal behaviour of optically pure polylactides and their blends.
Peinemann JC, Rhee C, Shin SG, Pleissner D. Non-sterile fermentation of food waste with indigenous consortium and yeast – effects on microbial community and product spectrum. Bioresour Technol. 2020;306:123175. https://doi.org/10.1016/j.biortech.2020.123175.
John, R. P.; Nampoothiri, M.; Pandey, A. (2006). Simultaneous saccharification and fermentation of Cassava Bagasse for L-(+)-lactic acid production using lactobacilli.
Tang J, Wang XC, Hu Y, Zhang Y, Li Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour Technol. 2017;224:544–52. https://doi.org/10.1016/j.biortech.2016.11.111.
Beaulieu M, Perreault V, Mikhaylin S, Bazinet L. How overlimiting current condition influences lactic acid recovery and demineralization by electrodialysis with nanofiltration membrane: comparison with conventional electrodialysis. Membranes. 2020;10(6):1–19. https://doi.org/10.3390/membranes10060113.
Laube H, Schneider R, Venus J. Investigation of spiral-wound membrane modules for the cross-flow nanofiltration of fermentation broth obtained from a pilot plant fermentation reactor for the continuous production of lactic acid. Bioresour Bioprocess. 2017;4(1) https://doi.org/10.1186/s40643-016-0133-5.
Komesu A, Maciel MRW, Filho RM. Separation and purification technologies for lactic acid: a brief review. BioResources. 2017;12(3):6885–901. https://doi.org/10.15376/biores.12.3.6885-6901.
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol. 2008;6(8):579–91. https://doi.org/10.1038/nrmicro1931.
Condrad R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: a mini review. Pedosphere Soil Sci Soc China. 2020;25–39 https://doi.org/10.1016/S1002-0160(18)60052-9.
Kabir MM, Forgács G, Sárvári Horváth I. Enhanced methane production from wool textile residues by thermal and enzymatic pretreatment. Process Biochem. 2013;48(4):575–80. https://doi.org/10.1016/j.procbio.2013.02.029.
Kheyrandish M, Asadollahi MA, Jeihanipour A, Doostmohammadi M, Rismani-Yazdi H, Karimi K. Direct production of acetone-butanol-ethanol from waste starch by free and immobilized Clostridium acetobutylicum. Fuel. 2014;142:129–33. https://doi.org/10.1016/j.fuel.2014.11.017.
Qureshi N, Li XL, Hughes S, Saha BC, Cotta MA. Butanol production from corn fiber xylan using Clostridium acetobutylicum. Biotechnol Prog. 2006;22(3):673–80. https://doi.org/10.1021/bp050360w.
Luo H, Ge L, Zhang J, Ding J, Chen R, Shi Z. Enhancing acetone biosynthesis and acetone-butanol-ethanol fermentation performance by co-culturing Clostridium acetobutylicum/Saccharomyces cerevisiae integrated with exogenous acetate addition. Bioresour Technol. 2016;200:111–20. https://doi.org/10.1016/j.biortech.2015.09.116.
Wen Z, Li Q, Liu J, Jin M, Yang S. Consolidated bioprocessing for butanol production of cellulolytic Clostridia: development and optimization. J Microbial Biotechnol. 2020;13(2):410–22. https://doi.org/10.1111/1751-7915.13478.
Xin X, Cheng C, Du G, Chen L, Xue C. Metabolic engineering of histidine kinases in Clostridium beijerinckii for enhanced butanol production. Front Bioeng Biotechnol. 2020;8:1–8. https://doi.org/10.3389/fbioe.2020.00214.
Zhang J, Jia B. Enhanced butanol production using Clostridium beijerinckii SE-2 from the waste of corn processing. Biomass Bioenergy. 2018;115:260–6. https://doi.org/10.1016/j.biombioe.2018.05.012.
Xu M, Zhao J, Yu L, Tang IC, Xue C, Yang ST. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl Microbiol Biotechnol. 2015;99(2):1011–22. https://doi.org/10.1007/s00253-014-6249-7.
Ezeji TC, Qureshi N, Blaschek HP. Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Microbiol Biotechnol. 2004;63(6):653–8. https://doi.org/10.1007/s00253-003-1400-x.
Probst M, Walde J, Pümpel T, Wagner AO, Schneider I, Insam H. Lactic acid fermentation within a cascading approach for biowaste treatment. Appl Microbiol Biotechnol. 2015;99(7):3029–40. https://doi.org/10.1007/s00253-015-6414-7.
Kwan TH, Hu Y, Lin CSK. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J Clean Prod. 2018;181:72–87. https://doi.org/10.1016/j.jclepro.2018.01.179.
Gaida SM, Liedtke A, Jentges AHW, Engels B, Jennewein S. Metabolic engineering of Clostridium cellulolyticum for the production of n-butanol from crystalline cellulose. Microb Cell Fact. 2016;15(1):1–11. https://doi.org/10.1186/s12934-015-0406-2.
Wen Z, Ledesma-amaro R, Lin J, Jiang Y. Improved n-butanol production from clostridium cellulovorans by integrated metabolic and evolutionary engineering. Appl Environ Microbiol. 2019;85(7):1–17. https://doi.org/10.1128/aem.02560-18.
Fedorova I, Arseniev A, Selkova P, Pobegalov G, Goryanin I, Vasileva A, Musharova O, Abramova M, Kazalov M, Zyubko T, Artamonova T, Artamonova D, Shmakov S, Khodorkovskii M, Severinov K. DNA targeting by Clostridium cellulolyticum CRISPR-Cas9 Type II-C system. Nucleic Acids Res. 2020;48(4):2026–34. https://doi.org/10.1093/nar/gkz1225.
Jiang Y, Lv Y, Wu R, Lu J, Dong W, Zhou J, Zhang W, Xin F, Jiang M. Consolidated bioprocessing performance of a two-species microbial consortium for butanol production from lignocellulosic biomass. Biotechnol Bioeng. 2020;117(10):2985–95. https://doi.org/10.1002/bit.27464.
Liu J, Jiang Y, Chen J, Yang J, Jiang W, Zhuang W, Ying H, Yang S. Metabolic engineering and adaptive evolution of Clostridium beijerinckii to increase solvent production from corn stover hydrolysate. J Agric Food Chem. 2020;68(30):7916–25. https://doi.org/10.1021/acs.jafc.0c03048.
Yousuf A, Bastidas-Oyanedel JR, Schmidt JE. Effect of total solid content and pretreatment on the production of lactic acid from mixed culture dark fermentation of food waste. Waste Manag. 2018;77:516–21. https://doi.org/10.1016/j.wasman.2018.04.035.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shoaf, T.J., Engelberth, A.S. (2022). Recycling of Multiple Organic Solid Wastes into Chemicals via Biodegradation. In: Fang, Z., Smith Jr., R.L., Xu, L. (eds) Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste. Biofuels and Biorefineries, vol 11. Springer, Singapore. https://doi.org/10.1007/978-981-16-6162-4_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-6162-4_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6161-7
Online ISBN: 978-981-16-6162-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)