Abstract
In this work, acetone–butanol–ethanol (ABE) fermentation characteristics of cassava starch and cassava chips when using Clostridium saccharoperbutylacetonicum N1-4 was presented. The obtained results in batch mode using a 1-L fermenter showed that C. saccharoperbutylacetonicum N1-4 was a hyperamylolytic strain and capable of producing solvents efficiently from cassava starch and cassava chips, which was comparable to when glucose was used. Batch fermentation of cassava starch and cassava chips resulted in 21.0 and 19.4 g/L of total solvent as compared with 24.2 g/L of total solvent when using glucose. Solvent productivity in fermentation of cassava starch was from 42% to 63% higher than that obtained in fermentation using corn and sago starches in the same condition. In fermentation of cassava starch and cassava chips, maximum butanol concentration was 16.9 and 15.5 g/L, respectively. Solvent yield and butanol yield (based on potential glucose) was 0.33 and 0.41, respectively, for fermentation of cassava starch and 0.30 and 0.38, respectively for fermentation using cassava chips.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of increasing oil prices, running out of fossil fuels and constant conflicts in the oil-supply region of the world, various research works have been initiated to produce biofuels such as ethanol and butanol all over the world. Butanol, a fermentation product of some Clostridium species, is a superior fuel to ethanol [1]. In addition to fuel applications, butanol can be used in the manufacture of plastics, and extraction of food flavors [2]. Butanol can be produced from renewable agricultural resources including molasses, agricultural biomass, wood hydrolysate, starchy materials (corn, wheat, rice, rye, and cassava starches), dairy industry waste, etc. [3].
The economics of butanol production is affected by raw materials used, the type of bioreactor, the butanol recovery techniques, byproduct credit, yield, solvent concentration, and productivity [2]. One of the most important economic factors in solvent fermentation is the cost of substrate. Thus, the availability of an inexpensive raw material is essential if solvent fermentation is to become economically viable.
Most abundantly available, cost-effective raw materials used for fermentation industry are starchy materials. In South East Asia, cassava represents an alternative cheap carbon source for fermentation processes that is attractive in both economic and geographical considerations. Cassava is widely grown for its enlarged starch-filled roots, which contain nearly the maximum theoretical concentration of starch on a dry weight basis among food crops. Besides that, cassava is able to grow in poor soils on marginal lands with minimal amounts of fertilizer, pesticides, and water [4]. Therefore, cassava is a promising crop for biofuel production from renewable resources. Furthermore, for many countries in South East Asia, cassava is not the staple food; therefore, the food security concerns would be minimized.
Traditionally, since cassava is a starchy substrate, an additional pretreatment process is required for its liquefaction and saccharification. In most cases, it is obligatory to perform a two-step process that includes saccharification by acids or enzymes, followed by microbial fermentation. The production of amylolytic enzymes by a single strain that also metabolizes the sugars produced to the desired end product has an advantage over those systems where hydrolysis and subsequent fermentation of starch occur separately [5]. By doing this strategy, the enzymatic hydrolysis step for the conversion of starch to fermentable sugars can be eliminated and hence, would reduce the production cost greatly.
The strain Clostridium saccharoperbutylacetonicum N1-4 was developed several years ago to produce acetone and butanol. This strain can produce more than four times as much as acetone by fermentation with the yield of butanol being more than 0.25 on the basis of supplied sugars [6]. Besides superior properties in producing butanol, this strain possesses also a hyperamylolytic activity to hydrolyze starch. Substantial activities of amylolytic enzymes during active growth phase will hydrolyze the starch to fermentable sugars that are subsequently used for production of solvents. Thus, the use of amylolytic enzyme-producing microorganisms such as C. saccharoperbutylacetonicum N1-4 in solvent production by direct fermentation of starchy materials is very important in term of economizing the process for biofuel production.
However, to the best of our knowledge, there have been few reports thus far regarding direct fermentation of cassava for acetone–butanol production. In this work, acetone–butanol–ethanol (ABE) fermentation characteristics of cassava starch and cassava chips when using C. saccharoperbutylacetonicum N1-4 is presented.
Materials and Methods
Bacterial Strain
C. saccharoperbutylacetonicum N1-4 ATCC 13564 was used in this study. The culture was maintained in the form of spores in fresh potato glucose medium (PG medium) at 4 °C. To prepare the seed culture, 1 mL of spore suspension was aseptically transferred into 9 mL of PG medium. Then, this mixture was subjected to heat shock by placing it in boiling water for 1 min and was subsequently cultivated at 30 °C for 24 h.
Starchy Materials
Cassava starch
Starch is the main constituent of cassava. About 25% starch may be obtained from mature, good-quality roots. Cassava starch is extracted primarily by the wet milling of fresh cassava roots. Extraction of starch from fresh cassava roots can be divided into five main stages: preparation (peeling and washing), rasping/pulping/grating, purification (starch washing), dewatering and drying, and finishing (milling and packaging). For cassava, the process of starch extraction is relatively simple as there are only small amounts of secondary substances, such as protein, in the roots. Cassava starch is easy to extract using a simple process, when compared to other starches that can be carried out on a small-scale with limited capital. In this work, cassava starch was kindly supplied by a local factory in Vietnam.
Cassava chip
The chips are dried irregular slices of cassava roots which vary in size but should not exceed 5 cm in length, so that they can be stored in silos. Therefore, the chips contain all components of cassava roots including impurities such as sand or soil. Cassava chips are the most common form in which dried cassava roots are marketed and most exporting countries produce them. In the ethanol industry, cassava chips are grinded to cassava chip flour and this flour is used directly as raw material for the process. In this work, cassava chip flour was kindly supplied by a bioethanol plant in Vietnam, where this flour has been used as raw material.
Corn starch and sago starch
Corn and sago starches were used as referenced materials. Corn starch was purchased from Hinode Seifun Co., Ltd, Japan and sago starch was purchased from Sin Eng Seng Sendirian Berhad, Malaysia.
Preparation of Medium
The fresh potato medium (PG medium) contained the following substances per liter of distilled water [7]: 150 g grated fresh potato, 10 g glucose, 0.5 g (NH4)2SO4, and 3 g CaCO3. After mixing the above substances, the medium was incubated in boiling water for 1 h with mixing every 10 min. After that, the medium was filtered through a gauze. The medium was sterilized at 121 °C for 1 h.
Tryptone–yeast extract–acetate medium (TYA medium) was used for the pre-culture [8]. The medium per liter of distilled water consisted of 20 g glucose, 2 g yeast extract (Difco Laboratories, Detroit, MI, USA), 6 g tryptone (Difco Laboratories), 3 g CH3COONH4, 0.3 g MgSO4·7H2O, 0.05 g or 0.5 g KH2PO4, and 10 mg FeSO4·7H2O. The medium was sterilized at 115 °C for 15 min.
The main fermentation medium contained starchy materials (from 40 to 70 g/L on the basis of starch concentration), CH3COONH4 (3 g/L), MgSO4·7H2O (0.3 g/L), KH2PO4 (0.5 g/L) and FeSO4·7H2O (7 mg/L; all from Wako Pure Chemical Industries, Japan). Gelatinized starches were prepared by heating slightly slurry at 80 °C for 30 min. The medium was then autoclaved at 115 °C for 15 min. After autoclaving, the fermentation broth was sparged with oxygen-free nitrogen until the temperature of medium dropped to around 30 °C to achieve anaerobiosis. The medium was cooled down by sparging oxygen-free nitrogen gas. At 30 °C, tenfold concentrated media containing yeast extract (20 g/L) and tryptone (60 g/L), which was separately autoclaved at 115 °C for 15 min, was added to gelatinized starch media (45 mL of this solution to 360 mL of autoclaved medium containing starch and salts).
Batch Fermentation
Batch fermentation was performed in a 1-L stirred-tank fermenter with a working volume of 0.45 L. The pH of the broth was adjusted to 6.2 at the beginning of fermentation. Flushing by nitrogen gas was limited to the fermenter before and after inoculation and was terminated as soon as the cells started to produce the fermentation gases. The batch fermentation was initiated by inoculating a 10% (v/v) actively growing cell in TYA medium, which was 16 h vegetative culture grown in test tubes. In all experiments, the temperature was maintained at 30 °C and samples were periodically withdrawn for ABE, acids, and starch analysis. Experiments were duplicated and the presented data were the average values.
Enzymatic Hydrolysis of Starch
Two enzymes, Kleistase T10S (Daiwa Kasei, K.K. Ltd., Osaka, Japan), which is an amylase from Bacillus subtilis with an activity of 13,100 Lj/g (Lj = amylase unit), and Gluczyme (Amano Pharmaceutical Co., Ltd., Nagoya, Japan) from Rhizopus niveus with an activity of 6,000 U/g were used. The method for hydrolysis of cassava chip hydrolysis was as follows: 90 g of cassava chip flour was suspended in water. The volume of starch suspension was 400 mL. The liquefaction was carried out by adding 0.3% (v/v) of Kleistase T10S to the slurry at pH 6.0 and incubating at 95 °C for 2 h. Saccharification was carried out by adding 0.5% (v/v) of Gluczyme at pH 4.5 and maintained at 58 °C for 15 h. The obtained glucose solution was kept at 4 °C as stock for further use.
Analytical Procedures
Cell concentration was determined using a modified method of Soni et al. [9]. A portion of 20 µL Kleistase T10S was added to 1 mL of culture medium and then incubated at 90 °C for 2 h to hydrolyze starch in the medium to soluble dextrin. Samples, which were contained in pre-weighted Eppendorf tubes, were centrifuged at 20,000×g for 10 min at 4 °C. The supernatant was discharged and the remaining solid was redissolved with water and once centrifuged. After discharging the supernatant, the cells, which was free from starchy substances, was dried at 105 °C for 4 h for determination of dry cell weight. Fresh medium was used as a blank sample.
ABE and acids (acetic and butyric) were measured using a gas chromatography (7890A GC-System, Agilent Technologies, USA) equipped with a flame ionization detector and a 15 m capillary column (Innowax; i.d. 0.53 mm; 19095N-121; Agilent Technologies). The oven temperature was programmed to increase from 50 °C to 170 °C at the rate of 10 °C/min. The injector and detector temperatures were set at 250 °C. Helium was the carrier gas and was set at a flow rate of 3.7 mL/min.
Maltose and glucose concentration in fermentation broth was determined by using a high-performance liquid chromatography (LaChrom Elite, Hitachi High Technologies, Tokyo, Japan) equipped with a SUGAR SH 1011 column (Shodex, Tokyo, Japan) using 3 mM HClO4 as the mobile phase at flow rate of 1.0 mL/min and 50 °C.
Starch concentration of the samples was determined using a modified method of Holm et al. [10]. A portion of 20 µL Kleistase T10S was added to 1 mL of culture medium and then incubated at 90 °C for 3 h to hydrolyze starch in the medium to soluble dextrin. After that, 8,880 µL of 0.1 M acetate buffer pH 4.5 and 100 µL of Gluczyme were added to the solution and then incubated at 58 °C for 4 h. The solution was allowed to cool down to room temperature and then transferred to a 10-mL volumetric flask followed by filling it with distilled water to the volume. Glucose concentration of this solution was determined using the HPLC method. In parallel, enzymatic method (Mutarotase—GOD method) using Wako Glucose C2 Kit (Japan) was carried out as a referenced method: a portion of the solution (20 µL) was mixed with color reagent (3.0 mL) and incubated at 37 °C for 5 min. Standard solutions of anhydrous d-glucose containing 1–5 g/L of glucose in distilled water were prepared. Twenty microliters of each of the standard solution was mixed with color reagent (3.0 mL) and incubated at 37 °C for 5 min. A blank (phosphate buffer pH 7.1) was also incubated with the reagent and was used for zero adjustment of the spectrophotometer. After 5 min, the absorbance was measured at 505 nm using a spectrophotometer (V-530; JASCO, Japan) and the glucose content in the sample was calculate based on the standard curve. Starch concentration in fermentation broth was calculated as follows:
where a is dilution factor and b is correction factor for glucose to starch.
Results and Discussions
ABE Fermentation Using Cassava Chip Hydrolysate
Even if it was noted that dry substances of cassava contains mostly starch [4], some possible trace components could affect the fermentation using cassava starch-based materials. In order to examine the possible inhibitory effects of unidentified components of cassava chips on solvent production by C. saccharoperbutylacetonicum N1-4, an experiment was carried out with cassava chip hydrolysate. In order to compare results obtained in these studies, control fermentation was conducted in which glucose was used as a substrate.
Production of ABE from glucose and cassava chip hydrolysate is shown in Fig. 1. Glucose concentration in both cases was around 65 g/L. It can be seen in general that the fermentation profiles are similar. It seems that there is no inhibitory effect of unidentified components of cassava chip on the growth of C. saccharoperbutylacetonicum N1-4. After 16 h, maximum concentration of dry cell weight of 2.5 g/L was reached in both experiments. The initial pH 6.2 of fermentation broth in both experiments decreased to pH 5.1 after 16 h and then increased to final pH 5.8 after 72 h. In the same time, glucose consumption in both experiments was similar when final glucose concentration was about 2.6 g/L.
During the fermentation with cassava chip hydrolysate, maximum ABE concentration of 23.1 g/L was produced in 36 h. This included 6.0 g/L acetone, 16.4 g/L butanol, and 0.7 g/L ethanol. A solvent productivity of 0.64 g/L h and solvent yield of 0.37 was achieved in this experiment. After 36 h, concentration of solvent started decreasing slightly due to possibly evaporative loss. At the end of fermentation, no butyric acid was detected while acetic acid was 0.7 g/L.
When glucose was used as carbon source, the culture produced 7.0 g/L acetone, 16.2 g/L butanol, and 1.0 g/L ethanol over the course of 36 h, resulting in a total ABE concentration of 24.2 g/L. A solvent productivity of 0.67 g/L h and solvent yield of 0.38 was achieved in this experiment. Regarding organic acid produced, similar result was observed in fermentation using cassava chip hydrolysate when no butyric acid was detected while acetic acid was 0.5 g/L. In terms of butanol and ethanol, there was very little difference in comparison with fermentation using cassava chip hydrolysate when maximum butanol and ethanol concentration was 16.2 and 1.0 g/L, respectively. However, regarding acetone produced and solvent productivity there was significant difference between two experiments. It is interesting to note that acetone produced in fermentation using glucose was about 16.7% higher than that produced in fermentation using cassava chip hydrolysate. This fact led to a slightly higher solvent concentration when glucose was used instead of cassava chip hydrolysate. In addition, when glucose was used, fermentation time was shorter than when using cassava chip hydrolysate. This fact resulted in a significantly higher solvent productivity in fermentation of glucose.
It was interesting to note that the results obtained in simple batch fermentation using glucose and cassava chip hydrolysate by C. saccharoperbutylacetonicum N1-4 was comparable to that obtained by C. beijerinckii BA101 in gas-stripping integrated batch fermentation [11]. As can be seen in Table 1, in simple batch fermentation using 60 g/L glucose, C. saccharoperbutylacetonicum N1-4 could produce as efficiently as C. beijerinckii BA101 did in gas-stripping integrated batch fermentation. In comparison with simple batch fermentation by C. beijerinckii BA101, C. saccharoperbutylacetonicum N1-4 showed much better fermentation performance. In previous work, C. beijerinckii BA101 produced 5.3 g/L acetone, 11.9 g/L butanol, and 0.5 g/L ethanol over the course of 60 h, resulting in a total solvent concentration of 17.7 g/L (37% lower) and solvent productivity of 0.29 (116% lower) as compared to solvent concentration of 24.2 g/L and solvent productivity of 0.67 for fermentation of glucose using C. saccharoperbutylacetonicum N1-4.
ABE Fermentation Using Cassava Starch
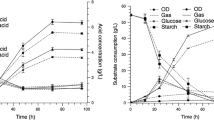
To evaluate fermentability of cassava starch, fermentation trials were run with about 70.0 g/L of cassava starch powder using C. saccharoperbutylacetonicum N1-4 in TYA medium. This produced a medium containing 60.0 g/L total starch, which was equal to a medium containing 66 g/L glucose with an assumption that all starch was converted to glucose. A typical time course of solvent fermentation by C. saccharoperbutylacetonicum N1-4 using cassava starch is shown in Fig. 2. It can be seen that this strain could grow in medium containing starch as well as in medium containing starch hydrolysate. The maximum dry cell weight concentration in both cases was around 2.5 g/L and reached after 20 h.
Direct fermentation of cassava starch to solvent by C. saccharoperbutylacetonicum N1-4 can be divided into two phases, the acidogenic phase and the solventogenic phase. An acidogenic phase was observed during the first 12 h of fermentation where C. saccharoperbutylacetonicum N1-4 grew rapidly with production of organic acids (acetic and butyric acids) which caused reduction in medium pH from 6.2 to 5.6. The fermentation entered the solventogenic phase when growth reached stationary phase (i.e., after 20 h). During this phase, the metabolism of cells undergoes a shift to produce solvent by reassimilation of organic acid. During this solventogenic phase, acetic and butyric acid was maintained at around 0.6 g/L.
During the early stages of growth, cassava starch was hydrolyzed into maltose and glucose by α-amylase and glucoamylase enzymes. From this experiment, it was observed that C. saccharoperbutylacetonicum N1-4 hydrolyzed starch to glucose faster than they utilized the latter for cell growth, maintenance, and ABE production. This strain hydrolyzed starch efficiently and there was free glucose as well as free maltose in the medium throughout the fermentation. More interesting was the strain’s ability to hydrolyze starch even during the initial phase of cell growth (at 4 h) when there was 0.3 g/L glucose present. At 4 h of fermentation, it was observed that the viscosity of medium decreased significantly. Concentration of glucose and maltose at 24 h of fermentation was 0.4 g/L and 0.7 g/L, respectively. At 48 h, glucose and maltose concentration was 1.0 g/L and 0.7 g/L, respectively. At the end of the experiment, even if no solvent was produced, amylolytic activities still remained, hence resulting in approximately 2.5 g/L glucose and 1.2 g/L maltose in fermentation broth. These results indicated that the extracellular amylases and glucoamylases continued hydrolyzing starch throughout the fermentation and C. saccharoperbutylacetonicum N1-4 was is a hyperamylolytic-producing strain.
As can be seen in Fig. 2, starch was not all consumed at the end of fermentation. The experiment was carried out in 96 h (data not shown), however, fermentation was actually finished at 48 h, when the concentration of the solvent as well as butanol reached maximum. This fermentation time was much shorter than any results reported previously for fermentation using starches as carbon sources. At this moment, concentration of starch was 11.5 g/L while glucose and maltose residues were 1.0 g/L and 0.7 g/L, respectively. During 48 h of fermentation, the strain produced 21.0 g/L total solvent including 16.9 g/L butanol, 3.6 g/L acetone, and 0.5 g/L ethanol, which resulted in a solvent productivity of 0.44 g/L h. During the fermentation, 46.9 g/L of starch was utilized, resulting in a solvent yield of 0.41 and a butanol yield of 0.33, on the basis of potential glucose present in the medium (with assumption that all starch was converted to glucose, 1 g starch would produce 1.1 g glucose). In comparison with fermentation using cassava chip hydrolysate, solvent yield was about 11% higher (0.41 vs. 0.37) while solvent productivity was about 45% lower (0.44 g/L h vs. 0.64 g/L h). In terms of butanol production, C. saccharoperbutylacetonicum N1-4 can utilize cassava starch as efficient as glucose, even better. Butanol concentration of 16.9 g/L was slightly higher than that obtained in fermentation using glucose. However, it was interesting to note that acetone produced in direct fermentation of starch was about 42–50% less than that obtained in fermentation of cassava chip hydrolysate (3.5 vs. 6.0 g/L) or glucose (3.5 vs. 7.0 g/L).
Effect of Cassava Starch Concentration
In order to investigate the effect of cassava starch concentration on ABE fermentation, different experiments were carried out using starch concentrations varying from 40 to 70 g/L. Experiments using 80 g/L starch were also investigated but it was very difficult to carry out fermentation since high pseudoplastic behavior and the apparent viscosity of medium increased drastically. In general, the profiles of fermentation using different concentration of cassava starch were similar to Fig. 2. All fermentations were finished at 48 h. The performance of solvent fermentation by C. saccharoperbutylacetonicum N1-4 using different concentrations of cassava starch is shown in Table 2. It can be seen that there was no different in growth of C. saccharoperbutylacetonicum N1-4 when starch concentration varied from 40 g/L to 70 g/L. Maximum dry cell weight concentration was not significantly different when it varied in the range of 2.5 to 2.6 g/L.
In fermentation using 40 g/L of cassava starch, the residual concentration of starch was 6.1 g/L, indicating that 84.8% starch (33.9 g/L) was utilized during the fermentation. During this fermentation, neither glucose nor maltose was detected. This indicated that at starch concentration as low as 40 g/L, the strain hydrolyzed starch to glucose slower than they utilized the latter for fermentation. It was possible that at a starch concentration of 40 g/L and lower, fermentation could be deficient in substrate.
When starch concentration increased from 50 to 70 g/L, glucose and maltose concentration in medium increased correspondingly, from 0 to 4.9 g/L for glucose and from 0.3 to 2.7 g/L for maltose. In the same time, residual concentration of starch increased from 7.1 to 15.0 g/L indicating that amount of starch utilized during fermentation decreased from 85.2% to 68.6%. It can be concluded that when starch concentration above 50 g/L, starch utilization degree started to decrease. Reduction in utilization of starch at high starch concentrations may be due to an increase in apparent viscosity, which in turn, limits the mass transfer for enzymatic hydrolysis and microbial reactions.
From Table 2, it was found that solvent yield and butanol yield decreased from 0.46 and 0.37 to 0.39 and 0.31, respectively, with the increase of starch concentration from 40 to 70 g/L. There was no significant difference in the concentration of solvents and therefore in solvent productivity at different starch concentrations, except fermentation using 40 g/L starch. Solvent productivity was around 0.43 g/L h for all fermentations as compared to 0.36 for fermentation using 40 g/L starch. Results in Table 2 also indicate that maximum concentration of butanol in direct fermentation of cassava starch would not be higher than 17.0 g/L. Table 2 also shows that the concentration of butyric acid, which is important for the onset of butanol production [12], drastically decreased when starch concentration increased from 40 to 50 g/L. In this case, maximum butyric acid concentration dropped from 1.5 to 0.5 g/L. On the other hand, butyric acid as well as acetic concentration was maintained at around 0.5 g/L for both acids in all fermentations using 50 to 70 g/L starch. It was reported that solvent production was not stimulated by the high amount of acetic and butyric acid accumulated during the fermentation but high solvent production was much more related to the low amount of butyric acid accumulated [13].
Direct Fermentation of Various Starchy Materials to ABE
The effect of different types of starchy materials using C. saccharoperbutylacetonicum N1-4 on fermentation is shown in Table 3. The initial concentration of starch was fixed at around 60 g/L, except for corn starch. However, consumption of starch by this strain was not the same for different starches. While fermentation using cassava and corn starches as well as cassava chips showed a similar starch utilization rate (78.2–78.6%), fermentation of sago starch presented a relatively low starch utilization rate (69.1%).
The maximum dry cell weight concentration was more or less the same (about 2.5 g/L). In previous works, Madihah et al. [13] reported that C. acetobutylicum P262 showed the highest growth (2.5 g/L) in fermentation of sago starch, followed by corn starch (2.0 g/L), potato starch (1.9 g/L), and cassava starch (1.1 g/L). Not only growth, it also seemed that amylolytic activities produced by C. saccharoperbutylacetonicum N1-4 were maintained more or less the same in all fermentations using different types of starches. Total glucose and maltose in media was maintained at level of about 1.6–3.5 g/L. From these results, it can be seen that C. saccharoperbutylacetonicum N1-4 can grow well in different starchy materials.
The highest total solvent production (21.0 g/L) was obtained when cassava starch was used and followed by corn starch (20.7 g/L). Total concentration of solvent obtained in fermentation using sago starch was comparable to that obtained by fermentation of cassava chips (around 19.5 g/L). There was no significant difference in acetone production with the different types of starches except for fermentation of corn starch, when acetone was produced at 4.0 g/L, slightly more than 3.5 g/L obtained in fermentation using cassava chips, cassava, and sago starches. In terms of butanol production, highest concentration of 16.9 g/L was obtained in fermentation using cassava starch. Ethanol production was more or less the same in fermentations using different starches when ethanol concentration in medium was in the range of 0.4–0.6 g/L. In fermentation using cassava chips and sago starch, butanol was produced at the same level, which was about 5% lower than that produced in fermentation using corn starch (15.5 g/L vs. 16.2 g/L). The highest solvent productivity (0.44 g/L h) was obtained in fermentation using cassava starch, followed by fermentation using cassava chips (0.40 g/g/L h). However, the solvent productivity for fermentations using corn and sago starches was from 42% to 63% lower compared to fermentation using cassava starch. It is interesting to note that solvent yield, based on potential glucose, in fermentation of cassava chips was similar to that observed in fermentation of glucose. On the other hand, solvent yield obtained in direct fermentation of starches was from 7.8% (for cassava starch) to 23.7% (for corn starch) higher than in fermentation using glucose.
Regarding organic acid production, it can be seen that fermentation using cassava chips resulted in a significantly high amount compared to fermentation using cassava, corn, or sago starches. While total acetic and butyric acid concentration in fermentation of cassava chips was 3.3 g/L, it was maintained as low as 0.4–1.2 g/L in fermentation using cassava, corn, or sago starch. Especially, no butyric acid was detected at the end of fermentation using corn and sago starches. The reason for this could be due to unidentified components in cassava chips. It was reported that the accumulation of intermediate organic acids (acetic and butyric acids) greatly influenced on solvent production. Higher amounts of organic acid accumulated during the fermentation of cassava chips appear to have inhibited cell growth, sugar consumption and, hence, reduced the total solvent production [13]. Total solvent concentration obtained in fermentation using cassava chips was lower compared to fermentation of cassava starch (19.4 vs. 21.0 g/L).
However, the most significant difference among fermentations using different types of starch was the fermentation time. While fermentation using both cassava starch and cassava chips was finished at 48 h, fermentation using corn and sago starches was terminated at 66 and 72 h, respectively. This resulted in a lower solvent productivity when corn and sago starches were used instead of cassava starch or cassava chips. The explanation for this observation may be due to the difference in physico-chemical properties of starches. Corn starch has an average granule size of 15 µm [14] and contains approximately 28% amylose [15]. Sago starch has an average granule size of 30 µm [16] and contains 24–31% amylose [15, 17], while average granule size of cassava starch is 13–15 µm [18, 19] and amylose content is about 18.6–23.6% [20]. The smaller granule sizes improve the digestibility by enzymes because smaller granules have a greater surface area and are more rapidly digested by amylases. The relationship between granule size and digestibility was previously reported [21, 22].
Conclusions
From this work, it was found that C. saccharoperbutylacetonicum N1-4 was able to grow well not only on cassava starch, but also on cassava chips as well as on other starchy materials such as corn and sago starches. The results obtained in this work demonstrated that C. saccharoperbutylacetonicum N1-4 was a hyperamylolytic strain and capable of producing solvents from cassava starch which was comparable to when glucose was used. A plot of starch utilization by C. saccharoperbutylacetonicum N1-4 during batch fermentation indicated that this strain did utilize cassava starch efficiently during the batch fermentation process. Moreover, fermentation using cassava chips demonstrated that production of solvent in general and production of butanol in particular was comparable to that obtained in fermentation using cassava starch. This fact would improve economy of butanol production from cassava since cassava chips can be used directly in fermentation like in ethanol industry. It should be noted that to produce cassava starch from cassava chips, energy and water costs, as well as the environment are matter of great concern.
The maximum concentration of total solvent (20.5–21.0 g/L) and the solvent yield based on glucose consumed (0.41–0.46) obtained in this work is comparable with those reported in the other works where corn starch was used [2, 11, 23]. The obtained results are much higher than those reported by Madihah et al. [13] when a solvent yield of 0.20–0.34 was observed in direct fermentation of cassava starch by C. acetobutylicum P262.
Production of solvents by C. saccharoperbutylacetonicum N1-4 using cassava starch may be limited at starch concentration above 70 g/L. At 70 g/L cassava starch, a substantial amount of starch remained at the end of the fermentation (31.2%). Even amylolytic activities during active growth were apparently sufficient to hydrolyze high starch concentration to fermentable sugars, high viscosity of fermentation broth resulted in a reduction in solvent production. Until 96 h of fermentation, maltose and glucose was continuously produced with 3.2 g/L maltose and 7.1 g/L glucose but no more solvent was produced compared to 48 h (data not shown). ABE fermentation using high cassava starch concentration may be affected by the mass transfer rate, which is a function of broth viscosity, with higher viscosity increasing diffusion of metabolized substances. Using a stirred-tank fermenter could be a solution to overcome this limitation. It is also interesting to note that acetone produced in fermentation using glucose or cassava chip hydrolysate was about twofold than that obtained in fermentation of starches.
Starches from different origins showed different susceptibilities to amylolytic enzymes. Starch susceptibility to enzyme attack is influenced by several factors such as amylose and amylopectin content, particle size, crystalline structure, and the presence of enzyme inhibitors [24, 25]. Among non-cereal starches, cassava starch has relatively higher enzyme susceptibility than other starches [26] while sago starch was a poor substrate for enzyme action compared to corn and cassava starches tested under the same conditions [27]. This could be a reason for relatively rapid fermentation of cassava starch over corn and sago starches.
References
Ladisch, M. R. (1991). Enzyme and Microbial Technology, 13, 280–283.
Qureshi, N., & Blaschek, H. P. (2001). Journal of Industrial microbiology & Biotechnology, 27, 292–297.
Campos, E. J., Qureshi, N., & Blaschek, H. P. (2002). Applied Biochemistry and Biotechnology, 98–100, 553–561.
Grace, M. R. (1977). Cassava processing. Rome: Food and Agriculture Organization of the United Nations (FAO).
Jesse, T. W., Ezeji, T. C., Qureshi, N., & Blaschek, H. P. (2002). Journal of Industrial microbiology & Biotechnology, 29, 117–123.
Hongo, M. (1960) US Patent 2945786
Lee, T. M., Ishizaki, A., Yoshino, S., & Furukawa, K. (1995). Biotechnological Letters, 17, 649–654.
Tashiro, Y., Takeda, K., Kobayashi, G., Sonomoto, K., Ishizaki, A., & Yoshino, S. (2004). Journal of Bioscience and Bioengineering, 98, 263–268.
Soni, B. K., Soucaille, P., & Goma, G. (1987). Applied Microbiology and Biotechnology, 25, 317–321.
Holm, J., Björck, I., Drews, A., & Asp, N. G. (1986). Starch/Stärke, 38, 224–226.
Ezeji, T. C., Qureshi, N., & Blaschek, H. P. (2003). World Journal of Microbiology & Biotechnology, 19, 595–603.
Monot, F., & Engasser, J. M. (1983). Biotechnological Letters, 5, 213–218.
Madihah, M. S., Ariff, A. B., Sahaid, K. M., Suraini, A. A., & Karim, M. I. A. (2001). World Journal of Microbiology & Biotechnology, 17, 567–576.
Ma, Y., Cai, C., Wang, J., & Sun, D. W. (2006). Journal of Food Engineering, 73, 297–303.
Ahmad, F. B., Williams, P. A., Doublierb, J. L., Durand, S., & Buleon, A. (1999). Carbohydrate Polymers, 38, 361–370.
Wang, W. J., Powell, A. D., & Oates, C. G. (1995). Carbohydrate Polymers, 26, 91–97.
Sandhu, K. S., & Singh, N. (2007). Food Chemistry, 101(4), 1499–1507.
Rao, M. A., & Tattiyakul, J. (1999). Carbohydrate Polymers, 38(2), 123–132.
Yuan, Y., Zhang, L., Dai, Y., & Yu, J. (2007). Journal of Food Engineering, 82(4), 436–442.
Defloor, I., Dehing, I., & Delcour, J. A. (1998). Starch/Stärke, 50, 58–64.
Cone, J. W., & Wolters, G. E. (1990). Starch/Stärke, 42, 298–301.
Franco, M. L. C., Preto, S. J. R., & Ciacco, C. F. (1992). Starch/Stärke, 44, 113–116.
Ezeji, T. C., Qureshi, N., & Blaschek, H. P. (2005). Journal of Biotechnology, 115, 179–187.
Cui, R., & Oates, C. G. (1997). Carbohydrate Polymers, 32, 65–72.
Shariff, Y. N., Karim, A. A., Fazilah, A., & Zaidul, I. S. M. (2009). Food Hydrocolloids, 23, 434–440.
Zhang, T., & Oates, C. G. (1999). Food Chemistry, 65, 157–163.
Wang, W. J., Powell, A. D., & Oates, C. G. (1996). Sago starch as a biomass source: raw sago starch hydrolysis by commercial enzymes. Bioresource Technology, 55(1), 55–61.
Acknowledgments
This work was financed by the Japan Society for Promotion of Science (JSPS). The authors would like to thank Dr. Nana Yokochi for the useful technical supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thang, V.H., Kanda, K. & Kobayashi, G. Production of Acetone–Butanol–Ethanol (ABE) in Direct Fermentation of Cassava by Clostridium saccharoperbutylacetonicum N1-4. Appl Biochem Biotechnol 161, 157–170 (2010). https://doi.org/10.1007/s12010-009-8770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8770-1