Abstract
Dye-containing wastewaters are produced in large quantities worldwide. The bright colors they provide, associated with their potential toxicity, carcinogenicity, and mutagenicity, consist of environmental threats. Therefore, remediation of these waste streams is essential. However, the low biodegradability shown by these compounds constitutes an obstacle to the application of effective and low-cost conventional biological wastewater treatment systems. In this context, other advanced biofilm technologies, such as the moving bed biofilm reactor (MBBR), may consist in an economically viable and eco-friendly alternative for achieving high removal of dyes, while also reaching high performance in the concomitant degradation of other pollutants, such as organic matter and nutrients. In this chapter, the MBBR technology is briefly explained, and recent investigations on the treatment of dye-containing wastewaters by this biofilm process are described. Moreover, the most influential operational parameters for color removal improvement are discussed. The use of sequential reactors in multistage processes and the association of MBBR with other technologies are also evaluated. Finally, the kinetics of dye degradation in MBBR is presented. Few studies were found in the literature on the application of MBBR for dye removal, mainly consisting of lab-scale or pilot-scale investigations. A single MBBR as a stand-alone technology does not seem to achieve complete dye mineralization. Nonetheless, the use of a series of MBBR or their combination with other physicochemical or biological processes seems to be a good alternative for dyes remediation. It is clear that more studies on MBBR process optimization are needed, especially for real dye-containing wastewaters, to guarantee the effectiveness and sustainability of large-scale treatment plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Dyes are natural or synthetic compounds responsible for giving color to a certain material. In their natural form, they have been used since antiquity to dye fabrics, ceramics, and leathers. The production of synthetic dyes, on the other hand, was only boosted in the mid-nineteenth century, after the industrial revolution and the synthesis of the first organic dye by William Perkin, leading to the development of the textile industry [50]. Currently, the annual production of dyes accounts for 8 × 105 t [4], supplying industries of different branches, such as paper and cellulose, plastics, paints, food, and textiles, the latter being the main consumer. The global market of textile dyes accounted for $9.4 billion in 2018 and may reach $15.5 billion in 2026 [61].
Overall, more than 280,000 t of dye-containing wastewaters are produced worldwide on a yearly basis, and generally constitute around 80% of the emissions generated by the textile industry [74]. The high quantities of water used mainly in the dyeing process result in the generation of large volumes of effluents. For example, the dyeing of 1 kg of cotton (most used fiber worldwide) requires 70–150 L of water, in addition to 0.6–0.8 kg of NaCl and 30–60 g of dyes [1, 2]. Thus, the large production and discharge of liquid effluents by this industrial sector threaten the environment [84]. If not properly treated and discharged in water bodies, dye-containing wastewaters may present several risks to the local biota. Even in small concentrations, dyes can provide bright colors, which may block sunlight penetration in aquatic systems, affecting photosynthetic processes and leading to changes in biological cycles [73].
Dyes are highly toxic to all forms of life and are potentially mutagenic and carcinogenic [36, 62]. Besides, they have complex organic aromatic structures, which are not only responsible for the fixation and durability of color but may also act as an obstacle to biodegradation [13]. In a dye, color is given by the electronic transition between several molecular orbitals. These compounds have a chromophore group (–N = N–, –C = C–, –C = O–) which is responsible for the color effect due to electron excitation. Dyes are also composed of an auxochrome (–OH, –NH2, –NR2), which promotes color fixation [25].
Dyes can be classified according to their chemical structure and the nature of the chromophores in “nitroso, nitro, monoazo, diazo, stilbene, diarylmethane, triarylmethane, xanthene, acridine, quinoline, methine, thiazole, indamine, indophenol, azine, oxazine, thiazine, aminoketone, anthraquinone, indigoid, phthalocyanine, and inorganic pigments” [52]. Among textile dyes, the azo type is the most used one, as it provides more intense colors than other classes. However, between 15 and 50% of azo dyes do not remain in the fabric during dyeing, being discarded as wastewaters generally used for irrigation in agriculture in developing countries [60].
Currently, azo dyes represent more than 60% of the dyes used in the textile industry [30]. Approximately 70% of all dyes used in this sector are azo dyes [47]. In view of the large volume of dyes that do not fixate onto the fabrics, color is often the main problem concerning textile effluents. However, the discoloration of these effluents is not a simple task since the color is often not removed by the conventional wastewater treatment processes [45]. Physicochemical methods have shown to be economically disadvantageous for the removal of dyes since they require high energy consumption, and the use of chemicals implies high costs. Furthermore, such methods do not completely remove recalcitrant azo compounds and the generated by-products, leading to the production of chemical sludge [66]. Coagulation/flocculation processes are effective mainly for removing sulfur and dispersant dyes, but they present low removal of acid, direct, reactive, and vat dyes [66]. Conventional aerobic biological processes (e.g., activated sludge), widely used to treat different types of wastewaters due to relatively low cost and effectiveness, are usually inefficient in degrading azo dyes [10, 16, 24]. Moreover, in the presence of dyes, activated sludge deflocculation may be observed [34].
In the activated sludge process, there is a complex composition of filamentous flocs and microorganisms, polymers, and metabolic excreta. Microorganisms can synthesize extracellular polymeric substances (EPS) that lead to the formation of flocs by agglomeration of bacteria. EPS provides a large surface area per volume for microorganism attachment and significantly affects floc settling [35]. Işık and Sponza [34] studied different means of cultivation of activated sludge flocs and observed that as the composition of EPS is influenced by the type of substrate and by the microorganisms in the activated sludge, the sedimentation characteristics also become strongly influenced by the reactor microenvironment, including the dynamics of the microbial population and degradable organic compounds. Flocs grown in wastewater containing easily degradable organics exhibited good sedimentation properties at low sludge volume index values. Flocs grown in wastewater containing organic substrates with greater difficulty in degradation, such as chemicals and dyes, exhibited worse sedimentation properties.
On the other hand, biofilm systems retain bacterial cells in appropriate quantities within the reactor by developing a biofilm adhered to fixed or mobile supports. The biofilm consists of a humid matrix and a variety of soluble and particulate components that include microorganisms and EPS [79]. The moving bed biofilm reactor (MBBR) is an example of a biofilm technology in growing expansion over the world. Recent studies have shown that this biofilm technology may be efficient for the treatment of dye-containing effluents [65, 71, 87]. However, the number of studies addressing the application of the MBBR process for this purpose is limited. Therefore, in this chapter, an overview of the MBBR technology is presented with the main focus on the application of this process on the removal of dyes. The factors affecting the effectiveness of dyes removal, optimum process conditions, and kinetic aspects were addressed in this contribution. The chapter is divided into seven main topics, including a brief description of biofilm reactors,the MBBR technology and its advantages over other biofilm systems; the removal of dyes in MBBR; factors affecting dye removal in MBBR; kinetics of dye removal in MBBR; process combinations; and a comparison between MBBR and other biological wastewater treatment systems for dye removal, considering technological, environmental, and economic aspects.
2 Biofilm Reactors
Biological processes are widely used for wastewater treatment, being divided into two classes: suspended or fixed biomass (biofilms) systems. In the first case, microorganisms cluster in the form of microbial flocs, with a predominance of bacteria. Examples of systems with suspended biomass are activated sludge, membrane bioreactors, stabilization ponds, among others. These processes are very efficient in removing organic matter and nutrients, but some have limitations such as the need for large areas and high sludge production [8].
In the case of biofilm reactors, microorganisms grow attached to a solid surface with a high surface area, leading to the accumulation of a high concentration of biomass in the reactor. A biofilm consists of three-dimensional heterogeneous microbial aggregates containing several microorganisms that compete for the available substrates. They are immobilized in a matrix of EPS, together with cellular products, and grow in a very compact way. As a result, a large amount of biomass can be accumulated in a small reactor volume, leading to high pollutant removal rates. Biofilms are resistant to dehydration and offer protection against predatory organisms, good stability, and resistance to shock loads [28].
Biofilm growth occurs through different processes: free cell transport from the liquid medium to the solid surface and initial fixation; growth, production, and excretion of EPS; fixation of cells to the already formed biofilm; erosion of small particles; and loss of larger aggregates [85]. Cells can detach from the biofilm due to shear forces and also when the environment becomes unfavorable. Due to a balance between the detachment of cells and microbial growth, biofilm thickness varies with time and position. The detached microorganisms start to occupy the bulk phase and, as the suspended microbial community increases, it may also play an important role in pollutants degradation [28].
The transport of the components from the bulk phase to the cells involves several sequential steps: adsorption onto the biofilm surface, diffusion through the stagnant liquid film at the interface between biofilm and liquid phase, and diffusion through the biofilm (Fig. 1). The encapsulated structure that maintains the biofilm cohesion may lead to concentration gradients for all substances. Therefore, ensuring an effective mass transfer in biofilms is an important factor for effective pollutant removal. If the substrate mass transfer is limited, reaction rates will be reduced, compromising the treatment process [46].

Source Adapted from Lin [46]
Substrate diffusion from the bulk phase to the biofilm layer.
Biofilm-based wastewater treatment systems provide great advantages to the treatment process compared to those with suspended growth. Since the microorganisms grow adhered to a surface and are not constantly removed from the reactor along with the liquid effluent, the hydraulic retention time (HRT) is decoupled from the sludge retention time (SRT), allowing the use of a high SRT regardless of the HRT, and without the need of sludge recycle from the secondary clarifier. In addition, by retaining the biomass inside the reactor for longer periods, this type of system facilitates the development of slow-growing organisms. The formation of biofilms also reduces the area required for the installation of the reactors since it simplifies the solid–liquid separation step [8].
Some examples of biofilm processes are trickling filter, submerged aerated filter, rotating biological contactors (RBC), moving bed biofilm reactors (MBBR), among others. The MBBR, in particular, is a relatively recent technology, which stands out over other biofilm systems as it does not present clogging problems and provides lower head loss [9]. Further details on this system are given next.
3 The Moving Bed Biofilm Reactor (MBBR)
The MBBR process was created in Norway between the 1980s and 1990s, being used ever since to treat domestic and industrial wastewaters as an alternative to conventional secondary treatment processes. The MBBR provides high removal rates of biodegradable organic matter and nitrogen with the advantage of allowing the use of smaller reactor volumes as compared to activated-sludge-based systems [12].
The MBBR technology uses low-density moving carriers, where biofilms are formed. The media is inserted in the reactor and moves freely throughout the reactor volume. It can be applied in both aerobic and anoxic/anaerobic environments, with the mixing and fluidization of the carriers being obtained by diffuse aeration (for aerobic reactors) or mechanical mixing (for anoxic/anaerobic reactors) [40, 42]. Agitation also enables the transport of the substrates to the biofilm and helps to control the biofilm thickness due to the action of shear forces [41]. A scheme of the MBBR process (for both aerobic and anoxic/anaerobic configurations) is displayed in Fig. 2. A sieve is used at the outlet of the reactor to retain the carriers and allow only the treated effluent to pass through.

Source Adapted from [63]
Scheme of aerated MBBR (a) and mechanically stirred MBBR (b).
MBBR has advantages over fixed biomass systems, such as low head loss, absence of clogging, use of moving carrier media with high specific biofilm surface area, and the entire volume of the system available for the biological conversions [8, 39]. Besides, the use of moving media excludes the necessity for sludge recycling and facilitates the subsequent step of solid–liquid separation easier [8]. By introducing carriers to an existing treatment facility, the sludge age can be increased without major changes in the plant, allowing the development of bacteria with low growth rates, such as nitrifiers. It may also favor the production of specific enzymes by the microorganisms, which are necessary for the degradation of certain recalcitrant compounds, such as dyes [40, 49]. Other characteristics of MBBR systems that may favor dye degradation are the presence of anoxic/anaerobic zones inside the biofilm, even for aerated systems, and the resistance to toxic compounds provided by the external protective EPS layer.
3.1 Removal of Dyes in Moving Bed Biofilm Reactors (MBBR)
Although MBBR is already an established technology for wastewater treatment, few studies have been conducted regarding the use of this biofilm process for the treatment of dye-containing wastewaters (Table 1). Among the studies published so far, most of them were conducted on a laboratory or pilot scale, with synthetic wastewater simulating a real scenario used to feed the bioreactors. Process associations have also been investigated, such as ozonation + aerobic MBBR [14, 15, 21, 22, 27, 59], Fenton or photo-Fenton + MBBR [3, 72], anaerobic MBBR + aerobic MBBR [23, 27, 39, 55, 68], upflow anaerobic sludge blanket (UASB) + MBBR [48], photo-rotating biological contactor (PRBC) + aerobic MBBR [80], granular-activated carbon bed (GAC) + MBBR [76].
3.2 Factors that Influence Dye Removal in MBBR
-
Dissolved oxygen (DO) concentration
Biological systems can be kept under anaerobic, anoxic, and aerobic conditions. The redox condition is a crucial factor influencing the biological removal of azo dyes since there are big differences between the physiology of microorganisms that grow in the presence and absence of oxygen, directly influencing the degradation mechanism [57].
The removal of dyes from wastewaters can occur via adsorption onto the biomass or via biodegradation. The biosorption mechanism, alone, has some limitations since the microbial biomass becomes saturated over time, and there are problems of final disposal of the adsorbent (sludge), which contains the undegraded toxic compounds. However, biosorption is generally the first stage of biodegradation [57].
There are several hypotheses to explain the mechanisms of biodegradation of dyes. Some of them involve intra- or extracellular enzymes or a non-specific extracellular reduction [18]. In one of the proposed mechanisms, it is suggested that electrons produced during the generation of adenosine 5′-triphosphate (ATP), in catabolic reactions, are transferred to the dye (by means of enzymes and coenzymes), which acts as a final electron acceptor, inducing the chromophore breakage (e.g., azo bond). The electron transfer can occur by enzymatic pathway directly (Fig. 3a) or indirectly by means of redox mediators (Fig. 3b), which are produced during the cellular metabolism of certain substrates or added to the system. These mechanisms will be further described in the sequence. Another hypothesis relates the chromophore breakage to the reducing action of end products of the cellular catabolism, such as inorganic compounds, leading to color removal (Fig. 3c) [54, 57]. For instance, when H2S is present in the reaction medium, it reacts with monoazo dyes in a molar proportion of 2:1, leading to the formation of 2 mol of sulfur (S0) and 2 mol of aromatic amines [88].
Fig. 3 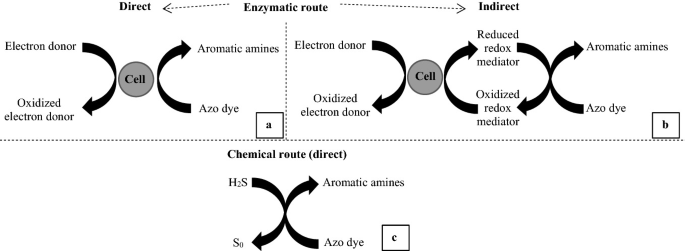
Source Adapted from [54]
Possible mechanisms related to the biological degradation of azo dyes: direct enzymatic route (a), indirect enzymatic route (b), and chemical route (c).
-
When the mechanism is intracellular, the removal of the dye depends on its diffusion across the cell membrane. This transport can be impaired when the dye has a high molar mass, and there are sulfonated groups in the molecule [18]. In such cases, it is likely that the mechanism is extracellular. Hence, for the azo bond to be broken, the cell must establish a link between the intracellular respiratory chain and the extracellular dye molecule. To this end, electron carriers must be located outside the cell, enabling contact with the dye [57].
-
The enzymes involved in reducing azo dyes are known as azo reductases. These enzymes catalyze the azo bond reduction only in the presence of reduction equivalents (coenzymes FADH, NADH, and NADPH) [70] (Fig. 4). When electrons are released during ATP production, the reduction equivalents promote the electron transfer to the azo reductases, which then catalyze azo dye reduction directly or by means of redox mediators. Azo reductases can be synthesized both in the presence and absence of oxygen [18]. However, for these enzymes to be produced in aerobic systems, a long adaptation period in the presence of a simple azo substance is required. After that period, a compound-specific azo reductase is produced, and the azo dye can then be removed in the presence of oxygen.
Fig. 4 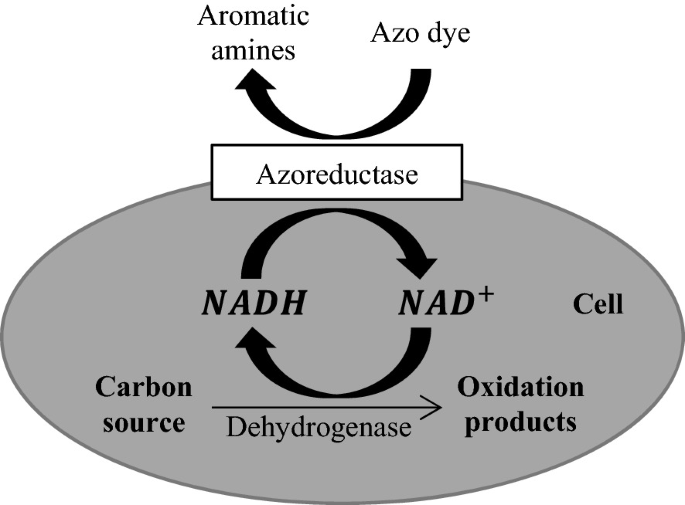
Source Adapted from [57]
Extracellular direct removal of azo dyes.
-
On the other hand, in anaerobic systems, the process is not specific to an azo compound. In that case, any added azo dye can be removed, with greater or lesser efficiency, depending on the dye molecular structure and reactor operating conditions used. Therefore, anaerobic processes are more used than aerobic for the purpose of biological dye removal [57].
-
Anthraquinones represent the second most important group of textile dyes (after azo dyes) [19, 90]. They are used for dyeing cellulosic fabrics, wool, and polyamide fibers. Another class of dyes, triphenylmethane, is used to dye nylon, nylon modified with polyacrylonitrile, wool, and silk. These dyes are usually resistant to light, temperature, and biodegradation, so they accumulate in the environment [64, 90]. In addition to azoreductases, laccases and peroxidases are the most important enzymes capable of degrading dyes.
-
Laccases are part of the family of multicopper oxidase enzymes that catalyze the oxidation of countless substrates in water, through a reaction mechanism involving radical formation [11]. These enzymes are mostly of fungal and vegetal origin, although some have been identified in bacteria and insects [6, 26]. The most useful and the most researched laccases in biotechnology applications are of fungal origin. Physiologically, laccases have several functions, such as lignolysis, pigment formation, detoxification, and pathogenesis, which result from the ability of enzymes to oxidize a wide variety of aromatic substrates (e.g., polyphenols and diamines) and inorganic compounds [26, 32].
-
Peroxidases are a group of multiple versatile and stable heme-containing enzymes that use hydrogen peroxide or electron acceptor of organic hydroperoxides (R-OOH) to catalyze the oxidation of various substances. Peroxidases show great potential to be environmental biocatalysts, being one of the most researched groups of enzymes. They can successfully degrade synthetic dyes with a high redox potential, such as anthraquinone and azo dyes [32].
-
According to Table 1, anaerobic conditions were used in most of the studies, in which biological color removal was achieved [15, 23, 27, 39, 48, 55, 65, 68, 81]. Castro et al. [14] and Park et al. [55] evaluated the performance of aerobic MBBRs in dye degradation and reported that no significant color removal was observed in the presence of oxygen. Therefore, the redox condition is a key factor to achieve the biological removal of dyes. For removing color, a favorable environment, usually in the absence of oxygen, is required to reduce the chromophores. Oxygen has a high redox potential and can replace the dye by acting as the final electron acceptor, inhibiting discoloration [57].
-
However, in some cases, the discoloration can be achieved in aerobic conditions, for instance, by using selected microorganisms, such as white-rot-fungi strains (Cerioporus squamosus and Phanerochaete chrysosporium) [56], Bacillus sp. [71], and the photosynthetic bacterium Rhodopseudomonas palustris [80]. Inoculating an MBBR with sludge from a WWTP treating dye-containing wastewaters is also a strategy to achieve aerobic color removal, possibly because of the existence of a microbial community adapted to dyes and, therefore, able to synthesize specific enzymes and use the dye as a carbon source [69, 87].
-
Agitation
Agitation is an important factor to be considered, especially in biofilm and anaerobic systems. The agitation speed can influence both biofilm thickness and mass transfer, affecting the biodegradation of dyes.
The intrinsic nature of MBBRs requires agitation to achieve bed fluidization. Agitation is also important to provide shear forces that are crucial to guarantee a good balance between biofilm attachment and detachment. If the biofilm becomes too thick, substrate transport to the inner biofilm layers can be affected. However, an excessively high stirring speed can enhance microbial detachment to such a level that the concentration of attached solids is substantially reduced. The detached biomass contributes to an increase in the concentration of suspended solids which, in the absence of sludge recycling, can be washed out (if the cell growth rate is slower than the rate of liquid effluent discharge from the reactor) [5].
Moreover, high agitation speed results in high mass transfer rates between the reaction medium and the cells, and also between the surrounding air and the medium [70]. At the same time that it may benefit the substrate transport within the biofilm, high agitation intensity may lead to increases in DO concentration, negatively affecting the mechanism of anaerobic dye removal [37]. Stirring speeds ranging from 30 to 500 rpm have been reported (Table 1). However, very few studies regarding the influence of this parameter on dye removal in MBBRs have been conducted. Considering the high molecular weight and size of many dyes, which can lead to low diffusion rates [29], the optimum coordination between agitation and biofilm thickness has to be defined to improve color removal.
-
Type of carrier media, material modifications, and filling ratio
The type of media used in the MBBR affects its performance. Two of the most influential carrier characteristics are its specific surface area and the material that it is made. Carriers showing a high specific surface area may provide higher concentrations of attached solids within the reactor and allow more microorganisms to be placed at the biofilm-bulk phase interface. In fact, the specific surface area available in the carriers for biofilm development has a greater impact on the performance of attached growth processes than the reactor volume itself [53]. The composition of such carriers can also offer more or less affinity toward microorganism attachment and can even act as a substrate source [83].
The carriers used in dye biodegradation in MBBRs are described in Table 1, being mostly made of plastic (e.g., polyethylene and polypropylene), which satisfy the low-density requirements, but usually present low hydrophilicity and low biological affinity, leading to low growth rates and cell detachment [17]. The most-reported biomedia used in MBBRs for dyes removal are the Kaldnes® K1 and polyurethane (PU) foams. Kaldnes® K1 media has a high specific surface area of 690 m2/m3 (total), or 500 m2/m3 (effective) [53], while PU cubic sponges may present specific surface areas of more than 4000 m2/m3 [48].
-
Wang et al. [83] reported new support made of high-density polyethylene (HDPE), Zn nanoparticles (NPs), and poly (lactic acid) (PLA). The authors observed that the presence of Zn NPs in concentrations of up to 20 wt% helped improving organic matter and ammonium nitrogen removals in an aerobic MBBR treating real pretreated textile wastewater. Dehydrogenase activity was also improved and the biodiversity increased. Zn ion was found to stimulate Planctomycetes, which possibly helped improving nitrogen removal. According to the authors, Zn is one of the essential micronutrients used for cofactors and enzymes production and could be beneficial to stimulate the growth of dye-degrading bacteria. At concentrations below the optimal, microbial activity can decrease, while toxic effects can be observed at high doses. Since it is difficult to provide optimum Zn concentrations to all biofilm layers, its incorporation into the biomedia material and its controlled release from it may be an alternative to improve the bioreactor performance.
Adopting an adequate media filling ratio is another important factor when operating MBBRs. The filling ratio is a parameter that indicates the volume of the reactor that is occupied by the carriers. A low filling ratio usually leads to low concentrations of attached solids within the reactor. However, it should also not exceed 70% (v/v) in order to avoid hydrodynamic problems. An excessive amount of carriers may hinder the effective homogenization of the reaction medium [53]. Sonwani et al. [71] studied the effect of this parameter on the bioremediation of Congo red dye, finding the optimum value of 45% (v/v). Percentages ranging from 30 to 67% (v/v) were found in this review.
-
pH
The azo dye color removal process is strongly dependent on the pH of the medium. Efficient discoloration usually occurs for pH between 6 and 10 [38], decreasing rapidly in strongly acidic or basic environments [89].
The pH of the medium may be linked to the transport of dye through the cell membrane (rate-limiting step for color removal) [38]. Most enzymes have an optimum pH at which their activity is maximum. The reaction rate decreases as the pH value moves away from the optimum value. These enzymes may possess ionic groups on their active sites, which are subjected to ionization (Eq. 1). By changing the pH, the equilibrium state is disturbed, causing a shift toward the right or left side of Eq. 1, according to Le Chatelier’s principle [5]. Hence, the enzymes may be found either in the acidic or basic state depending on the pH, which also happens with the dyes. The linkage between enzyme and substrate and, therefore, dye biodegradation, depends upon the formation of compatible ionic forms [71]. As the reduction of the azo bond tends to generate amines with a lower pH than the original dye, buffer solutions are normally used in the reaction medium [57].
$$HA\leftrightarrow {H}^{+}+{A}^{-}$$(1)The pH also affects the chemical equilibrium of other species in the solution, such as unionized and ionized ammonia (NH3/NH4+), and nitrite and nitrous acid (NO2−/HNO2). At high pH (above 9.4), NH3 formation is favored, while at low pH, the NH4+ compound is predominant. Considering that NH3 is a volatile molecule, nitrogen loss via stripping can occur at high pH, and the gaseous emissions of the process can increase. HNO2 formation, on the other hand, is favored in acidic conditions. Both NH3 and HNO2 can inhibit the activity of nitrifiers, hindering the performance of aerobic processes and leading to lower nitrification rates. When nitrification is envisaged, a pH around 7.0–8.0 is usually recommended [31].
Biofilm formation is also affected by the pH of the reaction medium. Variations in the pH value decrease EPS excretion and influence its structure and properties, ultimately leading to cell lysis and death [20].
-
Temperature
Temperature is a factor commonly known to influence diffusion, solubility, reaction rates, and cell and enzyme activities. Thus, it plays a crucial role in the microbial decolorization of dyes. As previously mentioned, an efficient substrate diffusion is essential to guarantee a satisfactory performance of MBBRs. By increasing temperature, the resistance to mass transfer decreases and diffusion rates increase. However, when dealing with biological systems, temperature control must be done very carefully. Temperatures above 45 ºC commonly lead to enzyme denaturation and loss of cell viability. According to Pearce et al. [57], the optimum temperature for color removal is between 35 and 45 ºC. Santos-Pereira et al. [65] reported an increase in the color removal of Direct Red 75 (DR75), in an anaerobic MBBR, from 45% at 21 ± 2 °C to 85% at 30 ± 0.5 °C (Table 1).
Carbon and nitrogen removals are affected by temperature variations as well. Li et al. [43] investigated how temperature affects the effectiveness of an aerobic MBBR treating real textile wastewater from a dyeing company. The following average COD removals were obtained: 60.7% (at 30 ºC), 63.2% (at 35 ºC), 69.8% (at 40 ºC), 54.2% (at 45 ºC), 70.1% (at 50 ºC), and 41.5% (at 55 ºC). Very low COD removal levels were reported for temperatures over 55 ºC. Thermotolerant microorganisms were eliminated by increasing temperature up to 40 ºC, but a new community of thermotolerant microbes was formed when the temperature was increased to 50 ºC. Average NH3-N removal efficiencies were 33.3% (at 30 ºC), 39.1% (at 35 ºC), 38.5% (at 40 ºC), 28.0% (at 45 ºC), 17.5% (at 50 ºC), and 11.5% (at 55 ºC). Hence, NH3-N removal was more affected by temperature than COD removal. The same authors observed that the total amount of soluble EPS increased up to 45 ºC when it reached maximum levels of 1,748 mg/L and then decreased. This decrease was associated with an inhibitory effect of high temperatures on EPS production and cell damage. Thermophilic communities changed with the increasing temperature, mainly including genera Caldilinea (from 35 ºC to 45 ºC) and Rubellimicrobium and Pseudoxanthomonas (>50 ºC). The thermophilic species Geobacillus thermoglucosidasius, Pseudoxanthomonas taiwanensis, Geobacillus thermo denitrificans, and Rubellimicrobium thermophilum were identified over 50 ºC and contributed to organic matter removal at 50 ºC and 55 ºC. The ammonium oxidizing bacteria (AOB) Nitrosomonas eutropha was only observed at 35 ºC and not at higher temperatures. Zunongwangia profunda, which can produce high amounts of EPS, was identified at 45–50 ºC.
In aerobic processes, temperature control is also important to guarantee adequate concentrations of dissolved oxygen (DO) and avoid ammonia nitrogen volatilization [43, 75]. Since biological degradation of pollutants is usually carried out at mild temperatures, the effect of temperature on DO concentrations is not commonly a factor of concern.
The sensitiveness of a biological process toward temperature variations can be measured by the factor Q10 (Eq. 2) [43].
$${Q}_{10}={\left(\frac{{R}_{1}}{{R}_{2}}\right)}^{\frac{10}{({T}_{2}-{T}_{1})}}$$(2)where \({Q}_{10}\) = temperature coefficient (measures the rate of change in a biological process, due to temperature variation); \({R}_{1}\) = rate of pollutant removal at the temperature \({T}_{1}\); \({R}_{2}\) = rate of pollutant removal at the temperature \({T}_{2}\).
-
Dye concentration
High concentrations of dye negatively affect color removal due to the toxic effects they may cause on bacteria and the blocking of active enzyme sites by the dye molecule. According to its concentration, there may also be an inappropriate proportion of cell biomass and dye. In addition, aromatic amines formed by azo bond breakage in anaerobic processes can have toxic and inhibitory effects on microorganisms. Therefore, by increasing the dye concentration, the formation of aromatic amines can increase, enhancing the toxic effects [58].
As a result of the toxicity of the dyes and their degradation products, the production of new cells remains low at high dye concentrations [38]. Koupaie et al. [39] observed a reduction in the biofilm mass of an anaerobic system with the increase in Acid Red dye 18 concentration. Wang et al. [81] also reported a decreased growth rate of R. palustris W1 by increasing the initial Reactive Black 5 (RB5) concentration. The same authors reported a decrease in the decolorization kinetic constant (K) value from 0.114 to 0.064 h−1 by increasing the dye concentration from 50 to 1,000 mg/L.
Sonwani et al. [71] modeled the effect of azo dye concentration, pH, and carrier filling ratio on color removal by Bacillus sp. in an aerobic MBBR. The dye concentration was found to be the most influential factor, having a strong negative coefficient on the modeled equation, which described dye removal efficiency. Castro et al. [15] reported that a low azo dye inlet concentration (5 mg/L of RO16) and an HRT of 12 h were necessary to achieve high color removals on an anaerobic MBBR. However, according to Pearce et al. [57], very low concentrations of the dye also influence the process, as the substrate identification by the specific microbial enzymes is hindered under this condition.
-
Dye structure
The efficiency of dye degradation is directly linked to the structure of the molecule. Simpler molecules with lower molar mass tend to be metabolized more easily. In this way, monoazo dyes, for example, are removed faster than those that have more than one azo bond (e.g., diazo and triazo dyes) [38]. Azo dyes also tend to release nitrogen after azo bond breakage. Therefore, after discoloration, an increase in nitrogen concentrations in the bulk is often observed [27, 48], while this may not happen for other types of dyes, having different chromophores.
Dyes with electronegative substituents, such as –SO3H groups and –SO2NH2 in the ortho and para positions (with respect to the azo bond), usually present higher removal rates, as they lead to a more effective resonance effect and make the azo bond more susceptible to reduction. In contrast, when the substituent is an electron donor, such as –NH-triazine, or when electronegative substituents are in the target position, removal becomes slower [70]. Azo compounds having hydroxyl or amino groups are more easily broken down than those containing methyl, methoxy, sulfo, or nitro groups [58].
Sulphonated groups can hinder dye removal if the mechanism is intracellular, as they can block the molecule’s passage through the cell membrane. Therefore, in this case, the higher the number of sulfonated groups, the lower the dye removal efficiency [57]. The steric effect must also be taken into account since the presence of voluminous substituents in the vicinity of the chromophore may hinder access to it [70].
In addition, the toxicity of dyes depends on their chemical nature and the characteristics of their degradation products. Such toxicity can affect not only dye removal but also other biochemical processes within the MBBR, such as nitrogen removal. While Castro et al. [15] reported that no long-term inhibition of nitrifiers was observed after feeding an aerobic MBBR with ozonated RO16 solutions in concentrations of up to 500 mg/L, Dias et al. [22] observed a completely opposite behavior for RR239: its ozonation products disturbed the activity of nitrifying bacteria, completely inhibiting nitratation, at much lower dye concentrations (50 mg/L). Furthermore, nitrifiers did not seem to adapt to the presence of RR 239 ozonation products, since ammonium removal remained low (41%), even after 90 days of reactor operation. In a subsequent study, Dias et al. [21] reported that low ammonium removal was associated with the reduced enzymatic activity of nitrifiers in the presence of chlorine-containing triazine compounds, resulting from RR 239 ozonation.
Dye toxicity can cause higher biofilm detachment rates, leading to small biofilm thickness and low contents of attached solids, therefore influencing removal patterns [14, 15, 21, 39]. The contents of polysaccharides and proteins, which are directly related to the biofilm characteristics, are also influenced by the properties of the dye and its degradation products. Shin et al. [68] observed lower EPS and protein contents in the first reactor of a series of three MBBRs (anaerobic + aerobic + aerobic), while pollutant removal was also lower.
Conversely, dye degradation products can also act as mediators, improving color removal (Fig. 3b). Wang et al. [80] identified the compound 1–2-7-triamino-8-hydroxy-3–6-naphthalinedisulfate (TAHNDS) in the effluent of an anaerobic photo-rotating biological contactor (PRBC) treating a mixed-dye system, containing RB5 and AR1. The effluent of the PRBC was subsequently fed to an aerobic MBBR. According to the authors, the TAHNDS can act as a mediator to improve azo bond breakage. 1-Amino-2-naphthol produced during the dye metabolization was further degraded into quinone, a more effective redox mediator. As a result, discoloration took place mostly in the MBBR. Quinones may also accelerate denitrification [44]. Therefore, denitrification may also take place even in aerobic MBBRs in the presence of such compounds, and due to the presence of anoxic zones within the biofilm [15].
-
Organic substrate (electron donor)
The carbon source in biological systems is essential for survival and microbial growth. The primary substrate also acts as a donor of electrons for the azo bond breakage. These electrons, derived from the substrate oxidation, are transferred to azo dyes, resulting in their reduction and discoloration [70]. Some widely used organic substrates are acetate, ethanol, yeast extract, and glucose [58].
The type of electron donor used can influence color removal. According to Khan et al. [38], the addition of glucose or acetate ions can induce azo bond breakage. Wang et al. [81] tested the effect of different carbon sources (formate, acetate, sodium lactate, propionate, butyrate, oxalate, and glutamine) on the removal of color from RB5-containing wastewater by the autotrophic bacterium Rhodopseudomonas palustris W1, isolated from an anaerobic MBBR. The highest discoloration rates were observed for lactate and glutamine.
The amount of substrate used is also important. There must be enough carbon to meet cellular needs and act as a donor of electrons for discoloration. However, excessive quantities may cause cells to consume the primary substrate rather than the dye [70]. According to van der Zee and Villaverde [78], two pairs of electrons are needed to reduce the azo bond, which results in a theoretical requirement of 32 mg of COD per mmol of azo dye.
Castro et al. [15] observed a decrease in the kinetic constant for Reactive Orange 16 (RO16) discoloration upon increasing initial glucose concentration in a batch trial. However, under continuous operation, color removal from RO16-containing wastewater in an anaerobic MBBR increased by increasing glucose concentration. The authors attributed this behavior to the increase in solids concentration within the reactor. Wang et al. [80] also observed an increase in color removal when the co-substrate (sucrose and sodium acetate) concentration was increased in a continuous system composed by a photo-rotating biological contactor (PRBC) followed by an aerobic MBBR (Table 1).
-
HRT
In general, the color removal rates in anaerobic systems are low [77]. As a result, higher HRT values are needed to achieve high color removal efficiency. This behavior may be related to the greater activity of enzymes responsible for the reduction of chromophores at higher HRT values. However, very high HRT can lead to toxic effects caused by the dye and its degradation products, thereby decreasing the discoloration efficiency. Moreover, it can affect the COD removal, hydrolysis efficiency, and biodegradability [27]. In the literature, a very wide range of HRT values is found for MBBRs treating dye-containing wastewaters (from 5 to 132 h) (Table 1). HRT lower than 11 h was mostly used in aerobic MBBRs.
The HRT also directly influences the organic load, therefore affecting microbial growth and color removal. According to Sonwani et al. [71], by reducing the HRT, the effective attached growth of microorganisms on the carrier media surface is hindered. Castro et al. [15] concluded that increasing the HRT from 6 to 12 h was essential to achieve a higher color removal of RO16 since it allowed higher biomass growth within the reactor.
Overall, MBBR seems to achieve better performances in terms of color removal than conventional activated sludge (CAS), for lower HRT, while COD and TSS removals also remain high. This allows the construction of more compact units and lowers energy consumption, reducing operational costs and environmental impacts [87].
-
Composition of the microbiota
Pure and mixed microbial communities have been applied in MBBRs for dye removal. Mixed cultures were mostly developed from inocula collected at municipal or industrial WWTP (Table 1). In particular, full-scale reactors treating dye-containing wastewaters are prone to naturally select microbial strains, which are resistant to the potentially toxic effect of dyes. Nonetheless, other sources of microorganisms may also be used. Santos-Pereira et al. [65] developed the microbiota of a lab-scale anaerobic MBBR by cultivating microbes from rice husks. Vaidhegi et al. [76] inoculated an MBBR with sludge from sewage treatment plants and dairy animals excrement. The use of a mixed culture in biofilm systems allows the coexistence of both heterotrophic and autotrophic communities, which are specialized in the removal of different pollutants. For instance, Liu et al. [48] treated real wastewater from a dyeing factory in an UASB-aerobic MBBR system using sludge from a municipal WWTP. The authors attributed the removal of dyes to Shewanella spp., both in the anaerobic and aerobic reactors. Nitrogen removal was associated with the presence of the ammonia-oxidizing archaea Nitrososphaera spp., the nitrite-oxidizing bacteria Arcobacter spp., and the denitrifying Hydrogenophaga spp. The presence of denitrifiers on the aerobic MBBR was also reported. Protocatella spp., Acetoanaerobium spp., and Proteiniclasticum spp. promoted the conversion of organic matter into acetic acid, which was further degraded into methane by the methanogenic archaea Methanothrix spp. and Methanosarcina spp.
By adopting pure cultures, on the other hand, the removal of a target pollutant can be optimized. White-rot fungi [56] and Bacillus sp. [71] are known for achieving high color removal efficiencies from wastewaters under aerobic conditions. Wang et al. [81] and [80] have also shown the effectiveness of the photosynthetic bacterium Rhodopseudomonas palustris in the discolorization of the azo dye Reactive Black 5.
-
Other factors
Additional factors that may influence biological color removal from dye-containing matrices include salt and micronutrient concentrations, as well as the presence or absence of a magnetic field.
Textile wastewaters are known to have high concentrations of salts. High salinity affects the osmotic pressure, causing cell dehydration, plasmolysis, and death. Moreover, by increasing salt concentrations, carrier media coverage by biofilms may decrease, as well as the charge of EPS, leading to biofilm compaction and porosity reduction. Shifts in bacterial communities may also occur [82]. High salt concentrations can also inhibit and denature enzymes (e.g., azoreductases) and reduce substrate transfer rates [81]. In addition, COD and ammonium removal may be affected [86].
High concentrations of micronutrients (e.g., Zn, Cu, Fe, Mn, Ni, Co, etc.) are also detrimental to dye biodegradation. However, at optimum quantities, their presence is essential since the cofactors act as an enzyme activator and therefore having a decisive role in enzymatic processes and metabolic pathways [5, 83]. In biofilm systems, since resistance to mass transfer may be observed, enzymatic processes can be slowed down due to the low availability of micronutrients. Wang et al. [83] demonstrated how color removal improved by incorporating Zn nanoparticles into the support material and ensuring its controlled release (Table 1).
Biological processes can also be affected by magnetic fields, which influence bacterial movement and physicochemical properties of the wastewater, inducing colloidal particles to agglomerate [72].
3.3 Kinetics of Dye Removal in MBBR
The MBBR is, essentially, a continuous reactor. The mass balance for the limiting substrate in a continuous bioreactor is given by Eq. (3) [67].
Equation (2) can be mathematically translated by Eq. (4).
where \(\frac{dS}{dt}\) is the rate of variation in the mass of substrate within the reactor; S is the limiting substrate concentration in the effluent of the reactor; t is time; Q is the volumetric flow rate; V is reactor volume; \({\mathrm{S}}_{0}\) is the inlet concentration of the limiting substrate; \({(-r}_{s})\) is the rate of substrate consumption; X is the cell concentration.
Correspondingly, the mass balance for the microorganisms is expressed by Eqs. (5) and (6).
where \(\frac{dX}{dt}\) is the rate of variation in the mass of cells within the reactor; X is the concentration of the cells in the effluent of the reactor; \({\mathrm{X}}_{0}\) is inlet concentration of cells; \({r}_{X}\) is the rate of cell growth.
If operated in a steady-state mode, which is usually the case for MBBRs running for a long time, and considering that no bacterial cells are fed to the reactor (\({X}_{0}=0\)) (the first reactor of a series, no recycle), Eqs. (4) and (6) become Eqs. (7) and (8):
Equations (7) and (8), when combined, result in Eq. (9).
The substrate consumption rate is described by different models in the literature, such as Monod model (Eq. 10)
where µmax is the maximum specific substrate removal rate; Ks is the half-velocity coefficient for the substrate.
This set of equations (Eqs. 7 to 10) allows designing an appropriate reactor for a given process. Equation (8) evidences the importance of defining \({r}_{X}\), through which the reactor volume can be calculated for a given flow rate. The rates \({r}_{X}\) and \({r}_{S}\) are related by Eq. (9), while \({r}_{S}\) depends on two reaction constants, \({\mu }_{max}\) and \({K}_{S}\), which can be estimated through batch tests by measuring the substrate and cell concentrations over time.
For a batch reactor, there are no influent or effluent flows. Therefore, Eq. (4) can be simplified and transformed into Eq. (11).
For a small period of time, the concentration of cells can be considered constant, and Eq. (11) can be simplified as Eq. (12).
with \({R}_{max}={\mu }_{max}X\), representing the maximum substrate removal rate.
For low substrate concentrations (S < < Ks), Eq. (12) can be simplified and integrated, resulting in a first-order reaction rate (Eq. 13). For low substrate concentrations (S > > Ks), Eq. (12) becomes a zero-order reaction (Eq. 14) [33].
where: \({S}_{t}\)= substrate concentration at reaction time t; \({S}_{0}\)= initial substrate concentration; \({k}_{1}\) = first-order kinetic constant (\(\frac{{R}_{max}}{Ks}\)); \({k}_{0}\) = zero-order kinetic constant (\({R}_{max}\));
Alternatively, Eqs. (7) and (10) can be put together (Eq. 15) and rearranged into a linear form (Eq. 16) (Modified Stover–Kincannon model), allowing the determination of kinetic parameters in a continuous mode operation [71].
Table 2 displays the studies in which the kinetics of dye degradation in MBBR was investigated.
3.4 Combination of Processes
In order to take advantage of the best characteristics of each type of process, many authors have used the combination of different methods for treating textile effluents (Table 1). Advanced oxidation processes (AOPs), for example, can achieve high levels of color removal in a short time [21] and may improve dye biodegradability [72]. However, the high cost associated with AOPs to reach the effluent discharge standards can restrict their application. AOPs have been applied both before the MBBR, for color removal and improvement of the wastewater biodegradability, and after it, for polishing (Table 1). When used as a pre-treatment, its operational conditions can affect the nature of by-products and dye toxicity. Dias et al. [21] reported an increase in biomass detachment from an aerobic MBBR when RR239 ozonation time was increased from 12 to 20 min, which contributed to reducing biofilm thickness. Therefore, an in-depth study of the best set of operational conditions is needed for each process.
Biological processes have a relatively low cost, but usually do not meet the disposal standards in one single step [10]. Thus, the combination of anaerobic–aerobic bioreactors has been widely found in the literature. During the anaerobic metabolism of azo dyes, potentially toxic aromatic amines are produced, which are usually not removed in the absence of oxygen. Therefore, a subsequent aerobic process is often used to mineralize azo compounds [78]. During the aerobic post-treatment, less aromatic and more polar substances are formed [39]. Moreover, the adoption of a series of MBBRs also leads to higher removals of organic matter and nitrogen. At high inlet organic matter concentrations, there is usually a predominance of heterotrophic bacteria over autotrophic bacteria, leading to higher COD removals. Meanwhile, at low organic matter concentrations, autotrophic communities such as nitrifiers develop well. Therefore, by adopting a series of two aerobic MBBRs, COD removal usually takes place in the first reactor, while ammonium removal rates are higher in the second one [7].
Gong [27] implemented a series of anaerobic MBBR–aerobic MBBR–ozonation–aerobic MBBR, and concluded that while the first MBBR was important for improving the biodegradability of real textile wastewater, the second and third MBBRs contributed to increasing COD and ammonium removals. Shin et al. [68] adopted a series of anaerobic MBBR–aerobic MBBR–aerobic MBBR–coagulation. Also, in this case, COD removal took place mainly in the aerobic MBBRs (56.4%), while the anaerobic MBBR and the coagulation processes were responsible for the removal of 82.8% of the color.
3.5 Comparison Between MBBR and Other Biological Wastewater Treatment Systems
The type of reactor used for dye remediation has a great influence on color removal efficiency. According to van der Zee and Villaverde [78], biological reactors with greater biomass retention capacity may perform better in the removal of azo dyes than those with less cell retention capacity. The growth of microorganisms in suspension or attached to a carrier can also influence the process. When the biomass grows adhered to a media, the microorganisms grow partially protected from external predators, load shocks, and temperature and pH variations [8, 57]. Thus, the performance of fixed biomass systems can be higher than those in which microorganisms grow in suspension.
Mohan et al. [51] conducted a comparative study on the use of these two types of processes in the treatment of wastewater containing the acid black azo dye 10B. The authors employed two sequencing batch reactors, operating separately: one with suspended biomass and the other with biofilm, with alternating cycles of anoxic–aerobic–anoxic conditions. It was observed that the biofilm system achieved greater color removal than that with suspended biomass. According to Mohan et al. [51], biofilms induce the formation of aerobic and anoxic zones along with the biofilm thickness, leading to a spatial distribution of microorganisms. In this type of system, besides the longer biomass residence times, a high level of metabolic activities is maintained in the reactor.
Yang et al. [87] recommended the use of MBBR over a conventional activated sludge process and MBR for the treatment of textile wastewaters due to satisfactory performance, relatively low costs, and environmental friendliness. The MBBR was the most economical technology at the industrial scale, saving 68.4% of the capital expenditures (CAPEX) and having the same operating expenditures (OPEX) as MBR. It also showed the lowest environmental impacts (15 out of 18 midpoint categories: ozone depletion, human toxicity, photochemical oxidant formation, particulate matter formation, ionizing radiation, terrestrial acidification, freshwater eutrophication, terrestrial ecotoxicity, marine ecotoxicity, agricultural land occupation, urban land occupation, natural land transformation, water depletion, metal depletion, fossil depletion,and 3 out of 3 endpoint categories: human health, ecosystems, and resources) and was selected as the most feasible technology for industrial scale. The treated effluent was reused for textile dyeing.
4 Conclusions and Future Perspectives
The intense global commercialization of textile dyes caused an increased emission of polluting wastewaters containing these harmful compounds into the receiving waters. Therefore, there is a major concern regarding the quality of such wastewaters that reaches the aquatic environment since most dyes are toxic and hardly biodegradable. Conventional biological methods, such as activated sludge, are usually not capable of completely removing dyes and their associated colors from the wastewaters and traditional aerobic biological processes are inefficient to promote dye degradation. Moreover, operating problems such as deflocculation may occur in suspended biomass-based reactors in the presence of these compounds, making solid–liquid separation more difficult.
In contrast, biofilm systems are more resilient to toxic loads and may enable a better degradation of dye molecules. The moving bed biofilm reactor (MBBR) stands out as a good alternative for the bioremediation of toxic wastewaters, being robust, effective, economical, and environmentally friendly. The key characteristics of MBBR, including the high concentration of specialized biomass within the reactor, high sludge age, the protected environment provided by the biofilm, and the presence of different redox zones (anoxic/anaerobic and aerobic) within the biofilms, even for aerated processes, may enhance the biodegradation of dyes. Color removal in MBBR can be achieved in both aerobic and anaerobic conditions, and depends on microbial adaptation period, microbial strains, hydraulic retention time (HRT), co-substrate concentration, and dye concentration and molecular structure. For mixed microbial cultures, color removal is higher in MBBRs subjected to anaerobic conditions. Other factors that influence dye biodegradation in these reactors are agitation, type of carrier, pH, temperature, HRT, solids concentrations, micronutrients, and salinity levels.
Due to the diversity of dyes and the wide concentration ranges they are found in real dye-containing wastewaters, difficulties are faced in the operation and process optimization. Therefore, in-depth studies on the kinetic and mechanism of dye biodegradation are needed, which may help define the best combination of process-specific parameters. In addition, identifying microbial strains capable of degrading specific dyes, and describing the effect of widely used dyes and their degradation products on microbial behavior is crucial for improving dye removal. Investigations on best process combinations are also recommended since a single MBBR as a stand-alone technology is not capable of mineralizing dyes. Future studies should also address technical–economic comparisons between different reactor associations to better understand their benefits and drawbacks in a holistic approach.
Abbreviations
- AOA:
-
Ammonium-Oxidizing Archaea
- AOB:
-
Ammonium Oxidizing Bacteria
- An-SBR:
-
Anaerobic Sequencing Batch Reactor
- AR18:
-
Acid Red 18
- ATP:
-
Adenosine 5'-triphosphate
- BOD:
-
Biochemical Oxygen Demand
- CAPEX:
-
Capital Expenditures
- CAS:
-
Conventional Activated Sludge
- COD:
-
Chemical Oxygen Demand
- DNB:
-
Denitrifying Bacteria
- DO:
-
Dissolved Oxygen
- DR75:
-
Direct Red 75
- EPS:
-
Extracellular Polymeric Substances
- FADH:
-
Flavin Adenine Dinucleotide
- GAC:
-
Granular Activated Carbon
- HDPE:
-
High-Density Polyethylene
- HNO2:
-
Nitrous Acid
- HRT:
-
Hydraulic Retention Time
- NaCl:
-
Sodium Chloride
- NADH:
-
Nicotinamide Adenine Dinucleotide
- NADPH:
-
Nicotinamide Adenosine Dinucleotide Phosphate
- NH3:
-
Unionized ammonia
- NH4+:
-
Ionized ammonia, or ammonium
- NO2−:
-
Nitrite
- NOB:
-
Nitrite Oxidizing Bacteria
- NPs:
-
Nanoparticles
- MBBR:
-
Moving Bed Biofilm Reactor
- MBR:
-
Membrane Bioreactor
- MB-SBR:
-
Aerobic Moving Bed Sequencing Batch Biofilm Reactor
- OPEX:
-
Operational Expenditures
- pH:
-
Potential of Hydrogen
- PLA:
-
Poly (lactic acid)
- PRBC:
-
Photo-Rotating Biological Contactor
- PU:
-
Polyurethane
- PU-AC:
-
Polyurethane-Activated Carbon
- PU-DSCM:
-
Polyurethane-Dyeing Sludge Carbonaceous Material
- PVC:
-
Polyvinyl Chloride
- RB-5:
-
Reactive Black-5
- RBC:
-
Rotating Biological Contactors
- RR 239:
-
Reactive Red 239 (RR 239)
- RO16:
-
Reactive Orange 16
- SS:
-
Suspended Solids
- TAHNDS:
-
1-2-7-Triamino-8-hydroxy-3-6-naphthalinedisulfate
- TDS:
-
Total Dissolved Solids
- TSS:
-
Total Suspended Solids
- UASB:
-
Upflow Anaerobic Sludge Blanket
- UF:
-
Ultrafiltration
- WWTP:
-
Wastewater Treatment Plant
References
Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J Environ Manag 69:229–238. https://doi.org/10.1016/j.jenvman.2003.09.005
Allégre C, Moulin P, Maisseu M, Charbit F (2006) Treatment and reuse of reactive dyeing effluents. J Membr Sci 269:15–34. https://doi.org/10.1016/j.memsci.2005.06.014
Anju F, Sosamony KJ (2016) Treatment of pre-treated textile wastewater using moving bed bio-film reactor. Proc Technol 24:248–255. https://doi.org/10.1016/j.protcy.2016.05.033
Ayadi I, Souissi Y, Jlassi I, Peixoto F, Mnif W (2016) Chemical synonyms, molecular structure and toxicological risk assessment of synthetic textile dyes: a critical review. J Dev Drugs 5:1–4. https://doi.org/10.4172/2329-6631.1000151
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw-Hill, New York
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242. https://doi.org/10.1111/j.1574-4976.2005.00010.x
Bassin JP, Dezotti M, Sant’Anna GL Jr (2011) Nitrification of industrial and domestic saline wastewaters in moving bed biofilm reactor and sequencing batch reactor. J Hazard Mater 185:242–248. https://doi.org/10.1016/j.jhazmat.2010.09.024
Bassin JP, Dezotti M (2018) Moving Bed Biofilm Reactor (MBBR). Advanced biological processes for wastewater treatment. In: Dezotti M, Lippel G, Bassin JP (eds) Advanced biological processes for wastewater treatment: emerging, consolidated technologies and introduction to molecular techniques. Springer International Publishing, pp 37–74. https://doi.org/10.1007/978-3-319-58835-3_3.
Bassin JP, Kleerebezem R, Rosado AS, Van Loosdrecht MCM, Dezotti M (2012) Effect of different operational conditions on biofilm development, nitrification, and nitrifying microbial population in moving-bed biofilm reactors. Environ Sci Technol 46:1546−1555. https://doi.org/10.1021/es203356z
Beyene HD (2014) The potential of dyes removal from textile wastewater by using different treatment technology, a review. Int J Environ Monit Anal 2(6):347–353. https://doi.org/10.11648/J.IJEMA.20140206.18.
Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S (1997) Reactivities of various mediators and laccases with Kraft pulp and lignin model compounds. Appl Environ Microbiol 63(12):4627–4632. https://doi.org/10.1128/AEM.63.12.4627-4632.1997
Calderón K, Martín-Pascual J, Poyatos JM, Rodelas B, González-Martínez A, González-López J (2012) Comparative analysis of the bacterial diversity in a lab-scale moving bed biofilm reactor (MBBR) applied to treat urban wastewater under different operational conditions. Biores Technol 121:119–126. https://doi.org/10.1016/j.biortech.2012.06.078
Calvete T, Lima EC, Cardoso NF, Vaghetti JCP, Dias SLP, Pavan FA (2010) Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: kinetic, equilibrium, and thermodynamic studies. J Environ Manag 91:1695–1706. https://doi.org/10.1016/j.jenvman.2010.03.013
Castro FD, Bassin JP, Dezotti M (2017) Treatment of a simulated textile wastewater containing the Reactive Orange 16 azo dye by a combination of ozonation and moving-bed biofilm reactor: evaluating the performance, toxicity, and oxidation by-products. Environ Sci Pollut Res 24(7):6307–6316. https://doi.org/10.1007/s11356-016-7119-x
Castro FD, Bassin JP, Alves TLM, Sant’Anna GL, Dezotti M (2020) Reactive Orange 16 dye degradation in anaerobic and aerobic MBBR coupled with ozonation: addressing pathways and performance. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-020-02983-8
Chan YJ, Chong MF, Law CL, Hassell DG (2009) A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18. https://doi.org/10.1016/j.cej.2009.06.041
Chen S, Cheng X, Zhang X, Sun D (2012) Influence of surface modification of polyethylene biocarriers on biofilm properties and wastewater treatment efficiency in moving-bed biofilm reactors. Water Sci Technol 65(6):1021–1026. https://doi.org/10.2166/wst.2012.915
Chengalroyen MD, Dabbs ER (2013) The microbial degradation of azo dyes: minireview. World J Microbiol Biotechnol 29:389–399. https://doi.org/10.1007/s11274-012-1198-8
Christie RM (2007) Environmental aspects of textile dyeing. Woodhead Publishing
di Biase A, Kowalski MS, Devlin TR, Oleszkiewicz JA (2020) Physicochemical methods for biofilm removal allow for control of biofilm retention time in a high rate. MBBR Environ Technol. https://doi.org/10.1080/09593330.2020.1843078
Dias NC, Alves TLM, Azevedo DA, Bassin JP, Dezotti M (2020) Metabolization of by-products formed by ozonation of the azo dye Reactive Red 239 in moving-bed biofilm reactors in series. Braz J Chem Eng 37:495–504. https://doi.org/10.1007/s43153-020-00046-6
Dias NC, Bassin JP, Sant’Anna GL, Dezotti M (2019) Ozonation of the dye Reactive Red 239 and biodegradation of ozonation products in a moving-bed biofilm reactor: revealing reaction products and degradation pathways. Int Biodeterior Biodegrad 144:104742. https://doi.org/10.1016/j.ibiod.2019.104742
Dong B, Chen H, Yang Y, He Q, Dai X (2013) Treatment of printing and dyeing wastewater using MBBR followed by membrane separation process. Desalin Water Treat 52(22–24):4562–4567. https://doi.org/10.1080/19443994.2013.803780
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971. https://doi.org/10.1016/j.envint.2004.02.001
Ghaly AE, Ananthashankar R, Alhattab M, Ramakrishnan VV (2014) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5(1). https://doi.org/10.4172/2157-7048.1000182
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67(3):369–385. https://doi.org/10.1007/s00018-009-0169-1
Gong X-B (2016) Advanced treatment of textile dyeing wastewater through the combination of moving bed biofilm reactors and ozonation. Sep Sci Technol 51(9):1589–1597. https://doi.org/10.1080/01496395.2016.1165703
Goode C (2010) Understanding biosolids dynamics in a moving bed biofilm reactor. PhD thesis, Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canadá
Guo CJ, de Kee D (1991) Effect of molecular size and free volume on diffusion in liquids. Chem Eng Sci 46(8) 2133–2141. https://doi.org/10.1016/0009-2509(91)80171-T
Gürses AM, Günes AK, Gürses MS (2016) Classification of dye and pigments. In: Gürses AM, Açikyildiz M, Günes AK, Gürses MS (eds) Dyes and pigments. Springer, Cham, pp 31–45. https://doi.org/10.1007/978-3-319-33892-7_3
Hoang V (2013). MBBR ammonia removal: an investigation of nitrification kinetics, biofilm and biomass response, and bacterial population shifts during long-term cold temperature exposure. Master Thesis. Department of Civil Engineering, University of Ottawa. https://ruor.uottawa.ca/bitstream/10393/24041/3/Hoang_Valerie_2013_thesis.pdf.
Hofrichter M et al (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87(3) 871–897. https://doi.org/10.1007/s00253-010-2633-0
Işik M, Sponza DT (2005) A batch study for assessing the inhibition effect of Direct Yellow 12 in a mixed methanogenic culture. Process Biochem 40:1053–1062. https://doi.org/10.1016/j.procbio.2004.03.011
Işik M, Sponza DT (2004) Decolorization of azo dyes under batch anaerobic and sequential anaerobic/aerobic conditions. J Environ Sci Health Part A 39(4):1107–1127. https://doi.org/10.1081/ese-120028417
Jorand F, Boue-Bigne F, Block JC, Urbain V (1998) Hydrophobicity/hydrophilic properties of activated sludge exopolymeric substances. Water Sci Technol 37(4–5) 307–315. https://doi.org/10.1016/S0273-1223(98)00123-1
Kabra AN, Khandare RV, Govindwar SP (2013) Development of a bioreactor for remediation of textile effluents and dye mixture: a plant-bacterial synergistic strategy. Water Res 47:1035–1048. https://doi.org/10.1016/j.watres.2012.11.007
Kalme S, Ghoda EG, Gov Ndwar S (2007) Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NC M 2112. Int Biodeterior Biodegrad 60:327–333. https://doi.org/10.1016/j.ibiod.2007.05.006
Khan R, Bhawana P, Fulekar MH (2013) Microbial decolorization and degradation of synthetic dyes: a review. Rev Environ Sci Biotechnol 12:75–97. https://doi.org/10.1007/s11157-012-9287-6
Koupaie EH, Moghaddam MRA, Hashemi SH (2011) Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195:147–154. https://doi.org/10.1016/j.jhazmat.2011.08.017
Leyva-Díaz JC, Calderón K, Rodríguez FA, González-López J, Hontoria E, Poyatos JM (2013) Comparative kinetic study between moving bed biofilm reactor-membrane bioreactor and membrane bioreactor systems and their influence on organic matter and nutrients removal. Biochem Eng J 77:28–40. https://doi.org/10.1016/j.bej.2013.04.023
Leyva-Díaz JC, Martín-Pascual J, González-López J, Hontoria E, Poyatos JM (2013) Effects of scale-up on a hybrid moving bed biofilm reactor – membrane bioreactor for treating urban wastewater. Chem Eng Sci 104:808–816. https://doi.org/10.1016/j.ces.2013.10.004
Li S, Cheng W, Wang M, Chen C (2011) The flow patterns of bubble plume in an MBBR. J Hydrodyn 23(4):510–515. https://doi.org/10.1016/S1001-6058(10)60143-6
Li C, Zhang Z, Li Y, Cao J (2015) Study on dyeing wastewater treatment at high temperature by MBBR and the thermotolerant mechanism based on its microbial analysis. Process Biochem 50(11):1934–1941. https://doi.org/10.1016/j.procbio.2015.08.007
Li H, Guo J, Lian J, Xi Z, Zhao L, Liu X, Zhang C, Yang J (2014) Study the biocatalyzing effect and mechanism of cellulose acetate immobilized redox mediators technology (CE-RM) on nitrite denitrification. Biodegradation 25(3) 395-404. https://doi.org/10.1007/s10532-013-9668-8
Lin SH, Lin CM (1993) Treatment of textile waste effluents by ozonation and chemical coagulation. Water Res 27(12):1743–1748. https://doi.org/10.1016/0043-1354(93)90112-U
Lin YH (2008) Kinetics of nitrogen and carbon removal in a moving-fixed bed biofilm reactor. Appl Math Model 32:2360–2377. https://doi.org/10.1016/j.apm.2007.09.009
Lipskikh OI, Korotkova EI, Khristunova YEP, Barek J, Kratochvil B (2018) Sensors for voltammetric determination of food azo dyes—a critical review. Electrochim Acta 260:974–985. https://doi.org/10.1016/j.electacta.2017.12.027
Liu Y, Wang N, Wei Y, Dang K, Li M, Li Y, Li Q, Mu R (2020) Pilot study on the upgrading configuration of UASB-MBBR with two carriers: Treatment effect, sludge reduction and functional microbial identification. Process Biochem. https://doi.org/10.1016/j.procbio.2020.09.007
Mannina G, di Trapani D, Viviani G, Ødegaard H (2011) Modelling and dynamic simulation of hybrid moving bed biofilm reactors: model concepts and application to a pilot plant. Biochem Eng J 56:23–36. https://doi.org/10.1016/j.bej.2011.04.013
Menda M (2011). Corantes e pigmentos. http://www.crq4.org.br/quimicaviva_corantespigmentos.
Mohan SV, Reddy CN, Kumar AN, Modestra JA (2013) Relative performance of biofilm configuration over suspended growth operation on azo dye based wastewater treatment in periodic discontinuous batch mode operation. Biores Technol 147:424–433. https://doi.org/10.1016/j.biortech.2013.07.126
Nikfar S, Jaberidoost M (2014) Dyes and Colorants. Encycl Toxicol 252–261. https://doi.org/10.1016/b978-0-12-386454-3.00602-3
Ødegaard H, Gisvold B, Strickland J (2000) The influence of carrier size and shape in the moving bed biofilm process. Water Sci Technol 41(4–5):383–391. https://doi.org/10.2166/wst.2000.0470
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Park HO, Oh S, Bade R, Shin WS (2010) Application of A2O moving-bed biofilm reactors for textile dyeing wastewater treatment. Korean J Chem Eng 27(3):893–899. https://doi.org/10.1007/s11814-010-0143-5
Park HO, Oh S, Bade R, Shin WS (2011) Application of fungal moving-bed biofilm reactors (MBBRs) and chemical coagulation for dyeing wastewater treatment. KSCE J Civ Eng 15(3):453–461. https://doi.org/10.1007/s12205-011-0997-z
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigm 58:179–196. https://doi.org/10.1016/S0143-7208(03)00064-0
Popli S, Patel UD (2015) Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol 12:405–420. https://doi.org/10.1007/s13762-014-0499-x
Pratiwi R, Notodarmojo S, Helmy Q (2018) Decolourization of remazol black-5 textile dyes using moving bed bio-film reactor. IOP Conf Ser: Earth Environ Sci 106:012089. https://doi.org/10.1088/1755-1315/106/1/012089
Rehman K, Shahzad T, Sahar A, Hussain S, Mahmood F, Siddique MH et al (2018) Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ 6:e4802. https://doi.org/10.7717/peerj.4802
Reports and Data (2019). Textile Dyes Market To Reach USD 10.13 Billion By 2026. https://www.globenewswire.com/news-release/2019/09/12/1914626/0/en/Textile-Dyes-Market-To-Reach-USD-10-13-Billion-By-2026-Reports-And-Data.html
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile efluent: a critical review on current treatment technologies with a proposed alternative. Biores Technol 77:247–255. https://doi.org/10.1016/S0960-8524(00)00080-8
Rusten B, Kolkinn O, Ødegaard H (1997) Moving bed biofilm reactors and chemical precipitation for high efficiency treatment of wastewater from small communities. Water Sci Technol 35(6). https://doi.org/10.1016/s0273-1223(97)00097-8
Rys P, Zollinger H (1972) Fundamentals of the chemistry and application of dyes. Wiley-Interscience, New York, p 196
Santos-Pereira GC, Corso CR, Forss J (2019) Evaluation of two different carriers in the biodegradation process of an azo dye. J Environ Health Sci Eng 17(2):633–643. https://doi.org/10.1007/s40201-019-00377-8
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157. https://doi.org/10.1016/j.jtice.2010.06.006
Schmidell W, Lima UA, Aquarone E, Borzani W (2001). Biotecnologia industrial, vol 2. Editora Edgard Blücher Ltda, São Paulo
Shin DH, Shin WS, Kim Y-H, Ho Han M, Choi SJ (2006) Application of a combined process of moving-bed biofilm reactor (MBBR) and chemical coagulation for dyeing wastewater treatment. Water Sci Technol 54(9):181–189. https://doi.org/10.2166/wst.2006.863
Soler CR, Xavier CR (2015) Tratamento de efluente de indústria têxtil por reator biológico com leito móvel. RBCIAMB 38:21–30. https://doi.org/10.5327/Z2176-947820155714
Solís M, Solís A, Perez HI, Manjarrez N, Flores M (2012) Microbial decolouration of azo dyes: a review. Process Biochem 47:1723–1748. https://doi.org/10.1016/j.procbio.2012.08.014
Sonwani RK, Swain G, Giri BS, Singh RS, Rai BN (2020) Biodegradation of Congo red dye in a moving bed biofilm reactor: performance evaluation and kinetic modeling. Biores Technol 122811. https://doi.org/10.1016/j.biortech.2020.122811
Sosamony KJ, Soloman PA (2018) Treatment of pretreated textile wastewater using modified Mbbr. Int J Eng Technol 7(3.8):106. https://doi.org/10.14419/ijet.v7i3.8.16843.
Sponza DT, Işik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744. https://doi.org/10.1016/j.procbio.2004.12.016
Talha MA, Goswami M, Giri BS, Sharma A, Rai BN, Singh RS (2018) Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Biores Technol 252:37–43. https://doi.org/10.1016/j.biortech.2017.12.081
Tromans D (1998) Temperature and pressure dependent solubility of oxygen in water: a thermodynamic analysis. Hydrometallurgy 48:327–342. https://doi.org/10.1016/S0304-386X(98)00007-3
Vaidhegi K, Selvam SA, Kumar AM (2018). Treatment of dye waste water using Moving Bed Biofilm Reactor & Granular Activated Carbon [MBBR-GAC]. J Adv Res Dyn Control Syst 10:08-Special Issue
van der Zee FB (2001) Anaerobic azo dye reduction. PhD thesis, University of Wageningen, Holland. https://edepot.wur.nl/121282
van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes—a short review of bioreactor studies. Water Res 39:1425–1440. https://doi.org/10.1016/j.watres.2005.03.007
WEF/ASCE/EWRI (2009) Design of municipal wastewater treatment plants, 5ª ed, Manual of Practice No. 8, Water Environment Federation, Alexandria, Virginia
Wang X, Cheng X, Sun D (2011) Interaction in anaerobic biodecolorization of mixed azo dyes of Acid Red 1 and Reactive Black 5 under batch and continuous conditions. Colloids Surf, A 379(1–3):127–135. https://doi.org/10.1016/j.colsurfa.2010.11.065
Wang X, Cheng X, Sun D, Qi H (2008) Biodecolorization and partial mineralization of Reactive Black 5 by a strain of Rhodopseudomonas palustris. J Environ Sci 20(10):1218–1225. https://doi.org/10.1016/s1001-0742(08)62212-3
Wang J, Liu Q, Wu B, Hu H, Dong D, Yin J, Ren H (2020) Effect of salinity on mature wastewater treatment biofilm microbial community assembly and metabolite characteristics. Sci Total Environ 711:134437. https://doi.org/10.1016/j.scitotenv.2019.134437
Wang F, Zhou L, Zhao J (2018) The performance of biocarrier containing zinc nanoparticles in biofilm reactor for treating textile wastewater. Process Biochem 74:125–131. https://doi.org/10.1016/j.procbio.2018.08.022
Wang DM (2016) Environmental protection in clothing industry. In: Zhu L (ed) Sustainable development: proceedings of the 2015 International Conference on sustainable development (ICSD2015), pp 729–735. World Scientific Publishing Co Pte Ltd., Singapore. https://doi.org/10.1142/9789814749916_0076
Xavier JB, Picioreanu C, Almeida JS, van Loosdrecht MCM (2003). Monitorização e modelação da estrutura de biofilmes, Biomatemática—Modelação da estrutura de biofilmes. Boletim de biotecnologia 76:2–13. http://biofilms.bt.tudelft.nl/pdf/2002_jxavier_biofilmes.pdf
Xu M, Zhou W, Chen X, Zhou Y, He B, Tan S (2020) Analysis of the biodegradation performance and biofouling in a halophilic MBBR-MBR to improve the treatment of disinfected saline wastewater. Chemosphere 22:128716. https://doi.org/10.1016/j.chemosphere.2020.128716
Yang X, López-Grimau V, Vilaseca M, Crespi M (2020) Treatment of Textile Wastewater by CAS, MBR, and MBBR: a comparative study from technical, economic, and environmental perspectives. Water 12(5):1306. https://doi.org/10.3390/w12051306
Yoo ES, Libra J, Adrian L (2001) Mechanism of decolorization of azo dyes in anaerobic mixed culture. J Environ Eng 127(9):844–849. https://doi.org/10.1061/(asce)0733-9372(2001)127:9(844)
Zhao M, Sun PF, Du LN, Wang G, Jia XM, Zhao YH (2014) Biodegradation of methyl red by Bacillus sp. strain UN2: decolorization capacity, metabolites characterization, and enzyme analysis. Environ Sci Pollut Res 21:6136–6145. https://doi.org/10.1007/s11356-014-2579-3
Zollinger H (2003) Color chemistry: syntheses, properties, and applications of organic dyes and pigments. 3rd rev. ed. Verlag Helvetica Chimica Acta; Wiley-VCH. Zurich, Weinheim, p 637. https://doi.org/10.1002/anie.200385122
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Castro, F.D., Lemos, F.R., Bassin, J.P. (2022). Removal of Dyes from Wastewaters in Moving Bed Biofilm Reactors: A Review of Biodegradation Pathways and Treatment Performance. In: Muthu, S.S., Khadir, A. (eds) Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-16-5932-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-5932-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5931-7
Online ISBN: 978-981-16-5932-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)