Abstract
In this work, the biodegradation of by-products formed by ozonation of the azo dye Reactive Red 239 was evaluated using two MBBRs in series. Two ozone dosages were applied and low carbon removals and increasing ozone consumption observed after discoloration show the formation of oxidation resistant by-products. Five by-products were identified by GC/MS. High COD removal (90%) was observed in the biological process. However, nitrification inhibition was observed with ammonium removal of only 40%. This inhibition was probably caused by 4-amino-6-chloro-1,3,5-triazine-2-ol, which passed unscathed in the MBBRs. The nitrifying activity of the biofilm was restored when the MBBRs in series were fed with synthetic effluent (without by-products), proving the inhibition of nitrifying bacteria by ozonation by-products. The association of ozonation with the biological process was efficient in RR 239 color removal and degradation of some by-products. Higher ozone dosages are required for triazine oxidation, which probably inhibited nitrification in the MBBRs in series. The importance of identifying by-products formed by ozonation and their metabolization or not in a biological process is clear. In addition to partially inhibiting nitrification, special attention should be paid to chemicals that pass undegraded through a biological process and can be released into receiving bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthetic dyes commonly found in the wastewater of the textile industry emerged in the eighteenth century. It is estimated that currently over 10,000 textile dyes are produced and 800,000 tons of dyes per year are used (Bazin et al. 2012; Ayadi et al. 2016). In the textile industry, 1000 to 3000 m3 of wastewater is generated per day for processing 10 to 20 tons of fibers (Ghaly et al. 2014). Azo dyes present in effluents cause the reduction of photosynthesis and dissolved oxygen concentration in the receiver body. These dyes may be toxic to fish, may reduce the embryonic survival, cause morphological deformities and are genotoxic to fish erythrocytes at the concentration of 10 mg L−1 and may still be carcinogenic to humans (Kabra et al. 2011; Jungtanasombut et al. 2014; Al-Sabti 2000).
Biological processes have been extensively used in the treatment of textile effluents (Adabju 2013). However, the degradation of an azo dye by biological process is impaired due to the azo group (–N=N–), linked to at least one but usually two aromatic groups, which makes these dyes resistant to biodegradation (Wang et al. 2002; Solís et al. 2012).
In aerobic biological processes, microorganisms require a long adaptation period for degradation of a simple azo dye, whereas in anaerobic processes azo dye degradation is easily accomplished (Firmino et al. 2011; Baêta et al. 2016; Victral et al. 2016). However, the breakdown of the azo bond in anaerobic processes generally forms toxic products such as aromatic amines (Chengalroyen and Dabbs 2013).
Oxidative processes, such as ozonation, are able to oxidize organic substances difficult to degrade and are efficient in the degradation of conjugated chains, which are responsible for the color of the dye. The oxidation by ozone occurs via molecular ozone or HO· and HO2· radicals, depending on the pH of the aqueous medium (Turhan and Ozturkcan 2013; Rice 1996). Studies on the color removal of azo dyes by ozonation are abundant (Muhammad et al. 2008; Turhan and Ozturkcan 2013; Günes et al. 2012; Shen et al. 2017; Zhang et al. 2015; Castro et al. 2016; Zheng et al. 2016; Xian-Bing et al. 2014; Larouk et al. 2017). However, complete mineralization of azo dyes is difficult to achieve due to the generation of stable by-products (Zheng et al. 2016).
Ozonation and biological processes can be associated with the goal of removing color and biodegradation of the by-products, as reported in the literature by Montaño et al. (2008), Lu et al. (2009), and Punzi (2015). In these studies, biological processes make use of suspended biomass and aim to remove organic matter. Only one study on ozonation of reactive red azo dye 239 was published and the focus of that work was on color removal (Günes et al. 2012).
Biological processes with adhered biomass have been used for the degradation of azo dyes (Koupaie et al. 2011; Spagni et al. 2010; Cirik et al. 2013; Castro et al. 2016). The moving-bed biofilm reactor (MBBR) is a compact system that can withstand high hydraulic loads and has high efficiency in removing organic matter and nitrogen (Li et al. 2011; Calderón et al. 2012; Bassin et al. 2012). In biofilm systems, the transport of nitrogen into the biofilm occurs by diffusion, allowing the formation of microhabitats with the presence of nitrifying bacteria throughout the biofilm's internal matrix (Young 2017). However, nitrifying bacteria are sensitive and can be inhibited by the presence of aromatic and chlorinated compounds, for example (McCarthy 1999).
This study aimed to evaluate the association of azo dye Reactive Red 239 (RR 239) ozonation until complete color removal with subsequent aerobic biological treatment (two MBBR reactors in series) for the biodegradation of ozonation by-products. The impact of these by-products on the nitrification process was assessed.
Materials and methods
The azo dye Reactive Red 239 (RR 239, 95% purity) was purchased from Oficina de Tintas (local company in Brazil) and has a molecular weight of 1026.37 g mol−1, CAS number 89157-03-9 and molecular formula C31H24ClN7O19S6 (Fig. 1). All the chemicals used in the analytical determinations and effluents preparation were of analytical grade.
Synthetic effluent
The RR 239 solution was prepared in buffered medium (0.1 M phosphate buffer) at a concentration of 50 mg L−1. The pH was adjusted to 7 with a 0.1 M sodium hydroxide solution. This solution was ozonized.
The synthetic effluent was prepared with the following compounds: glucose as a carbon source providing a COD of 400 mg L−1, 30 mg L−1 of NH4+–N as a nitrogen source, 270 mg L−1 of NaHCO3 to supply the alkalinity required for the nitrification process and 4.45 mg L−1 of K2HPO4, 4.55 mg L−1 of KH2PO4 as a source of phosphorus. 0.5 mL/L of a solution containing micronutrients was added to the synthetic effluent (Vishniac and Santer 1957).
The MBBRs feed effluent was prepared by adding these compounds to the after-ozonated dye solution.
Ozonation assays
Ozonation was carried out using buffered solutions containing 50 mg L−1 RR 239 at pH 7. Only the dye was present in the aqueous matrix to allow the identification of products resulting only from dye degradation. Figure S1a (Supplementary Material) depicts the ozonation set-up, consisting of an ozone generator (Ozone & Life, model 3.0 RM), a cylindrical glass ozonation column (2 L) containing a porous gas diffuser, and two gas scrubbers (200 mL, useful volume) filled with a solution of potassium iodide (2% m/v). This solution was used to quantify the ozone consumed after chemical determination of iodine, which was formed in the reaction with the residual ozone. This procedure is used to capture the residual ozone (method 2350E), as recommended in APHA (2005). All the ozonation experiments were conducted at room temperature (25 ± 2 °C).
The ozone concentration applied at the reactor inlet was 20 mg L−1. Ozonation was performed until complete color removal of the solution, which corresponded to 12 min of ozonation with an average ozone consumption of 88.5 mgO3 L−1 under the experimental conditions used.
After ozonation and complete color removal, the organic and inorganic compounds were added to the medium. This synthetic effluent was fed to the MBBR reactors.
Operation of moving-bed biofilm reactors in series
The degradation of the by-products formed in RR 239 dye ozonation was performed in a system with two moving-bed biofilm reactors (MBBR) in series, as shown in Fig. 2. In a previous work, only a cylindrical MBBR glass reactor with a useful volume of 300 mL was used, but the results for ammonium removal were not satisfactory (Dias et al. 2019). In this study, which aimed to achieve higher ammonium removals, the MBBR system consisted of two cylindrical glass reactors in series, with a useful volume of 150 mL each reactor. Biomedias with biofilm already adapted to the effluent from the previous work were used in this work. Aeration of the reactors was performed by a porous diffuser with compressed air flow of 1 mL min−1, resulting in an approx. dissolved oxygen concentration of 5 mg L−1. The biological reactor was fed by a Longer Pump model BT100-2 J peristaltic pump and operated at 20 ± 2 °C. The total hydraulic retention time (HRT) applied was of 6 h (3 h for each reactor). AnoxKaldnes™ carriers (K1 type) were employed and occupied 40% of the useful volume of each MBBR.
Three operational runs were evaluated, shown in Table 1. The MBBR1 and MBBR2 reactors were fed with the following effluents: synthetic effluent + ozonation by-products (12 min of ozonation) (run 1), synthetic effluent + ozonation by-products (20 min of ozonation) (run 2) and synthetic effluent (without ozonated dye solution) (run 3).
In all runs, the COD and ammonium of the synthetic influent were approx. 400 mg L−1 and 40 mgNH4+ L−1, respectively. In runs 1 and 2, where MBBR1 was fed with synthetic effluent + ozonation by-products, COD and ammonium concentrations also remained approx. 400 mg L−1 and 40 mgNH4+ L−1, respectively, even with the small carbon and nitrogen from the by-products of the ozonated dye solution (DOC = 12.36 mg L−1 and Nteo = 4.77 mg L−1).
Analytical determinations
The color removal of the RR 239 solution was quantified by measuring the reduction of absorbance at 542 nm using a Hach spectrophotometer (model DR 2800).
Dissolved organic carbon (DOC) was determined after filtration through a 0.45 μm membrane, using a Shimadzu TOC analyzer (model PCN/TMN-1). Chemical oxygen demand (COD) and ammonium were determined according to the methods 5220D and 4500-NH3C, respectively (APHA 2005). Nitrate and nitrite levels were assessed by spectrophotometry using Hach kits. Suspended solids in the MBBR were determined by the gravimetric method 2540D (APHA 2005). Some carriers were eventually withdrawn from the reactor and the attached solids were removed with a fine brush and further vigorously washed with distilled water. The resulting suspension was used to determine the mass of solids attached to the carriers by the gravimetric method used for suspended solids determination.
The identification of dye ozonation products (after ozonation and in the MBBR2 effluent) was made by gas chromatography coupled with a mass spectrometer (GC/MS, Agilent model 7890B/5977A). 30 mL samples were acidified to pH 2 with hydrochloric acid and saturated with sodium sulfate. Extraction was performed with 5 mL of dichloromethane (3 times), followed by solvent evaporation (dichloromethane) by purging the extract with N2 and derivatization with 100 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) for 10 min at 60 °C. 5 µL of the derivatized solution were injected (splitless mode) into the GC/MS fitted with HP-5MS column (30 m × 0.25 mm × 0.25 μm) under the following conditions: initial temperature set at 100 ºC for 2 min and then it was increased by 10 °C per min until 310 °C; Hydrogen was used as carrier gas (1.6 mL min−1) and the column pressure was 7.9 psi (54.5 MPa).
Additional analytical determination of organic nitrogen compounds was made using a high-resolution mass spectrometer Q Exactive Plus Orbitrap system (Thermo Scientific), m/z range of 50 to 800 Da, resolution of 140,000, equipped with an external electrospray ionization source (ESI). Data acquisition and processing was made employing the software Xcalibur™ (Thermo Scientific). The statistical analysis of the data was performed in the software OriginPro 8.5 by means of the analysis of one-way variance (ANOVA) and TUKEY test, with significance level of 0.05.
Results and discussion
Ozonation of the azo dye Reactive Red 239 (RR 239)
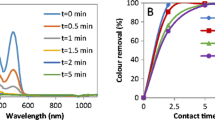
The color, COD and DOC removals and ozone consumption at different ozonation times of a solution containing 50 mg L−1 of RR 239 at pH 7 are shown in Fig. 3. It can be observed that there was total color removal with 12 min of ozonation (88.5 mgO3 L−1 consumed). Azo dye removal by ozone occurs by electrophilic attack in regions with high negative charge density of the molecule, such as –N=N– bonds (Turhan and Ozturkcan 2013). Studies published in the literature indicate that color removal occurs quickly at basic pH. At basic pH, molecular ozone decomposes to form the hydroxyl radical (HO·) which has greater oxidation potential than molecular ozone (O3) (Rice 1996; CRC Handbook 1985). Results presented by Günes et al. (2012) report 90% of color removal of a solution containing 500 mg L−1 from each of the Reactive Red 239 and Reactive Yellow 176 azo dyes in 90 min of ozonation with 16.6 mgO3 min−1 at pH 11. Total color removal was obtained by Zhang et al. (2015) on ozonation of the azo dye Reactive Red 195 at a concentration of 100 mg L−1, pH 7.9 and 3.47 LO3 min−1 for 10 min. For the azo dye Reactive Red X-3B, Shen et al. (2017) obtained 92% of color removal by applying 0.66 LO3 h−1 for 6 min at pH 6.5.
COD and DOC removals obtained after 20 min of ozonation (106.9 mgO3 L−1 consumed) were 62 and 35% (Fig. 3), respectively. The statistical analysis of the COD and DOC removal data shows significant differences between the 0–6 min interval and the 12–20 min interval, COD and DOC removal of 62 and 35%, with p-values of 0.0003 and 0.00008, respectively. Increasing ozone consumption after total color removal from the solution containing RR 239 and low COD and DOC removals indicate the generation of stable by-products. Results in the literature also report low carbon removals in azo dye ozonation. Castro et al. (2016) obtained 48% of DOC removal after 5 min ozonation of a solution containing 100 mg L−1 of Reactive Orange 16 dye applying 51 mgO3 L−1. Zhang et al. (2014) observed 21% of DOC removal in the dye Reactive Red 2 (500 mg L−1) ozonation in 60 min reaction with 75 mgO3 L−1. Zheng et al. (2016) observed 20% of DOC removal on dye Reactive Black 5 (200 mg L−1) ozonation in 60 min reaction applying 53.3 mgO3 min−1. The reduction in COD/TOC ratio from 2.96 to 1.75 at the end of 20 min of ozonation, according to previous work (Alvares et al. 2001), confirms the partial oxidation of RR 239 and the incorporation of oxygen into the ozonation products. Therefore, ozonation is capable of breaking the azo bond and other parts of the dye molecule structure, but does not mineralize the by-products to CO2 and H2O under the conditions applied (12 min ozonation, 88.5 mgO3 L−1 consumed). The RR 239 ozonation by-products, with 12 min (88.5 mgO3 L−1 consumed) and 20 min (consumed ozone 106.9 mgO3 L−1) of ozonation, are shown in Table 2. The compounds are aniline (C6H7N), phenol (C6H6O), catechol (C6H6O2), 4-amino-6-chloro-1,3,5-triazine-2-ol (C3H2ClN4O) and phthalic acid (C8H6O4). These RR 239 ozonation by-products have also been identified by other authors (Turhan and Ozturkcan 2013; Xian-Bing et al. 2014; Song et al. 2007; Shen et al. 2017; Zhang et al. 2015). Phthalic acid, catechol and phenol compounds are present in the mechanisms proposed by Zhang et al. (2015) and Shen et al. (2017), which indicate that, after 12 and 20 min of ozonation, there was the degradation of naphthalene rings and aromatic sulfonate. Pelizzetti et al. (1990) and Yixin et al. (2014) also reported the difficulty of opening the triazine ring by HO· oxidation and attributed this behavior to the high stability of the triazine ring.
Moving-bed biofilm reactors in series
The results obtained in a previous work, which was performed with only one MBBR, presented a low efficiency of 40% ammonium removal and 90% COD removal (Dias et al. 2019). Therefore, it was decided to operate two MBBRs in series in order to favor the nitrifying consortium and create an environment that is protected from by-products, as shown in Fig. 4. Thus, the MBBR1 received the effluent containing potentially inhibitory by-products and removed most of the COD and these by-products. MBBR2 would then be responsible for the removal of ammonium. This configuration of MBBRs in series has been extensively studied by several authors and with great success (Pellicer-Nàcher et al. 2013; Bassin et al. 2011; Lim et al. 2011; Ma et al. 2017; Casas et al. 2015; Jaroszynski and Oleszkiewicz 2011).
The degradation of ozonation by-products and ammonium removal was carried out in 3 operational runs with MBBR1 and MBBR2 in series. The operating conditions are presented in Table 1 and COD and ammonium removals are shown in Fig. 4a, b.
The results obtained for a single MBBR (previous work) and for the two serial MBBRs show that there was a good adaptation of heterotrophic bacteria and high COD removals were observed. However, nitrification inhibition of the RR 239 ozonation by-products persisted in MBBR1 and especially in MBBR2, where high ammonium removal was expected.
COD removals of 91% by MBBR1 and 94% by MBBR1 + MBBR2 were observed in run 1. It should be considered that the concentration of organic matter in the MBBR2 influent was very small (32 mg L−1). These results show that there was a slight increase in COD removal when changing the setting from only 1 MBBR (90%) to 2 MBBRs in series (94%), applying the same HRT (6 h) and volume of reactor filling with carriers (40% v/v). Studies from the literature that associated ozonation with biological processes in the degradation of azo dyes reported total color removal and 50–90% COD removals, ratifying the results obtained in this work (Montaño et al. 2008; Lu et al. 2009; Punzi 2015).
In run 2, MBBR1 was fed with ozonated effluent for 20 min (consumed ozone 106.9 mgO3 L−1) in order to reduce the impact of by-products on the microbiota, since by-products formed during ozonation for more time are more oxidized. However, a reduction in COD removal from 91 to 83% by MBBR1 and from 94 to 90% after MBBR1 + MBBR2 was observed. This result suggests the generation of less biodegradable ozonation by-products or with some toxicity when longer ozonation time was applied. Zhang et al. (2007) also reported an increase in toxicity from 1.86 to 36.65% for Vibrio fischeri assays after 60 min of ozonation of the dye Reactive Red 120 at a concentration of 200 mg L−1 and applying 12.8 mgO3 L−1. Wang et al. (2011) observed increased toxicity from 45 to 75% in Vibrio fischeri assays after 30 min of ozonation (20.5 mgO3 L−1) of Remazol Black 5 dye at a concentration of 2 g L−1. These results contrast with those presented by Khadhraoui et al. (2009), who observed in Vibrio fischeri assays the absence of toxicity of the Congo Red dye (300 mg L−1) after 2 min of ozonation (2.7 gO3 L−1) and stated that association of the ozonation with biological degradation is an alternative for the treatment of effluents containing azo dyes.
Average ammonium removals for runs 1 and 2 were 40%, showing incomplete nitrification (Fig. 4b). The conversion percentages of influent ammonium to nitrite were 36% and 29% after MBBR1 in runs 1 and 2, respectively (Fig. 5) and, therefore, the formation of nitrite and nitrate was minimal in MBBR2. These results indicate that the increase in RR 239 ozonation time generated by-products that inhibit nitrification. Low ammonium removal obtained is possibly related to a reduction in the enzymatic activity of nitrifying bacteria caused by triazine compounds containing chlorine, one of the RR 239 ozonation by-products that passed unharmed or is poorly degraded in the biological process (Tomnlinson et al. 1966; McCarthy 1999). Results obtained by Ong et al. (2010) and He and Bishop (1994) report a reduction in ammonium removal in wetland and activated sludge systems, respectively, when in the presence of the dye Acid Orange 7 in concentrations of 50 and 5 mg L−1. Spagni et al. (2010) observed nitrite peaks resulting from inhibition of the second nitrification step in activated sludge treatment of an effluent containing Reactive Orange 16 dye at a concentration of 25 mg L−1.
In order to verify the adverse effects caused by the RR 239 ozonation by-products and to verify the presence of nitrifying bacteria in the biofilms of both reactors, the MBBRs in series were fed only with synthetic effluent in run 3 (without by-products). Overall COD and ammonium removals of 90% and 83%, respectively, were obtained, and almost all influent ammonium was converted to nitrate. The COD and ammonium removal data (Fig. 3a, b) are statistically different for the 3 runs evaluated, except between run 2 and 3 for COD removal, with p values < 0.00001. However, the incapability to achieve high nitrification in the presence of by-products formed by RR 239 ozonation was proved. These results are extremely important when the focus is on the association of ozonation with a later biological process. It is observed that the impact of ozonation by-products on nitrification was extensive and that some by-products, which have high toxicity such as triazine rings, for example, can pass unharmed through the biological process and be released into the receiver bodies. One of the ozonation by-products of RR 239, 4-amino-6-chloro-1,3,5-triazine-2-ol, has a chemical structure similar to atrazine. Atrazine is an herbicide and a persistent organic pollutant (Solís et al. 2012; Yixin et al. 2014). Results obtained by Sanchis et al. (2014) and Pathak et al. (2018) on atrazine degradation by activated sludge and in a baffled osmotic membrane bioreactor-microfiltration hybrid system, respectively, showed a slight degradation of atrazine, confirming its persistence and probable inhibition of biological process.
Concentrations of suspended and adhered solids are shown in Table 3. In runs 1 and 2, when MBBRs were fed with ozonated synthetic effluent for 12 and 20 min, a decrease in total suspended solids concentration (TSS) from 0.38 to 0.32 g L−1 in MBBR1 and a TSS increase from 0.39 to 0.47 g L−1 in MBBR2 can be observed. The concentration of total solids attached (TSA) also decreased between runs 1 and 2 from 1.56 to 0.44 g L−1 in MBBR1 and from 0.82 to 0.48 gL−1 in MBBR2. These results show the biomass detachment in MBBR2 when the reactors were fed with ozonated effluent for 20 min and that the increase in the RR 239 ozonation time contributed to the reduction in the biofilm thickness. Zhang et al. (1995). and Castro et al. (2016) also observed a reduction in biofilm thickness in a MBBR fed with effluents containing ozonated azo dyes. However, the mean volatile solids attached/total solids attached (VSA/TSA) ratios of 0.77 in MBBR1 and 0.66 in MBBR2 suggest that there is a sufficient amount of microorganisms in the biofilm for degradation of organic and inorganic matter.
The products identified in MBBR2 effluent in runs 1 and 2, shown in Table 2, are formaldehyde (CH2O), oxalic acid (C2H2O4), 1,3-dihydro-2-bezofuran-1,3-diol (C8H8O3) and 4-amino-6-chloro-1,3,5-triazine-2-ol (C3H2ClN4O). The MBBRs in series provided the degradation of phenol, aniline and catechol. The 4-amino-6-chloro-1,3,5-triazine-2-ol shown in Table 2 passed unharmed or was poorly degraded by the biological process, confirming the likely inhibition of the nitrifying bacteria by the presence of the triazine ring. However, other by-products may have been formed and were not identified by the analytical techniques used and may also have an inhibitory effect on the removal of organic matter and especially on the nitrification process.
Conclusions
Total color removal of the dye RR 239 was achieved within minutes of ozonation. Increasing ozone consumption over time and COD removal (35%) after 20 min of ozonation suggest the formation of by-products of difficult oxidation. The by-products identified from RR 239 ozonation were aniline, catechol, phenol, phthalic acid and 4-amino-6-chloro-1,3,5-triazine-2-ol. The MBBR system with two reactors in series removed COD (90%) and degraded phenol, aniline, catechol and phthalic acid. A low ammonium removal (40%) persisted in MBBR1 and MBBR2, probably due to inhibition of nitrification by 4-amino-6-chloro-1,3,5-triazine-2-ol, which has a chemical structure similar to atrazine. This paper shows the importance of evaluating the metabolization of the by-products formed and the possible inhibitions of the biological process when ozonation or other AOP is used as an effluent pretreatment.
References
Adabju S (2013) Specific moving bed biofilm reactor for organic removal from synthetic municipal wastewater. M.Sc. Thesis, University of Technology, Sydney
Al-Sabti K (2000) Chlorotriazine reactive azo red 12 textile dye induces micronuclei in fish. Ecotoxicol Environ Saf 47:149–155
Alvares AB, Diaper C, Parsons AS (2001) Partial oxidation by ozone to remove recalcitrance of wastewaters—a review. Environ Technol 22:409–427
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington, DC
Ayadi I, Souissi Y, Jlassi I, Peixoto F, Mnif W (2016) Chemical synonyms, molecular structure and toxicological risk assessment of synthetic textile dyes: a critical review. J Dev Drugs 5:1–4
Baêta BEL, Lima DRS, Queiroz Silva S, Aquino SF (2016) Influence of the applied organic load (OLR) on textile wastewater treatment using submerged anaerobic membrane bioreactors (SAMBR) in the presence of redox mediator and powdered activated carbon (PAC). Braz J Chem Eng 33:817–825
Bassin JP, Dezotti M, Sant’Anna GL Jr (2011) Nitrification of industrial and domestic saline wastewaters in moving bed biofilm reactor and sequencing batch reactor. J Hazard Mater 185:242–248
Bassin JP, Kleerebezem R, Rosado AS, van Loosdrecht MCM, Dezotti M (2012) Effect of different operational conditions on biofilm development, nitrification, and nitrifying microbial population in moving-bed biofilm reactors. Environ Sci Technol 46:1546–1555
Bazin I, Hassine AIH, Hamouda YH, Mnif W, Bartegi A, Lopez-Ferber M, Waard MD, Gonzalez C (2012) Estrogenic and anti-estrogenic activity of 23 commercial textile dyes. Ecotoxicol Environ Saf 85:131–136
Calderón K, Martín-Pascual J, Poyatos JM, Rodelas B, González-Martínez A, González-López J (2012) Comparative analysis of the bacterial diversity in a lab-scale moving bed biofilm reactor (MBBR) applied to treat urban wastewater under different operational conditions. Biores Technol 121:119–126
Casas ME, Chhetri RK, Ooi G, Hansen KMS, Litty K, Chistensson M, Kragelund C, Andersen HR, Bester K (2015) Biodegradation of pharmaceuticals in hospital wastewater by stage moving bed biofilm reactors (MBBR). Water Res 83:293–302
Castro FD, Bassin JP, Dezotti M (2016) Treatment of a simulated textile wastewater containing Reactive Orange 16 azo dye by a combination of ozonation and oving-bed biofilm reactor: evaluating the performance, toxicity and oxidation by-products. Environ Sci Pollut Res 24:6307–6316
Chengalroyen MD, Dabbs ER (2013) The microbial degradation of azo dyes: mini review. World J Microbiol Biotechnol 29:389–399
Cirik K, Kitis M, Çinar Ö (2013) Effect of nitrate on anaerobic azo dye reduction. Bioprocess Biosyst Eng 36:69–79
CRC (1985) Handbook CRC. In: West RC, Astle MJ, Beyer WH (eds) Handbook of chemistry and physics. CRC Press, Inc., Boca Raton
Dias NC, Sant’Anna GL Jr, Bassin JP, Dezotti M (2019) Ozonation of the dye Reactive Red 239 and biodegradation of ozonation products in a moving-bed biofilm reactor: revealing reaction products and degradation pathways. Int Biodeterior Biodegrad 144:104742–104751
Firmino PIM, da Silva MER, Mota FSB, dos Santos AB (2011) Applicability of anthraquinone-2,6-disulfonate (AQDS) to enhaced colour removal in mesophilic UASB reactor treating textile wastewater. Braz J Chem Eng 28:617–623
Ghaly AE, Ananthashankar R, Alhattab M, Ramakrishnan VV (2014) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5:1–19
Günes Y, Atav R, Namirti O (2012) Effectiveness of ozone in decolorization of reactive dye effluents depending on the dye chromophore. Text Res J 82:994–1000
He Y, Bishop PL (1994) Effect of acid orange 7 on nitrification process. J Environ Eng 120:108–121
Jaroszynski LW, Oleszkiewicz JA (2011) Autotrophic ammonium removal from reject water: partial nitrification and anamox in one-reactor versus two-reactor systems. Environ Technol 32:289–294
Jungtanasombut W, Preeprem P, Kovitvadhi S, Kovitvadhi U, Hannongbua S (2014) Effects of Reactive Red 239 on developing Zebrafish (Danio rerio) embryos. Kasetsart J Nat Sci 48:619–628
Kabra AN, Khandare RV, Waghmode TR, Govindwar SP (2011) Differential fate of metabolism of a sulfonated azo dye Remazol Orange 3R by plants Aster amellus Linn., Glandularia pulchella (Sweet) Tronc. and their consortium. J Hazard Mater 190:424–431
Khadhraoui M, Trabelsi H, Ksibi M, Bouguerra S, Elleuch B (2009) Discoloration and detoxification of a Congo red dye solution by means of ozone treatment for a possible water reuse. J Hazard Mater 161:974–981
Koupaie EH, Moghaddam MRA, Hasheim SH (2011) Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195:147–154
Larouk S, Ouargli R, Shahidi D, Olhund L, Shiao TC, Chergui N, Sehili T, Roy R, Azzouz A (2017) Catalytic ozonation of Orange-G through highly interactive contributions of hematite and SBA-16–To better understand azo-dye oxidation in nature. Chemosphere 168:1648–1657
Li S, Cheng W, Wang M, Chen C (2011) The flow patterns of bubble plume in an MBBR. J Hydrodyn 23:510–515
Lim J, Seng C, Lim P, Ng S, Sujari AA (2011) Nitrogen removal in moving bed sequencing batch reactor using polyurethane foam cubes of various sizes as carrier materials. Bioresour Technol 102:9876–9883
Lu X, Yang B, Chen J, Sun R (2009) Treatment of wastewater containing azo dye reactive brilliant red X-3B using sequential ozonation and up flow biological aerated filter process. J Hazard Mater 161:241–245
Ma W, Han Y, Ma W, Han H, Zhu H, Xu C, Li K, Wang D (2017) Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour Technol 244:84–91
McCarthy GW (1999) Modes of action of nitrification inhibitors. Biol Fertil Soils 29:1–9
Montaño JG, Domènech X, García-Hortal JA, Torrades F, Peral J (2008) The testing of several biological and chemical coupled treatments for Cibacron Red FN-R azo dye removal. J Hazard Mater 154:484–490
Muhammad A, Shafeeq A, Butt MA, Rizvi ZH, Chughtai MA, Rehman S (2008) Decolorization and removal of COD and BOD from raw and biotreated textile dye bath effluent through advanced oxidation processes (AOPS). Braz J Chem Eng 25:453–459
Ong SA, Uchiyama K, Inadama D, Ishida Y, Yamagiwa K (2010) Treatment of azo dye Acid Orange 7 containing wastewater using up-flow constructed wetland with and without supplementary aeration. Bioresour Technol 101:9049–9057
Pathak N, Li S, Kim Y, Chekli L, Phuntsho S, Jang A, Ghaffour N, Leiknes T, Shon HK (2018) Assessing the removal of organic micropollutants by a novel baffled osmotic membrane bioreactor-microfiltration hybrid system. Bioresour Technol 262:98–106
Pellicer-Nàcher C, Franck S, Gülay A, Ruscalleda M, Terada A, Al-Soud WA, Hansen MA, Sørensen SJ, Smets BF (2013) Sequentially aerated membrane biofilm reactors for autotrophic nitrogen removal: microbial community composition and dynamics. Microb Biotechnol 7(1):32–43. https://doi.org/10.1111/1751-7915.12079
Pelizzetti E, Maurino V, Minero C, Carlin V, Pramauro E, Zerbinati O (1990) Photocatalytic degradation of atrazine and other s-triazine herbicides. Environ Sci Technol 24:1559–1565
Punzi M (2015) Treatment of textile wastewater by combining biological processes and advanced oxidation. Ph. D. Thesis, Faculty of Engineering, Lund University, Sweden
Rice RG (1996) Applications of ozone for industrial wastewater treatment—a review. Ozone Sci Eng J Int Ozone Assoc 18:477–515
Sanchis S, Polo AM, Tobajas M, Rodriguez JJ (2014) Strategies to evaluate biodegradability: application to chlorinated herbicides. Environ Sci Pollut Res 21:9445–9452
Shen Y, Xu Q, Wei R, Ma J, Wang Y (2017) Mechanism and dynamic study of Reactive Red X-3B dye degradation by ultrasonic-assisted ozone oxidation process. Ultrason Sonochem 38:681–692
Solís M, Solís A, Perez HI, Manjares N, Flores M (2012) Microbial decolouration of azo dyes: a review. Process Biochem 47:1723–1748
Song S, Ying H, He Z, Chen J (2007) Mechanism of decolorization and degradation of CI Direct Red 23 by ozonation combined with sonolysis. Chemosphere 66:1782–1788
Spagni A, Grilli S, Casu S, Mattiolu D (2010) Treatment of a simulated textile wastewater containing the azo-dye reactive orange 16 in an anaerobic-biofilm anoxic-aerobic membrane bioreactor. Int Biodegrad Biodegrad 64:676–681
Tomlinson TG, Boon AG, Trotman GNA (1966) Inhibition of nitrification in the activated sludge process of sewage disposal. J Appl Bacteriol 29:266–291
Turhan K, Ozturkcan SA (2013) Decolorization and degradation of reactive dye in aqueous solution by ozonation in a semi-batch bubble column reactor. Water Air Soil Pollut 224:1353
Victral DM, Aquino SF, Silva SQ, Baêta BEL (2016) Application of residual yeast as a source of redox mediators for the anaerobic decolorization of a model azo dye. Braz J Chem Eng 33:705–711
Vishniac W, Santer M (1957) The thiobacilli. Bacteriol Rev 21:195–213
Wang C, Yediler A, Lienert D, Wang Z, Kettrup A (2002) Toxicity evaluation of reactive dyestuffs, auxiliaries and selected effluents in textile finishing industry to luminescent bacteria Vibrio fischeri. Chemosphere 46:339–344
Wang J, Qiai M, Wei K, Ding J, Liu Z, Zhang K, Huang X (2011) Decolorizing activity of Malachite Green and its mechanisms involved in dye biodegradation by Achromobacter xylocoxidans MG1. J Mol Microbiol Biotechnol 20:220–227
Xian-Bing Z, Wen-Yi D, Fei-Yun S, Wei Y, Jiao D (2014) Degradation efficiency and mechanism of azo dye RR2 by a novel ozone aerated internal micro-electrolysis filter. J Hazard Mater 276:77–87
Yixin Y, Hongbin C, Pai P, Hongmiao B (2014) Degradation and transformation of atrazine under catalysed ozonation process with TiO2 as catalyst. J Hazard Mater 279:444–451
Young B (2017) Nitrifying MBBR performance optimization in temperate climates through understanding biofilm morphology and microbiome. Ph.D. Thesis. Department of Civil and Environmental Engineering, Faculty of Engineering, University of Ottawa, Canada
Zhang TC, Fu YC, Bishop PL, Kupferle M, Fitzgerald S, Jiang HH, Harmer C (1995) Transport and biodegradation of toxic organics in biofilms. J Hazard Mater 41:267–285
Zhang F, Yediler A, Liang X (2007) Decomposition pathways and reaction intermediate formation of the purified, hydrolysed azo reactive dye C.I. Reactive Red 120 during ozonation. Chemosphere 67:712–717
Zhang XB, Dong WY, Sun FY, Yang W, Dong J (2014) Degradation efficiency and mechanism of azo dye RR2 by a novel ozone aerated internal micro-electrolysis filter. J Hazard Mater 276:77–87
Zhang R, Yuan DX, Liu BM (2015) Kinetics and products of ozonation of C.I. Reactive Red 195 in a semi-batch reactor. Chin Chem Lett 26:93–99
Zheng Q, Dai Y, Han X (2016) Decolorization of azo dye C.I. Reactive Black 5 by ozonation in aqueous solution: influencing factors, degradation products, reaction pathway and toxicity assessment. Water Sci Technol 73:1500–1510
Acknowledgements
N. C. Dias gratefully acknowledges her doctoral scholarships supported by CNPq and Capes (142269/2015-8 and PROEX 0070041) and FAPERJ (reference E-26/202.995/2015). The authors thank the Technological Development Support Laboratory (LADETEC), Chemistry Institute, Federal University of Rio de Janeiro and Bioprocess Laboratory, COPPE, Federal University of Rio de Janeiro for the analytical by-products determinations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dias, N.C., Alves, T.L.M., Azevedo, D.A. et al. Metabolization of by-products formed by ozonation of the azo dye Reactive Red 239 in moving-bed biofilm reactors in series. Braz. J. Chem. Eng. 37, 495–504 (2020). https://doi.org/10.1007/s43153-020-00046-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00046-6