Abstract
Heavy metal pollution poses a grave environmental threat. Some of the most toxic metals are highly mobile and, therefore, easily transported through ground water systems, thus, affecting large areas. Over the last decade, adsorption has been greatly focused on as a strategy for contaminated water treatment. Its versatility and relative ease of application have been a major determinant of its preference. Nanosized adsorbents have high surface areas and are size tunable and, hence, have been favored in adsorption applications. The magnetic properties of nanosized magnetite (Fe3O4) have made them particularly favorable. Magnetite composites with various materials have widely been applied in the adsorptive treatment of real and synthetic water containing heavy metal pollutants. This review outlines the application of Fe3O4 nanoparticles and Fe3O4 organic composites in the adsorption of heavy metal ions in aqueous solution. The reviewed articles indicate that the formation of Fe3O4 inorganic–organic composites improves the adsorption efficiencies of the composites and improves their applicability by providing magnetic separability. The presence of Fe3O4 nanoparticles in the composite materials also provides for improved reusability of the adsorbent. Generally, the formation of these composites tends to make adsorption a more viable alternative to conventional water treatment options for heavy metal pollutants in water.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The environmental accumulation of heavy metals is of great concern owing to their non-biodegradability [5, 31, 64]. Heavy metal pollution occurs primarily through either of the following anthropogenic processes: manufacturing, mining, burning of fossil fuels, and agriculture [20, 66]. Although anthropogenic activities contribute the greater extent of heavy metal pollution, natural phenomena, e.g., erosion and weathering of rocks also contribute to the pollution burden [48]. According to the US EPA, the most toxic heavy metals are arsenic and lead with a maximum contaminant level goal (MCLG) of 0 mg L−1 (US EPA 2009; [21, 63]. Other listed toxic heavy metals are copper, chromium, mercury, nickel, and cadmium. Heavy metals may be toxic even at low concentrations resulting in poisoning or genetic disorders as they have the potential to interfere with biological processes [12, 22]. As information on the toxicity of heavy metals increases, the regulatory limits are adjusted to lower concentrations making remediation more challenging [62]. Techniques like electrochemical and photocatalytic oxidation, chemical coagulation, ion exchange, bio- and phyto-remediation, and adsorption have been employed for the adsorption of heavy metal pollution control [9, 40].
Adsorption is considered favorable due to its efficiency, versatility, simplicity of operation, zero sludge production, and relatively lower costs [42, 70]. Adsorption at the solid-solution interface provides a possibility to control pollution due to liquid waste [20]. Through consistent improvement efforts, several adsorbents have been developed with current technologies focusing on nanosized adsorbents due to the uniqueness of the properties owing to their nanometer sizes. Some of the most investigated nanomaterials are iron oxides as a result of their stability, pollutant affinity, and relatively low toxicity compared to other metal containing nanoparticles [61]. Magnetite has received great consideration because it offers superior advantages such as surface areas >100 m2 g−1 and superparamagnetism (~90 emu g−1 for bulk magnetite) as the size reduces to nanoscale [27, 61]. The removal of pollutants through adsorption methods is highly dependent on the adsorbent’s surface charge and the adsorbate’s speciation and degree of ionization [20]. The presence of both ferrous (Fe2+) and ferric (Fe3+) ions allows Fe3O4 nanoparticles to participate in redox-coupled adsorption processes which are particularly useful in the sequestration of multi-valent ions. The magnetic properties of Fe3O4 make them easily recoverable after treatment, a challenge while using many nanometer sized materials [4, 20]. The recovered particles can be reused, therefore, reducing the economic burden of the treatment process [4, 12].
2 Magnetite
Iron-based nanoparticles have recently been applied in the adsorptive treatment of polluted water [65]. Of the reported iron-based nanoparticles, zero-valent iron has received the greatest attention [26, 32]. Nanosized iron oxides composition varies depending the iron species present and the magnetic properties; of the known iron oxides, hematite (α-Fe2O3) maghemite (γ-Fe2O3), and magnetite (Fe3O4) have been considered in the adsorption of heavy metals [29, 35, 38, 57]. Superparamagnetic iron oxides (magnetite; Fe3O4) are commonly applied because of the ease of post-adsorption retrieval using an external magnetic field. Upon removal of the magnetic field, the particles are demagnetized since they do not possess residual magnetization [25, 48].
A wide range of synthetic methods including solvothermal [29, 37], laser co-vaporization [54], sol–gel [23, 51], thermal decomposition [2, 52], and chemical co-precipitation [20, 43] has been used in the production of Fe3O4 nanoparticles. Chemical co-precipitation the most favored method because it is simple, efficient, and relatively cheaper than the above-mentioned methods [1, 68]. Chemical co-precipitation of Fe3O4 takes place in alkaline media, and the formation of Fe3O4 follows the reaction steps outlined in Eqs. 1–4 below [68].
Apart from magnetite nanoparticles synthesized at the point of application, commercial magnetite nanoparticles are readily available and have also been applied in heavy metal adsorption. Iconaru et al. [20] synthesized 14 nm magnetite nanoparticles and compared their properties with those of commercial magnetite of 90 nm average diameters [20]. The surface area ratio of the commercial to synthesized magnetite was 7%; however, the synthesized sample showed lower crystallinity [15, 28]. When applied in the adsorption of As(V) and Cu(II), it was evident that the as-synthesized smaller particles provided better adsorption efficiencies for both species [20, 36]. The results obtained from As(V) and Cu(II) adsorption on both nanoparticle batches were modeled following a theoretical calculation of the packing density. Data from adsorption on commercial nanoparticles provided a better accuracy than synthesized sample, while As(V) data had 50% higher accuracy than Cu(II) adsorption data. The results pointed to more uniform distribution of commercial nanoparticles as compared to synthesized nanoparticles with a higher affinity for As(V) than Cu(II) resulting from differences in complexation energies in the adsorption process [19].
Further, Kumari et al. [29] studied Cr(VI) and Pb(II) adsorption on mesoporous Fe3O4 nanospheres synthesized using a solvothermal method [29]. Hollow nanospheres consist of a shell-like morphology of nanoparticles with a hollow core providing low densities. The hollow nanospheres were synthesized using a solvothermal method. In the solvothermal method, the solvent acts as a reducing medium reducing a small amount of the Fe3+ precursor to Fe2+. The structure directing salt initiates nucleation to form spheres in the presence of the surfactant with the solvent controlling the size of the spheres. Ostwald ripening results in small inner spheres forming larger ones on the outer side increasing the size of the inner cavities. This results in the formation of a hollow interior with larger nanocrystals forming the outer surface. The particle diameters were determined to be 31 nm with surface areas of 11 m2 g−1. Adsorption of Cr(VI) and Pb(II) ions resulted in modifications on the adsorbent surface of the with the initially rough surface appearing smooth in post-adsorption analyzes.
Luther et al. [34] synthesized Fe3O4 nanoparticles and studied the effects of pH and interfering anions on As(III) and As(V) adsorption [34]. The synthesized Fe3O4 nanomaterials had diameters of 17 nm, and the optimum pH used for adsorption studies was pH 6 since it was within the optimum range for both As species. The As(III) adsorption capacity was consistently higher than As(V) capacity after 1 h and 24 h contact time; however, a decreased binding capacity with increased contact time was observed and attributed to redox dissolution. Interference studies indicated that the presence of SO42− affected the binding of As(III) decreasing it by up to 50% at concentrations greater than 1000 ppm, while As(V) binding of was completely eliminated at similar concentrations. The presence of PO43− had insignificant effects on the adsorption capacity of either As species, while the presence of CO32− decreased As(III) and As(V) binding of by up to 15% and 50%, respectively. From the highlighted studies, Fe3O4 has been portrayed as an efficient adsorbent for the sequestration of heavy metal ions in water. The particle size, pH, and competing ions have been identified as important factors influencing the adsorption process. Table 1 summarizes the efficiency of magnetite adsorbents in the sequestration of heavy metals.
3 Magnetite Composites
Pristine Fe3O4 nanoparticles commonly face challenges of oxidation during preparation, handling, and adsorption resulting in changes in their dispersion and magnetic properties [46]. Similarly, the achievement of size control during Fe3O4 synthesis presents a challenge due to agglomeration resulting from high surface energies resulting in broad particle size distribution, insufficient dispersion, and difficulty in mass production. One of the most studied methods to control Fe3O4 properties during synthesis is the formation of composite materials, and composites retain the properties of both materials, therefore, providing a more versatile adsorbent. Fe3O4 inorganic–organic composite adsorbents are favored over pristine Fe3O4 as they incorporate the high surface areas, mechanical strength, and magnetism of the inorganic Fe3O4 component and provide functional groups from the organic material [43]. The organic functional groups provide multiple advantages of anchoring the Fe3O4 surfaces, surface passivation, as well as sequestration of various pollutants including heavy metals [14, 42]. In this section, inorganic–organic composites of Fe3O4 with some selected organic materials are reviewed with a focus on their application in heavy metal adsorption.
3.1 Magnetite-polymer Composites
The modification of Fe3O4 nanoparticle surfaces with organic ligands presents an avenue for both surface passivation and functionalization allowing for the targeted adsorption of desired pollutants [61]. Organic ligands control particle growth resulting in smaller particles, hence, large accessible surface areas, therefore, improving the adsorption capacities [16]. Zarnegar and Safari [68] studied polymer stabilization effects on Fe3O4 nanoparticle properties. They prepared Fe3O4 composite materials with polyethylene glycol (PEG) and polycitric acid (PCA) [68]. The synthesis was carried out in two stages; firstly, PCA-PEG-PCA copolymer macromolecules were prepared followed by the co-precipitation of ferric and ferrous ions in the presence of the copolymers. During the co-precipitation, ferric and ferrous salts were first stirred with the polymers resulting in the formation of a complex structure with surface carboxylic acid groups. Upon the addition of a base, the carboxylic acid groups promoted nucleation, while the copolymers controlled the nanoparticles growth thereby providing size control and resulting in the formation of particles of 5–10 nm. The dendritic nature of the macromolecules provided repulsion aiding in particle dispersion providing uniformly dispersed particles. The polymer-coated particles were spherical and monodisperse with 5–10 nm diameters and 66.54 emu g−1 saturation magnetization compared to 15–30 nm and 62.76 emu g−1, respectively, for uncoated Fe3O4. Polymer stabilization improved the size distribution and magnetic properties of Fe3O4 as a result of improved crystallinity of the smaller nanoparticles [68].
Guan and co-workers prepared a core–shell nano-adsorbent consisting of a nano-magnetite core and a polyacrylic acid shell for the adsorption of Cr(III) ions from tannery effluent. A silane coupling agent aided the grafting of polyacrylic acid onto the surface of the magnetite nanoparticles. The synthesized composite material had a core size of 21 ± 5 nm and specific surface areas of 41.4 ± 0.6 m2 g−1. The saturation magnetization decreased in the order pristine Fe3O4 > silane/Fe3O4 > polyacrylic acid/silane/Fe3O4. The decrease is resulted from the encapsulation of the Fe3O4 in a polymeric shell; however, the resulting composite retained sufficient magnetism to facilitate magnetic separation within 5 min of adsorption completion. Chromium(III) adsorption was most favorable at pH 6 resulting in a percentage removal of 92.5%. The results indicated that Cr(III) ions were coordinated with the carboxyl groups on the polyacrylic acid shell.
Bhaumik et al. [8] reported on the synthesis of polypyrolle-magnetite (PPY/Fe3O4) nanocomposite for Cr(VI) adsorption [8]. The composite synthesis was carried out in situ through chemical oxidative polymerization [7]. Fe3O4 nanoparticles were spherical but appeared aggregated, but after polymerization with polypyrolle, the particles were spherical with larger particle sizes resulting from polypyrolle encapsulation of the particles. The nanocomposite presented superior adsorption properties compared to its constituents in the order PPY/ Fe3O4 > PPY > Fe3O4. Adsorption of Cr(VI) on the nanocomposite was determined to be through ion exchange and reduction [44]. The appearance of Cr(III) species on the spent adsorbent surface indicated that a portion of the bound Cr(VI) ions was reduced by the electron-rich polypyrolle groups in the composite material. The adsorbent was tested for reusability, and two cycles were deemed optimum with a 17% reduction in capacity observed in the third cycle.
Burks et al. [10] studied the characterization and chromium adsorption properties of mercaptopropionic acid-coated magnetite nanoparticles. Calculations from TGA measurements indicated that the coverage of mercaptopropionic acid on SPION surface was approximately 2.5 μmol m−2 [10], while FTIR results revealed that mercaptopropionic acid formed surface bonds with the SPION using the carboxylate end leaving the thiol group exposed [41]. Bands attributed to sulfonate groups indicated oxidation of the thiol groups during air drying. From the isotherm fitting, the obtained data pointed to a multilayer adsorption on a heterogenous surface. At low Cr(VI) concentrations, the reaction was controlled by diffusion to the adsorbent surface; however, as concentrations increased, chemisorption was the rate limiting step. Multiple rate controlling steps were confirmed by a plot of qt against t1/2 (intraparticle diffusion kinetic model) [43]. The adsorption mechanism was illustrated to be via the bonding of HCrO4− ions to -SO3H groups on the 3-MPA surface.
Alqadami et al. [1] studied the application of 5–10 nm Fe3O4@TSC (magnetite@tri-sodium citrate) nanocomposite in the adsorption of Cr3+ and Co2+ ions [1]. The presence of Cr–O and Co–O bonds on the spent adsorbent surface was attributed to electrostatic attraction to the electron rich acetate groups. Adsorption of Cr3+ was faster than that of Co2+; thus, the equilibrium time for Co2+ was considered as the optimal contact time, and pH 6 was considered as optimal above which the formation of metal hydroxides resulted in decreased adsorption efficiency. Langmuir isotherm and pseudo-second-order kinetics model accurately described >97% of the observed results, and the adsorption process was determined to be exothermic. A decrease in adsorption with temperature was attributed to weakening adsorbent-adsorbate and adsorbate–adsorbate forces.
A ternary composite of magnetite nanoparticles (Fe3O4 NPs), reduced graphene oxide sheets (rGO), and poly-N-phenylglycine nanofibers (d-PPG NFs) was prepared for Cu(II) adsorption [27]. The formation of Fe3O4 (270 ± 30 nm) on GO sheets opened the spaces between the sheets, while the grafting of PPG NFs nearly doubled the composite’s surface area. The nanofibers ultrafine morphology was responsible for the increased surface area. Copper adsorption was more efficient on the ternary composite as compared to the binary composite as a result of increased affinity by PPG nanofibers and higher surface areas. The COO− group in the nanofibers was responsible for the increased cation affinity by electrostatic attraction. Formation of a stable copper-carboxylate complex led to preferential copper adsorption in bimetal solutions with cobalt ions.
In 2010, Warner and co-workers demonstrated the synthesis of lauric acid capped Fe3O4 followed by a single step ligand exchange reaction to alter the surface and produce nanoparticles with affinities for a variety of heavy metal pollutants [61]. High-temperature decomposition was applied to generate a magnetite core and lauric acid shell resulting in the formation of 8 nm particles with surface areas >100 m2 g−1. Surface-modified nanoparticles were applied in the adsorption of Hg, Pb, Cd, Ag, Co, Cu, and Tl in spiked river water to determine their efficiency. After ligand exchange, core sizes remained unaffected and the particles were superparamagnetic with no remnant coercivity. Adsorption efficiencies of the functionalized particles for the tested metal pollutants were consistently higher than those of activated carbon with the exception of Ag where activated carbon had the highest distribution coefficient.
Studies on organic ligand stabilized Fe3O4 nanoparticles have concluded that their presence does not alter the nanoparticles magnetic properties and in fact increases the particles affinity for specific heavy metal pollutants while maintaining the high surface areas and superparamagnetism [27, 68].
3.2 Magnetite-biosorbent Composites
Biological materials with the capability of binding pollutants on their surfaces (adsorption) are referred to as biosorbents. In the process of biosorption, heavy metals (pollutants) are adsorbed through a metabolically passive process which occurs on non-living tissues [67]. Biomaterials do not pose a threat to the environment since they are organic in nature and are biodegradable [47]. Several biosorbents have been applied in heavy metals adsorption due to the abundance of functional groups capable of heavy metal sequestration [13, 50, 60]. Despite the adsorption potentials of biomaterials, they face challenges such as low porosity, surface areas, and difficulty in post-treatment separation [39, 66]. The incorporation of nanomaterials on the surfaces of biosorbents has been confirmed to improve surface areas and porosity of adsorbents [27, 69]. Fe3O4 nanoparticles when deposited on biosorbents incorporate magnetic properties on the composite adsorbent allowing for the application of magnetic separation. In this section, we review the application of Fe3O4-biosorbent composites in the adsorption of heavy metals.
3.2.1 Magnetite-chitosan Composites
Chitosan is the second most naturally available polymer after cellulose, and it contains –NH2 and OH functional groups which sequester ions through coordination forming a mesh-like cage-shaped structure [18, 64]. However, the reusability of traditional chitosan adsorbents poses a challenge; therefore, the formation of magnetic composites has been considered. The chitosan-magnetite composites faced some challenges due to low sorption capacities owing to their large sizes leading [64] to explore the formation of polyethylene modified polystyrene/Fe3O4/chitosan (PS/Fe3O4/CS-PEI) of sub-micron sizes for Cu(II) adsorption [64]. The adsorbents had an average size of 300 nm with Fe3O4 nanoparticles of ~10 nm immobilized on the surface. The composite retained its magnetic properties and was easily recovered by magnetic separation, and it was confirmed that all the constituents of the composite material were present in the adsorbent. The mechanism of Cu(II) adsorption on PS/Fe3O4/CS-PEI was attributed to the surface complexation between Cu(II) ions and N atoms from nitrogen containing groups on the adsorbent surface.
Haldorai et al. [18] demonstrated the efficiency of <30 nm Fe3O4/chitosan (Fe3O4/CS) for the adsorption of Lanthanum (La3+) ions from aqueous solutions [18]. Successful adsorption of La3+ on the adsorbent surface was confirmed by scanning electron microscopy. Response surface methodology (RSM) was applied to optimize the factors affecting the adsorption process. The Box-Behnken model (BBM) was used to determine the parameters’ effects on the adsorption efficiency. The investigated parameters were solution pH, adsorbent dosage, reaction time, and temperature. The quadratic model which explained 87% of the total variables predicted the efficient removal of La3+ for the studied parameters. The adsorption efficiency was highly dependent on the solution pH, and the optimum pH was observed to be pH 11. Reaction time and temperature had insignificant effects on La3+ adsorption efficiency. Increasing the adsorbent dosage provided more adsorption sites thereby increasing the adsorption efficiency. The Freundlich isotherm model fitted the adsorption data pointing to adsorption on heterogenous sites.
Chitosan-modified biochar was employed for the adsorption of dissolved As(V) by [33] to improve the separation ability of the chitosan/biochar composite, and chitosan was coated with magnetic Fe3O4 fluid during the composite formation [33]. Although the synthesized magnetic chitosan biochar (MCB) exhibited a lower saturation magnetization (16.67 emu g−1) compared to the magnetic fluid (67 emu g−1), it was sufficient to provide magnetic separation. The As(V) adsorption capacity of the binary and ternary composites improved threefold compared to biochar indicating the contribution of chitosan and Fe3O4 during adsorption. In the presence of competing anions, As(V) adsorption efficiency was significantly altered by the presence of PO43−, CO32−, and SO42−, while Cl and NO3− had no significant impact.
3.2.2 Magnetite-agricultural Biosorbent Composites
Plant tannin is a natural polyphenol capable of reductively adsorbing heavy metal ions, including Ag(I), Au(III), Cr(VI), and Pd(II), due to the large number of hydroxyl groups it contains [14]. Microspheres consisting of a magnetic Fe3O4 core and silica shell are favorable as the magnetic core provides for simple magnetic retrieval, while the silica shell passivates the core and provides active sites allowing for further modification. Persimmon tannin (PT) was immobilized on the Fe3O4@SiO2 spheres to create an organic–inorganic composite material and applied in the sequestration of Au(III) and Pd(II) [14]. The PT was immobilized onto the spheres via a two-step method involving the reduction of FeCl3 in ethylene glycol to form Fe3O4 and sol–gel method to prepare the silica coating [14]. Solution pH between 1 and 5 was investigated for Au(III) and Pd(II) adsorption. There was an observed increase in Au(III) adsorption with an increase in pH which was attributed to the more favorable adsorption of hydrolyzed chlorogold (AuCl3(OH)− and AuCl2(OH)2−) as compared to AuCl4− which is the dominant species below pH 3. The decreased adsorption below the point of zero charge (pHPZC) at pH 1.6 resulted from competition for the available with Cl− ions in solution. The optimum adsorption of Pd(II) was determined to be pH 3 despite the observed slight increase in adsorption capacity at pH 5 which was attributed to the formation of Pd(OH)42− whose adsorption is less favorable than that of PdCl3. The transfer and sharing electrons between the Fe3O4@SiO2@PT and metal ions were determined to be the mechanism for adsorption. Au(III) and Pd(II) adsorption onto Fe3O4@SiO2@PT proceeded via a fast adsorption phase with electrostatic adsorption and intraparticle diffusion controlling the process followed by a slower second phase resulting forms the relatively time-consuming redox process. Evidence of the redox process was obtained from the post-adsorption XPS analysis, and the spectra indicated that Au(III) was reduced to metallic gold, while Pd(II) was chelated by oxygen-containing surface groups of the adsorbent. Au(III) adsorption was overall faster than Pd(II) adsorption indicating a higher affinity of the adsorbent for Au(III). The Fe3O4@SiO2@PT composite demonstrated selective adsorption for Au(III) despite interference from other metal ions, while the selectivity for Pd(II) was lower due to competition for adsorption sites with Au(III). Higher concentrations of Cl− ions also decreased Pd(II) adsorption efficiency.
Magnetite-tea waste composite was prepared by [66] for the adsorption of Pb2+ from rainwater, groundwater, and freshwater [66]. Tea leaves contain numerous polar aliphatic and aromatic functional groups allowing it to be good adsorbent for heavy metals [55]. The magnetite-tea waste composite was prepared via co-precipitation of iron-loaded tea waste in aqueous media resulting in a sixfold increase in the tea waste surface area with a slight reduction in pore size. The prepared composite retained the superparamagnetism of Fe3O4 with saturation magnetism values of 7 and 32 emu g−1 for the composite and Fe3O4, respectively. Formation of the composite prevented Fe leaching in the studied water samples. Unmodified tea waste showed consistent higher Pb2+ adsorption efficiencies which is largely attributed to the presence of -NH2 and -COOH functional groups which sequester Pb2+ ions, while the presence of humic acid resulted in the formation of Pb-humate complexes, therefore, lowering Pb2+ concentration in groundwater samples.
The calcination effects of Fe3O4—honeycomb briquette cinders (HBC) —composite on arsenic (As(III) and As(V)) adsorption was studied by [6]. HBC are waste biomass materials from cylindrical stoves. Arsenic adsorption on the Fe3O4—HBC—composite surface proceeded via a ligand exchange process and formed inner-sphere complexes [6, 45]. Electrostatic repulsion led to decreased adsorption at higher pH ranges since the adsorbent surface became increasingly negatively charged.
3.2.3 Magnetite-cellulose Composites
Cellulose is a renewable, biodegradable, and inexpensive raw material as a result of its abundance in nature; in fact, it has often been cited as the most abundant organic raw material on the planet [56]. The challenge cellulose-based adsorbents face is difficulty in recovery, and magnetization of the cellulose adsorbents through the formation of composites with superparamagnetic magnetite nanoparticles, therefore, provides a simple solution to this challenge. Several authors have investigated the formation of composite materials with either pure cellulose or cellulosic materials for the adsorption of heavy metals from water, and some of their findings are presented in this section.
Cellulose-magnetite composites were synthesized for aqueous Cr(VI) adsorption by [53] and [56]. The nanoparticles with sizes ranging between 10 and 40 nm were attached by the bacterial cellulose (BC) nanofibrils forming a composite material with saturation magnetization values of 40 emu g−1 [53]. The composite was determined to be superparamagnetic, and the observed results were attributed to the small sizes of the composite particles. Response surface methodology (RSM) was used to better understand the influence of the factors and their interactions on Cr(VI) sequestration. Solution pH and its interaction with the adsorbate concentration were the factors that most significantly influenced the adsorption process. The optimum pH for adsorption was determined to be pH 4 from the influence of the factors on the removal efficiency of chromium. XPS analysis pointed to adsorption followed by Cr(VI) reduction to Cr(III) by a heterogeneous redox process as the adsorption mechanism.
Amino-functionalized magnetite-silica-cellulose (Fe3O4@SiO2@cellulose) nanocomposite was prepared in a multi-step synthesis by [56]. The composite preparation proceeded firstly by magnetite nanoparticle synthesis by co-precipitation followed by deposition of silica onto the Fe3O4 nanoparticles, and the Fe3O4@SiO2 particles were suspended in a cellulose solution to form Fe3O4@SiO2@cellulose composite. Amino-functionalization was achieved through grafting of glycidyl methacrylate followed by reaction with ethylenediamine. Cr(VI) adsorption studies indicated that the capacity was highly affected by the solution pH as reported in other studies [42, 53]. The adsorbent showed promising results for Cr(VI) adsorption, and reusability tests confirmed its potential to be applied in up to five cycles while retaining its efficiency. Gupta et al. [17] also reported improved adsorption capacities for Cr(III) adsorption after the formation of composites of multiwalled carbon nanotubes and magnetic iron oxide.
Other carbon-based materials that have been used in the formation of composites with magnetite nanoparticles for adsorption include activated carbon [30, 49], starch [3], wheat straw [58], palm shell [24], and pine cone [42]. From the reports, it was established that the presence of Fe3O4 nanoparticles in the composites resulted in ease of magnetic retrieval of the spent adsorbent, while the nanoscale sizes of magnetite generally improved the accessible surface areas in the adsorbents thereby improving their efficiency [17]. The functional groups from organic components of the composites contribute greatly to the sequestration of heavy metal pollutants as previously discussed.
Table 2 summarizes the adsorption capacities for some of the composites discussed in this review.
4 Conclusion
Heavy metal contamination of ground water poses challenges in environmental management, and strategies to improve the remediation efficiency are greatly desired. The adsorption process provides an alternative to complex treatment strategies. Adsorption provides ease of operation, selectivity, and wide applicability. The use of different adsorbents provides selectivity for pollutants and increased adsorption efficiency. The review established that the formation of composites of various organic materials with Fe3O4 nanoparticles provided high affinities for heavy metal pollutants and increased surface areas and magnetic separability which provided efficient remediation. The presence of interfering ions minimally affected the adsorption process owing to high affinity of Fe3O4 for the studied pollutants. Although numerous studies on magnetite and its various composites for the adsorption of pollutants from wastewater, most studies utilize synthetic wastewater and are often conducted in batch mode. Reports on the application of these materials in continuous flows reactors using real wastewater are still limited and would be crucial to the applications of these composite materials in industrial applications.
References
Alqadami AA, Naushad M, Abdalla MA, Ahamad T, Abdullah Alothman Z, Alshehri SM (2016) Synthesis and characterization of Fe3O4 @TSC nanocomposite: highly efficient removal of toxic metal ions from aqueous medium. RSC Adv 6:22679–22689. https://doi.org/10.1039/C5RA27525C
Amara D, Margel S (2011) Solventless thermal decomposition of ferrocene as a new approach for the synthesis of porous superparamagnetic and ferromagnetic composite microspheres of narrow size distribution. J Mater Chem 21:15764–15772. https://doi.org/10.1039/c1jm11842k
An B, Liang Q, Zhao D (2011) Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res 45:1961–1972. https://doi.org/10.1016/j.watres.2011.01.004
Attia TMS, Hu XL, Yin DQ (2014) Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J Exp Nanosci 9:551–560. https://doi.org/10.1080/17458080.2012.677549
Badruddoza AZM, Shawon ZBZ, Tay WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym 91:322–332. https://doi.org/10.1016/j.carbpol.2012.08.030
Baig SA, Sheng T, Sun C, Xue X, Tan L, Xu X (2014) Arsenic removal from aqueous solutions using Fe3O4-HBC composite: effect of calcination on adsorbents performance. PLoS ONE 9:e100704. https://doi.org/10.1371/journal.pone.0100704
Bhaumik M, Leswifi TY, Maity A, Srinivasu VV, Onyango MS (2011) Removal of fluoride from aqueous solution by polypyrrole/F3O4 magnetic nanocomposite. J Hazard Mater 186:150–159. https://doi.org/10.1016/j.jhazmat.2010.10.098
Bhaumik M, Maity A, Srinivasu VV, Onyango MS (2011) Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J Hazard Mater 190:381–390. https://doi.org/10.1016/j.jhazmat.2011.03.062
Bhowmick S, Chakraborty S, Mondal P, Van Renterghem W, Van den Berghe S, Roman-Ross G, Chatterjee D, Iglesias M (2014) Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism. Chem Eng J. https://doi.org/10.1016/j.cej.2013.12.049
Burks T, Avila M, Akhtar F, Göthelid M, Lansåker PCC, Toprak MSS, Muhammed M, Uheida A (2014) Studies on the adsorption of chromium(VI) onto 3-mercaptopropionic acid coated superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci 425:36–43. https://doi.org/10.1016/j.jcis.2014.03.025
Darezereshki E, Darban A, Abdollahy M, Jamshidi-Zanjani A (2018) Influence of heavy metals on the adsorption of arsenate by magnetite nanoparticles: Kinetics and thermodynamic. Environ Nanotechnol Monit Manag 10:51–62. https://doi.org/10.1016/j.enmm.2018.04.002
Dave PN, Chopda LV (2014) Application of iron oxide nanomaterials for the removal of heavy metals. J Nanotechnol
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229. https://doi.org/10.1016/j.jhazmat.2008.01.024
Fan R, Min H, Hong X, Yi Q, Liu W, Zhang Q, Luo Z (2019) Plant tannin immobilized Fe3O4@SiO2 microspheres: a novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J Hazard Mater 364:780–790. https://doi.org/10.1016/j.jhazmat.2018.05.061
Gonzalez-Moragas L, Yu SM, Murillo-Cremaes N, Laromaine A, Roig A (2015) Scale-up synthesis of iron oxide nanoparticles by microwave-assisted thermal decomposition. Chem Eng J 281:87–95. https://doi.org/10.1016/j.cej.2015.06.066
Guan X, Chang J, Chen Y, Fan H (2015) A magnetically-separable Fe3O4 nanoparticle surface grafted with polyacrylic acid for chromium(iii) removal from tannery effluents. RSC Adv 5:50126–50136. https://doi.org/10.1039/C5RA06659J
Gupta VK, Agarwal S, Saleh TA (2011) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45:2207–2212. https://doi.org/10.1016/j.watres.2011.01.012
Haldorai Y, Rengaraj A, Ryu T, Shin J, Huh YS, Han Y-K (2015) Response surface methodology for the optimization of lanthanum removal from an aqueous solution using a Fe3O4/chitosan nanocomposite. Mater Sci Eng B 195:20–29. https://doi.org/10.1016/j.mseb.2015.01.006
Horst MF, Lassalle V, Ferreira ML (2015) Nanosized magnetite in low cost materials for remediation of water polluted with toxic metals, azo- and antraquinonic dyes. Front Environ Sci Eng 9:746–769. https://doi.org/10.1007/s11783-015-0814-x
Iconaru SL, Guégan R, Popa CL, Motelica-Heino M, Ciobanu CS, Predoi D (2016) Magnetite (Fe3O4) nanoparticles as adsorbents for As and Cu removal. Appl Clay Sci 134:128–135. https://doi.org/10.1016/j.clay.2016.08.019
Ihsanullah AA, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161. https://doi.org/10.1016/j.seppur.2015.11.039
Inoue K (2011) Heavy metal toxicity. J Clin Toxicol s3:1–2. https://doi.org/10.4172/2161-0495.S3-007
Itoh H, Sugimoto T (2003) Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J Colloid Interface Sci 265:283–295. https://doi.org/10.1016/S0021-9797(03)00511-3
Jais FM, Ibrahim S, Yoon Y, Jang M (2016) Enhanced arsenate removal by lanthanum and nano-magnetite composite incorporated palm shell waste-based activated carbon. Sep Purif Technol 169:93–102. https://doi.org/10.1016/j.seppur.2016.05.034
Khalighyaan N, Hooshmand N, Razzaghi-Asl N, Zare K, Miri R (2014) Response surface strategy in the synthesis of Fe3O4 nanoparticles. Int J Nano Dimens 5:351–363
Khatoon N, Khan AH, Pathak V, Agnihotri N, Rehman M (2013) Removal of hexavalent chromium from synthetic waste water using synthetic Nano Zero Valent Iron (NZVI) as adsorbent. Int J Innov Res Sci Eng Technol 2:6140–6149
Kim HJ, Choi H, Sharma AK, Hong WG, Shin K, Song H, Kim HY, Hong YJ (2021) Recyclable aqueous metal adsorbent: synthesis and Cu(II) sorption characteristics of ternary nanocomposites of Fe3O4 nanoparticles@graphene–poly-N-phenylglycine nanofibers. J Hazard Mater 401:123283. https://doi.org/10.1016/j.jhazmat.2020.123283
Kolen’Ko YV, Bañobre-López M, Rodríguez-Abreu C, Carbó-Argibay E, Sailsman A, Piñeiro-Redondo Y, Cerqueira MF, Petrovykh DY, Kovnir K, Lebedev OI, Rivas J (2014) Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J Phys Chem C 118:8691–8701. https://doi.org/10.1021/jp500816u
Kumari M, Pittman CU, Mohan D (2015) Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J Colloid Interface Sci 442:120–132. https://doi.org/10.1016/j.jcis.2014.09.012
Kwon JH, Wilson LD, Sammynaiken R (2014) Synthesis and characterization of magnetite and activated carbon binary composites. Synth Met 197:8–17. https://doi.org/10.1016/j.synthmet.2014.08.010
Lasheen MR, El-Sherif IY, Sabry DY, El-Wakeel ST, El-Shahat MF (2016) Adsorption of heavy metals from aqueous solution by magnetite nanoparticles and magnetite-kaolinite nanocomposite: equilibrium, isotherm and kinetic study. Desalin Water Treat 57:17421–17429. https://doi.org/10.1080/19443994.2015.1085446
Latif A, Sheng D, Sun K, Si Y, Azeem M, Abbas A, Bilal M (2020) Remediation of heavy metals polluted environment using Fe-based nanoparticles: mechanisms, influencing factors, and environmental implications. Environ Pollut 264:114728. https://doi.org/10.1016/j.envpol.2020.114728
Liu S, Huang B, Chai L, Liu Y, Zeng G, Wang X, Zeng W, Shang M, Deng J, Zhou Z (2017) Enhancement of As(V) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv 7:10891–10900. https://doi.org/10.1039/C6RA27341F
Luther S, Borgfeld N, Kim J, Parsons JG (2012) Removal of arsenic from aqueous solution: a study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchem J 101:30–36. https://doi.org/10.1016/j.microc.2011.10.001
Mahdavian AR, Mirrahimi MA-S (2010) Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem Eng J 159:264–271. https://doi.org/10.1016/j.cej.2010.02.041
Mayo JTT, Yavuz C, Yean S, Cong L, Shipley H, Yu W, Falkner J, Kan A, Tomson M, Colvin VLL, Shiple H, Yu W, Falkner J, Kan A, Tomson M, Colvin VLL (2007) The effect of nanocrystalline magnetite size on arsenic removal. Sci Technol Adv Mater 8:71–75. https://doi.org/10.1016/j.stam.2006.10.005
Minitha CR, Suresh R, Maity UK, Haldorai Y, Subramaniam V, Manoravi P, Joseph M, Rajendra Kumar RT (2018) Magnetite nanoparticle decorated reduced graphene oxide composite as an efficient and recoverable adsorbent for the removal of Cesium and strontium ions. Ind Eng Chem Res 57:1225–1232. https://doi.org/10.1021/acs.iecr.7b05340
Monárrez-Cordero B, Amézaga-Madrid P, Antúnez-Flores W, Leyva-Porras C, Pizá-Ruiz P, Miki-Yoshida M (2014) Highly efficient removal of arsenic metal ions with high superficial area hollow magnetite nanoparticles synthetized by AACVD method. J Alloys Compd 586:S520–S525. https://doi.org/10.1016/j.jallcom.2012.12.073
Ofomaja AE, Naidoo EB, Modise SJ (2009) Removal of copper(II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J Hazard Mater 168:909–917. https://doi.org/10.1016/j.jhazmat.2009.02.106
Okoli CP, Ofomaja AE (2019) Development of sustainable magnetic polyurethane polymer nanocomposite for abatement of tetracycline antibiotics aqueous pollution: response surface methodology and adsorption dynamics. J Clean Prod. https://doi.org/10.1016/J.JCLEPRO.2019.01.157
Ouma ILA, Mushonga P, Madiehe AM, Meyer M, Dejene FB, Onani MO (2014) Synthesis, optical and morphological characterization of MPA-capped PbSe nanocrystals. Phys B Condens Matter 439:130–132. https://doi.org/10.1016/j.physb.2013.10.057
Ouma ILA, Naidoo EB, Ofomaja AE (2017) Iron oxide nanoparticles stabilized by lignocellulosic waste as green adsorbent for Cr(VI) removal from wastewater. Eur Phys J Appl Phys 79:30401. https://doi.org/10.1051/epjap/2017160406
Ouma ILA, Naidoo EB, Ofomaja AE (2018) Thermodynamic, kinetic and spectroscopic investigation of arsenite adsorption mechanism on pine cone-magnetite composite. J Environ Chem Eng 6:5409–5419. https://doi.org/10.1016/j.jece.2018.08.035
Ouma ILA, Naidoo EB, Ofomaja AE (2019) An insight into the adsorption mechanism of hexavalent chromium onto magnetic pine cone powder. Chemistry for a clean and healthy planet. Springer International Publishing, Cham, pp 185–195
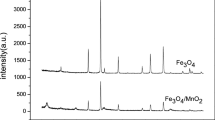
Ouma L, Ofomaja A (2020) Probing the interaction effects of metal ions in Mnx Fe(3–x)O4 on arsenite oxidation and adsorption. RSC Adv 10:2812–2822. https://doi.org/10.1039/C9RA09543H
Peng X, Xu F, Zhang W, Wang J, Zeng C, Niu M, Chmielewská E (2014) Magnetic Fe3O4 @ silica–xanthan gum composites for aqueous removal and recovery of Pb2+. Colloids Surfaces A Physicochem Eng Asp 443:27–36. https://doi.org/10.1016/j.colsurfa.2013.10.062
Pholosi A, Ofomaja AE, Naidoo EB (2013) Effect of chemical extractants on the biosorptive properties of pine cone powder: influence on lead(II) removal mechanism. J Saudi Chem Soc 17:77–86. https://doi.org/10.1016/j.jscs.2011.10.017
Ray PZ, Shipley HJ (2015) Inorganic nano-adsorbents for the removal of heavy metals and arsenic: a review. RSC Adv 5:29885–29907. https://doi.org/10.1039/C5RA02714D
Salam MA, El-Shishtawy RM, Obaid AY (2014) Synthesis of magnetic multi-walled carbon nanotubes/magnetite/chitin magnetic nanocomposite for the removal of Rose Bengal from real and model solution. J Ind Eng Chem 20:3559–3567. https://doi.org/10.1016/j.jiec.2013.12.049
Shafique U, Ijaz A, Salman M, Zaman WU, Jamil N, Rehman R, Javaid A (2012) Removal of arsenic from water using pine leaves. J Taiwan Inst Chem Eng 43:256–263. https://doi.org/10.1016/j.jtice.2011.10.006
Shaker S, Zafarian S, Chakra S, Rao VK (2013) Preparation and characterization of magnetite nanoparticles. Int J Innov Res Sci Eng Technol 2:2969–2973
Sharma G, Jeevanandam P (2013) Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv 3:189–200. https://doi.org/10.1039/c2ra22004k
Stoica-Guzun A, Stroescu M, Jinga SI, Mihalache N, Botez A, Matei C, Berger D, Damian CM, Ionita V (2016) Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int J Biol Macromol 91:1062–1072. https://doi.org/10.1016/j.ijbiomac.2016.06.070
Stötzel C, Kurland HD, Grabow J, Müller FA (2015) Gas phase condensation of superparamagnetic iron oxide-silica nanoparticles—control of the intraparticle phase distribution. Nanoscale 7:7734–7744. https://doi.org/10.1039/c5nr00845j
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: a review. Bioresour Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Sun X, Yang L, Li Q, Zhao J, Li X, Wang X, Liu H (2014) Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chem Eng J 241:175–183. https://doi.org/10.1016/j.cej.2013.12.051
Teja AS, Koh P-Y (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater 55:22–45. https://doi.org/10.1016/j.pcrysgrow.2008.08.003
Tian Y, Wu M, Lin X, Huang P, Huang Y (2011) Synthesis of magnetic wheat straw for arsenic adsorption. J Hazard Mater 193:10–16. https://doi.org/10.1016/j.jhazmat.2011.04.093
US EPA (2009) National primary drinking water regulations
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Warner CL, Addleman RS, Cinson AD, Droubay TC, Engelhard MH, Nash MA, Yantasee W, Warner MG (2010) High-performance, superparamagnetic, nanoparticle-based heavy metal sorbents for removal of contaminants from natural waters. Chemsuschem 3:749–757. https://doi.org/10.1002/cssc.201000027
Warner CL, Chouyyok W, Mackie KE, Neiner D, Saraf LV, Droubay TC, Warner MG, Addleman RS (2012) Manganese doping of magnetic iron oxide nanoparticles: tailoring surface reactivity for a regenerable heavy metal sorbent. Langmuir 28:3931–3937. https://doi.org/10.1021/la2042235
Wu L-K, Wu H, Zhang H, Cao H, Hou G, Tang Y, Zheng G-Q (2018) Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem Eng J 334:1808–1819. https://doi.org/10.1016/j.cej.2017.11.096
Xiao C, Liu X, Mao S, Zhang L, Lu J (2017) Polystyrene/Fe3O4/chitosan magnetic composites for the efficient and recyclable adsorption of Cu(II) ions. Appl Surf Sci 394:378–385. https://doi.org/10.1016/j.apsusc.2016.10.116
Yan W, Lien H-L, Koel BE, Zhang W (2013) Iron nanoparticles for environmental clean-up: recent developments and future outlook. Environ Sci Process Impacts 15:63. https://doi.org/10.1039/c2em30691c
Yeo SY, Choi S, Dien V, Sow-Peh YK, Qi G, Hatton TA, Doyle PS, Thio BJR (2013) Using magnetically responsive tea waste to remove lead in waters under environmentally relevant conditions. PLoS ONE 8:e66648. https://doi.org/10.1371/journal.pone.0066648
Zabochnicka-Światek M, Krzywonos M (2014) Potentials of biosorption and bioaccumulation processes for heavy metal removal. Polish J Environ Stud 23:551–561
Zarnegar Z, Safari J (2017) Modified chemical coprecipitation of magnetic magnetite nanoparticles using linear-dendritic copolymers. Green Chem Lett Rev 10:235–240. https://doi.org/10.1080/17518253.2017.1358769
Zhang J, Liu W, Zhang M, Zhang X, Niu W, Gao M, Wang X, Du J, Zhang R, Xu Y (2017) Oxygen pressure-tuned epitaxy and magnetic properties of magnetite thin films. J Magn Magn Mater 432:472–476. https://doi.org/10.1016/j.jmmm.2017.02.032
Zhou Z, Liu Y, Liu S, Liu H, Zeng G, Tan X (2017) Sorption performance and mechanisms of arsenic (V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231. https://doi.org/10.1016/j.cej.2016.12.113
Acknowledgements
The authors acknowledge South Africa National Research Foundation (NRF) and the University of the Western Cape for funding this work.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ouma, L., Onani, M. (2022). Sequestration of Heavy Metal Pollutants by Fe3O4-based Composites. In: Lichtfouse, E., Muthu, S.S., Khadir, A. (eds) Inorganic-Organic Composites for Water and Wastewater Treatment. Environmental Footprints and Eco-design of Products and Processes. Springer, Singapore. https://doi.org/10.1007/978-981-16-5916-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-5916-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5915-7
Online ISBN: 978-981-16-5916-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)