Abstract
The extensive spatial variabilities in the reservoir conditions including lithology, porosity, permeability, crude oil composition and state of stress (oxygen and nutrient availability, water content) lead to heterogeneity in oil reservoir temperature, pressure, water saturation, pH, salinity and formation water chemistry. These aspects are major factors governing each reservoir's significant microbial community composition along with their relative abundances. Such heterogeneous subsurface environmental conditions assist in maintaining the distinctive microbial population in each environment, constructing a relatively stable local community arrangement over a prolonged period. Continuous researches have established microbial sustainability in the deep subsurface to be extremely divergent and broadly dispersed. The foremost expertise to extract the genetic and taxonomic information from the microbes existing in an environmental niche is DNA sequencing. However, the constraints of DNA sequencing could be overcome by modern innovations in sequencing knowledge (i.e. high‐throughput next‐generation sequencing). The complete microbial population of numerous environmental samples can nowadays be sequenced instantaneously with high precision in a single sequencing run. A number of such advanced microbial strategies have been discussed for detecting indigenous microbes. The established reservoir conditions have also been detailed country-wise for exploring and accomplishing success in-situ MEOR.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The implementation of the in-situ MEOR procedure is dependent on the various intrinsic and extrinsic factors including the type of the reservoirs, appropriate screening of prospective microbial community, the viable functioning of the microbes at the internal reservoir environment, amount of metabolites produced and mobilizing residual oil along with cost-effective aspects (Jang et al. 1983; Zhang et al. 2020b). Worldwide oil reservoirs are full of diversity in terms of temperature, salinity, traits of crude oil as well as the oil–water proportion of the reservoir. Because of the reservoir heterogeneity, the occurrence of microbes variation is obvious compared to the native population to the individual oil wells (Phukan et al. 2019; Rabiei et al. 2013; Rathi et al. 2018; Saha et al. 2018a, b).
Oil reservoirs host numerous indigenous microorganisms which can endure high pressure, salinity and temperature conditions. In-situ MEOR encompasses stimulating inherent reservoir microorganisms or inoculating specifically screened microbes into the oil reserve to induce certain metabolic activities. This promotes the biosynthesis of different metabolites including biosurfactants to improve oil retrieval. MEOR utilizes microbes or their metabolites for residual oil repossession with low permeability or high viscosity. Microbes utilize two types of nutrients: subsurface crude oil or additives to the injected fluids (Safdel et al. 2017; Wood 2019). However, the incorporation of biosurfactant-synthesizing bacteria and the necessary supplements into the reservoir have been mainly examined in the MEOR method. The injected strains need to survive, nurture and be metabolically dynamic in the extreme internal reservoir environment of elevated temperature and pressure. In this regard, indigenous bacteria isolated from reservoir soil, formation water or crude oil sample are assumed to be the ideal candidates over the extremophiles from other sites (Miyazaki et al. 2012).
The sustainability and success of the reservoir ecosystems are controlled by complex and cooperative microbial activity. Microbes are the most diverse group of living organisms on the planet; hence it is very important to understand both diversity and metabolic proficiencies in a specified range of environmental factors for illustrating their potential for recovery of residual oils. Even though microbes have been reported to prefer the oxygen-rich environment theoretically, advanced investigations have revealed some exclusive and effective biodegradation pathways are followed by microbes under sub-oxic (i.e., extremely low dissolved oxygen; occasionally coexisting with sulfides) environments inside the reservoirs.
The holistic procedure involves the selection of strains that would thrive under the reservoir conditions and mobilize the crude oil by synthesizing biosurfactants or by biotransformation. This requires many efforts for the identification, incubation, and potential assessment of the strains at the lab scale. The next step involves properly formulation of injection bio-slug, which facilitates the exogenous bacteria to effectively build a colony inside the reservoir. Whenever nutrients are introduced, they should be adequate to enhance the long-term metabolic activity of the microbes. Finally, the oil wells, relevant accessories and other amenities should be cautiously equipped before MEOR operations. These additional facilities need to be treated with steam for removing debris and undesirable microbes to ensure the injection of only the desired microbial species into the reservoir (Gao 2018).
This chapter lists key reservoir environmental conditions including lithology, porosity, permeability, crude oil composition, temperature, pressure, pH and salinity and their influence on the microbial community. Different culture-dependent and independent microbiological approaches for detecting inherent microorganisms are summarized. Various microbial populations isolated from different locations worldwide oil reservoirs and explored for their suitability for MEOR applications are detailed. Important reservoir environmental conditions for the screening and conducting MEOR trials are outlined.
2 Influence of Reservoir Environmental Conditions
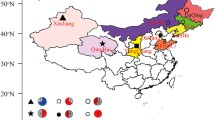
Oil reservoirs are one of the main modules of the extensive biosphere, where intrinsic microbial inhabitants have survived over a prolonged period (Gao et al. 2018; Li et al. 2017). The activity of the microbes is influenced by environmental as well as geological parameters of the reservoirs like lithological composition, reservoir type, internal temperature, porosity, permeability, crude oil gravity and fluid attributes. These factors directly or indirectly control microbial growth, movement and metabolism (Dhanarajan et al. 2017; Hong et al. 2019; Sharma et al. 2019a, b; Verma et al. 2020). Temperature is one of the prime significant parameters, which governs the bacterial growth and the functionalities of the strains (Aditama et al. 2017). Porosity and permeability mainly control the migration of the microbes or internally produced metabolites and very small pores leave a negative influence on this progression (Gabitto and Barrufet 2005). Salinity is one of the most imperative influencer assuming saline soil and fluid samples. At elevated salinity, biological activity is reduced due to less cell functioning and plasmolysis of microbes, which affected the survival of most microorganisms (Hadibarata et al. 2017). Besides these, pH, pressure and crude oil viscosity furthermore influence the microbes for its application in MEOR practice drastically (Bachmann et al. 2014; Kamari et al. 2014; Sari et al. 2019). The environmental parameters as well as the reservoir conditions are pictorially represented in Fig. 1.
2.1 Properties of Crude Oil
Petroleum is a naturally occurring, complex organic mixture, comprising numerous components which can be mainly classified into the following types: alkanes (paraffins), cycloalkanes (naphthenes), aromatics, and more complex asphaltenes and resins. Crude oil is usually categorized into bitumens, heavy oils, medium oil and light oils based on American Petroleum Institute (API) gravity (Santos et al. 2014). Heavy crude oils are well-defined as liquid petroleum possessing 10–22.3° API gravity or >100 mPas viscosity in normal reservoir environments. Medium crude oil possesses API gravity of 22.3–31.1° and 33.4–100 mPas viscosity; light oil has an API gravity of >31° and viscosity <33.4 mPas (Zhang et al. 2020a). The sequential degradation usually occurs following the trend of hydrocarbon susceptibility to bacterial degradation from light to heavy chain contents. The degradation rates of aliphatic hydrocarbons were established to be higher than aromatic hydrocarbons, which can be expressed in this order: aliphatic (linear > branched) > light aromatic (mono aromatics) > cycloalkane > heavy aromatic (substituted or poly aromatics) > asphaltenes and resins (Sharma and Pandey 2020; Van Hamme et al. 2003).
MEOR technology was implemented mostly in the light conventional oil reserves largely in the USA, Russia and China from 1980 to 2010 (Belyaev et al. 2004; Youssef et al. 2009). But relatively less MEOR work has been done in heavy crude oil (density of 920–1000 kg/m3) reservoirs. The difference between heavy crude oil and traditional conventional oil are mainly high density, more complex composition possessing increased asphaltenes, resins, sulfur, nitrogen, and metal-containing compounds as well as low gas content and low hydrogen/carbon ratio. Heavy oil reservoirs can store about seven times more than conventional oil reservoirs (Leon and Kumar 2005). The largest heavy oil reservoir is located at the Orinoco oil belt of Venezuela. There are a number of heavy oil fields in Oman, where crude oil recovery is complex and expensive because of its high viscosity (Leon and Kumar 2005). The viscosity reduction of heavy oil facilitates improving oil retrieval from a reservoir due to improving the flow behaviour and minimizing the pressure drop (Santos et al. 2014). Different physical methods like heating and dilution are employed for this purpose (Santos et al. 2014).
The reduction in viscosity is also achieved majorly by two microbial mechanisms: either bioconversion of heavy into light oil fractions or by the production of microbial metabolites (e.g., biosurfactants) that modify the physical attributes of the oil, such as lowering its IFT. Several microbial isolates and produced enzymes contribute to the degradation of saturate and aromatic aerobically or anaerobically (Mbadinga et al. 2011; Nie et al. 2014; Rojo 2009; Widdel and Rabus 2001). Indigenous microflora can improve the fluidity of heavy crude oil by altering its high viscosity and subsequently forming lighter oil components (Pineda-Flores and Mesta-Howard 2001). Oil viscosity can also be reduced by produced CO2 gas (a byproduct of microbial metabolic activity), which creates pressure within the reservoir and push the crude oil upwards by fractional degradation of the large molecular components of crude oil. The produced biomass gathers between the oil and the well-rock surface and displaces the oil, improving the mobility for easier recovery from the well (Marchant and Banat 2012; Safdel et al. 2017). However, there are very few reported literature that addressed the microbial systems with the capability of biological degradation or biotransforming heavy oil fractions (asphaltenes) and in turn improve the oil recovery by reducing the oil viscosity.
2.2 Rock Lithology
The physical characteristics of rocks regulate the occurrence of indigenous microbes and the adsorption behaviour of metabolites, i.e., surfactants. Microorganisms are classified depending on their occurrence in the reservoir rock and their role in the biogenic weathering of the reservoirs. Endolithic microbes are alive inside the rock or in the pores between mineral grains, while epiliths survive firmly on the rock surfaces. Chasmoendoliths accumulate in rock fractures or excavated formations. Euendoliths accumulate on carbonate surfaces by penetrating rock strata via dissolution and form the borings through active digging (Hoppert et al. 2004). The biosurfactant quantity essential for recovering oil is dependent on the adsorption capacity on the rock surface. Various rock formations have dissimilar absorption values. Generally, sandstone can adsorb 0.1–1 mg surfactant/g of rock, however, this also depends on the initial concentration of the surfactant. In the sandstone reservoirs, the anionic surfactant flooding is more competent than the carbonate reservoirs due to the high adsorption capacity of carbonates (Nikolova and Gutierrez 2020). However, in the carbonate reservoirs, the wettability alteration (from oil-wet state to water-wet state) plays a more prominent role in oil recovery. Water-wet state is comparatively favourable for improved oil recovery as oils in carbonate rocks are concentrated in bigger pores, which can be easily accessed during flooding (Kowalewski et al. 2006).
2.3 Reservoir Temperature and Pressure
The highest reservoir temperature has been projected to be about 137 °C at a depth of 4 km by the researchers and the surfactants need to be functional at such a high temperature (Kargarpour 2017). The reservoir depth is not a restrictive parameter for microbial flooding as long as it is complying with the reservoir temperature limit (Sheng 2013). There is an optimal temperature for microbial growth, which is adversely affected when the optimal temperature limit is surpassed (Chen et al. 2001). Arthrobacter paraffineus, thermophilic Bacillus and Pseudomonas strain DSM-2874 are comparatively temperature-sensitive. The composition of the produced biosurfactant is also controlled by temperature (Roy 2017). Temperature increase results in better solubility of hydrophobic components, decline viscosity, enhances long-chain n-alkanes diffusion and transfer from solid phase to fluid phase. The pressure in oil reservoirs varies in the range of 10–100 MPa. The effects of pressure on microbes are also systematically connected with temperature as elevated pressures in natural environments are related to temperature differences (Marshall 2008). Thus, pressure also affects microbial survival and metabolite production. The microbial decay rate was related to the pressure and exposure time (Jeong et al. 2021).
2.4 Environmental pH
The growth profile of the microbes along with their produced biometabolites is controlled by the pH of the environment. Biosurfactants production from different strains like Bacillus sp., Pseudomonas sp., T. bombicola, and N. corynebacteroides was found to be dependent on pH (Datta et al. 2020; Sharma and Pandey 2020). pH value influences the interfacial adsorption properties as well as CMC, ST, emulsifying activity and coefficient of elasticity of rhamnolipids (Özdemir et al. 2004). The carboxyl group that is responsible for the anionic nature of rhamnolipid molecules is largely influenced by the pH. The dissociation of the Rhamnosyl structure takes place at higher pH values (pH > 11) and leads to different behaviours. The alkaline conditions enhance rhamnolipid solubilization, and therefore cell permeability is increased, which significantly escalates the levels of extracellular carbohydrates, proteins and metabolites (Özdemir et al. 2004). In fact, the emulsifying property is greatly influenced by the pH of surfactin and its emulsions are stabilized quite well above a pH value of 7.4. Approximately, 98% oil emulsification ratio was achieved at pH 11; whereas this emulsification property got quickly and entirely vanished when pH was decreased below 3. This revealed the significance of pH in selecting the suitable reservoir to conduct biosurfactant-mediated EOR (Long et al. 2017).
2.5 Fluid Salinity
The salinity of the injected fluid must be close to the reservoir water salinity, so that the lower most IFT between oil and fluid could be obtained at the optimal salt concentration, which is dependent on several factors including types of crude oil and surfactants. For most of the surfactants, the optimal salinity is not too high (Sheng 2015). In a study, the significance of salt concentration was explained where it was stated that 41% crude oil degradation could be achieved after 4 months of incubation in soil samples without the addition of salt, while only 12% crude oil biodegradation was observed after 4 months incubation in the same soil samples when excess salt (50 g/L NaCl) was supplemented (Minai-Tehrani et al. 2006). However, the effect of salinity is reported to decrease the oil/water IFT values (Rostami et al. 2019).
2.6 Permeability
Low-permeability reservoirs are projected to possess decreasing production in comparison with high-permeability reservoirs. Thus permeability improvement is expected to result in improved oil reclamation. Organic acids (acetate, butyrate) production by bacteria (e.g., Clostridium, Enterobacter aerogenes) in in-situ mode can dissolve formation rocks and expose additional pore volume, specifically in carbonate reserves, and consequently, the permeability and fluids flow are improved (Van Hamme et al. 2003). Imbibition of surfactant, brine and surfactant was explored as an important means for the better recovery of oil from low permeability reservoirs (Xu et al. 2019).
3 Microbiological Approaches for Detecting Inherent Microorganisms
Microbial activity is a universal and significant part of the petroleum reserve systems; nevertheless, present information about the composition, assortment of active microflora in offshore petroleum reservoirs remains inadequate, which is vital to understand metabolic activities to employ suitable exploration strategies in the oil reservoirs. The variation of the microbial community of production wells is very diverse and at the same time complex. There are an extensive collection of microbes explored in MEOR, which can be classified broadly into two distinct classes (Youssef et al. 2009). The first one is autochthonous or indigenous microbes that already exist in oil reserves, and the second one is allochthonous or exogenous microbes, which are developed purposely by injecting into reservoirs. Exogenous microbes imply suitable microflora screened by employing reservoir-like conditions to increase oil reclamation by its proliferation and production of metabolites (Cheng et al. 2006; She et al. 2019).
Conventionally, culture-dependent techniques have been utilized to enrich and isolate microbial species from petroleum reserves (Singh et al. 2014). The cultivation-based approach assists in the improvement of the physiological potentials of some indigenous microbes that perform MEOR processes. However, this is not the only approach that reflects the actual environmental microbial diversity. As huge proportions of environmental bacteria cannot be cultured, the culture-independent approach is preferred during analyzing microbial population in reservoirs (Bordoloi and Konwar 2008; Brown and Vadie 1997; Wang et al. 2014).
As only a small portion of microbes could be grown using culture-dependent techniques so it is quite difficult to evaluate the inclusive reservoir microflora composition. In order to get rid of these cultivation restrictions, culture-independent methods have been established for characterizing the complex microbial population. Advanced microbiological and molecular strategies for detecting inherent microbial populations are pictorially described in Fig. 2. Particularly, some molecular techniques such as DNA extraction, polymerase chain reaction (PCR)-based approaches, 16S rRNA and functional genes sequencing and next-generation sequencing (NGS) have improved overall proficiency for identifying microbes in petroleum reserves. RNA-based sequencing methods could also be another substitution for analyzing active and inactive species. The internal microbial community could be identified by complex high-tech biotechnological culture-independent techniques (She et al. 2019) including terminal restriction fragment length polymorphism (T-RFLP), denaturing gradient gel electrophoresis (DGGE) (Xingbiao et al. 2015), gene bank (Liu et al. 2010), clone library and most probable number (MPN) (Cheng et al. 2006). Thus, metagenomics or microbial community investigation using whole metagenome-based strategy provides suitable insight into the inherent microbiome structurally and functionally. Therefore, expansion from culture-based strategy to metagenomics, metatranscriptomics or single-cell genomics have commenced a paradigm shift in perceptive of microbial assortment and genomic collection of intrinsic microbes (Gupta et al. 2019). Advanced metagenomics strategies in combination with metaproteomics, metabolomics, stable isotope probing and single-cell sequencing apprise innovative viewpoint in the microbial ecophysiology of the subsurface ecosystems (Ismail et al. 2017).
Advanced microbiological approaches for detecting indigenous microbial communities [Adapted from (Philips et al. 2020)]
4 Microbial Diversity in Worldwide Oil Reservoirs
Hydrocarbon degrading microorganisms are termed as hydrocarbonoclastic. The indigenous hydrocarbonoclastic community of a reservoir is anticipated to possess specific benefits over other microorganisms or exogenous microbial consortiums due to better adaptability of the former in the internal reservoir conditions. The primary adaptations include their utilization of hydrocarbons, extreme endurance in high temperature, salinity and pressure, as well as their metabolic activity anaerobically.
The common hydrocarbon-degrading microbial genera are: Bacillus, Pseudomonas, Acinetobacter, Micrococcus luteus, Nocardia, Rhodococcus, Streptomyces, Vibrio, Xanthomonas maltophilia (Mariano et al. 2007). Acinetobacter, Burkholderia, Paraburkholderia and Luteibacter mainly contributed to petroleum hydrocarbons degradation under optimal conditions (Cui et al. 2020). However, the proficient oil-byproduct degrading bacterial genera are: Acidovorans, Acinetobacter, Agrobacterium, Alcaligenes, Aeromonas, Arthrobacter, Beijemickia, Burkholderia, Comomonas, Corynebacterium, Cycloclasticus, Flavobacterium, Gordonia, Microbacterium, Moraxella, Mycobacterium, Neptunomonas, Paracoccus, Pasteurella, Polaromonas, Ralstonia, Sphingomonas and Stenotrophomonas (Tonini et al. 2010). Marine microbes, Alcanivorax, Oleiphilus, Oleispira, Thalassolituus, Planomicrobium alkanoclasticum MAE2 uses a variety of saturated hydrocarbons, however, Cycloclasticus could utilize a range of polycyclic aromatic hydrocarbons (Head et al. 2006). Some microbes from the marine environment such as Pseudomonas, Pseudoalteromonas, Marinomonas, Oceanobacillus, Halomonas, Rhodococcus, Sphingomonas and Cobetia have been designated as biosurfactant synthesizers (Schultz and Rosado 2020).
Anaerobic microbes are more widespread species in oil reservoirs (Fardeau et al. 2004). Bacillus, Pseudomonas, Mycobacterium, Micrococcus and Rhodococcus can oxidize hydrocarbon present in oil reserves (Saravanan et al. 2020). Methanogenesis is an obligatory anaerobic biological mechanism prevalent in oil reservoirs (Zhou et al. 2020). Methanogenic pathways could be categorized into hydrogenotrophic, acetoclastic and methylotrophic methanogenesis based on the utilizing substrate. Methane could be produced by hydrogenotrophic (Methanocalculus, Methanoplanus, Methanothermobacter and Methanolinea), acetoclastic (Methanosaeta), methylotrophic (Candidatus Methanomethylicus and Methanomassiliicoccaceae, genera Methanosarcina, Methanhalophiluus and Methermicoccus) and other (Candidatus Methanofastidiosum) methanogens (Meslé et al. 2013; Youssef et al. 2009; Zhou et al. 2020). The phyla, namely Acidobacteria, Atribacteria, Chloroflexi, Fusobacteria, Nitrospira, Planctomycetes, Spirochaetes, Synergistetes and Thermodesulfobacteria were found in much less amount in oil reserves. Other less commonly identified archaeal diversity comprised of Desulfurococcales, Fervidicoccales, Haloferacales, Methanocellales, Methanomassiliicoccales, Thermoproteales, Thermoplasmatales and Sulfolobales (Li et al. 2017).
Three thermotolerant hydrocarbonoclastic strains of Bacillus, Geobacillus, and Petrobacter were isolated from high-temperature oilfield and described to endure 55 °C anaerobically and degrade hydrocarbon (Shibulal et al. 2014; Wang et al. 2008), signifying their appropriateness to participate MEOR process. Mesophilic genera such as Arcobacter, Clostridium, Desulfuromonas, Geobacter, Marinobacter and Pseudomonas could also be traced in large propensity in comparatively lower temperature oil fields (Hubert et al. 2012; Okpala et al. 2017; Zhang et al. 2012).
The primary bacterial and archaeal lineages detected in oil reservoirs to carry out in-situ MEOR are shown in Fig. 3. Different phylum, class and family of bacterial and archaeal lineages can be found in detail elsewhere (Rosenberg et al. 2014). Proteobacteria are a major phylum of Gram-negative bacteria and are distributed into nine different classes including alpha to zeta-proteobacteria. The phylum Deferribacteres are Gram-negative anaerobic bacteria. The phylum Bacteroidetes are non-spore-forming Gram-negative and facultative bacteria commonly spread in the environment. The family Firmicutes are a phylum of Gram-positive bacteria. Actinobacteria are a group of unicellular filamentous Gram-positive bacteria and occur in terrestrial or aquatic environments. The phylum Thermotogae include Gram-negative anaerobic and thermophilic/hyperthermophilic bacteria. Archaea are also unicellular organisms and the most ancient. Many archaea are extremophiles, but a few mesophiles.
Reservoir inherent hydrocarbon utilizing microbial population. Prepared based on the information from the reference (Youssef et al. 2009)
4.1 Various Microbial Populations in Worldwide Oil Reservoirs
Oil reserves are distributed across the globe and mainly concentrated in Saudi Arabia, Venezuela, Russia and Iran (Tong et al. 2018). Similarly, various microbes have been isolated from different locations worldwide and explored for their suitability for MEOR applications. A few of the potential isolates from oil reservoirs are discussed here.
Molecular approaches analysis of formation water of a high-temperature oil reservoir in Russia (Samotlor) showed the presence of certain genus such as Thermoanaerobacter, Thermotoga, Geobacillus, Petrotoga, Thermococcus and Thermosipho including some previously unidentified taxa Desulfurobacterium. Thermovibrio cluster of Desulfurococcus and Thermus genera were also identified via targeted oligonucleotide microchip analysis (Bonch-Osmolovskaya et al. 2003). Physicochemical and biotechnological properties of formation waters of low-temperature heavy oil reservoirs of Russia (particularly Chernoozerskoe, Severo- Bogemskoe and Yuzhno-Suncheleevskoe oilfields) harboring limited microbial species, as well as the other (Vostochno-Anzirskoe and Cheremukhovskoe) oilfields comprising comparatively diverse microbial community were studied (Nazina et al. 2017). Sulphate reduction and methanogenesis were not observed in the samples from water-flooded sections. Metagenomics study and 16S rRNA sequencing of microbial gene fragments in the population of injection fluid displayed the presence in the order of descending quantity consisted of Proteobacteria, Betaproteobacteria, Alphaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Epsilonproteobacteria, as well as some Firmicutes, Bacteroidetes, and Archaea. DGGE examination of mcrA genes in the microbial population of injection fluid showed the occurrence of methanogens genera including Methanobacterium, Methanothrix, Methanospirillum, Methanoregula, Methanosarcina, and Methanoculleus, along with undetected Thermoplasmata. Pure strains of Cellulomonas, Gordonia, Pseudomonas, Rhodococcus genera accomplished biosurfactant synthesis by supplementing with heavy oil. Fermentative bacteria supplemented with enrichment media containing sacchariferous substrates produced noteworthy amounts of volatile organic acids (VFA) (acetic, butyric and propionic), which were dissolved in the carbonates of oil-bearing rock proficiently (Nazina et al. 2017).
In the Tatariya carbonate oil field (Russia), Clostridium increased the oil production by 28–46% (Sakthipriya et al. 2017). The low-temperature heavy oil fields (Russia) accommodated a microbial community that was competent enough to produce oil-displacing metabolic compounds. Aerobic strains Rhodococcus erythropolis HO-KS22 and Gordonia amicalis 6–1 isolated from Russian oil reserves reported to oxidize heavy crude oil to produce biosurfactants which considerably reduced ST and IFT as well as exhibited prospects for paraffin degradation, MEOR and the hydrocarbon bioremediation (Nazina et al. 2020).
B. subtilis, P. aeruginosa, and Bacillus cereus isolated from oil-contaminated sites of Iran could withstand extreme reservoir surroundings (120 °C, pH 4, 25 g/L salinity) and ST was decreased from 72 to about 26 mN/m because of the production of biosurfactants (Amani et al. 2010; Bachmann et al. 2014). Bacillus mojavensis were isolated in Masjed-I Soleyman carbonate field (Iran) having a pressure of 3–4 MPa. The produced biosurfactant reduced the ST to 26.7 mN/m and a temperature of 42 °C (Ghojavand et al. 2012). Bacillus licheniformis was isolated from the Zilaei oil reservoir in southwest Iran, which grew optimally and produce biosurfactant at 50 °C which reduced the ST from 72 to 23.8 mN/m along with the IFT from 36.8 to 0.93 mN/m (Daryasafar et al. 2016). This same species was further isolated from the Niage field of the Egyptian Western Desert, which exhibited considerably good surface properties including a highest emulsifying index of 96% and ST reduction to 36 mN/m when incubated for 72 h at 45 °C (El-Sheshtawy et al. 2015).
Among several isolated spore-forming bacteria from soil samples of Oman oil fields, an autochthonous strain, Paenibacillus ehimensis BS1 was reported to improve heavy oil recovery due to a great endurance in stressful conditions with long dormancy period, in high thermal condition, drying and presence of acid. The isolate exhibited utmost growth when supplemented with elevated heavy oil and incubated for four days. Biotransformation of heavy crude oil (API 4.57°) to light aliphatic and aromatic constituents along with its prospects in EOR was accessed aerobically and anaerobically (Shibulal et al. 2017). Five dominant identified genera of Suwaihat oilfield, Oman were Halomonas, Cenothrix, Methylobacter, Burkholderia and Crenarchaeote. The most commonly detected bacterial genera were Rhodococcus followed by Petrotoga, Diaphorobacter, Thermotoga and Actinobacterium in Wafra Oil Wells, Kuwait, identified by gene sequencer (culture-independent technique) (Al-Wahaibi et al. 2013).
Marinobacter hydrocarbonoclasticus was isolated from the upper segment of a Vietnamese oil well, which grew on high salinity of 5% NaCl. It could biodegrade n-hexadecane, pristane along with some other crude oil constituents (Huu et al. 1999). The microbiological aspects along with functional gene analysis were predicted from metagenomics 16S rRNA gene data of the microbial population from onshore Mae Soon Reservoir, Thailand, which signified the prospects of the native microbes to facilitate the MEOR processes by synthesizing biosurfactants. The biosurfactant-synthesizing bacteria from the oil-bearing sandstone reservoir was identified as Bacillus licheniformis MS5-16 and the produced biosurfactant decreased ST from 72 to 32 mN/m. Promising gene sequences accountable for biosurfactant production were (licA3 0.26 kb), lipase generation (lipP1 0.63 kb) and catechol 2,3-dioxygenase for hydrocarbon assimilation (C23O 1.27 kb), which also indicated their applicability to oil recovery. The genes encrypting MEOR-associated functional proteins for alkane degradation were enoyl-CoA hydratase, alcohol dehydrogenase and alkane 1-monooxygenase (Phetcharat et al. 2019).
The whole genome of hydrogenotrophic thermophilic methanogen Methanococcus maripaludis strain X1, isolated from an offshore Malaysian oilfield subsurface production fluid, was reconstructed from high-quality metagenomics datasets (Wang et al. 2011). The microflora was targeted by certain genes such as dissimilatory sulphate reductase (dsrA/B) and dissimilatory nitrate reductase (narG/napA). The subsequent biodegradation process includes the methanogens, that convert hydrogen and CO2 (hydrogenotrophic) or acetate to methane (CH4) (acetogenic), maintaining equilibrium during the course of syntrophic biodegradation of hydrocarbons to methane, by eliminating the intermediates (hydrogen and CO2). This population is detected by the methyl coenzyme-A reductase (mcr) gene (Gupta et al. 2019). The isolated and characterized native strains of a heavy oil reservoir of South Sumatra could effectively degrade heavy crude oil constituents and subsequently reduced oil viscosity. Three candidates were chosen as G3, G7, and N6 which reduced oil viscosity up to 22.67%, 23.14% and 24.36%, respectively. Isolate G3 which was identified as Pseudoxhantomonas taiwanensis degraded 38% aromatics along with 29% resin, isolate G7-Brevibacillus agri degraded 61% aromatic fraction while N6, identified as Bacillus subtilis degraded 52% asphaltenes fraction. This implied the suitability of these isolates for MEOR technology (Purwasena et al. 2018).
Institute of Reservoir Studies (IRS), India, indigenously formulated consortia, namely IRSM-1 and IRSM-2 were anaerobic thermophilic and halophilic (3% salt concentration) bacterial mixture comprising small cocci and short rod size (1.5–2 μm), with pH endurance of 6–8.5 and thermal tolerant up to 65 °C. This microbial system could produce suitable metabolites like fatty acids, biosurfactants and biogases in the oil fields. The MEOR field trials via huff and puff were conducted in Badarpur (3 wells), Kosamba (1well) and Padra (1well) of Mehsana asset of Cambay basin. IRS also prepared two more anaerobic consortia, NJS7-91 and NJS4-96 developed from the microbes of formation waters of Nandej and Sobhasan oil wells of Ahmedabad and Mehsana oil fields. These consortia were hyperthermophilic (grew at 91 and 96 °C) and halophilic (grew in 7% and 4% salt concentration) (Patel et al. 2015).
A bacterial isolate (Garciaella petrolearia) from Mumbai, India, could grow well-exploiting asphaltenes substrate and favorably degraded asphaltenes as well as aromatics present in crude oil (Lavania et al. 2012). The different strains were isolated from the formation water of the Assam oil reservoir field based on crude oil degradation and biosurfactant production. These strains were identified using 16S rRNA sequencing and found to be Stenotrophomonas sp. MG520349, Bacillus subtilis MG520348 and Bacillus subtilis MG495086. Among isolates, Bacillus subtilis MG495086 under optimal conditions i.e. 3.8% (v/v) of light-paraffin oil as a sole carbon source at 62.4 °C and pH 7.7 produced 6.3 ± 0.1 g/L of lipopeptide biosurfactant in 96 h and reduced ST to 29.85 mN/m (Datta et al. 2018). In another study, Bacillus tequilensis MK 729017 and Bacillus subtilis MK729018 were isolated from the reservoir soil of the Assam oil reservoir field. Among isolated strains, Bacillus tequilensis MK 729017 was chosen based on the better surface-active properties. The produced lipopeptide, surfactin was found to be thermal and colloidal stable and reduced ST to 30 ± 2 mN/m. It also decreased the wettability of hydrophobic rock surface from 90 ± 1° to 26 ± 1 (Datta et al. 2020).
Similarly, the microbial population of other reservoirs production fluid was also scrutinized and many of them were found to be biosurfactants producers. Initially, the dominant genera present in the production well were found to be Arcobacter, Flexistipes, Hyphomonas, Parvibaculum, Pseudomonas, Syntrophus, Treponema and Wolinella. During the bioaugmentation process, dominant genera somehow changed to Pseudomonas, Arcobacter, Acinetobacter, Shewanella, Enterococcus, Flavobacterium. However, Pseudomonas aeruginosa DQ3 strain remained the most dominant in Daqing oil reservoirs and could produce maximum biosurfactant of 228 mg/L anaerobically at reservoir temperature (42 °C), which was sufficient because the minimum biosurfactant concentration necessary for mobilizing the entrapped oil from the sandstone reservoir was already known to be only 10 mg/L (Youssef et al. 2007, 2013). Brevibacillus brevis and Bacillus cereus were also employed at Daqing low permeability high-temperature oil field at 65 °C which could reduce 40% of oil viscosity. Enterobacter, Bacillus licheniformis utilized at Fuyu oil field of China (1.95–2.95 MPa, 28 °C) and increased oil recovery by twofold (Sakthipriya et al. 2017).
G. amicalis LH3, isolated from oil-contaminated water samples of the Chinese Jidong oilfield, could degrade 18% (2% w/v) paraffin anaerobically at a rate of 4.4 mg/d after 10 days of cultivation at 40 °C with 5% NaCl. The strain could also reduce 45% of oil viscosity and degraded 10.5% (w/w) oil aerobically after 7 days of incubation (Hao et al. 2008). Two Pseudomonas aeruginosa strains (Gx and Fx) were isolated from oil-saturated soils of Yanchang oilfield, China, and produced biosurfactants using crude oil heavy components as their substrate. The oil displacement prospects of Gx and Fx were examined by the degradation capability of pure asphalt and crude oil asphaltenes where almost 10% of pure asphalt and 59–72% of asphaltenes were biodegraded utilizing bacterial supernatants showing the oil-displacing diameter of 15–17 cm. The lighter fractions (saturates and aromatics, maximum 11%) content were augmented while the heavier fractions (resins and others, maximum 75%) contents were bio-transformed with approximately 50% reduction of the oil viscosity (Gao et al. 2017).
Several microbes were detected and isolated from the production water samples of Jilin oilfields of China for the formulation of crude oil and asphaltenes-degrading microcosm based on dissimilar bacterial types. After two or three weeks of enrichment period, Gas chromatography-mass spectrometry (GC–MS) and Fourier transform infrared spectroscopy (FTIR) results proved the biodegradation of crude oil and asphaltene. The leading genera which formed crude oil-degrading microcosm were Alcanivorax, Devosia, Hydrogenophaga, Parvibaculum, Pseudomonas and Dietzia and similarly asphaltenes-degrading microcosm was prepared by combining Alcanivorax, Flavobacterium, Hyphomonas, Parvibaculum and Reyranella. This imparted an innovative dimension to understand the microbial miscellany in reservoir production fluid and its prospective function for oil degradation (Song et al. 2018). The microbial diverse community and their relative abundance were scrutinized in consecutive indigenous MEOR (IMEOR) metabolic phases. Pseudomonas, Citrobacter and Burkholderia were displayed to be dominant genera in the aerobic, facultative and early anaerobic stages whereas Bacillus, Achromobacter, Rhizobiales, Alcaligenes and Clostridium became prevailing in the later anaerobic phase which demonstrated the unique characteristics of microbial succession stimulated by wheat bran supplementation in the Chinese oilfields (Zhan et al. 2017).
Culture-independent DGGE and clone-library-based examination of production and formation water from high temperature (85 °C) offshore petroleum reserve in the Norwegian Sea focused on the microbial miscellany and recognized a common bacteriological assembly including Arcobacter, Halomonas and Pseudomonas (Brakstad et al. 2008). A pyrosequencing-based entire metagenomic investigation of Norwegian shelf oil fields samples (2.5 km deep, 85 °C, 250 bar) designated the microflora composition by mainly sulfate-reducing bacteria (SRB) from δ or ε Proteobacteria (Campylobacterales, Desulfovibrionales and Desulfuromonadales) along with Thermotogales and methanogenic archaeal Methanococcus (Kotlar et al. 2011). Kosmotoga olearia, an anaerobic, thermophilic, heterotrophic strain from the Troll B oil platform in the North Sea could grow in the temperature range of 20 to 80 °C, pH of 5.5 to 8.0 and 10–60 g/L of salinity (DiPippo et al. 2009). Moderately thermophilic and halophilic SRB, Petrotoga halophila, was isolated from an offshore oil well of Congo, West Africa, capable of hydrocarbon degradation (Miranda-Tello et al. 2007).
A culture-independent metagenomics analysis in formation water from the North Alaska slope oil field exhibited the dominance of thermophilic Thermoanaerobacter, Desulfonauticus and Archeaoglobus at a high temperature of 80 to 83 °C and 2.5 km depth site Ivishak (Hu et al. 2016). It was reported from a DGGE-based investigation on heavy oil carbonate reservoir in Cordoba platform, Veracruz, Mexico, that the consortia consisting thermophilic anaerobic, acetogenic Thermoanaerobacter were functional at 60–80 °C temperature and 535 g/L salinity, respectively (Castorena-Cortés et al. 2012a; Castorena-Cortés et al. 2012b). A bacterial consortium (Bacillus sp., Corynebacterium sp., Brevibacillus sp. and Staphylococcus sp.) was formulated which consumed asphaltenes of Mexico’s Maya crude oil as their only carbon substrate (Pineda-Flores et al. 2004). The indigenous microflora in the Gulf of Mexico beach sands denoted the predominance of Gammaproteobacteria and Alphaproteobacteria as the chief contributor in oil biodegradation (Kostka et al. 2011).
A dual culturable and 16S rRNA gene clone-library-based study of the microbial assortment of biodegraded and non-biodegraded terrestrial oil from Potiguar basin, Brazil, identified the occurrence of Actinobacteria, Firmicutes, Proteobacteria (culturable representatives) and Thermotogae in both samples (Silva et al. 2013). Additionally, the prominent candidates were Bacteroidetes, Deferribacteres, Spirochaetes, and Synergistetes in the biodegraded sample and Chloroflexi and Thermotoga in the non-biodegraded sample along with archaeal family Methanomicrobiaceae. Similar investigation on three other oil fields of Brazil implied a core microbiome comprising of three bacterial (γ-proteobacteria, Clostridia and Bacteroidia) and one archaeal (Methanomicrobia) classes (Sierra-Garcia et al. 2017). Nine bacterial groups have been reported from Brazilian oil reservoirs samples, such as: Acinetobacter, Arcobacter, Bacillus, Halanaerobium, Leuconostoc, Marinobacter, Propionibacterium, Streptomyces and Streptococcus (Sette et al. 2007; Souza et al. 2014). A fungal strain, Neosartorya fischeri, isolated from Venezuela exploited asphaltenes substrate and efficiently degraded asphaltenes as well as aromatics present in crude oil (Uribe‐Alvarez et al. 2011).
Comparative analysis to develop a better understanding of the microbial population was conducted by collecting the production fluid from four deep subsurface high-temperature oil reservoirs which revealed surprising resemblance among geographically distant oil reservoirs. The similarity was observed in oil wells situated in the Segno oilfield near Houston, USA (80–85 °C) and Crossfield oilfield, Canada (75 °C), as a preliminary investigation (Lewin et al. 2014). A similar study was carried out in four oil secluded reservoirs (China, Lousiana, Norwegian Sea, Danish North Sea). The pH range of the four production fluids was 5.53 to 8. The major species consisted of acetoclastic and hydrogenotrophic methanogens (Methanosaetaceae, Methanoculleus and Methanobacterium), along with Clostridiaceae and Thermotogaceae families, which were also found to be thermotolerant and/or spore-forming. The major microbial taxa were Pseudomonadales, Clostridiales, Burkholderiales, Methanococcales, Rhizobiales and Synergistales. A strong concurrence association was noticed among the orders Bacteroidales, Sphaerochaetales, Desulforomonadales and Oceanospirillales, and all were obligatory anaerobic hydrocarbon-utilizing organisms (Kim et al. 2018).
5 Reservoir Environmental Screening Parameters to Conduct MEOR Trials
The efficiency of the MEOR procedure is dependent upon the following constraints; reservoir temperature, crude oil viscosity and API gravity, brine salinity, porosity, permeability, water cut, pressure, residual oil saturation, reservoir depth, wax content along with indigenous microbial content and diversity of the reservoir systems (Sen 2008). The constructive outcome or the success rate of the MEOR process significantly depends upon the microbial consortia present in reservoirs along with the reservoir category. The MEOR progression involves primarily hydrocarbon-consuming microbes (Saravanan et al. 2020). The suitable reservoir conditions to implement the MEOR process have been described in various literature, which could be generalized as the temperature below 93 °C, salinity 100,000 ppm and permeability 75 mD (Yernazarova et al. 2016). The inoculum size and oxygen availability were also found to affect the MEOR process through cellular activities. MEOR has been attempted in more challenging Chinese reservoirs possessing high temperature, high salt concentration, low permeability and heavy crude oil. In some of the cases, reservoir temperature was reported to be as high as 80 °C, salt tolerance up to 46,000 ppm, oil viscosity of 43,000 cP, and reservoir permeability as low as 25 mD. Field-scale assessments proved that MEOR attained accomplishments even under such adverse environments (Gao 2018). Due to the variation in the reservoir environmental conditions, different organizations such as the US Department of Energy (US-DOE) (Bryant and Douglas 1988), China National Petroleum Corporation (CNPC) (Guo et al. 2015; She et al. 2019) and Institute of Reservoir Studies (IRS), India, (Patel et al. 2015) proposed standard parameter ranges for wells selection to carry out MEOR process. The detailed information of the screening parameters of the reservoirs for an individual country to perform the MEOR process is represented in Table 1.
6 Conclusion
The MEOR process performance is tough to anticipate due to the inducing environmental situations inside the oil reservoir which influence the microbial growth and metabolic product formation. As the environmental factors fluctuate in different seasons in a reservoir and are dissimilar among various reservoirs, the MEOR procedure needs to be tailored for precise settings in each of the reservoirs to increase its success rate. At present, oil-producing companies consider MEOR as a high-risk technology for achieving competent and predictable oil retrieval. While modelling strategies for foreseeing consistent oil repossession under replicated reservoir environments shed a ray of assurance and advancement. Though it had been revealed to be a relatively slow development. The probable progression of a “universal” formulation, comprising a combination of nutrients, particular microbes and biosurfactants is an optimistic elucidation subjected to future research in the field scales.
References
Aditama, P., E. Avbelj, S. Reimann, N. Dopffel, E. Mahler, M. Poulsen, W. Jelinek, and H. Alkan. 2017. Design and execution of an MEOR Huff and Puff pilot in a wintershall field. In SPE Europec featured at 79th EAGE conference and exhibition. OnePetro. Society of Petroleum Engineers.
Al-Wahaibi, Y.M., A. Al-Bemani, S. Joshi, and Y. Sugai. 2013. Microbial consortia in Oman oil fields: a possible use in enhanced oil recovery. Journal of Microbiology and Biotechnology 23(1): 106–117.
Amani, H., M.H. Sarrafzadeh, M. Haghighi, and M.R. Mehrnia. 2010. Comparative study of biosurfactant producing bacteria in MEOR applications. Journal of Petroleum Science and Engineering 75 (1–2): 209–214.
Awan, A.R., R. Teigland, and J. Kleppe. 2008. A survey of North Sea enhanced-oil-recovery projects initiated during the years 1975 to 2005. SPE Reservoir Evaluation & Engineering 11 (03): 497–512.
Bachmann, R.T., A.C. Johnson, and R.G. Edyvean. 2014. Biotechnology in the petroleum industry: An overview. International Biodeterioration & Biodegradation 86: 225–237.
Belyaev, S., I. Borzenkov, T. Nazina, E. Rozanova, I. Glumov, R. Ibatullin, and M. Ivanov. 2004. Use of microorganisms in the biotechnology for the enhancement of oil recovery. Microbiology 73 (5): 590–598.
Bonch-Osmolovskaya, E.A., M.L. Miroshnichenko, A.V. Lebedinsky, N.A. Chernyh, T.N. Nazina, V.S. Ivoilov, S.S. Belyaev, E.S. Boulygina, Y.P. Lysov, and A.N. Perov. 2003. Radioisotopic, culture-based, and oligonucleotide microchip analyses of thermophilic microbial communities in a continental high-temperature petroleum reservoir. Applied and Environmental Microbiology 69 (10): 6143–6151.
Bordoloi, N., and B. Konwar. 2008. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids and Surfaces B: Biointerfaces 63 (1): 73–82.
Brakstad, O.G., H.K. Kotlar, and S. Markussen. 2008. Microbial communities of a complex high-temperature offshore petroleum reservoir. International Journal of Oil, Gas and Coal Technology 1 (3): 211–228.
Brown, L.R., and A.A. Vadie. 1997. The utilization of the microflora indigenous to and present in oil-bearing formations to selectively plug the more porous zones thereby increasing oil recovery during waterflooding. Annual report, January 1, 1996--December 30, 1996. Hughes Eastern Corp., Jackson, MS (United States).
Bryant, R.S., and J. Douglas. 1988. Evaluation of microbial systems in porous media for EOR. SPE Reservoir Engineering 3 (02): 489–495.
Castorena-Cortés, G., T. Roldán-Carrillo, J. Reyes-Avila, I. Zapata-Peñasco, M. Mayol-Castillo, and P. Olguín-Lora. 2012a. Coreflood assay using extremophile microorganisms for recovery of heavy oil in Mexican oil fields. Journal of Bioscience and Bioengineering 114(4): 440–445.
Castorena-Cortés, G., I. Zapata-Peñasco, T. Roldán-Carrillo, J. Reyes-Avila, M. Mayol-Castillo, S. Román-Vargas, and P. Olguín-Lora. 2012b. Evaluation of indigenous anaerobic microorganisms from Mexican carbonate reservoirs with potential MEOR application. Journal of Petroleum Science and Engineering 81: 86–93.
Chen, Z.-Y., Q.-X. Feng, and R.-L. Liu. 2001. Development and application of thermophilic microorganism species in oil recovery. Acta Petrolei Sinica 22 (6): 59–62.
Cheng, H.-Y., X.-L. Wang, D.-T. Xu, G.-D. Ma, and W.-D. Wang. 2006. Experiments on EOR employing indigenous microorganisms. Shiyou Kantan Yu Kaifa(Petroleum Exploration and Development) 33(1): 91–94.
Cui, J., H. Chen, M. Sun, and J. Wen. 2020. Comparison of bacterial community structure and function under different petroleum hydrocarbon degradation conditions. Bioprocess and Biosystems Engineering 43 (2): 303–313.
Daryasafar, A., M. Jamialahmadi, M.B. Moghaddam, and B. Moslemi. 2016. Using biosurfactant producing bacteria isolated from an Iranian oil field for application in microbial enhanced oil recovery. Petroleum Science and Technology 34 (8): 739–746.
Datta, P., P. Tiwari, and L.M. Pandey. 2018. Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresource Technology 270: 439–448.
Datta, P., P. Tiwari, and L.M. Pandey. 2020. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. Journal of Petroleum Science and Engineering 195: 107612.
Dhanarajan, G., V. Rangarajan, C. Bandi, A. Dixit, S. Das, K. Ale, and R. Sen. 2017. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. Journal of Biotechnology 256: 46–56.
DiPippo, J.L., C.L. Nesbø, H. Dahle, W.F. Doolittle, N.-K. Birkland, and K.M. Noll. 2009. Kosmotoga olearia gen. nov., sp. nov., a thermophilic, anaerobic heterotroph isolated from an oil production fluid. International Journal of Systematic and Evolutionary Microbiology 59 (12): 2991–3000.
El-Sheshtawy, H., I. Aiad, M. Osman, A. Abo-ELnasr, A. Kobisy. 2015. Production of biosurfactants by Bacillus licheniformis and Candida albicans for application in microbial enhanced oil recovery. Egyptian Journal of Petroleum 25 (3): 293–298.
Fardeau, M.-L., M.B. Salinas, S. l'Haridon, C. Jeanthon, F. Verhé, J.-L. Cayol, B.K. Patel, J.-L. Garcia, and B. Ollivier. 2004. Isolation from oil reservoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. International Journal of Systematic and Evolutionary Microbiology 54(2): 467–474.
Gabitto, J., and M. Barrufet. 2005. Combined microbial surfactant-polymer system for improved oil mobility and conformance control. In SPE Annual Technical Conference and Exhibition. OnePetro. Prairie View A&M State University.
Gao, C. 2018. Experiences of microbial enhanced oil recovery in Chinese oil fields. Journal of Petroleum Science and Engineering 166: 55–62.
Gao, C.H., and A. Zekri. 2011. Applications of microbial-enhanced oil recovery technology in the past decade. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 33 (10): 972–989.
Gao, H., J. Zhang, H. Lai, and Q. Xue. 2017. Degradation of asphaltenes by two Pseudomonas aeruginosa strains and their effects on physicochemical properties of crude oil. International Biodeterioration & Biodegradation 122: 12–22.
Gao, P., G. Li, J. Le, X. Liu, F. Liu, and T. Ma. 2018. Succession of microbial communities and changes of incremental oil in a post-polymer flooded reservoir with nutrient stimulation. Applied Microbiology and Biotechnology 102 (4): 2007–2017.
Ghojavand, H., F. Vahabzadeh, and A.K. Shahraki. 2012. Enhanced oil recovery from low permeability dolomite cores using biosurfactant produced by a Bacillus mojavensis (PTCC 1696) isolated from Masjed-I Soleyman field. Journal of Petroleum Science and Engineering 81: 24–30.
Guo, H., Y. Li, Z. Yiran, F. Wang, Y. Wang, Z. Yu, S. Haicheng, G. Yuanyuan, J. Chuyi, and G. Xian. 2015. Progress of microbial enhanced oil recovery in China. In SPE Asia Pacific enhanced oil recovery conference. OnePetro. Society of Petroleum Engineers.
Gupta, A., J. Sarkar, and P. Sar. 2019. Understanding the structure and function of extreme microbiome through genomics: Scope and challenges. In Microbial Diversity in the Genomic Era, 581–610. Academic Press. Elsevier.
Hadibarata, T., A.B. Khudhair, R.A. Kristanti, and H. Kamyab. 2017. Biodegradation of pyrene by Candida sp. S1 under high salinity conditions. Bioprocess and Biosystems Engineering 40(9): 1411–1418.
Hao, D.-H., J.-Q. Lin, X. Song, J.-Q. Lin, Y.-J. Su, and Y.-B. Qu. 2008. Isolation, identification, and performance studies of a novel paraffin-degrading bacterium of Gordonia amicalis LH3. Biotechnology and Bioprocess Engineering 13 (1): 61–68.
He, J., Y. Wang, and G. Liang. 2018. Emerging strategic technology of the oilfield development. Petroleum Industry Press.
Head, I.M., D.M. Jones, and W.F. Röling. 2006. Marine microorganisms make a meal of oil. Nature Reviews Microbiology 4 (3): 173–182.
Hong, E., M.S. Jeong, and K.S. Lee. 2019. Optimization of nonisothermal selective plugging with a thermally active biopolymer. Journal of Petroleum Science and Engineering 173: 434–446.
Hoppert, M., C. Flies, W. Pohl, B. Günzl, and J. Schneider. 2004. Colonization strategies of lithobiontic microorganisms on carbonate rocks. Environmental Geology 46 (3): 421–428.
Hu, P., L. Tom, A. Singh, B.C. Thomas, B.J. Baker, Y.M. Piceno, G.L. Andersen, and J.F. Banfield. 2016. Genome-resolved metagenomic analysis reveals roles for candidate phyla and other microbial community members in biogeochemical transformations in oil reservoirs. MBio 7 (1): e01669–15.
Hubert, C.R., T.B. Oldenburg, M. Fustic, N.D. Gray, S.R. Larter, K. Penn, A.K. Rowan, R. Seshadri, A. Sherry, and R. Swainsbury. 2012. Massive dominance of Epsilonproteobacteria in formation waters from a Canadian oil sands reservoir containing severely biodegraded oil. Environmental Microbiology 14 (2): 387–404.
Huu, N.B., E.B. Denner, D.T. Ha, G. Wanner, and H. Stan-Lotter. 1999. Marinobacter aquaeolei sp. nov., a halophilic bacterium isolated from a Vietnamese oil-producing well. International Journal of Systematic and Evolutionary Microbiology 49 (2): 367–375.
Ismail, W.A., J.D. Van Hamme, J.J. Kilbane, and J.-D. Gu. 2017. Petroleum microbial biotechnology: Challenges and prospects. Frontiers in Microbiology 8: 833.
Jang, L.-K., P.W. Chang, J.E. Findley, and T.F. Yen. 1983. Selection of bacteria with favorable transport properties through porous rock for the application of microbial-enhanced oil recovery. Applied and Environmental Microbiology 46 (5): 1066–1072.
Jeong, M.S., Y.W. Lee, H.S. Lee, and K.S. Lee. 2021. Simulation-based optimization of microbial enhanced oil recovery with a model integrating temperature, pressure, and salinity effects. Energies 14 (4): 1131.
Kamari, A., M. Nikookar, L. Sahranavard, and A.H. Mohammadi. 2014. Efficient screening of enhanced oil recovery methods and predictive economic analysis. Neural Computing and Applications 25 (3): 815–824.
Kargarpour, M.A. 2017. Investigation of reservoir temperature in a gas reservoir in Middle East: Case study. Journal of Petroleum Exploration and Production Technology 7 (2): 531–541.
Kim, D.D., C. O’Farrell, C.R. Toth, O. Montoya, L.M. Gieg, T.H. Kwon, and S. Yoon. 2018. Microbial community analyses of produced waters from high-temperature oil reservoirs reveal unexpected similarity between geographically distant oil reservoirs. Microbial Biotechnology 11 (4): 788–796.
Kostka, J.E., O. Prakash, W.A. Overholt, S.J. Green, G. Freyer, A. Canion, J. Delgardio, N. Norton, T.C. Hazen, and M. Huettel. 2011. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Applied and Environmental Microbiology 77 (22): 7962–7974.
Kotlar, H.K., A. Lewin, J. Johansen, M. Throne‐Holst, T. Haverkamp, S. Markussen, A. Winnberg, P. Ringrose, T. Aakvik, and E. Ryeng. 2011. High coverage sequencing of DNA from microorganisms living in an oil reservoir 2.5 kilometres subsurface. Environmental Microbiology Reports 3(6): 674–681.
Kowalewski, E., I. Rueslåtten, K. Steen, G. Bødtker, and O. Torsæter. 2006. Microbial improved oil recovery—Bacterial induced wettability and interfacial tension effects on oil production. Journal of Petroleum Science and Engineering 52 (1–4): 275–286.
Lavania, M., S. Cheema, P.M. Sarma, A.K. Mandal, and B. Lal. 2012. Biodegradation of asphalt by Garciaella petrolearia TERIG02 for viscosity reduction of heavy oil. Biodegradation 23 (1): 15–24.
Leon, V., and M. Kumar. 2005. Biological upgrading of heavy crude oil. Biotechnology and Bioprocess Engineering 10 (6): 471–481.
Lewin, A., J. Johansen, A. Wentzel, H.K. Kotlar, F. Drabløs, and S. Valla. 2014. The microbial communities in two apparently physically separated deep subsurface oil reservoirs show extensive DNA sequence similarities. Environmental Microbiology 16 (2): 545–558.
Li, X.-X., S.M. Mbadinga, J.-F. Liu, L. Zhou, S.-Z. Yang, J.-D. Gu, and B.-Z. Mu. 2017. Microbiota and their affiliation with physiochemical characteristics of different subsurface petroleum reservoirs. International Biodeterioration & Biodegradation 120: 170–185.
Liu, J.-Y., K. Shen, Z.-W. Huang, H.-N. Huai, and F.-L. Chen. 2010. A pilot study on MEOR in Ansai extra-low permeability oil field. Xinjiang Petroleum Geology 31 (6): 634–636.
Long, X., N. He, Y. He, J. Jiang, and T. Wu. 2017. Biosurfactant surfactin with pH-regulated emulsification activity for efficient oil separation when used as emulsifier. Bioresource Technology 241: 200–206.
Marchant, R., and I.M. Banat. 2012. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends in Biotechnology 30 (11): 558–565.
Mariano, A.P., A.P.D.A.G. Kataoka, D.D.F.D. Angelis, and D.M. Bonotto. 2007. Laboratory study on the bioremediation of diesel oil contaminated soil from a petrol station. Brazilian Journal of Microbiology 38(2): 346–353.
Marshall, S.L. 2008. Fundamental aspects of microbial enhanced oil recovery: A literature survey. National Research Flagships wealth from Oceans.
Mbadinga, S.M., L.-Y. Wang, L. Zhou, J.-F. Liu, J.-D. Gu, and B.-Z. Mu. 2011. Microbial communities involved in anaerobic degradation of alkanes. International Biodeterioration & Biodegradation 65 (1): 1–13.
Meslé, M., G. Dromart, and P. Oger. 2013. Microbial methanogenesis in subsurface oil and coal. Research in Microbiology 164 (9): 959–972.
Minai-Tehrani, D., A. Herfatmanesh, F. Azari-Dehkordi, and S. Minuoi. 2006. Effect of salinity on biodegradation of aliphatic fractions of crude oil in soil. Pakistan Journal of Biological Sciences 9(8): 1531–1535.
Miranda-Tello, E., M.-L. Fardeau, C. Joulian, M. Magot, P. Thomas, J.-L. Tholozan, and B. Ollivier. 2007. Petrotoga halophila sp. nov., a thermophilic, moderately halophilic, fermentative bacterium isolated from an offshore oil well in Congo. International Journal of Systematic and Evolutionary Microbiology 57(1): 40–44.
Miyazaki, M., O. Koide, T. Kobayashi, K. Mori, S. Shimamura, T. Nunoura, H. Imachi, F. Inagaki, T. Nagahama, and Y. Nogi. 2012. Geofilum rubicundum gen. nov., sp. nov., isolated from deep subseafloor sediment. International Journal of Systematic and Evolutionary Microbiology 62 (5): 1075–1080.
Nazina, T., D.S. Sokolova, T. Babich, E. Semenova, A. Ershov, S.K. Bidzhieva, I. Borzenkov, A. Poltaraus, M. Khisametdinov, and T. Tourova. 2017. Microorganisms of low-temperature heavy oil reservoirs (Russia) and their possible application for enhanced oil recovery. Microbiology 86 (6): 773–785.
Nazina, T., D. Sokolova, D. Grouzdev, E. Semenova, T. Babich, S. Bidzhieva, D. Serdukov, D. Volkov, K. Bugaev, and A. Ershov. 2020. The potential application of microorganisms for sustainable petroleum recovery from heavy oil reservoirs. Sustainability 12 (1): 15.
Nie, Y., C.-Q. Chi, H. Fang, J.-L. Liang, S.-L. Lu, G.-L. Lai, Y.-Q. Tang, and X.-L. Wu. 2014. Diverse alkane hydroxylase genes in microorganisms and environments. Scientific Reports 4 (1): 1–11.
Nikolova, C., and T. Gutierrez. 2020. Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Frontiers in Microbiology 10: 2996.
Niu, J., Q. Liu, J. Lv, and B. Peng. 2020. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. Journal of Petroleum Science and Engineering 192: 107350.
Nnaemeka, O., N. Franklin, and O. Stanley. 2018. A review of microbial enhanced oil recovery applications projects. Oil Gas Res 4 (152): 2472–0518.
Okpala, G.N., C. Chen, T. Fida, and G. Voordouw. 2017. Effect of thermophilic nitrate reduction on sulfide production in high temperature oil reservoir samples. Frontiers in Microbiology 8: 1573.
Özdemir, G., S. Peker, and S. Helvaci. 2004. Effect of pH on the surface and interfacial behavior of rhamnolipids R1 and R2. Colloids and Surfaces A: Physicochemical and Engineering Aspects 234 (1–3): 135–143.
Patel, J., S. Borgohain, M. Kumar, V. Rangarajan, P. Somasundaran, and R. Sen. 2015. Recent developments in microbial enhanced oil recovery. Renewable and Sustainable Energy Reviews 52: 1539–1558.
Phetcharat, T., P. Dawkrajai, T. Chitov, W. Mhuantong, V. Champreda, and S. Bovonsombut. 2019. Biosurfactant-producing capability and prediction of functional genes potentially beneficial to microbial enhanced oil recovery in indigenous bacterial communities of an onshore oil reservoir. Current Microbiology 76 (3): 382–391.
Philips, C.A., P. Augustine, P.K. Yerol, G.N. Ramesh, R. Ahamed, S. Rajesh, T. George, and S. Kumbar. 2020. Modulating the intestinal microbiota: therapeutic opportunities in liver disease. Journal of Clinical and Translational Hepatology 8 (1): 87.
Phukan, R., S.B. Gogoi, and P. Tiwari. 2019. Alkaline-surfactant-alternated-gas/CO2 flooding: Effects of key parameters. Journal of Petroleum Science and Engineering 173: 547–557.
Pineda-Flores, G., G. Boll-Argüello, C. Lira-Galeana, and A.M. Mesta-Howard. 2004. A microbial consortium isolated from a crude oil sample that uses asphaltenes as a carbon and energy source. Biodegradation 15(3): 145–151.
Pineda-Flores, G., and A.M. Mesta-Howard. 2001. Petroleum asphaltenes: generated problematic and possible biodegradation mechanisms. Revista Latinoamericana de microbiología 43(3): 143–150.
Purwasena, I., D. Astuti, R. Fatmawati, and Q. Afinanisa. 2018. Isolation and characterization of oil-degrading bacteria from one of South Sumatera’s oilfield. In IOP Conference Series: Materials Science and Engineering 288 (1): 012123.
Rabiei, A., M. Sharifinik, A. Niazi, A. Hashemi, and S. Ayatollahi. 2013. Core flooding tests to investigate the effects of IFT reduction and wettability alteration on oil recovery during MEOR process in an Iranian oil reservoir. Applied Microbiology and Biotechnology 97 (13): 5979–5991.
Rathi, R., M. Lavania, V. Kukreti, and B. Lal. 2018. Evaluating the potential of indigenous methanogenic consortium for enhanced oil and gas recovery from high temperature depleted oil reservoir. Journal of Biotechnology 283: 43–50.
Rojo, F. 2009. Degradation of alkanes by bacteria. Environmental Microbiology 11 (10): 2477–2490.
Rosenberg, E., E.F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson. 2014. The prokaryotes: Other major lineages of bacteria and the archaea. Springer, Heidelberg. ISBN: 978-3-642-38955-9.
Rostami, P., M.F. Mehraban, M. Sharifi, M. Dejam, and S. Ayatollahi. 2019. Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: A mechanistic study. Petroleum 5 (4): 367–374.
Roy, A. 2017. Review on the biosurfactants: Properties, types and its applications. Journal of Fundamentals of Renewable Energy and Applications 8: 1–14.
Safdel, M., M.A. Anbaz, A. Daryasafar, and M. Jamialahmadi. 2017. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renewable and Sustainable Energy Reviews 74: 159–172.
Saha, R., R.V. Uppaluri, and P. Tiwari. 2018. Influence of emulsification, interfacial tension, wettability alteration and saponification on residual oil recovery by alkali flooding. Journal of Industrial and Engineering Chemistry 59: 286–296.
Saha, R., R.V.S. Uppaluri, and P. Tiwari. 2018. Effects of interfacial tension, oil layer break time, emulsification and wettability alteration on oil recovery for carbonate reservoirs. Colloids and Surfaces A: Physicochemical and Engineering Aspects 559: 92–103.
Sakthipriya, N., M. Doble, and J.S. Sangwai. 2017. Enhanced microbial degradation of waxy crude oil: A review on current status and future perspective. International Journal of Oil, Gas and Coal Technology 16 (2): 130–165.
Santos, R.G., W. Loh, A. Bannwart, and O. Trevisan. 2014. An overview of heavy oil properties and its recovery and transportation methods. Brazilian Journal of Chemical Engineering 31(3): 571–590.
Saravanan, A., P.S. Kumar, K.H. Vardhan, S. Jeevanantham, S.B. Karishma, P.R. Yaashikaa, and P. Vellaichamy. 2020. A review on systematic approach for microbial enhanced oil recovery technologies: opportunities and challenges. Journal of Cleaner Production 258: 120777.
Sari, C., R. Hertadi, M. Gozan, and A. Roslan. 2019. Factors affecting the production of biosurfactants and their applications in Enhanced Oil Recovery (EOR). A review. In IOP conference series: Earth and environmental science 353 (1): 012048. IOP Publishing.
Schultz, J., and A.S. Rosado. 2020. Extreme environments: A source of biosurfactants for biotechnological applications. Extremophiles 24 (2): 189–206.
Sen, R. 2008. Biotechnology in petroleum recovery: The microbial EOR. Progress in Energy and Combustion Science 34 (6): 714–724.
Sette, L.D., K.C. Simioni, S.P. Vasconcellos, L.J. Dussan, E.V. Neto, and V.M. Oliveira. 2007. Analysis of the composition of bacterial communities in oil reservoirs from a southern offshore Brazilian basin. Antonie Van Leeuwenhoek 91 (3): 253–266.
Sharma, S., P. Datta, B. Kumar, P. Tiwari, and L.M. Pandey. 2019a. Production of novel rhamnolipids via biodegradation of waste cooking oil using Pseudomonas aeruginosa MTCC7815. Biodegradation 30(4): 301–312.
Sharma, S., and L.M. Pandey. 2020. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresource Technology 307: 123261.
Sharma, S., R. Verma, and L.M. Pandey. 2019b. Crude oil degradation and biosurfactant production abilities of isolated Agrobacterium fabrum SLAJ731. Biocatalysis and Agricultural Biotechnology 21: 101322.
She, H., D. Kong, Y. Li, Z. Hu, and H. Guo. 2019. Recent advance of microbial enhanced oil recovery (MEOR) in China. Geofluids 2019.
Sheng, J.J. 2013. Foams and their applications in enhancing oil recovery. In Enhanced Oil recovery field case studies, 251–280. Gulf Professional Publishing Elsevier.
Sheng, J.J. 2015. Status of surfactant EOR technology. Petroleum 1 (2): 97–105.
Shibulal, B., S.N. Al-Bahry, Y.M. Al-Wahaibi, A.E. Elshafie, A.S. Al-Bemani, and S.J. Joshi. 2014. Microbial enhanced heavy oil recovery by the aid of inhabitant spore-forming bacteria: An insight review. The Scientific World Journal 2014.
Shibulal, B., S.N. Al-Bahry, Y.M. Al-Wahaibi, A.E. Elshafie, A.S. Al-Bemani, and S.J. Joshi. 2017. The potential of indigenous Paenibacillus ehimensis BS1 for recovering heavy crude oil by biotransformation to light fractions. PloS One 12(2): e0171432.
Sierra-Garcia, I.N., B.M. Dellagnezze, V.P. Santos, R. Capilla, E.V.S. Neto, N. Gray, and V.M. Oliveira. 2017. Microbial diversity in degraded and non-degraded petroleum samples and comparison across oil reservoirs at local and global scales. Extremophiles 21(1): 211–229.
Silva, T., L. Verde, E.S. Neto, and V. Oliveira. 2013. Diversity analyses of microbial communities in petroleum samples from Brazilian oil fields. International Biodeterioration & Biodegradation 81: 57–70.
Singh, A., J.D. Van Hamme, R.C. Kuhad, N. Parmar, and O.P. Ward. 2014. Subsurface petroleum microbiology. In Geomicrobiology and biogeochemistry, 153–173. Springer.
Song, W.-F., J.-W. Wang, Y.-C. Yan, L.-Y. An, F. Zhang, L. Wang, Y. Xu, M.-Z. Tian, Y. Nie, and X.-L. Wu. 2018. Shifts of the indigenous microbial communities from reservoir production water in crude oil-and asphaltene-degrading microcosms. International Biodeterioration & Biodegradation 132: 18–29.
Souza, E.C., T.C. Vessoni-Penna, and R.P. de Souza Oliveira. 2014. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. International Biodeterioration & Biodegradation 89: 88–94.
Tong, X., G. Zhang, Z. Wang, Z. Wen, Z. Tian, H. Wang, F. Ma, and Y. Wu. 2018. Distribution and potential of global oil and gas resources. Petroleum Exploration and Development 45 (4): 779–789.
Tonini, R.M.C.W., C.E. de Rezende, and A.D. Grativol. 2010. Degradation and bioremediation of petroleum compounds by bacteria: a review. Oecology Australis 14 (4): 1025–1035.
Uribe-Alvarez, C., M. Ayala, L. Perezgasga, L. Naranjo, H. Urbina, and R. Vazquez-Duhalt. 2011. First evidence of mineralization of petroleum asphaltenes by a strain of Neosartorya fischeri. Microbial Biotechnology 4 (5): 663–672.
Van Hamme, J.D., A. Singh, and O.P. Ward. 2003. Recent advances in petroleum microbiology. Microbiology and Molecular Biology Reviews 67 (4): 503–549.
Verma, R., S. Sharma, L.M. Kundu, and L.M. Pandey. 2020. Experimental investigation of molasses as a sole nutrient for the production of an alternative metabolite biosurfactant. Journal of Water Process Engineering 38: 101632.
Wang, J., T. Ma, J. Liu, Q. Liu, L. Zhao, F. Liang, and R. Liu. 2008. Isolation of functional bacteria guided by PCR-DGGE technology from high temperature petroleum reservoirs. Huan Jing ke Xue= Huanjing Kexue 29(2): 462–468.
Wang, L.-Y., W.-J. Ke, X.-B. Sun, J.-F. Liu, J.-D. Gu, and B.-Z. Mu. 2014. Comparison of bacterial community in aqueous and oil phases of water-flooded petroleum reservoirs using pyrosequencing and clone library approaches. Applied Microbiology and Biotechnology 98 (9): 4209–4221.
Wang, X., P. Greenfield, D. Li, P. Hendry, H. Volk, and T.D. Sutherland. 2011. Complete genome sequence of a nonculturable Methanococcus maripaludis strain extracted in a metagenomic survey of petroleum reservoir fluids. Journal of Bacteriology 193 (19): 5595–5595.
Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Current Opinion in Biotechnology 12 (3): 259–276.
Wood, D.A. 2019. Microbial improved and enhanced oil recovery (MIEOR): Review of a set of technologies diversifying their applications. Advances in Geo-Energy Research 3 (2): 122–140.
Xingbiao, W., X. Yanfen, Y. Sanqing, H. Zhiyong, and M. Yanhe. 2015. Influences of microbial community structures and diversity changes by nutrients injection in Shengli oilfield, China. Journal of Petroleum Science and Engineering 133: 421–430.
Xu, D., B. Bai, H. Wu, J. Hou, Z. Meng, R. Sun, Z. Li, Y. Lu, and W. Kang. 2019. Mechanisms of imbibition enhanced oil recovery in low permeability reservoirs: Effect of IFT reduction and wettability alteration. Fuel 244: 110–119.
Yernazarova, A., G. Kayirmanova, A. Baubekova, and A. Zhubanova. 2016. Microbial enhanced oil recovery. In Chemical Enhanced Oil Recovery (cEOR)— A practical overview 147–167. InTech.
Youssef, N., M.S. Elshahed, and M.J. McInerney. 2009. Microbial processes in oil fields: Culprits, problems, and opportunities. Advances in Applied Microbiology 66: 141–251.
Youssef, N., D. Simpson, K. Duncan, M. McInerney, M. Folmsbee, T. Fincher, and R. Knapp. 2007. In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Applied and Environmental Microbiology 73 (4): 1239–1247.
Youssef, N., D.R. Simpson, M.J. McInerney, and K.E. Duncan. 2013. In-situ lipopeptide biosurfactant production by Bacillus strains correlates with improved oil recovery in two oil wells approaching their economic limit of production. International Biodeterioration & Biodegradation 81: 127–132.
Zhan, Y., Q. Wang, C. Chen, J.B. Kim, H. Zhang, B.A. Yoza, and Q.X. Li. 2017. Potential of wheat bran to promote indigenous microbial enhanced oil recovery. Journal of Industrial Microbiology & Biotechnology 44 (6): 845–855.
Zhang, F., Y.-H. She, L.-J. Chai, I.M. Banat, X.-T. Zhang, F.-C. Shu, Z.-L. Wang, L.-J. Yu, and D.-J. Hou. 2012. Microbial diversity in long-term water-flooded oil reservoirs with different in situ temperatures in China. Scientific Reports 2 (1): 1–10.
Zhang, J., H. Gao, and Q. Xue. 2020a. Potential applications of microbial enhanced oil recovery to heavy oil. Critical Reviews in Biotechnology 40(4): 459–474.
Zhang, Y., A.E. Dekas, A.J. Hawkins, A.E. Parada, O. Gorbatenko, K. Li, and R.N. Horne. 2020b. Microbial community composition in deep‐subsurface reservoir fluids reveals natural interwell connectivity. Water Resources Research 56(2): e2019WR025916.
Zhou, L., Y.-W. Lu, D.-W. Wang, S.-L. Zhang, E.-G. Tang, Z.-Z. Qi, S.-N. Xie, J. Wu, B. Liang, and J.-F. Liu. 2020. Microbial community composition and diversity in production water of a high-temperature offshore oil reservoir assessed by DNA-and RNA-based analyses. International Biodeterioration & Biodegradation 151: 104970.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Datta, P., Tiwari, P., Pandey, L. (2022). Effect of Reservoir Environmental Conditions and Inherent Microorganisms. In: Pandey, L., Tiwari, P. (eds) Microbial Enhanced Oil Recovery. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-16-5465-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-5465-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5464-0
Online ISBN: 978-981-16-5465-7
eBook Packages: EnergyEnergy (R0)