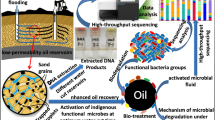
Abstract
Further exploitation of the residual oil underground in post-polymer flooded reservoirs is attractive and challengeable. In this study, indigenous microbial enhanced oil recovery (IMEOR) in a post-polymer flooded reservoir was performed. The succession of microbial communities was revealed by high-throughput sequencing of 16S rRNA genes and changes of incremental oil were analyzed. The results indicated that the abundances of reservoir microorganisms significantly increased, with alpha diversities decreased in the IMEOR process. With the intermittent nutrient injection, microbial communities showed a regular change and were alternately dominated by minority populations: Pseudomonas and Acinetobacter significantly increased when nutrients were injected; Thauera, Azovibrio, Arcobacter, Helicobacter, Desulfitobacterium, and Clostridium increased in the following water-flooding process. Accompanied by the stimulated populations, higher oil production was obtained. However, these populations did not contribute a persistent level of incremental oil in the reservoir. In summary, this study revealed the alternative succession of microbial communities and the changes of incremental oil in a post-polymer flooded reservoir with intermittent nutrient stimulation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer flooding is a method of tertiary oil recovery for high water-cut reservoirs. This technique generally employs polyacrylamide to improve the flooding area by sealing mainstream channels and increasing the viscosity of displacing phase (Goodyear et al. 1995). However, large pore paths usually form during and after long-term polyacrylamide flooding (Le et al. 2014). As a result, the injected displacing fluid flows out of oil-bearing strata quickly, with the water content of the produced liquid maintained at a very high level. Further exploitation of the residual oil underground in post-polymer flooded high water-cut reservoirs is becoming increasingly challengeable for oil industry.
Indigenous microbial enhanced oil recovery (IMEOR) may be a promising alternative oil-recovery technique for the post-polymer flooded reservoirs. IMEOR is driven by the synergistic effects of reservoir microorganisms and their metabolites, which can lower oil viscosity and interfacial tension and block undesired flow paths to improve the recovery of residual crude oil (Ivanov et al. 1993; Magot et al. 2000; Youssef et al. 2009; Voordouw 2011; Zhu et al. 2013). As was previously proved by Ivanov et al. (1993), in the case of the biotechnology based on the oxidation of residual oil by oilfield microorganisms, injection of dissolved oxygen (as a water-air mixture) and of nitrogen and phosphorus mineral salts initially stimulated the growth of aerobic hydrocarbon-degrading bacteria. The products of oil oxidation (fatty acids, alcohols, and surface-active agents), as well as nutrients, are transported in the reservoir strata along with the injected water flow. At this stage, fermentative and methanogenic microorganisms further degrade these compounds producing microbial biomass and gas, which improve oil recovery via reservoir re-pressurization, oil swelling, decrease of oil viscosity, and modification of the oilfield hydrodynamic flows. In other biotechnologies, nitrates are added in order to activate the growth of denitrifying bacteria to control microbial involvement in oil souring (Gieg et al. 2011; Gassara et al. 2015; Suri et al. 2017).
Compared with polyacrylamide flooding, IMEOR is of low energy consumption, low environmental impact, and cost-effective (Youssef et al. 2009; Voordouw 2011). This technique has been intensively developed and successfully applied in water-flooded reservoirs worldwide (Wang et al. 2014; Li et al. 2014; Gassara et al. 2015; Le et al. 2015; You et al. 2016); yet, little information is available for post-polymer flooded high water-cut reservoirs. In this study, considering the existing large pore paths of post-polymer flooded reservoirs, an improved indigenous microbial enhanced oil recovery (IMEOR) process with small polymer and nutrient packages alternately and intermittently pumped into oil strata was designed and tested in a post-polymer flooded reservoir located in Daqing Oil Field, Northeast China. IMEOR benefits from increasing understanding of the assemblages of microbial communities underground. Therefore, the succession of microbial communities during the intermittent IMEOR process was analyzed by 16S rRNA high-throughput sequencing. The changes of incremental oil were analyzed to reveal the relationships with the stimulated microbial communities during the long-term IMEOR process.
Materials and methods
Reservoir information and IMEOR strategy
The reservoir is a homogeneous sandstone reservoir located in Daqing Oil Field, Northeast China. The tested block includes injection well N2-2-P40 and oil production wells N2-D2-P40, N2-2-P140, N2-2-P141, and N2-D3-P40 in a relatively closed site (0.12 km2), with an average inter-well spacing of 250 m (Fig. 1a). The temperature of the oil-bearing strata is 44.6 °C. The average permeability of the oil-bearing strata is 0.414 μm2. The block was exploited by polymer flooding from April 1999 to March 2005. Subsequently, water-flooding was employed to recover the remaining oil from April 2005. When the IMEOR process was performed (November 2011), approximately 61.89% oil reserve had been extracted, and water content of the produced liquid reached 93.29%. The injection water and the formation production fluid in the test area were 6130 and 4540 mg/L, respectively.
Tested block and study design. a Location of the field trial block and distribution of the targeted oil production wells. b Sampling timeline. The solid red circles indicate the sampling time points. c Polymer and nutrient injection information. Before nutrient injection, a small amount of polyacrylamide was firstly injected into oil strata to seal the mainstream channels to avoid the injected nutrients rapidly flow out along with the large pore paths
According to the culture-dependent experiments and physical simulations of oil displacement carried out in the laboratory, an optimal nutrient medium, which consisted of 14 g corn steep powder, 1.5 g (NH4)2HPO4, and 2.5 g NaNO3, was selected (Le et al. 2014). To avoid the injected nutrients rapidly flowing out along the large pore paths, a small amount of polyacrylamide (2000 mg L−1) was first injected into the oil strata through the injection well to seal the mainstream channels. From 7 December 2011 to 30 April 2012 and 5 December 2012 to 30 April 2013, two rounds with a total of 5387 m3 (0.00175 PV) polyacrylamide and 15,605 m3 (0.065 PV) nutrient medium prepared by formation brine were alternately and intermittently injected into the block (Fig. 1b, c).
Sample collection and DNA extraction
Microbial communities and production performances of the tested block were constantly monitored from August 2011 to October 2014 (Fig. 1b). Oil–water mixture samples were taken using the sampling valves located at the wellhead of the four oil production wells. The collected samples completely filled 10-L sterilized plastic buckets, which were immediately sealed with screw caps to avoid contamination and oxygen intrusion. DNA extraction was performed within 24 h after sample collection. Microbial cells were collected from 2-L water samples by centrifugation at 4 °C and 10,000×g for 15 min in a high-speed centrifuge (Beckman, USA). Total genomic DNA was extracted using a bead shaker treatment and the AxyPrep™ Genomic DNA Miniprep Kit (Axygen, USA) as previously described (Gao et al. 2016).
16S rRNA gene sequencing and statistical analyses
The universal primer set 515f (5′-GTG CCA GCM GCC GCG GTAA-3′) and 806r (5′-GGA CTA CHV GGG TWT CTA AT-3′) were used to amplify the microbial 16S rRNA gene V4 region (300–350 bp), according to the previously described protocol (Caporaso et al. 2011; Caporaso et al. 2012). PCR products in triplicate of the same sample were mixed to avoid PCR artifacts. Amplicon sequencing on the Illumina MiSeq platform was performed by Novogene Co., Beijing, China. Pairs of reads from the original DNA fragments were merged using fast length adjustment of short reads (FLASH) (Magoc and Salzberg 2011). Sequences were then demultiplexed and quality filtered using the Quantitative Insights into Microbial Ecology (QIIME) software package (Caporaso et al. 2010). An average of 40,698 16S rRNA gene fragments was obtained for each sample. To reduce sequencing deviation, 16,650 sequences were drawn out at random for each sample to calculate the microbial alpha diversity, which included observed operational taxonomic units (OTUs), Shannon, and Chao1 indices. The OTU clustering pipeline UPARSE was used to select OTUs with 97% similarity (Edgar 2013). The alpha diversity indices Chao1, Shannon, and observed OTUs were calculated using QIIME. The representative sequence sets were aligned and given a taxonomic classification using the Ribosomal Database Project at an 80% confidence level (Wang et al. 2007). Principal coordinate analysis (PCoA) was implemented using QIIME based on weighted-UniFrac distances and was used to investigate the distribution of microbial communities. Linear discriminative analysis (LDA) effect size (LEfSe) analysis was used to determine the populations with statistically significant differences http://huttenhower.sph.harvard.edu/galaxy (Segata et al. 2011).
Quantitative PCR
The 16S rRNA gene was used as a molecular marker to quantify the abundances of microbial communities during the IMEOR field trial as previously described (Li et al. 2014). Quantitative PCR (qPCR) was performed using the FastStart Universal SYBR Green Master PCR mix (Roche Applied Science, Germany) in a Bio-Rad iQ5 sequence detection system (Applied Biosystems, California, USA). The microbial 16S rRNA gene was amplified with the primer set 8F (5′-AGA GTT TGA T(CT)(AC) TGG CTC-3′) and 338R (5′-GCT GCC TCC CGT AGG AGT-3′).
Data accessibility
The raw reads were deposited in the GenBank at the National Center for Biotechnology Information (BioProject ID: PRJNA 349240, https://submit.ncbi.nlm.nih.gov/subs/bioproject/SUB2027000/overview).
Results
Abundances and diversities of microbial communities in the nutrient injection process
Based on 16S rRNA gene sequencing, microbial alpha diversities, including the observed OTUs, Shannon, and Chao1 indices, were calculated. As shown in Table S1, there were obvious changes in alpha-diversity indices along with the nutrient injection process (Fig. 2a). Compared with the water samples obtained before nutrient injection, fewer species and lower Chao1 and Shannon indices were observed when nutrients were injected. After the IMEOR process, microbial alpha diversity increased. qPCR indicated that the community abundances obviously increased during the nutrient stimulation process (Fig. 2b). Assuming that one bacterial cell contains 3.6 copy numbers of 16S rRNA genes (Li et al. 2010), the total number of microorganisms reached 10–100-folds.
Temporal trends in microbial communities with the nutrient injection process. a Microbial Shannon indices, P < 0.05 represent there is significant difference between the compared samples. Shannon index \( {\mathrm{H}}^{\hbox{'}}=\frac{}{}\sum \limits_{i=1}^S{p}_i\ln {p}_i \), S represents the number of total species, pi represents the proportion of species i in the total species. b Microbial 16S rRNA gene abundances
Succession of microbial communities with the intermittent nutrient injection process
Based on phylogenetic analysis, 17 dominant microbial classes were detected during the IMEOR field trial. These taxa accounted for an average of 97.82% of the whole community in every sample, while the other classifiable classes only accounted for an average of 1.56% (Fig. 3). Compared with the control samples (August 2011), Gammaproteobacteria obviously increased in the water of each production well after nutrient injection (March 2012 and May 2012), with an average increase in relative abundance of 22.8%. Epsilonproteobacteria obviously decreased in the water of each production well, with an average decrease in relative abundance of 8.5%. In the following water-flooding process, particularly in July 2012, Gammaproteobacteria and Alphaproteobacteria obviously decreased, with an average decrease in relative abundance of 43.85 and 15.87%, respectively. At this stage, Betaproteobacteria, Epsilonproteobacteria, and Deltaproteobacteria obviously increased, with an average increase in relative abundance of 27.19, 27.38, and 5.58%, respectively. When the nutrients were injected again (February 2013 and April 2013), the microbial communities changed again, but had similar community structures to the samples from the first round of nutrient injection (March 2012 and May 2012). Gammaproteobacteria and Alphaproteobacteria significantly increased, while Betaproteobacteria, Epsilonproteobacteria, and Deltaproteobacteria significantly decreased. In the following water-flooding process (July 2013 and December 2013), Gammaproteobacteria and Alphaproteobacteria significantly decreased, while Betaproteobacteria, Epsilonproteobacteria, and Clostridia significantly increased. In Gammaproteobacteria, Pseudomonas and Acinetobacter were the dominant genera. Thauera and Azovibrio were the dominant Betaproteobacterial genera. In Alphaproteobacteria, Hyphomonas and Phenylobacterium were the dominant genera. In Epsilonproteobacteria, Arcobacter and Helicobacter were the dominant genera. In Clostridia, Desulfitobacterium and Clostridium were the dominant genera.
PCoA based on weighted UniFrac distances were performed to reveal the succession of the microbial communities along with the intermittent nutrient injection process (Fig. 4 and Fig. S1). In the PCoA plots, sample points that are close together are more similar in their community composition than those that are far apart. PCoA indicated that the reservoir microorganisms showed responses to the injected nutrient solution, with observed changes in the community structures between the nutrient injection (March 2012, May 2012, February 2013, and April 2013) and following water-flooding processes (July 2012, July 2013, and December 2013). Correlation analysis showed that the fractions of microbial populations showed low correlation between the nutrient injection and following water-flooding processes (r = 0.42, P < 0.01, Fig. 5a, and r = 0.51, P < 0.01, Fig. 5f). Moreover, the microbial communities regularly changed with the intermittent nutrient injection. As shown in Fig. 5, the fractions of microbial populations in the first round of nutrient injection showed positively correlated with those in the second round of nutrient injection (r = 0.95, P < 0.01, Fig. 5b). Similarly, positive correlation was observed between the first round and second round of following water-flooding process (r = 0.89, P < 0.01, Fig. 5e). These observations suggest the controllability and variability of microbial communities along with nutrient injection process.
Dominant microbial populations before and after the nutrient injection process
LEfSe analysis was performed to determine the populations with significant differences before and after the nutrient injection processes (Fig. 6). The results indicated that Gammaproteobacteria, Alphaproteobacteria, Betaproteobacteria, Epsilonproteobacteria, Deltaproteobacteria, and Clostridia were the dominant different biomarkers with the intermittent nutrient injection (P < 0.05). For example, Gammaproteobacteria and Alphaproteobacteria in nutrient injection phase (Mar. 2012, May. 2012, Feb. 2013, and Apr. 2013) were found significantly different with those in the following water-flooding phase (Jul. 2012, Nov. 012, and Jul. 2013), while Epsilonproteobacteria and Deltaproteobacteria in the following water-flooding phase showed significantly different with those in the nutrient injection phase (Fig. 6b, d). Among them, Pseudomonas, Hyphomonas, and Arcobacter were the dominant different genera between the nutrient injection phase and following water-flooding phase (P < 0.05). Overall, these determined biomarkers showed a high consistency with the succession of the community compositions in the IMEOR process.
Distribution and representation of the taxa contributing to the different microbial communities in the IMEOR process. a–c LEfSe taxonomic cladograms. Significantly discriminant taxon nodes are colored. Branch areas are shaded according to the highest ranked group for that taxon. The yellow-colored nodes represent the taxa with no significant differences among sample habitats. d Histogram of LDA score showing the discriminant taxon between the nutrient injection phase and the following water-flooding phase
Shared microbial populations during the IMEOR filed trial
Despite the diverse community structures, representatives of 21 microbial taxa were found always existed and dominated the tested block before and after the IMEOR process (Fig. 3b). These populations accounted for an average of 63.22% of the whole community in each sample, while the other classifiable Prokaryotes only accounted for an average of 6.86%. These shared populations contained representatives of the genera (such as Pseudomonas, Acinetobacter, Thauera, Phenylobacterium, Arcobacter, and Helicobacter) that dominated the changes of microbial communities with intermittent nutrient injection process.
Oil production performances with the intermittent nutrient injection process
The oil production performances were compared before and after the intermittent nutrient injection processes (Fig. 7 and Fig. S2). Before the nutrient stimulation (from October 2010 to December 2011), oil production of the tested block was gradually decreasing, while the water content was gradually increasing. During the IMEOR process, a clear improvement in oil production performances was observed. The incremental oil in the first round of nutrient injection and the following water-flooding process were more remarkable than those in the second round of nutrient injection. Compared with the control (from October 2010 to December 2011), the oil production was maintained at a relatively high level after the nutrient injection (from December 2013 to June 2015). Based on the decline curve, a cumulative incremental oil production of 6234 t was obtained by the end of June 2015. Based on the oil production before the IMEOR process (November 2011), a total of 3593 t incremental oil was obtained.
Oil production curve and water content curve during the IMEOR field trial. The black trend lines were drawn based on data from October 2010 to November 2011 and were used to predict the trends of water content and oil production of the field trial block. The blue trend line was drawn based on oil production from October 2010 to June 2015
Discussion
This study reveals the alternative succession of microbial communities and the changes of incremental oil during intermittent nutrient injection process in a post-polymer flooded reservoir. When the IMEOR process was performed in the post-polymer flooded reservoir, only 38.11% oil reserve remained underground, with an average water content of produced liquid of 93.29%. Considering the existed large pore paths in the post-polymer flooded reservoir, a small amount of polymer was first injected to avoid the injected nutrients rapidly flowing out along the large pore paths. The nutrient solutions were intermittently injected to investigate the succession of microbial communities and the changes of incremental oil.
Microbial alpha diversity decreased during the nutrient stimulation process. Although reservoirs harbor diverse microbial populations, it seems that the injected nutrients only enriched some microbial populations, resulting in them dominating in communities. The growth of these stimulated populations made other low-abundance populations hard to detect using routine sequencing techniques. This is consistent with the results of community quantification, which showed that the total number of microorganisms obviously increased. Community analysis also demonstrated that minority microbial populations were selectively activated when nutrients were injected into the reservoir.
IMEOR studies center on how microbial communities change with nutrient injection. Studies have demonstrated that microbial communities showed positive responses to nutrient stimulation in laboratory and field trials (Liu et al. 2005; Li et al. 2014; Wang et al. 2015; Chai et al. 2015; Xiao et al. 2016). In this study, the microbial communities, in particular, the dominant microbial populations, regularly changed with the intermittent nutrient injection process. When the eutrophic solutions were injected into the reservoir (March 2012, February 2013, and April 2012), Pseudomonas and Actinobacteria increased in each production well, while Arcobacter and Helicobacter decreased. At this stage, Thauera, Azovibrio, Arcobacter, Helicobacter, Desulfitobacterium, and Clostridium also existed with lower relative abundances. In the following water-flooding process (July 2012 and July 2013), Pseudomonas, Actinobacteria, Hyphomonas, and Phenylobacterium significantly decreased, while anaerobic microorganisms, such as Thauera, Azovibrio, Arcobacter, Helicobacter, Desulfitobacterium, and Clostridium, increased. The results indicated the alternative succession and controllability of microbial communities during long-term IMEOR process. The stability of microbial communities may contribute a persistent level of incremental oil in IMEOR process.
Environmental variables, such as temperature, chemical composition of the formation brine, spatial isolation, low permeability of reservoir strata, and stochastic processes (Stegen et al. 2012; Zhou et al. 2014; Ren et al. 2015; Gao et al. 2016; Nie et al. 2016; Song et al. 2017), have all been found to be important drivers of distinct microbial assemblages in oil reservoirs. Despite of the highly diverse community structures, some microbial populations are always found across different reservoir environments (Gao et al. 2016). Here, shared microbial populations were detected in the tested reservoir block, implying that these populations play key roles in recycle of substance and energy in IMEOR process (Gray et al. 2010). The phenomena have also been reported in marine environments (Gibbons et al. 2013) and sewage treatment plants (Zhang et al. 2012). Can these shared populations drive the IMEOR process? As reported previously, many species affiliated with Pseudomonas, Rhodobacter, Parvibaculum, and Pelotomaculum are able to degrade hydrocarbon or produce surfactants. However, we did not observed the markedly decrease in surface tension of the produced liquids. The phenomenon may be related with the amount of surfactant production and dilution effect of displacing fluid when flowing into oil production wells. On the other hand, the acetate in produced liquids increased after 60-day nutrient stimulation. Species from Thauera, Azoarcus, Azorhizophilus, and Azovibrio play important roles in nitrogen cycle. Thauera can also anaerobically degrade aromatic compounds and is among the important genera in nitrate-based souring control strategies (Gieg et al. 2011). Tepidimonas are aerobic organotrophic bacteria (Nazina et al. 2017) and facultative sulfur-oxidizing bacteria. Desulfovibrio and Desulfomicrobium are generally detected sulfate-reducing bacteria in reservoirs. In addition, there are Hydrogenophaga, a type of facultative hydrogen autotrophic bacteria, and syntrophic propionate-oxidizing bacteria Pelotomaculum and Syntrophus. These populations may play important roles in supporting the growth and metabolism of methanogens in reservoirs. It seems that these populations can conduct the biogeochemical cycles of oil reservoirs, such as the carbon, nitrogen, and sulfur cycles. More importantly, these shared microbial populations with greater abundance may contribute to a more robust function of the reservoir microbial ecosystem (Wittebolle et al. 2009).
Although IMEOR has been successfully applied in improving oil recovery in oil field, our knowledge about the successions of microbial communities and changes of incremental oil with intermittent nutrient injection process is very limited. Here, despite injection of a larger amount of nutrients and similar community compositions, the incremental oil in the second round of nutrient injection and the following water-flooding process were significantly lower than those in the first round of nutrient injection process. The result indicated that higher oil increment mainly appeared in the initial stage of the IMEOR process in the post-polymer flooded reservoir. This phenomenon suggests that the enhancement of oil recovery is not only driven by the stimulated microorganisms, but also influenced by the distribution of residual oil and the complex geological features of the oil-bearing strata.
In summary, this study demonstrated the application potential of IMEOR process in a post-polymer flooded reservoir. The results revealed the alternative succession of microbial communities and the changes of incremental oil during a long-term IMEOR process in a post-polymer flooded reservoir.
References
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Meth 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6(8):1621–1624. https://doi.org/10.1038/ismej.2012.8
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108(Suppl 1):4516–4522. https://doi.org/10.1073/pnas.1000080107
Chai LJ, Fan Z, She YH, Banat IM, Hou DJ (2015) Impact of a microbial-enhanced oil recovery field trial on microbial communities in a low-temperature heavy oil reservoir. Nat Environ Pollut Technol 14(3):455–462. https://doi.org/10.1007/s00253-011-3717-1
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Meth 10(10):996–998. https://doi.org/10.1038/nmeth.2604
Gao PK, Tian HM, Wang YS, Li YS, Li Y, Xie JX, Zeng B, Zhou JF, Li GQ, Ma T (2016) Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. SCI REP-UK 6(1):20174. https://doi.org/10.1038/srep20174
Gassara F, Suri N, Stanislav P, Voordouw G (2015) Microbially enhanced oil recovery by sequential injection of light hydrocarbon and nitrate in low- and high-pressure bioreactors. Environ Sci Technol 49(20):12594–12601. https://doi.org/10.1021/acs.est.5b03879
Gibbons SM, Caporaso JG, Pirrung M, Field D, Knight R, Gilbert JA (2013) Evidence for a persistent microbial seed bank throughout the global ocean. Proc Natl Acad Sci U S A 110(12):4651–4655. https://doi.org/10.1073/pnas.1217767110
Gieg LM, Jack TR, Foght JM (2011) Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92(2):263–282. https://doi.org/10.1007/s00253-011-3542-6
Goodyear SG, Mead BJ, Woods CL (1995) A novel recovery mechanism for polymer flooding in gravity-dominated viscous oil reservoirs. SPE Reserv Eng 10(04):259–265. https://doi.org/10.2118/27772-PA
Gray ND, Sherry A, Hubert C, Dolfing J, Head IM. 2010. Chapter 5 - methanogenic degradation of petroleum hydrocarbons in subsurface environments: remediation, heavy oil formation, and energy recovery. Elsevier Science & Technology
Ivanov MV, Belyaev SS, Borzenkov IA, Glumov IF, Ibatulin BP (1993) Additional oil production during field trials in Russia. Dev Pet Sci, Microbial enhancement of oil recovery: recent advances 39:373–381. https://doi.org/10.1016/S0376-7361(09)70074-3
Le JJ, Liu F, Zhang JY, Bai LL, Wang R, Liu XB, Hou ZW, Lin WX (2014) A field test of activation indigenous microorganism for microbial enhanced oil recovery in reservoir after polymer flooding. Acta Pet Sin 35:99–106. https://doi.org/10.7623/syxb201401011
Le JJ, Wu XL, Wang R, Zhang JY, Bai LL, Hou ZW (2015) Progress in pilot testing of microbial-enhanced oil recovery in the Daqing oilfield of north China. Int Biodeter Biodegr 97:188–194. https://doi.org/10.1016/j.ibiod.2014.10.014
Li GQ, Gao PK, Wu YQ, Tian HM, Dai XC, Wang YS, Cui QS, Zhang HZ, Pan XX, Dong HP, Ma T (2014) Microbial abundance and community composition influence production performance in a low-temperature petroleum reservoir. Environ Sci Technol 48(9):5336–5344. https://doi.org/10.1021/es500239w
Li H, Chen S, Mu BZ, Gu JD (2010) Molecular detection of anaerobic ammonium-oxidizing (Anammox) bacteria in high-temperature petroleum reservoirs. Microb Ecol 60(4):771–783. https://doi.org/10.1007/s00248-010-9733-3
Liu JF, Ma LJ, Mu BZ, Liu RL, Ni FT, Zhou JX (2005) The field pilot of microbial enhanced oil recovery in a high temperature petroleum reservoir. J Pet Sci Eng 48(3-4):265–271. https://doi.org/10.1016/j.petrol.2005.06.008
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Magot M, Ollivier B, Patel BKC (2000) Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 77(2):103–116. https://doi.org/10.1023/a:1002434330514
Nazina TN, Shestakova NM, Semenova EM, Korshunova AV, Kostrukova NK, Tourova TP, Min L, Feng Q, Poltaraus AB (2017) Diversity of metabolically active bacteria in water-flooded high-temperature heavy oil reservoir. Front Microbiol 8:707. https://doi.org/10.3389/fmicb.2017.00707
Nie Y, Zhao JY, Tang YQ, Guo P, Yang Y, Wu XL, Zhao F (2016) Species divergence vs. functional convergence characterizes crude oil microbial community assembly. Front Microbiol 7:1254. https://doi.org/10.3389/fmicb.2016.01254
Ren HY, Xiong SZ, Gao GJ, Song YT, Cao GZ, Zhao LP, Zhang XJ (2015) Bacteria in the injection water differently impacts the bacterial communities of production wells in high-temperature petroleum reservoirs. Front Microbiol 6:505. https://doi.org/10.3389/fmicb.2015.00505
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Song Z, Yao Z, Zhao F, Sun G, Zhu W (2017) Wellhead samples of high-temperature, low-permeability petroleum reservoirs reveal the microbial communities in wellbores. Energy Fuel 31(5):4866–4874. https://doi.org/10.1021/acs.energyfuels.7b00152
Stegen JC, Lin X, Konopka AE, Fredrickson JK (2012) Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J 6(9):1653–1664. https://doi.org/10.1038/ismej.2012.22
Suri N, Voordouw J, Voordouw G (2017) The effectiveness of nitrate-mediated control of the oil field sulfur cycle depends on the toluene content of the oil. Front Microbiol 8:956. https://doi.org/10.3389/fmicb.2017.00956
Voordouw G (2011) Production-related petroleum microbiology: progress and prospects. Curr Opin Biotechnol 22(3):401–405. https://doi.org/10.1016/j.copbio.2010.12.005
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. https://doi.org/10.1128/aem.00062-07
Wang WD, Lin JZ, Geng XL, Wang J, Li XM, Jiang Y, Zhao FM (2014) MEOR field test at block Luo801 of Shengli oil field in China. Petrol Sci Technol 32(6):673–679. https://doi.org/10.1080/10916466.2011.601507
Wang XB, Xue YF, Yuan SQ, Huang ZY, Ma YH (2015) Influences of microbial community structures and diversity changes by nutrients injection in Shengli oilfield, China. J Pet Sci Eng 133:421–430. https://doi.org/10.1016/j.petrol.2015.06.020
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N (2009) Initial community evenness favours functionality under selective stress. Nature 458(7238):623–626. https://doi.org/10.1038/nature07840
Xiao M, Sun SS, Zhang ZZ, Wang JM, Qiu LW, Sun HY, Song ZZ, Zhang BY, Gao DL, Zhang GQ, Wu WM (2016) Analysis of bacterial diversity in two oil blocks from two low-permeability reservoirs with high salinities. Sci Rep-UK 6(1):19600. https://doi.org/10.1038/srep19600
You J, Wu G, Ren F, Chang Q, Yu B, Xue Y, Mu B (2016) Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl Microbiol Biotechnol 100(3):1469–1478. https://doi.org/10.1007/s00253-015-7073-4
Youssef N, Elshahed MS, McInerney MJ (2009) Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol 66:141–251. https://doi.org/10.1016/S0065-2164(08)00806-X
Zhang T, Shao M-F, Ye L (2012) 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6:1137–1147. https://doi.org/10.1038/ismej.2011.188
Zhou JZ, Deng Y, Zhang P, Xue K, Liang YT, Van Nostrand JD, Yang YF, He ZL, Wu LY, Stahl DA, Hazen TC, Tiedje JM, Arkin AP (2014) Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci U S A 111(9):E836–E845. https://doi.org/10.1073/pnas.1324044111
Zhu H, Carlson HK, Coates JD (2013) Applicability of anaerobic nitrate-dependent Fe(II) oxidation to microbial enhanced oil recovery (MEOR). Environ Sci Technol 47:8970–8977. https://doi.org/10.1021/es401838b
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grants No. 31500414 and 41373074) and National High Technology Research and Development Program of China (Grant No. 2013AA064402).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 227 kb)
Rights and permissions
About this article
Cite this article
Gao, P., Li, G., Le, J. et al. Succession of microbial communities and changes of incremental oil in a post-polymer flooded reservoir with nutrient stimulation. Appl Microbiol Biotechnol 102, 2007–2017 (2018). https://doi.org/10.1007/s00253-018-8766-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8766-2