Abstract
Cichorium intybus, commonly called chicory, is a biennial herb belonging to family Asteraceae. The plant is considered to originate 4000 years ago in Europe and grows in Asia, America, and Africa. Italy is known to cultivate chicory on large scale for the production of seeds. Ayurvedic system of medicine considers the plant as an essential medicinal herb. Various systems of medicine like Unani, Siddha, and Ayurveda utilize the medicinal herb as remedy for anorexia, disorders of renal system, and dyspepsia. Leaves are considered to contain high levels of total phenolic and total flavonoid content as compared to other parts of chicory plant. Roots possess near about 40% inulin. Chicory is considered to possess numerous active phytochemicals like vitamins, flavonoids, sesquiterpenes, chicoric acid, chicorin and caffeic acid, etc. that are responsible for its bioactivity. Due to the presence of such active phytoconstituents, it has been traditionally used in folklore medication in numerous parts of the world. The plant is reported to be the best substituent for coffee. Ancient Egyptians have cultivated chicory as medicinal plant and since decades it had been used medicinally in regions where the plant has been adopted as well as in indigenous regions. Reported literature on the plant evidences a number of pharmacological activities including antidiabetic, anti-inflammatory, hepatoprotective, antimalarial, etc. Besides these pharmacological activities, it has been found to be highly potent against gram-positive bacteria, fungi, and helminths. The basic rationale of the chapter is to provide a comprehensive review of various therapeutic activities of the plant and phytochemical moieties responsible for medicinal repute of C. intybus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Cichorium intybus, a biennial herb, belonging to family “Asteraceae” is commonly known as chicory. It is called as “Kasani” in Sanskrit (Zafar and Ali 1998). The plant’s name has been obtained from Latin as well as from Greek, Cichorium corresponds to “domain” whereas, intybus means “to crack” (in Greek) and tubus indicating “empty stem” (in Latin). The two different names for intybus are attributed to the leaves (Ema 2010). The plant is considered to be one of the most essential medicinal plant in the Ayurvedic system of medicine (Rizvi et al. 2014). It is a herbal plant consisting of 14 species. It is extensively grown in temperate parts of the world and mainly in the Mediterranean region, northern Asia, and north Africa. The plant is indigenous to Europe (Sastri 1962). In indigenous medicines, plants of the genus Cichorium (Asteraceae) are used abundantly (DerMarderosian and Beutler 2002). In Unani, Siddha, and Ayurvedic systems of medicine, this medicinal plant is used to treat renal system disorders, hepatobiliary system, dyspepsia, and anorexia (Tyler et al. 1988; Crellin and Philpott 1990). The plant possesses rosette leaves and tuberous taproot and is usually upright and glandular (Zafar and Ali 1998). It is a tiny biennial aromatic herb, possessing blue or white flowers (Nandagopal and Kumari 2007). The plant tolerates a vast range of climatic and soil conditions, is considered a cosmopolitan weed (Simon et al. 1996).
The most popular reference sources like Physicians Desk Guide for Herbal Medicines and German Commission e-monographs reported that the C. intybus, due to the existence of sesquiterpene lactones, cinnamic acid derivatives, and flavonoids, can be used for negative chronotropic and inotropic effects and against loss of appetite (Fleming 2000). Chicory extract has been rated as “generally considered safe” by the Food and Drug Administration (FDA) and described in “Everything Added to Food in the United States” (Schmidt et al. 2007). The existence of vitamins, such as ascorbic acid (Gilani and Janbaz 1994), thiamine, riboflavin, retinol, carotenoids and niacin (Wills et al. 1986), inulin, sesquiterpenes, that is, esculin, esculetin, cichorin A, lactucin, zinc, hydroxycinnamic acid, and lactucopicrin was discovered in comprehensive phytochemical work on the plant material (Vg and Dranik 1972). Caffeic acid, cichoric acid as a quinic acid monoester, and isorhamnetin as a variety of flavonoids are contained in the leaves of plant. Cichoriolide A; cichorioside A, B, and C; along with some other known sesquiterpenes are contained in roots of the plant (Bais and Ravishankar 2001). The bitter taste of chicory is ascribed to the presence of these sesquiterpene lactones (Peters and van Amerongen 1998). As chicory is a hardy plant, thus during both vegetative and reproductive growth stages it can survive extreme temperatures (Bais et al. 2000). All parts of the plant transude milky latex on breakage (Van Wyk et al. 1997). C. intybus is being cultivated for a wide variety of applications, and hence according to the use, plant has been divided into four major varieties or cultivation classes:
-
1.
Root chicory or industrial chicory is mainly confined to northwestern part of Europe, Chile, India, and some regions of South Africa. These regions cultivate conical root which is used as substituent in coffee manufacture and for extraction of inulin.
-
2.
Witloof chicory or Brussels is usually grown within Europe for the production of buds that are etiolated.
-
3.
Aerial (leaf) chicory has been used fresh or sometimes cooked as vegetables.
-
4.
Silage chicory, originally obtained from chicory that’s usually wild variety, is more often found by the side of roadways and barren land. This kind of chicory has been used to increase acquisition of herbage in perennial pastures for livestock since the mid-1970s (Cadalen et al. 2010).
However, various plant parts have global utilization pertaining to conventional medicine due to its widespread distribution (Süntar et al. 2012). The basic constituents are contained in the root but some of the important phytochemical constituents have been found to be present throughout the plant (Bais et al. 2000).
12.2 Historical Background and Distribution
C. intybus belonging to Asteraceae family, originated 4000 years ago in Europe, several parts of Asia, America, and Africa. Egyptians used C. intybus in medicinal practices and its use in folk medicine is widely recorded. Although the beginning of the cultivation of chicory is not established exactly, but a Roman historian called it “Plinius” and registered it with lettuce about 50 AD (Kiers et al. 1999). In the North of Europe, it was used as fodder until the seventeenth century. The use of chicory for pasture in England was pioneered by Elliott. In New Zealand, chicory was first reported in the year 1867. In Pennsylvania, until 1993, chicory was classified as a noxious weed (Jung et al. 1996). Historically, chicory was cultivated as curative herb, veggies crop, and coffee replacement by the ancient Egyptians and was sometimes used for animal feed. It was discovered in the 1970s that 40% of the inulin was contained in roots of C. intybus, that possess marginal effect on plasma glucose and hence considered to be ideal for the treatment of diabetes (Judžentienė and Būdienė 2008). Up to date, C. intybus is grown on an industrial scale for the production of inulin (van Arkel et al. 2012). It is one of the most frequently used herbal regimen and a multipurpose edible plant in east Anatolia known as kanej, tahlisk, or hindiba. Turkey’s eastern Anatolia area is very mountainous and highly fractured, so it provides favorable conditions for diverse plant growth (Özgökçe and Özçelik 2004). Wild chicory can be found in coastal areas and in the mountains in Italy. Demands are growing in Italy at the moment and some seed companies have begun to grow wild chicory seeds. It is also well known not only in the different regions of Italy (Guarrera and Savo 2013), but also in India, north and south Europe (Bais et al. 2000), and in Spain (Benítez et al. 2010). For thousands of years, chicory has been a component of natural grasslands in many parts of the world, but as a forage crop, it only has a relatively recent past. This plant is considered to be a nutritious forb that is used in the summer for grazing ruminants to create available forage with high nutritional value (Barry 1998).
12.3 Morphology
Cichorium, a plant species comprising diploid cells (2n = 18) belonging to the family of Asteraceae, subfamily of Cichoriodeae, tribe of Lactuceae or Cichorieae (Funk et al. 2005), is generally referred to as witloof chicory. Wild chicory species is perennial, but as a biennial species, the crop has been selected for cultivation (Kiers et al. 1999). Cichorium is an upright arboreous plant extended to about 90–100 cm length and possessing 75 cm long fleshy taproot and wide basal leaves (Bais et al. 2000; Van Wyk et al. 1997). This plant also possesses stout tap root that is roughly hairy or glabrous. The length of stem is usually 15–150 cm. In short, the basal leaves are petiolate, oblanceolate, toothed to runcinate. The cauline leaves are found to be sessile. The capitulum usually 2.5–3.5 cm wide is contained in axillary. The outer phyllaries of the plant are ovate whereas inner phyllaries are lanceolate and usually two to three times longer than ovate (Yıldırımlı 1999). The color of the leaves is usually red and the color of flowers varies from bright blue to white or pink. The fleshy root of the plant grows up to 75 cm and fresh buds are often found near the surface of soil. The flowers open at the beginning of the day and close during the afternoon. On the basis of flecked or multicolored leaves or more or less uniformly colored red blades, the Italian red variety of chicory was determined (Roustakhiz and Majnabadi 2017).
12.4 Traditional Uses
All over the world, medicinal plants have been used for millennia, and in order to meet the primary health care needs, various communities still depend on indigenous medicinal plants. In traditional cultures, the informative knowledge of plant-based remedies is likely to progress by trial and error and the most informative knowledge of plant-based remedies and major therapies have precisely moved sequentially through generations (Gurib-Fakim 2006). Long ago, ancient Egyptians have cultivated chicory as a medicinal plant and since decades it had been used medicinally in the region where the plant has been adopted as well as in the indigenous region (Wang and Cui 2011). The numerous customary or regional names that identify this plant may be attributed to the extensive usage of unique folklore communities. Various preparations of chicory plant are used for the treatment of different ailments (1). It is said that juice acts as a therapeutic to cure uterine cancers and neoplasm (Judžentienė and Būdienė 2008). Leaves and roots have been used to prepare tea as remedy for jaundice in South Africa; although it is considered a common herb, syrup made from the chicory plant is used as tonic and as purifying drug for babies (Van Wyk et al. 1997). Turkey has formulated an ointment from the leaves of chicory for wound healing (Sezik et al. 2001). Whole plant or sometimes only individual plant parts have been used traditionally to prepare decoction of chicory. As per the data in European Monograph, roots of chicory have been traditionally used to solace the indications associated with disorders of digestive system (e.g., distended abdomen, borborygmus, and sluggish assimilation) and reduced appetite (Sile et al. 2020). The flowers (Cichorii flos) of this plant are considered to be herbal remedy for regular illnesses like appetite stimulants and analeptic; in addition, these flowers are also used for the treatment of gallstones, bruises and cuts, gastroenteritis, and sinus issues (Judžentienė and Būdienė 2008). The whorls in Italy are turned into a decoction and used as a depurative (Pieroni 2000). Jigrine, an Indian commercial commodity which is used as therapeutic for numerous liver ailment, contains Cichorium seeds as one of the key ingredients (Ahmed et al. 2003). Owing to extensive distribution, various plant parts have been possibly used worldwide in conventional medicine, including in Turkey. The roots and leaves are used for diverse purposes in Turkish folk medicine. Chicory roots have been utilized to form decoction that can be consumed against cancer and kidney stones. In other parts of the world, various other health benefits have also been reported. In Afghanistan, aqueous extract of chicory roots had been used against malaria (Bischoff et al. 2004). In Iran this plant was used as therapy for warts (Syed et al. 2008). In Poland, it is used for the treatment of digestive ailment and liver disease (Kisiel and Michalska 2002). In Italy and Serbia, it is consumed as a diuretic and laxative (Pushparaj et al. 2007). In Pakistan, its roots are utilized to form a poultice which is used for the relief of pain (Shah et al. 2006). Similar to Turkey, dried chicory root is also used in Belgium, France, and the USA to prepare coffee-like drinks and as stomachic (Mulinacci et al. 2001). In India, aqueous seed extract is used for the treatment of liver disease and diarrhea (Gadgoli and Mishra 1997), while fresh shoots are eaten as food and used for stomach ache and urinary tract infections (Shah et al. 2006). Biological activity assessment studies have revealed that the complete plant extract of C. intybus exhibits antidiabetic and hepatoprotective activity (Pushparaj et al. 2007; Gadgoli and Mishra 1997), whereas highest antioxidant potential, anthelmintic and antimicrobial potential is possessed by the aerial parts of the plant (Foster et al. 2011). On the other hand, various other pharmacological benefits, such as analgesic, antimalarial, anti-inflammatory, anti-ulcerogenic, and anticarcinogenic activities have been reported for the plant roots (Wesołowska et al. 2006; Conforti et al. 2008).
12.5 Pharmacological Activities
12.5.1 Hepatoprotective Activity
The disorders of liver have been categorized in the high priority regions of healthcare system. As reported by World Health Organization, it has been estimated that approximately 500 million humans are affected by aliments of liver, most often chronic hepatitis (Al-Asmari et al. 2014). Medicines that have originated from plants may also function as practicable remedy for triumphing liver problems due to their safety, less complicated availability, being environment friendly, and for their price effectiveness (Izzo et al. 2016).
As per numerous studies, chicory had a long history of restorative use and especially it is being used as a tonic for ailments of liver and digestive tract (Street et al. 2013). One of the studies reported the decreased levels of serum enzymes (aspartate transaminase and alanine aminotransferase) and bilirubin in carbon tetrachloride (CCl4) that prompted hepatic damage due to increased levels of serum enzyme and bilirubin; on the other hand, the tiers of albumin and proteins reduced in rats treated with C. intybus root callus and natural root extracts. One more study proposes that the ingredients from cultured chicory, cells are greater powerful antihepatotoxic as compared with that of herbal root extract in opposition to carbon tetrachloride (CCl4)-prompted hepatic harm (Elgengaihi et al. 2016). Furthermore, seeds of chicory are used in biliary disorders together with jaundice and are substances used in several recipes prescribed by means of traditional healers to reduce hepatobiliary proceedings (Said 1982). Yet another study evaluated that the hydro-methanolic extract of C. intybus shoots afforded safety against acetaminophen-triggered hepatotoxicity in rats (Gilani et al. 1993). However, a scientific study on the effect of seeds in liver harm is missing. In the same investigation, crude extract of chicory seeds were examined against acetaminophen as well as toward carbon tetrachloride (CCI4), which caused liver injuries, to further authenticate the conventional use of this plant in hepatic harm.
12.5.2 Anti-Inflammatory Activity
Inflammation is defined as protection reaction of body to perilous stimuli along with allergens and/or harm to the tissues; however, out-of-control inflammatory reaction is the main cause of an enormous sequence of problems inclusive of hypersensitive reactions, cardiovascular dysfunctions, metabolic syndrome, most cancers, and autoimmune sicknesses, forcing vast economic burden on individuals and therefore on the society (Bagad et al. 2013). Inflammation and oxidative stress are rigorously interlinked processes that involve the mechanism of releasing numerous nuclear factor ĸB (NF-ĸB)-mediated seasoned inflammatory mediators (Keshk et al. 2017). The process of inflammation involves regulation of extensively merged signals that are mediated through AMP-activated protein kinase (AMPK) and NF-κB. AMPK, a multisubstrate serine or threonine protein kinase, plays regulatory roles in oxidative pressure, irritation, autophagy, mitochondrial dysfunction, and cell destiny (Chen and Zhu 2016).
One of the in vitro research has reported the anti-inflammatory activity of C. intybus roots (Cavin et al. 2005).Various models of inflammation have been characterized to evaluate the anti-inflammatory activity of experimental compounds but carrageenan-induced inflammation based on molecular mechanism is widely used. The production of histamine, leukotrienes, and cyclooxygenase merchandise are associated with early phase of carrageenan edema, even as the behind schedule segment for carrageenan-induced response of inflammation is related to infiltration of neutrophils and the release of neutrophil-extracted unfastened molecules, along with superoxide radical, hydrogen peroxide, and hydroxyl radicals, and also to the release of further neutrophil-extracted inflammatory agent (Vinegar et al. 1969). Another research study on Cichorium roots concluded full-sized, dose-based decrease in paw edema in carrageenan-triggered paw edema technique. Chicory roots reduced the serum TNF-α, IL-6, and IL-1 stages. They also significantly diminished malonyl aldehyde ranges and elevated the sports of catalase and glutathione peroxidase in paw tissue. Similarly, chicory roots confirmed an extensive lower in granuloma formation in cotton pellet brought about granuloma technique. The roots of C. intybus contain anti-inflammatory activity, and this might be because of the inhibition of various cytokines, antioxidants, and their loose radical scavenging pastime (Huang et al. 2012).
12.5.3 Gastroprotective Activity
One of the key issues of contemporary gastroenterology is the treatment of gastroduodenal ulcers (Krylova et al. 2015). Although there are numerous preventive measures and modern therapeutic methods, recurrence occurs in about 30–80% cases despite using highly effective antiulcer regimen. In about 25–40% sufferers, there are chances of complicated peptic ulcers and 14–20% sufferers have been found to be impervious to maximum up-to-date treatment options. Furthermore, traditional regimen motive side effects of numerous types in nearly one-third of patients (Krylova et al. 2015). During the ulcerogenic situation there is domination of aggressive factors even though the protecting factors are reduced. The underlying cause of both the parameters include disorder in metabolism and synthesis of nucleic acids and proteins (Ivashkin et al. 2003). As per the study conducted on dry C. intybus root extract (CRE), it has been found to be a favorable approach for the treatment of gastrointestinal illnesses of various etiology. This extract is received through earliest technology, its efficiency is justified on diverse version systems of experimental gastroenterology (Ivashkin et al. 2003). In yet another study, the incidence of ulcer reduced three to nine times after pretreatment with chicory root extract (25 and 50 mg/kg) within the rat gastric mucosa (Krylova et al. 2015). There has been no change in the volume of gastric secretion by using chicory root extract (CRE) in a dose of 25 mg/kg; at the same time, there was reduction of 44% secretion tension indistinguishable to that in response to famotidine using 50 mg/kg dose of CRE. Same study evaluated remarkable increase of pH values in reaction to both doses, and famotidine indicated a significant decrease of gastric acidity in rats with ulcers and thus it was concluded that CRE has a remarkable inhibitory effect on acid peptic factor (Krylova et al. 2015).
12.5.4 Antidiabetic Activity
Diabetes mellitus is a group of metabolic disorders that outline an elevated level of blood glucose with decreased insulin degrees, and has been regularly associated with insulin resistance, high blood pressure, dyslipidemia, and obesity (Saltiel 2001). Obesity is fundamentally the idea of insulin resistance in type 2 diabetes, that’s characterized through insulin resistance and beta-cell disorder of pancreas (Masters et al. 2010). M1 macrophages and activated M2 macrophages are the basic two types of adipose tissue macrophages (Fujisaka et al. 2009).When there is an imbalance in the ratio of M1/M2 macrophages, there is weight gain that causes obesity and hence there occurs stimulation of M1 macrophages and downregulation of M2 macrophages, leading to persistent infection and the propagation of metabolic dysfunction inflicting diabetes (Kraakman et al. 2014). Traditionally, C. intybus provides an expansion of fit-to-be-eaten products and is extensively used as an essential medicinal herb to treat diverse ailments, including diabetes (Li et al. 2014). Pharmacologically, the roots of C. intybus had been proposed to possess antidiabetic activity (Pushparaj et al. 2007). Evident from various studies type 2 diabetes can be prevented by omega-3 fatty acids through the inhibition of NOD-, LRR-, and pyrin domain–containing protein 3 (NLRP3) inflammasome activation (Yan et al. 2013). Thus, one of the studies on this plant proposed that the plant extract inhibited high-fat diet (HFD)-prompted IL-1b manufacturing via inhibition of HFD-induced NLRP3 inflammasome activation. Also, there is an impairment of insulin receptor signaling due to HFD-induced interleukin (Wen et al. 2011). Thus, this study suggested that chicory remedy might improve the HFD-prompted insulin resistance and hence decreased HFD-brought about IL-1b production. One of the examiner analyzed the effect of C. intybus methanolic (CME) extract on glucose delivery and adipocyte differentiation in 3T3-L1 cells by reading the radiolabeled uptake of glucose. The radiolabeled glucose uptake assay was used to evaluate different extracts (hexane, ethyl acetate, and methanol) of C. intybus. The maximum glucose uptake was shown by methanolic extract. CME exhibited dose-established growth in glucose uptake and concentration of 100 ng/ml was found to be the ideal dose showing maximum glucose uptake (Nam et al. 2001). Another study investigated the effect of leaves of C. intybus in inhibiting protein tyrosine phosphatase 1B (PTP1B), and evaluated the key markers involved in insulin cellular signaling and adipogenesis by utilizing 3 T3-L1 adipocytes (Byon et al. 1998). Purification studies guided by bioactivity enhanced the additive outcomes of chlorogenic acid.
One more study on the plant proposed that methanolic extract of C. intybus contains chlorogenic acid in combination with other caffeic acid derivatives. The 2-deoxy-d-three [H]-glucose uptake was enhanced when methanolic extract and chlorogenic acid was incubated with 3 T3-L1 adipocytes and also there was an inhibition of adipogenesis by altering markers of adipogenesis and signaling of insulin. The in vivo studies have evaluated the effect of CME on insulin sensitivity in diabetic rats. The insulin sensitivity as well as plasma metabolic profile was restored on supplementation of CME for 2 weeks. Same study concluded that the caffeoyl derivatives of leaves of C. intybus had promising pharmacological effect on homeostasis (Gum et al. 2003).
12.5.5 Antimicrobial Activity
The antibacterial potential of naturally affluent acid extract of C. intybus has been investigated on periodontopathic bacteria consisting of Prevotella intermedia, Streptococcus mutans, and A. naeslundii. Oxalic acid, shikimic acid, quinic acid, and succinic acid are active compounds isolated from Cichorium extract. Adhesion of microorganism to the cells and biofilm formation was lowered using these natural acids with one of a kind tiers of efficacy (Gazzani et al. 2000). One of the promising study on C. intybus proposed that the crude aqueous and natural seed extracts possess significant antimicrobial activity in opposition to various pathogenic bacteria, specifically, Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, and extract of the roots had mentioned outcomes for B. subtilis, Staphylococcus aureus, Salmonella typhi, Micrococcus luteus, and Escherichia coli (Rub and Sasikumar 2016). Chicory leaf extract has also confirmed a moderate interest in opposition to multidrug resistant S. typhi (Rani and Khullar 2004). Root extracts rich in guaianolides have proven antifungal residences against anthropophilic fungi. Phytoalexin cichoralexin, a sesquiterpenoid remoted from this plant, manifested strong antifungal interest in opposition to Pseudomonas cichorii (Monde et al. 1990). One of the studies on ethanol, ethyl acetate, and aqueous extract of C. intybus has shown significant antibacterial activity, but ethyl acetate extract has notably shown the maximum activity. The growth of Agrobacterium tumefaciens, Erwinia carotovora, Pseudomonas fluorescens, and Pseudomonas aeruginosa was inhibited by aqueous extract. Comparative studies suggest that ethyl acetate extract has the best pastime with respect to all examined bacterial species. P. aeruginosa become the maximum sensitive and had the widest zones of inhibition. Root extracts have greater extensive antibacterial activity than extracts from complete plant (Keles et al. 2001). Another research proposes hydroalcoholic and ethanol extracts of chicory (15 mg/mL) showed the significant activity towards S. aureus. On the opposite hand antifungal activity was shown by aqueous extract of C. intybus whereas ethyl acetate extract lagged antifungal activity (Rehman et al. 2014).
12.5.6 Antioxidant Activity
The foundation motive of the development and continuation of several diseases is oxidative stress. An optimistic way of fighting the undesirable results of reactive oxygen species (ROS) triggered oxidative damage can be diminished using exogenous antioxidants or boosting endogenous antioxidants. The attenuation of ROS due to oxidative harm can be reduced by wide range of nonenzymatic antioxidants synthesized by plants. Antioxidants notably put off oxidation of oxidizable substrates when the substrate concentration is higher than antioxidants (Halliwell 2007). Antioxidants like reduced glutathione (GSH) and superoxide dismutase (SOD) are synthesized in vivo and some are obtained from dietary supplements (Halliwell 2007; Sies 1997). Exogenous antioxidants are mostly obtained from plants. It is reported that among all the plant species existing on earth, majority of plant species have medicinal importance, and first-rate antioxidant capability is shown by almost all the plant species (Krishnaiah et al. 2011). One of the study reported that the extracts of red chicory possess cytoprotective, antioxidant, and antiproliferative sports in Caco-2 intestinal cell fashions. A modulating impact at the oxidative strain caused via 4-tert-octylphenol and hepatotoxicity was shown by extracts of red chicory.
A huge boom within the tiers of thiobarbituric acid reactive materials (TBARS) and bilirubin, aspartate aminotransferase, alanine transaminase, alkaline phosphatase, and gamma-glutamyl transpeptidase was observed in rats receiving 4-tert-octylphenol. The C. intybus extract modulated the abnormalities on account of the harm due to 4-tert-octylphenol and also caused the reduction in superoxide dismutase, glutathione, and catalase which is an endogenous antioxidant enzyme. Various biochemical and antioxidant parameters were improved and TBARS levels were reduced (Saggu et al. 2014). The antioxidant activity of C. intybus was confirmed by extracts rich in polyphenols and morphological modifications in Caco-2 cells was also validated in the extract due to the presence of polyphenols. The above study also confirmed that the 17 μmol/L concentration of polyphenol fraction possessed mild antioxidant activity, whereas cytotoxic consequences, reduced transepithelial electric resistance, elevated permeability, and altered epithelium were confirmed at concentrations of 70 μmol/L and 34 μmol/L respectively. Oxidative strain and cellular harm was reduced by the extracts of C. intybus and also in vitro Caco-2 cellular version was triggered (Azzini et al. 2016).
According to a study conducted by (Lante et al. 2011), the red chicory extract was found to contain highest anthocyanin content, that is, 313.1 μg/g (31.31 mg/g of sample). Another study was conducted by (Sahan et al. 2017), and they proposed C. intybus contained free phenolic compounds. There was a marked distinction inside the phenolic compound content material as the sample used for the study of antioxidant activity produced significant effervescence. For the optimized crimson chicory extract, the EC50 value was determined in correlation with anthocyanin concentration. From the remaining percent of DPPH as a feature of the attention ratio of the anthocyanins, the EC50 value was calculated and promising results were obtained and thus radical scavenging capacity was found to be associated with a decrease EC50 fee (Brand-Williams et al. 1995).
One more study for antioxidant evaluation was conducted on C. intybus juices using the centrifugation of the plant via micellar model device linoleic acid/b-carotene and hence it was concluded that it has antioxidant activity. The pro-oxidant components had been thermally instable because the boiled juice has shown promising antioxidant activity. Juice additives from C. intybus have been fractionated with the aid of sequential dialysis. The presence of numerous antioxidant compounds having different molecular weight and polar features have been noticeably evaluated from C. intybus by reversed-phase high-performance liquid chromatography (RP-HPLC) technique. The fraction of C. intybus extract that contributes to antioxidant activity is retained by molecular weight 300,000 Da dialysis membrane (Papetti et al. 2002). In yet another study the polyphenols-wealthy fraction of C. intybus was subjected to DPPH radical scavenging activity (Heimler et al. 2009). Polyphenol content and evaluation of antioxidant activity of this plant was determined by means of artificial 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl radical by the reaction catalyzed with the aid of relevant enzymatic assets for reactive oxygen species, particularly, diaphorase, xanthine oxidase, and myeloperoxidase. Evaluation of antioxidant activity was done by synthetic radical and the enzyme-catalyzed reactions and thus total phenolics were extensively correlated with antioxidant activity (Lavelli 2008). Another research proposed dose-dependent inhibition of xanthine oxidase enzyme using hydroalcoholic extract of aerial parts of C. intybus (Pieroni et al. 2002). Additionally, chelated ferrous ion and inhibited hydrogen peroxide were observed for DPPH radical scavenging activity of C. intybus (Nehir El and Karakaya 2004).
12.5.7 Antimalarial Activity
The antimalarial use of C. intybus has been reported on the basis of its traditional use in Afghanistan as the fresh roots of plant have been reported to be used as regime for malarial fevers. Isolated bitter compounds namely lactucopicrin, lactucin, and guaianolide sesquiterpenes from aqueous extract of C. intybus have been reported to possess significant antimalarial activity. One of the studies conducted on plasmodium falciparum reports inhibition of the HB3 a dead ringer for strain Honduras-1 of Plasmodium falciparum using 10 μg/mL and 50 μg/mL concentration of lactucin and lactucopicrin respectively (Bischoff et al. 2004). The Walter Reed Army Medical Research Institute validated the inhibitory action of lactucin (complete inhibition at 10 μg/mL) and lactucopicrin (complete inhibition at 50 μg/mL) as the parallel outcomes were observed for a light unprotected crude ether extract of chicory root. One more study proposes the use of ether extract of chicory against W-2 strain of Plasmodium falciparum and D-6 strain (clone of Africa). The ether extract has been observed to be moderately energetic in opposition to the W-2 strain of Plasmodium falciparum (Indochina clone, IC50 = 243.4) and weakly energetic against the D-6 strain (clone of Africa). These mixed consequences supply credence to the Afghan claim of a light-sensitive plant treatment for malaria. Different structurally associated sesquiterpene lactones and isolated compounds like lactucin and lactucopicrin are likely additives of an aqueous extract and hence presence of such compounds in fresh aqueous extract might show various degrees of antimalarial activity and that the collective activity may also provide an cheaper, quite simply available alternative or adjunct remedy to the affliction (Kisiel and Zielińska 2001).
12.5.8 Anthelmintic Activity
One of the most generic and economically vital pathogens in cattle across the world are nematode parasites of the gastrointestinal tract, particularly in animals which can be grazed outside (Fitzpatrick 2013). For the evaluation of anthelmintic potential of subsidiary metabolites found in chicory plant, various studies are conducted. Thus, several studies reported that grazing of animals on C. intybus had better overall performance based index, also the incidence of nematode in gastrointestinal tract decreased. Enormous studies on the plant have shown promising results of anthelmintic activity due to the presence of condensed tannins and sesquiterpene lactones (Miller et al. 2011).
Anthelmintic activity of this plant has been additionally observed for lambs. The study proposed that the lambs consuming this plant had lesser number of abomasal helminths (Marley et al. 2003). Larval migration inhibition assay has been used to evaluate the efficacy of sesquiterpene-rich extract and condensed tannins in opposition to deer lungworm larvae, Dictyocaulus viviparus and some larvae of gastrointestinal nematode. Another study investigated on both lungworm and gastrointestinal nematodes, C. intybus was found to produce a dose-dependent decrease in larval motility (Molan et al. 2003). Egg hatching of Haemonchus contortus was also inhibited using sesquiterpene lactone-rich extracts of C. intybus. Significant reduction in survival of third-stage larvae of Ascaris suum has been reported using purified C. intybus extract (Williams et al. 2014).
12.5.9 Analgesic Activity
Analgesic activity of C. intybus was evaluated using hot plate and tail-flick test. In both the tests analgesic movement in mice was exhibited using lactucopicrin, 11β, 13-dihydrolactucin, and lactucin. In the recent study, all three compounds exerted an analgesic effect was exerted by all the three compounds, but the compound lactucopicrin produced maximum effect. Evaluation using tail-flick test, 30 mg/kg dose of the tested compounds produced antinociception effect akin to 60 mg/kg dose of ibuprofen. As glaring from the reduced spontaneous locomotor activity in mice compounds like lactucopicrin and lactucin were also reported to possess sedative action (Wesołowska et al. 2006).
12.5.10 Tumor Inhibitory Activity
C. intybus has been evaluated for tumor inhibitory activity. One of the studies proposed that ethanolic crude extract of roots of chicory produced widespread hampering of Ehrlich neoplasm in mice. Also, 70% growth inside lifestyle stretch become discovered using intraperitoneal dose of tested extract equal to 500 mg/kg/day (Hazra et al. 2002). Antiproliferative impact on amelanotic melanoma C32 mobile strains was exerted by aqueous alcoholic macerate of C. intybus leaves (Conforti et al. 2008). Compounds like Magnolialide, 1β-hydroxyeudesmanolide remoted from chicory roots constraint various tumor mobile traces and differentiation of human leukemia U-937 and HL-60 cells to cells resembling monocytes and macrophages was also prompted (Lee et al. 2000).
12.5.11 Antiparasitic Activity
Gastrointestinal (GI) parasites are responsible for causing infections in grazing farm animals worldwide, along with scientific and subclinical sicknesses due to which agricultural economies and food manufacturing could be markedly impaired (Fitzpatrick 2013). In 1980s, C. intybus was selected for feeding farm animals and thus the first industrial forage variety (Grasslands Puna) was released. Henceforth, there has been the development of various forage C. intybus cultivars (Rumball et al. 2003). Authentic evidence of C. intybus as an antiparasitic has been furnished through novel research involving in vitro assays and excessive outturn chemical profiling of the tested extracts of the plant. As described by Foster et al., sesquiterpene lactone–rich extracts from two forage C. intybus cultivars (“Grasslands Puna” and “Forage Feast”) have been reported to bring about a dose-dependent inhibition of egg hatching in unfastened-living degrees of H. contortus. One of the researches identified the main guaianolide of C. intybus in the tested extracts and reported multiplied ovicidal pastime of the Grasslands Puna extract (Foster et al. 2011). Recent studies have proven that the C. intybus extract containing sesquiterpene lactone have effective and dose-dependent in vitro pastime against parasitic levels of livestock nematodes (grown up Cooperia oncophora and Ostertagia ostertagi), that are predicted to be important targets of nutritional supplements in the host (Pena-Espinoza et al. 2015).
12.5.12 Renal Impairment
A wholesome kidney is vital for glucose homeostasis. The glucose is filtered by the kidney and then the filtered glucose is either reabsorbed by the kidney or excreted in the urine. Glucose uptake is essential for energy requirement and the newly synthesized glucose via gluconeogenesis is also released into the circulation (Marsenic 2009). Unfavorable changes in the kidney tissue are usually generated due to metabolic syndrome, obesity, and diabetes leading to altered kidney function. The main cause of chronic kidney disorder (CKD) is hyperglycemia that can lead to diabetic nephropathy, which in turn is main cause of end-stage renal ailment (Rebić et al. 2015).
Recent research has proved to be beneficial in comparing hypoglycemia and antihyperlipidemic consequences of lyophilized C. intybus seed extract (CSE). One of the study proposed the usefulness of CSE in preventing diabetes-induced kidney damage (McMahon and Waikar 2013). Another study was carried out on diabetic animals with early type 2 diabetes and late type 2 diabetes. In early type 2 diabetes, CSE was found to possess ameliorating effects on urea, BUN, alpha-1-microglobulin, sodium, and potassium in serum. One more study evaluated the urine of early type 2 diabetes after using Cichorium seed extract and the promising effect was observed on the levels of alpha-1-microglobulin, as urine alpha-1-microglobulin was substantially reduced. In yet another study serum uric acid levels decreased in late type 2 diabetes by using C. intybus seed extract. Hence, from both the studies it can be concluded that the C. intybus benefitted each kind of diabetes with reference to histology-reduced glomerular diameter and serum uric acid in late type 2 diabetes and lowered urinary alpha-1-microglobulin in early type 2 diabetes (Ghamarian et al. 2012).
12.6 Phytochemistry
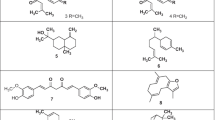
Phytochemical screening of C. intybus revealed that various parts of the plant contain different constituents such as sesquiterpene lactones, derivatives of caffeic acid like chicoric acid, chlorogenic acid, isochlorogenic acid, and dicaffeoyltartaric acid), inulin, proteins, sugars, flavonoids, alkaloids, coumarins, hydroxy derivatives of coumarins, terpenoids, essential and volatile oils, polyenes, and numerous vitamins (Al-Snafi 2016). The structures of various isolated compounds have been shown in Fig. 12.1.
12.6.1 Phytochemistry of Root
A study was conducted to isolate numerous compounds from the roots of the plant. Upon transformed root culture, sesquiterpenes of germacrane and guaiane type such as lactucopicrin, 8-desoxylactucin were isolated from the roots along with glycosides of sesquiterpene lactone (sonchuside A, ixerisoside D, and crepidiaside B). Chicory roots contained higher amount of tannins but lesser total phenolic content.
The methanolic extract of chicory root was studied using GC-MS chromatographic technique, which reveals the presence of 22 compounds that were later identified and exhibits several peaks indicating the presence of different characterized constituents using National Institute of Standards & Technology (NIST) library database. The major group in these compounds was aldehyde, fatty acid, hydrocarbon, ester, steroid, and terpenoid and the compounds which were identified include tetradecanoic acid; 2-(ethylhexyl)salicylate; 1,2-benzenedicarboxylic acid; bis(2-methylpropyl)ester; 2-decenal (Z); 2,4-decadienal (E,E); 9-octadecenoic acid; hexadecanoic acid; cis-9-Hexadecenoic acid; n-hexadecanoic acid; octadecanoic acid; eicosatrienoic acid (Z,Z,Z), mono(2-ethylhexyl)ester; cyclopropane; hexadecane; 1,1-dichloro-2,2,3,3-trimethyl-9,12 octadecadienoic acid (Z,Z); 2-hydroxy-1-(hydroxymethyl)ethylester; docasone; stigmasta-dien-3-ol; beta-sitosterol; lupeol (Malik et al. 2016).
Other studies conducted on chicory root reveal the presence of several phytoconstituents like inulin (a polysaccharide similar to starch), flavonoids, coumarins, sesquiterpene, lactones (e.g., lactucopicrin and lactucin), tannins, vitamins, minerals, alkaloids, and volatile oils. Inulin, a fructose polymer with beta glycosidic linkage, is contained in 68% of compounds isolated from chicory roots (Nwafor et al. 2017). A study conducted by Soobo reported that root of chicory is high in oligofructose and fructan-containing inulin (Shim 2005). Chemically inulin has been converted to two fragments, that is, glucose and fructose by the process of hydrolysis as it is a polydisperse(2,1) fructan (Peters and van Amerongen 1998). Numerous sesquiterpenes are found to be accountable for the bitter taste of chicory and this was confirmed by isolating sesquiterpene lactones like lactucin, 8-deoxylactucin and lactucopicrin. One more study on roasted chicory roots reported that it contain various other compounds like phenols, furfural, vanillin, pyrazine, benzothiazoles, aldehydes, phenyl acetic acid, 2-acetylpyrrole, furans, aromatic hydrocarbons, organic acids, and insole alkaloid (like carboline) in traces (de Kraker et al. 1998). Chicory root extract in which the insoluble fraction was removed using filtration and centrifugation was found to contain alkaloids, volatile oils, fixed oils, fatty acids (oleic and palmitic acid),triterpenes, tannins, sugars (mannose and fructose), and saponins (Nandagopal and Kumari 2007).
12.6.2 Phytochemistry of Flower
For the study of phytoconstituents present in the flower of chicory, the technique of column chromatography was used. The study conducted by Norbaek used subsequent preparative HPLC with Amberlite XAD-7 for carrying out column chromatography which isolated anthocyanins from the plant (Nørbæk et al. 2002).
In yet another study, the methanolic extract of chicory flower was evaluated using GC-MS technique and hence different bioactive compounds were identified, some of the important bioactive compounds include ketones (4h-pyrone; 4-(1-hydroperoxy-2,2-dimethyl-6-methylene-cyclohexyl)-pent-3-en-2-one; 6-Dodecanone, 2-Heptadecanone); Aldehyde (5-(hydroxymethyl)-2-furaldehyde), Fatty acids (octadecanoic acid, tetradecanoic acid, n-hexadecanoic acid and heptadecanoic acid); hydrocarbons; esters (2-propenyl nonanoate, cyclohexanol, etc.), sugar, steroids (Malik et al. 2016).
One more study proposed that the flowers also contain cichoriin in addition to intybin, lactucin, and a crystalline colorless glucoside (Shaikh et al. 2010). Saccharides, methoxy-coumarin cichorine, essential oils, and flavonoids were also found to be present in chicory flowers (Street et al. 2013).
12.6.3 Phytochemistry of Seed
The study on phytochemical evaluation on seeds of the plant reported that the chicory seed extract contained significant amount of phenolics content (51.7–284 GAE mg/100 g of dry sample) and flavonoids (42.2–152 CE mg/100 g of dry sample) (Al-Snafi 2016).
From analysis of Bisma Malik, the major compounds that are present in the seed extract are fatty acids (pentadecanoic acid, n-hexadecanoic acid, tetradecanoic acid, octadecanoic acid, and 9-octadecenoic acid); esters (1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester, hexadecanoic acid, methyl ester, 9,12-octadecanoic acid); hydrocarbons (octacosane, docosane); terpenoids (verrucarol, lupeol); steroids (stigmasterol), and ketones.
According to the study of (WenYing and Jin-Gui 2012), chicory seeds are rich of nutrients which are beneficial for two types of nutrition, that is, monogastric and ruminant. They also determined that most chicory seed varieties possess crude protein in higher amounts which usually more than 19% of dry weight and these chicory seeds are 1.5–2.5 times more efficient than the standard grains, like rice, barley, corn, and wheat. These authors distinguished that chicory seeds are also considered to be the reliable source of nearly all essential amino acids like leucine, methionine, phenylalanine, lysine, isoleucine, etc.
In addition, the seeds are also considered to be the good source of saturated as well as unsaturated fatty acids of which linoleic acid, including monounsaturated acids like oleic acid, stearic acid, and palmitic acid is about 76%. On comparison with other plant parts, the seeds are found to be the main source of vital minerals like potassium, selenium, magnesium, zinc, and calcium. Moreover, from C. intybus seeds, some researchers have isolated a sesquiterpene glycoside, cichotyboside, which had been confirmed to possess a good hepatoprotective activity. From the above discussion it can be concluded that the chicory seeds are essential nutritional components for both humans as well as animals (WenYing and Jin-Gui 2012).
12.6.4 Phytochemistry of Stem
A number of compounds such as tannins, flavonoids, saponins, cardiac glycosides, terpenoids, and anthocyanins were seen in the stem of C. intybus after phytochemical analysis (Al-Snafi 2016). By using GC-MS different phytochemicals were identified and characterized by using NIST library database. The important phytochemicals identified from the stem of C. intybus are fatty acids (tetradecanoic acid, n-hexadecanoic acid, pentadecanoic acid); aldehydes (2-furancacarboxaldehyde, 5(hydroxymethyl)palmitaldehyde); sugar (beta-d-glucopyranose,1,6 anhydro); terpenoid (2-hexadecen-1-ol, lupeol, etc.); hydrocarbons; steroids (cholesta(4,6-dien)3-ol, acetoxystigmasta-4,6,22-triene, stigmasterol, gamma-sitosterol), and esters (Malik et al. 2016) (Table 12.1).
12.6.5 Phytochemistry of Leaf
The phytochemical analysis on the leaves of C. intybus conducted by (Al-Snafi 2016) reported that the total flavonoid content and total phenolic content is comparatively high than other parts of the plant and it was also determined that leaves have comparatively high reducing sugar and nonreducing sugar content. Chicory leaves are also considered to be rich source of usually free amino acids and proteins that are soluble in water. The list of identified compounds from methanolic extract of leaf as well as root using HPLC technique are given in Table 12.2.
References
Ahmad M, Qureshi R, Arshad M, Khan MA, Zafar M (2009) Traditional herbal remedies used for the treatment of diabetes from district Attock (Pakistan). Pak J Bot 41(6):2777–2782
Ahmed B, Al-Howiriny TA, Siddiqui AB (2003) Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol 87(2–3):237–240
Al-Asmari AK, Al-Elaiwi AM, Athar MT, Tariq M, Al Eid A, Al-Asmary SM (2014) A review of hepatoprotective plants used in Saudi traditional medicine. Evid-Based Complem Altern Med 2014:22
Al-Snafi AE (2016) Medical importance of Cichorium intybus—a review. IOSR J Pharm 6(3):41–56
van Arkel J et al (2012) Sink filling, inulin metabolizing enzymes and carbohydrate status in field grown chicory (Cichorium intybus L.). J Plant Physiol 169(15):1520–1529
Azzini E et al (2016) The potential health benefits of polyphenol-rich extracts from Cichorium intybus L. studied on Caco-2 cells model. Oxid Med Cell Longev 2016:1594616
Bagad AS, Joseph JA, Bhaskaran N, Agarwal A (2013) Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv Pharmacol Sci 2013:805756
Bais HP, Govindaswamy S, Ravishankar GA (2000) Enhancement of growth and coumarin production in hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow local) under the influence of fungal elicitors. J Biosci Bioeng 90(6):648–653
Bais HP, Ravishankar GA (2001) Cichorium intybus L—cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J Sci Food Agric 81(5):467–484
Barry TN (1998) The feeding value of chicory (Cichorium intybus) for ruminant livestock. J Agric Sci 131(3):251–257
Benítez G, González-Tejero MR, Molero-Mesa J (2010) Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J Ethnopharmacol 129(1):87–105
Bischoff TA, Kelley CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Arefi AG (2004) Antimalarial activity of Lactucin and Lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J Ethnopharmacol 95(2–3):455–457
Brand-Williams W, Cuvelier M-E, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30
Byon JCH, Kusari AB, Kusari J (1998) Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol Cell Biochem 182(1–2):101–108
Cadalen T et al (2010) Development of SSR markers and construction of a consensus genetic map for chicory (Cichorium intybus L.). Mol Breed 25(4):699–722
Cavin C et al (2005) Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem Biophys Res Commun 327(3):742–749
Chen B, Zhu H (2016) AMPK: a bridge between inflammation and metabolism. JSM Atheroscler 1(2):1008–1016
Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F (2008) Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol 46(10):3325–3332
Crellin JK, Philpott J (1990) A reference guide to medicinal plants: herbal medicine past and present. Duke University Press, Durham
DerMarderosian A, Beutler JA (2002) The review of natural products: the most complete source of natural product information., no. Ed. 3. Facts and Comparisons
Elgengaihi S, Mossa A-TH, Refaie AA, Aboubaker D (2016) Hepatoprotective efficacy of Cichorium intybus L. extract against carbon tetrachloride-induced liver damage in rats. J Diet Suppl 13(5):570–584
Ema H (2010) Community herbal monograph on Rosmarinus officinalis L., folium. EMA/HMPC/13633/2009, 15 July 2010
Fitzpatrick JL (2013) Global food security: the impact of veterinary parasites and parasitologists. Vet Parasitol 195(3–4):233–248
Fleming T (2000) PDR for herbal medicines. Medical Economics Company, New Jersey
Foster JG, Cassida KA, Turner KE (2011) In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus L.) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Vet Parasitol 180(3–4):298–306
Fujisaka S et al (2009) Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58(11):2574–2582
Funk VA et al (2005) Everywhere but Antarctica: using a supertree to understand the diversity and distribution of the compositae. Biol Skr 55:343–374
Gadgoli C, Mishra SH (1997) Antihepatotoxic activity of Cichorium intybus. J Ethnopharmacol 58(2):131–134
Gazzani G, Daglia M, Papetti A, Gregotti C (2000) In vitro and ex vivo anti-and prooxidant components of Cichorium intybus. J Pharm Biomed Anal 23(1):127–133
Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A (2012) Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU J Pharm Sci 20(1):56
Gilani AH, Janbaz KH (1994) Evaluation of the liver protective potential of Cichorium intybus seed extract on acetaminophen and CCl4-induced damage. Phytomedicine 1(3):193–197
Gilani AH, Janbaz KH, Javed MH (1993) Hepatoprotective activity of Cichorium intybus, an indigenous medicinal plant. Med Sci Res 21:151
Guarrera PM, Forti G, Marignoli S (2005) Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J Ethnopharmacol 96(3):429–444
Guarrera PM, Savo V (2013) Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: a review. J Ethnopharmacol 146(3):659–680
Gum RJ et al (2003) Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 52(1):21–28
Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med 27(1):1–93
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35(5):1147–1150
Hazra B, Sarkar R, Bhattacharyya S, Roy P (2002) Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia 73(7–8):730–733
Heimler D, Isolani L, Vignolini P, Romani A (2009) Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem 114(3):765–770
Huang G-J, Pan C-H, Wu C-H (2012) Sclareol exhibits anti-inflammatory activity in both lipopolysaccharide-stimulated macrophages and the λ-carrageenan-induced paw edema model. J Nat Prod 75(1):54–59
Ivashkin VT, Lapina TL, Maiev IV, Trukhmanov AS (2003) Rational pharmacotherapy of diseases of the digestive system. Literra, Moscow
Izzo AA, Hoon-Kim S, Radhakrishnan R, Williamson EM (2016) A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phyther Res 30(5):691–700
Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M (2001) Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North Centre region of Morocco (Fez–Boulemane). J Ethnopharmacol 77(2–3):175–182
Judžentienė A, Būdienė J (2008) Volatile constituents from aerial parts and roots of Cichorium intybus L. (chicory) grown in Lithuania. Chemija 19(2):25–28
Jung GA, Shaffer JA, Everhart JR, Varga GA (1996) Performance of ‘Grasslands Puna’chicory at different management levels. Agron J 88(1):104–111
Keles O, Bakirel T, Ak S, Alpmar A (2001) The antibacterial activity of some plants used for medicinal purposes against pathogens of veterinary importance. Folia Vet 45(1):26–31
Keshk WA, Zahran SM, Katary MA, Ali DA-E (2017) Modulatory effect of silymarin on nuclear factor-erythroid-2-related factor 2 regulated redox status, nuclear factor-κB mediated inflammation and apoptosis in experimental gastric ulcer. Chem Biol Interact 273:266–272
Kiers AM, Mes THM, Van Der Meijden R, Bachmann K (1999) Morphologically defined Cichorium (Asteraceae) species reflect lineages based on chloroplast and nuclear (ITS) DNA data. Syst Bot 24:645–659
Kisiel W, Michalska K (2002) A new coumarin glucoside ester from Cichorium intybus. Fitoterapia 73(6):544–546
Kisiel W, Zielińska K (2001) Guaianolides from Cichorium intybus and structure revision of Cichorium sesquiterpene lactones. Phytochemistry 57(4):523–527
Kokoska L, Polesny Z, Rada V, Nepovim A, Vanek T (2002) Screening of some Siberian medicinal plants for antimicrobial activity. J Ethnopharmacol 82(1):51–53
Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL (2014) Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol 5:470
de Kraker J-W, Franssen MCR, de Groot A, König WA, Bouwmeester HJ (1998) (+)-Germacrene A biosynthesis: the committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol 117(4):1381–1392
Krishnaiah D, Sarbatly R, Nithyanandam R (2011) A review of the antioxidant potential of medicinal plant species. Food Bioprod Process 89(3):217–233
Krylova S, Vymyatnina Z, Zueva E, Amosova E, Razina T, Litvinenko V (2015) Effects of Cichorium intybus L. root extract on secretory activity of the stomach in health and ulcer disease. Bull Exp Biol Med 159(5):638–641
Lante A, Nardi T, Zocca F, Giacomini A, Corich V (2011) Evaluation of red chicory extract as a natural antioxidant by pure lipid oxidation and yeast oxidative stress response as model systems. J Agric Food Chem 59(10):5318–5324
Lavelli V (2008) Antioxidant activity of minimally processed red chicory (Cichorium intybus L.) evaluated in xanthine oxidase-, myeloperoxidase-, and diaphorase-catalyzed reactions. J Agric Food Chem 56(16):7194–7200
Lee K-T, Kim J-I, Park H-J, Yoo K-O, Han Y-N, Miyamoto K (2000) Differentiation-inducing effect of magnolialide, a 1β-hydroxyeudesmanolide isolated from Cichorium intybus, on human leukemia cells. Biol Pharm Bull 23(8):1005–1007
Li G-Y, Gao H-Y, Huang J, Lu J, Gu J-K, Wang J-H (2014) Hepatoprotective effect of Cichorium intybus L., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol 20(16):4753
Loi MC, Maxia L, Maxia A (2005) Ethnobotanical comparison between the villages of Escolca and Lotzorai (Sardinia, Italy). Int J Geogr Inf Syst 11(3):67–84
Malik B, Pirzadah TB, Tahir I, Abdin MZ, Rehman RU (2016) Phytochemical studies on Cichorium intybus L.(chicory) from Kashmir Himalaya using GC-MS. J Pharm Res 10(11):715–726
Marley CL, Cook R, Keatinge R, Barrett J, Lampkin NH (2003) The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vet Parasitol 112(1–2):147–155
Marsenic O (2009) Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 53(5):875–883
Masters SL et al (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11(10):897–904
McMahon GM, Waikar SS (2013) Biomarkers in nephrology: core curriculum 2013. Am J Kidney Dis 62(1):165–178
Miller MC, Duckett SK, Andrae JG (2011) The effect of forage species on performance and gastrointestinal nematode infection in lambs. Small Rumin Res 95(2–3):188–192
Miraldi E, Ferri S, Mostaghimi V (2001) Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran). J Ethnopharmacol 75(2–3):77–87
Molan AL, Duncan AJ, Barry TN, McNabb WC (2003) Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitol Int 52(3):209–218
Mona IM, Wafaa AA, Elgindy AA (2009) Chemical and technological studies on chicory (Cichorium intybus L.) and its applications in some functional food. J Adv Agric Res 14(3):735–742
Monde K, Oya T, Shirata A, Takasugi M (1990) A guaianolide phytoalexin, cichoralexin, from Cichorium intybus. Phytochemistry 29(11):3449–3451
Mulinacci N, Innocenti M, Gallori S, Romani A, La Marca G, Vincieri FF (2001) Optimization of the chromatographic determination of polyphenols in the aerial parts of Cichorium intybus L. Chromatographia 54(7–8):455–461
Nam S, Smith DM, Dou QP (2001) Tannic acid potently inhibits tumor cell proteasome activity, increases p27 and Bax expression, and induces G1 arrest and apoptosis. Cancer Epidemiol Prev Biomarkers 10(10):1083–1088
Nandagopal S, Kumari BDR (2007) Phytochemical and antibacterial studies of Chicory (Cichorium intybus L.)—a multipurpose medicinal plant. Adv Biol Res (Rennes) 1(1–2):17–21
Nehir El S, Karakaya S (2004) Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int J Food Sci Nutr 55(1):67–74
Nørbæk R, Nielsen K, Kondo T (2002) Anthocyanins from flowers of Cichorium intybus. Phytochemistry 60(4):357–359
Nwafor IC, Shale K, Achilonu MC (2017) Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci World J 60:357–359
Özgökçe F, Özçelik H (2004) Ethnobotanical aspects of some taxa in East Anatolia, Turkey. Econ Bot 58(4):697
Papetti A, Daglia M, Gazzani G (2002) Anti-and pro-oxidant activity of water soluble compounds in Cichorium intybus var. silvestre (Treviso red chicory). J Pharm Biomed Anal 30(4):939–945
Pena-Espinoza M, Boas U, Williams AR, Thamsborg SM, Simonsen HT, Enemark HL (2015) Sesquiterpene lactone containing extracts from two cultivars of forage chicory (Cichorium intybus) show distinctive chemical profiles and in vitro activity against Ostertagia ostertagi. Int J Parasitol Drugs Drug Resist 5(3):191–200
Peters AM, van Amerongen A (1998) Relationship between levels of sesquiterpene lactones in chicory and sensory evaluation. J Am Soc Hortic Sci 123(2):326–329
Pieroni A (2000) Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J Ethnopharmacol 70(3):235–273
Pieroni A, Janiak V, Dürr CM, Lüdeke S, Trachsel E, Heinrich M (2002) In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phyther Res 16(5):467–473
Pieroni A, Quave C, Nebel S, Heinrich M (2002) Ethnopharmacy of the ethnic Albanians (Arbëreshë) of northern Basilicata, Italy. Fitoterapia 73(3):217–241
Pushparaj PN, Low HK, Manikandan J, Tan BKH, Tan CH (2007) Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol 111(2):430–434
Rani P, Khullar N (2004) Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phyther Res 18(8):670–673
Rebić D, Hadžović-Džuvo A, Valjevac A (2015) Chronic kidney disease and endothelium. EMJ Nephrol 3(1):111–117
Rehman A, Ullah N, Ullah H, Ahmad I (2014) Antibacterial and antifungal study of Cichorium intybus. Asian Pacific J Trop Dis 4:S943–S945
Rizvi W et al (2014) Anti-inflammatory activity of roots of Cichorium intybus due to its inhibitory effect on various cytokines and antioxidant activity. Anc Sci Life 34(1):44
Roustakhiz J, Majnabadi JT (2017) Cultivation of chicory (Cichorium intybus L), an extremely useful herb. Int J Farming Allied Sci 6(1):14–23
Rub RA, Sasikumar S (2016) Antimicrobial screening of Cichorium intybus seed extracts. Arab J Chem 9:S1569–S1573
Rumball W, Keogh RG, Miller JE, Claydon RB (2003) ‘Choice’forage chicory (Cichorium intybus L.). New Zeal J Agric Res 46(1):49–51
Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H (2014) Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol 72:138–146
Sahan Y, Gurbuz O, Guldas M, Degirmencioglu N, Begenirbas A (2017) Phenolics, antioxidant capacity and bioaccessibility of chicory varieties (Cichorium spp.) grown in Turkey. Food Chem 217:483–489
Said HM (1982) Diseases of the liver: Greco-Arab concepts. Hamdard Foundation Press, Karachi
Saltiel AR (2001) New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104(4):517–529
Sastri BN (1962) The wealth of India, p 425. CSIR, New Delhi
Šavikin K et al (2013) Ethnobotanical study on traditional use of medicinal plants in South-Western Serbia, Zlatibor district. J Ethnopharmacol 146(3):803–810
Schmidt BM, Ilic N, Poulev A, Raskin I (2007) Toxicological evaluation of a chicory root extract. Food Chem Toxicol 45(7):1131–1139
Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T (2001) Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol 75(2–3):95–115
Shah SRU, Gul H, Abdur R, Imtiaz A (2006) Ethnobotanical studies of the flora of district Musakhel and Barkhan in Balochistan, Pakistan. Pak J Weed Sci Res 12(3):199–211
Shaikh T, Mujum A, Wasimuzzama K, Rub RA (2010) An overview on phytochemical and pharmacological profile of Cichorium intybus Linn. Br J Pharmacol 2:298–307
Shim S (2005) Effects of prebiotics, probiotics and synbiotics in the diet of young pigs. PhD thesis.
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol Transl Integr 82(2):291–295
Sile I, Romane E, Reinsone S, Maurina B, Tirzite D, Dambrova M (2020) Medicinal plants and their uses recorded in the Archives of Latvian Folklore from the nineteenth century. J Ethnopharmacol 249:112378
Simon L, Martin HW, Adriano DC (1996) Chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Web.) as phytoindicators of cadmium contamination. Water Air Soil Pollut 91(3–4):351–362
Street RA, Sidana J, Prinsloo G (2013) Cichorium intybus: traditional uses, phytochemistry, pharmacology, and toxicology. Evid-Based Complem Altern Med 2013:579319
Süntar I, Akkol EK, Keles H, Yesilada E, Sarker SD, Baykal T (2012) Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: stepwise isolation of an active component by in vivo bioassay and its mode of activity. J Ethnopharmacol 143(1):299–309
Syed NA, Hasan TN, Aalam SMM (2008) Evaluation of wound healing potential of Chicorium intybus in rats as animal model. Iran J Pharmacol Ther 7(2):180–181
Tyler VE, Brady LR, Robbers JE (1988) Pharmacognosy, 9th edn. Lea Fabiger, Philadelphia
Van Wyk B-E, van Oudtshoorn B, Gericke N (1997) Medicinal plants of South Africa. Briza, Pretoria
Vg D, Dranik LI (1972) Oxycinnamic acids of Cichorium-Intybus, Khimiya Prirodnykh Soedinenii, no. 6. Akademiya Nauk Uzbekskoi Ssr Ul Kuibysheva 15. Tashkent, Uzbekistan, pp 796–797
Vinegar R, Schreiber W, Hugo R (1969) Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 166(1):96–103
Wang Q, Cui J (2011) Perspectives and utilization technologies of chicory (Cichorium intybus L.): a review. Afr J Biotechnol 10(11):1966–1977
Wen H et al (2011) Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12(5):408–415
WenYing G, Jin-Gui L (2012) Chicory seeds: a potential source of nutrition for food and feed. J Anim Plant Sci 13(2):1736–1746
Wesołowska A, Nikiforuk A, Michalska K, Kisiel W, Chojnacka-Wójcik E (2006) Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J Ethnopharmacol 107(2):254–258
Williams AR, Ropiak HM, Fryganas C, Desrues O, Mueller-Harvey I, Thamsborg SM (2014) Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasit Vectors 7(1):518
Wills RBH, Lim JSK, Greenfield H (1986) Composition of Australian foods. 32. Leafy, stem and other vegetables. Food Technol Aust 10:416–417
Yan Y et al (2013) Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38(6):1154–1163
Yıldırımlı Ş (1999) The chorology of the Turkish species of Asteraceae family. Ot Sist Bot Derg 6(2):75–123
Zafar R, Ali SM (1998) Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J Ethnopharmacol 63(3):227–231
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Qadir, I. et al. (2022). Cichorium intybus: A Comprehensive Review on Its Pharmacological Activity and Phytochemistry. In: Masoodi, M.H., Rehman, M.U. (eds) Edible Plants in Health and Diseases . Springer, Singapore. https://doi.org/10.1007/978-981-16-4959-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-4959-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4958-5
Online ISBN: 978-981-16-4959-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)