Abstract
Cola belongs to the family Malvaceae and contains 125 Cola plants. Among the Cola species, Cola acuminata and Cola nitida are the most studied for their pharmacology effects. Cola contains phytochemicals such as alkaloids, caffeine, theobromine, theophylline, quinic acid, chlorogenic acid, purine, (−)-epicatechin, (+)-catechin, sterols, anthraquinones, flavonoid glycosides, cardenolides, tannins, rostratanic acid, bauerenol, lupeol, acotatarone A, lignoceric acid, betulinic acid, friedelanone, friedelan, stigmasterol, and nonanedioc acid, among others. These secondary metabolites are responsible for the various pharmacological activities such as antioxidant, antibacterial, antifungal, antimalarial, anti-inflammatory, antidiabetic, antidiarrheal, antiviral, anticancer, antimycobacterium, and antiatherosclerotic and hypolipidaemic. Economically, Cola has been used in both manufacturing and pharmaceutical industries to produce energy drinks, flavoring agents, wine, chocolates, animal feeds, medicine, food, disinfectant, pomade, organic fertilizers, candles, detergents, and as dyes in textiles. This review work aimed to review the report published up to 2019 describing the traditional uses, phytochemistry, and pharmacological activities of Cola species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional medicine has been in existence for many years and is been used in the treatment of various ailments in the developing countries. Medicinal plants provides alternative sources of bioactive ingredients for the manufacture of drugs by the pharmaceutical industries [1]. Cola, known as African tropical plant belongs to the family Sterculiaceae [2]. The word Cola is a household name in Africa because of the traditional uses of the seed, kola nut. The stem, seeds, nuts, roots, and leaves of the plants in this family are used for various healthcare needs particularly in Nigeria. Because of the importance of traditional medicine practiced in Africa, ethnomedicinal applications of this plants have been extended internationally [3]. Medicinal plants have been used for centuries to treat ailments because of their phytochemical contents [4]. The presence of these phytochemicals in the cola plants, makes them highly valued as medicinal plants [5]. The literature reports of the pharmacological activities and toxicological effects of kola nuts have been published [6]. According to Adeleye et al. 2015, Cola acuminata, Cola verticillata, and Cola nitida are the most used species among the cola plants. This report was validated by Adenuga et al. [7]. The seeds of cola plants grow up to 3 cm long and 3 cm in width containing a minute embryo which houses the cotyledons. C. acuminata has between 3 and 6 cotyledons, while C. nitida has 2 cotyledons with the cotyledons producing the unique splitting pattern in the seeds [8]. The two closely related cola plants, C. nitida, and C. acuminata are differentiated by the number of cotyledons, leaves, and pods [9]. Kola nuts have been used by the various groups of individuals as stimulants. Surprisingly, there are no reports of side effects of the usage of this nut. Conspicuously noticed however on the uses of kola nut is it antecedent brown stain on the teeth. It is on the account of the uses of Cola plants that this review was aimed at investigating the medicinal and biological activities of the plants in the cola family to validate the huge medicinal potential of these plants.
Taxonomy
Kingdom: plantae, order; Malvales, family: Malvaceae, Subfamily: Sterculioideae, genus: Cola, Species: Cola species
Botanical classification
The genus Cola grows in the tropical forests of Africa and belongs to the family Malvaceae (subfamily Sterculioideae). They are commonly known as the kola nut (kola tree). Cola is evergreen plant, grows up to 20 m in height, leaves are about 30 cm long, while the fruits are star-shaped containing lobes (between 2 and 6 lobes). The genus is made up of between 100 and 125 different plants, some are tropical trees, shrubs, or even herbs [10]. Cola species thrive mostly in dry areas of the rain forest, however, Cola gagantea and Cola millenii are widely distributed in both dry and wet forests [11]. C. acuminata, Cola nitida, and Cola verticillata are the notable fruit-bearing species of the Cola species, the others are plants with other economic relevance [12]. The C. acuminata and C. nitida are propagated via the seeds [13].
Traditional uses of Cola species
As part of cultural practices, kola nut is chewed during occasions in many West African region [14]. Some individuals believe that chewing kola nut brings good omen. The spiritual uses of kola nut cannot be overemphasized, as the nuts are used extensively during burial and wedding ceremonies. Students, drivers, and hunters have used kola nuts as stimulants and for its ability to ease hunger pangs [12]. The leaves, nuts, and stem bark of C. acuminata have been used traditionally in the form of juice extract or decoction. In addition, C. acuminata has been a good appetite suppressant and stimulant. It has also been used also in the treatment of respiratory infections, hypertension, as antiparasitic, and as an aphrodisiac [15]. The leaves of C. acuminata has been used as a stimulant and the bark used in the treatment of abdominal disorder [16]. C. acuminata, Cola caricaefolia, Cola rostrata, Cola gabonensis, and Cola pachycarpa were used as an aphrodisiac [17]. The decoction of C. acuminata has been used as a cicatrizant, while the mashed roots were used to treat wounds. Similarly, the macerated bark and leaves of C. verticillata were used to treat cough [18]. C. acuminata and C. nitida have been used as tonic and stimulants [19]. C. acuminata has been used traditionally to enhance memory [20], and as stimulants [21]. Idu et al. [22], reported that among the Idoma people of Benue State, Nigeria, the seeds of cola laurifolia were masticated and used orally in the treatment of anemia in five houses [22]. The stem bark and twig of C. laurifolia were used to treat toothache [23]. Cola acuminata has been used to treat cancer, as stimulant, and as an antidote against poisons [24]. Tannins derived from C. laurifolia was used to treat hemorrhoids, diarrhea, and as dye [25]. The leaves of Cola cordifolia (Cav.) R. Br prepared by the decoction method has been used to treat hypertension successfully [26]. Extracts of the leaf, fruit, and seed of C. acuminata, C. caricaefolia, C. gabonensis, C. nitida, C. pachycarpa, and C. rostrata were used as aphrodisiacs [27]. Furthermore, fruit of C. acuminata has been used effectively as abscesses/ inflammation [28], anti-aging agent [29]. Cola nuts are used for the treatment of fever, whooping cough, malaria, asthma, and as stimulants [30]. Okeke et al. [31] reported that the stimulating property of the seed of C. acuminata was due to its high alkaloid content (0.26 + 0.11%). Aside from being a stimulant, caffeine has been established to help in weight reduction (burns fat) [31, 32]. Individuals take the nut of C. nitida to keep them awake due to its caffeine content [33]. The macerated seeds of C. nitida have been used to treat cough successfully [34], vomiting control in pregnant women [35, 36], and as food [37]. Cola (nuts) are used as stimulants; which enhance physical energy and alertness in humans (euphoria action). In addition, fruits, leaves, and other parts of C. acuminata have been used to treat cough, dysentery, vomiting, chest pain, and diarrhea [38]. Kola nuts are rich in alkaloids, caffeine, kolanin, and theobromine which are used in pharmaceuticals. The leaves of C. milenii has been used to treat gonorrhea, dysentery, ringworm effectively [39]. The leaves of C. nitida have been used to treat wound [40], the stem used to treat rheumatism and arthritis [41], fruits as stimulants, and seeds used in healing rituals [42]. Raymond et al. [43] reported the use of the bark and fruits of C. nitida in the treatment of typhoid fever and respiratory tract infections [43]. Extracts of C. acuminata have been used traditionally to treat microbial ailments [44]. Traditionally, kola nut has been reported to cure morning sickness, migraine, and metabolic disorders [45], asthma, dysentery, and whooping cough [46]. The uses of the seeds of C. nitida in folk medicine to clean teeth and gums have been established [47]. The stem bark of C. nitida has been used to ease child delivery [48]. Furthermore, seeds of C. nitida have been used to corerect weak erection and low sperm count (Nduche and Okwulehie 2015). The extracts of the seed and bark of C. nitida have been reported to strengthen fetuses, treat cough, fontanels, in circumcision healing, and facilitation of childbirth [49], fracture [7], dysentery and diarrhea [50]. The extracts of C. cordifolia (stem bark, leaves) and C. nitida (root bark, fruit) have been used traditionally to treat wounds [51]. C. gigantea has been a good plant used to treat skin infections, sores, and pains [52]. Choi et al. [53] reported that the root of Cola clavata Mast was used to stop bleeding, while the root of Cola usambarensis Engl was used to treat hernia. The maceration extract of the stem of C. cordifolia (Cav.) R.Br. was used to treat tuberculosis [54]. Austarheim et al. [55] reported that C. cordifolia was used mainly to treat wounds and pains [55]. The bark of C. cordifolia (Cav.) R Br has been used to treat dysentery, chest-pain, constipation, while the leaves were used to treat eye problems [10]. The seeds and stem of C. cordifolia (Cav.) R.Br has also been used in the treatment of anemia, blood disorders, and eye diseases, while the extract of the seeds of Cola nitida (Vent.) Schott & Endl were used to cure intestinal problems [37]. The aqueous extract of the leaves of C. latertia has been used to treat malarial [56]. Fruits, leaves, and roots of C. gabonensis were used to cure scabies, migraine and as an aphrodisiac. Also, leaves and fruits of C. pachycarpa have been reported to cure internal heat and cough [42]. C. lepidota has been very effective in the treatment of oxidative stress and other health disorders [57]. The decoction of the leaves of millenii K. Schum and Cola nitida Schott & Endl. have been used to treat liver damage [58, 59]. C. millenii leaves were used to treat venereal diseases [60], infections, fever, and pains [61]. The methanolic extract of C. nitida has been found to induced diuresis in Wistar rats [45]. Despite the widely published information of the medicinal uses of Cola species of the family Malvaceae, there is no evidence of the uses of plants in the Aryuvedic or Unani system. The various medicinal uses of the Cola species are summarized in Table 1.
Economic uses
Both C. nitida and C. acuminata were reported to be of economic importance [9, 62, 63]. The caffeine and flavor contents from the extract of C. acuminata are used in the production of energy drink for sporting activities [24]. In the food industries, kola nut extracts are used as flavoring agents. They are also useful in methyl xanthine-based pharmaceuticals and in the treatment of fatigue. In the U.S.A for instance, kola nuts extracts have been used as beverages [6]. Various kola nuts and their by-products have been used as kola drinks, chocolates, wines, animal feeds, and medicinal products [62]. C. nitida and C. acuminata have been used locally as dyes in textiles, medicine, and food [64]. The fruit of C. nitida has been used as a disinfectant and pomade [65]. Kola nuts have been used in the production of beverage drinks, dyes, organic fertilizers, candles, and detergents [66]. Also, the seeds of C. pachycarpa K. Schum are edible, while the plant is used as a timber product [67]. Barks of C. lepidota Schott and Endl. are used to produce bags and baskets [68].
Phytochemistry
The leaves of C. nitida and C. acuminata are rich in phenols, flavonoids, alkaloids, saponins, tannins [16], steroids, cardiac glycosides, and terpenoids [69]. C. acuminata was found to contain sugar [38]. Chigozie et al. [25] reported that C. laurifolia from Nigeria contained alkaloids (4.10%), saponins (4.6%), and tannins (180 mg/100 g). kola nuts are sources of anthocyanins and phenolics that have been used as antioxidants and as an antidote (tannic acid precipitates toxins in the gut) [24]. The extracts from C. nitida, C. millenii, and C. acuminata contained steroidal triterpenes, alkaloids, reducing sugars, tannins, and saponins [70]. The chloroform and ethanol extracts of the seeds of C. nitida were found to contain tannins, phlobatannins, reducing sugar, alkaloids, flavonoids, saponins, cardiac glycosides, and anthraquinones [71]. The phytochemical analysis of the methanol extract of the seeds of C. nitida led to the isolation of hexadecanoic acid and Caffeine [72]. Furthermore, phytochemical studies of the aqueous, ethanol and n-hexane extracts of the leaves and stem bark of C. gigantea revealed the presence tannins, flavonoids, phenol, cardiac glycoside, steroids, and alkaloids. The steroids and alkaloids were reported to be present in a higher amount [73]. In another study, tannin was also derived from the leaf extract of C. greenwayi Brenan [41]. Similarly, the fruits of C. parchycarpa contain cysteine, lysine and leucine, vitamin B, tocopherols, and ascorbic acid. This result indicates the nutritional values of the fruits of cola plants [74]. Edible fruit pulp and seeds extracts of C. lepidota and C. rostrata were found to contain saponnins, alkaloids, carbohydrates, flavonoids, and terpenoids [75, 76], while the fruits of C. lepidota contain phenols [77]. Alkaloids, tannins, and saponins were derived from the stem bark, seed, leaf, pulp, and root of C. millenii [78], while phytosterols, steroids, alkaloids, saponins, volatile oils, saponin glycosides, triterpenoids, glycosides, hydrolysable tannins, and phenols were found in the fruits [79].
Proximate analysis
The proximate analysis of C. nitida and C. acuminata showed that C. acuminata has more ash, fat, and protein content (booster or energy supplier), low moisture content of 9.73% (responsible for its good storage capacity and the moderate antimicrobial activities), 7.30% crude fiber (aids digestion of food and makes food well absorbed by the body). The proximate analysis of C. nitida and C. acuminata as well as the method of analysis are presented in Table 2 [80]. A similar study showed that C. nitida has the highest moisture content (65.27%) and low ash content (1.84%), while C. acuminata contains the highest crude fiber (10.08%) and protein (413.62%) content (Table 3) [81]. This later report validated the initial result of the proximate analysis.
Mineral content
The results of the mineral content analysis of C. acuminata and C. nitida showed that the plants contain important minerals elements such as Na, Ca, K, Mg, Fe, Zn, P, S, Cu, and Vitamins (Table 3). Cl and Mn were not detected in the plants. The Na (51.47%) and Cu (0.98%) have the highest mineral content and were detected in C. acuminata, while the least values of the mineral content were detected in C. nitida, except for the Na and Cu [81]. The seeds of C. parchycarpa were reported to contain a high content of K, Mn, and Zn, while Mg, Fe, and Ca were found dominant in the fruits [74]. The metal compositions of the fruit’s extract of C. lepidota were found to be Fe (1.79 mg/100 g), Zn (0.27 mg/100 g), and Mn (0.57 mg/100 g). However, heavy metals such as Pb, Cr, and Cd were absent in the fruits. This makes the fruits safe and good for human consumption [82]. The seeds of C. lepidota were found to contain Na, Zn, Ca, Mg, and Mn [83], while the fruits epicarp contain Ca, Na, Zn, Mg, Mn, and Cu. K and Fe were found in the seed and aril, respectively [84]. The seed and pulp of Cola millenii contain Ca, Mg, Fe, Zn, Mn, Na, K, and Cu [85]. These essential elements make the seeds of C. lepidota good for the human body. Adetola and Akinyemi reported that the concentrations of Pb, Cr, and Cd were low in the pods of C. millenii [86]. These results provide conformational bases to justify the medicinal and other uses of Cola species.
Essential oil
Atolani et al. [87] reported the presence of acetylenic fatty acid, palmitic, sterolic acid, and linoleic acids as well as campesterol, stigmasterol, cholesterol, and ß-sitosterol as the essential oils from the seed of C. gigantea. The seed oil of C. millenii was reported to contain acid (32.00 mgKOH/g), peroxide (35.00 meqKOH/g), FFA (916.09%), and iodine (96.57 gI2/100 g) [88]. The essential oil has been reported for its useful bioactivities among which are antibacterial, antifungal, antioxidant, antidiabetic activities [89].
Phytochemical constituents
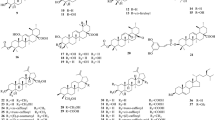
Two flavonoids, epicatechin, and catechin as well as two alkaloids, caffeine and theobromine were derived from the seeds of C. nitida, C. acuminata, and Cola anomala [1]. Caffeine and theobromine are reported to be the most important phytochemicals in kola nuts [6, 90]. Caffeine acts as a stimulant, while theobromine is responsible for the chocolate flavor in kola nuts. C. acuminata and C. nitida contain theophylline, quinic acid, and chlorogenic acid [19] as well as purines [91]. Procyanidin B1 and B2 have been isolated from C. acuminata [15]. Kola nuts contain theobromine, kolanin, and caffeine which are used as stimulants [8]. Pharmacologically, caffeine as a stimulant increases norepinephrine secretion [92]. The HPLC analysis of the aqueous extracts of nuts of C. acuminata and C. nitida showed the presence of caffeine, (−)-epicatechin, (+)-catechin, and procyanidin B1 and B2 [93]. The European Medicine Agency [94] reported the presence of colanin, colatin, colatein in Cola seeds. The report also showed that Cola seed contains amines such as piperidine, isopentylamine, dimethylamine, methylamine, isobutylamine, ethylamine, and pyrrolidine [94]. Proanthocyanidins, fatty acids, sugars, sterols, and alcohols have been identified from the extracts of C. nitida [72] as well as phlobatannins and anthraquinones [91]. The phytochemical investigation of the methanol extract of the leaves of C. caricifolia led to the isolation of caffeoyl derivatives, phytosterols, caffeic acid derivatives, and flavonoid glycosides [95]. Similarly, phytochemical analysis of the ethanol extracts of C. millenii K. Schum, C. acuminata, and C. gigantea A.Chev revealed the presence of cardenolides, tannins, alkaloids, and saponins [96]. A new rostratanic acid was derived from the dichloromethane-methanol extract of the roots of C. rostrata K. Schum. alongside known bauerenol, lupeol, acotatarone A, lignoceric acid, botulin, betulinic acid, friedelanone, friedelan, herranone, arjunolic acid, stigmasterol, nonanedioc acid, β − sitosterol, and β − sitosterol-3-O-β-D-glucopyranoside [97]. Furthermore, the ethanol extract of the seed of C. lepidota contains fatty acids [98]. The structures of the chemical compounds from the Cola species are presented in Fig. 1.
Toxicological studies of Cola species
The seed and leaves C. acuminata were reported to be nontoxic. This justifies the traditional uses of this plant for various purposes [99]. Further toxicity study has shown that there are no serious toxic effects of the uses of C. acuminata [38]. The major ingredient in kola nut is caffeine and presently, there are limited data on the toxicity of extracts of the seeds. However, the safety history of the uses of kola nut-based beverages and other products has been established. Therefore, the consumption of foods containing kola nut extracts is safe [6]. Salahdeen et al. [32] reported that the ethanol extract of kola nuts from C. nitida contains high caffeine content. Therefore, prolong and frequent uses of the nuts produced toxic effects manifested in weight loss. Though C. nitida is safe for consumption, however, individuals with stomach ulcer should avoid eating the nuts as it contains tannin and caffeine [47]. Kola nuts contain high tannin; hence prolonged usage could affect the color of teeth. Presently, there are no reports of death from uses of kola nut. In Nigeria, particularly in the northern region, there have been cases of usage of the kola without side effects. However, prolonged usage could lead to over-dose intake of caffeine that could result in sleeplessness. This effect of caffeine has been observed among students studying for examinations. In a similar study, the aqueous extracts of the leaves and bark of C. cordifolia produced no toxicity on the brine shrimps used [55]. The toxicity study of the crude methanol extract of the root bark of C. rostrata did not show mortality even at dosage of 8.0 g/kg on the Swiss albino mice used. This result showed that the root bark of the plant is safe [100], confirming the use of the plant as an aphrodisiac [17]. In another study, the ethanol extracts the seed of C. millenii K. Schum possessed non-cytotoxicity on the Wistar albino rats used [101]. Also, the leaf and stem bark of C. millenii did not show toxicity on the rats [102]. However, it is advisable to use these extracts in moderate concentration. As the acute toxicity study of the aqueous extract of the seed of C. millenii showed a dose-dependent hepatotoxic effect with LD50 of 1250 mg/kg on Wistar rats after 21 days [103].
The pharmacological activity of Cola species
Anti-bacterial activity
The methanol, ethanol, and aqueous extracts of C. nitida and C. acuminata exhibited moderate activity against S. aureus, E. coli, lactobacillus, P. aeruginosa and K. pneumoniae, with MIC value of 1–4 mg/ml [69]. In another research, the aqueous and ethanol extracts of C. nitida and C. acuminata exhibited considerable activity against P. aeruginosa (LHC181), A. fumigates (LUML56), and P. mirabilis (LHC201) [104]. Furthermore, the ethanol extract of the leaf of C. acuminata exhibited significant antimicrobial activity [99]. The acetone and methanol extracts of the stem of C. acuminata showed antibacterial activity, with MIC values of 5–100 mg/mL [105]. Various extracts of C. nitida were reported to inhibit the growth of Pseudomonas spp, Shigella spp, and Salmonella spp [4]. In addition, the aqueous and methanol extracts of the seed of C. acuminata showed remarkable antibacterial activities [106]. The in vitro antimicrobial study of the ethanol extract of C. acuminata showed activity against K. pneumoniae, with a MIC of 90 mg/ml [107]. In a study, the methanol and aqueous extracts of C. nitida demonstrated antibacterial activity against S. typhi, E. coli, S. aureus, and K. pneumonia [108]. In a similar study, the ethyl acetate and ethanol extracts of the bark of C. nitida exhibited potent antibacterial activity against P. vulgaris, with the zone of inhibition diameter (9.5 ± 0.7 mm), a MIC (5.00 mg/mL), and an MBC (20 mg/mL) [109]. The acetone extract of C. acuminata showed tremendous antibacterial activity against B. subtilis, S. aureus, and E. faecalis, with ED50 values of 3.04 ± 0.08 μg/mL, 4.3 ± 0.03 μg/mL, and 6.72 ± 0.03 μg/mL, respectively. The extract also displayed antibacterial activity, with MIC values of 14–50 μg/mL [44]. Furthermore, the methanolic extract of the seeds of C. nitida showed antimicrobial activity against K. pneumonia, E. coli, S. aureus, and S. pneumonia, with MIC values (6.25–200 mg/ml) [110]. The methanol-water extract of the seeds of C. nitida exhibited antibacterial activity against S. marcescens, S. typhi, B. cereus, S. epidermidis, and P. vulgaris, with the zone of inhibition diameter (13.00 ± 0.577 to 24.33 ± 0.667) mm [47]. In addition, C. nitida extract has demonstrated remarkable activities against C. sporogenes, Pseudomonas Spp., C. pyogenes, C. sporogenes, and Shigella Spp [72]. Similarly, the stem bark of C. nitida has shown activity against MRSA and MLSB [111]. [46] studied the in vitro antibacterial activity of the water-ethanol extract of C. anomala pods. The finding was that the extract exhibited a bactericidal effect in the diarrhetic rats used. The result showed a MIC value of 2.0 mg/mL (P < 0.01). This confirmed the use of this plant in the treatment of gastrointestinal diseases [46].
The ethanol extracts of the leaf and stem bark of C. gigantea exhibited significant antibacterial activity against S. aureus, E. coli, B. subtilis, and P. aeruginosa, with MIC values of 0.125–2.75 mg/mL [52]. The aqueous leaves extract of C. gigantea have shown antimicrobial activities against E. coli, B. subtilis, and S. aureus [73]. Al Muqarrabun, Ahmat [10] reported the biological activities of the ethyl acetate extract of the leaves of C. greenwayi. The result should that the extract displayed moderate antibacterial activities against K. pneumoniae and S. aureus, with a MIC of 0.78 mg/ml and 0.39 mg/ml, respectively. However, the leaf extract of C. natalensis was inactive against S. aureus, B. subtilis, K. pneumonia, and E. coli [10]. Sonibare et al. [96] carried out in vitro antimicrobial activity of ethanol extract of the leaf of Cola Schott & Endl. C. nitida (Vent) Schott & Endl, C. millenii K. Schum and C. acuminata (P. Beauv.) Schott & Endl. The ethanol extract of the leaf of C. acuminata showed remarkable activity against K. pneumoniae, with a MIC value of 90 mg/ml [96]. While the aqueous extract of the leaves of C. gigantea exhibited the highest antibacterial activities against S. aureus, B. subtilis, and E. coli [73]. Similarly, the n-hexane extract of the leaf and bark of C millenii exhibited higher activity against E. coli [112]. The methanol extract of the epicarp of Cola millenii K.Schum displayed excellent activity against S. aureus, P. aeruginosa, and B. subtilis [113].
Anti-inflammatory activity
Dayo et al. [33] studied the anti-inflammatory activity of the methanol extract of C. nitida. The extract exhibited remarkable anti-inflammatory activity at a dosage of 200 mg/kg even better than the aspirin used. Alkaloids derived from the seed of C. laurifolia showed anti-inflammatory activities [25]. The ethanol extract of the stem bark of C. gigantea has shown significant anti-inflammatory activity [52]. In addition, the ethanolic extracts of the twigs and leaves of C. greenwayi, showed remarkable anti-inflammatory activity, with a degree of reduction of 78% and 75%, respectively. Furthermore, the dichloromethane extract of twigs of C. greenwayi showed good anti-inflammatory activity at reduction degree of 78% [10]. Reid et al. [41] reported that the ethanol and dichloromethane extracts of the leaf of C greenwayi showed excellent cyclooxygenase 1 inhibition possibly due to the tannin content in the extracts.
Antioxidant activity
The tannins, alkaloids, and flavonoids found in kola nuts have shown antioxidants activities [114]. [15] reported the antioxidant effects of the methanol extract of the stem bark of C. acuminata. The study showed that a concentration of 500 mg/kg administered to alloxan-induced diabetic rats decreased the blood glucose levels [15]. The seed (ethanol and ethyl acetate) extract of C. acuminata has shown to be a good antioxidant [99]. In addition, the alkaloids from C laurifolia quenched the singlet oxygen in brucine and strychnine. Alkaloids protect the living tissues against singlet oxygen which damages the living tissues in light (UV). Because the antioxidant activity of alkaloids has been established [25], this result confirmed the use of C. laurifolia as natural antioxidants. [115] studied the antioxidant activity of C. nitida and C. acuminata (kola nuts). The result showed that the nut contained high antioxidant activity (13.0–53.21 μmol) [115]. Studies have shown that the extracts of C. nitida contain alkaloids, tannins, and flavonoids that are responsible for the antioxidants activity in the plants [35]. The in vitro studies of the aqueous extracts of nuts of C. acuminata and C. nitida showed significant antioxidant activity, with the IC50 values of 2.74–4.08 mg/mL and 1.70–2.83 mg/mL for the 2-deoxyguanosine HPLC-based assays and hypoxanthine/xanthine oxidase, respectively [93].
The essential oil from the C. gigantea showed potent antioxidant activity in ABTS assay, with an IC50 value of 44.19 ± 6.27 mg/mL [87]. Sut et al. [95] reported that the methanol extract of the leaves of C. caricifolia exhibited excellent antioxidant activity. In one study, the aqueous extract of the stem bark of C. gigantea var. glabrescens displayed good antioxidant activity, with an IC50 value <50 μg/mL [116]. The fruit pulp and seeds of the methanol extracts of C. lepidota and C. rostrata showed significant antioxidant activity, with IC50 values of 60.0–63.0 μg/mL and 50–66.5 μg/mL, respectively [76]. This result justifies the reports of the uses of the fruits and seeds of C. lepidota and C. rostrata as natural antioxidants. In another research, the chloroform extract of the leaf extract of C. lepidota showed higher antioxidant activity, with an IC50 of 50 μg/ml vis á vis the methanol extract, with an IC50 value of 190 μg/ml [117]. The methanol/chloroform extract of the leaf of C. lepidota K. Schum. exhibited antioxidant activity, with an IC50 value of 190/50 μg/mL [118]. While the methanol extract of the seed of C. lepidota exhibited significant antioxidant activity with the radical scavenging percentage (40%) [119]. The methanol extract of the epicarp of C. millenii K.Schum showed strong antioxidant activity [113].
Antifungal activity
The ethyl acetate extract of C. acuminata at 1.8 μg/ml inhibited the rate of mycelia growth in F. verticillioide [99]. In another study, the methanol extract of C. acuminata nuts showed an MFC value of 250 μg/ml against C. albicans [38]. The aqueous and methanol extracts of C. acuminata and C.nitida were found to reduce the mycelia growth of A. fumigates, R. oryzae, A. niger, and M. recemosus [39]. Furthermore, the ethanol extracts of C. millenii, C. acuminata, and C. gigantea were active against C. albicans, with a MIC value of 120 mg/ml [107]. The ethanolic extract of the stem bark of C. nitida was highly active against T. tonsurans and T. rubrum, with a MIC value <100 μg/mL. This result confirmed the traditional uses of the plant in Nigeria to treat skin diseases (Olakunle 2017). In addition, both the aqueous stem extract of C. gigantea showed antifungal activity against C. albicans, but the aqueous extract exhibited the highest antifungal activity against the fungus [73]. In a study, the extracts of C. gigantea A.Chev, C. acuminate, and C. millenii K. Schum showed antifungal activity against C. albicans, with a MIC value of 120 mg/ml [96].
Antiviral activity
Alkaloids isolated from the seed of C. laurifolia showed antiviral activities [25]. While the fruits of C. millenii K.Schum showed good antiviral activity [79].
Antimalarial activity
In Africa, malaria is one disease too many because of the havoc it has caused the population. Literature reports have confirmed the magnitude of death caused by this disease yearly. According to WHO, malaria is one of the diseases that have the highest epidemics [120]. Okello [121] reported that between one to two million Africans died of malaria annually. The study conducted by Verde et al. [105] revealed that malaria is prevalent in 100 countries in South America, Africa, and Southeast Asia. One of the commonest causes of death in Nigeria is malarial [122], a major tropical disease [123] whose symptoms are anemia and fever [124]. The causative agent of malaria in human, plasmodium falciparum, a protozoan parasite produced by infected female anopheles mosquitoes had been reported to be resistant to many antimalarial drugs. Consequently, many pharmaceutical companies producing antimalarial drugs use combine action in the formulation of these drugs. Many of these synergetic drugs have been very effective in the treatment of malaria. For instance, artemisinin-based antimalarial drugs have been adopted by many countries as combine therapy [120]. However, prediction on the assurance of the everlasting efficacy of these antimalarial drugs in circulations today may not be guaranteed because of the resistant nature of P. falciparum over time. The popular antimalarial drug, chloroquine is no longer effective in the most endemic region, especially in the tropics. To beat the resistant ability of this P. falciparum, sustained and concerted efforts are required to formulate drugs that could eradicate malaria. To achieve this, more searches should be focused on plants base active constituents especially those that have not been studied previously.
Saponnins isolated from the seed of C. laurifolia has been effective in the treatment of malaria [25]. However, a study on the antimalarial effects of C. nitida in humans showed that eating the plant enhances plasmodium survival and its transmission in endemic areas [125]. But the stem bark of C. acuminata prepared by the decoction method exhibited remarkable antimalarial activity [3]. The aqueous leaf extract of C. acuminata was reported to show good antimalarial activity against Plasmodium berghei [126]. Also, the extract of C. acuminata prepared by decoction methods showed antimalarial activity [127]. Furthermore, the in vitro studies of the aqueous extract of C. caricaefolia against P. falciparum showed significant antiplasmodium activity [128] in agreement with the reported activity of the plant against P. falciparum [129]. On the other hand, the aqueous and ethanol extracts of the leaves of C. nitida have shown excellent antimalarial activity against P. berghei in Swiss albino mice [130], hence the traditional uses of the plant in the treatment of malarial in Nigeria. The pentane extract of the stem of C. caricaefolia showed antiplasmodium activity against P. falciparum [29]. While the leaves of C. cordifolia (Cav.)R.Br prepared by decoction method exhibited good antimalarial activity [131]. The Antimalarial activity and analgesic properties of the leafy stem of C. millenii K.Schum prepared by the decoction have been established [132].
Anticancer activity
Cancer refers to a disease in which abnormal cells divide uncontrollably and destroy body tissue. Anticancer agents, on the other hand, are substances that are effective in the treatment of cancerous diseases. Saponnins for instance, have been effective in the treatment of cancer [25]. The extract of C. acuminata prepared by decoction methods showed valuable anticancer activity against breast cancer [127].
Anti-mycobacterium activity
Adeniyi et al. [70] studied the antimycobacterium properties of the extracts of C. nitida, C. acuminata, and C. milleni against M. vaccae and M. bovis (ATCC 35738) using both broth micro dilution and radiometric (BACTEC) methods, respectively. The results showed that the methanol extract of root of C. milleni and C. nitida were potent against M. vaccae and M. bovis (ATCC 35738), with a MIC value of 125 μg/ml.
Anti-diabetic activity
Sangodele and Okere [133] studied the antidiabetic properties of cold water extract of the seeds of C. acuminata. The result showed that C. acuminata reduced the blood sugar level from 599–0.667 mg/dl to 59–1.202 mg/dl in the rats tested. This showed that the plant was a better potent antidiabetic agent vis á vis the Glanil (the known antidiabetic drug). In another study, the leaf extract of C. acuminata prepared by decoction method exhibited antidiabetic activity [134]. Imam-fulani et al. [135] reported that the acetone extract of the seeds of C. nitida exhibited a hypoglycemic effect on diabetic female Wistar rats. Furthermore, the hot water extract of C. nitida showed antidiabetic activity against the two rats tested [90].
Anti-diarrheal activity
Doe et al. [71] studied the antidiarrheal activity of the chloroform and ethanol extracts of the seeds of C. nitida. The extracts showed antidiarrheal activity by decreasing the gastrointestinal motility and the frequency of defecation in the Wistar albino rats used.
Anti-atherosclerotic and hypolipidaemic activities
C. lepidota ethanol seeds extracts have been reported to show anti-atherosclerotic and hypolipidaemic activities [136]. The various pharmacological uses of the Cola species are shown in Table 4.
Parts of Cola species used for traditional medicine and pharmacological activities
The different parts of Cola species were used traditionally to treat various ailments as well as stimulants (Fig. 2). From this study, the leaves (30%), stem bark (22%), and the seeds (22%) were the most used part for the preparation of the herbal extracts. The percentages of the extracts from the root, fruits, and nuts of the plants are 13%, 11%, and 2%, respectively. Similarly, the pharmacological activities of the different parts of the Cola species are presented (Fig. 3). The leaves (30%), stem bark (25%), and seeds (21%) were the most parts of the plants used. In addition, the fruits, roots, and nuts of the plants used pharmacologically are 13%, 11%, and 2%, respectively. The higher usage of the leaves to prepare the extracts could be attributed to the fact that photosynthesis activities are higher in the leaves consequently, more phytochemicals are present in the leaves that may be responsible for the curative effects [48]. Figure 4 gives the number of plants used for their pharmacological activities. This result showed that most of the Cola plants were used as antioxidants. Antioxidants are found in the human diet and help in protecting the cells against free radical peroxidation [137].
Future perspective
Drugs from natural sources are becoming more popular because they are less expensive, have fewer or no side effect, and better patient tolerance [138]. Plants offer alternative sources of active secondary metabolites for the manufacture of drugs [1]. In ethnomedicine, Cola species offer wide range of applications in the treatment of different diseases. There are also reports of the pharmacological activities and toxicological effects of the extracts from these plants. However, a few member of this genus have been studies. Biological activities of the secondary metabolites derived from the plants are limited. Therefore, a revival of interest in the phytochemistry and pharmacology of the Cola species could lead to the manufacture of lead drugs. Random clinical trial as well as pharmacokinetics of these plants could provide possibility of producing effective curative agents in this regard. This requires isolation of the bioactive metabolites and pharmacological activities of the plant extracts; carry out clinical trial, pharmacokinetics, and then toxicological analyses.
Conclusion
The Cola species are made up of about 125 Cola plants. However, only a few members have been studied for their phytochemical constituents and pharmacological activities. Available toxicological studies also confirmed that extracts of Cola plants are safe for human consumption except for the aqueous extract of seed of C. millenii which showed hypo toxicity. Given the wide uses of these plants, more researches about the toxicity, pharmacology, and phytochemicals of these species are required perhaps to promote their medicinal uses. Cola plants contain alkaloids, caffeine, theobromine, proanthocyanidins, theophylline, quinic acid, chlorogenic acid, purine, (−)-epicatechin, (+)-catechin, rostratanic acid, bauerenol, lupeol, acotatarone A, lignoceric acid, botulin, betulinic acid, friedelanone, friedelan, herranone, arjunolic acid, stigmasterol, nonanedioc acid, among others. These bioactive secondary metabolites are responsible for the various pharmacological activities such as antioxidant, antibacterial, antifungal, antimalarial, anti-inflammatory, antidiabetic, antidiarrheal, antiviral, anticancer, antimycobacterium, and antiatherosclerotic, and hypolipidaemic. Economically, Cola has been used in manufacturing and pharmaceutical industries to produce energy drink, flavoring agents, wine, chocolates, animal feeds, medicine, food, disinfectant, pomade, organic fertilizers, candles, detergents, and dyes in textiles. The reports published up to 2019 on Cola species provide justification on their ethnomedicinal uses.
References
Leitão F, Guimarães S, Stern V, Machline I, Martins K. Original article medicinal plants traded in the open-air markets in the State of Rio de Janeiro, Brazil: an overview on their botanical diversity and toxicological potential. Rev Bras Farmacogn. 2014;24:225–47. https://doi.org/10.1016/j.bjp.2014.04.005.
Niemenak N, Onomo PE, Lieberei R, Ndoumou DO. Purine alkaloids and phenolic compounds in three Cola species and Garcinia kola grown in Cameroon. South Afr J Bot. 2008;74:629–38. https://doi.org/10.1016/j.sajb.2008.03.003.
Iyamah PC, Idu M. Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J Ethnopharmacol. 2015;173:287–302. https://doi.org/10.1016/j.jep.2015.07.008.
Ezeigbo OR, Ejike EN, Nwachukwu I, Ejike BU. Comparative antibacterial activity of methanolic, ethanolic and aqueous extract of Garcinia kola (Bitter kola) and Cola nitida (Kola nut). Int J Plant Res. 2016;6:53–6. https://doi.org/10.5923/j.plant.20160603.01.
Adesoye AI, Fasola TR, Akinro LA. Extraction of high quality DNA from Cola nitida and Cola acuminata. Res Plant Biol. 2014;4:21–6.
Burdock GA, Carabin IG, Crincoli CM. Safety assessment of kola nut extract as a food ingredient. Food Chem Toxicol. 2009;47:1725–32. https://doi.org/10.1016/j.fct.2009.04.019.
Adenuga OO, Mapayi EF, Olasupo FO, Olaniyi OO, Oyedokun AV. Nigeria’s cola genetic resources: the need for renewed exploration. Asian J Agric Sci. 2012;4:177–82.
Forest M, Technology BE, Polytechnic DS. Effects of variety and different storage structures on the quality of Cola acuminata and Cola nitida in storage. IOSR J Environ Sci Toxicol Food Technol. 2015;9:57–61. https://doi.org/10.9790/2402-09615761.
Olapade EO (1996) FAO International Technical Conference. Indigenous Plant Genetic Resources. pp 1–107.
Al Muqarrabun LMR, Ahmat N. European Journal of Medicinal Chemistry Medicinal uses, phytochemistry and pharmacology of family Sterculiaceae: a review. Eur J Med Chem. 2015;92:514–30. https://doi.org/10.1016/j.ejmech.2015.01.026.
Shaturaev J. Fermenting infusion of Cola Nitida and Cola Acuminata Husk and Testa. Int J Innov Technol Explor Eng. 2020;9:283–7. https://doi.org/10.35940/ijitee.D1375.029420.
Dah-nouvlessounon D, Adoukonou-sagbadja H, Nafan D, Adjanohoun A. Indigenous knowledge and socio- economic values of three Kola Species (Cola Nitida, Cola Acuminata, and Garcinia Kola) used in Southern Benin. Eur Sci J. 2015c;11:1857–431.
Dah-nouvlessounon D, Adoukonou-sagbadja H, Diarrassouba N, Sina H, Adjanohoun A, Inoussa M, et al. Phytochemical analysis and biological activities of Cola nitida Bark. Biochem Resaerch Int. 2015a;2015:1–12. https://doi.org/10.1155/2015/493879.
Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J Ethnopharmacol. 2014;155:857–24. https://doi.org/10.1016/j.jep.2014.05.055.
Eromosele OJ, Kehinde OM. Phytochemical study of underutilized leaves of Cola acuminata and C. nitida. Am Res J Biosci. 2018;4:1–7.
Singh R, Singh S, Jeyabalan G, Ali A. An overview on traditional medicinal plants as aphrodisiac agent. J Pharmacogn Phytochem. 2012;1:43–56.
Akendengue B, Louis AM. Medicinal plants used by the Masango people in Gabon. J Ethnopharmacol. 1994;41:193–200.
Kukula-Koch WA, Widelski J (2017) Alkaloids. Pharmacognosy. p 9.
Babawale OP, Taiye FR, Adetunji OS. Ethnobotanical survey of plants used as memory enhancer in three states of southwestern Nigeria. J Appl Pharm Sci. 2016;6:209–14. https://doi.org/10.7324/JAPS.2016.60931.
Martin LQ, Matimele H, Banze A, Lawrence P. Cola species of the limestone forests of Africa, with a new endangered species, Cola cheringoma (Sterculiaceae), from Cheringoma, Mozambique. KEW Bull. 2019;74:1–15. https://doi.org/10.1007/s12225-019-9840-3.
Idu M, Erhabor JO, Ovuakporie-uvo O. Ethnomedicinal plants used by the idoma. Am J Ethnomed. 2014;1:72–88. 2014.
El-ghani MMA. Traditional medicinal plants of Nigeria: an overview. Agric Biol J North Am. 2016;7:220–47. https://doi.org/10.5251/abjna.2016.7.5.220.247.
Lowe HIC, Watson CT, Badal S, Peart P, Toyang NJ, Bryant J. Promising efficacy of the Cola acuminata plant: a mini review. Adv Biol Chem. 2014;4:240–5.
Chigozie ME, Chukwuma SE, Augustine NE. Determination of physical and phytochemical constituents of some tropical timbers indigenous to niger delta area of Nigeria. Eur Sci J. 2014;10:247–70. https://doi.org/10.19044/esj.2014.v10n18p%25p.
Kabine O, Samba BM, Fatoumata B, Namagan K, Luopou HN, Mamadou BA. Anti-oxidative activity of fruit extracts of some medicinal plants used against chronic diseases (diabetes, hypertension) in Kankan, Guinea. J Plant Sci. 2015;3:1–5. https://doi.org/10.11648/j.jps.s.2015030102.11.
Singh B, Gupta V, Bansal P, Singh R, Kumar D. Pharmacological potential of plant used as aphrodisiacs. Int J Pharm Sci Rev Res. 2010;5:104–13.
Fonge BA, Egbe EA, Fongod AGN, Focho DA, Tchetcha DJ, Nkembi LN, et al. Ethnobotany survey and uses of plants in the Lewoh-Lebang communities in the Lebialem highlands, South South West Region, Cameroon. J Med Plants Res. 2012;6:855–65. https://doi.org/10.5897/JMPR11.1494.
Menan H, Banzouzi JT, Hocquette A, Pelissier Y, Blache Y, Kone M, et al. Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. Plantaphile. 2006;65:29.
Salahdeen HM, Omoaghe AO, Isehunwa GO, Murtala BA, Alada ARA. Gas chromatography mass spectrometry (GC-MS) analysis of ethanolic extracts of kolanut (Cola nitida) (vent) and its toxicity studies in rats. J Med Plants Res. 2015;9:56–70. https://doi.org/10.5897/JMPR2014.5711.
Dayo L, Olumide A, Olubanjo F, Adetoyin B, Adedayo J, Joshua T, et al. Neurobiology of pain methanol extract of Cola nitida ameliorates inflammation and nociception in experimental animals. Neurobiol Pain. 2019;5:100027 https://doi.org/10.1016/j.ynpai.2019.100027.
Koffi NG, Chimène A, Henri KK, Botanique I, Andokoi A. Ethnobotanical study of antitussive plants used in traditional medicine by Abbey and Krobou populations, in the South of Côte d’Ivoire. Int J Adv Pharm Biol Chem. 2015;4:513–22.
Nyamien Y, Adje F, Niamké F, Chatigre O, Adima A, Biego GH. Caffeine and phenolic compounds in Cola nitida (Vent.) Schott and Endl and Garcinia kola Heckel Grown in Côte d’Ivoire. Br J Appl Sci Technol. 2014;4:4846–59. https://doi.org/10.9734/BJAST/2014/11561.
Nyamien Y, Coulibaly A, Belleville M, Petit E, Adima A, Biego GH. Simultaneous determination of Caffeine, Catechin, Epicatechin, Chlorogenic and Caffeic acid in Cola nitida dried nuts from Côte d’Ivoire using HPLC. Asian J Biotechnol Bioresour Technol. 2017;1:1–7. https://doi.org/10.9734/AJB2T/2017/34800.
Catarino L, Havik PJ, Romeiras MM. Medicinal plants of Guinea-Bissau: therapeutic applications, ethnic diversity and knowledge transfer. J Ethnopharmacol. 2016;183:71–94. https://doi.org/10.1016/j.jep.2016.02.032.
Kenneth EN, Bola AD, Kingsley CI, Mahady G. Phytochemical and antimicrobial properties of crude n-hexane and methanol extracts of Cola acuminata Nuts. Br J Pharm Res. 2014;4:920–8.
Kanoma AI, Muhammad I, Ibrahim ID, Shehu K, Maishanu HM, Isah AD. Phytochemical screening of various species of cola nut extracts for antifungal activity against phytopathogenic fungi. Am J Biol Life Sci. 2014;2:18–23.
Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol. 2009;125:393–403. https://doi.org/10.1016/j.jep.2009.07.024.
Reida KA, Jägera AK, Lighta ME, Mulholland DA, Stadena JV. Phytochemical and pharmacological screening of Sterculiaceae species and isolation of antibacterial compounds. J Ethnopharmacol. 2005;97:285–91. https://doi.org/10.1016/j.jep.2004.11.010.
Cousins D, Huffman MA. Medicinal properties in the diet of gorillas: an ethno-pharmacological evaluation. Afr Study Monogr. 2002;23:65–89.
Raymond G, Tchouya F, Souza A, Claude J, Yala J, Boukandou M, et al. Ethnopharmacological surveys and pharmacological studies of plants used in traditional medicine in the treatment of HIV/AIDS opportunistic diseases in Gabon. J Ethnopharmacol. 2015;162:306–16. https://doi.org/10.1016/j.jep.2014.12.052.
Telles C, Mckinsey C, Thomas B, Gray W. The antimicrobial property of the acetone extract of Cola acuminata. Toxicology. 2019;4:1–10. https://doi.org/10.19080/OAJT.2019.04.555631.
Adeosun OI, Olaniyi KS, Amusa OA, Jimoh GZ, Oniyide AA. Methanolic extract of Cola nitida elicits dose-dependent diuretic, natriuretic and kaliuretic activities without causing electrolyte impairment, hepatotoxicity and nephrotoxicity in rats. Int J Physiol Pathophysiol Pharm. 2017;9:231–9.
Wambe H, Aim P, Archange M, Tagne F, Fondjo F, Fankem O, et al. Anti-shigellosis activity of Cola anomala water/ethanol pods extract on shigella flexneri-Induced diarrhea in rats. Biomed Res Int. 2019;2019:1–9.
Obey JK, Swamy TA. Original research article antibacterial activity of methanolic extracts of Cola nitida seeds on selected pathogenic organisms. Int J Curr Micriobiology Appl Sci. 2014;3:999–1009.
Appiah KS, Oppong CP, Mardani HK, Omari RA, Kpabitey S, Amoatey CA, et al. Medicinal plants used in the Ejisu-Juaben ethnobotanical study. Medicines. 2019;6:1–27. https://doi.org/10.3390/medicines6010001.
Vliet EV (2012) Medicinal plants for women’s and children’s health in urban and rural areas of Gabon, pp 1–38.
Tamo SPB, Essama SHR, Etoa FX. Plants used in Bandjoun village (La’Djo) to cure infectious diseases: An ethnopharmacology survey and in-vitro Time- Kill Assessment of some of them against Escherichia coli. J Phytopharm. 2016;5:56–70.
Diallo D, Sogn C, Samaké FB, Paulsen BS, Michaelsen TE, Keita A. Wound healing plants in Mali, the Bamako region. an ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm Biol. 2002;40:117–28. https://doi.org/10.1076/phbi.40.2.117.5846.
Agyare C, Koffuor GA, Boamah VE, Adu F, Mensah KB, Adu-amoah L. Antimicrobial and anti-inflammatory activities of Pterygota macrocarpa and Cola gigantea (Sterculiaceae). Evid-Based Complement Alter Med. 2012;2012:1–9. https://doi.org/10.1155/2012/902394.
Choi CW, Song SB, Oh JS, Kim YH. Antiproliferation effects of selected Tanzania plants. Afr J Tradit Complement Alter Med. 2015;12:96–102.
Diatta K, Diatta W, Fall AD, Ibra S, Dieng M, Mbaye AI, et al. Ethnobotanic survey of aids opportunistic infections in the Ziguinchor District, Sénégal. Asian J Res Med Pharm Sci. 2019;8:1–10. https://doi.org/10.9734/AJRIMPS/2019/v8i1-230130.
Austarheim I, Mahamane H, Sanogo R, Togola A, Khaledabadi M. Anti-ulcer polysaccharides from Cola cordifolia bark and leaves. J Ethnopharmacol. 2012;143:221–7. https://doi.org/10.1016/j.jep.2012.06.027.
Karunamoorthi K. Role of traditional antimalarial plants in the battle against the Global Malaria Burden. Vector-Borne Zoonotic Dis. 2013;13:521–44. https://doi.org/10.1089/vbz.2011.0946.
Nwankpa P, Chukwuemeka OG, Ugwuezumba PC, Ekweogu CN, Etteh CC, Emengaha FC, et al. Assessment of anti-oxidative potential of ethanol seed extract of Cola lepidota in high fat fed female Wistar Albino Rats. East Afr Sch J Med Sci. 2018;1:47–53.
Gbekley HE, Karou SD, Katawa G, Tchacondo T, Batawila K, Ameyapoh Y, et al. Ethnobotanical survey of medicinal plants used in the management of hypertension in the Maritime Region of Togo. Afr J Tradit Complement Alter Med. 2018;15:85–97.
Kpodar MS, Karou SD, Katawa G, Anani K, Gbekley HE, Adjrah Y, et al. An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J Ethnopharmacol. 2016;181:263–73. https://doi.org/10.1016/j.jep.2015.12.051.
Uzodimma DE. Medico-ethnobotanical inventory of Ogii, Okigwe Imo State, South Eastern Nigeria-I. Glob Adv Res J Med Plants. 2013;2:30–44.
Agbongiarhuoyi AE, Mokwunye FC, Ndagi I, Adebiyi S, Ndubuaku TCN. The challenges of Kola nuts processing, trade and export from Nigeria and other Sub-Saharan African countries. Int J Sci Nat. 2012;3:6–11.
Oladokun MAO (2000) KOLA: The Tree of Life, 1–37.
Valere P, Fokou T, Kwadwo A, Appiah-Opong R, Rachel L, Yamthe T, et al. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J Ethnopharmacol. 2015;172:297–311. https://doi.org/10.1016/j.jep.2015.06.024.
Adebayo S, Oladele OI. Medicinal values of Kolanut in Nigeria: implication for extension service delivery. Life Sci J. 2012;9:887–91.
Biosci IJ, Harrison EA, Science F, State D, Biosci IJ. Tree species of Lekki Conservation Center, Lagos State, Nigeria. Int J Biosci. 2019;15:308–14.
Fernando KS, Antony S. Echinoderm diversity in Mudasal Odai and Nagapattinam coast of south east India. Int J Biodivers Conserv. 2014;6:1–7.
Omwirhiren EM, Abass AO, James SA. The phytochemical constituents and relative antimicrobial activities against clinical pathogens of different seed extracts of Cola nitida (Vent.), Cola acuminata (Beauvoir) and Garcinia kola (Heckel) grown in South West, Nigeria. J Pharmacogn. Phytochem. 2017;6:493–501.
Doe P, Ametepey NK, Mshelia VC, Otchere DB, Fofana O, Amissah AA, et al. Anti-diarrheal activity of ethanol and chloroform seed extract of Cola nitida in experimentally induced diarrhea. Univers J Pharm Res. 2019;4:17–21.
Adesanwo JK, Ogundele SB, Akinpelu DA, Mcdonald AG. Chemical analyses, antimicrobial and antioxidant activities of extracts from Cola nitida seed. J Explor Res Pharm. 2017;2:67–77. https://doi.org/10.14218/JERP.2017.00015.
Steve AC, Chidinma OL, Emmanuella NE, Chukwuebuka AK, Sunday AN, Chidi OB, et al. Phytochemical and antimicrobial screening of Cola gigantea leaves, stem and bark. Univers J Microbiol Res. 2016;4:49–54. https://doi.org/10.13189/ujmr.2016.040203.
Essien EE, Udousoro II. Cola parchycarpa K. Schum: chemical evaluation of amino acids, vitamins and other nutritional factors in seed, fruit mesocarp and epicarp. UK J Pharm Biosci. 2017;5:23–9.
Okafor PN, Nwankpa P, Ekweogu CN, Etteh CC, Ugwuezenmba PC. Assessment of the weight reducing potentials of ethanolic seed extract of Cola lepidota K Schum in high fat fed female Albino Wistar Rats. Am J Phytomedicine Clin Ther. 2018;6:1–10. https://doi.org/10.21767/2321-2748.1003.
Emmanuel E. Essien NSP and SMA. Chemical composition and antioxidant property of two species of Monkey Kola (Cola rostrata and Cola lepidota K. Schum) extracts. Eur J Med Plants. 2015;7:31–7. https://doi.org/10.9734/EJMP/2015/15976.
Okudu HO, Asumugha VU, Umoh EJ. Evaluation of the nutrients and phytochemical composition of two varieties of Monkey Kola membrane (Cola parchycarpa and Cola lepidota). Direct Res J. 2016;4:320–5.
Adewumi FIA, Lanre B. Evaluation of phytochemical components of various parts of Cola millenii. Ovidius Univ Ann Chem. 2018;29:29–35. https://doi.org/10.2478/auoc-2018-0004.
Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease. Aftican J Tradit Complement Alter Med. 2013;10:210–29.
Dewole EA, Dewumi DFA, Alabi JYT, Adegoke A. Proximate and phytochemical of Cola nitida and Cola acuminata. Pak J Biol Sci. 2013;16:1593–6.
Nwaokobia K, Olorode EM, Ogboru RO. Some mechanical and nutritive properties of Cola acuminata (P. Beauv.) Schott & Endl. and Cola Nitida (Vent.) Schott & Endl. Am J Environ Eng Sci. 2019;6:1–10.
Oranusi S, Onibokun A, Afolabi O, Okpalajiaku C, Seweje A, Olopade B, et al. Chemical, microbial and antioxidant activity of Cola lepidota K.Schum fruits. Czech J Food Sci. 2020;38:11–9.
Oni SO, Oladimeji AO, Owoade RO, Obon RE, Akinlabi KA. Preliminary investigation, phytochemical profile and proximate composition of Cola lepidota (karl schum) seed. J Chem Soc Niger. 2020;45:86–93.
Imaobong I, Udousoro EEE. Amino acids, vitamins and other nutritional and anti-nutritional components of Cola lepidota (Monkey Kola). Am Assoc Sci Technol. 2017;4:12–8.
Borokini FB, Abitogun A, Olumayede EG. Nutritional, antinitritional and antimicrobial activities of seed and Pulp of Cola Millenii. J Appl Chem. 2014;7:113–8.
Koenig KD, Adetola OO, Akinyemi OE. Comparative analysis of the mineral elements constituent of Cola millenii K. Schum and Blighia. World Nat Sci. 2019;24:350–6.
Atolani O, Oguntoye H, Areh ET, Adeyemi OS, Kambizi L, Oguntoye H, et al. Chemical composition, anti-toxoplasma, cytotoxicity, antioxidant, and anti-inflammatory potentials of Cola gigantea seed oil. Pharm Biol. 2019;57:154–60. https://doi.org/10.1080/13880209.2019.1577468.
Iyun RA. Nigerian Journal of Pharmaceutical and Applied Science Research. Niger J Pharm Appl Sci Res. 2018;7:1–6.
Bansal TKH. Benefits of essential oil. J Chem Pharm. 2016;8:143–9.
Erukainure OL, Sanni O, Ijomone OM, Ibeji CU, Chukwuma CI, Islam S. The antidiabetic properties of the hot water extract of kola nut (Cola nitida (Vent.) Schott & Endl.) in type 2 diabetic rats. J Ethnopharmacol. 2019;242:112033 https://doi.org/10.1016/j.jep.2019.112033.
Reid KA (2002) Pharmacological properties of members of the Sterculiaceae p 20.
Mcclatchey WC, Mahady GB, Bennett BC, Shiels L, Savo V. Pharmacology & therapeutics ethnobotany as a pharmacological research tool and recent developments in CNS-active natural products from ethnobotanical sources. Pharm Ther. 2009;123:239–54. https://doi.org/10.1016/j.pharmthera.2009.04.002.
Atawodi SE-O, Pfundstein B, Haubner R, Spiegelhalder B, Bartsch H, Owen RW. Content of polyphenolic compounds in the Nigerian stimulants Cola nitida ssp. alba, Cola nitida ssp. rubra A. Chev, and Cola acuminata Schott & Endl and their antioxidant capacity. J Agric Food Chem. 2007;55:9824–8.
Europen medicinal Agency. Assessment report on Cola nitida (Vent.) Schott et Endl. and its varieties and Cola acuminata (P. Beauv.) Schott et Endl. Eur Medicinal Agency. 2011;16:1–25.
Sut S, Dall S, Ibrahime K, Bene K, Kumar G, Fawzi M, et al. Industrial crops & products Cola caricifolia (G. Don) K. Schum and Crotalaria retusa L. from Ivory Coast as sources of bioactive constituents. Ind Crop Prod. 2020;147:112246. https://doi.org/10.1016/j.indcrop.2020.112246.
Mubo AS, Soladoye MO, Esan OO, Sonibare OO. Phytochemical and antimicrobial studies of four species of Cola Schott & Endl. (Sterculiaceae). Afican J Tradit. Complement Alter Med. 2009;6:518–25.
Dongmo W, Tsopgni T, Guy A, Azebaze B, Emmanuel J, Teinkela M, et al. New unsaturated fatty acid and other chemical constituents from the roots of Cola rostrata K. Schum. (Malvaceae). Biochem Syst Ecol. 2019;86:103913. https://doi.org/10.1016/j.bse.2019.103913.
Chukwuemeka OG, Okafor PN, Nwankpa P, Etteh CC, Ekweogu CN. Qualitative phytochemical screening and GCMS-derived fatty acid composition of ethanolic seed extract of Cola lepidota K. Schum. Int J Curr Microbiol Appl Sci. 2018;7:12–24.
Nouvlessounon DDAH, Sagbadja HA, Diarrassouba N, Sina H, Noumavo PA, Moussa FB, et al. (2015) Antimicrobial, Antioxidant, Cytotoxic Activities and Phytochemical Assessment of Cola Acuminata used in Benin. Int J Pharm Pharm Sci. 7.
Ubon JA, Akpanabiatu MI, Akpanyung EO, Ufot FU. Effects of ethanolic extracts of Cola millenii K. Schum seed on biochemical and toxicological indices of male wistar albino rats. J Pharmacogn Phytochem. 2017;6:160–6.
Akinnibosun FI, Adewumi LB. Study of acute and sub-acute toxicity of Cola millenii. Not Sci Biol. 2019;11:358–62. https://doi.org/10.15835/nsb11410548.
Oloye MT, Adedugbe OF, Ilesanmi OS, Arobasalu J, Fasuyi FO. Effects of aqueous extracts of Cola millenii K. Schum seed on toxicological indices of White Male Albino Rats. Int J Biotechnol Biochem. 2020;16:17–24.
Nwonuma CO, Adelani-akande TA, Olaniran AF, Osemwegie OO, Adeyemo TA. Comparative study of in vitro antimicrobial potential and phytochemicals of some medical plants. F1000 Res. 2019;8:1–16.
Verde C, Tomé S, Silva JRDA, Ramos ADS, Machado M, De Moura DF, et al. A review of antimalarial plants used in traditional medicine in communities in Portuguese-Speaking countries: Brazil, Mozambique. Antimalar Plants Based Knowl Tradit Med. 2011;106:142–58.
Adam S, Salih M. Antimicrobial activity of the masticatory Cola acuminata Nut (Gooro). Curr Res J Biol Sci. 2011;3:357–62.
Cam. Research paper. Afr J Tradit Complement Alter Med. 2009;6:518–25.
Indabawa II, Arzai AH. Antibacterial activity of Garcinia Kola and Cola nitida seed extracts. Bayero J Pure Appl Sci. 2011;4:52–5. https://doi.org/10.4314/bajopas.v4i1.11.
Dah-nouvlessounon D, Adoukonou-sagbadja H, Diarrassouba N, Sina H, Adjanohoun A, Inoussa M, et al. Phytochemical analysis and biological activities of Cola nitida Bark. Biochem Res Int. 2015b;1:1–12.
John D, Okwubie L, Njemanze IO. Antimicrobial activity of methanolic seeds extract of Cola nitida (Kolanut) against microorganisms Isolated from the oral cavity isolation of microorganisms. Int J Pharmacogn Phytochem Res. 2018;10:151–6. https://doi.org/10.25258/phyto.10.4.5.
Kagoyire KMW, Atindehou K (2009) West African plants and related phytocompounds with anti-multidrug-resistance activity. New Strateg Combating Bact Infect. 137–163.
Nwankwo CE (2017) Antimicrobial and Spectroscopic Analyses of Cola millenii K.Schum, 61–82.
Orisakeye OT, Ojo AA. Antimicrobial and antioxidant evaluation of various parts of Cola milleni K.Schum plant. Afr J Pharm Pharm Full-. 2013;7:1–4. https://doi.org/10.5897/AJPP2013.3416.
Yves N, Henri G, Biego M. Caffeine and phenolic compounds in Cola nitida (Vent.) Schott and Endl and Garcinia kola Heckel Grown in Côte d’Ivoire. Br J Appl Sci Technol. 2014;4:4846–59. https://doi.org/10.9734/BJAST/2014/11561.
Ogunlade I, Awosanmi IA, Osukoya OA. Antioxidant activity and total phenolic content of some nuts commonly consumed in South-Western Nigeria. J Phytopharm. 2014;3:248–53.
Offoumou MR, Kipre GR, Kigbafori DS, Camara D, Sylla Y, Djaman AJ, et al. In vitro antioxidant activity of crude extracts of four medicinal plants used in the treatment of malaria in Ivory Coast. Indo Am J Pharm Sci. 2018;05:6202–6.
Oghenerobo VI, Falodun A. Antioxidant activities of the leaf extract and fractions of Cola lepidota K. Schum (sterculiaceae). Niger. J Biotechnol. 2013;25:31–6.
Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clin Phytoscience. 2016;2:7–66. https://doi.org/10.1186/s40816-016-0037-0.
Nwidu LL, Cheriose P, Alikwe N, Elmorsy E, Carter WG. An investigation of potential sources of nutraceuticals from the Niger Delta Areas, Nigeria for attenuating oxidative stress. Medicines. 2019;6:1–16. https://doi.org/10.3390/medicines6010015.
Christopher R, Mgani QA, Nyandoro SS, Rousseau AL, Van Vuuren SF, Isaacs M, et al. Antitrypanosomal, antiplasmodial, and antibacterial activities of extracts from selected Diospyros and Annonaceae species. J Complement Med Res. 2018;7:161–70.
Okello D. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid-Based Complement Alter Med. 2019;2019:1–27.
Alaribe AAA, Ejezie GC, Ezedinachi ENU. The role of Kola Nut (Cola Nitida) in the etiology of malaria morbidity the role of Kola Nut (Cola Nitida) in the etiology of malaria morbidity. Pharm Biol. 2003a;41:458–62. https://doi.org/10.1076/phbi.41.6.458.17835.
Guédé NZ, N’guessan K, Dibié TE, Grellier P. Ethnopharmacological study of plants used to treat malaria, in traditional medicine by Bete Populations of Issia (Côte d’Ivoire). J Pharm Sci Res. 2010;2:216–27.
David S, Attemene D, Beourou S, Tuo K, Alloh A, Abibatou G, et al. Antiplasmodial activity of two medicinal plants against clinical isolates of Plasmodium falciparum and Plasmodium berghei infected mice. J Parasit Dis. 2017;1:1–8. https://doi.org/10.1007/s12639-017-0966-7.
Alaribe AAA, Ejezie GC, Ezedinachi ENU. The role of Kola Nut (Cola Nitida) in the etiology of malaria morbidity. Pharm Biol. 2003b;41:458–62.
Zailani HA, Banyawa M, Muhammad AA. Effects of aqueous leaf extract of Cola acuminata on parasitaemia, haematological and liver function parameters in plasmodium berghei infected mice. Direct Res J Heal Pharm. 2016;4:14–20.
Afolayan FID, Sulaiman KA, Okunade WT. Ethnobotanical survey of plants used in cancer therapy in Iwo and Ibadan, South-Western of Nigeria. J Pharm Pharmacogn Res. 2020;8:346–67.
Komlaga G, Agyare C, Akosua R, Lincoln M, Mensah K, Loiseau PM, et al. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol. 2015;172:333–46. https://doi.org/10.1016/j.jep.2015.06.041.
Komlaga MG (2016) Search for antiplasmodial compounds from Ghanaian medicinal plants, 1–128.
Omoya FO. The in vivo assessment of antiplasmodial activities of leaves and stem bark extracts of Mangifera indica (linn) and Cola nitida (linn). Int J Infect Dis. 2016;45:373.
Traore MS, Baldé MA, Diallo MST, Baldé ES, Diané S, Camara A. Ethnobotanical survey on medicinal plants used by Guinean traditional healers in the treatment of malaria. J Ethnopharmacol. 2013;150:1145–53. https://doi.org/10.1016/j.jep.2013.10.048.
Dénoua A, Koudouvo K, Togola A, Aziati KY, Esseh J, Ajavon CA, et al. Traditional knowledge on antimalarial plants having analgesic properties, used in Togo Maritime Region. J Ethnobiol Tradit Med Phot. 2019;126:1160–70.
Sangodele J, Okere S. Phytochemical constituents and hypoglycemic properties of Cola acuminata seed on alloxan-induced diabetic rats. Nord Pharm Soc Basic Clin Pharm Toxicol. 2014;115:92–61.
Abo KA, Fred-jaiyesimi AA, Jaiyesimi AEA. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J Ethnopharmacol. 2008;115:67–71. https://doi.org/10.1016/j.jep.2007.09.005.
Imam-fulani AO, Sanusi KO, Owoyele BV. Effects of acetone extract of Cola nitida on brain sodium-potassium adenosine triphosphatase activity and spatial memory in healthy and streptozotocin-induced diabetic female Wistar rats. J Basic Clin Physiol Pharm. 2018;1:8–13.
Chukwuemeka OGOP. Effect of ethanolic seed extract of Cola lepidota on the lipid profile of high fat fed albino wistar rats. Food Nutr Open Access. 2018;2:1–7.
Akter J, Hossain A, Takara K, Islam Z. Antioxidant activity of di ff erent species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp Biochem Physiol Part C. 2019;215:9–17. https://doi.org/10.1016/j.cbpc.2018.09.002.
Idris AO, Kolawole F, Luqman A, Oyedeji AO. Neuropharmacological activities of ethanolic extract of Cola millenii dried leaf in rats. Eur J Med Plants. 2016;16:1–12. https://doi.org/10.9734/EJMP/2016/28718.
Djidomi L, Kinsou C, Assogba MF, Dominique M, Zinsou C, Goudjo AIM, et al. Review of literature and phytochemistry screening of medicinal plants used in traditional treatment of brain diseases in Africa. Int J Phytopharm. 2019;9:1–33. https://doi.org/10.7439/ijpp.v9i6.5285.
Nduche MU, Omosun G, Okwulehie IC. Ethnobotanical survey of plants used as remedy for fertility conditions in Ebonyi State of Nigeria. Sch Acad J Biosci. 2015;3:214–21.
Mickymaray S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019;8:257.
Uwabunkeonye CO, Ezeabara CA, Horoiheoma C, Denis UC, Aziagba BO. Comparative proximate and phytochemical compositions of Cola acuminata (P. Beauv.) Schott and Cola nitida (Vent) Schott and Endl. Plant. 2015;3:26–9. https://doi.org/10.11648/j.plant.20150303.12.
Sunderland TCH, Clark LE, Vantomme P (1998) Current research issues and prospects for conservation and development, Food and agriculture organization of the United Nations Rome,1–278.
Akram M, Tahir IM, Shah SMA, Mahmood Z, Altaf A, Ahmad K, et al. (2018) Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phyther Res 1–12. https://doi.org/10.1002/ptr.6024.
Christian A, David DO, Yaw DB, Newman O (2013) Anti-inflammatory and analgesic activities of African medicinal plants. Medicinal Plants Research in Africa, 725–52.
Adeniyi BA, Groves MJ, Gangadharam PRJ. In vitro anti-mycobacterial activities of three species of Cola plant extracts (Sterculiaceae). Phyther Res. 2004;18:414–8.
Emmanuel EO, Ching FP, Abiodun F, Sunday AA. Cola rostrata: phytochemical and toxicity studies. J Appl Sci Environ Manag. 2013;17:603–7.
Mebude OO, Lawal TO, Adeniyi BA. Anti-dermatophytic potential of formulated extract of Cola nitida (Vent.) Schott. J Clin Exp Dermatol Res. 2017;8:1–7. https://doi.org/10.4172/2155-9554.1000379394.
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekalu, A., Habila, J.D. Phytochemistry, pharmacology and medicinal uses of Cola (Malvaceae) family: a review. Med Chem Res 29, 2089–2105 (2020). https://doi.org/10.1007/s00044-020-02637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02637-x