Abstract
The catalytic converter is used to reduce the emission from the vehicles. Long run reduces the efficiency of the catalytic converter. Pollution is increasing due to the increase in the number of cars. The cars emit harmful gases. These gases are affecting human beings and the environment in different ways. CO, HC, SOx, NOx, and PM 2.5 are harmful emission present in the exhaust emission. A new novel device is introduced in this research to reduce diesel engine emissions. The device uses a freezer gel pack, carbon black, and alkali solution (NH4OH), in association with the catalytic convertor. For performing the experiments, an experimental setup is fabricated. For measuring the emission, AVL DIGAS 444 Automobile Exhaust Gas Analyzer is using a probe. NOx is reducing by 63%, CO2 by 70%, and PM2.5 by 99% with the use of the proposed novel system.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The world is changing very quickly. Many airborne diseases are spreading nowadays in the world. Different virus and bacteria are transmitting via air, but pollution is in itself enough to create a pandemic. Everyone is using cars worldwide, and it is increasing exponentially from 45 million to over 650 million. The rise is cars will be around 2 billion by 2021 [1]. Increase in the vehicles globally is impacting the environment. Due to this reason, it is crucial to focus on the area of exhaust emission [2]. The emission from the exhaust is very harmful to nature [3]. Carbon monoxide (CO), nitrous oxide gases (NOx), unburned hydrocarbon (HC), sulfur oxides (HC), and particulate matter (PM) are the primary harmful pollutants from emission. Cars are the primary CO source in the urban areas contributing to half of all human-caused air pollutants [4]. The major sources of health problems like respiratory diseases, cancer, etc., are emissions [5]. The diesel engine produces large and harmful emission as compared to the petrol engine. Research is going on from past to present, and people invent the devices to reduce harmful emissions.
One invention is the catalytic converter, and it is found in all the vehicles nowadays. French engineer named Eugene Houdry invented the catalytic convertor. For reducing the exhaust emissions, catalytic converter is a beneficial device. Inside the catalytic converter, a chemical reaction occurs, which is reducing the harmful emission to less harmful emission [6,7,8]. The materials used inside are platinum, palladium, and rhodium, which are noble elements. These elements act as a catalyzer for the chemical reaction, and temperature plays a vital role in activating them. At a temperature range of 350–500 °C, the catalytic converter activates. Before the catalytic converter attains this temperature range, many harmful emissions are coming out from the exhaust. This is the cold start phase. Many researchers are preheating the catalytic convertor to reduce this cold start time and increasing its efficiency [9,10,11]. Equations 1 and 2 shows the reducing behavior of platinum and rhodium as a catalyst.
Equations 3 and 4 shows the reactions which are taking place in the second chamber. Platinum and palladium are acting as a catalyst for the oxidation in this chamber.
Long time running of cars decreases the effectiveness of the catalytic converter. In this research, a novel device is proposed, fitted in addition to the catalytic converter. An experimental setup of the proposed device and the catalytic converter is assembled. Effectiveness of the proposed device is known by comparing the results when the device is fitted and not fitted.
2 Experimental Setup
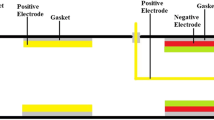
Figure 1 shows the proposed device is an association with the catalytic converter.
Figure 1b shows the experimental setup. Two layers of freezer gel are enfolding the engine exhaust pipe. The exhaust pipe is joining the aluminum alloy container, containing the black carbon particles of size equal to 0.8 μm. To avoid the converter’s choking, mesh filter of lattice size 0.5 μm is placed before the exhaust pipe is connected to the 3-way catalytic converter. One layer of freezer gel pack is again enfolding the exhaust pipe. This exhaust pipe is connecting to ammonium hydroxide solution (NH4OH) contained in an aluminum chamber. At an exit also a mesh filter of lattice size of 0.8-μm is placed before the exhaust pipe exit. The exhaust gases are measured using the AVL DIGAS 444 Automobile Exhaust Gas Analyzer.
Nitrogen oxides are forming inside the combustion chamber when the reaction between O2 and N2 occurs at a temperature range of 1000–1200 °C. Before the exhaust gases enter the chamber containing the carbon black, its temperature reduces up to 400 °C using the freeze gel pack (carboxymethyl cellulose and propylene glycol). The carbon black is reacting or absorbing with the oxygen gas and producing carbon–oxygen surface compounds that further decompose into CO2. More nitrous oxide gas is reducing into nitrogen gas because a higher concentration of oxygen is decreasing in the previous stage. All the HC and CO gets oxidize when passing through the 3-way catalytic converter.
Before entering the chamber of NH4OH solution, the freezer gel pack reduces the exhaust gas temperature to 50–70 °C. Ammonium carbonate ((NH4)2CO3) is formed by absorbing CO2 emitted from the convertor. The system, at the end, uses a mesh filter having lattice of 0.8 μm to stop fine particles producing from burning of fuel. Table 1 displays the engine specification, and Table 2 displays the 3-way catalytic convertor properties.
3 Result and Discussion
At different times of the day, the experiments are repeated, and the data were recorded and graphed. NOx, CO2, and PM 2.5 level in the exhaust decrease with the proposed device’s use. The comparison of the default system and the proposed novel system is shown in Figs. 2, 3, and 4. Figure 1 shows the NOx level which decrease by 63%; Fig. 2 shows CO2 level which reduces by 70%, and Fig. 3 shows PM 2.5 level which decreases by 99%.
A proposed novel method is very efficient and effective as shown by the experimental result and fitting in the vehicles can reduce pollution.
References
Sperling D, Gordon D (2009) Two billion cars: driving toward sustainability. Oxford University Press, New York
Calvert JG, Heywood JB, Sawyer RF, Seinfeld JH (1993) Achieving acceptable air quality: some reflection on controlling vehicle emissions. Science 261:37–45
Heck RM, Farrauto RJ (2001) Automobile exhaust catalysts. Appl Catal A 221(1–2):443–457
Flachsbart PG, Howes JE, Mack GA, Rodes CE (1987) Carbon monoxide exposures of Washington commuters. J Air Pollut Control Assoc 37(2):135–142
US Department of Health Education and Welfare (1970) Air quality criterion for carbon monoxide. Washington, DC, pp 6–22
Wright M (2006) What exactly is a catalytic converter? About.com
http://autorepair.about.com/od/glossary/ss/how-it_catalyti.htm
Pundir BP (2007) Engine emissions pollutant formation and advances in control technology. Narosa Publishing House, New Delhi
Bera A, Hegde MS (2010) Recent advances in auto exhaust catalysis. J Indian Inst Sci 90(2):299–325
Houdry EJ, Ann (1956) Catalytic structure and composition. US Patent-US2742437, filed September 1952
Shelef M, McCabe RW (2000) Twenty-five years after introduction of automotive catalysts: what next? Catal Today 62:35–50
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhandwal, M., Tyagi, R.K. (2022). Emission Reduction from Diesel Engine Using Alkali Solution, and Carbon Black in Union with Catalytic Convertor. In: Govindan, K., Kumar, H., Yadav, S. (eds) Advances in Mechanical and Materials Technology . EMSME 2020. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-2794-1_125
Download citation
DOI: https://doi.org/10.1007/978-981-16-2794-1_125
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2793-4
Online ISBN: 978-981-16-2794-1
eBook Packages: EngineeringEngineering (R0)