Abstract
The exhaust emissions from the compression ignition engines are harmful to both human beings and the environment. After-treatment devices placed in the exhaust are designed to reduce these emissions. These devices have significant conversion efficiency but have various drawbacks such as the cost and availability of the precious catalyst for catalytic converters. In this work, an emission reduction setup was developed that can reduce NO, HC, CO and smoke simultaneously. The emission reduction setup is based on the concept of an electrostatic precipitator (ESP) and plasma generation by corona discharge technique. Both diesel and waste cooking oil biodiesel (WCO) were separately used for the test. The results show that HC emissions at full load with ESP system reduced from 0.71 to 0.27 g/kWh for diesel and for WCO it reduced from 0.81 to 0.31 g/kWh. Similarly, the CO emissions reduced from 1.50 to 0.6 g/kWh for diesel and from 1.95 to 0.92 g/kWh for WCO. The smoke emission and NO emission were also reduced by 30.86 and 29.3% for diesel and WCO and 17 and 18% for diesel and WCO, respectively. However, the carbon dioxide emissions were found to increase as the HC and CO generated were also converted to CO2. The study shows that the emission reduction setup can effectively reduce the emissions without any effect on the engine performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transportation sector and the agricultural sector are dependent upon the diesel engines due to their higher fuel economy, initial torque and low maintenance cost (Geo et al. 2017). However, the main problem with the use of diesel engines is the release of hazardous pollutants in the exhaust namely oxides of nitrogen emissions (NOx), unburnt hydrocarbon emissions (HC), carbon monoxide emissions (CO) and particulate matter. These pollutants not only affect the environment, they also affect the health of human beings (Subramanian et al. 2017). The diesel engines emit particulate matter in the small particle form at a concentration above 10 million particulates per cm3. The diameter of the primary carbon particles lies in the range of 0.01 to 0.08 μm, whereas the diameter of the agglomerated particles is in the range of 0.08 to 0.25 μm with nearly 90% of the particles having a diameter less than 1 μm. Several government agencies have classified the particulate matter as “human carcinogen” or “probable human carcinogen”. They increase the risk to the heart and respiratory diseases such as asthma and bronchitis. Presence of two or more benzene ring hydrocarbons in the particulate matter also called poly-nuclear aromatic hydrocarbons (PAHs) are known human carcinogens. The soluble organic fraction of the particulate matter containing four and five rings is the most harmful compounds (Losacco and Perillo 2018). To tackle the problem of pollutants, the emissions norms all over the world are becoming increasingly stringent. India has also taken a big leap by migrating from Bharat Stage IV (BS-IV) to BS-VI by 2020. The Indian automobile industry now has a new challenge in finding the optimal solution for migrating to BS-VI norms for its existing portfolio of BS-IV norms engine.
The existing exhaust after-treatment devices can effectively reduce the regulated emissions, but there are several drawbacks to their use. The diesel particulate filter placed in the exhaust pipe is used to trap PM emissions; however, the back pressure increases due to its use. There are also problems of regeneration and their efficiency of collecting small particulates is low and the maintenance cost is high (Nguyen Huu Nhon et al. 2004; Stratakis et al. 2002). Similarly, NOx is reduced by selective catalytic reduction (SCR) which has issues like catalyst poisoning, slip of ammonia and low collection efficiency at low loads. These systems are not cost effective and the catalyst used in these systems is not readily available in the aftermarket and if available their cost is prohibitive (Subramaniam et al. 2016).
The electrostatic precipitators (ESP) are used all over the world to control the fly ash in coal-fired power plants. In 1800s, Oliver Lodge gave the idea of ESP and developed it with Alfred Walker for a lead smelter. However, due to high voltage power supply and high resistivity of the lead oxide fumes, the ESP did not work. Later on, in the 1900s, Dr. F. G. Cottrell developed an ESP on industrial scale and patented it (Huang et al. 2015). In ESP a high voltage electric field is generated between two oppositely charged electrodes. The ESP works on the principle of plasma reactions by corona discharge, where electrodes produce high energy electrons which when collides with the molecules present in the air or exhaust gas leads to the formation of new species including ions, atoms and free radicals. The ionized particles migrate towards the oppositely charged electrode. The most significant advantages of ESP is it does not obstruct the flow of air thereby the back pressure is unaffected, since the ionized particles are pulled through the system because of the electrostatic force developed (Parker 1997). Moreover, in the mechanical filters, the gas is passed through some porous media and the gas streamlines are bent based on the separation concept, resulting in pressure drop as soon as the gas hits the surface of the filter (Sudrajad and Yusof 2015). Another advantage is the requirement of less power for operating the ESP (Thonglek et al. 2011). They are widely employed in the industries to collect the fly ash particles.
Many researchers have worked upon removal of soot particles from the diesel engine exhaust using ESP, but no such product is available commercially due to some inherent drawbacks. The particulate matter has low resistance resulting in the particle re-entrainment and hence poor collection efficiency. A two-stage ESP was used by Zukeran et al. (2000). In the first stage, DC was used for charging the particles, and in the second stage AC field with trapezoidal waveforms was used for the collection of particles. Another way of increasing the collection efficiency is the use of agglomerators. Nakajima and Sato (2003) used acoustic waves for strong vibration of the particles along with electrostatic polarization of the particles. Due to acoustic waves, the smaller particles move relatively higher than coarser particles which increase the chance of collision, thereby increasing the agglomeration. Ji et al. (2004) agglomerated the particles by first bipolar charging and then passing the particles through an AC electric field and a separate DC electric field. The authors observed 25–29% reduction in particles smaller than 1 μm with AC frequency. Ozone regeneration by corona discharge is one of the best methods to incinerate the particles deposited on the electrode (Kuwahara et al. 2013; Umemoto et al. 2008). Subramaniam et al. (2016) designed an ESP containing spike-shaped charging electrode and a three-layered collecting electrode. The authors used a corona discharge technique for the generation of ozone resulting in the simultaneous reduction of HC, NOx and smoke.
Many methods have been used for removal of NOx such as injection of a radical for NOx oxidation and reduction. Some of the radicals are ammonia (Nishida et al. 2001), ozone (Okubo et al. 2008; Sano and Yoshioka 2003), methane (Chang et al. 1998; Kanazawa et al. 1997), etc. Another method is the use of plasma for reducing NOx in a gas stream. In one study, a system consisting of plasma and Ag, Au/Al2O3 catalyst was used for reducing NOx (Ryu et al. 2002). With model exhaust gas, the NOx reduction efficiency varied from 40 to 90% in the temperature range of 250 to 400 °C. With an engine test, the NOx removal efficiency was 46% at 364 °C. The system also showed remarkable NOx reduction tendency at 100 to 250 °C. In another study, non-thermal plasma along with SCR has been used to enhance the rate of NOx removal (Sato et al. 2011). The ammonia gas was supplied in the SCR system. With simulated gas of a diesel engine exhaust, large NOx reduction was observed. The authors also tested the system on a heavy duty engine over JE05 Japanese cycle and observed significant NOx reductions. On the laboratory scale, also numerous studies were done to reduce the NOx level (Talebizadeh et al. 2014; Tonkyn et al. 2003; Tran et al. 2004; Ueda et al. 2004). Almost all these studies use plasma along with lean burn internal combustion engines.
The literature shows that the existing after-treatment systems are effective in reducing emissions but they have their own drawbacks. Such as the presence of sulphur in the fuel poisons the catalysts present in the after-treatment system. The diesel particulate filter has various issues like high back pressure, regeneration and poor collection efficiency of small-sized particles. In the present study a plasma-assisted exhaust after-treatment system was developed for a single cylinder diesel engine. The system contains one electrostatic precipitator and one non-thermal plasma generator working on pulse corona discharge technique. The ESP was used for reducing the exhaust smoke and the plasma generator was used to reduce other emissions. The novelty of the work lies in the use of Ni/Al2O3 catalyst which has not been used so far by any of the researchers. The catalyst was coated on the surface of the negative electrode of the plasma generator for improving the emission conversion efficiency. The study aims to simultaneously reduce the hazardous pollutants from the engine exhaust without the drawbacks of the existing after-treatment systems and without affecting the engine performance. The engine tests were conducted with diesel, and to ascertain that the after-treatment system works well with fuels other than diesel, engine tests were also conducted with neat waste cooking oil biodiesel.
Waste cooking oil biodiesel production
Waste cooking oil is readily available all over the world. It can be used as an additive for the preparation of fodder and in the soap manufacturing. However, vast quantities of WCO are either dumped in rivers or wastelands thus harming the environment (Senthil Kumar and Jaikumar 2014). In India, there are many roadside shops which use cooking oil for making food. The shop owners use the oil two to three times before throwing it in the drains which are harmful both for the humans by eating the food prepared in reused oil and the environment as the used oil is thrown in the drain. Instead, the oil can be converted to biodiesel and used as fuel in internal combustion engines (Arslan and Ulusoy 2018).
Waste cooking oil was collected from roadside shop owners in and around Delhi. The oil was initially filtered to remove the impurities and heated to 110 °C so that all the water (if present) is evaporated. The purified WCO was placed in a batch-type reactor which is a round bottom flask having three necks. The central neck has a stirrer connected to a DC motor. One of the necks has a thermometer so that the temperature of the reactants can be continuously monitored. The flask was placed on a heating jacket for heating the flask so that the temperature of the reactants was maintained accordingly. A mixture of 185 g of methanol and 4.6 g of potassium hydroxide (optimized) was prepared separately in a beaker. The mixture was then added to the reactant under constant stirring and 65–70 °C was maintained for 45 min. After the reaction was complete, the mixture was settled for 12–24 h in a separating funnel. After 24 h, two layers were formed: the lower layer was darker in colour and it consisted of glycerine and other products, while the upper layer was light in colour and consisted mainly of biodiesel. The two layers were then separated and the methyl ester of waste cooking oil was purified by washing with water. The biodiesel was heated to above 100 °C, to evaporate any water left. The conversion rate of waste cooking oil into biodiesel was nearly 92%. Some of the properties of the obtained biodiesel are shown in Table 1.
Construction and working of the ESP
The after-treatment setup consists of two electrostatic precipitators and a catalyst layer attached to the negative electrode of the second ESP. A gasket was placed in between the electrodes and the outer pipe. The gasket acts as an insulator. Figure 1 shows the schematic of the ESP. Nickel supported on aluminium oxide was used as the catalyst. The catalyst was made by preparing nickel and aluminium precursor (salt) solutions in ethanol. The nickel-aluminium solution was then coated on thin aluminium plates pre-coated with silica. The plates were then dried at room temperature followed by calcination at 350 °C.
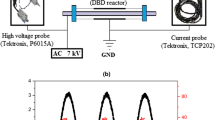
The exhaust gas first passes through the wire mesh with negative potential. The particles become negatively charged by passing through the electric field of the wire mesh. As the positive electrode is placed just after the wire mesh, the particles are attracted to the positive electrode which is cylindrical in shape. The positive electrode acts as a collector wherein some of the negatively charged particles are collected. In the second precipitator, a rod is placed in the centre of the pipe, and the rod has positive potential. The catalyst is placed on the surface of the negative electrode. Positive polarity pulse is used in the present work, since in the earlier works also positive polarity resulted in higher removal efficiency of gaseous pollutants (Kinoshita 2002; Thomas et al. 2001). As the streams take longer time in a positive pulse corona discharge, the ionization of more substantial volume of gas takes place in comparison to negative pulse corona discharge resulting in better performance (Li et al. 2000). A 13-kV AC power was applied between the two electrodes which helped in the generation of ozone (for few seconds). As the particles are attracted towards the negative layer, the catalyst present there provides the surface area for the oxidation of carbon monoxide and particulate matter, and reduction of hydrocarbons and oxides of nitrogen by ozone.
Experimental setup
In the present work, a Kirloskar make TAF-1 engine is used. The engine is four-stroke, air-cooled, direct injection, single cylinder; its specifications are given in Table 2. The engine was loaded in steps of 25% of full load using a DC generator connected to a load bank. To switch between diesel and WCO, a two-position, three-way manually operated directional control valve was used. An AVL di-gas analyser was used to measure NO, CO, CO2 and HC emissions in the exhaust gas and an AVL smoke meter was used for measuring the smoke opacity (Fig. 2).
The experiments were conducted at a constant engine speed of 1500 rpm and the loads were varied in steps of 25% of 100% load. Using a 50-cm3 burette and a stopwatch, the volumetric fuel flow rate was measured. Brake thermal efficiency and exhaust emissions were used to compare the engine performance. In the first phase, baseline was formed by conducting the experiments without the ESP. In the second phase, the experiments were conducted with the ESP. Only when the engine reached steady state condition, the readings were taken in triplicate and averaged. The uncertainty of the instruments used in this study is given in Table 3. The total uncertainty of the experiment was found using propagation of error techniques and was observed to be ± 3.2%. Appendix 1 shows the sample calculation for the uncertainty analysis.
Results and discussion
Figure 3 shows the effect of load and electrostatic precipitator on NO emission. The oxides of nitrogen are formed due to the high temperature of combustion which facilitates oxidation of nitrogen present in the air (Varuvel et al. 2018a). The NO emission level is higher with diesel fuel engine operation as compared to WCO engine operation at all loads. At full load, the NO emission with diesel and WCO engine operation is 7.26 and 7.01 g/kWh, respectively. The NO is formed mainly during the premixed combustion phase. WCO has lower NO emission since the premixed combustion phase is reduced, due to poor spray characteristics resulting in low fuel/air mixture. Hence less fuel is prepared for combustion during the initial phase (Boopathi et al. 2018). When the ESP is placed in the exhaust pipe, at full load, the NO emission decreases by 17% with diesel, and 21% with WCO as compared to engine operation with baseline diesel without ESP. The reduction of NOx occurs in two steps. First, the plasma oxidizes the NO to NO2 in the presence of hydrocarbons. The reaction for the oxidation is given by Eq. 1 (Penetrante et al. 1998; Puchkarev et al. 1999).
Here HC refers to hydrocarbons and HC-products refers to hydrocarbons that are partially oxidized. In the second step, as the formed NO2 and products of HC are attracted towards the negative potential, the catalyst with the help of hydrocarbons convert NO2 to N2 using Eq. 2.
Both NO2 and HC are absorbed on the surface of the catalyst wherein the hydrocarbon species present near to the sites where NO2 is absorbed chemically removes the oxygen through the formation of transient species and then the nitrogen molecule exits the catalyst. In the process, the hydrocarbon is converted to carbon monoxide and carbon dioxide. The basic chemistry of the reaction is thus confirmed, although there is no clarity on the possible transient species formed. The reaction also shows that the presence of sufficient hydrocarbon level is necessary for the reactions to occur. Theoretically equal amount of NO and HC are required for the reaction. However, higher ratios of hydrocarbon will increase the probability of NO reduction (Herling et al. 2000). Figure 3 also shows a higher reduction of NO emissions with WCO engine operation as compared to diesel engine operation mainly due to the presence of higher amount of unburnt hydrocarbons in the exhaust gas (Fig. 4) which helps in the reduction of NO by the process previously discussed.
The variation in HC emission with load for the test fuels is portrayed in Fig. 4. The HC emission with WCO engine operation is higher than diesel operation due to incomplete combustion. The density and viscosity of WCO are higher than diesel resulting in improper penetration of the spray thereby causing poor mixing of fuel and air and formation of partially burnt hydrocarbons (Geo et al. 2018). The HC emission level at full load without the electrostatic precipitator for diesel and WCO is 0.71 and 0.82 g/kWh, respectively. With ESP in place, the emission level reduces for both the test fuels. The HC emissions with ESP reduced to 0.27 and 0.31 g/kWh for diesel and WCO engine operation, respectively. The emission reduction with ESP can be attributed to the use of HC emissions for converting NO to N2, as previously discussed. Also, the emissions are oxidized during the ozone regeneration process in the plasma along with the catalyst, which speeds up the oxidation process, to carbon monoxide according to Eq. 3 (Subramaniam et al. 2016).
The CO emissions are generally lower in CI engines as compared to SI engines as the CI engines run on lean mixtures. Partial oxidation of the fuel molecules and reduced flame temperatures are the primary reasons for the formation of the intermediate carbon monoxide (Thiyagarajan et al. 2019). The variation in CO emission with load for the conducted tests is shown in Fig. 5. At 100% load, the CO emission is 1.5 and 1.95 g/kWh for diesel and WCO, respectively. The fuel molecules unburnt during the initial stages are surrounded by their own products of combustion in the later stages of combustion thus they do not have enough oxygen for their oxidation. Also, by the end of the expansion stroke, the CO that has formed does not have enough time for its oxidation along with lower temperature (Geo et al. 2017). As the ESP is placed in the exhaust pipe, the carbon monoxide emission levels reduced. At full load, as compared to diesel operation without ESP, the emission levels reduced by 60 and 40% with diesel and WCO engine operation, respectively. In the plasma, generated by the corona discharge, the ozone is formed according to the Eqs. 4 and 5. The ozone reacts with the carbon monoxide present in the exhaust gas according to the Eqs. 6 and 7 (Li et al. 2000), thus forming carbon dioxide. Figure 5 confirms that the complete conversion of carbon monoxide is not possible probably due to less residence time.
The variation in carbon dioxide emissions with load is shown in Fig. 6. Carbon dioxide is formed when the fuel containing hydrocarbons is completed burned. The amount of carbon that the fuel contains and the fuel consumption play a significant role in the formation of CO2 (Thiyagarajan et al. 2018a, b). The carbon dioxide emissions are lower with waste cooking oil biodiesel. Although WCO has higher oxygen content and the fuel consumption is also higher, the CO2 emissions are lower on account of slower combustion due to high viscosity and lower combustion temperature. The CO2 emissions at full load for diesel and WCO engine operation without ESP are 8.19 and 7.28 g/kWh, respectively. Whereas, with ESP placed in the exhaust pipe, the carbon dioxide emission at full load is 9.42 and 8.86 g/kWh with WCO and diesel engine operation, respectively. The carbon dioxide emission without the emission reduction setup is lower than with the setup in place. The plasma generated in the corona discharge setup and the catalyst placed on the negative electrode of the corona discharge setup facilitate the conversion of unburned hydrocarbons and carbon monoxide to carbon dioxide, according to Eqs. 2, 3 and 7, thereby increasing the CO2 emission levels.
The modulation in smoke opacity with various engine loads is shown in Fig. 7. The combustion in diesel engines is heterogeneous resulting in air-fuel ratio being rich in some parts of the combustion chamber. The fuel molecules in these regions do not get enough oxygen and are thermally cracked resulting in the formation of large amount of smoke (Edwin Geo et al. 2019). Waste cooking oil biodiesel engine operation resulted in higher smoke opacity than diesel on account of large quantity of fuel injected into the combustion chamber due to its lower calorific value. Also, the viscosity of the fuel is high and its volatility is poor which makes the combustion inferior. Combining these two effects results in the partial oxidation of the carbon atoms thereby increasing the formation of particulate matter. The smoke opacity is lower with the emission reduction system in place. The first electrostatic precipitator tends to reduce the particulate emission by charging the particles which are collected on the positive electrode of the electrostatic precipitator. When the exhaust gas moves further into the second ESP, the ozone generated in the plasma oxidizes some of the carbon particles according to Eq. 8.
Figure 7 also shows that the particulate reduction efficiency is high at higher loads in comparison to lower loads. It is observed that at 25% load the particulates reduction efficiency is 30.7 and 30.3% for diesel and WCO, respectively. Whereas at full load the reduction efficiency increases to 37.3 and 38.2% for diesel and WCO, respectively. With varying load conditions, a significant change occurs in the concentration of mass and composition of the particles. The particle mass concentration at high loads is less, but the number concentration is high resulting in a shift of size distribution towards smaller sized particles (Subramaniam et al. 2016). The data observed shows that the efficiency of ESP in collecting the small-sized particles is more in comparison to relatively bigger sized particles.
The engine efficiency is expected to decrease with catalytic converter placed in the exhaust pipe due to the back pressure caused by the obstruction to the flow of the exhaust gas. The advantage that the emission reduction setup used in the present work has over conventional catalytic converter is that the exhaust gas is not obstructed and the engine efficiency is the same, shown in Fig. 8. Moreover, the brake thermal efficiency with waste cooking oil engine operation is lower than that of diesel. At full load, the efficiency is 30.86 and 29.3% for diesel and WCO, respectively. The decrease in efficiency is due to poor spray characteristics caused by high viscosity resulting in poor mixing leading to inferior combustion (Varuvel et al. 2018b). Therefore, the heat released in premixed combustion is less due to longer ignition delay resulting in slow combustion and the flame front reaching the exhaust valves (Thiyagarajan et al. 2018a, b).
The variation in exhaust gas temperature (EGT) with engine load is shown in Fig. 9. It is seen that the EGT increases as the load on the engine increases. WCO engine operation resulted in higher exhaust temperature than diesel engine operation. The increase in EGT with WCO is due to higher heat release in the diffusion combustion phase which is not utilized in useful work but leaves the engine as waste heat (Boopathi et al. 2018). The exhaust temperature was similar with after-treatment system and without the system for both the fuels. This shows that the after-treatment system has no effect on the EGT. However, as shown in Fig. 3, the NO removal efficiency of the system decreases as the load on the engine increases. This effect is mainly attributed to the reduction in HC emissions at high loads (and high EGT), since HC emissions play a role in the reduction of NO emission. It is also seen that there is some improvement in the conversion efficiency of other emissions, since the catalyst efficiency improves with the increase in temperature. Therefore, it can be considered that temperature of the exhaust gas plays minuscule role in the working of the after-treatment system.
Conclusions
In the present work, the ESP was developed to reduce the pollutants namely HC, CO, NO and smoke from the exhaust of a compression ignition engine fuelled with diesel and WCO. The results show that NO emission is reduced by 20.8 and 16.9% for WCO and diesel, respectively, with ESP placed in the exhaust line. The plasma formed in the ESP along with unburnt hydrocarbons in the exhaust gas helps in the conversion of NO to nitrogen. The catalyst placed on the negative electrode of the ESP facilitates the faster conversion of NO to N2. The HC emissions are reduced by 61.9 and 62.5% with diesel and WCO engine operation. The ozone present in the plasma oxidizes the unburnt hydrocarbon to carbon dioxide along with the use of HC emissions in converting NO to N2, thereby reducing the HC emissions. The ozone and the catalyst also facilitate the faster conversion of CO to CO2. At full load, the CO emission reduced by 60.4 and 52.89% with diesel and WCO engine operation, respectively. Since the unburnt hydrocarbons and carbon monoxide are converted to CO2 by the emission reduction setup, the emission level at full load increased by 9.18 and 9.89% with WCO and diesel engine operation, respectively. The particulate matter emission is also reduced by the ESP. The removal efficiency is higher at high loads when the particle size is small. The brake thermal efficiency with and without the ESP shows that the ESP does not affect the engine performance. Thus it can be concluded that the ESP can reduce the pollutants from the exhaust gas of an engine without any modifications of the engine.
Future scope and limitation
In an electrostatic precipitator, due to the deposition of the smoke particles on the electrodes, its efficiency starts decreasing after certain hours of running. Therefore, it is necessary to regenerate the electrodes so that the ESP works with the same efficiency. In the present study, the efficiency of the ESP was not affected as after every test the electrodes were cleaned. Similarly, the efficiency of the catalyst can reduce after certain period of time. The future scope of the study is to devise a mechanism through which the electrodes of the ESP can be regenerated after certain hours of running of the vehicle. Also, the amount of energy required for operating an ESP is generally high. Therefore, a study can be carried out for finding the energy spent on operating the ESP and the optimization of the power required so that a balance exists between the emission reduction efficiency and the energy requirement of the system.
References
Arslan R, Ulusoy Y (2018) Utilization of waste cooking oil as an alternative fuel for Turkey. Environ Sci Pollut Res 25(25):24520–24525. https://doi.org/10.1007/s11356-017-8899-3
Boopathi D, Thiyagarajan S, Edwin Geo V, Madhankumar S, Gheith R (2018) Effect of geraniol on performance, emission and combustion characteristics of CI engine fuelled with gutter oil obtained from different sources. Energy 157:391–401. https://doi.org/10.1016/J.ENERGY.2018.05.129
Chang JS, Urashima K, Arquilla M, Ito T (1998) Reduction of NOx from combustion flue gases by corona discharge activated methane radical injections. Combust Sci Technol 133(1–3):31–47. https://doi.org/10.1080/00102209808952025
Edwin Geo V, Sonthalia A, Nagarajan G, Nagalingam B, Aloui F (2019) Effect of port premixed liquefied petroleum gas on the engine characteristics. J Energy Resour Technol 141(11). https://doi.org/10.1115/1.4043698
Geo VE, Sonthalia A, Nagarajan G, Nagalingam B (2017) Studies on performance, combustion and emission of a single cylinder diesel engine fuelled with rubber seed oil and its biodiesel along with ethanol as injected fuel. Fuel 209:733–741. https://doi.org/10.1016/j.fuel.2017.08.036
Geo VE, Sonthalia A, Aloui F, Femilda Josephin JS (2018) Study of engine performance, emission and combustion characteristics fueled with diesel-like fuel produced from waste engine oil and waste plastics. Front Environ Sci Eng 12(4):8–9. https://doi.org/10.1007/s11783-018-1063-6
Herling D, Smith M, Baskaran S, Kupe J (2000) Application of non-thermal plasma assisted catalyst technology for diesel emission reduction. SAE Technical Paper Series 2000-01–3088. https://doi.org/10.4271/2000-01-3088
Huang Y, Huang S, Zheng Q, Shen X, Wang S, Han P et al (2015) Recent progress of dry electrostatic precipitation for PM2.5 emission control from coal-fired boilers. International Journal of Plasma Environmental Science and Technology 9(2):69–126
Ji J-H, Hwang J, Bae G-N, Kim Y-G (2004) Particle charging and agglomeration in DC and AC electric fields. J Electrost 61(1):57–68. https://doi.org/10.1016/j.elstat.2003.12.003
Kanazawa S, Chang JS, Round GF, Sheng G, Ohkubo T, Nomoto Y, Adachi T (1997) Removal of NOx from flue gas by corona discharge activated methane radical showers. J Electrost 40–41:651–656. https://doi.org/10.1016/S0304-3886(97)00072-7
Kinoshita K (2002) Study on removal of PM and NOx in diesel exhaust by using DC corona discharge. SAE Technical Paper 2002-01–16(724). https://doi.org/10.4271/2002-01-1660
Kuwahara T, Nishii S, Kuroki T, Okubo M (2013) Complete regeneration characteristics of diesel particulate filter using ozone injection. Appl Energy 111:652–656. https://doi.org/10.1016/J.APENERGY.2013.05.041
Li X, Yang L, Lei Y, Wang J, Lu Y (2000) A method for removal of CO from exhaust gas using pulsed corona discharge. J Air Waste Manag Assoc 50(10):1734–1738. https://doi.org/10.1080/10473289.2000.10464215
Losacco C, Perillo A (2018) Particulate matter air pollution and respiratory impact on humans and animals. Environ Sci Pollut Res 25(34):33901–33910. https://doi.org/10.1007/s11356-018-3344-9
Nakajima Y, Sato T (2003) Electrostatic collection of submicron particles with the aid of electrostatic agglomeration promoted by particle vibration. Powder Technol 135–136:266–284. https://doi.org/10.1016/S0032-5910(03)00165-7
Nguyen Huu Nhon Y, Mohamed Magan H, Petit C (2004) Catalytic diesel particulate filter: evaluation of parameters for laboratory studies. Appl Catal B Environ 49(2):127–133. https://doi.org/10.1016/J.APCATB.2003.12.005
Nishida M, Yukimura K, Kambara S, Maruyama T (2001) Reduction of nitrogen oxide in N2 by NH3 using intermittent dielectric barrier discharge. J Appl Phys 90(6):2672–2677. https://doi.org/10.1063/1.1394902
Okubo M, Arita N, Kuroki T, Yoshida K, Yamamoto T (2008) Total diesel emission control technology using ozone injection and plasma desorption. Plasma Chem Plasma Process 28(2):173–187. https://doi.org/10.1007/s11090-008-9121-7
Parker KR (1997) Milestones in the history of precipitation. In: Parker KR (ed) Applied electrostatic precipitation. Blackie Acad.. & Professional, London, pp 11–12
Penetrante BM, Brusasco RM, Merritt BT, Pitz WJ, Vogtlin GE (1998) Plasma-assisted catalytic reduction of NOx. SAE Technical Paper Series 982508
Puchkarev V, Kharlov A, Gundersen M, Roth G (1999) Application of pulsed corona discharge to diesel exhaust remediation. Digest of Technical Papers. 12th IEEE International Pulsed Power Conference. (Cat. No.99CH36358) 1(October):511–514. https://doi.org/10.1109/PPC.1999.825522
Ryu J, Jeong D, Chun B, Lee D, Lee K, Lee H, Chun KM (2002) Experimental study on DeNOx performance by plasma-catalyst (Ag, Au/Al2O3) system. SAE International (2002-01–2705)
Sano K, Yoshioka Y (2003) Effect of oxygen injection and HC addition on NO removability of ozone injection method. IEEJ Trans Fundam Mater 123(10):1030–1036. https://doi.org/10.1541/ieejfms.123.1030
Sato S, Kawada Y, Sato S, Hosoya M (2011) The study of NOx reduction using plasma-assisted SCR system for a heavy duty diesel engine. SAE International (2011-01–0310). https://doi.org/10.4271/2011-01-0310
Senthil Kumar M, Jaikumar M (2014) A comprehensive study on performance, emission and combustion behavior of a compression ignition engine fuelled with WCO (waste cooking oil) emulsion as fuel. J Energy Inst 87(3):263–271. https://doi.org/10.1016/j.joei.2014.03.001
Stratakis GA, Psarianos DL, Stamatelos AM (2002) Experimental investigation of the pressure drop in porous ceramic diesel particulate filters. Journal of Automobile Engineering 216:773–784
Subramaniam MK, Pachamuthu S, Arulanandan J, Muthiya J (2016) Simultaneous reduction of HC, NOx and PM by using active regeneration technique. SAE International 2016-01–0912
Subramanian T, Varuvel EG, Martin LJ, Beddhannan N (2017) Effect of lower and higher alcohol fuel synergies in biofuel blends and exhaust treatment system on emissions from CI engine. Environ Sci Pollut Res 24(32):25103–25113. https://doi.org/10.1007/s11356-017-0214-9
Sudrajad A, Yusof AF (2015) Review of electrostatic precipitator device for reduce of diesel engine particulate matter. Energy Procedia 68:370–380. https://doi.org/10.1016/j.egypro.2015.03.268
Talebizadeh P, Babaie M, Brown R, Rahimzadeh H, Ristovski Z, Arai M (2014) The role of non-thermal plasma technique in NOx treatment: a review. Renew Sust Energ Rev 40(x):886–901. https://doi.org/10.1016/j.rser.2014.07.194
Thiyagarajan S., Geo VE, Martin LJ, Nagalingam B (2018a) Combined effect of fuel-design and after-treatment system on reduction of local and global emissions from CI engine. Environmental Technology (United Kingdom) 1–11. https://doi.org/10.1080/09593330.2018.1453871
Thiyagarajan S, Varuvel EG, Martin LJ, Beddhannan N, Geo VE, Martin LJ, Nagalingam B (2018b) Mitigation of carbon footprints through a blend of biofuels and oxygenates, combined with post-combustion capture system in a single cylinder CI engine. Renew Energy 130:1067–1081. https://doi.org/10.1016/j.renene.2018.07.010
Thiyagarajan S, Sonthalia A, Edwin Geo V, Ashok B, Nanthagopal K, Karthickeyan V, Dhinesh B (2019) Effect of electromagnet-based fuel-reforming system on high-viscous and low-viscous biofuel fueled in heavy-duty CI engine. J Therm Anal Calorim 138(1):633–644. https://doi.org/10.1007/s10973-019-08123-w
Thomas SE, Shawcross JT, Gillespie R, Raybone D, Martin AR (2001) The role of NO selective catalysts in the plasma enhanced removal of NOx and PM from diesel exhaust. Sae Technical Paper Series 724:2001–3568. https://doi.org/10.4271/2001-01-3568
Thonglek N, Dechthummarong C, Kiatsiriroat T (2011) Soot treatment by using high voltage pulse energized electrostatic precipitator. Energy Procedia 9:292–298. https://doi.org/10.1016/J.EGYPRO.2011.09.031
Tonkyn RG, Barlow SE, Hoard JW (2003) Reduction of NOx in synthetic diesel exhaust via two-step plasma-catalysis treatment. Appl Catal B Environ 40(3):207–217. https://doi.org/10.1016/S0926-3373(02)00150-9
Tran DN, Aardahl CL, Rappe KG, Park PW, Boyer CL (2004) Reduction of NOx by plasma-facilitated catalysis over In-doped γ-alumina. Appl Catal B Environ 48(2):155–164. https://doi.org/10.1016/j.apcatb.2003.10.008
Ueda M, Itoh Y, Shinjoh H, Nakakita K, Arakawa M (2004) A concept of plasma assisted catalyst system using a DeNOx catalyst for an automobile diesel engine. SAE Technical Papers 724. https://doi.org/10.4271/2004-01-1834
Umemoto H, Hayashi H, Takashima K, Mizuno A (2008) Electrostatic precipitators for cleaning diesel exhaust. In 2008 IEEE Industry Applications Society Annual Meeting (pp. 1–4). https://doi.org/10.1109/08IAS.2008.97
Varuvel EG, Sonthalia A, Subramanian T, Aloui F (2018a) NOx-smoke trade-off characteristics of minor vegetable oil blends synergy with oxygenate in a commercial CI engine. Environ Sci Pollut Res 25(35):35715–35724. https://doi.org/10.1007/s11356-018-3484-y
Varuvel EG, Sonthalia A, Subramanian T, Aloui F (2018b) NOx-smoke tradeoff characteristics of minor vegetable oil blends synergy with oxygenate in a commercial CI engine. Environ Sci Pollut Res 25(35):35715–35724
Zukeran A, Ikeda Y, Ehara Y, Ito T, Takahashi T, Kawakami H, Takamatsu T (2000) Agglomeration of particles by ac corona discharge. Electrical Engineering in Japan 130(1):30–37. https://doi.org/10.1002/(SICI)1520-6416(200001)130:1<30::AID-EEJ5>3.0.CO;2-F
Acknowledgements
The authors are thankful to Dr. B. P. Kiran from Synthesis with Catalysts Pvt. Ltd. for providing the catalyst.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Sample calculation for the uncertainty analysis
Uncertainty in brake power = \( \sqrt{{\left(\frac{\Delta V}{V}\right)}^2+{\left(\frac{\Delta I}{I}\right)}^2} \) = 1.2%
Uncertainty in fuel consumption = \( \sqrt{{\left(\frac{\Delta A}{A}\right)}^2+{\left(\frac{\Delta t}{t}\right)}^2} \) = 1.5%
Uncertainty in brake thermal efficiency =\( \sqrt{{\left(\frac{\Delta \mathrm{BP}}{\mathrm{BP}}\right)}^2+{\left(\frac{\Delta \mathrm{FC}}{\mathrm{FC}}\right)}^2} \) = 1.12%
Uncertainty in oxides of nitrogen =\( \sqrt{{\left(\frac{\Delta \mathrm{BP}}{\mathrm{BP}}\right)}^2+{\left(\frac{\Delta \mathrm{NO}}{\mathrm{NO}}\right)}^2} \) = 1.1%
Uncertainty in carbon monoxide =\( \sqrt{{\left(\frac{\Delta \mathrm{BP}}{\mathrm{BP}}\right)}^2+{\left(\frac{\Delta \mathrm{CO}}{\mathrm{CO}}\right)}^2} \) = 1.12%
Uncertainty in unburnt hydrocarbon =\( \sqrt{{\left(\frac{\Delta \mathrm{BP}}{\mathrm{BP}}\right)}^2+{\left(\frac{\Delta \mathrm{HC}}{\mathrm{HC}}\right)}^2} \) = 1.14%
Uncertainty in carbon dioxide =\( \sqrt{{\left(\frac{\Delta \mathrm{BP}}{\mathrm{BP}}\right)}^2+{\left(\frac{\Delta {\mathrm{CO}}_2}{{\mathrm{CO}}_2}\right)}^2} \) = 1.15%
Uncertainty in smoke = 1.0%
Where V—the voltage available from the generator, I—the current available from the generator, A—graduated burette, t—time, BP—brake power, FC—fuel consumption, NO—oxides of nitrogen, CO—carbon monoxide, HC—unburnt hydrocarbon, and CO2—carbon dioxide.
Rights and permissions
About this article
Cite this article
Sonthalia, A., Garg, S., Sharma, R. et al. Effect of electrostatic precipitator on exhaust emissions in biodiesel fuelled CI engine. Environ Sci Pollut Res 28, 11850–11859 (2021). https://doi.org/10.1007/s11356-019-07359-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07359-1