Abstract
Patients with ischemia and non-obstructive coronary artery (INOCA) often have coronary microvascular dysfunction (CMD), and they are at high risk for adverse cardiac events. Nevertheless, the management of CMD represents a major unmet need because the lack of large, randomized studies makes it difficult to generate evidence-based recommendations. Recently, it was demonstrated that stratified medical therapy guided by an interventional diagnostic procedure improves health status of patients with INOCA. Accordingly, the latest guidelines state that treatment of CMD should address the dominant mechanism of microcirculatory dysfunction. In patients with impaired microcirculatory conductance and a negative acetylcholine (ACh) provocation test, beta-blockers, ACE inhibitors, and statins, along with lifestyle modifications and weight loss, are indicated. On the other hand, patients developing ECG changes and angina in response to ACh testing but without severe epicardial coronary vasoconstriction (all suggestive of microvascular spasm) may be treated mainly by calcium channel blockers. However, in patients with INOCA, coronary functional abnormalities, including epicardial coronary spasm, reduced microvascular vasodilatation, and increased microvascular resistance, frequently coexist in various combinations. Thus, in everyday clinical practice, a combination of several types of vasodilators, such as a beta-blocker and a long-acting dihydropyridine calcium channel blocker, should constitute the second step when a single drug fails to success. In cases with refractory symptoms which seriously limit life quality, analgesic drugs or non-pharmacological interventions, including rehabilitation exercise programs, spinal cord simulation, and/or psychological treatments, might be helpful. In this section, we will discuss the treatment options for CMD, taking into consideration currently accepted pathogenic mechanisms of the disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Patients with ischemia and non-obstructive coronary artery (INOCA) often have coronary microvascular dysfunction (CMD) and are diagnosed as having microvascular angina (MVA). Recent studies demonstrated that they are at high risk for adverse cardiac events, including cardiac death, non-fatal myocardial infarction, heart failure, and hospitalization due to unstable angina [1, 2]. Nevertheless, the management of CMD represents a major unmet need because the lack of large, randomized studies involving homogeneous patient groups makes it difficult to generate evidence-based recommendations. Indeed, the guidelines of the European Society of Cardiology and the Japanese Circulation Society confirm relatively low levels of evidence for treatment of patients with CMD and no large randomized outcome trials [3, 4]. Thus, the treatment for CMD has so far been empirical because its pathophysiology appears to be multifactorial, with overlapping phenotypes that often coexist. On the other hand, recent papers have discussed the management of those patients and suggested potential therapies for CMD [5,6,7]. Targets for those therapies include conventional coronary risk factors and endothelial dysfunction, myocardial ischemia due to impaired coronary microvascular dilatory function or microvascular spasm, and chest pain–increased nociception [5,6,7]. The therapeutic aims are to improve myocardial ischemia addressing its causes, improve quality of life, and improve long-term prognosis. In this section, we will discuss the treatment options for CMD, taking into consideration currently accepted pathogenic mechanisms of the disorder.
2 Control of Risk Factors for Coronary Microvascular Dysfunction
The presence of CMD in patients with cardiovascular risk factors can be predictive of future development of macrovascular atherosclerosis [8]. Especially, those using intravascular ultrasound have also shown that non-obstructive coronary artery disease (CAD) is noted in a large proportion of patients with CMD [9]. Thus, aggressive management of all modifiable conventional risk factors is of paramount importance in the CMD patients [5, 10]. Smoking cessation, weight loss, adequate control of blood pressure, diabetes and metabolic abnormalities, lipid management, improved nutrition, and regular exercise may be applicable [11]. It has been demonstrated that anti-hypertensive drugs are able to improve CMD in patients with hypertension, although some differences among classes of medications may exist [12,13,14]. For instance, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have been shown to improve or even normalize endothelium-independent coronary microvascular function in hypertensive patients [12]. Furthermore, olmesartan, but not amlodipine, has been shown to improve endothelium-dependent coronary vasodilatation in hypertensive patients irrespective of blood pressure (BP) reduction [13]. In contrast, another study showed that verapamil, but not enalapril, was able to improve myocardial blood flow (MBF) during atrial pacing despite a similar BP reduction [14]. These findings suggest that the favorable effects of antihypertensive drugs on CMD mainly depend on mechanisms other than hypotensive effect, including direct effects on vascular smooth muscle cells, an improvement of oxidative state, endothelial function, and diastolic function, as well as effects on autonomic nervous system. Statins, alone or in combination with ACE inhibitors, have been shown to exert beneficial effects in patients with coronary endothelial and/or vascular smooth muscle dysfunction despite non-obstructive CAD [15,16,17]. Unlike the cases with hypertension or hypercholesterolemia, the effect of glycemic control on CMD in diabetic patients remains to be elucidated. Indeed, weight loss in obese patients has also been reported to improve microvascular function with increased adiponectin levels [18, 19]. Thromboxane A2 (TXA2) could cause microvascular constriction, platelet aggregation, and vascular injury. Thus, low-dose aspirin, which is a TXA2 inhibitor, could provide microvascular protection against oxidative injury in the microcirculation [20].
CMD is also initiated by classical cardiovascular risk factors that also maintain a low-grade inflammation [21, 22]. Additionally, chronic systemic inflammation is associated with CMD possibly mediated through C-reactive protein (CRP), which levels were related to coronary flow reserve impairment in patients with a chest pain syndrome without risk factors for CAD and angiographically normal epicardial arteries [23]. Anti-inflammatory agents block associated endothelial dysfunction that plays a key role in the pathogenesis of CMD. Specific approaches to modify inflammation in CMD are difficult to assess since essentially all effective anti-ischemic and anti-atherosclerosis agents modify inflammation to some degree [24].
3 Pharmacological Symptomatic Therapies for Coronary Microvascular Dysfunction
3.1 Beta Blockers
The European Society of Cardiology guidelines for patients with MVA recommend beta-blockers as first-line and calcium channel blockers if the former are not tolerated or efficacious [25]. Beta-blockers are able to reduce myocardial oxygen consumption and to improve coronary perfusion by prolonging diastolic time. In particular, beta-blocker therapy may be considered to provide therapeutic benefit for MVA patients with exercise-induced symptoms and those with increased sympathetic nervous activity as evidenced by elevated blood pressure-rate response to exercise [26, 27]. Actually, propranolol reduced the number of episodes of ST-segment depression during 24-h ECG Holter monitoring as compared to verapamil [28]. The use of atenolol has been shown to reduce the number of angina episodes and also improve the ischemic threshold [29, 30]. Carvedilol has been shown to improve endothelial function [31]. Furthermore, nebivolol, which is a highly selective beta-1 blocker with vasodilatory effects via nitric oxide (NO) production, has beneficial effects on angina and exercise capacity in patients with CMD [32]. Notably, nebivolol improved left ventricular filling pressure and coronary flow reserve (CFR) in uncomplicated arterial hypertension, suggesting the involvement of enhanced myocardial NO production and improvement of coronary microvascular function [32]. However, the effects of beta-blocker therapy on symptoms of chest pain are variable in MVA patients ranging from 19% to 60% [26]. Additionally, caution should be exercised in the use of beta-blockers in patients with microvascular spasm because they could exacerbate coronary vasoconstriction by unmasking α-adrenoceptors in the coronary circulation [5, 7].
3.2 Calcium Channel Blockers
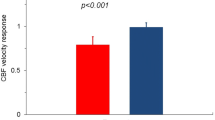
Calcium channel blockers (CCBs) have potent vasodilatory effects and are therefore expected to improve the increased resistance of coronary microcirculation. However, while dihydropyridine CCBs can reduce systemic blood pressure rapidly, they might simultaneously cause a reflex increase in adrenergic activity that antagonizes their favorable vasodilatory effects. In contrast, non-dihydropyridine CCBs could decrease myocardial oxygen consumption by the negative chronotropic and inotropic effects. In a clinical setting, CCBs are widely used in patients with non-obstructive CAD and coronary vasomotor disorders including vasospastic angina (VSA). In particular, benidipine, a long-acting dihydropyridine, showed beneficial prognostic impacts in VSA patients [33]. Additionally, with the hope of improving reduced vasodilator capacity of the coronary microcirculation and reducing cardiac afterload, CCBs are often used for patients with CMD, which is supported by an experimental study showing that amlodipine improves inward remodeling in CMD [34]. In expert consensus, CCBs are likely to represent the first-line agents for patients with documented microvascular spasm or abnormal CFR and those with mainly exercise-related symptoms if beta-blockers are without effects [6, 7, 25]. However, CCBs have shown variable results in the previous trials with INOCA patients [28, 29, 35, 36]. It has been reported that intracoronary diltiazem does not improve CFR in patients with MVA [35]. Furthermore, no significant improvement in angina was noted with amlodipine in INOCA patients, [29] and verapamil failed to reduce spontaneous episodes of ischemic ST-segment changes in another study [28]. On the other hand, patients with abnormal vasodilator reserve can have improved symptoms, less nitrate use, and improved exercise tolerance with verapamil or nifedipine [36]. Moreover, long-acting nifedipine exerted cardiovascular protective effects through inhibition of vascular inflammation and improvement of endothelial function in CAD patients with vasomotor dysfunction (Fig. 8.1) [37, 38]. Thus, long-acting L-type calcium channel blockers appear to be more effective for coronary microcirculation compared with short-acting ones. Importantly, it should also be noted that some patients may paradoxically experience worsening of symptoms on CCBs with a resultant withdrawal [7, 25].
The ENCORE II trial demonstrated that coronary vasodilator responses to intracoronary acetylcholine were improved only in the group treated with nifedipine but not in the placebo group. (Reproduced from Luscher et al. [37])
3.3 Nitrates
Nitrates are one of the classical drugs that have been widely used for cardiovascular diseases. Nitrates act via NO signaling pathways and exert endothelium-independent vasodilatation, leading to an increase in coronary perfusion and reductions in cardiac pre- and post-load [39, 40]. With these pharmacological features, nitrates acutely improve cardiac conditions, such as angina attacks and acute heart failure. However, chronic exposure to nitrates results in a rapid development of tolerance, blunting their anti-ischemic and hemodynamic efficacy [39, 40]. Furthermore, their potential harm for cardiovascular patients, such as generation of reactive oxygen species with resultant endothelial dysfunction, [41] sympathetic nerve activation, [42] and increase in sensitivity to vasoconstrictors [43], has also been reported. Since nitrate therapy acutely improves vasospastic symptoms, [44] they are often used mainly as a concomitant therapy with CCBs in VSA patients [4, 45]. However, the effects of nitrates on the coronary microcirculation seem to be variable and rather limited. Indeed, sublingual short-acting nitrates, which are the first-line drugs to treat angina attacks in patients with MVA as well as those with obstructive CAD or epicardial spasm, were found to be effective in only about a half of patients [46]. The previous studies suggested that sublingual nitrate therapy worsened or failed to improve exercise tolerance in patients with syndrome X [47, 48]. Furthermore, chronic oral nitrate therapy with isosorbide-5-mononitrate (40 mg) also failed to improve symptoms and quality of life over a period of 4 weeks in those patients, [29] and ISDN was not helpful for patients with CMD [49]. On the basis of these results, long-acting nitrates have generally presented no positive effect and thus may not be recommended as first-line drugs for patients with CMD.
3.4 Nicorandil
Nicorandil has the dual properties of nitrate and KATP channel agonist, showing the cardiovascular protective effects without tolerance development [50]. This agent opens ATP-sensitive potassium channels, thereby causing dilatation of coronary resistant arterioles and possesses a nitrate moiety which dilates epicardial coronary arteries. In fact, nicorandil could cause vascular relaxation without intracellular cGMP accumulation through opening potassium channels in the plasma membrane with resultant hyperpolarization of vascular smooth muscle cells. Importantly, a functional role of KATP channels in response to nicorandil becomes more apparent when cyclic GMP formation is suppressed as in the case of nitrate tolerance [51]. A previous study demonstrated that intravenous administration of nicorandil could lead to significant improvements in scintigraphy results as well as anginal symptoms and ST-segment depression during exercise [52]. Furthermore, in another randomized placebo-controlled trial, a 2-week therapy with nicorandil in patients with microvascular angina resulted in significant improvement in exercise-induced myocardial ischemia and exercise tolerability [53]. Accordingly, where available, nicorandil should be taken into account in the treatment of patients with CMD, in particular as an alternative to nitrates.
3.5 ACE Inhibitors
Local tissue angiotensin II is involved in the regulation of coronary microvascular structure and function, and it also enhances the effects of sympathetic nervous system on coronary microvascular tone. Thus, renin-angiotensin system inhibition has been considered to be an appropriate therapy for patients with CMD. Furthermore, ACE inhibitors could benefit coronary vascular bed by restoring endothelial function and may improve coronary flow reserve (CFR) by bradykinin-mediated, NO-dependent mechanisms [54]. Indeed, enalapril has been demonstrated to improve CMD through increase of NO availability and reduction of oxidative stress in MVA patients [55]. It also has been demonstrated that enalapril and cilazapril reduce the magnitude of ST-segment depression and increasing the total exercise duration and time to 1 mm of ST-segment depression in MVA patients with reduced coronary flow [56, 57]. Moreover, improvements of angina symptoms and exercise capacity have been noted with the use of several kinds of ACE inhibitors [16, 58, 59]. Thus, since available studies assessing the effects of ACE inhibitors in MVA patients have generally shown beneficial results, more proactive use of the agents should be recommended in patients with CMD.
3.6 Ranolazine
Ranolazine is an anti-ischemic dug that acts via inhibiting the transmembrane late sodium current, resulting in reduction of intracellular calcium levels and prevention of calcium overload during ischemia [60]. Thus, ranolazine is considered to be able to improve myocardial relaxation and left ventricular diastolic function [60]. The effect of ranolazine on CMD has been conflicting in the pilot placebo-controlled trials, [61, 62] whereas a recent large randomized crossover trial of ranolazine vs. placebo found no difference in symptoms or cardiac magnetic resonance imaging-myocardial perfusion reserve [63]. However, in a pre-defined subgroup who had CFR assessed invasively, symptomatic patients with CFR <2.5 and non-obstructive CAD showed improved angina and myocardial perfusion with ranolazine, indicating that ranolazine provides a promising management option for patients with CMD and low CFR [64].
3.7 Ivabradine
Selective If-channel blockade using ivabradine is a specific bradycardic agent that selectively reduces sinus node activity through inhibition of the If current [65]. In contrast to β-blockers, ivabradine does not cause vasoconstriction or negative inotropic effects [65]. Beneficial effects of ivabradine in IHD are mediated by its indirect effects to improve exercise tolerance, prolong time to ischemia during exercise, and reduce angina severity and frequency compared with other antianginal agents in patients with stable angina [65, 66]. Ivabradine improved angina in patients with MVA but coronary microvascular function did not change, suggesting that symptomatic improvement could be attributed to heart-rate-lowering effect [62]. However, others have found that ivabradine improves CFR in non-obstructed coronary arteries of patients with stable CAD at both baseline and paced heart rates identical to that before treatment [67]. Thus, ivabradine may improve CFR in patients with stable CAD. These effects persist even after heart rate correction, indicating improved microvascular function [68]. Thus, it is possible that ivabradine and/or perhaps some other If-channel inhibitors have a role in CMD patients, although further studies are needed.
3.8 Xanthine Antagonists
Xanthine derivatives are considered to have favorable effects on nociception in MVA patients. They were suggested to have analgesic effects that result from antagonizing stimulation of cardiac nerve pain fibers through adenosine, a major mediator of ischemic chest pain [69]. They may also have anti-ischemic actions through attenuation of the coronary microvascular steal phenomenon observed in MVA patients [70]. Aminophylline may improve exercise tolerance and exercise-induced myocardial ischemia in patients with INOCA [71, 72]. Clinically, these drugs represent a bailout option in completely refractory patients before more invasive methods such as spinal cord stimulation may be considered.
4 Expectation for Rho-Kinase Inhibitor, Fasudil, as a Therapeutic Option for CMD
Enhanced Rho-kinase activity plays important roles in the pathogenesis of both epicardial coronary and microvascular spasm [73]. In particular, the pathogenetic mechanisms of CMD appear to be heterogeneous, and many confounding cardiovascular risk factors cause both endothelial dysfunction and VSMC hyperconstriction, where activated Rho-kinase pathway plays important roles (Fig. 8.2). Furthermore, Rho-kinase pathway has also been shown to be substantially involved in inflammatory cell accumulation in blood vessel adventitia, [74] and a pathogenetic mechanism in patients with chest pain and non-obstructive CAD [75]. Rho-kinase enhances myosin light chain phosphorylation through inhibition of myosin-binding subunit of myosin phosphatase, leading to vascular smooth muscle hypercontraction (Fig. 8.3) [76]. Fasudil, a specific Rho-kinase inhibitor, is highly effective in preventing acetylcholine-induced coronary spasm and resultant myocardial ischemia (Fig. 8.3) [77]. Indeed, intracoronary fasudil is effective not only for patients with epicardial coronary spasm [77] but also for approximately two thirds of MVA patients [78]. Specifically in the latter, Mohri et al. studied consecutive 18 patients with angina and normal epicardial coronaries in whom intracoronary ACh induced myocardial ischemia (defined as ischemic electrocardiographic changes, myocardial lactate production, or both) without angiographically demonstrable epicardial coronary vasospasm. All patients underwent a second ACh challenge test after pretreatment with either saline (n = 5) or fasudil (4.5 mg intracoronarily, n = 13). While myocardial ischemia was reproducibly induced by ACh in the saline group, 11 of the 13 patients pretreated with fasudil had no evidence of myocardial ischemia during the second infusion of ACh (P < 0.01). The lactate extraction ratio (median value [interquartile range]) during ACh infusion was improved by fasudil pretreatment, from −0.16 (−0.25 to 0.04) to 0.09 (0.05 to 0.18) (P = 0.0125) (Fig. 8.4). These results strongly indicate that fasudil is able to ameliorate myocardial ischemia in patients who were most likely having coronary microvascular spasm. Furthermore, Fukumoto et al. examined whether Rho-kinase is involved in coronary microvascular constriction in patients with obstructive CAD [79]. In brief, intracoronary administration of fasudil (300 mg/min for 15 min) significantly increased oxygen saturation in coronary sinus vein from 37 ± 3% to 41 ± 3% (P < 0.05) but not in six age-matched controls (from 42 ± 3% to 43 ± 3%, P=NS). Importantly, intracoronary fasudil significantly ameliorated pacing-induced myocardial ischemia in patients with obstructive CAD (magnitudes of symptom, 1.5 ± 0.6 to 0.6 ± 0.4, P < 0.01; ischemic ST-segment depression, 1.8 ± 0.3 to 1.0 ± 0.2 mm, P < 0.01; percent lactate production, 50 ± 17% to 0.4 ± 7%, P < 0.01) without significant hemodynamic changes [78]. These results provide the evidence that Rho-kinase is substantially involved in the pathogenesis of CMD associated with myocardial ischemia in patients with obstructive CAD, suggesting that fasudil could be a therapeutic option for CMD with obstructive CAD. Myocardial hypertrophy induced by pressure overload leads to myocardial dysfunction, CMD, and ischemia possibly due to oxidative stress, enhanced vasoconstriction to endothelin-1, and compromised endothelial NO function via elevated Rho-kinase signaling [80]. Fasudil may be effective in a wide variety of CMD where Rho-kinase plays an important role.
Pathogenesis of coronary microvascular dysfunction and important role of Rho-kinase in it. The pathogenetic mechanisms of coronary microvascular dysfunction appear to be heterogeneous, and many confounding cardiovascular risk factors cause both endothelial dysfunction and VSMC hyperconstriction, where activated Rho-kinase pathway may play an important role. CV cardiovascular, ET-1 endothelin-1
Roles of the Rho/Rho-kinase signaling pathway in VSMC hyperconstriction. Contraction is induced by increased phosphorylation of MLC. The agonist-induced activation of G-protein-coupled receptors leads to the stimulation of MLCK through an increase in intracellular Ca2+ concentration and inhibition of MLCPh. Following stimulation by various agonists, the Rho/Rho-kinase-mediated pathway is activated, resulting in the inhibition of MLCPh (through phosphorylation of its MBS), with a resultant increase in MLC phosphorylation. This Rho-kinase-mediated contraction of VSMC can occur independently of intracellular Ca2+ levels and is known as “calcium sensitization.” Rho-kinase can also increase MLC phosphorylation and contractility by inactivating MLCPh after phosphorylation of CPI-17 or by direct phosphorylation of MLC. ACh acetylcholine, Ang II angiotensin II, Cat catalytic subunit, ET-1 endothelin-1, IP3 inositol (1,4,5)-trisphosphate, M20 20-kDa subunit, NE norepinephrine, PLC phospholipase C, PDGF platelet-derived growth factor, Uro II urotensin II. Stimulation is denoted by +; inhibition is denoted by −. (Reproduced from Shimokawa et al. [76])
Clinical findings in a patient with microvascular angina. Representative coronary angiography and ECG recordings (left) and group data comparison of the lactate extraction ratio during acetylcholine (ACh) infusion with (n = 13, fasudil group) and without pre-treatment of fasudil (n = 5, saline group) (right). Intracoronary administration of ACh caused no appreciable vasoconstriction of epicardial coronary arteries, whereas ECG changes and myocardial lactate production indicated the occurrence of myocardial ischemia. Intracoronary pre-treatment with fasudil abolished the ACh-induced myocardial ischemia. F fasudil, ISDN isosorbidedinitrate. (Reproduced from Masumoto et al. [77])
5 A Rational Approach for the Management of CMD Patients
Considering the results of the CorMicA trial, [81] the latest ESC guidelines state that treatment of microvascular angina should address the dominant mechanism of microcirculatory dysfunction (Fig. 8.5). In patients with impaired microcirculatory conductance with abnormal CFR <2.0 or IMR ≥25 units, and a negative acetylcholine provocation test, beta-blockers, ACE inhibitors, and statins, along with lifestyle modifications and weight loss, are indicated. On the other hand, patients developing ECG changes and angina in response to acetylcholine testing but without severe epicardial vasoconstriction (all suggestive of microvascular spasm) may be treated mainly by CCBs like VSA patients. However, as demonstrated by our group, in patients with INOCA, coronary functional abnormalities, including epicardial coronary spasm, reduced microvascular vasodilatation, and increased microvascular resistance, frequently coexist in various combinations [75]. Thus, in everyday clinical practice, the first-line medication is represented by beta-blockers or long-acting dihydropyridine CCBs, while a combination of them should constitute the second step when single drugs fail to success. In some cases, long-acting nitrates could be added, although there is less evidence of their actual efficacy. A proposed treatment algorithm for patients with MVA is shown in Fig. 8.5. All patients should receive optical risk control. If symptoms are not well controlled, addition of traditional and non-traditional anti-ischemic drugs is recommended. Ivabradine can be added when beta-blockers are scarcely tolerated, while ranolazine should be considered in MVA patients with reduced CFR. In cases with refractory symptoms that seriously limit quality of life, analgesic drugs or non-pharmacological interventions including rehabilitation exercise programs, spinal cord simulation, psychological treatments, and shock wave therapy [82] might be helpful.
References
Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (women’s ischemia syndrome evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32. https://doi.org/10.1016/j.jacc.2010.01.054.
Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58(5):510–9. https://doi.org/10.1016/j.jacc.2010.11.078.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. https://doi.org/10.1093/eurheartj/ehz425.
JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013). Circ J. 2014;78(11):2779–801. https://doi.org/10.1253/circj.cj-66-0098.
Lim TK, Choy AJ, Khan F, Belch JJ, Struthers AD, Lang CC. Therapeutic development in cardiac syndrome X: a need to target the underlying pathophysiology. Cardiovasc Ther. 2009;27(1):49–58. https://doi.org/10.1111/j.1755-5922.2008.00070.x.
Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–92. https://doi.org/10.1161/CIRCULATIONAHA.116.024534.
Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–70. https://doi.org/10.1093/cvr/cvaa006.
Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, Altin C, Atar I, Muderrisoglu H. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis. 2008;201(1):112–6. https://doi.org/10.1016/j.atherosclerosis.2008.02.016.
Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored women’s ischemia syndrome evaluation (WISE). J Interv Cardiol. 2010;23(6):511–9. https://doi.org/10.1111/j.1540-8183.2010.00598.x.
Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. https://doi.org/10.1056/NEJM200007063430103.
Eriksson BE, Tyni-Lenne R, Svedenhag J, Hallin R, Jensen-Urstad K, Jensen-Urstad M, Bergman K, Selvén C. Physical training in syndrome X: physical training counteracts deconditioning and pain in syndrome X. J Am Coll Cardiol. 2000;36(5):1619–25. https://doi.org/10.1016/s0735-1097(00)00931-1.
Motz W, Strauer BE. Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension. 1996;27(5):1031–8. https://doi.org/10.1161/01.hyp.27.5.1031.
Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, Tamaki N, Tsutsui H. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. J Am Coll Cardiol. 2007;50(12):1144–9. https://doi.org/10.1016/j.jacc.2007.06.013.
Brush JE Jr, Cannon RO 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319(20):1302–7. https://doi.org/10.1056/NEJM198811173192002.
Caliskan M, Erdogan D, Gullu H, Topcu S, Ciftci O, Yildirir A, Muderrisoglu H. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30(9):475–9. https://doi.org/10.1002/clc.20140.
Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X: role of superoxide dismutase activity. Circulation. 2004;109(1):53–8. https://doi.org/10.1161/01.CIR.0000100722.34034.E4.
Guethlin M, Kasel AM, Coppenrath K, Ziegler S, Delius W, Schwaiger M. Delayed response of myocardial flow reserve to lipid-lowering therapy with fluvastatin. Circulation. 1999;99(4):475–81. https://doi.org/10.1161/01.cir.99.4.475.
Nerla R, Tarzia P, Sestito A, Di Monaco A, Infusino F, Matera D, Greco F, Tacchino RM, Lanza GA, Crea F. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr Metab Cardiovasc Dis. 2012;22(8):626–34. https://doi.org/10.1016/j.numecd.2010.10.004.
Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, Di Marzo V, Ratib O, Mach F, Golay A, Schindler TH. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34(27):2063–73. https://doi.org/10.1093/eurheartj/eht085.
Chiang CY, Chien CY, Qiou WY, Chang C, Yu IS, Chang PY, Chien CT. Genetic depletion of thromboxane A2/thromboxane-prostanoid receptor signalling prevents microvascular dysfunction in ischaemia/reperfusion injury. Thromb Haemost. 2018;118(11):1982–96. https://doi.org/10.1055/s-0038-1672206.
Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17(3):192–205. https://doi.org/10.1111/j.1549-8719.2009.00015.x.
Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33(22):2771–82b. https://doi.org/10.1093/eurheartj/ehs246.
Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30(15):1837–43. https://doi.org/10.1093/eurheartj/ehp205.
Lucas AR, Korol R, Pepine CJ. Inflammation in atherosclerosis: some thoughts about acute coronary syndromes. Circulation. 2006;113(17):e728–32. https://doi.org/10.1161/CIRCULATIONAHA.105.601492.
Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, DiMario C, Ferreira R, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, CJM V. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. https://doi.org/10.1093/eurheartj/eht296.
Kaski JC, Valenzuela Garcia LF. Therapeutic options for the management of patients with cardiac syndrome X. Eur Heart J. 2001;22(4):283–93. https://doi.org/10.1053/euhj.2000.2152.
Fragasso G, Chierchia SL, Pizzetti G, Rossetti E, Carlino M, Gerosa S, Carandente O, Fedele A, Cattaneo N. Impaired left ventricular filling dynamics in patients with angina and angiographically normal coronary arteries: effect of beta adrenergic blockade. Heart. 1997;77(1):32–9. https://doi.org/10.1136/hrt.77.1.32.
Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63(5):286–90. https://doi.org/10.1016/0002-9149(89)90332-9.
Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84(7):854–6., A8. https://doi.org/10.1016/s0002-9149(99)00450-6.
Leonardo F, Fragasso G, Rossetti E, Dabrowski P, Pagnotta P, Rosano GM, Chierchia SL. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia. 1999;44(12):1065–9.
Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140(5):753–9. https://doi.org/10.1067/mhj.2000.110093.
Togni M, Vigorito F, Windecker S, Abrecht L, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. 2007;21(2):99–108. https://doi.org/10.1007/s10557-006-0494-7.
Nishigaki K, Inoue Y, Yamanouchi Y, Fukumoto Y, Yasuda S, Sueda S, Urata H, Shimokawa H, Minatoguchi S. Prognostic effects of calcium channel blockers in patients with vasospastic angina—a meta-analysis. Circ J. 2010;74(9):1943–50. https://doi.org/10.1253/circj.cj-10-0292.
Sorop O, Bakker EN, Pistea A, Spaan JA, VanBavel E. Calcium channel blockade prevents pressure-dependent inward remodeling in isolated subendocardial resistance vessels. Am J Physiol Heart Circ Physiol. 2006;291(3):H1236–45. https://doi.org/10.1152/ajpheart.00838.2005.
Sutsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52(2):135–43. https://doi.org/10.1016/0167-5273(95)02458-9.
Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56(4):242–6. https://doi.org/10.1016/0002-9149(85)90842-2.
Luscher TF, Pieper M, Tendera M, Vrolix M, Rutsch W, van den Branden F, Gil R, Bischoff KO, Haude M, Fischer D, Meinertz T, Münzel T. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease: the ENCORE II study. Eur Heart J. 2009;30(13):1590–7. https://doi.org/10.1093/eurheartj/ehp151.
Tsuburaya R, Takahashi J, Nakamura A, Nozaki E, Sugi M, Yamamoto Y, Hiramoto T, Horiguchi S, Inoue K, Goto T, Kato A, Shinozaki T, Ishida E, Miyata S, Yasuda S, Shimokawa H, NOVEL Investigators. Beneficial effects of long-acting nifedipine on coronary vasomotion abnormalities after drug-eluting stent implantation: the NOVEL study. Eur Heart J. 2016;37(35):2713–21. https://doi.org/10.1093/eurheartj/ehw256.
Munzel T, Daiber A, Gori T. More answers to the still unresolved question of nitrate tolerance. Eur Heart J. 2013;34(34):2666–73. https://doi.org/10.1093/eurheartj/eht249.
Daiber A, Wenzel P, Oelze M, Munzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol. 2008;97(1):12–20. https://doi.org/10.1007/s00392-007-0588-7.
Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49(12):1289–95. https://doi.org/10.1016/j.jacc.2006.10.074.
Gori T, Floras JS, Parker JD. Effects of nitroglycerin treatment on baroreflex sensitivity and short-term heart rate variability in humans. J Am Coll Cardiol. 2002;40(11):2000–5. https://doi.org/10.1016/s0735-1097(02)02532-9.
Heitzer T, Just H, Brockhoff C, Meinertz T, Olschewski M, Munzel T. Long-term nitroglycerin treatment is associated with supersensitivity to vasoconstrictors in men with stable coronary artery disease: prevention by concomitant treatment with captopril. J Am Coll Cardiol. 1998;31(1):83–8. https://doi.org/10.1016/s0735-1097(97)00431-2.
Rizzon P, Scrutinio D, Mangini SG, Lagioia R, de Toma L. Randomized placebo-controlled comparative study of nifedipine, verapamil and isosorbide dinitrate in the treatment of angina at rest. Eur Heart J. 1986;7(1):67–76. https://doi.org/10.1093/oxfordjournals.eurheartj.a061960.
Takahashi J, Nihei T, Takagi Y, Miyata S, Odaka Y, Tsunoda R, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Momomura S, Yasuda S, Ogawa H, Shimokawa H, Japanese Coronary Spasm Association. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2015;36(4):228–37. https://doi.org/10.1093/eurheartj/ehu313.
Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–14. https://doi.org/10.1016/0735-1097(94)00507-M.
Radice M, Giudici V, Pusineri E, Breghi L, Nicoli T, Peci P, Giani P, De Ambroggi L. Different effects of acute administration of aminophylline and nitroglycerin on exercise capacity in patients with syndrome X. Am J Cardiol. 1996;78(1):88–92. https://doi.org/10.1016/s0002-9149(96)00231-7.
Lanza GA, Manzoli A, Bia E, Crea F, Maseri A. Acute effects of nitrates on exercise testing in patients with syndrome X. Clinical and pathophysiological implications. Circulation. 1994;90(6):2695–700. https://doi.org/10.1161/01.cir.90.6.2695.
Russo G, Di Franco A, Lamendola P, Tarzia P, Nerla R, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther. 2013;27(3):229–34. https://doi.org/10.1007/s10557-013-6439-z.
Horinaka S. Use of nicorandil in cardiovascular disease and its optimization. Drugs. 2011;71(9):1105–19. https://doi.org/10.2165/11592300-000000000-00000.
O'Rourke ST. KATP channel activation mediates nicorandil-induced relaxation of nitrate-tolerant coronary arteries. J Cardiovasc Pharmacol. 1996;27(6):831–7. https://doi.org/10.1097/00005344-199606000-00010.
Yamabe H, Namura H, Yano T, Fujita H, Kim S, Iwahashi M, Maeda K, Yokoyama M. Effect of nicorandil on abnormal coronary flow reserve assessed by exercise 201Tl scintigraphy in patients with angina pectoris and nearly normal coronary arteriograms. Cardiovasc Drugs Ther. 1995;9(6):755–61. https://doi.org/10.1007/bf00879868.
Chen JW, Lee WL, Hsu NW, Lin SJ, Ting CT, Wang SP, Chang MS. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. 1997;80(1):32–8. https://doi.org/10.1016/s0002-9149(97)00279-8.
Nikolaidis LA, Doverspike A, Huerbin R, Hentosz T, Shannon RP. Angiotensin-converting enzyme inhibitors improve coronary flow reserve in dilated cardiomyopathy by a bradykinin-mediated, nitric oxide-dependent mechanism. Circulation. 2002;105(23):2785–90. https://doi.org/10.1161/01.cir.0000017433.90061.2e.
Camici PG, Marraccini P, Gistri R, Salvadori PA, Sorace O, L'Abbate A. Adrenergically mediated coronary vasoconstriction in patients with syndrome X. Cardiovasc Drugs Ther. 1994;8(2):221–6. https://doi.org/10.1007/bf00877330.
Kaski JC, Rosano G, Gavrielides S, Chen L. Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J Am Coll Cardiol. 1994;23(3):652–7. https://doi.org/10.1016/0735-1097(94)90750-1.
Nalbantgil I, Onder R, Altintig A, Nalbantgil S, Kiliccioglu B, Boydak B, Yilmaz H. Therapeutic benefits of cilazapril in patients with syndrome X. Cardiology. 1998;89(2):130–3. https://doi.org/10.1159/000006768.
Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90(9):974–82. https://doi.org/10.1016/s0002-9149(02)02664-4.
Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, Pepine CJ. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute women’s ischemia syndrome evaluation (WISE). Am Heart J. 2011;162(4):678–84. https://doi.org/10.1016/j.ahj.2011.07.011.
Hasenfuss G, Maier LS. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol. 2008;97(4):222–6. https://doi.org/10.1007/s00392-007-0612-y.
Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, Gill E, Minissian M, Shaw LJ, Slomka PJ, Slivka M, Berman DS, Bairey Merz CN. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4(5):514–22. https://doi.org/10.1016/j.jcmg.2011.03.007.
Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112(1):8–13. https://doi.org/10.1016/j.amjcard.2013.02.045.
Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, Thomson LE, Berman DS, Shaw LJ, Petersen JW, Brown GH, Anderson RD, Shuster JJ, Cook-Wiens G, Rogatko A, Pepine CJ. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37(19):1504–13. https://doi.org/10.1093/eurheartj/ehv647.
Rambarat CA, Elgendy IY, Handberg EM, Bairey Merz CN, Wei J, Minissian MB, Nelson MD, Thomson LEJ, Berman DS, Shaw LJ, Cook-Wiens G, Pepine CJ. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: women’s ischemia syndrome evaluation-coronary vascular dysfunction ancillary study. Int J Cardiol. 2019;276:8–13. https://doi.org/10.1016/j.ijcard.2018.09.081.
Borer JS, Fox K, Jaillon P, Lerebours G, Ivabradine IG. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107(6):817–23. https://doi.org/10.1161/01.cir.0000048143.25023.87.
Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, Investigators I. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26(23):2529–36. https://doi.org/10.1093/eurheartj/ehi586.
Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215(1):160–5. https://doi.org/10.1016/j.atherosclerosis.2010.11.035.
Camici PG, Gloekler S, Levy BI, Skalidis E, Tagliamonte E, Vardas P, Heusch G. Ivabradine in chronic stable angina: effects by and beyond heart rate reduction. Int J Cardiol. 2016;215:1–6. https://doi.org/10.1016/j.ijcard.2016.04.001.
Crea F, Pupita G, Galassi AR, el-Tamimi H, Kaski JC, Davies G, Maseri A. Role of adenosine in pathogenesis of anginal pain. Circulation. 1990;81(1):164–72. https://doi.org/10.1161/01.cir.81.1.164.
Crea F, Gaspardone A, Araujo L, Da Silva R, Kaski JC, Davies G, Maseri A. Effects of aminophylline on cardiac function and regional myocardial perfusion: implications regarding its antiischemic action. Am Heart J. 1994;127(4 Pt 1):817–24. https://doi.org/10.1016/0002-8703(94)90548-7.
Elliott PM, Krzyzowska-Dickinson K, Calvino R, Hann C, Kaski JC. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X). Heart. 1997;77(6):523–6. https://doi.org/10.1136/hrt.77.6.523.
Yoshio H, Shimizu M, Kita Y, Ino H, Kaku B, Taki J, Takeda R. Effects of short-term aminophylline administration on cardiac functional reserve in patients with syndrome X. J Am Coll Cardiol. 1995;25(7):1547–51. https://doi.org/10.1016/0735-1097(95)00097-n.
Shimokawa H. 2014 Williams Harvey lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014;35(45):3180–93. https://doi.org/10.1093/eurheartj/ehu427.
Ohyama K, Matsumoto Y, Takanami K, Ota H, Nishimiya K, Sugisawa J, Tsuchiya S, Amamizu H, Uzuka H, Suda A, Shindo T, Kikuchi Y, Hao K, Tsuburaya R, Takahashi J, Miyata S, Sakata Y, Takase K, Shimokawa H. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71(4):414–25. https://doi.org/10.1016/j.jacc.2017.11.046.
Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, Sakata Y, Shimokawa H. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74(19):2350–60. https://doi.org/10.1016/j.jacc.2019.08.1056.
Shimokawa H, Sunamura S, Satoh K. RhoA/Rho-kinase in the cardiovascular system. Circ Res. 2016;118(2):352–66. https://doi.org/10.1161/CIRCRESAHA.115.306532.
Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105(13):1545–7. https://doi.org/10.1161/hc1002.105938.
Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41(1):15–9. https://doi.org/10.1016/s0735-1097(02)02632-3.
Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91(3):391–2. https://doi.org/10.1136/hrt.2003.029470.
Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res. 2017;113(11):1329–37. https://doi.org/10.1093/cvr/cvx103.
Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–55. https://doi.org/10.1016/j.jacc.2018.09.006.
Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, Takeshita A, Sunagawa K, Shimokawa H. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Corona Artery Dis. 2006;17(1):63–70. https://doi.org/10.1097/00019501-200602000-00011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Takahashi, J., Shimokawa, H. (2021). Treatment of Coronary Microvascular Dysfunction. In: Shimokawa, H. (eds) Coronary Vasomotion Abnormalities. Springer, Singapore. https://doi.org/10.1007/978-981-15-7594-5_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-7594-5_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7593-8
Online ISBN: 978-981-15-7594-5
eBook Packages: MedicineMedicine (R0)