Abstract
Several methods are available for the production of nanofibres, such as rotary jet spinning, melt fibrillation, electrospinning, sol-gel method, phase separation, gas-jet technique, self-assembly and template synthesis. However, electrospinning surpasses all of them in terms of efficiency and effectiveness. Electrospinning enables the production of continuous nanofibres from a wide range of materials. It also facilitates precise control over several fibre/membrane properties like diameter, morphology, composition, porosity, etc., that too by using simple equipment and even simpler procedure. Nanofibres produced from electrospinning technique exhibit diverse features, such as high porosity, high surface area-to-volume ratio, small pore size, low weight and good mechanical properties. Considering these favourable characteristics, electrospun membranes are extensively used to fabricate bioactive products for application in various areas like healthcare, energy harvesting and storage, biomedical, environmental engineering, defence and security. This chapter focuses on the potential use of various natural, synthetic, functionalised, encapsulated and composite electrospun nanofibrous membranes for antibacterial bioactive applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology is an interesting interdisciplinary area with potential applications in almost all fields of science and technology: healthcare, material science, energy studies, environmental studies, mechanics, optics, electronics, plastics, aerospace and so on. Some typical morphologies of nanomaterials that have been researched upon extensively are nanofibres, nanowires, nanorods, nanotubes, nanoparticles, etc. Among these, nanofibres have recently gained substantial interest of the research community owing to their large surface area-to-volume ratio, feasibility of generating diverse surface functionalities and superior mechanical properties that render them suitable for use in myriad applications. Out of the numerous existing techniques for spinning nanofibres, electrospinning has emerged as most potent of producing continuous nanofibres from a variety of natural as well as synthetic polymers (Panthi et al. 2015; Joshi et al. 2008).
Over the years, bioactive and antibacterial/antimicrobial materials are gaining rising importance not only in healthcare sector but also in other applications like, sportswear, defence clothing, protective and industrial textiles, geomembranes, etc. These are important for creating a healthy and hygienic atmosphere at home, workplace and in general environment. In order to tackle bacterial infections, composite nanofibres, i.e. nanofibres integrated with powerful antibacterial agents such as silver, copper, copper oxide (CuO), zinc oxide (ZnO) in the form of nanoparticles are being developed (Ditaranto et al. 2018; Gadkari et al. 2017a; Saquing et al. 2009). In general, fabrication of antibacterial nanofibres via electrospinning technique involves integration of biocides in fibres. This can be achieved by various methods, such as using a homogenous blend of antibacterial agent in the polymer solution as feed for electrospinning, adding nanostructures of antibacterial substance(s) in feed solution for electrospinning fibres embedded with nanostructures, enclosing an antibacterial agent in the core of nanocomposite fibre by a sheath of other polymer, treating the electrospun nanofibres with antibacterial agent(s), etc. (Xue et al. 2017). However, the rising concern related to the adverse effects of synthetic antibacterial agents on the health of the human population and on the environment has driven the research on development of electrospun antibacterial fibres towards usage of natural plant extracts and essential oils having bactericidal property. The present chapter presents an overview of some significant studies focused upon the electrospinning of such antibacterial nanofibres or nanofibrous membranes.
2 Electrospinning Process
Electrospinning is the most popular and vastly used technology for making nanoscale fibres from both natural and synthetic polymers. Traditionally, synthetic fibres are obtained via melt spinning, which results in fibre diameter ranging between 5 μm to 200 μm, whereas the diameter of electrospun fibres falls within a range of several nanometres (Brown et al. 2016; Bhardwaj and Kundu 2010; Stankus et al. 2006). Moreover, production of polymeric fibres via melt spinning uses mechanical force to extract the melt polymer through spinnerets, whereas electrospinning is an electro-hydrodynamic process that uses electric potential to draw polymer solution into fibres. The electrospinning phenomenon was first observed way back in 1600 century by W. Gilbert, an English Physicist. He observed a water droplet placed over a dry surface reshaping in a conical form on being brought into proximity of a piece of rubbed amber (Brown et al. 2016). In 1749, Nollet demonstrated separation of water drops from charged water jet falling into a collector set at different electric potential. Later, Rayleigh (1897), Zeleny (1914) and Formhals (1934) also observed electrospraying effect (Bhardwaj and Kundu 2010). All of these studies became the basis for Antonin Formhals in 1934 to fabricate the first system for producing electrospun yarn using a voltage of 57 kV, whereafter a thorough research on electrospinning gained momentum.
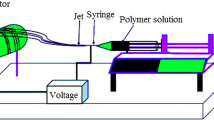
Typically, an electrospinning set-up consists of three basic units: a variable high DC voltage supply, a metallic spinneret (syringe) with a very fine orifice and a collector plate with some conductive element (Fig. 1). The granules of polymer to be electrospun are generally dissolved in some solvent to prepare a solution with requisite viscosity. A very high voltage is applied between the drop of polymer (held inside the spinneret tip by its surface tension) and a collector plate or target electrode placed at certain distance from the spinneret tip. Application of high voltage results in development of an electrostatic force on the polymer drop, which deforms the polymer drop into a conical shape called Taylor cone. When this electrostatic force reaches a critical value, it overcomes the surface tension, resulting in ejection of a charged jet from Taylor cone towards the collector plate. The solvent evaporates leaving behind the solid polymer in fibrous form, which gets deposited onto the collector forming nonwoven fibrous webs or mats (Persano et al. 2013; Balamurugan et al. 2011; Zhang et al. 2005; Zaikov 2016; Baker et al. 2006; Tamura and Kawakami 2010). Besides, during the stretching of polymer stream and simultaneous evaporation of solvent, the size of polymeric strand can be drastically decreased (up to six orders of magnitude), resulting in nano-sized fibres (Tamura and Kawakami 2010). The continuous deposition of fibres generates 2D nanofibrous web of very high porosity (even 90%), thus making them suitable for use in high efficiency filters, scaffolds for tissue engineering, artificial implants, wound dressings, masks, etc. Other than these applications, electrospun nanofibres are also used in energy harvesting and storage devices for smart textiles, solar cells, fuel cells, chemical and biological protection sensors, etc. (Ramakrishna et al. 2006; Riboldi et al. 2005; Reneker and Chun 1996; Reneker et al. 2000; Rho et al. 2006).
Conventional electrospinning set-ups are horizontal or vertical (Bhardwaj and Kundu 2010; Persano et al. 2013; Balamurugan et al. 2011), though some researchers have also reported designing typical systems for the production of complex nanofibrous structures to suit specific requirements and applications. Balamurugan et al. (2011) reported a system combining electrospinning and electrospraying techniques for the fabrication of nanocomposite membranes. Stankus et al. (2006) also combined electrospraying with electrospinning to integrate smooth muscle cells into biodegradable fibre matrix. Baker et al. (2006) used a circular mandrel to produce 3D electrospun polystyrene scaffolds which could be used for cell growth and cell to cell interaction. Numerous other studies about modification of electrospinning set-ups like development of coaxial electrospinning system for highly concentrated solutions (Yu et al. 2011), combination of melt electrospinning and solution electrospinning for hybrid electrospinning of scaffolds (Il Yoon et al. 2013), using differently profiled collectors for creating different alignment of fibres (Stocco et al. 2017; Levitt et al. 2017), etc. are also available.
3 Bioactive Antibacterial Electrospun Nanofibres
For any antibacterial material to function efficiently, the bacteria should essentially have good contact with it. Thus, the high surface area-to-volume ratio of electrospun nanofibres enhances the bactericidal effect of materials developed from them (Li et al. 2015). Moreover, the fibrous mats developed by electrospinning can be easily tailored for different applications during or after the generation of fibres. In addition, various strategies such as doping of antimicrobial agent(s) in precursor solution, surface functionalisation (Su et al. 2012; Schiffman and Elimelech 2011), solution blending (Charernsriwilaiwat et al. 2012), coaxial spinning (Khalf and Madihally 2017; Liu et al. 2011), coating of antibacterial/antimicrobial agents on nanofibrous mat (Rieger et al. 2016; Kang et al. 2009) can also be easily adopted as per required applications.
3.1 Natural Polymer Based Nanofibres
Natural polymer based electrospun nanofibres have been extensively used for various medical applications, like wound healing, artificial tissues, medical implants and drug delivery. Natural polymers are chosen over synthetic ones due to better biocompatibility, biodegradability, poor immunogenicity and enhanced cell proliferation (Bhattarai et al. 2005; Wang et al. 2016). However, it is difficult to spin natural polymers in their pure form. For instance, in case of pure alginate, fibre formation is not possible for less concentrated solutions due to occurrence of gelation. On the other hand, at higher concentration, the solution becomes too viscous to be extruded through spinneret (Bhattarai et al. 2006). Similarly, electrospinning of pure chitosan solution poses problems due to its polycationicity, rigid chemical structure and typical inter- and intra-molecular interactions (Pakravan et al. 2011). Hence, natural polymers are often either blended with synthetic copolymers or chemically modified to be electrospun into nanofibres.
3.1.1 Chitosan Based Nanofibres
Chitosan is a deacetylated derivative of chitin, which is a natural polysaccharide found in crustaceans, insects and some fungi exoskeletons. Chitosan derivatives, nanoparticles and nanofibres exhibit antibacterial activity against several fungi, viruses and bacteria (Gadkari et al. 2017b). However, as already mentioned, it is difficult to electrospin it. Many researchers have reported combining chitosan with other polymers to enhance its spinnability. Jung et al. (2007) dissolved poly(ethylene terephthalate) (PET) and chitosan in trifluoroacetic acid and electrospun this polymeric blend solution to make PET/chitosan nanofibrous mats having fibres with 500–800 nm diameter. Homayoni et al. (2009) suggested that the difficulty associated with electrospinning of pure chitosan due to its high viscosity can be resolved using alkaline treatment for hydrolysing its polymer chains, and thus decreasing its molecular weight. They demonstrated successful production of chitosan nanofibres of appropriate quality and processing stability by electrospinning a solution of alkaline treated chitosan in aqueous acetic acid. They also reported an increase in the mean diameter of the chitosan nanofibres upon decreasing the acetic acid concentration in the solvent.
Nguyen et al. (2011) manufactured core/shell structured poly(lactic acid)(PLA)/chitosan fibres through coaxial electrospinning process at different core feed rates of 1 μL/min, 2 μL/min and 4 μL/min. The antibacterial performance of the nanofibrous mats thus produced against E. coli bacterium was found to be 99–100% for a bacterial concentration of 103 CFU/mL. Ahmed et al. (2018) fabricated electrospun fibrous mats from polyvinyl alcohol (PVA)/chitosan blend and PVA/chitosan/ZnO nanoparticles blend. On assessing them for their antibacterial activity against E. coli, P. aeruginosa, B. subtilis and S. aureus by disc diffusion method, they were found to create zone of inhibitions with average diameter of 14.1 ± 0.8 mm, 15.8 ± 1.0 mm, 13.0 ± 0.7 mm and 5.4 ± 0.5 mm, respectively, in case of PVA/chitosan mats and 20.2 ± 1.0 mm, 21.8 ± 1.5 mm, 15.5 ± 0.8 mm and 21.5 ± 0.5 mm, respectively, in case of PVA/chitosan/ZnO mats.
3.1.2 Protein Based Nanofibres
A protein is a linear polymer of amino acids and is known to exhibit miscellaneous functions. Over the years, researchers have been increasingly exploiting the structural and functional qualities of fibrous proteins in order to enhance the efficiency of synthetic biomaterials. A variety of proteins and their blends with other polymer(s) have been electrospun by several researchers. This section highlights the use of various protein fibres for application in antibacterial materials.
Lin et al. (2012) prepared electrospun fibrous mats from pure collagen, pure zein and their blend using 40% (w/v) solutions of these polymeric polymers in 70% (v/v) aqueous acetic acid. Though they could not process pure collagen into micro- or nanofibres, pure zein and collagen/zein blend could be efficiently electrospun into bead-free fibrous mats. Besides, they also observed an increase in average fibre diameter from 423 nm to 910 nm on increasing zein weight fraction in collagen/zein blend from 0.33 to 0.67. In order to impart an inherent bactericidal effect, the authors also incorporated berberine drug in the feed solution while electrospinning collagen/zein membrane. In a similar study, Wongkanya et al. (2017) electrospun sodium alginate (SA) and soy protein isolated (SPI) blend fibres with poly(ethylene oxide) (PEO) polymer using different weight percentages of SA, SPI and PEO. They also added vancomycin (Van) drug (0.1 wt.% of polymer), which resists Gram-positive type bacteria, to the feed solution. The zone of inhibition created by Van-loaded SA/PEO/SPI fibres against S. aureus bacteria was found to be 21–22.8 mm.
Zhou et al. (2017) used 10:1 (v/v) mixture of 8% collagen solution in hexafluoroisopropanol and bioactive glass (BG) precursor solution to electrospin collagen/BG nanofibres with 494 ± 153 nm average diameter, good thermal stability and hydrophilicity. These fibres were also found to inhibit adhesion and proliferation of S. aureus bacterium due to release of Ca, P and Si ions. Khajavi et al. (2016) explored the feasibility of electrospinning nanofibrous scaffolds from different blend compositions of keratin (extracted from quail feather wastes), PVA and silver nanoparticles (Ag-NPs) at 20 kV voltage, 15 cm tip to collector distance and 1 mL/h feed rate. Increase in process efficiency was reflected in formation of uniform nanofibres with fewer beads upon increase in proteinaceous or keratin content. Besides, these mats showed 93–98% antibacterial activity against S. aureus (higher for higher keratin content), and almost 100% against E. coli.
3.1.3 Cellulose Based Nanofibres
Cellulose is a biocompatible and biodegradable polysaccharide consisting of linear chains of β(1→4) linked d-glucose monomer units. Cellulose based electrospun nanostructures and their derivatives find enormous applications in pharmaceutical industry. This section presents examples of studies conducted on production of antibacterial nanofibres from different derivatives of cellulose.
Carboxymethyl cellulose (CMC) is a classic derivative of cellulose and is non-toxic, biodegradable, biocompatible, water soluble, and has reported usage in variety of biomedical applications, food, detergents, etc. However, alike other natural polymers, it is not suitable to electrospin it in its pure form and needs blending with synthetic copolymers. Shi et al. (2016) developed CMC/PEO membrane by electrospinning an aqueous solution of CMC and PEO in equal amount, at 22 kV with a solution feed rate of 2 mL/h. Subsequent to this, they immersed the membrane in AgNO3 solution for 2 h, followed by irradiation under ultraviolet (UV) lamp for deposition of silver nanoparticles (Ag-NPs) on fibres. The scanning electron micrographs of the membranes thus developed using AgNO3 solutions of different concentrations showed presence of several Ag-NPs on their surface as well as in between the adjacent nanofibres. Treatment with 0.10 mol/L AgNO3 solution was observed to facilitate uniform growth of Ag-NPs on membrane surface, as well as preserved the integrity of 3D membranous structure unlike AgNO3 solutions with concentration lower or higher than this. Besides, this membrane was also found to exhibit 100% activity against both S. aureus and E. coli bacteria.
Cellulose acetate (CA) is the only derivative of cellulose that can be processed in an electrospinning set-up in its pure form itself. Several studies are available demonstrating increasing interest in electrospinning CA based antibacterial nanofibres using various antibacterial compounds. In one such study, Sultana et al. (2016) electrospun CA nanofibrous from differently concentrated solutions of CA in acetic acid/acetone. Lower concentration of CA (10% w/v) yielded nanofibres with many beads, whereas bead-free nanofibres were obtained when concentration of CA was increased to 14% (w/v). In case of feed solution loaded with 2% (w/v) tetracycline hydrochloride drug, clear area of inhibition was observed against B. cereus and E. coli bacteria, unlike pure CA membrane that exhibited no inhibition zone.
Cyclodextrin (CD), another derivative of cellulose, has attracted attention of the research community for the development of antimicrobial nanofibres. Celebioglu et al. (2014) electrospun nanofibres from extremely concentrated (160%) aqueous suspensions of cyclodextrin inclusion complexes (CD-IC) loaded with an antibacterial agent triclosan, using two forms of chemically modified CD, namely hydroxypropyl-beta-cyclodextrin (HPβCD) and hydroxypropyl-gamma-cyclodextrin (HPγCD). Bead-free membranes were obtained with average fibre diameter as 520 ± 250 nm and 1100 ± 660 nm, corresponding to former and latter forms of CD, which also showed very good antibacterial effect against both E. coli and S. aureus.
3.2 Nanofibres Encapsulating Bioactive Plant Extract
Plants are sources of numerous chemical compounds that display antibacterial activity against a range of bacteria. For thousands of years, plant oils and extracts have been in use for a wide range of purposes (Hammer et al. 1999), antimicrobial functionality being the most popular. Additionally, the over-rising concerns about the hazardous side effects of conventionally used synthetic compounds in medication and nourishment industry, as well as the escalating resistance of pathogens to antibiotics have led to a growing interest among the research community to integrate natural extracts with polymers to electrospin nanofibres for various applications like wound dressings, scaffolds for tissue engineering, drug delivery and active food packaging systems (Sridhar et al. 2015; Khan and Shi Xiangyang 2018; Zhang et al. 2017a). Crude extracts can be easily obtained from fresh plants or from milled dried plants via organic solvent extraction. Numerous extracts of crude plants, for example Garcinia mangostana, grewia mollis, aloe vera, centella asiatica, tecomella undulata, baicalein, chamomile, memecylon edule, Indigofera aspalathoides and Azadirachta indica, have been reported to be successfully encapsulated in electrospun fibres (Motealleh et al. 2014; Suganya et al. 2011; Chan et al. 2017; Charernsriwilaiwat et al. 2013; Agnes Mary and Giri Dev 2015; Al-Youssef et al. 2013).
Ganesan et al. (Ganesan and Pradeepa 2017) electrospun 108–519 nm diameter fibres from a blend of 80% PVA and 20% tridax daisy (or Tridax procumbens) leaves extract. The developed nanofibrous mat created 45 mm and 36 mm zone of inhibition for S. aureus and E. coli, respectively, demonstrating strong resistivity against both bacteria. Yao et al. (2017) prepared an electrospun membrane from a mixture of 17% gelatin solution (in formic acid) and aqueous PVA, also containing gotu kola or centella asiatica extract and demonstrated it to be biodegradable, facilitate dermal wound healing and exhibit antibacterial activity against S. aureus, E. coli and P. aeruginosa with minimum inhibitory concentration (MIC) of 6.25 mg/mL for S. aureus and 25 mg/mL for both E. coli and P. aeruginosa. Yousefi et al. (2017) fabricated 90/10 chitosan/PEO nanofibrous mat loaded with Henna (Lawsonia inermis) leaves extract. Though Henna extract did not affect the electrospinnability of the precursor blend solution, but the fibre diameter was affected by its concentration. It was also observed that on increasing Henna extract loading from 1 to 2 wt%, the zone of inhibition against E. coli increased from 16 mm to 25 mm and from 14 mm to 18 mm for S. aureus, as seen in Fig. 2.
Antibacterial activity of electrospun nonwoven mats of chitosan/PEO/Henna extract: (a) chitosan/PEO (0 wt.% Henna extract), (b) 1 wt.% Henna extract loading and (c) 2 wt.% Henna extract loading (Yousefi et al. 2017)
Radusin et al. (2019) electrospun films from pure PLA solution and PLA solution loaded with 10 wt.% wild garlic or Allium ursinum L (AU) extract at 2000 μL/h flow rate and 14 kV applied voltage. After annealing the films under hydraulic press at 135 °C, without pressure for 5 ± 1 s, followed by air-cooling at room temperature, PVA and AU were observed to exhibit ‘island-and-sea’ morphology, indicating successful encapsulation of AU droplets of size 2.3 ± 0.5 μm in the PLA matrix (Fig. 3). The PVA/AU film was found to exhibit high antibacterial activity against E. coli, but just reasonable against S. aureus. Zeyohanness and Zulkifli (2018) successfully electrospun bead-free nanofibres from 10% PVA solution loaded with rose myrtle or Rhodomyrtus tomentosa extract (RTE) in different proportions (0.25%, 0.5%, 1.5% and 2.5%). The concentration of RTE was observed to affect the average fibre diameter as well as the antibacterial activity of the fibres against B. subtilis, E. coli, P. aeruginosa and E. faecalis bacteria.
Scanning electron micrographs of cross-section of electrospun films of (a) neat PLA, (b) PLA/AU (Radusin et al. 2019)
Essential oils (EOs) are usually derived from aromatic plants and are mixtures of various chemical compounds like linalool, pinene, eugenol and cymene, etc. They can be biosynthesised from various plant organs, including flowers, herbs, buds, leaves, fruits, bark, seeds, wood and roots (El Asbahani et al. 2015). EOs, for example cinnamon, lemongrass, candeia, tea tree, lavender and thyme have been extensively explored for integration of antibacterial property in electrospun fibrous mats. Zhang et al. (2017b) electrospun PLA solution loaded with different concentrations of tea tree and manuka oils at a flow rate of 2 mL/h, applied voltage of 18.5 kV and needle to collector distance of 15 cm to form antibacterial nanofibres. The used EOs not only improved the antibacterial activity of PLA fibres, but also their mechanical properties, i.e. elongation at break and tensile strength. The bactericidal action of EOs was attributed to the partition of hydrocarbons into the bacterial membrane, further causing damage to the cytoplasmic membranes, thus disrupting their functions and ultimately causing cell lysis. Jung et al. (2020) developed membranes of core/sheath nanofibres of cinnamon oil (4.9 wt.%)/PVA by emulsion electrospinning. Upon evaluation of their antibacterial activity against S. aureus under dynamic contact condition, a reduction of 99.9% in the number of bacterial colonies was observed in comparison with the inoculated buffer solution (Table 1). However, the same nanofibrous membranes did not exhibit any inhibitory effect against K. pneumonia because of more complex cell wall structure of this bacteria. Unalan et al. (2019) manufactured peppermint essential oil (PEP) loaded poly(ε-caprolactone) (PCL) electrospun fibrous mats with a smooth, uniform and bead-free morphology; and with average fibre diameter reducing from 1.6 μm to 0.9 μm with increase in concentration of PEP from 1.5 to 6% (v/v). Other than bringing reduction in fibre diameter, higher PEP concentrations also led to reduction in bacterial viability for S. aureus and E. coli strains. However, as E. coli consists of a double membrane with the outer membrane having a layer of lipopolysaccharide that prevents the penetration of certain antibacterial compounds (Burt and Reinders 2003), the PEP loaded fibrous mats were less effective against E. coli.
Sofi et al. (2019) fabricated composite nanofibres consisting of polyurethane (PU) loaded with different concentrations of lavender oil (0%, 5%, 10%, 15% and 20%) and Ag-NPs (0%, 1%, 2%, 5% and 7%). As seen in Fig. 4, the growth of E. coli and S. aureus strains was not inhibited by pure PU fibrous mats. However, all the mats containing fibres loaded with Ag-NPs and lavender oil were effective in suppressing the bacterial growth.
Zone of inhibition of pure PU fibre and PU/lavender oil/Ag-NPs composite fibre mats against E. coli and S. aureus; (a, b) Photos of agar plates from antibacterial testing; (c) average diameter of zone of inhibition (Sofi et al. 2019)
3.3 Composite Nanofibres
Numerous polymers have been electrospun into nanofibres over the past few decades. However, these single-component electrospun nanofibres typically have limited properties and cannot perform multiple functions (Gao et al. 2019; Sahay et al. 2012; Yang et al. 2014). Of late, many researchers have focused their work around inclusion of other nanoscaled structures in the nanofibres to create composite nanofibres with added functionalities. This section comprises studies related to functionalisation of electrospun nanofibres by incorporation of nanoparticles to improve their antibacterial activity.
Augustine et al. (2014) added ZnO nanoparticles of size ∼60 nm in polycaprolactone (PCL) solution, in varying proportions, to electrospin PCL/ZnO nanocomposite membrane. For higher concentration of ZnO nanoparticles, fibre diameter was less than that of pure PCL fibres (2500 nm), and roughening of fibre surface was observed due to their agglomeration. The antibacterial activity assessment of these membrane revealed that membranes with less than 5 wt.% ZnO nanoparticles showed no activity against both bacteria. Notably, the bactericidal effect of ZnO nanoparticles activates only when they come in direct contact with the walls of the bacterial cells. At lower concentrations, most of the nanoparticles were trapped inside the polymer only, with very few superficial nanoparticles being able to make direct contact with bacterial cell wall.
Zhang et al. (2016) electrospun PVA/Ag-NPs composite nanofibrous membranes with varying concentration of embedded Ag-NPs (1–5%). The antibacterial activity of these nanofibres against S. aureus and E. coli, as evaluated via UV absorption method, has been depicted in Fig. 5 through the variation curves between UV–vis absorbance intensity and PVA/Ag-NPs nanofibres with different Ag-NPs content. The solution of bacterial suspension without any antibacterial agent in the broth medium was observed to be very turbid after incubation, supporting high UV-vis absorption. Besides, the UV–vis absorbance of PVA/Ag-NPs nanofibres was found to decrease with increase in concentration of Ag-NPs, for both bacteria. It was also observed that antibacterial activity of these fibres was better against S. aureus than against E. coli.
Variation curves between UV–vis absorbance and PVA/Ag-NPs nanofibres with different Ag-NPs content against (a) E. coli and (b) and S. aureus (Zhang et al. 2016)
Tijing et al. (2012) electrospun mats from pure PU nanofibres and composite nanofibres of PU incorporated with tourmaline nanoparticles (TM-NPs) in varying proportion. Pure PU nanofibrous mat showed no zone of inhibition, i.e. absence of any antibacterial activity, against both E. coli and Streptococci bacterial strains. On the other hand, PU/TM-NPs composite mats showed distinct inhibition zone, with increasing average diameter corresponding to increasing TM-NPs content. It is important to note here that TU-NPs decrease the membrane fluidity of E. coli, leading to increase in cell membrane permeability, and subsequent cell death. Moreover, TM-NPs possess piezoelectric and pyroelectric effects, which facilitate in killing Gram-positive Streptococci bacterium.
4 Summary
The key attributes of electrospun nanofibres, such as high surface area-to-volume ratio, tuneable mechanical and physical properties, excellent porosity make them a popular choice for various applications. The last decade has seen an increased number of publications involving electrospinning of natural and synthetic nanofibres with bactericidal property. This number is, in fact, expected to rise even further in the upcoming years to fulfil the growing need for improvement in materials for wound dressings, dressings for dermal bacterial infections, artificial skin, implants and scaffolds for tissue engineering, membranes for air/water purification, protective masks, sportswear, food packaging material, etc. There is still a tremendous scope for quality enhancement by exploring new antibacterial materials and finding ways to make their electrospinning feasible. Besides, a lot can be done to upscale the electrospinning set-ups for mass production of nanofibrous webs, as well as to make the process cost-effective and the final products affordable.
References
Agnes Mary S, Giri Dev VR (2015) Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J Text Inst 106:886–895. https://doi.org/10.1080/00405000.2014.951247
Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, Augustine R, Hasan A (2018) Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol 120:385–393. https://doi.org/10.1016/j.ijbiomac.2018.08.057
Al-Youssef HM, Amina M, Hassan S, Amna T, Jeong JW, Nam KT, Kim HY (2013) Herbal drug loaded poly(D,L-lactide-co-glycolide) ultrafine fibers: interaction with pathogenic bacteria. Macromol Res 21:589–598. https://doi.org/10.1007/s13233-013-1062-1
Augustine R, Malik HN, Singhal DK, Mukherjee A, Malakar D, Kalarikkal N, Thomas S (2014) Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J Polym Res 21:6. https://doi.org/10.1007/s10965-013-0347-6
Baker SC, Atkin N, Gunning PA, Granville N, Wilson K, Wilson D, Southgate J (2006) Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials 27:3136–3146. https://doi.org/10.1016/j.biomaterials.2006.01.026
Balamurugan R, Sundarrajan S, Ramakrishna S (2011) Recent trends in nanofibrous membranes and their suitability for air and water filtrations. Membranes 1:232–248. https://doi.org/10.3390/membranes1030232
Bhardwaj N, Kundu SC (2010) Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28:325–347. https://doi.org/10.1016/j.biotechadv.2010.01.004
Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M (2005) Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 26:6176–6184. https://doi.org/10.1016/j.biomaterials.2005.03.027
Bhattarai N, Li Z, Edmondson D, Zhang M (2006) Alginate-based nanofibrous scaffolds: structural, mechanical, and biological properties. Adv Mater 18:1463–1467. https://doi.org/10.1002/adma.200502537
Brown TD, Dalton PD, Hutmacher DW (2016) Melt electrospinning today: an opportune time for an emerging polymer process. Prog Polym Sci 56:116–166. https://doi.org/10.1016/j.progpolymsci.2016.01.001
Burt SA, Reinders RD (2003) Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol 36:162–167. https://doi.org/10.1046/j.1472-765X.2003.01285.x
Celebioglu A, Umu OCO, Tekinay T, Uyar T (2014) Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloids Surf B Biointerfaces 116:612–619. https://doi.org/10.1016/j.colsurfb.2013.10.029
Chan WP, Huang KC, Bai MY (2017) Silk fibroin protein-based nonwoven mats incorporating baicalein Chinese herbal extract: preparation, characterizations, and in vivo evaluation. J Biomed Mater Res 105:420–430. https://doi.org/10.1002/jbm.b.33560
Charernsriwilaiwat N, Opanasopit P, Rojanarata T, Ngawhirunpat T (2012) Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm 427:379–384. https://doi.org/10.1016/j.ijpharm.2012.02.010
Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P (2013) Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm 452:333–343. https://doi.org/10.1016/j.ijpharm.2013.05.012
Ditaranto N, Basoli F, Trombetta M, Cioffi N, Rainer A (2018) Electrospun nanomaterials implementing antibacterial inorganic nanophases. Appl Sci 8:91643. https://doi.org/10.3390/app8091643
El Asbahani A, Miladi K, Badri W, Sala M, Addi EHA, Casabianca H, El Mousadik A, Hartmann D, Jilale A, Renaud FNR, Elaissari A (2015) Essential oils: from extraction to encapsulation. Int J Pharm 483:220–243. https://doi.org/10.1016/j.ijpharm.2014.12.069
Gadkari RR, Ali SW, Alagirusamy R, Das A (2017a) Silver nanoparticles in water purification: opportunities and challenges. In: Modern age environmental problems and their remediation. Springer, Cham, pp 229–237. https://doi.org/10.1007/978-3-319-64501-8_13
Gadkari R, Ali W, Das A, Alagirusamy R (2017b) Scope of electrospun chitosan nanofibrous web for its potential application in water filtration. In: Chitosan. Wiley, Hoboken, pp 431–451. https://doi.org/10.1002/9781119364849.ch16
Ganesan P, Pradeepa P (2017) Development and characterization of nanofibrous mat from PVA/Tridax Procumbens (TP) leaves extracts. Wound Med 19:15–22. https://doi.org/10.1016/j.wndm.2017.09.004
Gao X, Han S, Zhang R, Liu G, Wu J (2019) Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J Mater Chem B 7:7075–7089. https://doi.org/10.1039/c9tb01730e
Hammer KA, Carson CF, Riley TV (1999) Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86:985–990. https://doi.org/10.1046/j.1365-2672.1999.00780.x
Homayoni H, Ravandi SAH, Valizadeh M (2009) Electrospinning of chitosan nanofibers: processing optimization. Carbohydr Polym 77:656–661. https://doi.org/10.1016/j.carbpol.2009.02.008
Il Yoon Y, Park KE, Lee SJ, Park WH (2013) Fabrication of microfibrous and nano-/microfibrous scaffolds: Melt and hybrid electrospinning and surface modification of poly(L-lactic acid) with plasticizer. Biomed Res Int 2013:309048. https://doi.org/10.1155/2013/309048
Joshi M, Bhattacharyya A, Ali SW (2008) Characterization techniques for nanotechnology applications in textiles. Indian J Fibre Text Res 33:304–317
Jung KH, Huh MW, Meng W, Yuan J, Hyun SH, Bae JS, Hudson SM, Kang IK (2007) Preparation and antibacterial activity of PET/chitosan nanofibrous mats using an electrospinning technique. J Appl Polym Sci 105:2816–2823. https://doi.org/10.1002/app.25594
Jung Y, Yang H, Lee IY, Yong TS, Lee S (2020) Core/sheath-structured composite nanofibers containing cinnamon oil: Their antibacterial and antifungal properties and acaricidal effect against house dust mites. Polymers 12:1–18. https://doi.org/10.3390/polym12010243
Kang YO, Yoon I-S, Lee SY, Kim D-D, Lee SJ, Park WH, Hudson SM (2009) Chitosan-coated poly(vinyl alcohol) nanofibers for wound dressings. J Biomed Mater Res Pt B Appl Biomater 9999:31554. https://doi.org/10.1002/jbm.b.31554
Khajavi R, Rahimi MK, Abbasipour M, Brendjchi AH (2016) Antibacterial nanofibrous scaffolds with lowered cytotoxicity using keratin extracted from quail feathers. J Bioact Compat Polym 31:60–71. https://doi.org/10.1177/0883911515598793
Khalf A, Madihally SV (2017) Recent advances in multiaxial electrospinning for drug delivery. Eur J Pharm Biopharm 112:1–17. https://doi.org/10.1016/j.ejpb.2016.11.010
Khan AR, Shi Xiangyang XM (2018) Electrospinning of crude plant extracts for antibacterial and wound healing applications: a review. J Biomed Eng 4:1–8
Levitt AS, Knittel CE, Vallett R, Koerner M, Dion G, Schauer CL (2017) Investigation of nanoyarn preparation by modified electrospinning setup. J Appl Polym Sci 134:1–7. https://doi.org/10.1002/app.44813
Li R, Jiang Q, Ren X, Xie Z, Huang TS (2015) Electrospun non-leaching biocombatible antimicrobial cellulose acetate nanofibrous mats. J Ind Eng Chem 27:315–321. https://doi.org/10.1016/j.jiec.2015.01.006
Lin J, Li C, Zhao Y, Hu J, Zhang LM (2012) Co-electrospun nanofibrous membranes of collagen and zein for wound healing. ACS Appl Mater Interfaces 4:1050–1057. https://doi.org/10.1021/am201669z
Liu J, Wang C, Wang J, Ruan H, Fan C (2011) Peripheral nerve regeneration using composite poly(lactic acid-caprolactone)/nerve growth factor conduits prepared by coaxial electrospinning. J Biomed Mater Res Part A 96A:13–20. https://doi.org/10.1002/JBM.A.32946
Motealleh B, Zahedi P, Rezaeian I, Moghimi M, Abdolghaffari AH, Zarandi MA (2014) Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ε-caprolactone)/polystyrene blends. J Biomed Mater Res 102:977–987. https://doi.org/10.1002/jbm.b.33078
Nguyen TTT, Chung OH, Park JS (2011) Coaxial electrospun poly(lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr Polym 86:1799–1806. https://doi.org/10.1016/j.carbpol.2011.07.014
Pakravan M, Heuzey MC, Ajji A (2011) A fundamental study of chitosan/PEO electrospinning. Polymer 52:4813–4824. https://doi.org/10.1016/j.polymer.2011.08.034
Panthi G, Park M, Kim HY, Park SJ (2015) Electrospun polymeric nanofibers encapsulated with nanostructured materials and their applications: a review. J Ind Eng Chem 24:1–13. https://doi.org/10.1016/j.jiec.2014.09.011
Persano L, Camposeo A, Tekmen C, Pisignano D (2013) Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol Mater Eng 298:504–520. https://doi.org/10.1002/mame.201200290
Radusin T, Torres-Giner S, Stupar A, Ristic I, Miletic A, Novakovic A, Lagaron JM (2019) Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag Shelf Life 21:100357. https://doi.org/10.1016/j.fpsl.2019.100357
Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma Z, Ramaseshan R (2006) Electrospun nanofibers: solving global issues. Mater Today 9:40–50. https://doi.org/10.1016/S1369-7021(06)71389-X
Reneker DH, Chun I (1996) Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216–223. https://doi.org/10.1088/0957-4484/7/3/009
Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys 87:4531–4547. https://doi.org/10.1063/1.373532
Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, Roh S, Cho JJ, Park WH, Min BM (2006) Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 27:1452–1461. https://doi.org/10.1016/j.biomaterials.2005.08.004
Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S (2005) Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials 26:4606–4615. https://doi.org/10.1016/j.biomaterials.2004.11.035
Rieger KA, Porter M, Schiffman JD (2016) Polyelectrolyte-functionalized nanofiber mats control the collection and inactivation of Escherichia coli. Materials 9:297. https://doi.org/10.3390/ma9040297
Sahay R, Kumar PS, Sridhar R, Sundaramurthy J, Venugopal J, Mhaisalkar SG, Ramakrishna S (2012) Electrospun composite nanofibers and their multifaceted applications. J Mater Chem 22:12953–12971. https://doi.org/10.1039/c2jm30966a
Saquing CD, Manasco JL, Khan SA (2009) Electrospun nanoparticle-nanofiber composites via a one-step synthesis. Small 5:944–951. https://doi.org/10.1002/smll.200801273
Schiffman JD, Elimelech M (2011) Antibacterial activity of electrospun polymer mats with incorporated narrow diameter single-walled carbon nanotubes. ACS Appl Mater Interfaces 3:462–468. https://doi.org/10.1021/am101043y
Shi D, Wang F, Lan T, Zhang Y, Shao Z (2016) Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 23:1899–1909. https://doi.org/10.1007/s10570-016-0918-x
Sofi HS, Akram T, Tamboli AH, Majeed A, Shabir N, Sheikh FA (2019) Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int J Pharm 569:118590. https://doi.org/10.1016/j.ijpharm.2019.118590
Sridhar R, Lakshminarayanan R, Madhaiyan K, Barathi VA, Limh KHC, Ramakrishna S (2015) Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem Soc Rev 44:790–814. https://doi.org/10.1039/c4cs00226a
Stankus JJ, Guan J, Fujimoto K, Wagner WR (2006) Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials 27:735–744. https://doi.org/10.1016/j.biomaterials.2005.06.020
Stocco TD, Rodrigues BVM, Marciano FR, Lobo AO (2017) Design of a novel electrospinning setup for the fabrication of biomimetic scaffolds for meniscus tissue engineering applications. Mater Lett 196:221–224. https://doi.org/10.1016/j.matlet.2017.03.055
Su Y, Su Q, Liu W, Jin G, Mo X, Ramakrishn S (2012) Dual-drug encapsulation and release from core-shell nanofibers. J Biomater Sci Polym Ed 23:861–871. https://doi.org/10.1163/092050611X564137
Suganya S, Senthil Ram T, Lakshmi BS, Giridev VR (2011) Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: an excellent matrix for wound dressings. J Appl Polym Sci 121:2893–2899. https://doi.org/10.1002/app.33915
Sultana N, Zainal A (2016) Cellulose acetate electrospun nanofibrous membrane: fabrication, characterization, drug loading and antibacterial properties. Bull Mater Sci 39:337–343. https://doi.org/10.1007/s12034-016-1162-6
Tamura T, Kawakami H (2010) Aligned electrospun nanofiber composite membranes for fuel cell electrolytes. Nano Lett 10:1324–1328. https://doi.org/10.1021/nl1007079
Tijing LD, Ruelo MTG, Amarjargal A, Pant HR, Park CH, Kim DW, Kim CS (2012) Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem Eng J 197:41–48. https://doi.org/10.1016/j.cej.2012.05.005
Unalan I, Slavik B, Buettner A, Goldmann WH, Frank G, Boccaccini AR (2019) Physical and antibacterial properties of peppermint essential oil loaded poly (ε-caprolactone) (PCL) electrospun fiber mats for wound healing. Front Bioeng Biotechnol 7:1–11. https://doi.org/10.3389/fbioe.2019.00346
Wang Y, Li P, Xiang P, Lu J, Yuan J, Shen J (2016) Electrospun polyurethane/keratin/AgNP biocomposite mats for biocompatible and antibacterial wound dressings. J Mater Chem B 4:635–648. https://doi.org/10.1039/c5tb02358k
Wongkanya R, Chuysinuan P, Pengsuk C, Techasakul S, Lirdprapamongkol K, Svasti J, Nooeaid P (2017) Electrospinning of alginate/soy protein isolated nanofibers and their release characteristics for biomedical applications. J Sci Adv Mater Dev 2:309–316. https://doi.org/10.1016/j.jsamd.2017.05.010
Xue J, Xie J, Liu W, Xia Y (2017) Electrospun nanofibers: new concepts, materials, and applications. Acc Chem Res 50:1976–1987. https://doi.org/10.1021/acs.accounts.7b00218
Yang H, Gao PF, Wu WB, Yang XX, Zeng QL, Li C, Huang CZ (2014) Antibacterials loaded electrospun composite nanofibers: release profile and sustained antibacterial efficacy. Polym Chem 5:1965–1975. https://doi.org/10.1039/c3py01335a
Yao CH, Yeh JY, Chen YS, Li MH, Huang CH (2017) Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J Tissue Eng Regen Med 11:905–915. https://doi.org/10.1002/term.1992
Yousefi I, Pakravan M, Rahimi H, Bahador A, Farshadzadeh Z, Haririan I (2017) An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater Sci Eng C 75:433–444. https://doi.org/10.1016/j.msec.2017.02.076
Yu DG, Branford-White C, White K, Chatterton NP, Zhu LM, Huang LY, Wang B (2011) A modified coaxial electrospinning for preparing fibers from a high concentration polymer solution. Express Polym Lett 5:732–741. https://doi.org/10.3144/expresspolymlett.2011.71
Zaikov GE (2016) Nanostructured fibers via electrospinning (part I). Appl Nanotechnol Mater Appl 2016:253–280. https://doi.org/10.1201/9781315366333
Zeyohanness SS, Abd Hamid H, Zulkifli FH (2018) Poly(vinyl alcohol) electrospun nanofibers containing antimicrobial Rhodomyrtus tomentosa extract. J Bioact Compat Polym 33:585–596. https://doi.org/10.1177/0883911518801040
Zhang Y, Ouyang H, Chwee TL, Ramakrishna S, Huang ZM (2005) Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res 72:156–165. https://doi.org/10.1002/jbm.b.30128
Zhang Z, Wu Y, Wang Z, Zou X, Zhao Y, Sun L (2016) Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities. Mater Sci Eng C 69:462–469. https://doi.org/10.1016/j.msec.2016.07.015
Zhang W, Ronca S, Mele E (2017a) Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 7:1–17. https://doi.org/10.3390/nano7020042
Zhang W, Huang C, Kusmartseva O, Thomas NL, Mele E (2017b) Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React Funct Polym 117:106–111. https://doi.org/10.1016/j.reactfunctpolym.2017.06.013
Zhou T, Sui B, Mo X, Sun J (2017) Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int J Nanomedicine 12:3495–3507. https://doi.org/10.2147/IJN.S132459
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ali, W., Gadkari, R., Arora, S., Somkuwar, V., Chowdhury, A. (2021). Antibacterial Electrospun Nanofibres. In: Inamuddin, Ahamed, M.I., Prasad, R. (eds) Advanced Antimicrobial Materials and Applications. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-7098-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-7098-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7097-1
Online ISBN: 978-981-15-7098-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)