Abstract
In recent years, flame retardants (FRs) focused on eco-friendliness, eco-viable and durable, are in great social demand and one of the most growing area of research interest on account of increased awareness towards environmental concerns. In this regard, strategies are considered onto FRs for textiles as well as other substrates with their applicability and selectivity. Phosphorus-based FRs provide a foundation for the directed design of nontoxic FRs mainly because of its versatility, for example, it can act in both the condensed and gas phase, as an additive or as a reactive component, in various oxidization states, and in synergy with numerous adjuvant elements. Various P-moieties make valuable contribution and combinations including elemental, inorganic salts and organophosphorus compounds. This chapter highlights general insight into phosphorus-based flame retardants for polymeric systems with future R&D opportunities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
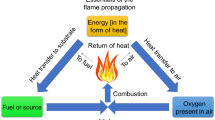
Flame-retardation ability to the polymeric substrates such as textiles (woven or non-woven), plastics, rubber and others, is vastly needed because they have been applied to a myriad of applications for general and engineering purposes. Specific chemical bonding was shown in several polymeric materials (i.e. cotton, linen, hemp; silk and wool; nylon; polypropylene; polyester etc.) acquired specificity towards implanting flame-retardancy. Textile materials have been applied worldwide in both civilian as well as military fields due to their inherent and excellent properties such as air-permeability, softness, comfortableness, hydrophilicity etc. In a general opinion, fire can be described as the combustion cycle which is illustrated by a fire triangle for which three components are necessary to be fire to occur; one is heated, secondly a combustible fuel and thirdly a combustive process. The most important factor in the combustive process is the air (oxygen). According to the fact that fire or flame is often initiated from the burning of the textile materials which subsequently results in burns and even loss of human life, causing serious damage to furniture, carpets, upholstery, buildings, properties, etc. [1, 2]. Thus, flame retardant (FR) finishing of textile substrates is extremely necessary for many applications for the prevention of fire and for protection of human life. Most of the polymeric materials such as cellulosics, wool, nylon, polyesters, polyurathanes possess higher flammability [3], and therefore, required high performance flame retardant finishes to overcome flammable aspects.
FRs provide fire resistance ability to the textiles through the heat absorbing, the covering effect, inhibition of chain reaction and gas dilution phase [4]. In general, there are many chemical treatments that are commonly employed to impart flame retardant finish for textile/polymeric materials. Main six categories of FRs are highly discussed and accepted, for example; halogenated, formaldehyde-based, P-based, N-based, Si-based and other mixed formulations [5]. However, the purpose of FR finishes is to reduce the amount of heat that is supplied to the polymer system to be below the level for flame stability [6]. Halogenated, phosphorus and formaldehydes based compounds such as Proban, THPC-TMM (Tetrakis(hydroxymethyl)phosphonium chloride-Trimethylolmelamine) and Pyrovatex CP, have been widely employed as the commonest FRs to impart durable fire-resistant ability to the cotton substrates [3,4,5,6,7].
In early 1990s serious environmental concerns have been noticed concerning halogenated FRs, especially brominated flame retardants (BFRs). It is found that under severe thermal stress or when they were burnt in accidental fires or uncontrolled combustion, BFRs could form halogen-based dioxins and furan derivatives [8, 9]. Furthermore, it is noteworthy that the environmental and health concerns limited not only of BFRs, but also of other types of flame retardants and have been studied extensively at a global scale. Several scientific meetings and conversations were organized in the late 90 s onto flame retardants: uses, risk assessments and safety globally until the transition to Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), a European Union Regulation Authority came existence in 2006. In 2008, REACH, [10] (REACH, 1907/2006/EC) entered “No data no market” slogan which requires the basic health and environmental data to be submitted for all chemicals before commercialization for their safety evaluation [10]. Halogenated FRs generate poisonous substances on fire and combustion [2] whereas formaldehyde-based FRs release formaldehyde which found carcinogenic [3]. They are found to have adverse health effects in animals and humans, including endocrine and thyroid disruption, immunotoxicity, reproductive toxicity, cancer, and adverse effects on fetal and child development and neurologic function [9,10,11,12,13].
As a result, health and environmental hazards associated with these FRs driven R&D for identifying and utilizing safer alternatives. Because of the social concerns onto eco-preservation using eco-friendly FRs, the new FRs needs to be halogen-free and formaldehyde-free. Phosphorus, nitrogen, and silicon-containing compounds are generally considered as environment-friendly FRs, because they do not generate harmful substances to human-ecosystem on burning with fire and their synergistic effects [6, 14]. P-based FRs are found very effective inert towards fire (most effective in high oxidation states) mainly because of its characteristics such as (i) low water solubility, (ii) low volatility, (iii) low dose requirement, (iv) less degradation to possibly hazardous substances, and (v) no toxic emissions [2, 6]. Mechanistically, phosphorous based flame retardants, during a fire form poly and meta-phosphoric acids which form an oxygen-barrier layer [15] and commonly used due to the environmental scrutiny halogenated and formaldehyde-free FRs.
The flame retardant mechanism described for phosphorus containing flame retardants includes both a condensed and a vapor phase mechanism depending on the type of phosphorus compound and the polymer. Specific applications for red phosphorus, organophosphates, chlorophosphates and bromophosphates are described. The use of triarylphosphates in PVC, modified polyphenylene oxide, and polycarbonate/ABS is described. The chlorophosphates are used in polyurethanes and the bromophosphates in engineering thermoplastics. Flammability and mechanical properties are given for specific polymers [16].
Phosphorous based flame resistant materials have long been used since the 1940s–1950s. P-based FRs exhibit excellent fire inertness ability and found effective both in the vapour and condensed phases. They vary in oxidation states (0 to +5) and can be classified into elemental, inorganic, and organic or organo-phosphorus [17] categories.
1.1 Elemental Phosphorus as FR
Phosphorus (P0) has several allotropic forms [18] out of which white phosphorus (WP) and red phosphorus (RP) are most common. WP is a white, soft, waxy solid consists of tetrahedral P4 molecules, in which each atom is bound to the other three atoms by a single bond. It ignites spontaneously and is very toxic and reactive in nature, and therefore cannot be used as FR [17].
Samples of WP always contain red phosphorus in a very little amount and accordingly appear yellow. On heating, WP can be converted into RP in the absence of air. It is harder, denser, more stable, less toxic, less reactive than WP and polymeric in structure with P4 units [19]. Although, it ignites easily but possesses thermal stability up to 450 °C (approx.) and thus, the ability to be used as sufficient FR agent [18]. RP is observed as an efficient FR especially for oxygen-containing polymers that work in the vapor and condensed phases [19, 20]. Among the high performance flame retardants (HFFR) additives, RP is a type of powerful FR and has been significantly used for polymeric moieties other than textiles such as polyethylene [21], poly(ethylene terephthalate) [22], nylon [23, 24] etc.
Moreover, the combination of RP with other HFFR additives, metal hydroxide or intumescent FR can improve the overall fire retardancy of highly flammable substrates like polyolefins (PO) blends have been investigated and reported as effective [25]. However, the main disadvantages of RP are because of poor thermostability, the evolution of highly toxic phosphine (PH3) during the reaction with moisture and the lack of compatibility with synthetic resins [26]. A novel technology was developed to prepare microencapsulated RP with suitable filling/supported agent to minimize the associated problems. Wu et al. [27] investigated microencapsulated RP as FR agent for synthetic polymers. In this study, they conclude that the microencapsulation of red phosphorus efficiently improved its water absorption, thermostability, ignition point, and decrease the amount of phosphine evolution with 5% amount [27]. A similar study was carried out by Liu and Wang [26]. In this study, a composite system of RP encapsulated by N-based FR was used for polyamide 6 (PA6) due to higher N-P synergistic effects. The action and mechanisms of the NFR-microencapsulated RPFR on PA6 were investigated in terms of limiting oxygen index (LOI) by using vertical burning experiment (UL94), thermogravimetric analysis (TGA), and scanning electron microscope (SEM) observations. It was concluded that the NFR-microencapsulated RPFR combination possessed desired flame retardancy because of effective char-formation of the condensed phase and it also showed satisfactory mechanical properties as the result of the good compatibility between flame retardant and PA6 resin.
1.2 Inorganic Phosphorus-Based FRs
Inorganic phosphorus-based FRs were developed and commonly used in the nineteenth century, mainly phosphates and polyphosphates. However, the great scientist Gay Lussac in 1821 used ammonium phosphate solution to impart flame retardancy of theater curtains [19, 20]. Ammonium phosphates (APs) possess fairly fire retarding ability and prevent afterglow. Monoammonium phosphate (NH4H2PO4) and diammonium phosphate ((NH4)2HPO4) or mixtures of these two phosphates have good water solubility and found very effective for many substrates as FR, for example, textiles, cellulosic fibers, wooden and paper products [19, 28]. With respect to susceptibility to bloom out of the material, matrix is a down-manner of APs. The low susceptibility of APs introduces ammonium polyphosphates (APPs) which have higher susceptibility. APPs are moderately soluble in water with several crystalline forms that differ in molecular weight ratio and particle size. APPs have been heated with a small amount of urea to enhance the solubility [19]. APPs are used as the principal ingredients in intumescent FR coatings because of their decomposition temperature (greater than 256 °C). The decomposition of APPs produces phosphorus acid that will interact with the carbon source to produce a carbonaceous char [19, 20]. APPs are cheaper, low toxic, quite thermally stable than their organic counterparts, and good thermal stability, and can be used for other non-textile materials such as plastics, rubber, paper, epoxy resins and wood [29].
1.3 Organic or Organophosphorus-Based FRs
Organophosphorus-based FRs gain increased attention preventing the risks of fire in recent times because of their significant efficacy and environmentally safer nature over halogenated and formaldehyde containing FRs [30]. Organophosphorus FRs have been used from last few decades and widely used for several various polymeric consumer products like plastics, textiles, polyurathanes (PU), polyamides (PA), polyethyleneterephthalate (PET), epoxy-materials etc. [31, 32]. Organophosphorus compounds containing P–C bonds are developed extensively as FR additives due to their excellent thermal and hydrolytic stability as well as ease of generation. In a mechanistic pathway it was observed that during the fire, phosphorus compounds break down to phosphorus acid which blocks polymer’s oxygen functionality, and therefore lead to the char-formation in the high ratio [33].
Wendels et al. [34] has published an exhausted and comprehensive review on organophosphorus compounds for the recent developments in organophosphorus flame retardants (OPFRs) having P–C bonding with their synthesis pathways and applications [34].
Nowadays, various products, large in numbers are commercially available based on organophosphorous compound. On the basis of carbon unit/moiety OPFRs can be broadly categorized into five main classes (Table 1) [17, 32,33,34,35,36]:
-
(i)
organophosphates,
-
(ii)
organophosphonates,
-
(iii)
organophosphinites,
-
(iv)
organoposphine oxide, and
-
(v)
organophosphites.
1.3.1 Organophosphate FRs
Organophosphate FRs with a wide range in their polarity, solubility and persistence have been produced and used as significant FRs in substitutes to the stringent regulation in the use of brominated FRs. These FRs are widely used as flame retardants in various consumer products such as textiles, electronics, industrial materials and furniture to prevent the high risk of fire [1, 32]. A large number of OPFRs have been fabricated with varied P–C assemblies. Some commonest OPFRs are shown in Table 1.
-
Aliphatic: P–Caliphatic bond containing organophosphorus compounds have been utilized as good FRs to polymeric materials such as Dimethyl phosphate (DMP), Diethyl phosphate (DEP), Trimethyl phosphate (TMP), Tripropyl phosphate (TPP), Tri-isopropyl phosphate (TIPP), Tri-n-butyl phosphate (TNBP) Tris(2-butoxyethyl) phosphate (TBOEP) etc.
-
Cl-aliphatic: Chlorine-based P–Caliphatic bond containing organophosphorus compounds have superior flame retardancy. Examples of this subclass include Tris(2,3-dibromopropyl) phosphate (TDBPP) and Tris(tribromoneopentyl) phosphate (TTBPP).
-
Br-aliphatic: Brromine-based P–Caliphatic bond containing FRs are found effective FRs, for example, Phenylpropan-2-ylhydrogen phosphate (PPHP), 2-Ethylhexyldiphenyl phosphate (EHDPP), Isodecyldiphenyl phosphate (IDPP) etc.
-
Aromatic: In the previous few decades aromatic phosphates possess strong flame-retardant properties [34]. Common examples are Triphenyl phosphate (TPP), Tricresyl phosphate (TCP), Triisopropyl phosphate (TIPP), Cresyl diphenylphosphate (CDPP), 6H-Dibenz[c,e] [1, 2] oxaphosphorin,6-oxide (DOPO), Bisphenol-A bis-(diphenylphosphate) (BPA-BDPP) etc.
1.3.2 Organophosphonate FRs
Organophosphonates were extensively developed and used as FRs. Many commercially accepted compounds based on organophosphonate specification have been generated and successfully employed as FRs and composed of two types:
-
Aliphatic: FRs with P–Caliphatic bond having phosphonate group are shown in Table 1 and commonly include Dimethyl hydrogen phosphonate (DMHP), Diethyl hydrogen phosphonate (DEHP), Dimethylethyl phosphonate (DMEP), Dimethylmethyl phosphonate (DMMP), Dimethylpropyl phosphonate (DMPP), Dimethylallyl phosphonate (DMAP) as well as a Cyclic tert-butyl phosphonate (TBP).
-
Aromatic: FRs with P–Caromatic bond having phosphonate group FRs were derived from the transformation of aliphatic moiety with aromatic units. A monosubstituted phosphorus compound (Ar-Phosphonate derivative 1, m.p. 184–187 °C) and a disubstituted phosphorus compound (Ar-Phosphonate derivative 2, m.p. 258–260 °C) were obtained as a mixture using modified separation and purification steps [37]. The phosphonate product diethyl-(2-hydroxy-5-vinylphenyl) phosphonate (Ar-Phosphonate derivative 4) was fabricated from diethyl-(4-vinylphenyl)phosphate (Ar-Phosphonate derivative 3) using ethoxy-P-acid [38]. Su et al. [39] patented the P-enabled esterification of phenol or polyhydroxybenzenes to develop several OPFRs. one example is compounds Ar-Phosphonate derivative 5 obtained from bisphenol A [39].
Another two phenolic white crystals/compounds (Ar-Phosphonate derivative 6, m.p. 169–171 °C and 7, m.p. 216–217 °C) were prepared through [1, 3]-sigmatropic rearrangement and were found to have high-performance against polymers [40].
1.3.3 Organophosphinites FRs
Recently, synthesis and applications of different phosphinic acid derivatives have been reported excel flame resistant abilities [41]. Examples of organophosphinites FRs are Diethylphosphinic acid (DEPA), Phenylphosphinic acid (PPA), p-Methoxyphenylphosphinic acid (PMPPA), Carboxyethyl-phenylphosphinic acid (CEPPA), Bis(2-cyanoethyl)phosphinic acid (BCEPA) etc.
1.3.4 Organoposphine Oxide FRs
Organoposphine oxide derivatives found to have limited FRs capabilities. Examples are Tris(hydroxymethyl) phosphineoxide (THMPO), Triphenylphosphineoxide (TPPO) and Bis(4-carboxyphenyl) phenylphosphine oxide (BCPPPO). In addition, Bis(4-aminophenyl) phenylphosphine oxide (BAPPO) was developed as a moderate water-soluble compound that found effectiveness towards Polyurathane-based materials with environmental susceptibility [42].
1.3.5 Organophosphites FRs
Like organoposphine oxide derivatives, organophosphites were also least responsive FRs because of their cholinergic neurotoxicity [43, 44]. Examples are Triisopropyl Phosphite (TIPP) and Tri-phenyl Phosphite (TPP).
2 Textile Applications of P-Based Flame Retardants
Organophosphorus compounds with P–C moieties have shown a wide range of design and development of exciting organophosphorus FRs due their high thermal and hydrolytic stability (P–C bond), ease of synthesis and suitability of processing even at high temperature. Extensive works have been published regarding the creation of P–C bond and their potential applications [1,2,3,4,5,6, 11,12,13, 22, 31,32,33,34,35]. These P–C containing organophosphorus compounds have shown several applications along with fire resistancy, for example, reagents, catalysts, pesticides, insecticides, herbicides, surfactants, lubricants and even more [1,2,3,4, 45].
Textiles and clothing are prepared from various fiber forming substrates either natural origin polymers such as cellulose and protein, or a wide variety of semi-synthetic and synthetic polymers such as cellulose acetate, polyesters, polyamides, polyolefins, polyacrylonitriles, polyaramids, polylactides, polyetherketones etc. All of these polymers are allocate a frequent limitation, combustible under normal environmental conditions and sometimes pose serious fire hazards in case of fire accidents.
In the present scenario, fire-caused deaths are a growing global problem. The Fire Administration Authority of US, has reported recently on the basis of 24 industrialized nations as the average rate of fire-related deaths and concluded that 10.7 per million populations every year have been observed [46, 47]. Additionally, The National Fire Protection Association claims that home structure involved fires are the main cause of fire-related death [47].
Textile materials provide an excellent source of fuel during the burning process, are found to be a rich source of inflammable or ideal fire carriers like hydrocarbons. The potential hazards and risks associated with textiles are described in depth by various researchers [1, 2, 7, 11]. In this prospect, textiles with lower flammability are still experiencing some changes like the improvement in effectiveness and the replacement of toxic chemical products with counterparts that have a low environmental impact and, more sustainable [4, 5]. Health and environmental concerns associated with halogenated as well as formaldehyde-based FRs driven R&D for identifying and utilizing safer alternatives. Because of the social concerns onto eco-preservation using eco-friendly FRs, the new FRs needs to be halogen-free and formaldehyde-free. Phosphorus, nitrogen, and silicon-containing compounds are generally considered as environment-friendly FRs, due to their safer-nature for human-ecosystem and synergistic effects [6, 14]. The effectiveness of P-based FRs towards fire mainly because of its characteristics, for example, low water solubility, low volatility, less dose requirement, less degradation to possibly hazardous substances, and no toxic emissions [2, 6, 31]. P-based FRs, play a key role possibly in combination with silicon- or nitrogen-containing structures, to the design of new and efficient FRs for textile substrates. Mechanistically, phosphorous based FRs, during a fire form poly and meta-phosphoric acids which form an oxygen-barrier layer [15].
Phosphorus based FRs have been found very reactive to inhibit fire and are used as thermosets for many substrates such as unsaturated epoxy resins, polyesters or polyurethanes. These type of substrates contain activated functional groups (i.e. halogens, alcohols, epoxy, amines etc.), which allow incorporation into the polymer matrix during the process [42, 43]. In case of cotton fibre, organic assembled phosphorus compounds (i.e. Pyrovatex CP and Pyrovatex CP New) can either with the cotton fabric to form cross-linked adducts/linked structures with the fibers [48]. In a study, a formaldehyde-free, inorganic-organic hybrid FR was developed and markedly found inferior FR performance compared with conventional formaldehyde-containing organic phosphorus FR. Lessan et al. [49] investigated the flame retardant behavior of sodium hypophosphite (SHP)—nano-TiO2 hybrid on woven cotton fabric through pad-dry-cure process. As a result, decreasing the flammability with increasing the char formation of the treated fabrics was observed [49].
Despite the use of toxic and not environmentally-friendly chemicals, high-molecular-weight proteins even DNA derived from animal or microbial sources have been investigated as ‘‘green’’ FRs for cotton fabrics [42, 49]. Current trends are made towards high-molecular-weight FRs based on P-moiety combined with polymeric/complex textile substrates impart multifunctional structures will aid in reducing flammability without a loss of their valuable properties. A novel organic phosphorus-based flame retardant has reported the enhancement of flame retardancy of cotton fabrics through the high-molecular-weight grafting of cellulose-phosphonic acid by Gao et al. [50] as an alternative to halogen-formaldehyde-based FRs [50]. In this study, an ammonium salt of hexamethylenediamine-N,N,N’,N’-tetra(methylphosphonic acid) (AHDTMPA), was fabricated using the reaction of urea with hexamethylenediamine-N,N,N’,N’-tetra(methylphosphonic acid) (HDTMPA). Further, new P–O–C covalent bonds were formed by this ammoniated salt reacted with the O-6 hydroxyls of glucose residues of cellulose. The resulted hybrid FR containing both P and N-moieties exhibited excellent flame retardant performance for cotton fabrics (70 g/L hybrid FR with limiting oxygen index (LOI) value of 36.0%) which remained relatively stable after 50 laundering cycles (70 g/L hybrid FR with LOI value 28.0%).
Some particular proteins such as phosphorus and sulphur-rich proteins (i.e. caseins and hydrophobins) derived from animal or microbial sources have been under investigations as a novel as well as green flame retardants for cotton fabrics. Alongi et al. [51] investigated caseins and hydrophobins as a novel and green flame retardants for cotton fabrics [51]. As a consequence, P-based-polymer matrix was achieved with improved flame retardancy, indicated by the increased total burning time as well as by the decreased total burning rate. In this study, the change in the flammability features of the fabric, favouring the dehydration of cellulose to form char as opposed to the depolymerization with further production of combustible volatile species were observed. The familiar results have been observed by the same research group when whey proteins were employed that homogeneously deposited on cotton fabric to impart FR properties, using the layer-by-layer technique [52].
In addition, bio-derived phytic acid exhibits the great potential to improve the flame retardancy of textile materials, but with low washing durability. To overcome the poor durability, Cheng et al. [53] investigated a reactive, efficient P-containing flame retardant using phytic acid, pentaerythritol and 1,2,3,4-butanetetracarboxylic acid [53]. The wool fabric treated with HPPHBTCA 0.14 mol/L HPPHBTCA had self-extinguishing performance even after 20 washing cycles during the vertical burning test, presenting good FR ability and resistance to washing with slight negligible effect on the whiteness, tensile strength and handle of wool fabric.
Therefore, phosphorus-containing compounds offer a novel route to prepare so-called green, eco-friendly and durable flame retardants for textiles or textile-based materials that inhibit or resist the spread of fire.
3 Conclusion and Future Outlook
Flame retardants have been added to the polymer systems to prevent the risk of fire and overall the use of FRs has substantially decreased the number of fires and fire fatalities in our social wardrobe. With increased awareness towards environmental concerns about FRs selectivity, phosphorus-based FRs provide a foundation for the directed design of nontoxic FRs mainly because of its versatility, for example, it can act in both the condensed and gas phase, as an additive or as a reactive component, in various oxidization states, and in synergy with numerous adjuvant elements.
Various P-moieties make a valuable contribution and various combinations including elemental, inorganic salts and organophosphorus compounds. Nowadays, various products, large in numbers are commercially available based on organophosphorus compound. Although the use of a new generation of chemicals known as Organophosphorus Flame Retardants (OPFRs) is a worthy goal for controlling household fires, on one hand, the other hand, it is also important to control or prevent their toxic effects. Based on carbon unit/moiety OPFRs can be broadly categorized into five main classes; organophosphates, organophosphonates, organophosphinites, organoposphine oxide, and organophosphites. A vast variety of P–C bond containing efficient FRs are being developed; however, further R&D works are needed in terms of their economical, renewability and green synthetic pathways, environmental impacts, long term durability, acute and chronic toxicity etc. without a loss of valuable properties at laboratory as well as their possible larger exploration.
References
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab 96:377–392
Horrocks AR (1986) Flame-retardant finishing of textiles. Rev Prog Color Relat Top 16(1):62. https://doi.org/10.1111/j.1478-4408.1986.tb03745.x
Liu W, Chen L, Wang YZ (2012) A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym Degrad Stabi 97(12):2487–2491
Gaan S, Salimova V, Rupper P, Ritter A, Schmid H (2011) Flame retardant functional textiles. In: Pan N, Sun G (eds) Functional textiles for improved performance, protection and health. Woodhead Publishing, Cambridge, UK, pp 98–130
Bocchini S, Camino G (2010) Chapter 4 halogen-containing flame retardants. In: Wilkie CA, Morgan AB (eds) Fire retardancy of polymeric materials, 2 edn. CRC Press, Florida, USA
Lu SY, Hamerton I (2002) Recent developments in the chemistry of halogen-free flame retardant polymers. Prog Polym Sci 27(8):1661–1712
Yusuf M (2018) A review on flame retardant textile finishing: current and future trends. Curr Smart Mater 3(2):99–108
Söderström G, Marklund S (2002) PBCDD and PBCDF from incineration of waste-containing brominated flame retardants. Environ Sci Technol 36(9):1959–1964
de Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46(5):583–624
https://ec.europa.eu/environment/chemicals/reach/reach_en.htm. Retrieved on 20 Sept 2019
Shaw S (2010) Halogenated flame retardants: do the fire safety benefits justify the risks? Rev Environ Health 25(4):261–306
Ülker OC, Ulker O (2019) Toxicity of formaldehyde, polybrominated diphenyl ethers (PBDEs) and phthalates in engineered wood products (EWPs) from the perspective of the green approach to materials: a review. BioResour 14(3):7465–7493
Castellano A, Colleoni C, Iacono G, Mezzi A, Plutino MR, Malucelli G, Rosace G (2019) Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen-and formaldehyde-free flame retardant finishing for cotton fabric. Polym Degrad Stab 162:148–159
Wang Y, Su Q, Wang H, Zhao X, Liang S (2019) Molded environment-friendly flame-retardant foaming material with high strength based on corn starch modified by crosslinking and grafting. J Appl Polym Sci 136(11):47193
Hull TR, Law RJ, Bergman Å (2014) Environmental drivers for replacement of halogenated flame retardants. In: Papaspyrides CD, Kiliaris P (eds) Polymer green flame retardants. Elsevier, Oxford, UK, pp 119–179
Green J (1992) A review of phosphorus-containing flame retardants. J Fire Sci 10(6):470–487
Morgan AB, Gilman JW (2013) An overview of flame retardancy of polymeric materials: application, technology, and future directions. Fire Mat 37(4):259–279
Holleman A, Wiberg N (1985) “XV 2.1.3”. Lehrbuch der Anorganischen Chemie (33rd edn). de Gruyter (ed.). ISBN 3-11-012641-9
Weil ED, Levchik SV (2017) Phosphorus flame retardants. Kirk-Othmer Encycl Chem Technol 2:1–34
Granzow A (1978) Flame retardation by phosphorus compounds. Acc Chem Res 11:177–183
Peters EN (1979) Flame‐retardant thermoplastics. I. Polyethylene–red phosphorus. J Appl Polym Sci 24(6):1457–1464
Yeh JT, Hsieh SH, Cheng YC, Yang MJ, Chen KN (1998) Combustion and smoke emission properties of poly (ethylene terephthalate) filled with phosphorous and metallic oxides. Polym Degrad Stab 61(3):399–407
Levchik GF, Vorobyova SA, Gorbarenko VV, Levchik SV, Weil ED (2000) Some mechanistic aspects of the fire retardant action of red phosphorus in aliphatic nylons. J Fire Sci 18(3):172–182
Levchik SV, Weil ED (2000) Combustion and fire retardancy of aliphatic nylons. Polym Int 49(10):1033–1073
Wang Z, Qu B, Fan W, Huang P (2001) Combustion characteristics of halogen‐free flame‐retarded polyethylene containing magnesium hydroxide and some synergists. J Appl Polym Sci 81(1):206–214
Liu Y, Wang Q (2006) Preparation of microencapsulated red phosphorus through melamine cyanurate self-assembly and its performance in flame retardant polyamide 6. Polym Eng Sci 46(11):1548–1553
Wu Q, Lü J, Qu B (2003) Preparation and characterization of microcapsulated red phosphorus and its flame-retardant mechanism in halogen-free flame retardant polyolefins. Polym Int 52(8):1326–1331
Xie R, Qu B (2001) Thermo-oxidative degradation behaviors of expandable graphite-based intumescent halogen-free flame retardant LLDPE blends. Polym Degrad Stab 71(3):395–402
Tan Y, Shao ZB, Yu LX, Long JW, Qi M, Chen L, Wang YZ (2016) Piperazine-modified ammonium polyphosphate as monocomponent flame-retardant hardener for epoxy resin: flame retardance, curing behavior and mechanical property. Polym Chem 7(17):3003–3012
Pantelaki I, Voutsa D (2019) Organophosphate flame retardants (OPFRs): a review on analytical methods and occurrence in wastewater and aquatic environment. Sci Total Environ 649:247–263
Salmeia K, Gaan S, Malucelli G (2016) Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polym 8(9):319
Keglevich G, Grün A, Bálint E, Kiss NZ, Bagi P, Tőke L (2017) Green chemical syntheses and applications within organophosphorus chemistry. Struct Chem 28(2):431–443
Camino G, Costa L (1988) Performance and mechanisms of fire retardants in polymers-a review. Polym Degrad Stab 20(3–4):271–294
Wendels S, Chavez T, Bonnet M, Salmeia K, Gaan S (2017) Recent developments in organophosphorus flame retardants containing P–C bond and their applications. Materials 10(7):784(1–32)
Gupta RC (2006) Classification and uses of organophosphates and carbamates. In: Gupta RC (ed) Toxicology of organophosphate and carbamate compounds. Academic Press, Burlington, pp 5–24
AbouDonia M, Abou-Donia MB, Salama M, Elgamal M, Elkholi I, Wang Q (2016) Organophosphorus flame retardants (OPFR): neurotoxicity. J Environ Health Sci 2(1). https://doi.org/10.15436/2378-6841.16.022
Benin V, Gardelle B, Morgan AB (2014) Heat release of polyurethanes containing potential flame retardants based on boron and phosphorus chemistries. Polym Degrad Stab 106:108–121
Dumitrascu A, Howell BA (2011) Flame-retarding vinyl polymers using phosphorus-functionalized styrene monomers. Polym Degrad Stab 96:342–349
Su WC, Sheng CS (2005) Method for preparing a biphenylphosphonate compound. U.S. Patent 20050101793
Finocchiaro P, Consiglio GA, Imbrogiano A, Failla S (2007) Synthesis and characterization of new organic phosphonates monomers as flame retardant additives for polymers. Phosphorus, Sulfur Silicon Relat Elem 182:1689–1701
Ma J, Yang J, Huang Y, Ke C (2012) Aluminum-organophosphorus hybrid nanorods for simultaneously enhancing the flame retardancy and mechanical properties of epoxy resin. J Mater Chem 22:2007–2017
Velencoso MM, Battig A, Markwart JC, Schartel B, Wurm FR (2018) Molecular firefighting—how modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew Chemie Int Ed 57(33):10450–10467
Carrington CD, Abou-Donia MB (1988) Triphenyl phosphite neurotoxicity in the hen: inhibition of neurotoxic esterase and a lack of prophylaxis by phenylmethylsulfonyl fluoride. Arch Toxicol 62(5):375–380
Wolschke H, Sühring R, Xie Z (2015) Organophosphorus flame retardants and plasticizers in the aquatic environment: a case study of the Elbe River. Germany Environ Poll 206:488–493
Demchuk OM, Jasinski R (2016) Organophosphorus ligands: Recent developments in design, synthesis, and application in environmentally benign catalysis. Phosphorus, Sulfur Silicon Relat Elem 191:245–253
TFRS Fire Death Rate Trends: An International Perspective (2016) vol. 12(8). https://www.usfa.fema.gov/downloads/pdf/statistics/v12i8.pdf. Accessed 1 Sep 2019
Fire Analysis and Research Statistical Reports (2014) NFPA. http://www.nfpa.org/News-and-Research/Fire-statistics-andreports/Fire-statistics/Fires-by-property-type/Residential/Home-structure-Fires. Accessed 1 Sep 2019
Horrocks AR, Kandola BK, Davies PJ, Zhang S, Padbury SA (2005) Developments in flame retardant textiles—a review. Polym Degrad Stab 88:3–12
Lessan F, Montazer M, Moghadam MB (2011) A novel durable flame-retardant cotton fabric using sodium hypophosphite, nano-TiO2 and maleic acid. Thermochim Acta 520:48–54
Gao WW, Zhang GX, Zhang FX (2015) Enhancement of flame retardancy of cotton fabrics by grafting, a novel organic phosphorous-based flame retardant. Cellulose 22(4):2787–2796
Alongi J, Carletto RA, Bosco F, Carosio F, Di Blasio A, Cuttica F, Antonucci V, Giordano M, Malucelli G (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stab 99:111–117
Bosco F, Carletto RA, Alongi J, Marmo L, Di Blasio A, Malucelli G (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 94(1):372–377
Cheng XW, Guan JP, Kiekens P, Yang XH, Tang RC (2019) Preparation and evaluation of an eco-friendly, reactive, and phytic acid-based flame retardant for wool. React Func Polym 134:58–66
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yusuf, M. (2020). Insights into Phosphorus-Containing Flame Retardants and Their Textile Applications. In: Shahid, M., Adivarekar, R. (eds) Advances in Functional Finishing of Textiles. Textile Science and Clothing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-15-3669-4_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-3669-4_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3668-7
Online ISBN: 978-981-15-3669-4
eBook Packages: EngineeringEngineering (R0)