Abstract
The thermal degradation of unsaturated polyester resin and composite fiberglass containing aluminum phosphate as additive flame retardant were investigated. A new phosphate type fire retardant is developed using aluminum phosphate embedded in unsaturated polyester resin with varies weight ratio (0–50%). The modified unsaturated polyester then combined with seven layer of fiberglass reinforcement to produce composite fiberglass boat panels. The effects of aluminum phosphate on structural and thermal properties were evaluated on both unsaturated polyester resin and composite fiberglass using Fourier Transform Infrared (FTIR) and thermogravimetric analysis (TGA). Addition of aluminum phosphate in modified unsaturated polyester observed increases in weight residue due to formation char layer. Thermal properties and structural bonding observed in both modified unsaturated polyester and composite fiberglass show that oxygen has a great influenced in the interaction between phosphate group, aluminum and silica in both systems.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Thermal degradation is a behavior of a material in a reaction with heat or at elevated temperature which causes a loss of its chemical, physical and mechanical properties. Common polymeric materials and its composites experienced a decomposition process due to chemical and physical reaction. The chemical reaction of polymer produces volatiles flammable products, meanwhile physical reaction produces melting and charring that change the decomposition and burning characteristic of materials. The decomposition of composite polymer starts from break down of molecules to smaller species with various different equilibrium. Lighter molecules will vaporize and heavier molecules will remain in the condensed phase either in solid or liquid. At higher temperature, the condense phase will further decomposed to lighter molecules and end as vapor. Some other composite polymer that does not break to lighter molecules will end as solid residues in the form of carbonaceous, inorganic or combination of both [1]. Char is a good thermal insulator, it significantly inhibit the flow of heat from the gaseous combustion zone back to the condensed phase and thus slowing down the decomposition process or decrease the flammability of a polymer through additive or reactive flame. Composite fiberglass boats are constructed from unsaturated polyester and fiberglass. High flammability of unsaturated polyester polymer is due to oxygen component which lead to high oxidation process. Flame retardants are chemicals which are added to flammable materials to improve their resistant from ignition from small heat source such as cigarette, candle or electrical faults. It delayed the spread of flame thus giving more time to save people and prevent severe losses. Flame retardant inhibits combustion process in a number of stages such as during heating, decomposition, ignition or flame spread. Phosphates type fire retardant is used to interrupt combustion process by formation of char layer and protect the heat flow on the surface of materials. The change in solid state decomposition and char layer are formed due incorporation of flame retardant that act by interfering with radical flame reaction. Phosphate additive reacts in the presence of heat source, releasing phosphoric acid that causes the material to char and form a thick glassy layer of carbon on the surface of specimen hence prevents the release of toxic gases and slow down the spreading of combustion [2]. Thermal stability and flame retardant properties of unsaturated polyester embedded with three different phosphorus types flame retardant additive observed that ammonium polyphosphate (APP) shows lower decomposition compared to melamine pyrophosphate (MPP) and silane-coated APP (S-APP) due to formation char layer [3]. APP also observed higher thermal stability, lower peak of heat release rate (PHRR) and lower heat combustion. Combination of APP with mineral additive, aluminum trihydroxide (ATH) in unsaturated polyester reduce the weight ratio of ATH, improved the thermal stability and flame properties due to occurrence of P–Al–O bond [4]. Aluminum phosphate is a new approach in unsaturated polyester and composite fiberglass system. Aluminum phosphate with 5 wt.% weight ratio shows good interaction with unsaturated polyester and composite fiberglass [5]. However the effect of aluminum phosphate on the thermal properties of unsaturated polyester and composite fiberglass were needed in order to have better understanding of its fire performance. Thus, this research is focused on the thermal degradation properties of unsaturated polyester and composite fiberglass embedded with aluminum phosphate.
2 Methodology
Unsaturated polyester resin Norsodyne 3338 W (Polynt Composite Malaysia Sdn. Bhd.) with 30–45% styrene monomer was modified with aluminum phosphate with various ratios of 0, 5, 10, 15, 20, 25 and 50 wt.%. Seven layers of composites fiberglass were prepared using the mixture of unsaturated polyester resin and aluminum phosphate on fiberglass mat from chopped strand mat and woven roving. Methyl Ethyl ketone Peroxide (MEKP) supplied by Pt Kawaguchi Kimia Indonesia was used as catalyst. Thermal analysis (TGA) of modified polyester and composite has been conducted in air atmosphere with heating rate of 10 °C/min and heated 0–1000 °C for both using Mettler Telode TGA/SDTA851 respectively. The sample used was 10–12 mg and the flow rate are 100 ml/min. Simultaneous differential thermal analysis (SDTA) provides the similar information as DSC/TGA. Sample preparation for modified unsaturated polyester and composite fiberglass are summarize in Table 1.
3 Results and Discussion
Figure 1 shows the FTIR spectra modified unsaturated polyester resin with various weight ratios of aluminum phosphate (0, 5, 10, 15, 20, 25 and 50 wt.%). Introduction of aluminium phosphate shows a significant improvement with occurrence and change spectra of 2928, 1718, 1254, 1119, 1065, 743, 701, 521 and 453 cm−1 peak that represent various band assignments C–H, C–O–Al, O–P–O–Al and O–Al respectively. These peaks become intense with increasing ratio of aluminum phosphate until 15 wt.% and then slowly reduced its intensity as observed broaden peak in sample with 50 wt.% aluminum phosphate. The amount of water also varies in the sample due to interaction with aluminum phosphate. All peak in the FTIR spectra shows good interaction between aluminium phosphate and unsaturated polyester resin that contribute to phosphate-carbonaceous char formation [6]. The FTIR spectra for unsaturated polyester composite fibreglass observed at 5 wt.% of aluminium phosphate ratio produce an optimum interaction between composited and aluminium phosphate have been reported earlier [5]. The oxygen element in unsaturated polymer and composite have interaction with phosphate and create bonding that reduced the oxygen available for a fire to ignite or propagate thus improve the fire retardant performance.
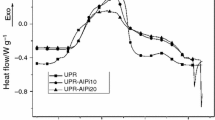
Figure 2 shows the TGA and SDTA pattern for modified unsaturated polyester with various aluminum phosphate ratios 0, 5, 10, 15, 20, 25 and 50 wt.%. The TGA pattern for both pure unsaturated polyester and modified polyester occur has simultaneously confirmed aluminum phosphate is an effective flame retardant and have good interaction with unsaturated polyester. Thermal degradation of modified unsaturated polyester produces a condensed phase mechanism which involves dehydration, crosslinking and char formation. The initial degradation observed below 300 °C was due to elimination of water, volatile materials and acetate groups as well as crosslinking and formation of double bond. The second degradation stage at temperature 300–550 °C contributes to 90 wt.% occurs due to spontaneous pyrolysis decomposition of pure unsaturated polyester backbones. The degradation of pure unsaturated polyester reported earlier started at temperature 320 °C was within the same range [7]. The third degradation stage and weight losses observed at range of temperature 550–625 °C due to oxidation process and formation of char layer. The remaining unoxidized residue remains as char layer at temperature higher than 700 °C. The weight residue of modified unsaturated polyester increases as the aluminum phosphate ratio increases. The weight residue of modified unsaturated polyester decreases to 32 wt.% as aluminum phosphate reaches to 50 wt.%. During thermal process, the dissociated of water increased the potential of re-associated phosphate group in unsaturated polyester. At lower temperature phosphate creates bond and transform to di-phosphate and tri-phosphate at higher temperature. Decomposition of pure unsaturated polyester breaks the complexes with aluminum phosphate. The decomposition produces phosphoric acid and polyphosphoric acid that catalyze dehydration and crosslinking reaction which contributed to the high char formation and weight residue [8]. DTA shows the exothermic peak shifted to lower temperature as aluminum phosphate increases. However for sample 5, 10 and 20 wt.% of aluminum phosphate ratio observed contras finding with exothermic peak shift slightly to higher temperature compared to virgin unsaturated polyester. The initial weight loss is observed below 150 °C, due to the evaporation of moisture. The first stage of degradation of virgin unsaturated polyester occurs between 150 and 450 °C due to elimination of volatile materials and acetate groups as well as crosslinking and formation of double bond. The maximum peak of exothermic has been observed for all samples at temperature range between 300 to 550 °C due to the formation of carbonaceous char by decomposition of carbon monoxide and carbon dioxide [9]. The modified unsaturated polyester observed less volatile product and higher char retention consequence of good interaction between unsaturated polyester with aluminum phosphate. Degradation of pure and modified unsaturated polyester occurs simultaneously within the same temperature range which confirmed that aluminum phosphate is an effective flame retardant [1].
Figure 3 shows the thermal properties of unsaturated polyester composite fiberglass embedded with various weight ratios of aluminum phosphate (0.5 and 10 wt.%). The TGA pattern for all samples shows similar degradation stage. The weigh residue increases as the aluminum phosphate increase. However at lower weight ratio of aluminum phosphate the weight residue for sample wit 5 wt.% shows lower value than sample with 0 wt.%. Exothermic peak for sample with 5 wt.% shift to higher temperature compare to both sample with 0 and 10 wt.% due to increase in crosslinking in the system [10]. However the sample with 5 wt.% observed less moisture with the occurrence of broaden exothermic at range below than 200 °C due interaction of composite with aluminum phosphate produce more Si–O–Al bond [11]. The decomposition of unsaturated polyester composite is affected with the present of oxygen due to breakage of bond with Si, Al and P, at higher temperature. However sample with 5 wt.% of aluminum phosphate needed higher temperature in order to break the complexes into lighter molecules before further degradation. At increasing temperature the complexes of phosphate, oxygen and Si found in fiberglass break to smaller component and decomposed. The oxygen content varies during the breakage and affected the performance of thermal degradation and formation of char layer. The addition of aluminum phosphate in 10 wt.% composite fiberglass encourage the ability of char layer to form in the materials during combustion [8]. The thermal decomposition of polymer may proceed by oxidative processes or simply by the action of heat. The TGA and DTA of modified polyester resin and composite fiberglass showed that the oxygen and water content plays and important roles to create bonding in polyester and composite fiberglass. Table 1 summarizes the thermal and flame retardant performance for both unsaturated polyester and composite fiberglass.
4 Conclusion
Thermal degradation for both unsaturated polyester and composite fiberglass embedded with aluminum phosphate show simultaneous degradation stage due to good interaction. Weight residue increases as the weight ratio of aluminum phosphate increases in unsaturated polyester due to the formation of more char layer. However, in composite fiberglass the residue varies due to the interaction with silica which is interesting to explore further. Thermal properties and structural bonding in both systems are influenced by the oxygen interaction with phosphate, aluminum and silica in fiberglass.
References
Beyler CL Hirschler MM (2005) Thermal decomposition of polymers. In: SFPE handbook of fire protection engineering, 3rd edn, pp 110–131
Leopoldshafen E, Dresden P, Str H (2012) Phosphorus polyesters as halogen-free polymeric flame retardants in poly(butylene terephthalate)—influence of the chemical structure
Ricciardi MR, Antonucci V, Giordano M, Zarrelli M (2012) Thermal decomposition and fire behavior of glass fiber-reinforced polyester resin composites containing phosphate-based fire-retardant additives. J Fire Sci 30(4):318–330
Hapuarachchi TD, Peijs T (2009) Aluminium trihydroxide in combination with ammonium polyphosphate as flame retardants for unsaturated polyester resin. Express Polym. Lett. 3(11):743–751
Saat AM, Malik AA, Azmi A, Latif MFA, Ramlee NE, Johan MR (2017) Effect of aluminum phosphate on structural and flame retardant properties of composites fibreglass. ARPN J Eng Appl Sci 12(4):1315–1318
Saat AM, Johan MR (2017) Enhanced thermal and structural properties of partially phosphorylated polyvinyl alcohol—aluminum phosphate (PPVA-AlPO4) nanocomposites with aluminium nitrate source. AIP Conf Proc 1901(130011):1–8
Bastiurea M, Rodeanu MS, Dima D, Murarescu M, Andrei G (2015) Thermal and mechanical properties of polyester composites with graphene oxide and graphite. Dig J Nanomater Biostructures 10(2):521–533
Pan LL, Li GY, Su YC, Lian JS (2012) Fire retardant mechanism analysis between ammonium polyphosphate and triphenyl phosphate in unsaturated polyester resin. Polym Degrad Stab 97(9):1801–1806
Al Bayaty SA, Farhan AJ (2015) Polyester and unsaturated polyester reinforcement by Toner Carbon Nano Powder (TCNP) composites. Int J Appl Innov Eng Mange 4(3):139–146
Siddharthan KS, Mohan S, Elayaperumal A (2014) Mechanical and thermal properties of glass/polyester composite with glycerol as additive. Int J Eng Trends Technol 7(2):61–64
Saat AM, Johan MR (2016) Effect of aluminum source on the structural and thermal properties of partially phosphorylated polyvinyl alcohol composite (PPVA)—aluminum phosphate (PPVA-AlPO4) nanocomposite. Key Eng Mater 701(3):291–294
Acknowledgements
This work was financially supported by UniKL Short Term Grant (UniKL/Cori/STRG/16035 & UniKL/Cori/STRG/15126). Author also appreciates of the collaboration with Nanotechnology and Catalyst Research, Universiti Malaya, Kuala Lumpur, Malaysia. The author gratefully acknowledge on both of support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Saat, A.M. et al. (2020). Thermal Degradation of Unsaturated Polyester and Composite Fiberglass Embedded with Aluminium Phosphate. In: Saw, C., Woo, T., a/l Karam Singh, S., Asmara Bin Salim, D. (eds) Advancement in Emerging Technologies and Engineering Applications. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-0002-2_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-0002-2_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0001-5
Online ISBN: 978-981-15-0002-2
eBook Packages: EngineeringEngineering (R0)