Abstract
Reduction of photosynthetic rates in plants under salt pressure is triggered primarily by reducing water potential and/or elevated levels of Na+ and/or Cl− accumulated in chloroplasts and chlorophyll that are essential for plant health. Much work has been done to create strategies to deal with salinity stress. The use of organic and inorganic compounds, nutrients, and seed priming is among the promising strategies adopted to alleviate the unfavorable consequences of salinity and improve plant yields and yield quality. Seed priming is a commercially used method to improve the germination and vigor of seeds. The purpose of this work is to review the recent literature on plant response to seed priming under salinity stress. A schematic diagram describing the chain reaction following the seed priming was proposed. The diagram illustrates the possible impact of the seed priming on different physiological processes, enhancing tolerance level and final yield. Several mechanisms have been proposed to explain how plants build up many physiological and biochemical adaptations to regulate themselves when using seed priming under saline conditions. The variation on photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 photosynthetic rate; ionic balance; K+:Na+ ratio difference; osmolyte accumulation, i.e., proline, glycine betaine, amino acids, and sugars; accumulation of enzymatic like superoxide dismutase, catalase, ascorbate peroxidase, peroxidase, and nonenzymatic antioxidants (i.e., total phenols, flavonoids, ascorbic acid, total carotenoids); membrane stability; and levels of H2O2 and malondialdehyde (MDA) contents are among the most important physiological and biochemical plant reaction mechanisms for seed priming that allow plants to cope under saline conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antioxidants
- Enzymes
- Growth
- Ionic balance
- Osmolytes
- Oxidative stress
- Photosynthesis
- Photosynthetic pigment
- Salinity
- Salt
- Seed priming
1 Introduction
Salinity is one of the most common environmental problems that limits the productivity of plants, particularly in arid and semiarid areas (Ashraf and Harris 2004). Based on the Food and Agriculture Organization (FAO) Soils portal (http://www.fao.org/soils-portal/soil-management/), soil is considered to be saline soil when electrical conductivity (EC) is >0.7 dS m−1. Nevertheless salt-affected soils are characterized by a specific electrical conductivity (EC) of extracts of saturated soil-paste above 4 dS m−1 (temp at 25 °C) according to the USDA Agriculture Handbook (1954). More recently, Bresler et al. (1982) stated that soils are rated salt affected beginning at an EC level of 2 dS m−1, as some vegetable, fruit, and ornamental crops undergo from salinity within the EC of 2–4 dS m−1.
Salt-affected soil refers to soils where, with normal plant growth, salts interfere. Depending on salt quantity, salt type, sodium present, and soil alkalinity, salt-affected soils can be divided into saline, saline-sodic, and sodic. There will be different characteristics for each type of salt-affected soil, which will also determine how it can be managed. Based on the FAO Soils portal using the world’s FAO/UNESCO soil map (1970–1980), the FAO estimated the total global saline soil area was 397 million hectares and the total sodic soils 434 million ha. However, Nelson and Mareida (2001) estimated approx. 12 million ha of irrigated land may possibly have disappeared from production accordingly of salinization.
Salinity results in many unfavorable effects on the growth of plants due to low osmotic soil potential (osmotic stress), ion effects (salt stress), element imbalances, or a combination of these causes (Ashraf 2004). All these factors cause physiological and biochemical unfavorable effects on plant growth and development (Munns and James 2003). To evaluate plant tolerance to salinity stress, the plant’s growth or survival is measured as it integrates many physiological mechanisms that occur within the plant. For plants growing in the saline medium, osmotic balance is essential.
The main strategies for the use of salt-affected soils are use of reclaiming and protective measures to make salt-affected soils suitable for agriculture and utilize salt-affected soils by growing halophytes or salt-tolerant crops/cultivars. The latter approach has been called the “biological approach” (Ashraf 1994) and has significant potential to mitigate the worldwide soil salinity problem. This chapter discuss biological strategies that can increase crop salinity tolerance. Strategies employed to relieve the impacts of salt stress in plants include applying chemical, biological, and physical treatments to seeds, seedlings, or plants prior to exposure to salinity stress. These treatments, mostly used before seed sowing, are anticipated to activate physiological and molecular pathways that allow the seed to react more quickly and/or more strongly after exposure to an environmental stress factor.
Seed priming is a knowledgeable, rational, and low-cost method for fast emergence, increased seedling strength, and improved plant yields under adverse environments (Jisha et al. 2013; Paparella et al. 2015). It is a restricted hydration method that activates metabolic processes before radicular protrusion at the early stage of germination (Hussain et al. 2015).
Under various environmental stresses, different methods of seed priming were utilized that include hydropriming, osmopriming, Chemopriming, nutrient priming, and hormonal priming (Jisha et al. 2013; Paparella et al. 2015). Seed priming promotes germination throughout the process by soaking seeds in solutions with exogenous molecules, i.e., salicylic acid (SA) and acetylsalicylic acid (ASA) (Khan et al. 2009a, b), or metals (Mirshekari et al. 2012), or hormones (Nakaune et al. 2012). Seed priming was proposed to trigger a sequence of physiological developments which increase plant performance grown in salt stress, including the introduction of antioxidant systems (Varier et al. 2010; Eisvand et al. 2010).
The purpose of this chapter is to present a wide overview of plant response under salt stress and the primary treatments in specific organic and inorganic substances and seed priming utilized to seeds/plants with a critical debate of the key accomplishments outlined in this area.
2 Plant Response to Salt Stress
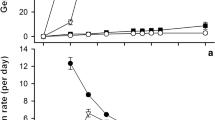
A soil is considered saline once the soil solution’s electrical conductivity (EC) is approximately 4 dS m−1 (corresponding to 40 mM NaCl), creating an osmotic pressure of approximately 0.2 MPa, which reduces most crop yields (Munns and Tester 2008). Salt stress induces ion toxicity, osmotic stress, nutrient deficiency, and oxidative stress on crops, thus restricting the absorption of soil water and eventually decreasing yield output (Awad et al. 2012; Hellal et al. 2012; Abdelhamid et al. 2013b; Rady et al. 2015, 2016b). Figure 1 shows response of soybean crop grown in soils with three salinity intensities, i.e., 1.12, 6.25, and 9.38 dS m−1, at Fayoum Governorate, Egypt.
Plant species vary for salt tolerance (Blaylock 1994; Bargaz et al. 2016). Salt-tolerant crops (plants less affected by salinity) can adjust the osmotic impacts of elevated levels of salt internally as compared to salt-sensitive crops. Also, salt-tolerant crops can absorb salt soil water more easily. On the other side, salt-sensitive crops have restricted ability for adjustment and are injured at comparatively small levels of salt. In reaction, in sensitive crop cultivars, reductions in yields caused by salinity look more widespread than in tolerant crop cultivars. Several authors report such a reduction in salinity yield (Blaylock 1994; Abdelhamid et al. 2013a; Dawood et al. 2016).
Global studies have shown that salinity affects germination and growth of seedlings in different crops, such as Bagayoko (2012). The effect of salt stress on plant can be attributed to the adverse effects of salinity on the cell cycle and differentiation. A general reduction in plant biomass was observed in all plant tissues under salt stress, but it is particularly noticeable in the aerial part (Abdelhamid et al. 2010). Different authors have associated a decrease in plant biomass with a decrease in leaf numbers or leaf abscissions (Dawood et al. 2014b) or a decrease in net assimilation rate and relative growth rate (Rady et al. 2015).
Salinity has several unfavorable effects on plant growth owing to low soil osmotic stress, ionic imbalances, or combined effects of these factors (Ashraf 2004). All these variables affect plant growth and development with adverse physiological and biochemical impacts (Munns and James 2003). The plant’s development or survival is evaluated by integrating many physiological processes that happen within the plant to assess the salinity stress tolerance of the plant.
The stress of salinity in the soil significantly reduces nutrient concentrations such as N, K, Ca, and Mg (Sadak et al. 2015; Talaat et al. 2015; Rady et al. 2016b). In addition, salinity reduces plant P uptake owing to precipitation of phosphate ions with Ca ions (Bano and Fatima 2009; Abdelhamid et al. 2010; Bargaz et al. 2016). Some elements have particular toxic effects on plants, such as sodium, chlorine, and boron. Excessive accumulation of Na+ in cell walls can rapidly trigger osmotic stress and cell death (Munns 2002). Furthermore, salinity has damaging impacts on photosynthesis, primarily due to decreased leaf area, chlorophyll content, and stomatal conductance, and to some degree owing to decreased effectiveness of photosystem II (Netondo et al. 2004).
There is no doubt that there has been noteworthy recognition of osmotic adjustment as a significant and efficient mechanism for resisting salinity in plants. Under saline environment, a positive growth relationship with osmotic adjustment was found, primarily because of building up more K+ and glycinebetaine, while a reduction of sugars (Ochiai and Matoh 2001). Correspondingly, Orabi and Abdelhamid (2016) found that salt-tolerant faba bean cultivar “Giza 843” was correlated with a superior capacity of osmotic adjustment by building up proline and ions, e.g., P, K+, Ca2+, and Mg2+, compared to the salt-sensitive cultivar “Giza 3.” Moreover, the salt-tolerant Phaseolus vulgaris genotype “RIL115” has been found to have superior osmotic adjustment by building up more soluble sugar, proline, amino acid, and ions, e.g., P, K+, Ca2+, Mg2+, and Mn2+, compared to the salt-sensitive genotype “RIL147” (Bargaz et al. 2016).
The accumulation of soluble carbohydrates in crops has been commonly recorded as a reaction to salinity (Murakeozy et al. 2003; Dawood et al. 2016). Amino acids were reported to accumulate under salinity stress in higher plants (vascular plants) (Mansour 2000). Di Martino et al. (2003) reported that building up amino acids and glycinebetaine caused osmoregulation, considered one of the predominant methods used by spinach crops to withstand salt stress. Several authors reported that in salt-stressed plants, proline accumulates more than other amino acids (Ashraf 1994; Bekheta et al. 2009; Abdelhamid et al. 2013a; Rady et al. 2016b).
Antioxidant metabolism, including antioxidant enzymes and nonenzymatic compounds, plays a critical role in salinity-induced detoxification of reactive oxygen species (ROS). Salinity tolerance is strongly associated with antioxidant enzyme activity, i.e., catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), and ascorbate peroxidase (APX), and nonenzymatic antioxidant, i.e., glutathione reductase (GR) (Gupta et al. 2005; Orabi and Abdelhamid 2016). Moreover, Orabi and Abdelhamid (2016) reported that faba bean plant is protected against the damaging impact of salinity primarily by enhancing the activity of enzymes, e.g., superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and phenylalanine acid lyase (PAL) enzymes.
It is well established in the literature that salinity causes severe decrease in plant yield. Several studies have already determined the fact that crops are adversely affected by this phenomenon, from planting to harvesting and even in storage. Salinity not only adversely affects seed yield and yield components in many plant species such as lupine (Dawood et al. 2016), common bean (Rady et al. 2016a), wheat (Ouda et al. 2015; Rady et al. 2016a), and faba bean (Orabi and Abdelhamid 2016) but also causes a nutritional imbalance in seed quality in terms of decreasing oil percentage, carbohydrates, and concentrations of 17 amino acids in lupine seeds (Dawood et al. 2016) and decreasing protein content in leaves and oat grains, with increasing level of salt in irrigation water (Kumar et al. 2010). In contrast, salinity raised carbohydrate and protein in seed yield of two faba bean cultivars (Orabi and Abdelhamid 2016) and increased protein, phenolic concentration, and lupine seed alkaloids (Dawood et al. 2016).
3 Techniques Used to Overcome Salt Stress in Plants
Salinity can be limited by salt leaching from the root rhizosphere, altering practices of farm management, and using salt-tolerant plants. Using new irrigation approaches such as deficit irrigation and using irrigation systems such as drip and sprinkler irrigation to optimize water use efficiently can sustain irrigation practices. Farming systems could also be modified to include better crop rotation in order to integrate perennials in crop rotation with annual crops and legumes within rotations and to adopt a precise agricultural approach. While using these tactics to sustainable agriculture that can alleviate the reduction of yields under the stress of salinity, the application is often limited due to the cost and availability of good-quality water or lack of water resources. Hence, developing an efficient, low-cost, easily adaptable technique to enhance plant performance under salt stress is a challenge. Globally, comprehensive study has been carried out to create strategies to deal with the stress of salinity, for example, the development of salt-tolerant varieties. Using organic and inorganic compounds, nutrients, and seed priming is one of the promising strategies adopted to alleviate the unfavorable outcomes of salinity and improve plant yields and yield quality.
3.1 Use of Osmoprotectants, Plant Growth Regulators (PGRs), Vitamins, and Nutrients
Much attention has been given to the approach of using osmolytes, osmoprotectants, plant growth regulators, and plant nutrient supply to overcome plant salt stress and boost crop production under saline conditions. Osmoprotectants or compatible solutes are greatly soluble compounds of low molecular weight, which can accumulate in cells without impairing cellular function at high levels. They safeguard crops against stress by contributing to cellular osmotic adjustment, detoxifying reactive oxygen species (ROS), protecting the structure of membranes, and stabilizing proteins (Ashraf and Foolad 2007). Exogenous use of glycinebetaine (GB) in perennial ryegrass significantly reduces salt stress negative impacts. The suggestion that GB increased salt tolerance in perennial ryegrass was primarily associated with increased SOD, CAT, and APX activity and cell membrane damage alleviation by reducing membrane lipid oxidation and improving ion homeostasis under salt stress (Alasvandyari et al. 2017). Exogenous proline applications alleviated oxidative stress and increased plant growth. Abdelhamid et al. (2013a) reported that proline increased the activity of antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), as well as carotenoid, ascorbic and endogenous proline, and P and K+ and K+:Na+ concentrations in salt-affected common bean plants. In addition, proline provided osmoprotection and facilitated the growth of salt-stressed faba bean plants (Dawood et al. 2014b). In order to improve their salt tolerance, a number of natural or synthetic PGRs were exogenously applied to crops. A foliar spray of indole-3-acetic acid at 150 ppm, for example, gave the highest plant growth values (flag leaf area and SPAD values), yield (grain and straw yield), and chemical composition (total crude protein, P and K percent) of wheat grown under salt stress conditions (El-Metwally et al. 2015). Khodary (2004) reported a significant increase in growth and development of maize under saline conditions due to applied 0.1 mM of salicylic acid to maize plants. A number of other organic compounds were also exogenously applied to test their effect on the plant’s response to saline conditions. In faba bean, for example, Semida et al. (2014) suggested that antioxidant α-tocopherol could activate antioxidants in plants and decrease oxidative damage resulting in physiological modifications in plants grown in saline soil. Furthermore, Dawood et al. (2016) concluded that the damaging impacts of salt stress on lupine plant growth and seed yield nutritional value were to some extent alleviated, mainly at 200 ppm α-tocopherol. In soybean, Rady et al. (2015) reported that 100 mg L−1 α-tocopherol exogenous application increased net assimilation rate, relative growth rate, chlorophyll, proline, free amino acids, soluble sugars, N, P, K+, and K+:Na+ ratios in soybean plants under salt stress. Exogenous use of 200 or 400 mg l−1 nicotinamide (vitamin B3) increased the nutrient concentration, sucrose, soluble sugars, free amino acids, lipid peroxidation, oxidative enzymes, photosynthetic pigments, plant growth, and seed yield and improved the quality of the seed yield, i.e., carbohydrates and protein of faba bean salt-stressed plants (Abdelhamid et al. 2013b).
Amino acids are well-known biostimulants that affect plant growth and yield positively, thus mitigating abiotic stress injuries significantly. For example, amino acid mixture on faba bean has been reported to have increased shoot length, leaf number per plant, fresh and dry shoot weight, photosynthetic pigments, total carbohydrates, polysaccharides, DNA, and nucleic acid RNA (Sadak et al. 2015). In addition, amino acid exogenous foliar application at rate of 500 or 1000 or 1500 mg L−1 enhanced salt tolerance of faba bean plants by increasing osmotic solutes, phenolic content, IAA, and endogenous polyamine content, while it reduced antioxidant enzymes and lipid peroxidation (Sadak and Abdelhamid 2015).
Salt-induced nutritional disorders can be alleviated by adding mineral nutrients to the growth medium, according to numerous reports. Adding N, for example, has improved growth and yield of sorghum crop (Esmaili et al. 2008). In addition, Rady et al. (2016b) recommended use of \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$3$}\right.{\mathrm{H}}_4{\mathrm{NO}}_3 \) at rate of 55 kg N ha−1 + cerealine (at rate of 4 kg ha−1 bio-fertilizer) + cattle manure (at rate of 10 t ha−1) in saline soils to improve wheat growth and nutritional yield quality with reduced heavy metal content. Also, in wheat, El-Lethy et al. (2013) reported important increases in photosynthetic pigments, antioxidant enzyme activity, soluble sugar, starch and total phenols, N, P, K+, and K+:Na+ ratio and wheat grain yield due to soil application of 150 mg K2O kg−1. It was also concluded that 150 mg K2O kg−1 soil reduced the unfavorable effects of NaCl salinity by enhancing photosynthetic pigments, antioxidant enzyme activity, osmoprotectant concentrations, and K+:Na+ ratio, which all were reflected in increasing the growth of common bean plants (Dawood et al. 2014a). Bargaz et al. (2016) found major increase in P, K+, Ca2+, and Mg2+ in leaves of both common bean recombinant inbred lines (RILs) and noteworthy increase in chlorophyll, carotenoids, soluble sugars, free amino acids and proline, and seed yields in all salinity intensities up to 60 kg ha−1 P. Like P, it is known that calcium (Ca) improves the unfavorable effects of salinity in plants, most probably by making possible superior selectivity of K+:Na+ ratios (Hasegawa et al. 2000). Foliar fertilization is generally a useful technique for supplementing plant-deficient nutrients when soil conditions restrict the availability of nutrients to roots (Mengel 2002). Under saline conditions, the absorption of K, Ca2+, and N through the roots and their supply to growing shooting regions is significantly impaired (Munns 2005), and thus the plant’s optimum concentration of essential nutrients is reduced (Marschner 1995). In this case, foliar fertilization can be used to promote mineral nutrition, suppress physiological disorders, and promote growth and yield of plant (Pervez et al. 2004). Efficiency of foliar applied nutrients depends on their mobility within the plant (Mengel 2002). Of all nutrients, N, K, and Mg are more phloem-mobile, whereas Ca and Fe are least phloem-mobile. Thus, foliar application of the latter two nutrients is not effective (Mengel 2002).
3.2 Seed Priming
Proof of seed priming benefits has been reported extensively. Seed priming is a water-based method that allows regulated seed rehydration to activate the generally activated metabolic classes during early germination but stops the transition of seed into complete germination. Priming is commonly used to treat vegetable seeds, mainly carrots, leek, onions, tomatoes, peppers, cucumbers, broccoli, lettuce, and okra (Dearman et al. 1987; Mahmoudi et al. 2012; Demirkaya 2014; Azooz et al. 2015; Hassini et al. 2017; Sarwar et al. 2017; Rinez et al. 2018), and field crop seeds, mostly wheat, canola lentil, alfalfa, bean, mung bean, common bean, safflower, barley, and maize (Jabeen and Ahmad 2013; Jamil et al. 2012; Al-Tawaha and Al-Ghzawi 2013; Azooz et al. 2013; Movaghatian and Khorsandi 2014; Younesi and Moradi 2014; Ali et al. 2017; Keshavarza and Moghadam 2017; Imran et al. 2018; Tabassum et al. 2018). Priming helps improve the quality of the product in flower seed industry that is usually utilized in best cultivars of Petunia (Momin 2013). Herbs, such as ajwain, dill, wild foxglove, rosemary, and scarlet sage, as well enjoy seed priming (Di Girolamo and Barbanti 2012; Mahdavi and Rahimi 2013; Ghassemi-Golezani and Nikpour-Rashidabad 2017; Masondo et al. 2018). Priming has also been used on Arabidopsis thaliana, which is the preferred model system for plant biology research (Jiménez-Arias et al. 2015). In seed conditioning in agriculture, several seed priming techniques have been successfully used to accelerate germination rates, improve seed uniformity, and improve yield and yield quality such as hydropriming, osmopriming, and halopriming (e.g., Jabeen and Ahmad 2013; Mahmoudi et al. 2012; Ouhibi et al. 2014; Maswada and Abd El-Kader 2016; Masondo et al. 2018; Panuccioa et al. 2018; Tabassum et al. 2018). Seed priming also promotes many plants to neutralize environmental stress negative impacts, such as salinity stress (see references in Table 1). A growing research has discussed and provided an outline of seed priming expertise, summarizing the currently available physical, chemical, and biological treatments (see recent reviews, e.g., Paparella et al. 2015; Ibrahim 2016; Mahmood et al. 2016). The following is a brief outline of seed priming methods:
3.2.1 Hydropriming
In hydropriming, seeds are soaked in water with or without aeration at optimum temperature (generally on or after 5–20 °C) and regularly for 6–24 h. This method is especially helpful in rural regions where crop production is damaged by unfavorable climatic environments, e.g., drought, and is required to enhance the efficiency of water use by decreasing chemical exposure (McDonald 2000). Hydropriming has long been known for its advantages, although it is now used less frequently than other methods. Harris (1992) suggested hydropriming as an inexpensive practice, explained for several crops in developing countries as “on-farm seed priming.”
3.2.2 Osmopriming
Osmopriming is known as osmotic priming or osmotic conditioning as well. It is a common pre-sowing practice that uses low-water-potential osmotic solutions to help manage water uptake. Osmopriming’s main goal is to limit the oxidative damage caused by ROS by preventing water from entering. As a result, the osmotic agent’s water potential is a critical factor (Heydecker and Coolbear 1977; Taylor et al. 1998). In osmopriming, seeds are soaked in osmotic agent solutions for a certain time period.
This technique mainly utilizes sugars, polyethylene glycol (PEG), glycerol, sorbitol, or mannitol, followed by air drying pre-sowing.
3.2.3 Solid Matrix Priming
The priming of solid matrix is also known as “matriconditioning.” It has been developed as a replacement to avoid high osmopriming costs, which require big volumes of osmotic solution and costly temperature management and aeration systems (Paparella et al. 2015). There are a number of natural materials that were used for solid priming as matrices, e.g., charcoal, sawdust, coal, calcined kaolin, and vermiculite, and marketable materials, e.g., Agro-Lig®. Solid matrix priming is best performed in an air circulation sealed container while avoiding too much evaporation.
3.2.4 Biopriming
Biopriming is a method using a combination of useful or bioactive particles. Is it well acknowledged that the mixture of plants with particular fungi or bacteria yields very appropriate outcomes as these microorganisms are capable of creating endophytic connections with the plant, leading to enhanced plant development and manufacturing of phytohormones and enhanced biotic/abiotic stress resistance (Waller et al. 2005). Bacillus spp., Enterobacter spp., Pseudomonas spp., and Trichoderma spp. are the most frequently used biopriming species (Niranjan Raj et al. 2004). In addition, biopriming is accomplished by adding secondary metabolites to the priming mixture and phytohormones (Hamayun et al. 2010).
3.2.5 Chemopriming
By addition of conventional decontaminators like sodium hypochlorite (NaOCl), hydrochloric acid (HCl), natural substances, and agrochemicals (e.g., herbicides, insecticides, fungicides, fumigants) to the priming solution for microbial pollution prevention, Chemopriming is accomplished (Parera and Cantliffe 1990). Natural compounds with a wide range of antimicrobial activity, including organic acids, essential oils, crude extracts of plants, and milk products, are mainly used for seed disinfection in organic farming (Van der Wolf et al. 2008). Additional environmentally acceptable means such as those made from insecticidal and fungicidal microencapsulated plant extracts are being tested (Gaidau et al. 2014).
3.2.6 Thermopriming
Thermopriming is a technique that seeds subject to different temperatures before sowing. This practice has been widely demonstrated to enhance germination effectiveness under negative environmental circumstances, decreasing seed germination thermo-inhibition (Huang et al. 2002). Thermopriming is accomplished by pre-sowing seed at different temperatures.
4 Mechanisms Induced by Seed Priming to Increase Plant Tolerance to Salt Stress
Seed priming has been shown to increase germination percentage, shoot and root length, shoot to root ratio, shoot to root weight, germination percentage, germination time, radicular length, radicular surface area, average radicle and radicle volume diameter, germination stress tolerance, seedling vigor, shoot and root length stress, fresh and root weight, and germination speed (Patade et al. 2009; Bajehbaj 2010; Movaghatian and Khorsandi 2014; Ghezal et al. 2016; Al-Tabbal 2017; Nimac et al. 2018); increased photosynthetic pigments in terms of chlorophyll, carotenoids, and chlorophyll fluorescence (Anwar et al. 2011; Demirkaya 2014; Azooz et al. 2015; Ghezal et al. 2016; Jisha and Puthur 2016; Abdel Latef et al. 2017; Panuccioa et al. 2018; Tabassum et al. 2018); increased plant water content (relative water content, osmotic and water potential, and root hydraulic conductivity) (Azooz 2009; Jamil et al. 2012; Ouhibi et al. 2014; Hassini et al. 2017; Tabassum et al. 2017; Yang et al. 2017; Jiménez-Arias et al. 2019); improved leaf photosynthetic rate and stomatal conductance (Iqbal and Ashraf 2013; Jisha and Puthur 2016; Sarwar et al. 2017; Yang et al. 2017; Panuccioa et al. 2018; Jiménez-Arias et al. 2019); increased osmoprotectants (soluble sugars, soluble proteins, and proline) (Oliveira et al. 2011; Jabeen and Ahmad 2013; Azooz et al. 2015; Abdel Latef et al. 2017; Ghassemi-Golezani and Nikpour-Rashidabad 2017); increased enzymatic antioxidant activity, i.e., catalase (CAT), peroxidase (POX), superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR) (Azooz 2009; Jabeen and Ahmad 2013; Oliveira et al. 2012; Abdel Latef et al. 2017; Alasvandyari et al. 2017; Mekawy et al. 2018); increased nutrient uptake, i.e., K+, Ca2+, and Mg2+ (Afzal et al. 2008; Iqbal and Ashraf 2010; Anwar et al. 2011; Demirkaya 2014; Azooz et al. 2015); increase plant growth (relative growth rate, unit leaf rate, leaf area ratio, specific leaf area) (Maswada and Abd El-Kader 2016); enhanced organic solutes, i.e., soluble sugar, soluble protein, free amino acids, proline, and betaine (Anwar et al. 2011; Oliveira et al. 2011; Azooz et al. 2015; Jiménez-Arias et al. 2015; Ghezal et al. 2016; Jisha and Puthur 2016; Alasvandyari et al. 2017; Ghassemi-Golezani and Nikpour-Rashidabad 2017; Sarwar et al. 2017; Keshavarza and Moghadam 2017; Tabassum et al. 2017); reduced leakage of electrolytes and H2O2 content and MDA content (Jisha and Puthur 2014; Alasvandyari et al. 2017; Mekawy et al. 2018; Rinez et al. 2018; Tabassum et al. 2018); and consequently increased plant yield (biomass, grain yield, yield attributes) (Iqbal et al. 2006; Iqbal and Ashraf 2010, 2013; Mohammadi et al. 2012; Ghezal et al. 2016) and accordingly is capable to boost plant performance and tolerance under salinity stress in several crops such as okra (Azooz et al. 2015), wheat (Iqbal and Ashraf 2010; Maswada and Abd El-Kader 2016), quinoa (Yang et al. 2017), and pepper (Rinez et al. 2018).
Table 1 shows the functions of seed priming in plant under salt stress. In Table 1, there are many plant species that were tested for seed priming under salt stress, namely, tomato (Solanum lycopersicum), sea fennel (Crithmum maritimum L.), quinoa (Chenopodium quinoa Willd.), chickpea (Cicer arietinum), pepper (Capsicum annuum L.), maize (Zea mays L.), rice (Oryza sativa L.), barley (Hordeum vulgare), wild foxglove (Ceratotheca triloba), sorghum (Sorghum bicolor), safflower (Carthamus tinctorius L.), sunflower (Helianthus annuus L.), lupine (Lupinus termis), dill (Anethum graveolens L.), cucumber (Cucumis sativus), broccoli sprouts (Brassica oleracea), common bean (Phaseolus vulgaris), pea (Pisum sativum L.), mung bean (Vigna radiata L.), okra (Hibiscus esculentus), Arabidopsis (Arabidopsis thaliana), faba bean (Vicia faba L.), alfalfa (Medicago sativa), lettuce (Lactuca sativa), lentil (Lens culinaris), Chinese cabbage (B. oleracea capitata), canola (Brassica napus), melon (Cucumis melo), sugarcane (Saccharum officinarum L.), ajwain (Carum copticum), and wheat (Triticum aestivum L.). These plant species were tested for seed priming techniques using many materials with different concentrations, namely, distilled water (dH2O), menadione sodium bisulfite (C11H9NaO5S), gibberellic acid (C19H22O6), saponin (C36H58O9), zinc sulfate (ZnSO4), salicylic acid (C7H6O3), apigenin (C15H10O5), validamycin A (C20H35NO13), phloroglucinol (C6H6O3), polyethylene glycol 6000 (PEG-6000) (HO(C2H4O)nH), glycine betaine (C5H11NO2), chitosan (C56H103N9O39), acetic acid (C2H4O2 or CH3COOH), potassium nitrate (KNO3), zinc oxide (ZnO nanoparticles), triacontanol (C30H62O), vitamin B12 (C63H88CoN14O14P), zinc sulfate (ZnSO4), β-amino butyric acid (C4H9NO2), jasmonic acid (C12H18O3), ascorbic acid (vitamin C) (C6H8O6 or HC6H7O6), nicotinamide (vitamin B3) (C6H6N2O), sodium nitroprusside (Na2[Fe(CN)5(NO)]), kinetin (C10H9N5O), indole acetic acid (C10H9NO2), indolebutyric acid (C12H13NO2), tryptophan (C11H12N2O2), calcium sulfate (CaSO4), calcium chloride (CaCl2), potassium chloride (KCl), sodium chloride (NaCl), putrescine (C4H12N2), spermidine (C7H19N3), spermine (C10H26N4), Zn-glutamine (C5H10N2O3), Zn-glycine (C4H8N2O4Zn), Zn-arginine (C6H14N4O2), and Zn-histidine (C6H9N3O2Zn), UV-C irradiation, Enterobacter spp. strain FD17, magnetic fields, aqueous extracts of Padina pavonica or Jania rubens or Typha angustifolia or Rosmarinus officinalis or Artemisia, commercial seaweed extract, and smoke-compound karrikinolide.
It has also been previously documented that plants are constructing many physiological and biochemical adaptations to adjust themselves under saline environments. With regard to using seed priming to alleviate the salt stress effects on plants, we proposed a schematic diagram describing the chain reaction following the seed priming (Fig. 2). The diagram illustrates the possible impact of the seed priming on different physiological processes, enhancing tolerance level and final yield.
4.1 Induced Salt Tolerance by Seed Priming Through Plant Physiological Response
Photosynthesis is the biochemical pathway through which plants transform solar energy into the chemical energy needed for growth. Under salt stress, reduced photosynthetic rates in plants are primarily due to reduced water potential and/or greater of Na+ and/or Cl− in chloroplasts that slow down chlorophyll (Iqbal and Ashraf 2007; Khayyat et al. 2014). Reducing the content of chlorophyll is common under salt stress and has been used in many reports as a sensitive indicator for cell metabolism (Chutipaijit et al. 2011). Reductions in chlorophyll a, chlorophyll b, carotenoids, and photosynthetic pigments have been recorded in faba bean following 44-day use of diluted seawater with 3.13 or 6.25 dS m−1, with reduced total pigments by 6.7% and 15.6%, respectively, using both seawater treatments compared to control crops (Dawood et al. 2014b). In another study in soybean plants that are exposed to diluted salinity of seawater, chlorophyll a, chlorophyll b, total carotenoids, and total concentrations of pigments gradually decreased with increased levels of salt stress (Rady et al. 2015). Membrane deterioration is considered one of several reasons which might affect negatively on the chlorophyll under stress (Mane et al. 2010). Moreover, increased chlorophyll a and chlorophyll b content was reported by Tabassum et al. (2018), which was correlated with increased cell membrane stability.
Photosystem II (PS II), when subjected to salt stress, is a fragile component of the photosynthetic structure (Allakhverdiev et al. 2000). The efficiency of PS II, the electron transportation chain (ETC), and the net rate of CO2 assimilation have been reported to be reduced under salt stress (Piotr and Grazyna 2005). Moreover, changes in Scenedesmus obliquus’ photosynthetic attributes resulting in reduced biomass accumulation were reported (Demetriou et al. 2007). There was no significant difference in salt tolerant-cultivar Vicia faba cv. Giza 843 a susceptible one Giza 4 under the saline condition in Fv/Fm (used to estimate PS II’s potential efficiency through darkly adapted measurements), while Giza 843 had a significantly higher PI (photosynthetic performance index) (Semida et al. 2014). Salt stress decreased the development of citrus by decreasing the net photosynthetic rate, stomatal behavior, PS II performance, and photosynthetic efficiency (Lòpez-Climent et al. 2008). It has been reported that salinity stress affects barley growth by altering chlorophyll fluorescence (PS II) and developing complex oxygen functions (Kalaji et al. 2011). In addition, salt stress decreased Brassica development by influencing PS II, electron transport rates, and D1 protein (Mittal et al. 2012). Moreover, under salt stress, there are other reasons that reduce net CO2 assimilation rates, such as cell membrane dehydration, which decreases carbon dioxide permeability, salt toxicity, increased senescence, variations in cytoplasmic structure-induced enzyme activity, and negative reaction to decreased sink activity (Iyengar and Reddy 1996).
As discussed above, a plant under salt stress faces a reduction in photosynthetic rates due to a decreasing in water potential accompanied/or not with too much concentration of Na+ and/or Cl− in the chloroplasts and chlorophyll. Therefore, any means that could increase plant water potential and exclude Na+ and Cl− may increase chlorophyll content and improve photosynthetic performance of PS II and consequently result in enhancing net CO2 photosynthetic rate and finally boost plant yield. In this regard, several authors reported that seed priming materials (e.g., saponin; dH2O; CaCl2; zinc nanoparticles (ZNPs); vitamin B12; NaCl; indole acetic acid; indolebutyric acid; tryptophan) increased photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 photosynthetic rate in several crops, e.g., quinoa, barley, lupine, common bean, mung bean, and wheat (Jisha and Puthur 2014; Abdel Latef et al. 2017; Keshavarza and Moghadam 2017; Yang et al. 2017; Tabassum et al. 2018). More details about the materials used for priming and their concentrations are shown in Table 1. A schematic diagram describing the chain reaction following the seed priming is shown in Fig. 2. The diagram illustrates the possible impact of the seed priming on different physiological processes, enhancing tolerance level and final yield.
It was reported that improved photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 assimilation rate by seed priming application (CaCl2; ZNPs; vitamin B12; aqueous leaf extracts of Typha angustifolia; β-amino butyric acid; NaCl; jasmonate sodium nitroprusside) were associated with decreased malondialdehyde and hydrogen peroxide in plants under salt stress (Jisha and Puthur 2014, 2016; Azooz et al. 2015; Ghezal et al. 2016; Maswada and Abd El-Kader 2016; Abdel Latef et al. 2017; Keshavarza and Moghadam 2017; Tabassum et al. 2018). Various studies (e.g., Sivritepe et al. 2005; Iqbal and Ashraf 2010; Azooz et al. 2013, 2015; Maswada and Abd El-Kader 2016; Ghezal et al. 2016; Tabassum et al. 2018) reported that enhanced photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 photosynthetic rate by seed priming application (CaCl2; chilling at 3 °C; NaCl; sodium nitroprusside; diluted seawater with 9.00 dS m−1; ascorbic acid; nicotinamide; jasmonate; aqueous leaf extracts of Typha angustifolia) were associated with increased contents of nutrients (P, K+, Ca2+, Mg2+, Ca2+, Zn), raised Ca2+:Na+ and K+:Na+ ratios, and reduced Na+ and Cl− in plants under salt stress. Enhanced photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 photosynthetic rate by seed priming application (CaCl2; NaCl; ZNPs; jasmonate; ascorbic acid; nicotinamide; EC 9.00 dS m−1; sodium nitroprusside) were correlated with increased organic solute content (proline and glycinebetaine, free amino acids, soluble sugar, soluble protein) under salt stress (Anwar et al. 2011; Azooz et al. 2013, 2015; Jisha and Puthur 2014; Maswada and Abd El-Kader 2016; Abdel Latef et al. 2017; Tabassum et al. 2018). Increased photosynthetic pigment (chlorophyll a, chlorophyll b, carotenoids), photosystem II, and net CO2 photosynthetic rate by seed priming application (ZNPs; vitamin B12; aqueous leaf extracts of Typha angustifolia; jasmonate; KNO3; dH2O; gibberellic acid) were associated with increased enzymatic antioxidants (SOD, CAT, POD, and APX, POX) and nonenzymatic antioxidants (total phenols, flavonoids, ascorbic acid, total carotenoids) in salt-stressed plants (Mahmoudi et al. 2012; Azooz et al. 2015; Ghezal et al. 2016; Abdel Latef et al. 2017; Keshavarza and Moghadam 2017).
4.2 Induced Salt Tolerance by Seed Priming Through Ionic Balance
When plants are stressed by salt, the absorption of essential mineral elements is interrupted. The increase in concentration of Na+ in the root rhizosphere directly affects negatively on the absorption of many necessary elements, such as K+, while Na+ shows opposite correlation with K+ (Kohler et al. 2009). In most plant species, salt tolerance was correlated with only tiny quantities of Na+ and Cl− in the shoots (Greenway and Munns 1980), while Cl− under saline conditions is the most prevalent anion. Noble and Rogers (1992) proposed shoot Cl− levels as selection standard for salt tolerance. For instance, in four Vicia faba cultivars, Abdelhamid et al. (2010) recorded a partial ion exclusion mechanism, stating that partial toxic ion exclusion was suggested as the main selection parameter for faba bean salt tolerance. They reported that faba bean salt-tolerant cultivars (Giza 429, Giza 843, Misr 1) had higher N, P, K+, Ca2+, and Mg2+ than susceptible cultivars (Giza 3), which showed lesser K+, Ca2+, and Mg2+ while higher Na+ and Cl−. Ions’ uptake in plants can be exploited as a marker of salt tolerance since they are genetically controlled but also influenced by the environment (Chaubey and Senadhira 1994). The Vicia faba salt-tolerant cultivar Giza 429 has been confirmed by Semida et al. (2014) that it had higher levels of N, P, K+, and Ca2+, whereas susceptible salt cultivar Giza 40 had lower levels of N, P, K+, and Ca2+ but higher levels of Na+. Furthermore, under saline conditions, high Na+:K+ and Na+:Ca2+ ratios occur in the soil due to excessive amounts of exchangeable Na+. Thus, plants grown in such environments have elevated Na+ levels, while K+ and Ca2+ take-up is decreased. For the integrity and functioning of cell membranes, rational quantities of both K+ and Ca2+ are requisite (Wenxue et al. 2003). For several major metabolic processes in plant cells, optimal K+ is essential (Tomar and Agarwal 2013). A good amount of K+:Na+ and Ca2+:Na+ ratios are also considered beneficial in maintaining ion balance in plant leaves and demonstrating salt tolerance in plant (Abdelhamid et al. 2010; Semida et al. 2014). Semida et al. (2014) confirmed that Vicia faba cv. Giza 429 salt-tolerant cultivar had higher K+:Na+ and Ca2+:Na+ ratio, while susceptible salt cultivar Giza 40 had lower ratios. In addition, Bargaz et al. (2016) reported that Phaseolus vulgaris recombinant inbred lines (RIL) RIL115, exhibited more salt tolerance with greater uptake of P, K+, Ca2+, Mg2+, and Mn2+ and higher uptake of K+:Na+ than susceptible salt tolerance of RIL147. However, contradictory results for different plant species were reported, where Ashraf et al. (1994) questioned ion exclusion mechanism validation. Moreover, salt-tolerant and salt-sensitive lines were found to vary a little in Na+ and Cl− shoot of the species Atylosia (Subbarao et al. 1990).
There are several reports examining how seed priming is affecting plant growth and mechanisms involved to help a plant to be more tolerant under salt stress condition. As discussed above that a plant under salt stress with greater elemental contents, i.e., K+ and Ca2+, and lower Na+ could be considered salt tolerant. In this regard, several authors reported that seed priming (namely, NaCl, CaCl2, gibberellic acid, PEG-6000, SA, glycinebetaine, ZnSO4, aqueous leaf extracts of Typha angustifolia, jasmonate, ascorbic acid, nicotinamide, UV-C irradiation, CaCl2, dH2O, seawater with 9.00 dS m−1, sodium nitroprusside, and CaSO4) improved K+, Ca2+, and the ratios of K+:Na+ and Ca2+:Na+, while it reduced Na+ in wheat (Triticum aestivum) (Afzal et al. 2008; Maswada and Abd El-Kader 2016; Tabassum et al. 2017), barley (Hordeum vulgare) (Anwar et al. 2011; Tabassum et al. 2018), rice (Oryza sativa L.) (Imran et al. 2016), sorghum (Sorghum bicolor) (Oliveira et al. 2011), dill (Anethum graveolens L.) (Ghassemi-Golezani and Nikpour-Rashidabad 2017), safflower (Carthamus tinctorius) (Alasvandyari et al. 2017), common bean (Phaseolus vulgaris) (Gulmezoglu et al. 2016), pea (Pisum sativum L.) (Ghezal et al. 2016), okra (Hibiscus esculentus) (Azooz et al. 2015), faba bean (Vicia faba L.) (Azooz et al. 2013), lettuce (Lactuca sativa) (Ouhibi et al. 2014), and melon (Cucumis melo) (Sivritepe et al. 2005). More details about the materials used for priming and their concentrations are shown in Table 1.
Seed priming has been shown to be capable of promoting plant growth under salinity stress through increased nutrient uptake of K+, Ca2+, and Mg2+ while lower uptake of Na+ and Cl− which could be considered as one of the seed priming approaches to mitigate salt stress (Afzal et al. 2008; Iqbal and Ashraf 2010; Anwar et al. 2011; Demirkaya 2014; Azooz et al. 2015). This could make seed priming useful to alleviate the plant’s salinity stress. Seed priming also participates with important function in regulating the ion transportation and membrane proteins, which manage plant ion homeostasis. For example, Yang et al. (2017) reported little Na+ and elevated K+ in quinoa plants grown under 400 mM NaCl stress primed with concentrations of 0.5, 2, 5, 10, 15, 25, and 35% saponin. In rice, 24-h seed priming with 10 ppm of apigenin reduced Na+ concentration in salt-stressed plants and helped maintain a superior K+:Na+ ratio in all plant parts compared to control plants, possibly by adjusting the appearance of confident central Na+ transporter encoding genes (Mekawy et al. 2018). Iqbal and Ashraf (2010) compared two salt-sensitive and salt-tolerant Triticum aestivum cultivars after priming their seeds with chilling at 3 °C for 2 weeks and distilled water hydropriming for 12 h at 22 ± 3 °C. They assigned the increased growth and yield to enhanced concentration of K+ and Ca2+ in the roots and decreased Na+ in wheat cultivars (Iqbal and Ashraf 2010). A schematic diagram describing the chain reaction following the seed priming is shown in Fig. 2. The diagram illustrates the possible impact of the seed priming on different physiological processes, enhancing tolerance level and final yield.
4.3 Induced Salt Tolerance by Seed Priming Through Osmolyte Organic Solutes
In order to combat stress, plant metabolism is altered in many respects by integrating compatible solute manufacturing to set proteins and cellular structures and/or retain cell turgor through osmotic adjustment and polymerization metabolism to eradicate ROS surpluses and resume the cellular redox equilibrium (Janska et al. 2010; Krasensky and Jonak 2012). Organic plant osmolytes are compounds of low molecular weight, methylated tertiary N and amino acids, and other metabolites of low molecular weight (Chen and Jiang 2010). Osmolytes have unique responses that can defend plant cells other than osmotically (Yancey 2005). Plant performance was also enhanced by the use of exogenous osmolytes under salt stress. A well-recognized adaptive mechanism in salt-stressed plants is the accumulation of osmolytes consisting of proline, glycine betaine, and sugar (Parida and Das 2005; Ashraf and Foolad 2007).
In many crop plants, e.g., safflower (Carthamus tinctorius), wheat (Triticum aestivum), sorghum (Sorghum bicolor), faba bean (Vicia faba), lupine (Lupinus termis), and common bean (Phaseolus vulgaris), and in reaction to salt stress, soluble sugars, free amino acids, proline, and glycine betaine build up (El-Lethy et al. 2013; Semida et al. 2014; Bargaz et al. 2016; Orabi and Abdelhamid 2016; Alasvandyari et al. 2017). The increment in accumulation of these organic osmolytes corresponded with a rise in the rate of salinity (Bargaz et al. 2016). Tolerant genotypes accumulated in some species more proline than susceptible one in reaction to salt stress, e.g., common bean salt-tolerant genotype RIL115 accumulated significant higher proline than the salt-susceptible RIL147 genotype (Bargaz et al. 2016), and in faba bean, the salt-tolerant cultivar Giza 843 had higher proline more than susceptible salt tolerance Giza 3 cultivar (Orabi and Abdelhamid 2016), while in wheat, salt-tolerant cultivar Sakha 93 accumulated significantly more soluble sugars than the salt-susceptible Gemiza 9 cultivar (El-Lethy et al. 2013). In addition, genotype 115 salt-tolerant faba beans appear to be associated with its capacity to accumulate more osmotic solutes than susceptible one (Azooz 2009). Proline therefore prevents crops from salt stress injury by keeping osmoregulation. Furthermore, the roles of proline as an osmolyte or osmoprotective compatible and its antioxidant characteristics to decrease ROS have become a strong plant safeguard against negative abiotic stress, including salt stress (Matysik et al. 2002).
Therefore, as discussed above, a plant under salt stress with greater compatible solute, e.g., glycine betaine, amino acids, soluble sugars, and proline, could be considered salt tolerant. In this regard, several authors reported that seed priming techniques with different materials, e.g., dH2O, CaCl2, PEG-6000, glycinebetaine, ZnO nanoparticles, gibberellic acid, triacontanol, vitamin B12, aqueous leaf extracts of Typha angustifolia, β-amino butyric acid, NaCl, jasmonate, menadione sodium bisulfite, soluble sugars, salicylic acid, glycine betaine, amino acids, and proline, in many crops i.e., in barley (Anwar et al. 2011), sorghum (Oliveira et al. 2011), safflower (Alasvandyari et al. 2017), lupine (Ghassemi-Golezani and Nikpour-Rashidabad 2017), cucumber (Sarwar et al. 2017), common bean (Keshavarza and Moghadam 2017), pea (Ghezal et al. 2016), mung bean (Jisha and Puthur 2016), okra (Azooz et al. 2015), Arabidopsis (Jiménez-Arias et al. 2015), and wheat (Tabassum et al. 2017). More details about the materials used for priming and their concentrations are shown in Table 1.
On the other hand, exogenous application of compatible solute might not only raise their levels but also protects components of cells, thus raising cellular osmotic pressure. Exogenous amino acids enhanced free amino acids, proline, and soluble sugars in salt-stressed faba bean (Sadak and Abdelhamid 2015). Furthermore, exogenous 5.0 mM proline applications increased proline in common bean plants in saline soil (Abdelhamid et al. 2013a). Exogenous use of glycinebetaine has been reported to improve CO2 assimilation, decrease stomatal conductance, and improve the efficiency of PS II in maize salt stress plants (Yang et al. 2005). Glycinebetaine functions an important job in protecting plant against salt stress (Ashraf and Foolad 2007) through protein and RuBisCO stabilization (Mäkelä et al. 2000), photosynthetic device protection (Cha-Um and Kirdmanee 2010), osmotic adjustment (Gadallah 1999), and ROS reduction (Ashraf and Foolad 2007). A schematic diagram describing the chain reaction following the seed priming is shown in Fig. 2. The diagram illustrates the effects of seed priming on different physiological processes, enhancing tolerance level and final yield.
Seed priming in 0, 10, 30, and 60 mM glycinebetaine increased salt tolerance in safflower, mainly linked with enhanced CAT and SOD enzyme activity, and protects cell membranes as a consequence of decreased lipid peroxidation and enhanced ion homeostasis (Alasvandyari et al. 2017). Tabassum et al. (2017) reported increased leaf proline and glycinebetaine, and this increase was positively correlated with increases in leaf area, water content, and grain yield while negatively associated with decreases in MDA and Na+ content when wheat primed with dH2O and CaCl2. The increased activity of POD and APX antioxidant enzymes with greater amount of osmoprotectants (soluble sugars, soluble proteins, and proline) resulted in protecting okra plants from oxidative injury induced by NaCl stress owing to 50 μM jasmonate seed priming (Azooz et al. 2015).
4.4 Induced Salt Tolerance by Seed Priming Through Antioxidant Defense Response
Salinity causes cell-level oxidative damage in plants. The oxidative burst findings since the molecule of oxygen (O2) occurs under stress circumstances as an electron acceptor, leading in the building up of reactive oxygen species (ROS) in subcellular compartments, especially in mitochondria and chloroplast. ROS contains superoxide radical (O2•−), singlet oxygen (1O2), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). These are oxidizing compounds that can cause injury to DNA, proteins, and lipids (Quiles and López 2004).
Most plant antioxidant studies show that plant production of antioxidants increases in response to salinity to counteract high levels of salt-induced ROS in cells. However, plants with elevated activities of antioxidants can scavenge/detoxify ROS and thus lead to higher salt tolerance (Wise and Naylor 1987; Garratt et al. 2002). Some of the antioxidant enzymes are directly engaged in salt-induced ROS detoxification, i.e., glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and ascorbate peroxidase (APX). Ascorbate, glutathione, and flavonoid are the main nonenzymatic antioxidants engaged in ROS detoxification by salinity (Van Oosten et al. 2013; Begara-Morales et al. 2014).
Significant differences in antioxidant expression for both enzymatic and nonenzymatic are apparent at interspecific or intraspecific concentrations in reaction to salt stress and may rely on germplasms, species, geographic distribution, climate variables, and seasons. For example, plants vary in antioxidant activity under salt environment, and this difference can be assigned to genotypic diversity and the degree of stomata closure that alters the CO2 fixation rate and avoids photoinhibition (Munns and Tester 2008). While determining if guaiacol peroxidase (POX), the SOD and CAT operate an important function in protecting and recovering cowpea from oxidative salt stress (Cavalcanti et al. 2004). It was reported, however, that the principal antioxidant mechanism, which includes CAT, SOD, and POX, in cowpea mature leaves did not interfere with salt-stressed plants’ survival (Cavalcanti et al. 2004).
Many studies confirm the positive association between elevated antioxidant activity and the salt tolerance grade in diverse plant species, even though in some other instances, this is not always right. For instance, the activity of PPO, POX, SOD, CAT, and APX antioxidant enzymes improved with higher concentrations of faba bean salt-stressed plants (Sadak and Abdelhamid 2015). Orabi and Abdelhamid (2016) recorded enhanced activity of antioxidant enzymes POX, CAT, SOD, and PAL in two faba bean cultivars under salt stress, and the greater activity was observed in Giza 843 salt-tolerant cultivar compared to Giza 3 susceptible one.
As discussed above that a plant under salt stress with greater antioxidants, both enzymatic and nonenzymatic could be considered a tolerant one. In this regard, several authors reported that seed priming techniques (e.g., vitamin B12; glycinebetaine; jasmonate; sodium nitroprusside; KCl; CaCl2; jasmonate; glycine betaine; ZNPs; chilling at 3 °C) increased antioxidants in many crops, e.g., wheat, broad bean, lupine, and safflower (Azooz 2009; Islam et al. 2015; Abdel Latef et al. 2017; Alasvandyari et al. 2017). More details about the materials used for priming and their concentrations are shown in Table 1. A schematic diagram describing the chain reaction following the seed priming is shown in Fig. 2. The diagram illustrates the effects of seed priming on different physiological functions, enhancing tolerance level and increased final yield.
The PEG-6000 solution used for seed priming with an osmotic potential of −0.86 Mpa increased CAT and POX activity, and these enzymes can protect sorghum seedlings from oxidative damage in nutrient solutions due to accelerated seed aging and salinity (Oliveira et al. 2012). In latest research by Mekawy et al. (2018), when rice seeds were submerged in a 10 ppm apigenin solution, apigenin pretreatment was connected with the induction of the antioxidant rice protection mechanism by increasing antioxidant CAT and APX activity in the roots, in addition to enhanced accumulation of nonenzymatic antioxidant carotenoids and flavonoids in the shoots, so apigenin pretreatment may reduce the damaging impacts of salinity on rice seedlings by probably triggering induction of antioxidant defense mechanism. Furthermore, Alasvandyari et al. (2017) found that priming with 60 mM glycinebetaine increased salt tolerance in the safflower, mainly owing to enhanced activity of CAT and SOD and decreased cell membrane damage due to decreased lipid peroxidation and enhanced ion homeostasis. Seed priming with ZNPs generally stimulate stressed plant growth, accompanied by increased ascorbic acid and Zn, plus enhanced activity of SOD, CAT, POD, and APX enzymes. Priming with ZNPs in stressed crops led to a decrease in MDA and Na+ content. These results therefore indicate that seed priming with ZNPs, especially 60 mg L−1 ZnO, is an effective method that can be utilized to enhance salt tolerance of lupine plants (Abdel Latef et al. 2017). Improved SOD, APX, and POX activity through seed priming (i.e., vitamin B12; glycine betaine; jasmonate; sodium nitroprusside; KCl; CaCl2) reduced levels of H2O2 and MDA in plants under salt stress (Azooz et al. 2015; Islam et al. 2015; Ali et al. 2017; Alasvandyari et al. 2017; Keshavarza and Moghadam 2017). Seed priming with jasmonate, glycinebetaine, ZNPs, and 3 °C chilling increased activity of SOD, APX, and POX which was associated with increased nutrient content of K+, Ca2+, Mg2+, Ca2+, and Zn in salt-stressed plants (Iqbal and Ashraf 2010; Azooz et al. 2015; Abdel Latef et al. 2017; Alasvandyari et al. 2017). Enhanced SOD, APX, and POX activities were associated with increased organic solutes of proline, free amino acids, soluble sugar, and soluble protein, in salt-stressed plants by seed priming application, i.e., vitamin B12, ZNPs, gibberellins, and salicylic acid (Abdel Latef et al. 2017; Ghassemi-Golezani and Nikpour-Rashidabad 2017; Keshavarza and Moghadam 2017). Moreover, Azooz (2009) reported that Vicia faba genotype 115 had a higher accumulation of CAT, POD, APX, and GR antioxidant enzyme activity than genotype 125, when grown in salt with 0 or 140 mM NaCl and seed priming with 0.2 mM salicylic acid; thus they concluded that the salt tolerance genotype 115 appears to be associated with increased antioxidant enzyme activity.
5 Concluding Remarks and Future Perspectives
In conclusion, salt stress is a condition shown in Fig. 1 where too many salts in the soil solution bring about plant growth inhibition or plant death. Salt stress presents a growing threat to plant farming. In order to regulate themselves under saline conditions, plants build up many physiological and biochemical adaptations. Seed priming is a technique that might advance seed performance under salt stress. Seed priming could build up various tolerance mechanisms in seeds against salt stress, for example, variation in photosynthetic pigment, osmotic adjustment, ionic balance, and ion exclusion and antioxidant protection system. A schematic diagram describing the chain reaction following the seed priming is shown in Fig. 2. The diagram illustrates the possible impact of the seed priming on different physiological processes, enhancing tolerance level and final yield. The up-to-date knowledge on seed priming management used in a variety of crops under saline condition is summarized in Table 1. The use of Chemopriming to boost plant tolerance against salt stress is highly promising. Because only limited useful information is available for a large number of plant species, further research is needed to study appropriate substances with precise concentration of priming agents that can guarantee successful seed germination and growth of plant seedling in saline environments. Future research should also focus on molecular, physiological, and metabolic changes caused by salt stress priming mediators. Moreover, further research in the directions proposed in this chapter would be a great extent support for establishment of this know-how in plant management under salt stress in the near future.
References
Abdel Latef AA, Abu Alhmad MF, Abdelfattah KE (2017) The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J Plant Growth Regul 36(1):60–70
Abdelhamid MT, Shokr M, Bekheta MA (2010) Growth, root characteristics, and leaf nutrients accumulation of four faba bean (Vicia faba L.) cultivars differing in their broomrape tolerance and the soil properties in relation to salinity. Commun Soil Sci Plant Anal 41(22):2713–2728
Abdelhamid MT, Rady M, Osman A, Abdalla M (2013a) Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. plants. J Hortic Sci Biotechnol 88:439–446
Abdelhamid MT, Sadak MSH, Schmidhalter U, El-Saady A (2013b) Interactive effects of salinity stress and nicotinamide on physiological and biochemical parameters of faba bean plant. Acta Biol Colomb 18:499–510
Afzal I, Rauf S, Basra SMA, Murtaza G (2008) Halopriming improve vigor, metabolism of reserves and ionic contents in wheat seedling under salt stress. Plant Soil Environ 54:382–388
Al-Tabbal JASM (2017) Germination and physiological traits to ascertain the ability of hormonal priming to improve salinity tolerance in Sorghum bicolor. J Agron 16:138–146
Al-Tawaha ARM, Al-Ghzawi ALA (2013) Effect of chitosan coating on seed germination and salt tolerance of lentil (Lens culinaris L.). Res Crops 14(2):489–491
Alasvandyari F, Mahdavi B, Hosseini SM (2017) Glycine betaine affects the antioxidant system and ion accumulation and reduces salinity-induced damage in safflower seedlings. Arch Biol Sci 69(1):139–147
Ali Q, Daud MK, Haider MZ, Ali S, Aslam N, Noman A, Iqbal N, Shahzad F, Rizwan M, Deeba F, Ali I, Jin ZS (2017) Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol Biochem 119:50–58
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N (2000) Inactivation of photosystems I and II in response to osmotic stress in Synechococcus. Contribution of water channels. Plant Physiol 122:1201–1208
Anwar S, Shafi M, Bakht J, Mohammad TJ, Yousaf H (2011) Effect of salinity and seed priming on growth and biochemical parameters of different barley genotypes. Afr J Biotechnol 10:15278–15286
Ashraf M (1994) Breeding for salinity tolerance in plants. Crit Rev Plant Sci 13:17–42
Ashraf M (2004) Some important physiological selection criteria fort salt tolerance in plants. Flora 199:361–376
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:207–216
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, Zafar ZU, Tufail M (1994) Intra-specific variation for salt tolerance in a potential oil-seed crop, brown mustard (Brassica juncea (L.) Czern & Coss.). Arch Agron Crop Sci 38:449–458
Awad N, Turky A, Abdelhamid M, Attia M (2012) Ameliorate of environmental salt stress on the growth of Zea mays L. plants by exopolysaccharides producing bacteria. J Appl Sci Res 8:2033–2044
Azooz MM (2009) Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int J Agric Biol 11:343–350
Azooz MM, Alzahrani AM, Yuoussef MM (2013) The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (Vicia faba L.). Aust J Crop Sci 7:291–2100
Azooz MM, Metwally A, Abou-Elhamd MF (2015) Jasmonate-induced tolerance of Hassawi okra seedlings to salinity in brackish water. Acta Physiol Plant 37:77
Bagayoko M (2012) Soil salinity alkalinity effects on germination and seedling growth of vegetable crops in the Office du Niger zone. J Res Environ Sci Toxicol 1(12):328–337
Bajehbaj AA (2010) The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. Afr J Biotechnol 9:1764–1770
Bano A, Fatima M (2009) Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol Fertil Soils 45:405–413
Bargaz A, Nassar RMA, Rady MM, Gaballah MS, Thompson SM, Brestic M, Schmidhalter U, Abdelhamid MT (2016) Improved salinity tolerance by phosphorus fertilizer in two Phaseolus vulgaris recombinant inbred lines contrasting in their phosphorus deficiency sensitivity. J Agron Crop Sci 202:497–507
Begara-Morales JC, Sanchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, López-Jaramillo J, Padilla MN, Carreras A, Corpas FJ, Barroso JB (2014) Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J Exp Bot 65:527–538
Bekheta MA, Abdelhamid MT, El-Morsi AA (2009) Physiological response of Vicia faba to prohexadione–calcium under saline conditions. Planta Daninha 27:769–779
Blaylock AD (1994) Soil salinity, salt tolerance, and growth potential of horticultural and landscape plants. Cooperative Extension Service, University of Wyoming, Department of Plant, Soil, and Insect Sciences, College of Agriculture, Laramie
Bresler E, McNeal BL, Carter DL (1982) Saline and sodics soils. Advanced series in agricultural series, 1st edn. Springer, Berlin, 236p
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571
Cha-um S, Kirdmanee C (2010) Effect of glycinebetaine on proline, water use, and photosynthetic efficiencies, and growth of rice seedlings under salt stress. Turk J Agric For 34:517–527
Chaubey CN, Senadhira D (1994) Conventional plant breeding for tolerance to problem soils. In: Yeo AR, Flowers TJ (eds) Soil mineral stresses, approaches to crop improvement. Springer, Berlin, pp 37–60
Chen H, Jiang JG (2010) Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ Rev 18:309–319
Chutipaijit S, Cha-um S, Sompornpailin K (2011) High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. indica. Aust J Crop Sci 5:1191–1198
Dawood MG, Abdelhamid MT, Schmidhalter U (2014a) Potassium fertiliser enhances the salt-tolerance of common bean (Phaseolus vulgaris L.). J Hortic Sci Biotechnol 89:185–192
Dawood MG, Taie HAA, Nassar RMA, Abdelhamid MT, Schmidhalter U (2014b) The changes induced in the physiological, biochemical and anatomical structure of Vicia faba by the exogenous application of proline under seawater stress. S Afr J Bot 93:54–63
Dawood MG, El-Metwally IM, Abdelhamid MT (2016) Physiological response of lupine and associated weeds grown at salt-affected soil to α-tocopherol and hoeing treatments. Gesunde Pflanzen 68:117–127
Dearman J, Brocklehust PA, Drew RLK (1987) Effect of osmotic priming and aging on the germination and emergence of carrot and leek seed. Ann Appl Biol 111:717–722
Demetriou G, Neonaki C, Navakoudis E, Kotzabasis K (2007) Salt stress impact on the molecular structure and function of the photosynthetic apparatus – the protective role of polyamines. Biochim Biophys Acta 1767:272–280
Demirkaya M (2014) Improvement in tolerance to salt stress during tomato cultivation. Turk J Biol 38:1–7
Di Girolamo G, Barbanti L (2012) Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital J Agric 7:e25
Di Martino C, Delfine S, Pizzuto R, Loreto F, Fuggi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158(3):455–463
Eisvand HR, Tavakkol-Afshari R, Sharifzadeh F, Maddah Arefi H, Hesamzadeh Hejazi SM (2010) Effects of hormonal priming and drought stress on activity and isozyme profiles of antioxidant enzymes in deteriorated seed of tall wheatgrass (Agropyron elongatum host). Seed Sci Technol 38:280–297
El-Lethy SR, Abdelhamid MT, Reda F (2013) Effect of potassium application on wheat (Triticum aestivum L.) cultivars grown under salinity stress. World Appl Sci J 26:840–850
El-Metwally IM, Ali OAM, Abdelhamid MT (2015) Response of wheat (Triticum aestivum L.) and associated grassy weeds grown in salt-affected soil to effects of graminicides and indole acetic acid. Agriculture (Poľnohospodárstvo) 61(1):1–11
Esmaili E, Kapourchal SA, Malakouti MJ, Homaee M (2008) Interactive effect of salinity and two nitrogen fertilizers on growth and composition of sorghum. Plant Soil Environ 54:537–546
Gadallah MAA (1999) Effects of kinetin on growth, grain yield and some mineral elements in wheat plants growing under excess salinity and oxygen deficiency. Plant Growth Regul 27:63–74
Gaidau C, Niculescu M, Stepan E, Epure D-G, Gidea M (2014) New mixes based on collagen extracts with bioactive properties, for treatment of seeds in sustainable agriculture. Curr Pharm Biotechnol 14:1389–2010
Garratt LC, Janagoudar BS, Lowe KC, Anthony P, Power JB, Davey MR (2002) Salinity tolerance and antioxidant status in cotton cultures. Free Radic Biol Med 33:502–511
Ghassemi-Golezani K, Nikpour-Rashidabad N (2017) Seed pretreatment and salt tolerance of dill: Osmolyte accumulation, antioxidant enzymes activities and essence production. Biocatal Agric Biotechnol 12:30–35
Ghezal N, Rinez I, Sbai H, Saad I, Farooq M, Rinez A, Zribi I, Haouala R (2016) Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. S Afr J Bot 105:240–250
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:49–90
Gulmezoglu N, Aydogan C, Turhan E (2016) Physiological, biochemical and mineral dimensions of green bean genotypes depending on Zn priming and salinity. Legum Res 39(5):713–721
Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56:2601–2609
Hamayun M, Khan SA, Khan AL, Tang DS, Hussain J, Ahmad B, Anwar Y, Lee IJ (2010) Growth promotion of cucumber by pure cultures of gibberellin-producing Phoma sp. GAH7. World J Microbiol Biotechnol 26:889–894
Harris D (1992) Staying in control of rainfed crops. In: Proceedings of the first annual scientific conference of the SADCC/ODA land and water management programme, Gaborone, Botswana, pp 257–262
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Phys 51:463–499
Hassini I, Martinez-Ballesta MC, Boughanmi N, Moreno DA, Carvajal M (2017) Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci Hortic 226:141–151
Hathout TA, El-Khallal SM, Abdelgawad ZA, Said EM, Al Mokadem AZ (2014) Enhancing rice salt stress tolerance by priming with validamycin A. Int J Bot 10:1–12
Hellal FA, Abdelhameid MT, Abo-Basha DM, Zewainy RM (2012) Alleviation of the adverse effects of soil salinity stress by foliar application of silicon on faba bean (Vicia faba L.). J Appl Sci Res 8:4428–4433
Heydecker W, Coolbear P (1977) Seed treatments for improved performance-survey and attempted prognosis. Seed Sci Technol 5:353–425
Huang YM, Wang HH, Chen KH (2002) Application of seed priming treatments in spinach (Spinacia oleracea L.) production. J Chin Soc Hortic Sci 48:117–123
Hussain S, Zheng M, Khan F, Khaliq A, Fahad S, Peng S (2015) Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci Rep 5:8101
Ibrahim EA (2016) Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol 192:38–46
Imran QM, Kamran M, Rehman SU, Ghafoor A, Falak N, Kim KM et al (2016) GA mediated OsZAT-12 expression improves salt resistance of rice. Int J Agric Biol 18:330–336
Imran M, Boelt B, Muhling K-H (2018) Zinc seed priming improves salt resistance in maize. J Agro Crop Sci 204:390–399
Iqbal M, Ashraf M (2007) Seed preconditioning modulates growth, ionic relations, and photosynthetic capacity in adult plants of hexaploid wheat under salt stress. J Plant Nutr 30(3): 381–396. https://doi.org/10.1080/01904160601171330
Iqbal M, Ashraf M (2010) Changes in hormonal balance: a possible mechanism of pre-sowing chilling-induced salt tolerance in spring wheat. J Agron Crop Sci 196:440–454
Iqbal M, Ashraf M (2013) Salt tolerance and regulation of gas exchange and hormonal homeostasis by auxin-priming in wheat. Pesqui Agropecu Bras 48:1210–1219
Iqbal M, Ashraf M, Rehman S, Rha ES (2006) Does polyamine seed pretreatment modulate growth and levels of some plant growth regulators in hexaploid wheat (Triticum aestivum L.) plants under salt stress? Bot Stud 47:239–250
İşeri ÖD, Sahin FI, Haberal M (2014) Sodium chloride priming improves salinity response of tomato at seedling stage. J Plant Nutr 37:374–392
Islam F, Yasmeen T, Ali S, Ali B, Farooq MA, Gill RA (2015) Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiol Plant 37:1–12
Iyengar ERR, Reddy MP (1996) Photosynthesis in high salt-tolerant plants. In: Pesserkali M (ed) Hand book of photosynthesis. Marshal Dekar, Baten Rose, pp 56–65. 08247 97086
Jabeen N, Ahmad R (2013) The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J Sci Food Agric 93:1699–1705
Jamil M, Ashraf M, Rha E (2012) Alleviation of salt stress using gibberellic acid in Chinese cabbage. Acta Agron Hung 60:345–355
Janska A, Marsik P, Zelenkova S, Ovesna J (2010) Cold stress and acclimation: what is important for metabolic adjustment? Plant Biol 12:395–405
Jiménez-Arias D, Pérez JA, Luis JC, Martín-Rodríguez V, Valdés-González F, Borges AA (2015) Treating seeds in menadione sodium bisulphite primes salt tolerance in Arabidopsis by inducing an earlier plant adaptation. Environ Exp Bot 109:23–30
Jiménez-Arias D, García-Machado FJ, Morales-Sierra S, Suárez E, Pérez JA, Luis JC, Garrido-Orduña C, Herrera AJ, Valdés F, Sandalio LM, Borges AA (2019) Menadione sodium bisulphite (MSB): beyond seed-soaking. Root pretreatment with MSB primes salt stress tolerance in tomato plants. Environ Exp Bot 157:161–170
Jisha KC, Puthur JT (2014) Halopriming of seeds imparts tolerance to NaCl and PEG induced stress in Vigna radiata (L.) Wilczek varieties. Physiol Mol Biol Plants 20:303–312
Jisha KC, Puthur JT (2016) Seed priming with BABA (β-amino butyric acid): a cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 253(2):277–289
Jisha KC, Vijayakumari K, Puthur JT (2013) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35:1381–1396
Kalaji MH, Govindjee BK, Kościelniak J, Żuk-Gołaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72
Karimi S, Eshghi S, Karimi S, Hasan-nezhadian S (2017) Inducing salt tolerance in sweet corn by magnetic priming. Acta Agric Slove 109:89–102
Kazemi JS, Aboutalebian MA, Hamzei J, Meskarbashee M (2018) Mycorrhiza and seed priming effect to improve the balance of sodium and potassium and some changes in antioxidants in the leaves of maize (Zea mays L.) under soil salinity. J Agron 17:18–27
Keshavarza H, Moghadam RSG (2017) Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere 3:143–149
Khan HA, Ayub CM, Pervez MA, Bilal RM, Shahid MA, Ziaf K (2009a) Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ 28:81–87
Khan HA, Pervez MA, Ayub CM, Ziaf K, Bilal RM, Shahid MA, Akhtar N (2009b) Hormonal priming alleviates salt stress in hot pepper (Capsicum annuum L.). Soil Environ 28:130–135
Khayyat M, Tehranifar A, Davarynejad GH, Sayyari-Zahan MH (2014) Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’ and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica 52:301–312
Khodary SEA (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int J Agric Biol 6:5–8
Kohler J, Hernández JA, Caravaca F, Roldán A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ Exp Bot 65:245–252
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Kumar A, Agarwal S, Kumar P, Singh A (2010) Effects of salinity on leaf grain protein in some genotypes of oat (Avena sativa L.). Res Sci Technol 2:85–87
Lòpez-Climent MF, Arbona V, Pérez-Clemente RM, Gòmez-Cadenas A (2008) Relationship between salt tolerance and photosynthetic machinery performance in citrus. Environ Exp Bot 62:176–184
Mahdavi B, Rahimi A (2013) Seed priming with chitosan improves the germination and growth performance of ajowan (Carum copticum) under salt stress. Eur Asia J Biosci 7:69–76
Mahmood A, Turgay OC, Farooq M, Hayat R (2016) Seed biopriming with plant growth promoting rhizobacteria: a review. FEMS Microbiology Ecology 92:fiw112. https://doi.org/10.1093/femsec/fiw112
Mahmoudi H, Ben Massoud R, Baatour O, Tarchoune I, Ben Salah I, Nasri N, Abidi W, Kaddour R, Hannoufa A, Lachaal M, Ouerghi Z (2012) Influence of different seed priming methods for improving salt stress tolerance in lettuce plants. J Plant Nutr 35:1910–1922
Mäkelä P, Kärkkäinen J, Somersalo S (2000) Effect of glycine-betaine on chloroplast ultrastructure, chlorophyll and protein content, and RuBPCO activities in tomato grown under drought or salinity. Biol Plant 43:471–475
Mane AV, Karadge BA, Samant JS (2010) Salinity induced changes in photosynthetic pigments and polyphenols of Cymbopogon Nardus (L.) Rendle. J Chem Pharm Res 2:338–347
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Masondo NA, Kulkarni MG, Finnie JF, Van Staden J (2018) Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol Environ Saf 147:43–48
Maswada HF, Abd El-Kader NIK (2016) Redox halopriming: a promising strategy for inducing salt tolerance in bread wheat. J Agron Crop Sci 202:37–50
Matysik J, Bhalu AB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
McDonald MB (2000) Seed priming. In: Black M, Bewley JD (eds) Seed technology and its biological basis. Sheffield Academic Press, Sheffield, pp 287–325
Mekawy AMM, Abdelaziz MN, Ueda A (2018) Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol Biochem 2018(130):94–104
Mengel K (2002) Alternative or complementary role of foliar supply in mineral nutrition. Acta Hortic 594:3348
Mirshekari B, Baser S, Allahyari S, Hamendanlu N (2012) ‘On-farm’ seed priming with Zn plus Mn is an effective way to improve germination and yield of marigold. Afr J Microbiol Res 6:5796–5800
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Mohammadi SK, Shekari F, Fotovat R, Darudi A (2012) Effect of laser priming on canola yield and its components under salt stress. Int Agrophys 26:45–51
Momin KC (2013) Challenges of the flower seed industry. Indian J Appl Res 3:244–245
Movaghatian A, Khorsandi F (2014) Salicylic acid effects on germination of mungbean (Vigna radiata L.) under salinity stress. Adv Environ Biol 8:566–570
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253:201–218
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 2008(59):651–681
Murakeozy EP, Nagy Z, Duhaze C, Bouchereau A, Tuba Z (2003) Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J Plant Physiol 160:395–401
Nakaune M, Hanada A, Yin Y, Matsukura C, Yamaguchi S, Ezura H (2012) Molecular and physiological dissection of enhanced seed germination using short-term low-concentration salt seed priming in tomato. Plant Physiol Biochem 52:28–37
Nelson M, Mareida M (2001) Environmental impacts of the CGIAR: an assessment, in Doc. No. SDR/TAC:IAR/01/11 presented to the mid-term meeting, 21–25 May, Durban, South Africa
Netondo GW, Onyango JC, Beck E (2004) Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci 44:806–811
Nimac A, Lazarević B, Petek M, Vidak M, Šatović Z, Carović-Stanko K (2018) Effects of salinity and seed priming on germination of sea fennel (Crithmum maritimum L.). Agric Conspec Sci 83(2):181–185
Niranjan Raj S, Shetty NP, Shetty HS (2004) Seed bio-priming with Pseudomonas fluorescens isolates enhances growth of pearl millet plants and induces resistance against downy mildew. Int J Pest Manag 50:41–48
Noble CL, Rogers ME (1992) Arguments for the use of physiological criteria for improving the salt tolerance in crops. Plant Soil 146:99–107
Ochiai K, Matoh T (2001) Mechanism of salt tolerance in the grass species, Anneurolepidium chinense—I. Growth response to salinity and osmotic adjustment. Soil Sci Plant Nutr 47:579–585
Oliveira ABD, Alencar NLM, Prisco JT, Gomes-Filho E (2011) Accumulation of organic and inorganic solutes in NaCl-stressed sorghum seedlings from aged and primed seeds. Sci Agric 68(6):632–637
Oliveira AB, Gomes-Filho E, Enéas-Filho J, Prisco JT, Alencar NLMA (2012) Seed priming effects on growth, lipid peroxidation, and activity of ROS scavenging enzymes in NaCl-stressed sorghum seedlings from aged seeds. J Plant Interact 7:151–159
Orabi SA, Abdelhamid MT (2016) Protective role of α-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J Saudi Soc Agric Sci 15:145–154
Ouda S, Noreldin T, Mounzer O, Abdelhamid MT (2015) CropSyst model for wheat irrigation water management with fresh and poor quality water. J Water Land Dev 27:41–50
Ouhibi C, Attia H, Rebah F, Msilini N, Chebbi M, Aarrouf J, Urban L, Lachaal M (2014) Salt stress mitigation by seed priming with UV-C in lettuce plants: growth, antioxidant activity and phenolic compounds. Plant Physiol Biochnol 83:126–133
Panuccioa MR, Chaabanib S, Roulab R, Muscoloa A (2018) Bio-priming mitigates detrimental effects of salinity on maize improving antioxidant defense and preserving photosynthetic efficiency. Plant Physiol Biochem 132(2018):465–474
Paparella S, Arau JSS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A (2015) Seed priming: state of the art and new perspectives. Plant Cell Rep 34:1281–1293
Parera C, Cantliffe D (1990) Improved stand establishment of sh2 sweet corn by solid matrix priming. Proceedings of the national symposium on stand establishment horticultural crops, Minneapolis, MN, USA, pp 91–96
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Patade VY, Sujata B, Suprasanna P (2009) Halopriming imparts tolerance to salt and PEG induced drought stress in sugarcane. Agric Ecosyst Environ 134:24–28
Patanè C, Cavallaro V, Cosentino SL (2009) Germination and radicle growth in unprimed and primed seeds of sweet sorghum as affected by reduced water potential in NaCl at different temperatures. Ind Crop Prod 30:1–8
Pervez H, Ashraf M, Makhdum MI (2004) Influence of potassium rates and sources on seed cotton yield and yield components of some elite cotton cultivars. J Plant Nutr 27:1295–1317
Piotr S, Grazyna K (2005) Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 125:31–40
Quiles MJ, López NI (2004) Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant growth effects on the chloroplast NADH dehydrogenase complex. Plant Sci 166:815–823
Rady MM, Sadak MS, El-Lethy SR, Abdelhamid EM, Abdelhamid MT (2015) Exogenous α-tocopherol has a beneficial effect on Glycine max (L.) plants irrigated with diluted sea water. J Hortic Sci Biotechnol 90:195–202
Rady MM, Semida WM, Hemida KA, Abdelhamid MT (2016a) The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int J Recycl Org Waste Agric 5:311–321
Rady MM, Mounzer OH, Alarcón JJ, Abdelhamid MT, Howladar SM (2016b) Growth, heavy metal status and yield of salt-stressed wheat (Triticum aestivum L.) plants as affected by the integrated application of bio-, organic and inorganic nitrogen-fertilizers. J Appl Bot Food Qual 89:21–28
Rinez I, Ghezal N, Rinez A, Farooq M, Dbara S, Saad I, Haouala R (2018) Improving salt tolerance in pepper by bio-priming with Padina pavonica and Jania rubens aqueous extracts. Int J Agric Biol 20:513–523
Sadak MS, Abdelhamid MT (2015) Influence of amino acids mixture application on some biochemical aspects, antioxidant enzymes and endogenous polyamines of Vicia faba plant grown under seawater salinity stress. Gesunde Pflanzen 67:119–129
Sadak MS, Abdelhamid MT, Schmidhalter U (2015) Effect of foliar application of amino acids on plant yield and physiological parameters in bean plants irrigated with seawater. Acta Biolo Colomb 20:141–152
Sarwar N, Yousaf S, Jamil FF (2006) Induction of salt tolerance in chickpea by using simple and safe chemicals. Pak J Bot 38:325–329
Sarwar M, Amjad M, Ayyub CM (2017) Alleviation of salt stress in cucumber (Cucumis sativus L.) through seed priming with triacontanol. Int J Agric Biol 19:771–778
Semida WM, Taha RS, Abdelhamid MT, Rady MM (2014) Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S Afr J Bot 95:24–31
Sivritepe HO, Sivritepe N, Eris A, Turhan E (2005) The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci Hortic 106:568–581
Subbarao GV, Johansen C, Jana MK, Kuma Rao JVDK (1990) Physiological basis of difference in salinity tolerance of pigeon pea and its related wild species. J Plant Physiol 137:64–71
Tabassum T, Farooq M, Ahmad R, Zohaib A, Wahid A (2017) Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol Biochem 118:362–369
Tabassum T, Ahmad R, Farooq M, Basra SMA (2018) Improving salt tolerance in barley by Osmopriming and biopriming. Int J Agric Biol 20:2455–2464
Talaat NB, Ghoniem AE, Abdelhamid MT, Shawky BT (2015) Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul 75:281–295
Taylor AG, Allen PS, Bennet MA, Bradford KJ, Burris JS, Misra MK (1998) Seed enhancements. Seed Sci Res 8:245–256
Tomar NS, Agarwal RM (2013) Influence of treatment of Jatropha curcas L. leachates and potassium on growth and phytochemical constituents of wheat (Triticum aestivum L.). Am J Plant Sci 4:1134–1150
Tsegay BA, Andargie M (2018) Seed priming with gibberellic acid (GA3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. J Crop Sci Biotechnol 21(3):261–267
USDA Agriculture Handbook (1954) Diagnosis and improvement of saline and alkaline soils: USDA agriculture handbook. No. 60. United States Department of Agriculture, Washington, DC, 160pp
Van der Wolf JM, Birnbaum Y, van der Zouwen PS, Grrot SPC (2008) Disinfection of vegetable seed by treatment with essential oils, organic acids and plant extracts. Seed Sci Technol 36:76–88
Van Oosten MJ, Sharkhuu A, Batelli G et al (2013) The Arabidopsis thaliana mutant air implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol Biol 83:405–415
Varier A, Vari AK, Dadlani M (2010) The subcellular basis of seed priming. Curr Sci India 99:451–456
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Huckelhoven R, Neumann C, Von-Wettstein D (2005) The endophytic fungus piriformis indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A 102:13386–13391
Wenxue W, Bilsborrow PE, Hooley P, Fincham DA, Lombi E, Forster BP (2003) Salinity induced differences in growth, ion distribution and partitioning in barley between the cultivar Maythorpe and its derived mutant golden promise. Plant Soil 250:183–191
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and endogenous antioxidants. Plant Physiol 83:278–282
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138:2299–2309
Yang A, Akhtar SS, Iqbal S, Qi Z, Alandia G, Saddiq MS, Jacobsen S-E (2017) Saponin seed priming improves salt tolerance in quinoa. J Agron Crop Sci 2018(204):31–39
Younesi O, Moradi A (2014) Effect of priming of seeds of Medicago sativa ‘bami’ with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J Hortic Res 22:167–174
Acknowledgments
The first author, Dr. Magdi Abdelhamid, would like to express his gratitude to his colleagues at the Department of Botany, National Research Center, Giza, Egypt, for their important contribution to this study in some phases.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abdelhamid, M.T. et al. (2019). Mechanisms of Seed Priming Involved in Salt Stress Amelioration. In: Hasanuzzaman, M., Fotopoulos, V. (eds) Priming and Pretreatment of Seeds and Seedlings. Springer, Singapore. https://doi.org/10.1007/978-981-13-8625-1_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-8625-1_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8624-4
Online ISBN: 978-981-13-8625-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)