Abstract
The beneficial effects of plant growth promoting fungi (PGPF) on plant growth and development are well documented. However, limited information is available on gibberellin (GA) production capacity of PGPF of endophytic origin. In current study, 11 fungal endophytes were isolated from cucumber roots and then screened on Waito-C rice, in order to identify plant growth promoting fungal strains. The fungal isolate GAH7 provided the maximum shoot length (11.3 cm) in comparison to control treatment (7.8 cm). In a separate experiment, bioassay of GAH7 significantly promoted growth attributes of cucumber. The GAH7 culture filtrate (CF) was found to contain physiologically active gibberellins in higher concentrations (GA1, 0.81 ng/ml; GA3, 4.34 ng/ml and GA4, 9.31 ng/ml) in conjunction with physiologically inactive GA9 (0.74 ng/ml), GA15 (0.97 ng/ml), GA19 (1.67 ng/ml) and GA20 (0.46 ng/ml). Isolate GAH7 produced higher amounts of GA3, GA4, GA9 and GA19 than wild type Fusarium fujikuroi, which was used as control for GA production. Gibberellins were analyzed through gas chromatograph/mass spectrometer (GC/MS) with selected ion monitoring (SIM). The fungal isolate GAH7 was later identified as a new strain of Phoma on the basis of sequence homology (99%) and phylogenetic analysis of 18S rDNA sequence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The beneficial role of certain rhizospheric fungi in plant growth promotion has been reported by many researchers (Hamayun et al. 2009a; Khan et al. 2009b). Plants infected with an endophyte often grow faster than non-infected ones (Cheplick et al. 1989). This growth promoting effect is at least in part due to the endophyte’s production of phytohormones, such as indole-3-acetic acid (IAA), cytokines and other plant growth-promoting substances (Zou and Tan 1999) and partly owing to the fact that endophytes could have enhanced the hosts’ uptake of nutritional elements such as nitrogen (Reis et al. 2000) and phosphorus (Malinowski and Belesky 1999). The endophytic fungi have also been shown to confer benefits to host plants, including tolerance to herbivory, heat, salt, disease, and drought (Marquez et al. 2007; Waller et al. 2005). Phoma (fungi imperfecti) is a cosmopolitan, dematiaceous filamentous fungus that inhabits the soil and plant material. Phoma is a ubiquitous saprophyte and toxigenic pathogen to plants and animals (Vishniac 1996) including human beings under some occasions (Taskinen et al. 1997).
Gibberellins (GAs) are diterpenoid plant hormones, first detected in the 1920s from culture filtrates of Gibberella fujikuroi, a known pathogen of rice plants (Ogas 2000). GAs appear to be involved in plant growth and development, but their most typical (and spectacular) property involves the enhancement of stem growth (Nishijima et al. 1995). GAs may modify the sex expression of flowers, induce the parthenocarpic development of fruit and delay senescence. They obviate the need for exposure to red light in the germination of seeds and spores, and the need for vernalisation in the growth of bulbs and tubers. They are associated with the breaking of winter dormancy and stimulate the formation of hydrolytic enzymes in germinating cereal grain (Martin 1983). Currently 136 GAs have been identified, while 12 fungi, pathogenic and non-pathogenic, associated with plants have been reported as GA producers (MacMillan 2002; Kawaide 2006; Vandenbussche et al. 2007). A new strain of Penicillium citrinum had also been reported as a GA producer (Khan et al. 2008).
Literature on gibberellins production by endophytic fungi is still limited, although fungal endophytes are already reported as rich sources of valuable secondary metabolites. They may be used as bio-fertilizers, as there is an increasing concern about the excessive use of fertilizer in agricultural fields and their subsequent negative impact on plant and environment. The aim of the current study was to select potential fungal inoculums for plant growth promotion in order to avoid the excessive use of fertilizer in the agriculture industry.
Materials and Methods
Sample collection and isolation of fungal strains
Endophytic fungi were isolated from the roots of cucumber (Cucumis sativus L.), grown under green-house conditions. Cucumber plants were grown in 5.5 l pots, containing sandy loam soil. Six cucumber plants were randomly selected and harvested after 4 weeks of germination and their roots were screened for the presence of endophytic fungi. The fine root pieces were thoroughly washed with tap water and the dirt was thus removed. These roots were then suspended in Tween 80 solution (2–3 drops in 50 ml of distilled water) and placed in a shaking incubator set at 120 rpm for 5 min at room temperature. Tween 80 was used as detergent and was removed from cucumber roots by washing them with distilled water. The cleaned samples were surface sterilized by suspending them in 50 ml of 1% perchloric acid, and placed in shaking incubator (120 rpm for 5 min). The roots were then washed with autoclaved distilled water, dried between sterilized filter papers and cut into 0.5 cm pieces. These pieces were cultured on Hagem media plates and incubated at 25°C till the emergence of fungal cells (Khan et al. 2009a). The effectiveness of surface sterilization was determined by imprinting sterilized root pieces on Hagem media plates. Absence of any microbial growth on imprinted plates after 4–7 days of incubation were considered enough for effective surface sterilization of roots (Khan et al. 2008). The Hagem minimal medium plates were supplemented with 80 μg/ml streptomycin (Yamada et al. 2001). Pure fungi culture was isolated, grown on potato dextrose agar (PDA) media plates and slants (Khan et al. 2008). The PDA slants were used for storage purpose. Czapek broth medium, containing 1% glucose and peptone, was used for GA production (Hasan 2002) by incubating the fungal isolate at 30°C and at 120 rpm for 7 days. The wild type strain of Fusarium fujikuroi, which was used as positive control for bioassay and GA production, was provided by the Korean Culture Center of Microorganisms (Hamayun et al. 2009b).
Bioassay on waito-c and cucumber
The culture filtrates (CFs) of isolated fungi were bioassayed on waito-c rice sprouts in order to identify their plant growth promoting capacity. The seeds of waito-c were surface sterilized with 1% perchloric acid and treated with 20 μg/ml uniconazol for 24 h, in order to check the GA biosynthesis. The treated seeds were then washed thoroughly and soaked in autoclaved distilled H2O for germination. Two waito-c seedlings were transplanted in each glass tube (50 ml), which already contained 20 ml of 0.8% water-agar medium. The glass tubes were transferred to a controlled growth chamber with a 16-h 30°C day and 8-h 20°C night regime and light intensity of 1,000 μmol m−2s−1 (Jang et al. 2008).
For bioassay experiment, the fungal isolates were grown in flasks containing Czapek medium. The flasks were placed on shaking incubator for 7 days at 30°C and 120 rpm. The fungal cultures (40 ml each) were then centrifuged at 5,000g at 4°C for 15 min and the resulting pellets and supernatants were immediately stored at −70°C and lyophilized (ISE Bondiro Freeze dryer). The lyophilized supernatant was mixed with 1 ml of autoclaved distilled water (DW) and 10 μl of supernatant solution was applied on the apical meristem of rice seedlings at the two leaf stage (Hamayun et al. 2009a; Khan et al. 2009b). The shoot lengths were observed 7 days after the application and compared with waito-c rice seedlings, that had been treated either with distilled water (negative control) or Czapek medium (positive control).
In regards to the cucumber bioassay, the seeds were surface sterilized with 5% NaClO for 15 min and then washed with distilled water. Seeds were sown in an autoclaved horticulture soil, under green house condition (30 ± 2°C). The horticulture soil contained peat moss (13–18%), perlite (7–11%), coco-peat (63–68%) and zeolite (6–8%), while the macro-nutrients were present as follows: NH4 +~90 mg/l; NO3 − ~205 mg/l; P2O5 ~350 mg/l and K2O ~100 mg/l. Fungal isolate GAH7 was selected for application, since it had caused maximum stem length promotion of waito-c rice. Two week old cucumber seedlings were treated with CF (10 ml) of GAH7 and the growth attributes i.e. plant length, shoot length, plant fresh weight and plant dry weight were recorded after 14 days of treatment. The growth promotion caused by CF of isolate GAH7 was compared with plants treated with same amounts of F. fujikuroi, Czapek medium and distilled water.
Extraction and quantification of gibberellins
Gibberellins were extracted from the CF of GAH7 by following an established protocol (Lee et al. 1998). GAs were chromatographed on a 3.9 × 300 mm Bondapak, C18 column (Waters Corp., Milford, MA, USA) and eluted at 1.5 ml/min with the following gradient: 0–5 min, isocratic 28% MeOH in 1% aqueous acetic acid; 5–35 min, linear gradient from 28 to 86% MeOH; 35–36 min, 86–100% MeOH; 36–40 min, isocratic 100% MeOH. Forty-eight fractions of 1.5 ml each were collected. The fractions were then prepared for gas chromatograph/mass spectrometer (GC/MS) with selected ion monitoring (SIM) (6890N network GC system, and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). For each GA, 1 μl of sample was injected in a 30 m × 0.25 mm i.d., 0.25 μm film thickness DB-1 capillary column (J & W Scientific Co., Folsom, CA, USA). The GC oven temperature was programmed for a 1 min hold at 60°C, then to rise at 15°C min−1 to 200°C followed by 5°C min−1 to 285°C. Helium carrier gas was maintained at a head pressure of 30 kPa. The GC was directly interfaced to a Mass Selective Detector with an interface and source temperature of 280°C, an ionizing voltage of 70 eV and a dwell time of 100 ms. Full scan mode (the first trial) and three major ions of the supplemented [2H2] GAs internal standards (obtained from Prof. Lewis N. Mander, Australian National University, Canberra, Australia) and the fungal gibberellins were monitored simultaneously. The retention time was determined using hydrocarbon standards to calculate the KRI (Kovats Retention Index) value, while the GAs quantification was based on the peak area ratios of non-deuterated (extracted) GAs to deuterated GAs.
Genomic DNA extraction and fungal identification
Genomic DNA isolation and PCR was performed according to an established protocol (Khan et al. 2009b). Fungal isolate was identified by sequencing the internal transcribed spacer (ITS) of 18S rDNA, using universal primers ITS-1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS-4 (5′-TCC TCC GCT TAT TGA TAT GC-3′). The BLASTn search program (http://www.ncbi.nlm.nih.gov/BLAST/) was used to look for nucleotide sequence homology. The sequences obtained were then aligned by ClustalW using MEGA version-4 software (Tamura et al. 2007) and the maximum parsimony tree was generated using the same software. The bootstrap replications (1 K) were used as a statistical support for the nodes in the phylogenetic tree.
Statistical analysis
The data was statistically analyzed for standard deviation, using MS-EXCEL software. The mean values were compared, using the Duncan’s multiple range test (DMRT) at P < 0.05 (ANOVA SAS release 9.1; SAS, Cary, NC, USA).
Results
Screening of fungal isolates for plant growth promotion
The CFs of eleven endophytic fungi were screened for plant growth promotion by applying them on waito-c rice. Nine fungal isolates promoted growth of waito-c rice while two isolates inhibited it. The fungal isolate GAH7 significantly promoted shoot length (11.3 cm) as compared to Czapek (9.05 cm) and distilled water (7.9 cm), respectively (Fig. 1).
Bioassay of fungal isolate GAH7 on cucumber
The CF (10 ml) of isolate GAH7 significantly increased growth attributes of cucumber seedlings as compared to Czapek and distilled water treated plants. The plant length (35.71 cm), shoot length (18.06 cm), plant fresh weight (4.98 g), and plant dry weight (0.35 g) were much higher than the Czapek medium and distilled water applied plants. However, the growth promotion capacity of isolate GAH7 was almost similar to that of F. fujikuroi (Table 1).
Analysis of CF of GAH7 for gibberellins
GA analysis of CF of isolate GAH7 showed the presence of bioactive GAs (GA1, 0.81 ng/ml; GA3, 4.34 ng/ml and GA4, 9.31 ng/ml), in conjunction with physiologically inactive GA9 (0.74 ng/ml), GA15 (0.97 ng/ml), GA19 (1.67 ng/ml) and GA20 (0.46 ng/ml). Fungal isolate GAH7 produced higher amounts of GA3, GA4, GA9 and GA19 than wild type F. fujikuroi during current investigations (Fig. 2).
Identification of fungal isolate GAH7
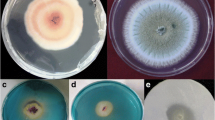
The phylogenetic analysis of fungal isolate GAH7 was carried out by maximum parsimony (MP) method. Consensus tree was constructed from 19 (18 references and 1 clone) aligned ITS sequences with 1,000 bootstrap replications. These strains were selected through BLAST search showing maximum sequence homology percentage and query coverage, and lowest E values. A. bisporus was used as out group. BLAST search showed that fungal isolate GAH7 has 99% sequence homology with Phoma sp. In the dendrogram, fungal isolate GAH7 formed a clade (58% bootstrap support) with Phoma glomerata (Fig. 3). On the basis of sequence homology and phylogenetic analysis, isolate GAH7 was thus identified as a new strain of Phoma sp. The 18S rDNA sequence was submitted to NCBI GenBank and was given accession no. FJ950743.
Phylogenetic tree constructed by the maximum parsimony method using the 18S rDNA sequence (ITS region) of Phoma sp. GAH7 and related fungi. GAH7 formed a clad (58% bootstrap support) with Phoma glomerata, which identify fungal isolate GAH7 as a new strain of Phoma species. Agaricus bisporous was taken as an out group
Discussion
Endophytism represents a new area of research based on the benefits of mutualistic interactions between host crops and non pathogenic fungi. The advantages conferred by endophytic fungi include their ability to promote plant growth and tolerance to abiotic and biotic stresses. As such, the practical applications of endophytes as potential sources of bioorganic nutrients and as biocontrol agents can significantly improve yields in an environmentally sound way (Diene and Narisawa 2009). In current study, we isolated fungal endophytes from cucumber plant and investigated their growth promotion capacity. The CF of fungal isolate with maximum shoot elongation potential was further bioassayed on cucumber plant, which promoted growth attributes of cucumber with respect to control treatments. The fungal isolate GAH7 was later checked for GA production, as screening of microbial CFs for the presence of secondary metabolites is an established method for the identification of biologically active molecules (Higgs et al. 2001). The microbial extracts had been and will continue to be an efficient source of novel secondary metabolites (Cragg et al. 1997).
The usage of water-agar growth media for rice helped in the determination of the sole effect of CF on rice seedling growth, as water-agar media was free of nutrients. We used waito-c rice for the screening experiment, as waito-c is a dwarf and GA deficient rice cultivar, due to blocked C13 hydroxylation pathway of GA biosynthesis. Our current screening results are in agreement with previous reports (Hamayun et al. 2009a; Khan et al. 2008). The CF of GAH7 was also bioassayed on cucumber, as we wanted to investigate the effect of GAH7 on its host plant. It was observed that growth attributes of cucumber was significantly promoted by such an application. Our present investigation confirmed an earlier report on growth promotion of host plant by an endophytic fungus (Hamayun et al. 2009b).
The plant growth promoting fungi (PGPF) are associated with plant roots and they secrete a number of secondary metabolites including gibberellins in the rhizosphere. Gibberellin secretion by PGPF was reported by several researchers (Kawaide 2006; Vandenbussche et al. 2007), which showed the importance of PGPF in plant growth and development, especially under nutrient deficient conditions. In present study, we reported the ability of Phoma sp. to produce 7 different gibberellins that also included bioactive GA1, GA3, and GA4. The bioactive GA3 and GA4 amounts were higher in CF of GAH7 than those of wild type F. fujikuroi, which demonstrated the favorable role of Phoma sp. in promoting growth of host plants. The fungus F. fujikuroi was selected as a positive control for GA production as it corresponds to the mating group C of the Gibberella fujikuroi species complex (O’Donnell et al. 1998), is the only organism capable of excreting GAs in industrially viable quantities (Takahashi et al. 1991).
Molecular and phylogenetic approaches are employed for the identification of fungi recently. DNA sequence analysis methods are objective, reproducible and rapid means of identification, and thus gaining importance. Many rDNA genes are highly conserved for members of the same taxonomic group, and therefore are used extensively for identification. ITS (I and II) had been employed more for fungal identification, although IGS and D1/D2, along with actin encoding genes is also gaining importance nowadays as they provide additional information on inter- and intra-specific identification (Kim and Lee 2000; Lee et al. 2001; Sugita and Nishikawa 2003). We used 5.8S gene and flanking ITS1/4 regions for identification of fungal isolate GAH7. It is because highly conserved 5.8S gene is suitable for higher taxonomic level analysis while highly variable ITS regions are useful for analysis at lower taxonomic levels. The phylogenetic tree construction is also crucial in molecular identification, since BLAST search alone cannot overcome the possibilities of statistical errors. Bootstrap consensus technique is applied in-order to read maximum sequence replications. On the basis of sequence homology and phylogenetic analysis results, isolate GAH7 was identified as a new strain of Phoma sp.
Our current study reports valuable information on the gibberellins producing capacity of an endophytic strain of Phoma sp. It also highlights the importance of fungi in growth promotion of their host plants, which may help in avoiding excessive use of fertilizer in agricultural fields. Further study is suggested on the identification and characterization of GA encoding gene cluster and the development of optimized GA producing media for this GA producing fungal strain.
References
Cheplick GP, Clay K, Marks S (1989) Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol 111:89–98
Cragg GM, Newman DJ, Snader KM (1997) Natural products in drug discovery and development. J Nat Prod 60:52–60
Diene O, Narisawa K (2009) The use of symbiotic fungal associations with crops in sustainable agriculture. J Dev Sust Agric 4:50–56
Hamayun M, Khan SA, Ahmad N et al (2009a) Cladosporium sphaerospermum as a new plant growth promoting endophyte from the roots of Glycine max (L.) Merr. World J Microbiol Biotechnol 25:627–632
Hamayun M, Khan SA, Kim HY et al (2009b) Gibberellin production and plant growth enhancement by newly isolated strain of Scolecobasidium tshawytschae. J Microbiol Biotechnol 19:560–565
Hasan HAH (2002) Gibberellin and auxin production plant root fungi and their biosynthesis under salinity-calcium interaction. Rostlinná Výroba 48(3):101–106
Higgs RE, James AZ, Jeffrey DG et al (2001) Rapid method to estimate the presence of secondary metabolites in microbial extracts. Appl Environ Microbiol 67(1):371–376
Jang SW, Hamayun M, Kim HY et al (2008) Effect of elevated nitrogen levels on endogenous gibberellins and jasmonic acid contents of three rice (Oryza sativa L.) cultivars. J Plant Nut Soil Sci 171:181–186
Kawaide H (2006) Biochemical and molecular analysis of gibberellin biosynthesis in fungi. Biosci Biotechnol Biochem 70(3):583–590
Khan SA, Hamayun M, Yoon HJ et al (2008) Plant growth promotion and Penicillium citrinum. BMC Microbiol 8:231
Khan SA, Hamayun M, Kim HY et al (2009a) Gibberellin production and plant growth promotion by a new strain of Gliomastix murorum. World J Microbiol Biotechnol 25:829–833
Khan SA, Hamayun M, Kim HY et al (2009b) A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellin production. Biotechnol Lett 31:283–287
Kim KS, Lee YS (2000) Rapid and accurate species-specific detection of Phytophthora infestans through analysis of ITS regions in its rDNA. J Microbiol Biotechnol 10:651–655
Lee IJ, Foster K, Morgan PW (1998) Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol 116:1003–1011
Lee HG, Lee JY, Lee DH (2001) Cloning and characterization of the ribosomal RNA gene from Gonyaulax polyerdra. J Microbiol Biotechnol 11:515–523
MacMillan J (2002) Occurence of gibberellins in vascular plants, fungi and bacteria. J Plant Growth Regul 20:387–442
Malinowski DP, Belesky DP (1999) Neotyphodium coenophialum-endophyte infection affects the ability of tall fescue to use sparingly available phosphorus. J Plant Nutr 22:835–853
Marquez LM, Redman RS, Rodriguez RJ et al (2007) A virus in a fungus in a plant-three way symbioses required for thermal tolerance. Science 315:513–515
Martin GC (1983) Commercial uses of gibberellins. In: Crozier A (ed) The biochemistry and physiology of gibberellins, vol 2. Praeger, New York, pp 395–444
Nishijima T, Koshioka M, Yamazaki H et al (1995) Endogenous gibberellins and bolting in cultivars of Japanese Radish. Acta Hort 394:199–206
O’Donnell K, Cigelnik E, Nirenberg HL (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycol 90:465–493
Ogas J (2000) Gibberellins. Cur Biol 10:R48
Reis VM, Baldani JI, Baldani VLD et al (2000) Biological nitrogen fixation in Gramineae and palm trees. Crit Rev Plant Sci 19:227–247
Sugita T, Nishikawa A (2003) Fungal identification method based on DNA sequence analysis. Reassessment of the methods of the pharmaceutical society of Japan and the Japanese pharmacopoeia. J Health Sci 49:531–533
Takahashi N, Phinney BO, MacMillan J (1991) Gibberellins. Springer, New York
Tamura K, Dudley J, Nei M et al (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Bio Evol 24:1596–1599
Taskinen T, Meklin T, Nousiainen M et al (1997) Moisture and mould problems in schools and respiratory manifestations in schoolchildren: clinical and skin test findings. Acta Paediatr 86:1181–1187
Vandenbussche F, Fierro AC, Wiedemann G et al (2007) Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol 7:65
Vishniac HS (1996) Biodiversity of yeasts and filamentous microfungi in terrestrial Antarctic ecosystems. Biodivers Conserv 5:1365–1378
Waller F, Achatz B, Baltruschat H et al (2005) The endophytic fungus Piriformis indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Nat Acad Sci 102:13386–13391
Yamada A, Ogura T, Degawa Y et al (2001) Isolation of Tricholoma matsutake and T. bakamatsutake cultures from field-collected ectomycorrhizas. Mycoscience 42:43–50
Zou WX, Tan RX (1999) Advances in plant science, vol 2. China Higher Education Press, Beijing, pp 183–190
Acknowledgments
This work was financially supported by the Korea Research Foundation Grant (KRF-521-F00001) and Brain Korea 21 Project, funded by Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamayun, M., Khan, S.A., Khan, A.L. et al. Growth promotion of cucumber by pure cultures of gibberellin-producing Phoma sp. GAH7. World J Microbiol Biotechnol 26, 889–894 (2010). https://doi.org/10.1007/s11274-009-0248-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0248-3