Abstract
Segmentation from colored retina images plays a vital role in stable feature extraction for image registration and detection in many ocular diseases. In this study, the authors will look at the segmentation of the blood vessels from fundus images which will further help in preparation of digital template. Here, images are passed through the preprocessing stages and then some of the morphological operators for thresholding are applied on the images for segmentation. Finally, noise removal and binary conversion complete the segmentation method. Then, a number count on blood vessels around the optic disk is done as a feature for further processing. The authors will ensure whether the segmentation accuracy, based on comparison with a ground truth, can serve as a reliable platform for image registration and ocular disease detection. Experiments are done on the images of DRIVE and VARIA databases with an average accuracy of 97.20 and 96.45%, respectively, for segmentation, and a comparative study has also been shown with the existing works.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
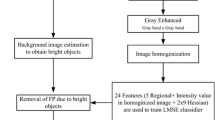
Automated analysis and segmentation from colored retinal images is a challenging task. It has a potential research impact in various areas like diagnosing of many diseases, person identification and security purposes due to significant advances in the field of digital image processing. Extraction of stable features from retina like bifurcation points, bifurcation angle, location of optic disk, width of the blood vessels, etc., needs special care. Thus, proper segmentation from colored retinal images is an essential task to accomplish the same. Manual segmentation of blood vessels is a cumbersome and time-consuming process. In a research setting, with proper technical support, automatic segmentation from retinal images provides better and faster solution. It also plays an important role in diagnosing many ocular diseases affecting retina [1, 2]. Blood vessels are indicative to many pathological changes caused due to hypertension, diabetes, arteriosclerosis, cardiovascular diseases. So observation on the vascular changes sometimes improves the disease diagnosis process. Apart from the medical diagnostics, the segmentation method has another potential application in person identification. The microvascular structure of human retina is unique for each individual and usually remains same during any one’s lifetime except surgical issues. With some existing distinct features on retinal vascular structure, it could be used as a secure template for identification. Many algorithms are there in literature to segment the blood vessel from colored retinal images. In this paper, a best suited segmentation approach has been proposed using some of the morphological operators which will further help in macula detection in the future. The proposed segmentation algorithm is subdivided into three categories, preprocessing, thresholding and blood vessel segmentation and post-processing. Preprocessing, like channel conversion, enhancement and noise removal techniques, helps in improving the quality of the image as most of them suffer from poor local contrast compared to background. Thresholding and binarization help in actual segmentation, and finally, noise present on the images is removed by applying 2-D median filtering in a \(3\times 3\) scanning window. Later, a count on blood vessels around optic disk [3] is done from the segmented images by looking at the number of transitions on binary images. The approach proves sensitivity and specificity as 0.9632 and 0.9470, respectively, and accuracy as 97%. Due to the inadequacy of available resources, the experiments are done only on all the images of DRIVE [4] and VARIA [5] databases. A precise view of the segmentation method is described in Fig. 1.
2 Background
Segmentation of a blood vessel from the retinal images using tracking-based approach is proposed in [6]. In this approach, fuzzy model of a one-dimensional vessel profile drives the segmentation process. One drawback of these approaches is that this method of segmentation depends upon the methods for identifying the seed pixel which is either the optic disk or the branch points which are detected subsequently. Gabor filter-based image processing methods were proposed for retinal vessel extraction in [7,8,9,10]. For segmentation of the blood vessels in the retinal images, optimized Gabor filters with local entropy thresholding had been used in those approaches. The drawback of those segmentation methodologies is that optimized Gabor filter methods fail to detect vessel of different widths and may sometimes detect blood vessels falsely. Also, detection process fails in case of defected retinal image caused by various retinal diseases. An automated texture-based blood vessel segmentation has been proposed in the paper [11]. In this paper, they have used Fuzzy c-Means (FCM) clustering algorithm for the classification between vessels and non-vessels depending on texture properties. This algorithm is having 84.37% sensitivity and 99.19% specificity.
3 Proposed Method
The existing algorithms suffer from problem of non-uniform illumination of the background in the retinal fundus image. The proposed method shows a way to overcome the limitations and to improve the effectiveness, accuracy and computational time.
Broadly, there are three principal stages of the segmentation method, preprocessing, blood vessel extraction and post-processing. Preprocessing technique has been applied, to increase the accuracy in recognizing and extracting the blood vessels. Green channel conversion of RGB images initiates the process as the green channel provides the highest vessel background contrast. Next, the boundary of the green channeled images is removed and contrast-limited adaptive histogram equalization (CLAHE) is applied over it to make a uniform illumination distribution. To this, morphological bottom hat operation with disk-shaped structuring element is applied to enhance the retinal blood vessels. Finally, to correct the illumination variation in the background of retinal image obtained from the previous stage, estimation of the background illumination and the contrast distribution is applied over it.
Image binarization and segmentation of the blood vessels are two essential tasks to be performed here with an empirically generated threshold value.
The primary task in post-processing stage is 2-D median filtering and morphological noise removal operations. This would remove the disconnected blood vessel and noise from the binary image and assist to accomplish the desired goal.
3.1 Image Preprocessing and Segmentation
The retinal images show non-uniform illumination of the background due to the presence of vitreous humor, which is a transparent gel that fills the interior of the eye. The blood vessels show variety in their thickness and contrast. So the darker vessels are clearly seen and easily detected. But it is difficult to extract the thin vessels having low contrast. It is easy to segment if the blood vessels appear as dark structure in the brighter background. So, difference in the contrast between the retinal blood vessels and the background is desirable. Hence, preprocessing is applied to the original retinal image to eliminate these anomalies and to prepare the image for the next steps of segmentation.
Channel conversion It is necessary to convert color (RGB) images into green channel as the green channel provides the best contrast between blood vessels and background of the RGB representation. As the red channel has low contrast and the blue channel has poor dynamic range, we are focusing only on green channel, refer Fig. 2b.
Boundary removal In this step, the outer border of the green component of the image is removed by suppressing the structures that have lower contrast than the surroundings and that are connected to the image border.
Uniform image illumination using AHE Fundus images suffer from background intensity variation due to non-uniform illumination which further deteriorate the segmentation result. Due to this issue, background pixels sometimes have higher gray-level values than the vessel pixels. Because of the difference in gray-level intensity and false vessel’s appearance, the global thresholding techniques cannot be applied in this phase. Contrast-limited adaptive histogram equalization (CLAHE) is applied here to enhance the contrast of the green channel retinal image, as shown in Fig. 2c. It redistributes the light value of the image within small regions of the image instead of the entire one. The process thus enhances the contrast of every smaller region of the image. This step also helps to reduce few false detection of blood vessels that would otherwise decrease the performance of blood vessel segmentation method.
Morphological transformation Sometimes, retinal blood vessels falsely appear as background due to poor intensity variation between the blood vessels and the image background. So to brighten the darker blood vessels in lighter background with an empirically tested disk-shaped structuring element of size \(8\times 8\), morphological bottom hat operation is applied here. Bottom hat filtering subtracts the input image from the result to perform a morphological closing operation on the image which is dilation followed by erosion. Dilation is an operation that grows or thickens the object in binary image. Erosion operation shrinks the object by eroding away the boundaries of regions of foreground pixels. After applying bottom hat, the changes in the result are shown in Fig. 2d.
Smoothing Illumination The image produced after the bottom hat operation has poor distribution of lightness value. To improve the distribution of intensity, again CLAHE is applied, so that the image is well prepared for thresholding and segmentation stages; see Fig. 2e.
3.2 Blood Vessel Segmentation
Preprocessing has enhanced the contrast of the original images. Now the images are being thresholded using Otsu’s algorithm by keeping the information below the threshold value and assigning rest of the image the same value as the threshold. Thus, cluster-based algorithm is used to perform clustering-based image thresholding and the preprocessed grayscale images are now reduced into the binary images (Fig. 2f).
3.3 Noise Removal
The clarity of the images obtained by the previous stages is not very good because of the presence of salt-and-pepper noise in it. Hence, a 2-D median filtering with \(3\times 3\) neighborhood size is applied to reduce the noise from the segmented image. Next, the small connected components, having lesser than 35 white pixels around it within the 8-connectivity window, are removed from the previously processed image. Desired image is shown in Fig. 2h.
3.4 Counting Vessels Around Optic Disk
Common parameters, like accuracy, specificity and sensitivity, are used to measure the strength of an algorithm and to compare the results with other segmentation algorithms. To compare the result of the proposed approach with gold standard images, count on the number of vessels is essential. Also, counting the number of blood vessels around optic disk helps in diagnosis of severe ocular disease called proliferative diabetic retinopathy (PDR). Abrupt changes in count on vessels are alarming for some unusual scenario on regular monitoring. Algorithm 1 explains the method of counting blood vessels in segmented images. Table 1 shows the number of blood vessels detected by the proposed algorithm on the images of DRIVE database.

4 Results and Performance Evaluation
A complete segmentation method, along with the intermediate steps, is shown in Fig. 2. To show the proficiency of the proposed algorithm, gold standard images which are manual segmented images from DRIVE [4] database have been used. Specificity, sensitivity and accuracy are calculated as measuring parameters to compare the results of the proposed algorithm with the other existing algorithms.
where TP is correctly detected positive values; TN is correctly detected negative values; FP is feature is negative, but detected as positive; and FN is feature is positive, but cannot detect (Figs. 3 and 4).
Please refer Table 2 for the performance measurement of the proposed algorithm. Table 3 compares the performance of the proposed algorithm with others.
5 Conclusion
In this paper, we have presented an automated approach for blood vessel segmentation from retinal fundus images. Proposed algorithm has been tested on two publicly available databases, DRIVE [4] and VARIA [5]. We have executed the proposed algorithm on 32-bit MATLAB R2013a version on 32-bit Windows 7 running on Intel Core 2 dual processor with 2 GB RAM. We have used various contrast enhancement techniques and the morphological operators which aim to provide better segmentation results including both minor and major vessels. This segmentation algorithm works efficiently for low-contrast images with less space and time. This algorithm can speed up the ocular disease diagnosis process for ophthalmologists and can be helpful in designing biometric templates.
References
J.L. Boulnois, Photo physical processes in recent medical laser developments. Lasers Med. Sci. 1, 47–66 (1986)
G.M. Bohigian, Lasers in medicine and surgery. JA-MA 256, 900–907 (1986)
M.D. Abrmoff, M. Niemeijer, The automatic detection of the optic disc location in retinal images using optic disc location regression, in 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS ’06 (2006)
The DRIVE database, Image sciences institute, university medical center utrecht, The Netherlands. http://www.isi.uu.nl/Research/Databases/DRIVE/. Last accessed 7 July 2007
VARIA Database, Department of Computer Science of the Faculty of Informatics of the University of Corua. http://www.varpa.es/varia.html
Y.A. Tolias, S.M. Panas, A fuzzy vessel tracking algorithm for retinal images based on fuzzy clustering. IEEE Trans. Med. Imaging 17, 263–273 (1998)
B. Dizdarog, E. Ataer-Cansizoglu, J. Kalpathy-Cramer, K. Keck, M.F. Chiang, D. Erdogmus, Structure-based level set method for automatic retinal vasculature segmentation. EURASIP J. Image Video Process. (2014). (Springer)
P.C. Siddalingaswamy, K.G. Prabhu, Automatic segmentation of blood vessels in colour retinal images using spatial gabor filter and multiscale analysis, in 13th International Conference on Biomedical Engineering, IFMBE Proceedings, vol. 23 (Springer, 2009), pp. 274–276
D. Wu, M. Zhang, J.C. Liu, W. Bauman, On the adaptive detection of blood vessels in retinal images. IEEE Trans. Biomed. Eng. 53(2), 341–343 (2006)
M.M. Fraz, P. Remagnino, A. Hoppe, S. Velastin, B. Uyyanonvara, S.A. Barman, A supervised method for retinal blood vessel segmentation using line strength, multiscale Gabor and morphological features, in IEEE International Conference on Signal and Image Processing Applications (ICSIPA) (2011), pp. 16–18
A. Bhuiyan, B. Nath, J. Chua, R. Kotagiri, Blood vessel segmentation from color retinal images using unsupervised texture classification, in IEEE International Conference, ICIP 2007, vol. 5 (2007)
A.M. Mendona, A. Campilho, Segmentation of retinal blood vessels by combining the detection of centerlines and morphological reconstruction. IEEE Trans. Med. Imaging 25, 1200–1213 (2006)
X. You, Q. Peng, Y. Yuan, Y. Cheung, J. Lei, Segmentation of Retinal Blood Vessels Using the Radial Projection and Semi-supervised Approach (Elsevier Science Inc., New York, NY, USA, 2011), pp. 2314–2324
D. Marin, A. Aquino, M.E. Gegundez-Arias, J.M. Bravo, A new supervised method for blood vessel segmentation in retinal images by using gray-level and moment invariants-based features. IEEE Trans. Med. Imaging 30(1), 146–158 (2010)
F.M. Villalobos-Castaldi, E.M. Felipe-Rivern, L.P. Snchez-Fernndez, A fast, efficient and automated method to extract vessels from fundus images. J. Visual. 13(3), 263–270 (2010). (Springer)
D.S. Fong, L. Aiello, T.W. Gardner, G.L. King, G. Blankenship, J.D. Cavallerano, F.L. Ferris, R. Klein, Diabetic retinopathy. Diabetes Care 26, 226229 (2003)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Goswami, S., Goswami, S., Roy, S., Mukherjee, S., Roy, N.D. (2020). An Automated Segmentation Approach from Colored Retinal Images for Feature Extraction. In: Mandal, J., Bhattacharya, D. (eds) Emerging Technology in Modelling and Graphics. Advances in Intelligent Systems and Computing, vol 937. Springer, Singapore. https://doi.org/10.1007/978-981-13-7403-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-7403-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7402-9
Online ISBN: 978-981-13-7403-6
eBook Packages: EngineeringEngineering (R0)