Abstract
In physiological condition, periostin is expressed in limited tissues such as periodontal ligament, periosteum, and heart valves. Periostin protein is mainly localized on extracellular collagen bundles and in matricellular space. On the other hand, in pathological condition, expression of periostin is induced in disordered tissues of human patients. In tumor development and progression, periostin is elevated mainly in its microenvironment and stromal tissue rich in extracellular matrix. Tumor stromal fibroblasts highly express periostin and organize the tumor-surrounding extracellular matrix architecture. In fibrosis in lung, liver, and kidney, proliferating activated fibroblasts express periostin and replace normal functional tissues with dense connective tissues. In inflammation and allergy, inflammatory cytokines such as IL-4 and IL-13 induce expression of periostin that plays important roles in pathogenesis of these diseases. The elevated levels of periostin in human patients could be detected not only in tissue biopsy samples but also in peripheral bloods using specific antibodies against periostin, because periostin secreted from the disordered tissues is transported into blood vessels and circulates in the cardiovascular system. In this chapter, I introduce the elevated expression of periostin in pathological conditions, and discuss how periostin could be utilized as a biomarker in disease diagnosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Detection of Periostin with Specific Antibodies
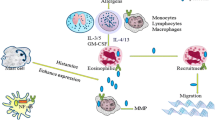
Periostin has been proposed as a biomarker for several diseases including tumor , fibrosis, inflammation and allergy [14, 16, 17, 23, 60, 70, 81, 85]. Elevated expressions of periostin were initially observed in microarray gene expression analyses [5, 22, 29, 33, 51, 82, 90]. Although gene expression of periostin could be evaluated with microarray or quantitative PCR with cDNA samples, these methods require total mRNA of the disordered tissues from biopsy samples of human patients . Biopsy is an invasive procedure that increases the burden on patients. Therefore, an alternative method to detect expression of periostin with minimal invasiveness on patients has been needed. As an alternative method, great attention has been paid to periostin in peripheral blood of patients with diseases [13, 24, 47, 48]. Periostin is a secretory protein mainly derived from fibroblastic cells and interacts with several extracellular matrix proteins such as fibronectin , tenascin-C , and collagens [31, 36]. On the other hands, a secreted periostin is transported into circulating blood through blood vessel, which would become permeabilized due to disease progression, and circulates in cardiovascular systems (Fig. 18.1). For example, neovessels formed in tumor tissue are permeable, and are easy to pass relatively large molecules including antibodies [45, 77, 91]. This permeabilization of blood vessels in tumor tissues is recognized as the enhanced permeability and retention (EPR) effect, by which molecules of appropriate sizes, such as liposomes, nanoparticles, and macromolecular drugs, accumulate in tumor tissue more than in normal tissues. Neovessels formed in inflamed tissue are also permeable. Thus, the circulating periostin in peripheral blood indicates existence of disordered tissue such as tumor microenvironment , fibrosis, and inflammation . The highly sensitive detection and quantification method of circulating periostin in peripheral blood of human patients has been evaluated as a minimally invasive procedure for disease diagnosis .
To utilize periostin as a biomarker for diseases, specific detection methods are required. Antibody has usually been used to specifically detect periostin in tissue thin section, blood, and whole body. In the earliest stage of periostin research, Kudo and colleagues developed rabbit polyclonal antibodies against the first FAS 1 domain (anti-RD1) and the carboxyl-terminal end of the CTD (anti-CT) [20, 80], and have revealed the spatiotemporal distribution of periostin in mouse and human tissues. Thereafter, a lot of polyclonal or monoclonal antibodies against periostin have been developed, and some of which are now commercially available. These antibodies against periostin were used to investigate periostin localization. Immunohistochemical analyses using these antibodies demonstrated that periostin physiologically localizes at collagen-dense areas in connective tissue, including the periodontal ligament [20, 30, 71, 84], periosteum [20, 71], cardiac valve [18, 57, 58], and alveolar wall in the lung [7, 35, 62]. Periostin has also been found to pathologically localize in infarcted myocardium [61, 80], fibrosis [22, 49, 62, 86, 92], the wound healing process [54, 56, 63, 98], and cancer-associated stroma [15, 28, 32, 33, 43, 55, 65, 68, 73, 83, 89, 94, 96]. Thus, expression of periostin is also closely associated with tissue regeneration post-injury [10]. In addition, function-blocking antibodies against periostin have been developed as rabbit polyclonal antibodies, PN1-Ab, PN21-Ab, PnAb, and mouse monoclonal antibody, MZ-1, OC-20, which were utilized in mouse disease models such as tumor growth and metastasis [39, 50, 52, 64, 88, 99]. These specific antibodies against periostin contribute to highly sensitive detection and quantification of periostin as a biomarker for disease progression.
Periostin-binding DNA aptamer has also been developed [40, 53, 93]. Nucleic acid-based aptamers comprise an emerging class of targeted therapeutic and diagnostic molecules [21, 69]. Aptamers are single-stranded DNAs or RNAs that are designed to bind to proteins with similar or better affinity and specificity, compared with antibodies or small molecules. Periostin-binding aptamers would also be useful in detection of periostin.
2 Detection of Periostin in Peripheral Blood
To detect and quantify the circulating periostin in peripheral blood samples, the sandwich enzyme-linked immunosorbent assay (ELISA) using antibodies against periostin has been developed [9]. An antibody against periostin is immobilized on wells of microtiter plate, which captures periostin protein in blood samples. The captured periostin is further labeled with the other antibody against periostin, which binds to the antigen distinct from the other one recognized by the antibody coated on the wells. The antibody on periostin was then recognized with an enzyme-conjugated secondary antibody. The captured periostin protein could be detected with the enzymatic activity as an output. As the enzymes conjugated on secondary antibody, alkaline phosphatase, horseradish peroxidase, and luciferase are utilized. Thus, ELISA enables highly sensitive and specific detection of periostin in peripheral blood samples.
At the beginning of researches on evaluation of periostin in peripheral blood samples, Ben et al . [6] developed ELISA using commercially available antibodies against periostin and measured serum periostin levels in peripheral bloods of human patients with cancers. The authors demonstrated that concentration of periostin in blood samples of patients with colorectal cancer was significantly higher than those of healthy volunteers and of patients with benign colorectal polyps or adenomas. The authors also showed that cancer cells were negative for periostin and their surrounding stromal tissues were positive, indicating that the circulating periostin in peripheral bloods is derived from the surrounding cancer stromal tissues. These results suggest that serum levels of periostin detected by ELISA are of clinical value in identifying patients who may be at a high risk for malignancy of colorectal cancer.
Yamaguchi et al . [97] also evaluated serum periostin levels in peripheral blood samples from patients with progressive skin sclerosis. Skin sclerosis is one of the symptoms of systemic sclerosis that is an autoimmune disorder. Autoimmune reaction causes tissue destruction, resulting in proliferation of activated fibroblasts and accumulation of collagenous extracellular matrix proteins under the skin. Periostin was strongly expressed in the affected dermis biopsy samples from patients with systemic sclerosis. Serum levels of periostin in patients with systemic sclerosis were significantly elevated compared with healthy subjects, indicating that periostin secreted in the affected dermis is transported to blood vessels and circulated in peripheral bloods. This report suggests that an elevated periostin level in patients with systemic sclerosis is associated with severity of skin sclerosis, and that periostin is a potential biomarker for progressive skin fibrosis.
Jia et al . [27] also demonstrated that periostin concentrations in peripheral blood samples from patients with asthma were significantly higher than those from healthy subjects. Asthma is a condition in which airway narrow and swell produce extra mucus, which makes breathing difficulty and trigger coughing, wheezing and shortness of breath. In a histological view of asthmatic airway, as a result from inflammation and remodeling processes, activated fibroblasts proliferate and deposit collagenous extracellular matrix , causing thickening and increasing density of the basement membrane. In this study, the authors examined several biomarker candidates in peripheral blood samples from patients with severe asthma , and concluded that the serum periostin level was the single best predictor of airway eosinophilia. Other reports also demonstrated periostin as a biomarker for asthma [47]. Thus, periostin is a systemic biomarker of airway eosinophilia in asthmatic patients and has potential utility in patient selection for asthma therapeutics.
In addition to the reports described above, elevated expression of periostin mRNA has been demonstrated in mice with the transient middle cerebral artery occlusion model that is similar to human cerebral ischemia [78, 79]. The elevated expression of periostin promotes neural stem cell proliferation and differentiation , which would contribute to regeneration of brain injury [44]. Furthermore, serum periostin concentrations were significantly increased in peripheral blood samples from patients with traumatic brain injury, compared with those from healthy controls [12]. These reports suggest that brain injury induces expression of periostin in the traumatic region, and that secreted periostin is transported into blood vessels and then circulates in the cardiovascular system [25, 85].
In analogy to these initially reported ELISA experiments , elevated periostin levels in peripheral blood samples from patients with diseases such as cancer, fibrosis, inflammation , allergy , and ischemia have been reported. Diseases with increased periostin expression in disordered tissues should be intended for periostin liquid biopsy as peripheral blood samples with minimal invasiveness, which is useful for patient selection in therapeutics.
Although periostin liquid biopsy is an alternative way having minimal invasiveness and informs us existence of the disordered tissues such as cancer, fibrosis, inflammation and allergy , it is impossible to visualize the disordered tissues. To overcome this problem, a molecular imaging strategy targeting periostin-positive disordered tissues has been examined.
3 Molecular Imaging Targeting Periostin in Living Animals
Antibody can be utilized in diagnosis with molecular imaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) (Fig. 18.2). PET is a nuclear medicine functional imaging method used to visualize localization of diagnosis probe molecules with radionuclide that emits positron. Positron-emitting radionuclides such as 11C (carbon-11), 18F (fluoride-18), and 64Cu (copper-64), are produced by nuclear reactions in cyclotron, in which hydrogen ions (proton) are accelerated under a high voltage in the magnetic field. These radionuclides are conjugated with probe molecules by chemical reactions. PET system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide, which is introduced into a biologically active molecule called a radioactive tracer. For example, small molecule tracers such as 18F-fluorodeoxyglucose (FDG) that is a glucose analogue, have been frequently utilized as diagnosis in clinical oncology for staging, restaging and evaluation of tumor response to treatment, because cancer cells rather than non-malignant cells uptake a lot of glucose as an energy source to proliferate (O’Connor et al. [59]). On the other hand, SPECT is able to visualize localization of radiolabeled diagnosis probe molecules in human patients, which detects gamma ray from radionuclide conjugated on the probe molecule. As gamma-emitting radionuclides, 99mTc (technetium-99m), 123I (iodine-123), 131I (iodine-131) are utilized. These radionuclides themselves could also be used to scan bone, myocardial perfusion, brain, and tumor.
In case of antibody , the small molecule metal chelator, such as DOTA, NOTA, and NODAGA, is covalently conjugated to antibody, with amine coupling reaction for example, and radionuclide metal ion such as 64Cu produced in cyclotron is then introduced to the chelators conjugated on antibody [72]. Radionuclide-labeled antibody is administrated into patients with disease such as cancer, who is thereafter scanned with PET camera and CT (computed tomography). For example, PET scan of patients with aggressive breast cancer were performed with 64Cu-labeled antibody against HER2 (epidermal growth factor receptor 2) (64Cu-trastuzumab) [37, 38, 74, 87]. 64Cu-trastuzumab was administrated to patients with metastatic HER2-positive breast cancer, and scanned with medical PET/CT camera, clearly visualizing its accumulation not only in the primary lesion of tumors but also in brain metastasis. Thus, PET imaging technique with radiolabeled antibody (Immuno-PET) would be useful for diagnosis of diseases [46].
Heidari et al . [19] have utilized an antibody against periostin in PET imaging with mice bearing the esophageal squamous cell carcinoma cell line expressing periostin. The authors used a commercially available anti-periostin monoclonal antibody, and prepared a F(ab’)2 fragment by enzymatic digestion. The F(ab’)2 fragment was then labeled with DOTA chelator and subsequently with 64Cu. PET imaging clearly showed specific accumulation of the radiolabeled antibody to tumor tissue derived from the inoculated cell line. Although this study demonstrated that periostin immuno-PET imaging is a powerful method to visualize periostin-positive tissues, it remains elusive whether periostin immuno-PET imaging could visualize the fibrotic region of dense connective tissues in tumor microenvironment , fibrosis, inflammation and allergy . Further studies on periostin immuno-PET imaging are required in order to visualize periostin-related diseases.
4 Clinical Imaging of Tenascin-C and Extracellular Matrix
Tenascin-C , which is one of the interacting proteins with periostin, has been targeted in PET or SPECT imaging studies [1, 3, 4, 34, 66, 67, 75, 76]. Akabani et al . [2] demonstrated that 131I-labeled anti-tenascin 81C6 murine monoclonal antibody accumulated lesion of patients with malignant gliomas in MRI/SPECT imaging. Jacobson et al . [26] developed a radiolabeled single-stranded DNA aptamer that targets tenascin-C and utilized it in PET imaging of mice bearing tenascin-C-positive tumor . Thus, molecular imaging techniques could target extracellular matrix proteins accumulated in the disordered tissues. It should be possible to visualize periostin in the extracellular matrix and matricellular space of the disordered tissues such as tumor microenvironment .
In addition to periostin and tenascin-C , the other extracellular matrix proteins and related transmembrane proteins have also been targeted in molecular imaging. Radiolabeled probes that recognize fibrin, fibronectin , collagens, MMPs, and integrins have been developed to visualize fibrosis and fibrogenesis [11]. These molecular probes including antibodies against periostin could be useful not only for diagnosis but also for the drug delivery system, which enables targeted delivery of drug only to the disordered tissues and reduces the adverse side effect of drug. For example, tumor microenvironment , which is rich in stromal fibroblasts and extracellular matrix proteins, is one of the crucial factors that cause tumor drug-resistance [8, 41, 42, 95]. Remodeling of tumor microenvironment by the drug delivery system targeting the extracellular matrix or transmembrane proteins described above would improve anti-tumor efficacy in drug treatment. Thus, targeting the fibrotic lesion in the disordered tissues is of importance in therapeutics as well as diagnosis .
5 Conclusion
Periostin should be a promising biomarker for diseases such as tumor, fibrosis, inflammation , and allergy . The advantage of periostin is to detect and quantify it in peripheral blood samples of human patients. This liquid biopsy could be performed with minimal invasiveness, which does not burden patients. Periostin in peripheral blood indicates existence of the disordered tissues, in which abnormally activated fibroblastic cells highly express and secret periostin that is transported into circulating blood through blood vessels. Basic researches on periostin in peripheral bloods have utilized ELISA in detection, whereas rapid detection methods, such as the quantitative immuno-chromatography, are required to expand its utility in routine clinical practice.
Antibodies against periostin could be also utilized in clinical immuno-PET diagnosis . Specific and high affinity antibodies against periostin have been developed and utilized for immunological detection, functional blocking, and molecular imaging. Immuno-PET diagnosis could visualize distribution of the disordered tissues in living patients with minimally invasiveness, which contributes to accurate operative treatment as well as evaluation of outcome from therapy. Because clinical molecular imaging such as immuno-PET is an emerging method, periostin in the disordered tissues would be targeted as a useful biomarker for disease diagnosis .
References
Ageyama N et al (2018) Successful inflammation imaging of non-human primate hearts using an antibody specific for tenascin-C. Int Heart J. https://doi.org/10.1536/ihj.17-734
Akabani G et al (2005) Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: a phase II study. J Nucl Med 46:1042–1051
Akabani G et al (1999) Dosimetry of 131I-labeled 81C6 monoclonal antibody administered into surgically created resection cavities in patients with malignant brain tumors. J Nucl Med 40:631–638
Aloj L et al (2014) Radioimmunotherapy with Tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging 41:867–877. https://doi.org/10.1007/s00259-013-2658-6
Asakura M, Kitakaze M (2009) Global gene expression profiling in the failing myocardium. Circ J 73:1568–1576
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F, Yuan YZ (2009) Circulating levels of periostin may help identify patients with more aggressive colorectal cancer. Int J Oncol 34:821–828
Bozyk PD et al (2012) Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One 7:e31336. https://doi.org/10.1371/journal.pone.0031336
Chen X, Song E (2018) Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-018-0004-1
Contie S, Voorzanger-Rousselot N, Litvin J, Bonnet N, Ferrari S, Clezardin P, Garnero P (2010) Development of a new ELISA for serum periostin: evaluation of growth-related changes and bisphosphonate treatment in mice. Calcif Tissue Int 87:341–350. https://doi.org/10.1007/s00223-010-9391-y
Conway SJ et al (2014) The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci 71:1279–1288. https://doi.org/10.1007/s00018-013-1494-y
Desogere P, Montesi SB, Caravan P (2018) Molecular probes for imaging fibrosis and fibrogenesis. Chemistry. https://doi.org/10.1002/chem.201801578
Dong XQ et al (2017) Serum periostin concentrations and outcomes after severe traumatic brain injury. Clin Chim Acta 471:298–303. https://doi.org/10.1016/j.cca.2017.06.020
Emson C, Pham TH, Manetz S, Newbold P (2018) Periostin and dipeptidyl peptidase-4: potential biomarkers of interleukin 13 pathway activation in asthma and allergy. Immunol Allergy Clin N Am 38:611–628. https://doi.org/10.1016/j.iac.2018.06.004
Francois H, Chatziantoniou C (2018) Renal fibrosis: recent translational aspects. Matrix Biol 68–69:318–332. https://doi.org/10.1016/j.matbio.2017.12.013
Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M (2008) Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol 21:1044–1053. https://doi.org/10.1038/modpathol.2008.77
Garnero P (2014) New developments in biological markers of bone metabolism in osteoporosis. Bone 66:46–55. https://doi.org/10.1016/j.bone.2014.05.016
Gonzalez-Gonzalez L, Alonso J (2018) Periostin: a matricellular protein with multiple functions in cancer development and progression. Front Oncol 8:225. https://doi.org/10.3389/fonc.2018.00225
Hakuno D et al (2010) Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest 120:2292–2306. https://doi.org/10.1172/JCI40973
Heidari P, Esfahani SA, Turker NS, Wong G, Wang TC, Rustgi AK, Mahmood U (2015) Imaging of secreted extracellular periostin, an important marker of invasion in the tumor microenvironment in esophageal cancer. J Nucl Med 56:1246–1251. https://doi.org/10.2967/jnumed.115.156216
Horiuchi K et al (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249. https://doi.org/10.1359/jbmr.1999.14.7.1239
Ireson CR, Kelland LR (2006) Discovery and development of anticancer aptamers. Mol Cancer Ther 5:2957–2962. https://doi.org/10.1158/1535-7163.MCT-06-0172
Ishikawa K et al (2014) Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J 28:131–142. https://doi.org/10.1096/fj.13-229740
Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, Yoshihara T (2017) Periostin in inflammation and allergy. Cell Mol Life Sci 74:4293–4303. https://doi.org/10.1007/s00018-017-2648-0
Izuhara K, Ohta S, Ono J (2016) Using periostin as a biomarker in the treatment of asthma. Allergy Asthma Immunol Res 8:491–498. https://doi.org/10.4168/aair.2016.8.6.491
Jabbarli R et al (2018) Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review. Neurosurg Rev. https://doi.org/10.1007/s10143-018-1037-y
Jacobson O et al (2015) PET imaging of tenascin-C with a radiolabeled single-stranded DNA aptamer. J Nucl Med 56:616–621. https://doi.org/10.2967/jnumed.114.149484
Jia G et al (2012) Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 130:647–654. https://doi.org/10.1016/j.jaci.2012.06.025
Kashima TG et al (2009) Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol 40:226–237. https://doi.org/10.1016/j.humpath.2008.07.008
Kashyap MK et al (2010) Overexpression of periostin and lumican in esophageal squamous cell carcinoma. Cancer 2:133–142. https://doi.org/10.3390/cancers2010133
Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A (2006) Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun 342:766–772. https://doi.org/10.1016/j.bbrc.2006.02.016
Kii I, Ito H (2017) Periostin and its interacting proteins in the construction of extracellular architectures. Cell Mol Life Sci 74:4269–4277. https://doi.org/10.1007/s00018-017-2644-4
Kikuchi Y et al (2008) Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem 56:753–764. https://doi.org/10.1369/jhc.2008.951061
Kikuchi Y et al (2014) The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol 184:859–870. https://doi.org/10.1016/j.ajpath.2013.11.012
Kobayashi N et al (2011) Toward in vivo imaging of heart disease using a radiolabeled single-chain Fv fragment targeting tenascin-C. Anal Chem 83:9123–9130. https://doi.org/10.1021/ac202159p
Kondoh H, Nishiyama T, Kikuchi Y, Fukayama M, Saito M, Kii I, Kudo A (2016) Periostin deficiency causes severe and lethal lung injury in mice with bleomycin administration. J Histochem Cytochem 64:441–453. https://doi.org/10.1369/0022155416652611
Kudo A, Kii I (2018) Periostin function in communication with extracellular matrices. J Cell Commun Signal 12:301–308. https://doi.org/10.1007/s12079-017-0422-6
Kurihara H et al (2015) (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res 5:8. https://doi.org/10.1186/s13550-015-0082-6
Kurihara H et al (2016) Molecular imaging using PET for breast cancer. Breast Cancer 23:24–32. https://doi.org/10.1007/s12282-015-0613-z
Kyutoku M et al (2011) Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med 28:181–186. https://doi.org/10.3892/ijmm.2011.712
Lee YJ et al (2013) Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther 21:1004–1013. https://doi.org/10.1038/mt.2013.30
Liang C et al (2017) Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp Mol Med 49:e406. https://doi.org/10.1038/emm.2017.255
Liang C et al (2018) Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer? Cell Mol Life Sci 75:1001–1012. https://doi.org/10.1007/s00018-017-2678-7
Liu AY, Zheng H, Ouyang G (2014) Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 37:150–156. https://doi.org/10.1016/j.matbio.2014.04.007
Ma SM et al (2015) Periostin promotes neural stem cell proliferation and differentiation following hypoxic-ischemic injury. PLoS One 10:e0123585. https://doi.org/10.1371/journal.pone.0123585
Man F, Lammers T, de Rosales TM (2018) Imaging nanomedicine-based drug delivery: a review of clinical studies. Mol Imaging Biol 20:683–695. https://doi.org/10.1007/s11307-018-1255-2
Massicano AVF, Marquez-Nostra BV, Lapi SE (2018) Targeting HER2 in nuclear medicine for imaging and therapy. Mol Imaging 17:1536012117745386. https://doi.org/10.1177/1536012117745386
Matsumoto H (2014) Serum periostin: a novel biomarker for asthma management. Allergol Int 63:153–160. https://doi.org/10.2332/allergolint.13-RAI-0678
Nagasaki T, Matsumoto H, Izuhara K, Ki HACRMG (2017) Utility of serum periostin in combination with exhaled nitric oxide in the management of asthma. Allergol Int 66:404–410. https://doi.org/10.1016/j.alit.2017.02.003
Naik PK et al (2012) Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Phys Lung Cell Mol Phys 303:L1046–L1056. https://doi.org/10.1152/ajplung.00139.2012
Nakama T et al (2016) Different roles played by periostin splice variants in retinal neovascularization. Exp Eye Res 153:133–140. https://doi.org/10.1016/j.exer.2016.10.012
Nakazawa T et al (2004) Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res 22:520–525. https://doi.org/10.1016/j.orthres.2003.10.007
Nakazawa Y et al (2018) Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci Rep 8:4013. https://doi.org/10.1038/s41598-018-22340-7
Nam BY et al (2017) Periostin-binding DNA aptamer treatment ameliorates peritoneal dialysis-induced peritoneal fibrosis. Mol Ther Nucleic Acids 7:396–407. https://doi.org/10.1016/j.omtn.2017.05.001
Nishiyama T et al (2011) Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One 6:e18410. https://doi.org/10.1371/journal.pone.0018410
Nitsche U et al (2016) Periostin and tumor-stroma interactions in non-small cell lung cancer. Oncol Lett 12:3804–3810. https://doi.org/10.3892/ol.2016.5132
Norris RA et al (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711. https://doi.org/10.1002/jcb.21224
Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR (2009a) The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 3:275–286. https://doi.org/10.1007/s12079-009-0063-5
Norris RA et al (2009b) Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn 238:1052–1063. https://doi.org/10.1002/dvdy.21933
O’Connor JP et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186. https://doi.org/10.1038/nrclinonc.2016.162
O’Dwyer DN, Moore BB (2017) The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci 74:4305–4314. https://doi.org/10.1007/s00018-017-2649-z
Oka T et al (2007) Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101:313–321. https://doi.org/10.1161/CIRCRESAHA.107.149047
Okamoto M et al (2011) Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 37:1119–1127. https://doi.org/10.1183/09031936.00059810
Ontsuka K et al (2012) Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol 21:331–336. https://doi.org/10.1111/j.1600-0625.2012.01454.x
Orecchia P et al (2011) Identification of a novel cell binding site of periostin involved in tumour growth. Eur J Cancer 47:2221–2229. https://doi.org/10.1016/j.ejca.2011.04.026
Oskarsson T, Massague J (2012) Extracellular matrix players in metastatic niches. EMBO J 31:254–256. https://doi.org/10.1038/emboj.2011.469
Paganelli G et al (1994) Pre-targeted immunodetection in glioma patients: tumour localization and single-photon emission tomography imaging of [99mTc]PnAO-biotin. Eur J Nucl Med 21:314–321
Popperl G, Gotz C, Gildehaus FJ, Yousry TA, Reulen HJ, Hahn K, Tatsch K (2002) Initial experiences with adjuvant locoregional radioimmunotherapy using 131I-labeled monoclonal antibodies against tenascin (BC-4) for treatment of glioma (WHO III and IV). Nuklearmedizin 41:120–128
Qin X et al (2016) TGFbeta3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep 6:20587. https://doi.org/10.1038/srep20587
Que-Gewirth NS, Sullenger BA (2007) Gene therapy progress and prospects: RNA aptamers. Gene Ther 14:283–291. https://doi.org/10.1038/sj.gt.3302900
Ratajczak-Wielgomas K, Dziegiel P (2015) The role of periostin in neoplastic processes. Folia Histochem Cytobiol 53:120–132. https://doi.org/10.5603/FHC.a2015.0014
Rios H et al (2005) periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144. https://doi.org/10.1128/MCB.25.24.11131-11144.2005
Roosenburg S et al (2014) PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with (64)Cu, (68)Ga, and (111)In. Mol Pharm 11:3930–3937. https://doi.org/10.1021/mp500283k
Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66:2219–2230. https://doi.org/10.1007/s00018-009-0013-7
Sasada S et al (2017) Visualization of HER2-specific breast cancer intratumoral heterogeneity using (64)Cu-DOTA-trastuzumab PET. Eur J Nucl Med Mol Imaging 44:2146–2147. https://doi.org/10.1007/s00259-017-3781-6
Sato M et al (2002) Detection of experimental autoimmune myocarditis in rats by 111In monoclonal antibody specific for tenascin-C. Circulation 106:1397–1402
Schold SC Jr et al (1993) Distribution and dosimetry of I-123-labeled monoclonal antibody 81C6 in patients with anaplastic glioma. Investig Radiol 28:488–496
Seymour LW (1992) Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst 9:135–187
Shimamura M et al (2012) Role of central nervous system periostin in cerebral ischemia. Stroke 43:1108–1114. https://doi.org/10.1161/STROKEAHA.111.636662
Shimamura M et al (2014) Long-term expression of periostin during the chronic stage of ischemic stroke in mice. Hypertens Res 37:494–499. https://doi.org/10.1038/hr.2014.36
Shimazaki M et al (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295–303. https://doi.org/10.1084/jem.20071297
Sirica AE, Almenara JA, Li C (2014) Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Exp Mol Pathol 97:515–524. https://doi.org/10.1016/j.yexmp.2014.10.007
Streit S, Michalski CW, Erkan M, Friess H, Kleeff J (2009) Confirmation of DNA microarray-derived differentially expressed genes in pancreatic cancer using quantitative RT-PCR. Pancreatology 9:577–582. https://doi.org/10.1159/000212084
Sung PL et al (2016) Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget 7:4036–4047. https://doi.org/10.18632/oncotarget.6700
Suzuki H et al (2004) Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol 281:1264–1275. https://doi.org/10.1002/ar.a.20080
Suzuki H, Nishikawa H, Kawakita F (2018) Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regen Res 13:1175–1178. https://doi.org/10.4103/1673-5374.235022
Takayama G et al (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118:98–104. https://doi.org/10.1016/j.jaci.2006.02.046
Tamura K et al (2013) 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 54:1869–1875. https://doi.org/10.2967/jnumed.112.118612
Taniyama Y et al (2016) Selective blockade of periostin exon 17 preserves cardiac performance in acute myocardial infarction. Hypertension 67:356–361. https://doi.org/10.1161/HYPERTENSIONAHA.115.06265
Tian Y et al (2015) Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLoS One 10:e0121502. https://doi.org/10.1371/journal.pone.0121502
Tsunoda T et al (2009) The increased expression of periostin during early stages of prostate cancer and advanced stages of cancer stroma. Prostate 69:1398–1403. https://doi.org/10.1002/pros.20988
Tuwahatu CA, Yeung CC, Lam YW, Roy VAL (2018) The molecularly imprinted polymer essentials: curation of anticancer, ophthalmic, and projected gene therapy drug delivery systems. J Control Release 287:24–34. https://doi.org/10.1016/j.jconrel.2018.08.023
Uchida M et al (2012) Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 46:677–686. https://doi.org/10.1165/rcmb.2011-0115OC
Um JE et al (2017) Periostin-binding DNA aptamer treatment attenuates renal fibrosis under diabetic conditions. Sci Rep 7:8490. https://doi.org/10.1038/s41598-017-09238-6
Underwood TJ et al (2015) Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol 235:466–477. https://doi.org/10.1002/path.4467
Venning FA, Wullkopf L, Erler JT (2015) Targeting ECM disrupts cancer progression. Front Oncol 5:224. https://doi.org/10.3389/fonc.2015.00224
Wang Z, Ouyang G (2012) Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell 10:111–112. https://doi.org/10.1016/j.stem.2012.01.002
Yamaguchi Y et al (2013) Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol 168:717–725. https://doi.org/10.1111/bjd.12117
Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ (2010) Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal 4:99–107. https://doi.org/10.1007/s12079-010-0090-2
Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, Slamon DJ (2011) Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther 10:1500–1508. https://doi.org/10.1158/1535-7163.MCT-11-0046
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kii, I. (2019). Practical Application of Periostin as a Biomarker for Pathological Conditions. In: Kudo, A. (eds) Periostin. Advances in Experimental Medicine and Biology, vol 1132. Springer, Singapore. https://doi.org/10.1007/978-981-13-6657-4_18
Download citation
DOI: https://doi.org/10.1007/978-981-13-6657-4_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6656-7
Online ISBN: 978-981-13-6657-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)