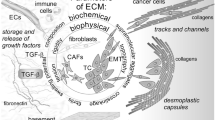
Abstract
Tumor microenvironment consists of tumor cells, stromal cells, extracellular matrix and a plethora of soluble components. The complex array of interactions between tumor cells and their surrounding tumor microenvironments contribute to the determination of the fate of tumor cells during tumorigenesis and metastasis. Matricellular protein periostin is generally absent in most adult tissues but is highly expressed in tumor microenvironments. Current evidence reveals that periostin plays a critical role in establishing and remodeling tumor microenvironments such as the metastatic niche, cancer stem cell niche, perivascular niche, pre-metastatic niche, fibrotic microenvironment and bone marrow microenvironment. Here, we summarize the current knowledge of the multifaceted role of periostin in the tumor microenvironments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tumor microenvironment is a specialized niche where tumor cells reside, which contains various growth factors, cytokines, extracellular matrix (ECM), stromal cells and tumor cells themselves. Tumor progression is strongly influenced by the complicated crosstalks between tumor cells and their surrounding stromal cells and also by the interactions occurring between cells and ECM or other non-cellular components in the tumor microenvironments [1, 2].
Periostin, a matricellular protein that mediates communications between cells and their extracellular microenvironment, is usually absent in normal adult tissues but is highly re-expressed in injured tissues, inflammatory sites and tumor stroma [3,4,5]. Periostin is overexpressed in various solid tumors and promote tumor growth and metastasis of breast, colon, lung, gastric, pancreatic and ovarian cancers as well as melanoma [5, 6]. In this review, we will summarize the roles of periostin in various tumor microenvironments including the metastatic niche [7], cancer stem cell niche [8], perivascular niche [9], pre-metastatic niche [10], fibrotic microenvironment [11] and bone marrow microenvironment [12].
Role of periostin in tumor microenvironments
Periostin in metastatic niche
Tumor metastasis is an inefficient cascade, and only a small number of tumor cells possess the potential to survive in the inhospitable new organ microenvironment and colonize in the metastatic niche [8, 13]. Cancer-associated fibroblasts (CAFs) are the predominant stromal cells that produce ECM components and can be activated by paracrine cues from nearby cancer cells and other stromal cells or by autocrine factors from themselves. CAFs act in concert with other stromal cells to establish and remodel the metastatic niche and promote tumor metastasis by secreting cytokines, growth factors or ECM proteins. Current evidence reveals that periostin is often secreted by the CAFs in metastatic tissues. We previously demonstrated that periostin is highly expressed in the liver metastatic niche and that overexpression of periostin promotes liver metastasis of colon cancer by augmenting cell survival and angiogenesis through the Akt pathway [7]. A recent finding illustrates that colonic fibroblast-derived periostin markedly promotes proliferation, invasion and chemo-resistance of colorectal tumor cells in a paracrine manner and promotes proliferation, migration and growth of fibroblasts themselves in colon cancer in an autocrine manner [14]. In the MMTV-PyMT mouse model of spontaneous breast cancer, periostin is highly expressed in CAFs of metastatic lungs and the knockout of periostin significantly decreases lung metastases but not primary tumors [8]. In a mouse model of head and neck cancer, periostin is highly expressed in the CAFs of metastatic lungs and significantly promotes the growth and invasion of head and neck cancer cells [15]. Furthermore, CAF-derived periostin may directly interact with other ECM proteins such as fibronectin and tenascin C to provide a supportive metastatic niche for tumor outgrowth [5].
Periostin in cancer stem cell niche
Cancer stem cell niches are adjacent local tumor microenvironments that maintain the pool of cancer stem cells and regulate their abilities of survival, self-renewal, differentiation and tumorigenesis. The disseminated cancer stem cells from the primary sites are a small number of tumor cells that can self-renew, differentiate and colonize in the secondary target sites. Using the MMTV-PyMT mouse breast cancer model, Malanchi et al. found that CD90+CD24+ breast cancer stem cells isolated from the primary tumors have the ability to metastasis to lung [8]. Moreover, the infiltrating breast cancer stem cells educate CAFs at the metastatic sites to produce periostin by TGF-β3 stimulation. Stromal periostin subsequently recruits Wnt ligands to cancer stem cells and thereby augments Wnt signaling inside cancer stem cells, which leads to cancer stem cell expansion and metastatic colonization in the lungs [8]. Our data reveal that periostin can endow human mammary epithelial cells and breast cancer cells with stem cell-like or cancer stem cell-like properties and that overexpression of periostin promotes lung metastasis of breast cancer cells [16]. Interestingly, Xu et al. demonstrated that periostin is highly expressed in CD44+CD24− breast cancer stem cells from clinical specimens. High level of periostin is significantly correlated with poor chemotherapy and can be used as an independent prognostic factor for breast cancer [17]. Another report further reveals that basal-like breast cancer cell-derived periostin forms a cancer stem cell niche to maintain the stemness of cancer stem cells via the IL-6/STAT3 signaling pathway [18]. These data demonstrate that periostin functions as a critical component in cancer stem cell niche to promote breast cancer stem cell maintenance and lung metastatic colonization.
Periostin in perivascular niche
Perivascular niche has been reported to play a critical role in the maintenance of hematopoietic stem cells [19] and cancer stem cells [20]. As a potent pro-angiogenic factor, periostin can bind with integrin αvβ3 and up-regulate the expression of the vascular endothelial growth factor receptor Flk-1/KDR via the FAK signaling pathway to promote tumor angiogenesis of breast cancer [21]. Our previous report demonstrated that overexpression of periostin in colon cancer cells promotes angiogenesis in metastatic livers [7]. Periostin has also been reported to promote angiogenesis in pancreatic [22] and oral cancers [23]. Interestingly, periostin is also involved in the remodeling of the perivascular niche. Bissell and colleagues identified two distinct perivascular niches in modulating breast tumor cell dormancy or micrometastatic outgrowth [9]. The authors found that dormant disseminated breast tumor cells reside on the stable microvasculature of lung, bone marrow and brain. Endothelial cells in the mature microvasculature express high level of thrombospondin-1 (TSP-1) and other secreted factors and form a perivascular niche to maintain breast cancer cell quiescence. However, endothelial cells in the sprouting neovasculature produce periostin, tenascin C, fibronectin, TGF-β and versican to establish a different perivascular niche to accelerate tumor metastatic outgrowth [9]. That is to say, periostin and other factors surrounding the tip cells of the neovasculature can awaken the dormant disseminated breast tumor cells and augment tumor outgrowth, while TSP-1 in the stable microvasculature maintains tumor cell dormancy [9]. Additionally, in the perivascular niche of glioblastoma, periostin secreted by glioblastoma stem cells is positively correlated with the recruitment of tumor-associated macrophages, which creates a tumor permissive microenvironment for glioblastoma malignant outgrowth in the brain [24]. These works highlight the important role of periostin in the perivascular niche during tumor progression.
Periostin in pre-metastatic niche
Accumulating data support the existence of pre-metastatic niches, which are created in organs that are targets of future metastases to permit the colonization and outgrowth of disseminated tumor cells. Pre-metastatic niche is triggered by systemic signals from primary tumor and tumor-mobilized bone marrow-derived cells, together with local stromal components, that are prior to the arrival of tumor cells in the predetermined metastatic locations [1, 25,26,27]. Bone marrow-derived VEGFR1-positive hematopoietic progenitor cells are recruited to target organ sites [25]. These hematopoietic progenitor cells and other cellular components alter the composition of the ECM to convert target sites into hospitable microenvironments for disseminated tumor cells.
In addition to bone marrow-derived VEGFR1-positive hematopoietic progenitor cells, myeloid-derived suppressor cells (MDSCs), which generally localize in the tumor microenvironment and exert immunosuppressive functions as an immature myeloid cell lineage, contribute to the formation of pre-metastatic niche for tumor metastatic outgrowth [10, 26]. Our recent work has demonstrated that periostin deficiency impairs the formation of the pre-metastatic niche during breast tumor metastasis by decreasing the accumulation and immunosuppressive functions of MDSCs in the lungs [10]. The accumulation of CD11b+Ly6G+Ly6CLow neutrophils and CD11b+Ly6G−Ly6CHigh monocytic cells is dramatically decreased in the lungs of MMTV-PyMT/periostin−/− mice. In an orthotopic pre-metastatic model, periostin is highly expressed in pre-metastatic lungs of mice bearing orthotopic tumor cells for 5 weeks, at which point disseminated breast tumor cells are not detectable in the lungs. Furthermore, periostin-knockout mice bearing orthotopic tumors exhibit decreased MDSC accumulation in pre-metastatic lungs. Moreover, the percentages of CD4+ and CD8+ T cells in lungs and blood of periostin-knockout mice bearing orthotopic MMTV-PyMT tumor cells are significantly higher than those of their wild-type counterparts. Immunosuppressive factors such as ADAM17, VEGF, TGF-β3 and iNOS are significantly reduced in MDSCs sorted from lungs of the tumor-bearing periostin-knockout mice. Therefore, CD11b+Ly6G+Ly6CLow neutrophils can be considered as the primary immune components in the pre-metastatic niche and in the metastatic niche during breast tumor metastasis. Periostin is a novel component of the immunosuppressive pre-metastatic niche, which is exploited by breast tumor cells to facilitate lung metastasis [10]. Another study reveals that periostin serves as a key component of pre-metastatic niche to promote remotely transplanted melanoma cell metastasis to wound sites where the expression of periostin is high [28]. Periostin may also be sent to the secondary metastatic lungs by exosomes and directly promote metastasis by shaping the landscape of the tumor microenvironment before the arrival of tumor cells [29]. This evidence underscores the functional requirement of stromal periostin for the establishment of pre-metastatic niche and tumor metastasis.
Periostin in fibrotic microenvironment
The tissue stroma is a dynamic mechanical microenvironment. The mechanical signals arising from increased ECM density in the tissue microenvironment can transduce signaling through mechanosensory proteins to modulate cell proliferation, differentiation, survival and motility. It has been reported that increased ECM deposition, crosslinking and remodeling contributes to an increase of stiffness which is associated with fibrosis and tumor progression [30]. As an important component of the tissue microenvironment, the ECM can be modulated by matricellular proteins, MMPs and other extracellular proteinases to maintain homeostasis or to be remodeled in tissue repair, fibrosis, tumorigenesis and metastasis.
Periostin has also been reported to be involved in the formation of fibrotic microenvironment. In pancreatic cancer, activated pancreatic stellate cells (PSCs) remodel the tumor microenvironment by enhancing the production of α-SMA, periostin, collagen I, fibronectin and TGF-β, and maintain the activation of PSCs themselves through an autocrine manner and promote cancer cell invasion in a paracrine manner [31]. Knockdown of periostin in PSCs reduces the PSCs activity and fibronectin production. Therefore, periostin is a key factor in regulating the mutual interactions between PSCs and pancreatic cancer cells by forming a positive feedback loop to alter the tumor microenvironment through ECM remodeling. Recently, Nielsen et al. further found that the recruitment of inflammatory monocytes to the liver is pivotal for liver metastasis of pancreatic cancer [11]. These macrophages secret granulin to activate hepatic stellate cells into myofibroblasts for increasing periostin expression. The increased deposition of periostin consequently leads to the formation of a fibrotic microenvironment for metastatic growth of pancreatic cancer cells in the livers [11]. We have previously found that periostin promotes acute and chronic hepatic inflammation, liver fibrosis and non-alcoholic steatohepatitis [32,33,34]. Periostin has the ability to directly interact with fibronectin and collagen I via its EMI domain and directly interact with tenascin-C and BMP-1 via its FAS1 domains. Moreover, periostin can recruit BMP-1 onto the fibronectin matrix to activate LOX for increasing collagen crosslinking [3, 4]. These data suggest that massive deposition of periostin in hepatic metastatic niche may act as a scaffold to aid collagen crosslinking and accelerate liver metastasis of pancreatic cancer by creating a collagen-rich fibrotic microenvironment in the metastatic livers.
Periostin in bone marrow microenvironment
Bone marrow is rich in vasculature structure which makes it a common site for tumor metastasis. Bone metastases from solid tumors will result in the dysregulation of bone marrow microenvironment and create a new supportive microenvironment for tumor outgrowth [35]. Interestingly, disseminated tumor cells usually take shelter in and occupy the niches for hematopoietic stem cell maintenance in the bone marrow and take similar strategies to those adopted by hematopoietic stem cells for homing to bone marrow [36]. Periostin has been reported to be involved in bone metastasis of breast cancers [37]; however, its mechanisms in promoting bone metastasis of solid tumors remain unknown. Recently, periostin has been shown to contribute to the maintenance of quiescent hematopoietic stem cells [38]. Our recent work demonstrates that periostin not only plays a critical role in the progression of various solid tumors and in the maintenance of hematopoietic stem cells, but also contributes to the development of non-solid tumors [12]. Periostin levels are significantly elevated in the bone marrow of patients and mice with B cell acute lymphoblastic leukemia (B-ALL) compared with their healthy counterparts. The accumulation of B-ALL cells in periostin-knockout mice is much less than in their wild-type counterparts. Moreover, the dissemination of leukemia cells to the limbs, liver and spleen is significantly decreased in periostin-knockout mice [12]. These data suggest that periostin may function as an important supportive component in the bone marrow microenvironment to enhance the growth and dissemination of B-ALL cells.
Discussion and conclusion remarks
The complex array of interactions between tumor cells and their surrounding tumor microenvironments contributes to the determination of the fate of tumor cells during tumorigenesis and metastasis. Periostin is mainly derived from CAFs or other bone marrow-derived mesenchymal stromal cells in tumor microenvironments. Current evidence indicates that stromal cell-derived periostin can remodel tumor microenvironments by interacting with its receptor integrins to activate downstream signaling pathways, by remodeling ECM via direct interaction with other ECM proteins to form a supportive microenvironment, by crosstalking with other signaling molecules and/or by recruiting inflammatory and immune cells [5]. Interestingly, other matricellular proteins such as tenascin C and TSP-1 are also involved in remodeling tumor microenvironments during tumor metastasis. Stem-like human breast cancer cell-derived tenascin C supports the survival of metastasis-initiating cells to promote metastatic growth in the lungs [39]. TSP-1 in the stable microvasculature maintains tumor cell dormancy, while periostin surrounding the tip cells of the neovasculature awakens the dormant disseminated breast tumor cells and augments tumor outgrowth [9]. However, how periostin orchestrates with other matricellular proteins through synergistic or antagonistic effects in distinct tumor microenvironments remains largely unknown. Whether and how periostin promotes the formation of inflammatory microenvironment in chronic inflammation-associated tumorigenesis remains unclear. The hypoxic niche is one of identified niches for the maintenance of cancer stem cells [40]. Whether periostin contributes to the hypoxic niche for cancer stem cells remains to be further investigated. In addition, the roles of different isoforms of periostin in distinct tumor microenvironments deserve further investigation. Together, the pivotal role of periostin in priming various tumor microenvironments for tumor progression made it a potential target for developing novel therapeutics for cancer treatment.
References
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17:302–317
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Kudo A (2011) Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68:3201–3207
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CT, Kudo A (2014) The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci 71:1279–1288
Liu AY, Zheng H, Ouyang G (2014) Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 37:150–156
Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66:2219–2230
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF (2004) Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5:329–339
Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481:85–89
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–817
Wang Z, Xiong S, Mao Y, Chen M, Ma X, Zhou X, Ma Z, Liu F, Huang Z, Luo Q, Ouyang G (2016) Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J Pathol 239:484–495
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC (2016) Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 18:549–560
Ma Z, Zhao X, Huang J, Jia X, Deng M, Cui D, Du Z, Fu G, Ouyang G, Xiao C (2017) A critical role of periostin in B-cell acute lymphoblastic leukemia. Leukemia 31:1835–1837
Wang Z, Ouyang G (2012) Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell 10:111–112
Xu X, Chang W, Yuan J, Han X, Tan X, Ding Y, Luo Y, Cai H, Liu Y, Gao X, Liu Q, Yu Y, Du Y, Wang H, Ma L, Wang J, Chen K, Ding Y, Fu C, Cao G (2016) Periostin expression in intra-tumoral stromal cells is prognostic and predictive for colorectal carcinoma via creating a cancer-supportive niche. Oncotarget 7:798–813
Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, Li Z, Wei W, Chen W (2016) TGFbeta3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep 6:20587
Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu X, Cai Y, Fang X, Lin S, Yuan L, Ouyang G (2013) Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One 8:e72962
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H, Lu P (2012) Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS One 7:e46670
Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, Gower AC, Reinhard BM, Abdolmaleky HM, Thiagalingam S (2016) Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol Cancer Res 14:103–113
Crane GM, Jeffery E, Morrison SJ (2017) Adult haematopoietic stem cell niches. Nat Rev Immunol 17:573–590
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82
Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF (2004) Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 24:3992–4003
Liu Y, Li F, Gao F, Xing L, Qin P, Liang X, Zhang J, Qiao X, Lin L, Zhao Q, Du L (2016) Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget 7:40148–40159
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T (2006) Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer 95:1396–1403
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S (2015) Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 17:170–182
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Wculek SK, Malanchi I (2015) Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528:413–417
Liu Y, Cao X (2016) Characteristics and significance of the pre-metastatic niche. Cancer Cell 30:668–681
Fukuda K, Sugihara E, Ohta S, Izuhara K, Funakoshi T, Amagai M, Saya H (2015) Periostin is a key niche component for wound metastasis of melanoma. PLoS One 10:e0129704
Vardaki I, Ceder S, Rutishauser D, Baltatzis G, Foukakis T, Panaretakis T (2016) Periostin is identified as a putative metastatic marker in breast cancer-derived exosomes. Oncotarget 7:74966–74978
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801
Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA, Friess H (2007) Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132:1447–1464
Huang Y, Liu W, Xiao H, Maitikabili A, Lin Q, Wu T, Huang Z, Liu F, Luo Q, Ouyang G (2015) Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am J Pathol 185:786–797
Li Y, Wu S, Xiong S, Ouyang G (2015) Deficiency of periostin protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis. J Hepatol 62:495–497
Wu T, Wu S, Ouyang G (2014) Periostin: a new extracellular regulator of obesity-induced hepatosteatosis. Cell Metab 20:562–564
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Wu T, Ouyang G (2014) Matricellular proteins: multifaceted extracellular regulators in tumor dormancy. Protein Cell 5:249–252
Contié S, Voorzanger-Rousselot N, Litvin J, Clézardin P, Garnero P (2011) Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases. Int J Cancer 128:352–360
Khurana S, Schouteden S, Manesia JK, Santamaria-Martínez A, Huelsken J, Lacy-Hulbert A, Verfaillie CM (2016) Outside-in integrin signalling regulates haematopoietic stem cell function via Periostin–Itgav axis. Nat Commun 7:13500
Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J (2011) Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17:867–874
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15:501–513
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81572598, 81372841), the Science and Technology Project of Natural Science Foundation of Fujian Province (No. 2016J01639), the Medical Innovations Topic in Fujian Province (No. 2016-CXB-8) and the Open Research Fund of State Key Laboratory of Cellular Stress Biology, Xiamen University (SKLCSB2017KF007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cui, D., Huang, Z., Liu, Y. et al. The multifaceted role of periostin in priming the tumor microenvironments for tumor progression. Cell. Mol. Life Sci. 74, 4287–4291 (2017). https://doi.org/10.1007/s00018-017-2646-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2646-2