Abstract
The rising population is closely related to the improvement and importance of transportation. Moreover, the rapid increasing Indian economy also entangles manufactures in enhancing the performance of internal combustion engine. The increasing number of vehicles with speedy advancement of technology is leading world to have around 2 billion vehicles by 2020. The exhaust emission from these vehicles is also contributing to myriad problems. The exhaust contains harmful gases like carbon monoxide (CO), hydrocarbon (HC), sulfur oxides (SOx), nitrogen oxides (NOx), and particulate matters PM2.5 and PM10. Keeping in mind the Environment Act, 1986 of India and The Air (prevention and control of pollution) Act, 1981 of India, this paper is prepared for the betterment of our environment, and related to this is an idea to introduce an exhaust system in addition to three-way catalytic convertor for reducing the gases such as sulfur dioxide (SO2), carbon dioxide (CO2), and particulate matter emitted from vehicles comprising a heat absorbing freezer gel pack chamber which would be immediately preceded by the catalytic converter. A chamber containing graphite electrodes in aqueous electrolyte water to absorb sulfur dioxide (SO2) and a chamber for absorbing carbon dioxides (CO2) in alkali solution and for trapping particulate emitted in exhaust by catalytic convertor filter layer is being used.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
A rapid increasing pollution is becoming a matter of concern worldwide. In India, due to its large population, air pollution is becoming a serious matter of thinking. Air pollution in the capital of India, Delhi is a serious threat. The amount of foreign particles in air, such as carbon dioxide, Sulfur dioxide, oxides of nitrogen, PM2.5, and PM10, is considerably very high and excessive intake of these may lead to severe diseases [1,2,3,4,5,6,7].
To overcome and to stop, mitigate such unavoidable issues in our ambience manufactures are doing great efforts. Nowadays in automobiles an anti-pollution device is installed known as catalytic convertor [6, 8, 9], which reduces the toxicity of emission coming out from internal combustion engine. The three main tasks of a catalytic convertor are to convert oxide of nitrogen, carbon monoxide, and unburnt hydrocarbon into nitrogen gas, carbon dioxide, and water vapor, respectively [10,11,12,13].
These reactions are
Catalytic convertor has various catalysts. They have certain efficiency to reduce harmful emissions from petrol-operated exhaust system of engines. Platinum and rhodium act as reduction catalyst while platinum and palladium act as oxidation catalyst.
This present idea is a small step in order to mitigate the problem discussed so far. Owing to the affinity toward certain chemicals and chemical reaction, pollutants like CO2, SO2, NOx, and CO [10, 14,15,16,17] could be absorbed. In present work, it would be tried to absorb as maximum as it would be possible for a particular pollutant. Various pollutants are discussed below.
1.1 Concept of Working
The temperature of the exhaust coming out from the engine is nearly about 750 °C and when it gets passed through catalytic convertor, the temperature of the same reaches to nearly about 350 °C. In order to mitigate this temperature, we are using two layers of freezer gel pack on exhaust outlet pipe of catalytic convertor. A freezer gel pack is made up of sodium carboxymethyl cellulose and propylene glycol. These layers of freezer gel pack will absorb the heat of the exhaust gases.
After cooling down the temperature of exhaust, it will pass through electrolytic solution (aqueous water) with two graphite electrodes in aluminum alloy chamber as it is corrosion resistance. The two electrodes will be energized by a 12 V battery for carrying out electrochemical reactions. In this reaction, it is expected that SO2 with desirable amount at anode can form a dil. solution of H2SO4 and H2 in trace which is harmless. Chemical reaction is shown below:
These treated exhausts then pass through another aluminum alloy chamber without getting any exposure to environment. One layer of freezer gel is also used for further reduction in temperature of the exhaust. The layer of freezer gel pack will reduce the temperature up to 50–70 °C. Now, the second container contains ammonium hydroxide solution (NH4OH) (3:1 concentration). This NH4OH solution will have affinity toward CO2. The NH4OH solution will absorb CO2 and form ammonium carbonate. The by-product of ammonium carbonate will not further decompose to ammonia and carbon dioxide gas at low temperature. Now this exhaust gas will pass through a very fine mesh with charcoal powder to absorb carbon dioxide gas and other fine particles.
2 Material and Methodology
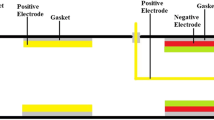
In order to perform experiment, fabrication of device is a must for us. An iron pipe of bore 152 mm and length 400 mm is taken, and this pipe is acting as the housing for aluminum container tightly screwed on the base on the pipe shell. For the fitting and placing of these two aluminum alloy containers, two cubic (101.6 × 101.6 mm) cuts are done. Two iron plates for coving the shell from both the ends are cut down of 152 mm diameter. A hole of 50.2 mm in diameter is made on each covering plate for exhaust pipe fitting. The two aluminum alloy containers are joined by an iron pipe having 25.4 mm diameter. At the outlet of exhaust, fine mesh is installed for trapping particulate matter. Figure 1a is representing the graphite electrodes used and Fig. 1b represents freezer gel pack used. Figure 2 shows the device.
2.1 Experimental Procedures
In order to check the performance and efficiency of the device, containing electrolytic solution with two graphite electrodes in one of aluminum alloy containers and NH4OH dilute solution (1:3) in another container of the same material, it is connected to the exhaust tailpipe of the water-cooled four-cylinder spark ignition engine.
An air flow analyzer AVL-DGAS-444 is employed for analysis of emission coming out from the engine The AVL-DGAS-444 has given the reading on hydrocarbon (HC), carbon monoxide (CO), carbon dioxide gas (CO2), oxide of nitrogen (NOx), and oxygen gas (O2). Though we have concentrated our study on the emission and control of NOx and CO2 only, in the beginning, the experiment engine is operated at 1500 rpm at no-load condition for 1 h in order to avoid cold starting problems [8, 17, 18] and any glitch. Readings are taken at 1500, 2000, 2500, and 3000 rpm at no-load condition (load due to friction is only considered).
3 Result and Discussion
Experiment is performed and readings are taken out on nitrogen oxide (NO) and carbon dioxide gas (CO2). Engine had run at different rpm with no-load condition (only load due to friction is shown here). Readings are shown in Table 1.
Mathematically, the % efficiency of the device is defined as
3.1 Effect on CO2 (Carbon dioxide Gas) Emission
Experimental reading shows a continuous decrease in the amount of CO2. Initially, there is no significant reduction in the amount of CO2, but later on as the experiment is carried out at higher rpm reduction in amount of CO2 is observed. Experimental reading on the emission of CO2 is shown in Table 1 with device and without device. Graphically, the reduction in amount of CO2 emission is also represented in Fig. 3.
3.2 Effect on NOx (Oxide of Nitrogen) Emission
Experimental reading shows a continuous decrease in NO emission. Initially, there is a rapid increase in the amount of NO, but later on as the experiment is carried out at higher rpm it reduces. Experimental reading of the emission of NO is shown in Table 1 with device and without device. Graphical representation is shown in Fig. 4.
4 Conclusion
From the above experiments and readings, it can be seen and said that there is affinity and influence of chemicals (used) on pollutants like NO and CO2 in the exhaust of engine and can be reduced. In future, use of such chemicals can enable us to reduce considerably the amount of emission of harmful gas from the exhaust of petrol-operated engine. This idea needs more R&D with its present condition for better results. Such devices can be used as a complementary to the present system of exhaust. The present idea and work is carried out for the noble cause of the society. Gradually increasing pollution in the capital and its territory is affecting our ecosystem and health of living organism.
References
Industries MH (2004) Environmental friendly diesel engine. UEC Eco-Engine 41(1):2–4
Elliott MA, Nebel GJ, Rounds FG (1955) The composition of exhaust gases from diesel, gasoline and propane powered motor coaches. J Air Pollut Control Assoc 5(2):103–108
Sharaf J (2013) Exhaust emissions and its control technology for an internal combustion engine. Int J Eng Res Appl 3(4):947–960. www.ijera.com
Saini B, Verma R, Himanshu SK, Gupta S (2013) Analysis of exhaust emissions from gasoline powered vehicles in a sub-urban Indian town. Environment 2(1):37–42
Rouphail NM, Frey HC, Colyar JD, Unal A (2000) Vehicle emissions and traffic measures: exploratory analysis of field observations at signalized arterials. In: TRB annual meeting, Nov 2000
Tong HY, Hung WT, Cheung CS (2000) On-road motor vehicle emissions and fuel consumption in urban driving conditions. J Air Waste Manag Assoc 50(4):543–554
Shiva Nagendra S, Khare M (2003) Principal component analysis of urban traffic characteristics and meteorological data. Transp Res Part D Transp Environ 8(4):285–297
Leman AM, Rahman F, Jajuli A, Zakaria S, Feriyanto D (2017) Emission treatment towards cold start and back pressure in internal combustion engine against performance of catalytic converter : a review. In: 9th International UNIMAS STEM Engineering Conference (ENCON 2016), 02021, p 7
Pesansky JD, Majiros NA, Sorensen CM, Thomas DL (2009) The effect of three-way catalyst selection on component pressure drop and system performance
Marsh P, Acke F, Konieczny R, Brück R (2001) Application guideline to define a catalyst layout for maximum catalytic efficiency. Society of Automotive Engineers
Amirnordin SH, Seri SM, Salim WSW, Rahman HA, Hasnan K (2011) Pressure drop analysis of square and hexagonal cells and its effects on the performance of catalytic converters. Int J Environ Sci Dev 2(3):239–247
Korin E, Reshef R, Tshernichovesky D, Sher E (1999) Reducing cold-start emission from internal combustion engines by means of a catalytic converter embedded in a phase-change material. Proc Inst Mech Eng Part D J Automob Eng 213(6):575–583
Soumelldis MI, Stobart RK, Jackson RA (2007) A chemically informed, control-oriented model of a three-way catalytic converter. Proc Inst Mech Eng Part D J Automob Eng 221(9):1169–1182
Subramani T (2012) Study of air pollution due to vehicle emission in tourism centre. Int J Eng Res Appl 2(3):1753–1763
Farrauto RJ, Heck RM (1999) Catalytic converters: state of the art and perspectives. Catal Today 51(3–4):351–360
Greenstone M, Hanna R (2014) Environmental regulations, air and water pollution, and infant mortality in India
Karkanis AN, Botsaris PN, Sparis PD (2004) Emission reduction during cold start via catalyst surface control. Proc Inst Mech Eng Part D J Automob Eng 218(11):1333–1340
Zhang J, Song C, Zhang J, Baker R, Zhang L (2013) Understanding the effects of backpressure on PEM fuel cell reactions and performance. J Electroanal Chem 688:130–136
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Pal, P., Sharma, P., Sharma, A., Bhandwal, M. (2019). A Novel System Based on the Principle of Electrochemical Treatment to Reduce Exhaust Emission from Gasoline-Operated Engine. In: Kumar, M., Pandey, R., Kumar, V. (eds) Advances in Interdisciplinary Engineering . Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-6577-5_20
Download citation
DOI: https://doi.org/10.1007/978-981-13-6577-5_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6576-8
Online ISBN: 978-981-13-6577-5
eBook Packages: EngineeringEngineering (R0)