Abstract
The members of the brassinosteroids family, defined as the 3-oxygenated (20β)-5α-cholestane-22α,23α-diols or their derived compounds isolated from plants, bearing additional alkyl or oxy substituents, are presented. Further, brassinosteroids are grouped into C27, C28, and C29 depending upon the number of carbons in their skeletons. Their structural variations occur due to the substitution in A and B-rings as well in the side chain. They occur in both free and conjugated forms to sugars, fatty and inorganic acids. Their presence in Algae, Bryophyta, Pteridophyta and Angiosperms indicates a ubiquitous distribution in the plant kingdom. The related brassinosteroids precursors, as well as their occurrence, are also presented. Brassinosteroids are considered as the 6th class of plant hormones which have been established after the discovery of brassinolide and other related compounds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Intrigued with previous reports of growth regulating properties of pollen extracts, Mitchell and Whitehead (1941) examined the growth responses and histological changes that resulted from the application of ethereal extracts of corn pollen on intact bean plants or on the cut surfaces of decapitated stems. They observed that the first internode of the plants where these extracts were applied grew significantly more and faster than the untreated ones or treated with some known auxins, as well as gained more fresh and dry weights than the controls. They demonstrated that these were light dependent phenomena, and due to cell elongation rather than cell division. When applied to tap roots, these extracts inhibited root elongation and provoked the appearance of small tumors distal to the application point. When these pollen extracts where applied to the cut surfaces of decapitated stems they caused pronounced radial elongation of epidermal, cortical parenchyma, and endothelial cells. Later Mitchell et al., reported that immature bean seeds also contained plant growth-stimulating hormones (Mitchell et al.1951) and that Brassica napus pollen contained new, yet unknown, hormones they called brassins (Mitchell et al.1970), all of them with properties similar to those reported earlier (Mitchell and Whitehead 1941).
About 60 kinds of pollen were then screened for plant growth activity in the bean second internode assay, and “a few samples, notably the pollen from rape plant (Brassica napus L.) and alder tree (Alnus glutinosa L.), produced an unusual response that combined elongation (the typical gibberellin response) with swelling and curvature” (Mandava 1988). At the same time, some experiments showed that application of brassins to young bean and Siberian elm tree plants promoted overall plant growth (Mitchell and Gregory 1972), what led United States Department of Agriculture (USDA) to initiate an effort aimed to explore the agricultural perspectives of brassins and to isolate their component(s). After processing 500 libers of rape pollen, finally, the USDA team announced the isolation and structure elucidation of the active principle, brassinolide (1) (Grove et al.1979), the first plant hormone of steroidal nature, presenting, unlike animal steroidal hormones, (i) a 22α,23α-dihydroxylated campestane side chain, (ii) a B-ring lactone, and, (iii) a 2α,3α-dihydroxylated ring A. Bean second internodes exhibited elongation, curvature, swelling and even splitting when treated with increasing amounts of brassinolide (1) (Grove et al.1979) (Fig. 1.1), a very distinct effect never observed with any other known plant hormone. Its isolation was followed by its partial synthesis (Fung and Siddall 1980; Ishiguro et al.1980) and of its analogues (Thompson et al.1979, 1981, 1982; Mori 1980; Takatsuto et al.1981; Sakakibara and Mori 1982; Sakakibara et al.1982; Mori et al.1982), some later recognized as plant hormones themselves.
The early synthetic work furnished many compounds with similar or weaker brassin activity, what prompted natural products chemists to search for brassinolide related compounds in plant species other than rape. To the first of them, the 6-ketosteroid castasterone (2) (Yokota et al.1982a), the putative biosynthetic precursor of brassinolide (1), followed that of dolicholide (3) (Yokota et al.1982b), dolichosterone (4) (Baba et al.1983), both with a 24-methylene-5α-cholestane structure, and 28-homodolichosterone (11) (Baba et al.1983), with a 24(E)-ethylidene-5α-cholestane skeleton instead of a 5α-campestane basis as in brassinolide (1) and castasterone (2), and then a multitude of brassinosteroids (BRs) of different side chain structures and oxygenation patterns were isolated, giving rise to the class of brassinosteroids phytohormones, the components of which will be described ahead.
2 Natural Brassinosteroids
About sixty compounds with structures related to that of brassinolide (1) were isolated from or detected in plant materials in the last forty years (see Table 1.1 and Fig. 1.1). They were found in 26 species of 6 families of Algae, in 2 species of 2 families of Bryophyte, in 15 species of 8 families of Pteridophyte, in 6 species of 4 families of Gymnospermae, in 74 species of 35 families of Angiospermae (in 18 species of 6 families of Monocotyledoneae and 56 species of Dicotyledoneae), and in some plant derived products. About 15 biosynthetic precursors of brassinosteroids, some presenting brassinosteroid activity themselves, were found in many plant species.
The finding that the rice lamina inclination assay, developed by Maeda (1965), to test for auxin activity could be used to detect the activity of brassinosteroids at even nanomolar or subnanomolar concentrations (Wada et al.1981) and the development of a microanalytical method for the quantification of 22α, 23α-dihydroxybrassinosteroids (Takatsuto et al.1982), allowed a rapid expansion of the number of known brassinosteroids. The first brassinosteroids isolated presented, as common features, (i) a 5α-cholestane or a 6,7-seco-5α-cholestane derived skeleton, (ii) ring A with one to three oxygen functions (one always at carbon 3), (iii) ring B fully saturated or with varying degree of oxidation at carbon 6, (iv) all-trans ring junctions and (v) 22α,23α-dihydroxylation. In this sense, 3-oxygenated (20β)-5α-cholestane-22α,23α-diols of plant origin, bearing additional alkyl or oxy substituents, were considered as natural brassinosteroids (Zullo and Adam 2002). A more restricted definition states that, in the biosynthetic route to a brassinosteroid lactone, “one would consider as brassinosteroids only those compounds originated after the 22α,23α-dihydroxylation (i.e., those between teasterone or 6-deoxoteasterone and brassinolide), and hence as brassinosteroid precursors those before dihydroxylation occurs (i.e., those compounds up to cathasterone and 6-deoxocathasterone)” (Zullo et al.2003; Zullo and Kohout 2004). After then some other brassinosteroids presenting 2,3-epoxy, 23-dehydro, 23-glycosidic, 23-ester functions, or 26-nor side chain or even 2,3-unsaturation were isolated, “allowing to consider as natural brassinosteroids the 3-oxygenated (20β)-5α-cholestane-22α,23α-diols or their derived compounds isolated from plants, bearing additional alkyl or oxy substituents” (Zullo 2018).
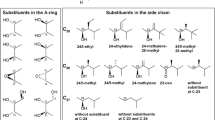
The unconjugated brassinosteroids so far isolated present 27 (C27), 28 (C28) or 29 (C29) carbons, with 5α-cholestane or 26-nor-5α-campestanes (= 26-nor-24α-methyl-5α-cholestane) structures for the C27 series, 5α-campestane (= 24α-methyl-5α-cholestane), 5α-ergostane (= 24β-methyl-5α-cholestane) or 24-methylene-5α-cholestane skeletons for the C28 series, and 5α-sitostane (= 24α-ethyl-5α-cholestane), 24(Z)-ethylidene-5α-cholestane, 25-methyl-5α-campestane and 24-methylene-25-methyl-5α-cholestane structures for the C29 series (Fig. 1.2). Only one of the side chains of isolated brassinosteroids is of a 26-nor sterol, although C26-demethylation of brassinosteroids have been demonstrated in metabolic studies with some species (Joo et al.2012, 2015; Kim et al.2000a, b). From the 12 different side chains of natural brassinosteroids, 9 of them present 22α,23α-dihydroxylation, while one presents a 22α-hydroxy-23-oxo group, another one presents conjugation of one glucose unit at the 23α-hydroxyl, and a last different side chain shows phosphorylation at the 23α-hydroxyl. Feeding studies shows that side chain glucosylation can occur at either C-23 (Poppenberger et al.2005) or C-22 (Soeno et al.2006), and also at C-25 or C-26 after hydroxylation at these carbons (Hai et al.1996). Phosphorylation (Kim et al.2015) and sulfonation (Rouleau et al.1999) have been demonstrated to occur at the side chain of brassinosteroids, but while the first occurs at C-23, the second occurs at C-22, at least with the actual experimental data available.
It is known that the bioactivity of brassinosteroids is dependent on the structure of the side chain and of the A/B rings (Takatsuto et al.1983b; Takatsuto et al.1983a; Brosa et al.1996; Takatsuto et al.1987; Mandava 1988; Liu et al.2017; Zullo and Adam 2002). Regarding to the side chain, as general rules, employing the rice lamina inclination assay on any of its versions (Maeda 1965; Wada et al.1981; Fujioka et al.1998a), for the same A/B ring structures, 22α,23α-dihidroxybrassinosteroids of the brassinolide series are so active as of the 28-homobrassinosteroids series (Takatsuto et al.1983a), and more active than those of 24-epi- or 28-norbrassinosteroids (Takatsuto et al.1983a; Wada et al.1983), which are more active than 26-norbrasssinosteroids (Kim et al.2000a; Watanabe et al.2001). 23-Dehydrogenation (Watanabe et al.2001), or conjugation at one of the side chain hydroxyls (Suzuki et al.1993b; Kim et al.2015; Rouleau et al.1999), diminishes (Yokota et al.1998; Suzuki et al.1993b) or abolishes the biological activity (Kim et al.2015; Rouleau et al.1999), an effect contrary to that observed with 25-methylation (Mori and Takeuchi 1988). It is to note that the relative biological activity of brassinosteroids vary according to the biological assay performed for their evaluation, not only in relation to the side chain but also to the other active sites of their molecules (Takatsuto et al.1983b; Watanabe et al.2001; Zullo and Adam 2002; Liu et al.2017).
A greater structural variation is observed in ring A, with 15 different structures reported, ranging from Δ2,3-unsaturated to trioxygenated and conjugated brassinosteroids: even so, this variation still does not reflect all the possible substructures at this ring, presumed either by efforts of large scale isolation of brassinosteroids (Kim 1991; Fujioka 1999), or by the study of the metabolism of brassinosteroids (Zullo 2018). The biological activity for brassinosteroids with A/B trans ring junctions increases as substitution in ring A changes in the order 3β-hydroxy < 3-oxo < 3α-hydroxy < 2α,3α-dihydroxy, and diminishes as deviates from these patterns (Mandava 1988; Zullo and Adam 2002; Liu et al.2017; Takatsuto et al.1987; Fujioka et al.1995a).
The structural variations in ring B reflect the main steps in the biosynthesis of brassinosteroids (Vriet et al.2013), being more active as its oxidation state increases (Mandava 1988) sequentially from the 6-deoxo to the 6α-hydroxy to the 6-oxo and to the 7-oxalactone types. Therefore, brassinosteroids can be classified, according to the B ring structure, as: (a) 6-oxo-7-oxalactonic brassinosteroids: (i) 2α,3α-dihydroxylated: brassinolide (1), dolicholide (3), 28-homodolicholide (10), 28-norbrassinolide (14), 28-homobrassinolide (17), 24-epibrassinolide (27), cryptolide (54); (ii) 2α, 3β-dihydroxylated: 3-epibrassinolide (51); (iii) 3α-hydroxylated: 2-deoxybrassinolide (7-oxatyphasterol, 43); (iv) 3β-hydroxylated: 3-epi-2-deoxybrassinolide (7-oxateasterone, 58); (b) 6-oxo (or 6-keto) brassinosteroids: (i) 2α,3α-dihydroxylated: castasterone (2), dolichosterone (4), 24-epicastasterone (9), 28-homodolichosterone (11), 28-homocastasterone (12), 28-norcastasterone (15), 25-methyldolichosterone (16), 25-methylcastasterone (33), 26-norcastasterone (59); (ii) 2β,3α-dihydroxylated: 2-epicastasterone (20), 2-epi-25-methyldolichosterone (24), 23-dehydro-2-epicastasterone (55); (iii) 2α,3β-dihydroxylated: 3-epicastasterone (21), 3,24-diepicastasterone (23); (iv) 2β,3β-dihydroxylated: 2,3-diepicastasterone (22), 2,3-diepi-25-methyldolichosterone (25); (v) 3α-monohydroxylated: typhasterol (7), 2-deoxy-25-methyldolichosterone (18), 28-homotyphasterol (37), 28-nortyphasterol (49); (vi) 3β-monohydroxylated: teasterone (8), 3-epi-2-deoxy-25-methyldolichosterone (19), 28-homoteasterone (34), 28-norteasterone (62); (vii) 1β,2α,3α-trihydroxylated: 1β-hydroxycastasterone (28); (viii) 1α,2α,3β-dihydroxylated: 1α-hydroxy-3-epicastasterone (29); (ix) 2α,3α-epoxide: 2,3-diepisecasterone (52); (x) 2β,3β-epoxide: secasterone (38), 24-episecasterone (46); (xi) Δ2-olefin: secasterol (53); (xii) 3β-conjugates: teasterone-3-myristate (35), teasterone-3-laurate (44), 3-O-β-D-glucopyranosylteasterone (48); (xiii) 23α-conjugates: 23-O-β-D-glucopyranosyl-25-methyldolichosterone (26), 23-O-β-D-glucopyranosyl-2-epi-25-methyldolichosterone (32), castasterone 23-phosphate (60); (xiv) 3-dehydro: 3-dehydroteasterone (36); (c) 6α-hydroxybrassinosteroids: 6α-hydroxycastasterone (47); (d) 6-deoxobrassinosteroids: (i) 2α,3α-dihydroxylated: 6-deoxocastasterone (5), 6-deoxodolichosterone (6), 6-deoxo-28-homodolichosterone (13), 6-deoxo-25-methyldolichosterone (31), 6-deoxo-28-norcastasterone (41), 6-deoxo-24-epicastasterone (42); (ii) 2α,3β-dihydroxylated: 3-epi-6-deoxocastasterone (30); (iii) 3α-monohydroxylated: 6-deoxotyphasterol (39), 6-deoxo-28-nortyphasterol (50), 6-deoxo-28-homotyphasterol (61); (iv): 3β-monohydroxylated: 6-deoxoteasterone (45), 6-deoxo-28-norteasterone (56); (v) 3-dehydro: 3-dehydro-6-deoxoteasterone (40), 3-dehydro-6-deoxo-28-norteasterone (57).
3 Brassinosteroids Precursors
A series of papers revealed the main steps of brassinosteroids biosynthesis, from the plant sterols to the brassinosteroid lactones, especially that from campesterol (CR) or campestanol (CN) to brassinolide (1). From these studies it became clear that, if the natural brassinosteroids can be easily recognized from their chemical structures, similar observation does not happen with their precursors (see Fig. 1.3 and Table 1.2). The first experiments established the biosynthesis of brassinolide (1) from teasterone (8) via, sequentially, 3-dehydroteasterone (36), typhasterol (7), and castasterone (2) (Suzuki et al.1993a, 1994a, c) (follow by Fig. 1.4). Soon after it was found that campesterol (CR) was converted to campestanol (CN) and to 6α-hydroxycampestanol (63), 6-oxocampestanol (64), 22α-hydroxy-6-oxocampestanol (65), named cathasterone, and this one to teasterone (8) (Fujioka et al.1995b). The complete biosynthetic sequence of brassinolide starting from campesterol (CR) via cathasterone (65) is known as the early C-6 oxidation pathway (a route in which C-6 oxidation occurs earlier than 22α,23α-dihydroxylation).
Biosynthesis of brassinolide (1) from campesterol (red: sterols; green: brassinosteroids precursors; blue: brassinosteroids). Adapted from Zullo 2018
The frequent isolation or detection of 6-deoxobrassinosteroids brought the suspicion that another biosynthetic route to brassinosteroid lactones could exist. Feeding experiments with labeled precursors established the sequence 6-deoxoteasterone (45), 3-dehydro-6-deoxoteasterone (40), 6-deoxotyphasterol (39), 6-deoxocastasterone (5), castasterone (2), brassinolide (1), which was called the late C-6 oxidation pathway (a route in which C-6 oxidation occurs later than 22α, 23α-dihydroxylation) (Choi et al.1997). It was further demonstrated the conversion of campestanol (CN) to 6-deoxoteasterone (45) through 6-deoxocathasterone (66) (Bishop et al.1999), and the presence of 3-epi-6-deoxocathasterone (67), a putative brassinosteroid precursor, in cultured cells of Catharantus roseus (Fujioka et al.2000b).
A thorough examination of the sterols present in cultured cells of C. roseus and in Arabidopsis seedlings, conjugated with metabolic studies with deuterated substrates, revealed that the conversion of campesterol (CR) to campestanol (CN) occurs through campest-4-en-3-one (4en3one) and campestan-3-one (3one) (Fujioka et al.2002). Moreover, it revealed the operation of intermediates in the conversion of campesterol (CR) to 6-deoxocathasterone (66), originating 22α-hydroxycampesterol (68), 22α-hydroxycampest-4-en-3-one (69), and 22α-hydroxy-5α-campestan-3-one (70) from, respectively, campesterol (CR), campest-4-en-3-one (4en3one) and campestan-3-one (3one). In the same extracts were found also the 28-norhomologues 22α-hydroxycholesterol (71), 22α-hydroxycholest-4-en-3-one (72), 22α-hydroxy-5α-cholestan-3-one (73), 6-deoxo-28-norcathasterone (74) and 3-epi-6-deoxo-28-norcathasterone (75). Later, studying the action of Arabidopsis CYP90C1 and CYP90D1, it was found that these enzymes act on 3-epi-6-deoxocathasterone (67), 22α-hydroxycampesterol (68), 22α-hydroxy-5α-campestan-3-one (70), and 22α-hydroxycampest-4-en-3-one (69) to yield, respectively, 6-deoxotyphasterol (39), 22α, 23α-dihydroxycampesterol (76), 3-dehydro-6-deoxoteasterone (40), and 22α, 23α-dihydroxycampest-4-en-3-one (77), revealing a new shortcut in the biosynthesis of brassinosteroids. Compounds 63-77, isolated from plant material, present side chains with no oxygen function or 22α-monohydroxylated or 22α,23α-dihydroxylated and rings A/B typical of common plant sterols (as 3β-hydroxy-Δ5-sterols or 3β-hydroxy-5α-stanols) or less usual ones [like Δ4-sten-3-ones (Franke et al.2004; Georges et al.2006; Pinto et al.2002) or 5α-stan-3-ones (Guillen and Manzanos 2001)] or reflecting the steps for the construction of typical A/B rings of brassinosteroids (5α-stan-3β,6α-diols, 5α-stan-3β-ol-6-one, 5α-stan-3α-ol) (Fig. 1.5). None of these fragments, per se, can be attributed exclusively to brassinosteroids (Zullo 2018).
It is to note that only brassinosteroids precursors of campestane and cholestane skeletons had been isolated to date, what does not exclude the possibility of similar biosynthetic reactions can occur at the remaining skeletons (ergostane, sitostane, 24-methylenecholestane, 24-ethylydenecholestane, 25-methylcampestane and 24-methylene-25-methylcholestane), for all the possible sequences in the grid (as shown in Fig. 1.4) or through conversions of skeletons while functionalizing them towards the synthesis of castasterone-like or brassinolide-like brassinosteroids, what could explain the isolation or detection of brassinosteroids of different skeletons in the same plant materials. The fact that total sterols usually comprise 2–3 × 10−3 g/g of plant dry weight (Benveniste 2004) and that brassinosteroids are present usually in 10−12–10−9 g/g fresh weight in plant material (Bajguz and Tretyn 2003; Takatsuto 1994), immersed in a matrix of tens of compounds of similar structure (and, hence, of similar polarity and similar chromatographic behavior), turns a very difficult task to determine the brassinosteroids profile of a given plant material, including the compounds of transient existence, like their precursors, can explain why precursors of different skeletons have not been isolated yet.
4 Brassinosteroids with Partially Elucidated Structure
A few natural brassinosteroids were isolated in pure state in enough amount to identify them by the usual spectroscopic methods, but usually they are detected by comparison with authentic compounds prepared by synthesis. Sometimes, due to small amounts of samples, to similar spectroscopic characteristics but different chromatographic behavior, it is not possible to determine the structure of all compounds present in a given brassinosteroids extract. Eventually the complete structure of one of these compounds is correctly elucidated.
One of the richest sources of brassinosteroids, the seeds of kidney beans, presents about 60 compounds of partially known structure (Hwang et al.2006), for which some of them were described (Yokota et al.1987c) (see Fig. 1.6). Among them is cited 1 isomer of 6-deoxo-28-homodolichosterone (78), 4 isomers of castasterone (79), 1 isomer of a hydroxylated castasterone (80), 2 isomers of 28-homocastasterone (81), 3 isomers of a homologue of dolichosterone (82), 1 isomer of a brassinolide derivative with 14 atomic units higher (83), 1 isomer of a brassinolide derivative with 44 atomic units higher (84), 1 isomer of dolicholide (85), 1 isomer of dolicholide with an extra oxygen (86), another one with an extra hydroxyl (87), a dolicholide derivative 28 atomic units higher (88), and another one with a carboxy group (89), an isomer of 28-homobrassinolide (90), an homologue of dolicholide (91) and its carbonyl derivative (92), a carbonyl homologue of dolicholide (93) (Yokota et al.1987c). Two other brassinosteroids were reported in Phaseolus vulgaris, ξ-epi-23-dehydrocastasterone (94) and an homologue with a carbonyl group (95) (Kim 1991). Three isomers of 28-homobrassinolide (90), four isomers of 23-dehydrobrassinolide (96), and one isomer of 28-homodolicholide (97) were reported in pollen and anthers of Cryptomeria japonica (Yokota et al.1998). 25-Methyldolichosterone (16) was later identified as one of the isomers of (82) (Kim et al.1987), as well as cryptolide (54) as one of the four isomers of 23-dehydrobrassinolide (96) (Watanabe et al.2000).
5 Occurrence of Brassinosteroids
Brassinosteroids have been isolated from different plant organs such as pollen, anthers, seeds, leaves, stems, roots, flowers, and grain as well as in insect and crown galls. The endogenous level of brassinosteroids varies from plant’s organ and the age of the plant. Pollen and immature seeds are found to have the highest concentration of brassinosteroids, however, young growing tissues contain higher levels of brassinosteroids than mature tissues. The presence of some bioactive brassinosteroids viz., castasterone (2, BR2), brassinolide (1, BR1), 6-deoxocastasterone (5, BR5), teasterone (8, BR8), typhasterol (7, BR7) and 3-dehydro-6-deoxoteasterone (40, BR40) was confirmed in at least 103, 71, 40, 34, 28 and 28 plant species, respectively. Brassinolide (1) and castasterone (2) are widely distributed in algae and flowering plants, but only castasterone (2) was detected in lower non-flowering plants (liverwort, moss, lycophytes and ferns). Their presence in so many species, from the simplest algae to the more complex phanerogams, as well as the increasing detection in many new species indicates their ubiquitous distribution in the plant kingdom, what is expected from their role as plant hormones.
Table 1.3 lists the occurrence of brassinosteroids in plant species and Table 1.4 the occurrence of the established brassinosteroids precursors. It does not discriminate from which organ they were isolated or detected, or the concentration which they were found, so, primary source of information must be retrieved for proper use of their data.
Brassinosteroids were also found in plant derived products, as 24-epibrassinolide (27) in biodiesel cakes of Brassica carinata A. Braun or Brassica napus L. (Bardi and Rosso 2015); brassinolide (1), castasterone (2), typhasterol (7), teasterone (8) and 28-homocastasterone (12) in a vermicompost leachate (Aremu et al.2015); and brassinolide (1), castasterone (2), 28-norbrassinolide (14) and 28-norcastasterone (15) in date (Phoenix dactilifera L.), medlar (Eryobotrya japonica Lindl.), milkvetch (Astragalus sp.), rape (Brassica napus L.) and robinia (Robinia pseudo-acacia L.) honeys, and also 28-homobrassinolide (17) in the last four honeys (Wang et al.2017).
References
Abe, H. (1991). Rice-lamina inclination, endogenous levels in plant tissues and accumulation during pollen development of brassinosteroids. In H. G. Cutler, T. Yokota, & G. Adam (Eds.), Brassinosteroids: Chemistry, bioactivity and applications (pp. 200–207). Washington: American Chemical Society. https://doi.org/10.1021/bk-1991-0474.ch017.
Abe, H., Morishita, T., Uchiyama, M., Marumo, S., Munakata, K., Takatsuto, S., & Ikekawa, N. (1982). Identification of brassinolide-like substances in Chinese cabbage. Agricultural and Biological Chemistry, 46, 2609–2611.
Abe, H., Morishita, T., Uchiyama, M., Takatsuto, S., Ikekawa, N., Ikeda, M., Sassa, T., Kitsuwa, T., & Marumo, S. (1983). Occurrence of three new brassinosteroids: Brassinone, (24S)-24-ethylbrassinone and 28-norbrassinolide, in higher plants. Experientia, 39, 351–353.
Abe, H., Morishita, T., Uchiyama, M., Takatsuto, S., & Ikekawa, N. (1984a). A new brassinolide-related steroid in the leaves of Thea sinensis. Agricultural and Biological Chemistry, 48, 2171–2172.
Abe, H., Nakamura, K., Morishita, T., Uchiyama, M., Takatsuto, S., & Ikekawa, N. (1984b). Endogenous brassinosteroids of the rice plant: Castasterone and dolichosterone. Agricultural and Biological Chemistry, 48, 1103–1104.
Abe, H., Honjo, C., Kyokawa, Y., Asakawa, S., Natsume, M., & Narushima, M. (1994). 3-Oxoteasterone and the epimerization of teasterone: Identification in lily anthers and Distylium racemosum leaves and its biotransformation into typhasterol. Bioscience, Biotechnology, and Biochemistry, 58, 986–989.
Abe, H., Takatsuto, S., Nakayama, M., & Yokota, T. (1995a). 28-Homotyphasterol, a new natural brassinosteroid from rice (Oryza sativa L.) bran. Bioscience, Biotechnology, and Biochemistry, 59, 176–178.
Abe, H., Takatsuto, S., Okuda, R., & Yokota, T. (1995b). Identification of castasterone, 6-deoxocastasterone, and typhasterol in the pollen of Robinia pseudo-acacia L. Bioscience, Biotechnology, and Biochemistry, 59, 309–310.
Antonchick, A. P., Schneider, B., Zhabinskii, V. N., Konstantinova, O. V., & Khripach, V. A. (2003). Biosynthesis of 2,3-epoxybrassinosteroids in seedlings of Secale cereale. Phytochemistry, 63, 771–776.
Antonchick, A., Svatos, A., Schneider, B., Konstantinova, O. V., Zhabinskii, V. N., & Khripach, V. A. (2005). 2,3-epoxybrassinosteroids are intermediates in the biosynthesis of castasterone in seedlings of Secale cereale. Phytochemistry, 66, 65–72.
Antonchick, A. P., Svatos, A., Konstantinova, O. V., Zhabinskii, V. N., Khripach, V. A., & Schneider, B. (2006). Reversible conversion in the brassinosteroid quartet castasterone, brassinolide and their 3β-epimers. Zeitschrift für Naturforschung. B, A Journal of Chemical Sciences, 61, 1039–1044.
Aremu, A. O., Stirk, W. A., Kulkarni, M. G., Tarkowska, D., Tureckova, V., Gruz, J., Subrtova, M., Pencik, A., Novak, O., Dolezal, K., Strnad, M., & Van Staden, J. (2015). Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regulation, 75, 483–492.
Arima, M., Yokota, T., & Takahashi, N. (1984). Identification and quantification of brassinolide-related steroids in the insect gall and healthy tissues of the chestnut plant. Phytochemistry, 23, 1587–1591.
Asahina, M., Tamaki, Y., Sakamoto, T., Shibata, K., Nomura, T., & Yokota, T. (2014). Blue light-promoted rice leaf bending and unrolling are due to up-regulated brassinosteroid biosynthesis genes accompanied by accumulation of castasterone. Phytochemistry, 104, 21–29.
Asakawa, S., Abe, H., Kyokawa, Y., Nakamura, S., & Natsume, M. (1994). Teasterone 3-myristate: A new-type of brassinosteroid derivative in Lilium longiflorum anthers. Bioscience, Biotechnology, and Biochemistry, 58, 219–220.
Asakawa, S., Abe, H., Nishikaa, N., Natsume, M., & Koshioka, M. (1996). Purification and identification of new acyl-conjugated teasterones in lily pollen. Bioscience, Biotechnology, and Biochemistry, 60, 1416–1420.
Baba, J., Yokota, T., & Takahashi, N. (1983). Brassinolide-related new bioactive steroids from Dolichos lablab seed. Agricultural and Biological Chemistry, 47, 659–661.
Bajguz, A. (2009). Isolation and characterization of brassinosteroids from algal cultures of Chlorella vulgaris Beijerinck (Trebouxiophyceae). Journal of Plant Physiology, 166, 1946–1949.
Bajguz, A., & Piotrowska-Niczyporuk, A. (2013). Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiology and Biochemistry, 71, 290–297.
Bajguz, A., & Piotrowska-Niczyporuk, A. (2014). Interactive effect of brassinosteroids and cytokinins on growth, chlorophyll, monosaccharide and protein content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiology and Biochemistry, 80, 176–183.
Bajguz, A., & Tretyn, A. (2003). The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry, 62, 1027–1046.
Bancos, S., Nomura, T., Sato, T., Molnar, G., Bishop, G. J., Koncz, C., Yokota, T., Nagy, F., & Szekeres, M. (2002). Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiology, 130, 504–513.
Bancos, S., Szatmari, A.-M., Castle, J., Kozma-Bognar, L., Shibata, K., Yokota, T., Bishop, G. J., Nagy, F., & Szekeres, M. (2006). Diurnal regulation of the brassinosteroid-biosynthetic CPD gene in Arabidopsis. Plant Physiology, 141, 299–309.
Bardi, L., & Rosso, F. (2015). Extraction and characterization of brassinosteroids from residues of the biodiesel chain. Industrial Crops and Products, 75 (Part A), 24–28.
Benveniste, P. (2004). Biosynthesis and accumulation of sterols. Annual Review of Plant Biology, 55, 429–457.
Best, N. B., Hartwig, T., Budka, J., Fujioka, S., Johal, G., Schulz, B., & Dilkes, B. P. (2016). nana plant2 encodes a maize ortholog of the Arabidopsis brassinosteroid biosynthesis protein DWARF1, identifying developmental interactions between brassinosteroids and gibberellins. Plant Physiology, 171, 2633–2647.
Beste, L., Nahar, N., Dalman, K., Fujioka, S., Jonsson, L., Dutta, P. C., & Sitbon, F. (2011). Synthesis of hydroxylated sterols in transgenic Arabidopsis plants alters growth and steroid metabolism. Plant Physiology, 157, 426–440.
Bhardwaj, R., Kaur, S., Nagar, P. K., & Arora, H. K. (2007). Isolation and characterization of brassinosteroids from immature seeds of Camellia sinensis (O) Kuntze. Plant Growth Regulation, 53, 1–5.
Bishop, G. J., Nomura, T., Yokota, T., Harrison, K., Noguchi, T., Fujioka, S., Takatsuto, S., Jones, J. D. G., & Kamiya, Y. (1999). The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proceedings of the National Academy of Sciences, 96, 1761–1766.
Brosa, C., Capdevila, J. M., & Zamora, I. (1996). Brassinosteroids: A new way to define the structural requirements. Tetrahedron, 52, 2435–2448.
Carland, F., Fujioka, S., & Nelson, T. (2010). The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiology, 153, 741–756.
Chen, M., Wang, R., Zhu, Y., Liu, M., Zhu, F., Xiao, J., & Chen, X. (2018). 4-Mercaptophenylboronic acid-modified spirally-curved mesoporous silica nanofibers coupled with ultra performance liquid chromatography-mass spectrometry for determination of brassinosteroids in plants. Food Chemistry, 263, 51–58.
Choe, S., Fujioka, S., Noguchi, T., Takatsuto, S., Yoshida, S., & Feldmann, K. A. (2001). Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. The Plant Journal, 26, 573–582.
Choe, S., Schmitz, R. J., Fujioka, S., Takatsuto, S., Lee, M. O., Yoshida, S., Feldmann, K. A., & Tax, F. E. (2002). Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3β-like kinase. Plant Physiology, 130, 1506–1515.
Choi, Y. H., Inoue, T., Fujioka, S., Saimoto, H., & Sakurai, A. (1993). Identification of brassinosteroid-like active substances in plant-cell cultures. Bioscience, Biotechnology, and Biochemistry, 57, 860–861.
Choi, Y. H., Fujioka, S., Harada, A., Yokota, T., Takatsuto, S., & Sakurai, A. (1996). A brassinolide biosynthetic pathway via 6-deoxocastasterone. Phytochemistry, 43, 593–596.
Choi, Y. H., Fujioka, S., Nomura, T., Harada, A., Yokota, T., Takatsuto, S., & Sakurai, A. (1997). An alternative brassinolide biosynthetic pathway via late C-6 oxidation. Phytochemistry, 44, 609–613.
Choi, S., Cho, Y. H., Kim, K., Matsui, M., Son, S. H., Kim, S. K., Fujioka, S., & Hwang, I. (2013). BAT1, a putative acyltransferase, modulates brassinosteroid levels in Arabidopsis. The Plant Journal, 73, 380–391.
Chung, H. Y., Fujioka, S., Choe, S., Lee, S., Lee, Y. H., Baek, N. I., & Chung, I. S. (2010). Simultaneous suppression of three genes related to brassinosteroid (BR) biosynthesis altered campesterol and BR contents, and led to a dwarf phenotype in Arabidopsis thaliana. Plant Cell Reports, 29, 397–402.
Deng, T., Wu, D., Duan, C., & Guan, Y. (2016). Ultrasensitive quantification of endogenous brassinosteroids in milligram fresh plant with a quaternary ammonium derivatization reagent by pipette-tip solid-phase extraction coupled with ultra-high-performance liquid chromatography tandem mass spectrometry. Journal of Chromatography. A, 1456, 105–112.
Dias, D. S., Ribeiro, L. M., Lopes, P. S. N., Munne-Bosch, S., & Garcia, Q. S. (2017). Hormonal profile and the role of cell expansion in the germination control of Cerrado biome palm seeds. Plant Physiology and Biochemistry, 118, 168–177.
Ding, J., Mao, L. J., Wang, S. T., Yuan, B. F., & Feng, Y. Q. (2013a). Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by high-performance liquid chromatography-tandem mass spectrometry. Phytochemical Analysis, 24, 386–394.
Ding, J., Mao, L. J., Yuan, B. F., & Feng, Y. Q. (2013b). A selective pretreatment method for determination of endogenous active brassinosteroids in plant tissues: Double layered solid phase extraction combined with boronate affinity polymer monolith microextraction. Plant Methods, 9, 13.
Ding, J., Jiang, L., & Feng, Y. (2014a). An automatic and sensitive method for determination of endogenous brassinosteroids in plant tissues by an online trapping-in situ derivatization-ultra performance liquid chromatography-tandem mass spectrometry system. Chinese Journal of Chromatography, 32, 1094–1103.
Ding, J., Wu, J. H., Liu, J. F., Yuan, B. F., & Feng, Y. Q. (2014b). Improved methodology for assaying brassinosteroids in plant tissues using magnetic hydrophilic material for both extraction and derivatization. Plant Methods, 10, 39.
Ding, J., Mao, L. J., Guo, N., Yu, L., & Feng, Y. Q. (2016). Determination of endogenous brassinosteroids using sequential magnetic solid phase extraction followed by in situ derivatization/desorption method coupled with liquid chromatography-tandem mass spectrometry. Journal of Chromatography. A, 1446, 103–113.
Dockter, C., Braumann, I., Gough, S. P., Lundqvist, J., Matyszczak, I., Muller, A. H., Zakhrabekova, S., Hansson, M., Gruszka, D., Kurowska, M., Marzec, M., Druka, A., Druka, I., Franckowiak, J., Janeczko, A., Lundqvist, U., Oklestkova, J., & Schulz, B. (2014). Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the “green revolution” genetic toolkit. Plant Physiology, 166, 1912–1927.
Franke, K., Nasher, A. K., & Schmidt, J. (2004). Constituents of Jatropha unicostata. Biochemical Systematics and Ecology, 32, 219–220.
Friebe, A., Volz, A., Schmidt, J., Voigt, B., Adam, G., & Schnabl, H. (1999). 24-Epi-secasterone and 24-epi-castasterone from Lychnis viscaria seeds. Phytochemistry, 52, 1607–1610.
Fujioka, S. (1999). Natural occurrence of brassinosteroids in the plant kingdom. In A. Sakurai, T. Yokota, & S. D. Clouse (Eds.), Brassinosteroids – Steroidal plant hormones (pp. 21–45). Tokyo: Springer.
Fujioka, S., & Sakurai, A. (1997). Brassinosteroids. Natural Product Reports, 14, 1–10.
Fujioka, S., Inoue, T., Takatsuto, S., Yanagisawa, T., Yokota, T., & Sakurai, A. (1995a). Biological activities of biosynthetically-related congeners of brassinolide. Bioscience, Biotechnology, and Biochemistry, 59, 1973–1975.
Fujioka, S., Inoue, T., Takatsuto, S., Yanagisawa, T., Yokota, T., & Sakurai, A. (1995b). Identification of a new brassinosteroid, cathasterone, in cultured cells of Catharanthus roseus as a biosynthetic precursor of teasterone. Bioscience, Biotechnology, and Biochemistry, 59, 1543–1547.
Fujioka, S., Choi, Y. H., Takatsuto, S., Yokota, T., Li, J. M., Chory, J., & Sakurai, A. (1996). Identification of castasterone, 6-deoxocastasterone, typhasterol and 6-deoxotyphasterol from the shoots of Arabidopsis thaliana. Plant & Cell Physiology, 37, 1201–1203.
Fujioka, S., Li, J. M., Choi, Y. H., Seto, H., Takatsuto, S., Noguchi, T., Watanabe, T., Kuriyama, H., Yokota, T., Chory, J., & Sakurai, A. (1997). The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell, 9, 1951–1962.
Fujioka, S., Noguchi, T., Takatsuto, S., & Yoshida, S. (1998a). Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry, 49, 1841–1848.
Fujioka, S., Noguchi, T., Yokota, T., Takatsuto, S., & Yoshida, S. (1998b). Brassinosteroids in Arabidopsis thaliana. Phytochemistry, 48, 595–599.
Fujioka, S., Noguchi, T., Sekimoto, M., Takatsuto, S., & Yoshida, S. (2000a). 28-norcastasterone is biosynthesized from castasterone. Phytochemistry, 55, 97–101.
Fujioka, S., Noguchi, T., Watanabe, T., Takatsuto, S., & Yoshida, S. (2000b). Biosynthesis of brassinosteroids in cultured cells of Catharanthus roseus. Phytochemistry, 53, 549–553.
Fujioka, S., Takatsuto, S., & Yoshida, S. (2002). An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiology, 130, 930–939.
Fukuta, N., Fujioka, S., Takatsuto, S., Yoshida, S., Fukuta, Y., & Nakayama, M. (2004). ‘Rinrei’, a brassinosteroid-deficient dwarf mutant of faba bean (Vicia faba). Physiologia Plantarum, 121, 506–512.
Fung, S., & Siddall, J. B. (1980). Stereoselective synthesis of brassinolide: A plant growth promoting steroidal lactone. Journal of the American Chemical Society, 102, 6580–6581.
Georges, P., Sylvestre, M., Ruegger, H., & Bourgeois, P. (2006). Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids, 71, 647–652.
Griffiths, P. G., Sasse, J. M., Yokota, T., & Cameron, D. W. (1995). 6-Deoxotyphasterol and 3-dehydro-6-deoxoteasterone, possible precursors to brassinosteroids in the pollen of Cupressus arizonica. Bioscience Biotechnology and Biochemistry, 59, 956–959.
Grove, M. D., Spencer, G. F., Rohwedder, W. K., Mandava, N., Worley, J. F., Warthen, J. D., Steffens, G. L., Flippenanderson, J. L., & Cook, J. C. (1979). Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature, 281, 216–217.
Gruszka, D., Szarejko, I., Janeczko, A., Dziurka, M., Pociecha, E., & Oklestkova, J. (2016). Barley brassinosteroid mutants provide an insight into phytohormonal homeostasis in plant reaction to drought stress. Frontiers in Plant Science, 7, 1824.
Guillen, M. D., & Manzanos, M. J. (2001). Some compounds detected for the first time in oak wood extracts by GC/MS. Sciences des Aliments, 21, 65–70.
Gupta, D., Bhardwaj, R., Nagar, P. K., & Kaur, S. (2004). Isolation and characterization of brassinosteroids from leaves of Camellia sinensis (L.) O. Kuntze. Plant Growth Regulation, 43, 97–100.
Habib, S. H., Ooi, S. E., Novak, O., Tarkowska, D., Rolcik, J., Dolezal, K., Syed-Alwee, S. S. R., Ho, C. L., & Namasivayam, P. (2012). Comparative mineral and hormonal analyses of wild type and TLS somaclonal variant derived from oil palm (Elaeis guineensis Jacq. var. tenera) tissue culture. Plant Growth Regulation, 68, 313–317.
Hai, T., Schneider, B., Porzel, A., & Adam, G. (1996). Metabolism of 24-epi-castasterone in cell suspension cultures of Lycopersicon esculentum. Phytochemistry, 41, 197–201.
Hartwig, T., Chuck, G. S., Fujioka, S., Klempien, A., Weizbauer, R., Potluri, D. P. V., Choe, S., Johal, G. S., & Schulz, B. (2011). Brassinosteroid control of sex determination in maize. Proceedings of the National Academy of Sciences, 108, 19814–19819.
He, J. X., Fujioka, S., Li, T. C., Kang, S. G., Seto, H., Takatsuto, S., Yoshida, S., & Jang, J. C. (2003). Sterols regulate development and gene expression in Arabidopsis. Plant Physiology, 131, 1258–1269.
Hou, S., Niu, H., Tao, Q., Wang, S., Gong, Z., Li, Z., Li, S., Weng, Y., Li, Z., Li, S., & Weng, Y. (2017). A mutant in the CsDET2 gene leads to a systemic brassinosteriod deficiency and super compact phenotype in cucumber (Cucumis sativus L.). Theoretical and Applied Genetics, 130, 1693–1703.
Huo, F., Wang, X., Han, Y., Bai, Y., Zhang, W., Yuan, H., & Liu, H. (2012). A new derivatization approach for the rapid and sensitive analysis of brassinosteroids by using ultra high performance liquid chromatography-electrospray ionization triple quadrupole mass spectrometry. Talanta, 99, 420–425.
Hwang, J. Y., Park, C. H., Namgung, H., & Kim, S. K. (2006). Identification of a new brassinosteroid, 23-dehydro-2-epicastasterone, from immature seeds of Phaseolus vulgaris. Journal of Plant Biology, 49, 409–412.
Hwang, J. Y., Park, C. H., & Kim, S. K. (2007). C-3 epimerization of 6-deoxocastasterone in Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 28, 175–176.
Ikeda, M., Takatsuto, S., Sassa, T., Ikekawa, N., & Nukina, M. (1983). Identification of brassinolide and its analogs in chestnut gall tissue. Agricultural and Biological Chemistry, 47, 655–657.
Ikekawa, N., & Takatsuto, S. (1984). Microanalysis of brassinosteroids in plants by gas chromatography/mass spectrometry. Journal of the Mass Spectrometry Society of Japan, 32, 55–70.
Ikekawa, N., Takatsuto, S., Kitsuwa, T., Saito, H., Morishita, T., & Abe, H. (1984). Analysis of natural brassinosteroids by gas chromatography and gas chromatography-mass spectrometry. Journal of Chromatography, 290, 289–302.
Ikekawa, N., Nishiyama, F., & Fujimoto, Y. (1988). Identification of 24-epibrassinolide in bee pollen of the broad bean, Vicia faba L. Chemical & Pharmaceutical Bulletin, 36, 405–407.
Ishiguro, M., Takatsuto, S., Morisaki, M., & Ikekawa, N. (1980). Synthesis of brassinolide, a steroidal lactone with plant-growth promoting activity. Journal of the Chemical Society, Chemical Communications, 20, 962–964.
Janeczko, A., & Swaczynova, J. (2010). Endogenous brassinosteroids in wheat treated with 24-epibrassinolide. Biologia Plantarum, 54, 477–482.
Jang, M. S., Han, K. S., & Kim, S. K. (2000). Identification of brassinosteroids and their biosynthetic precursors from seeds of pumpkin. Bulletin of the Korean Chemical Society, 21, 161–164.
Joo, S. H., Kim, T. W., Son, S. H., Lee, W. S., Yokota, T., & Kim, S. K. (2012). Biosynthesis of a cholesterol-derived brassinosteroid, 28-norcastasterone, in Arabidopsis thaliana. Journal of Experimental Botany, 63, 1823–1833.
Joo, S. H., Jang, M. S., Kim, M. K., Lee, J. E., & Kim, S. K. (2015). Biosynthetic relationship between C28-brassinosteroids and C29-brassinosteroids in rice (Oryza sativa) seedlings. Phytochemistry, 111, 84–90.
Kanwar, M. K., Bhardwaj, R., Arora, P., Chowdhary, S. P., Sharma, P., & Kumar, S. (2012). Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere, 86, 41–49.
Kanwar, M. K., Bhardwaj, R., Chowdhary, S. P., Arora, P., Sharma, P., & Kumar, S. (2013). Isolation and characterization of 24-epibrassinolide from Brassica juncea L. and its effects on growth, Ni ion uptake, antioxidant defense of Brassica plants and in vitro cytotoxicity. Acta Physiologiae Plantarum, 35, 1351–1362.
Kanwar, M. K., Poonam, & Bhardwaj, R. (2015). Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicology and Environmental Safety, 115C, 119–125.
Kasote, D. M., Ghosh, R., Kim, J., Bae, H., Chung, J. Y., & Bae, I. (2016). Multiple reaction monitoring mode based liquid chromatography-mass spectrometry method for simultaneous quantification of brassinolide and other plant hormones involved in abiotic stresses. International Journal of Analytical Chemistry, 2016, 7214087.
Katsumata, T., Hasegawa, A., Fujiwara, T., Komatsu, T., Notomi, M., Abe, H., Natsume, M., & Kawaide, H. (2008). Arabidopsis CYP85A2 catalyzes lactonization reactions in the biosynthesis of 2-deoxy-7-oxalactone brassinosteroids. Bioscience, Biotechnology, and Biochemistry, 72, 2110–2117.
Kim, S. K. (1991). Natural occurrences of brassinosteroids. In H. G. Cutler, T. Yokota, & G. Adam (Eds.), Brassinosteroids: Chemistry, bioactivity and appplications (pp. 26–35). Washington: American Chemical Society.
Kim, S. K., Yokota, T., & Takahashi, N. (1987). 25-Methyldolichosterone, a new brassinosteroid with a tertiary butyl group from immature seed of Phaseolus vulgaris. Agricultural and Biological Chemistry, 51, 2303–2305.
Kim, S. K., Abe, H., Little, C. H. A., & Pharis, R. P. (1990). Identification of two brassinosteroids from the cambial region of Scots pine (Pinus silverstris) by gas chromatography-mass spectrometry, after detection using a dwarf rice lamina inclination bioassay. Plant Physiology, 94, 1709–1713.
Kim, T. W., Chang, S. C., Choo, J., Watanabe, T., Takatsuto, S., Takao, Y., Lee, J. S., Kim, S. Y., & Kim, S. K. (2000a). Brassinolide and [26,28-2H6]brassinolide are differently demethylated by loss of C-26 and C-28, respectively, in Marchantia polymorpha. Plant & Cell Physiology, 41, 1171–1174.
Kim, T. W., Han, K. S., Joo, S. H., Kang, M. W., & Kim, S. K. (2000b). Metabolism of brassinolide in suspension cultured cells of Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 21, 1044–1046.
Kim, T. W., Park, S. H., Han, K. S., Choo, J., Lee, J. S., Hwang, S., & Kim, S. K. (2000c). Occurrence of teasterone and typhasterol, and their enzymatic conversion in Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 21, 373–374.
Kim, Y. S., Sup, Y. H., Kim, T. W., Joo, S. H., & Kim, S. K. (2002). Identification of a brassinosteroid, castasterone from Marchantia polymorpha. Bulletin of the Korean Chemical Society, 23, 941–942.
Kim, G. T., Fujioka, S., Kozuka, T., Tax, F. E., Takatsuto, S., Yoshida, S., & Tsukaya, H. (2005a). CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. The Plant Journal, 41, 710–721.
Kim, Y. S., Kim, T. W., & Kim, S. K. (2005b). Brassinosteroids are inherently biosynthesized in the primary roots of maize, Zea mays L. Phytochemistry, 66, 1000–1006. https://doi.org/10.1016/j.phytochem.2005.03.007.
Kim, H. B., Kwon, M., Ryu, H., Fujioka, S., Takatsuto, S., Yoshida, S., An, C. S., Lee, I., Hwang, I., & Choe, S. (2006a). The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiology, 140, 548–557.
Kim, Y. S., Joo, S. H., Hwang, J. Y., Park, C. H., & Kim, S. K. (2006b). Characterization of C29-brassinosteroids and their biosynthetic precursors in immature seeds of Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 27, 1117–1118.
Kim, Y. S., Kim, T. W., Chang, S. C., Pharis, R. P., Lee, J. S., Han, T. J., Takatsuto, S., Cheong, H., & Kim, S. K. (2006c). Regulation of castasterone level in primary roots of maize, Zea mays. Physiologia Plantarum, 127, 28–37.
Kim, B. K., Fujioka, S., Takatsuto, S., Tsujimoto, M., & Choe, S. (2008). Castasterone is a likely end product of brassinosteroid biosynthetic pathway in rice. Biochemical and Biophysical Research Communications, 374, 614–619.
Kim, M. K., Jang, M. S., Youn, J. H., Son, S. H., Lee, J. E., Kim, T. W., & Kim, S. K. (2015). Occurrence of phosphorylated castasterone in Arabidopsis thaliana and Lycopersicum esculentum. Physiologia Plantarum, 153, 58–67.
Koka, C. V., Cerny, R. E., Gardner, R. G., Noguchi, T., Fujioka, S., Takatsuto, S., Yoshida, S., & Clouse, S. D. (2000). A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiology, 122, 85–98.
Konstantinova, O. V., Antonchick, A. P., Oldham, N. J., Zhabinskii, V. N., Khripach, V. A., & Schneider, B. (2001). Analysis of underivatized brassinosteroids by HPLC/APCI-MS. Occurrence of 3-epibrassinolide in Arabidopsis thaliana. Collection of Czechoslovak Chemical Communications, 66, 1729–1734.
Lee, H. K., Kwon, M., Jeon, J. H., Fujioka, S., Kim, H. B., Park, S. Y., …Choe, S. (2006). An Arabidopsis short root and dwarfism mutant defines a novel locus that mediates both cell division and elongation. Journal of Plant Biology, 49, 61–69. https://doi.org/10.1007/BF03030789.
Lee, S. C., Hwang, J. Y., Joo, S. H., Son, S. H., Youn, J. H., & Kim, S. K. (2010). Biosynthesis and metabolism of dolichosterone in Arabidopsis thaliana. Bulletin of the Korean Chemical Society, 31, 3475–3478.
Lee, S. C., Joo, S. H., & Kim, S. K. (2011). Stereoisomers of castasterone, 3-epicastasterone and 2,3-diepicastasterone, in immature seeds of Phaseolus vulgaris. Journal of Plant Biology, 54, 10–14.
Li, H., Jiang, L., Youn, J. H., Sun, W., Cheng, Z., Jin, T., Ma, X., Guo, X., Wang, J., Zhang, X., Wu, F., Wu, C., Kim, S. K., & Wan, J. (2013). A comprehensive genetic study reveals a crucial role of CYP90D2/D2 in regulating plant architecture in rice (Oryza sativa). The New Phytologist, 200, 1076–1088.
Liu, J., Zhang, D., Sun, X., Ding, T., Lei, B., & Zhang, C. (2017). Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids, 124, 1–17.
Lv, T., Zhao, X. E., Zhu, S., Ji, Z., Chen, G., Sun, Z., Song, C., You, J., & Suo, Y. (2014). Development of an efficient HPLC fluorescence detection method for brassinolide by ultrasonic-assisted dispersive liquid-liquid microextraction coupled with derivatization. Chromatographia, 77, 1653–1660.
Maeda, E. (1965). Rate of lamina inclination in excised rice leaves. Physiologia Plantarum, 18, 813–827.
Mandava, N. B. (1988). Plant growth-promoting brassinosteroids. Annual Review of Plant Physiology and Plant Molecular Biology, 39, 23–52.
Mitchell, J. W., & Gregory, L. E. (1972). Enhancement of overall plant growth, a new response to brassins. Nature: New Biology, 239, 253–254.
Mitchell, J. W., & Whitehead, M. R. (1941). Responses of vegetative parts of plants following application of extract of pollen of Zea mays. Botanical Gazette, 102, 770–791.
Mitchell, J. W., Skraggs, D. P., & Anderson, W. P. (1951). Plant growth stimulating hormones in immature bean seeds. Science, 114, 159–161.
Mitchell, J. W., Mandava, N., Worley, J. F., Plimmer, J. R., & Smith, M. V. (1970). Brassins – a new family of plant hormones from rape pollen. Nature, 225, 1065–1066.
Mori, K. (1980). Synthesis of a brassinolide analog with high plant growth promoting activity. Agricultural and Biological Chemistry, 44, 1211–1212.
Mori, K., & Takeuchi, T. (1988). Synthesis of 25-methyldolichosterone, 25-methyl-2,3-diepidolichosterone, 25-methylcastasterone and 25-methylbrassinolide. Liebigs Annalen der Chemie, 1988, 815–818.
Mori, K., Sakakibara, M., Ichikawa, Y., Ueda, H., Okada, K., Umemura, T., Yabuta, G., Kuwahara, S., Kondo, Minobe, M., & Sogabe, A. (1982). Synthesis of (22S,23S)-homobrassinolide and brassinolide from stigmasterol. Tetrahedron, 38, 2099–2109.
Mori, M., Nomura, T., Ooka, H., Ishizaka, M., Yokota, T., Sugimoto, K., Okabe, K., Kajiwara, H., Satoh, K., Yamamoto, K., Hirochika, H., & Kikuchi, S. (2002). Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiology, 130, 1152–1161.
Morishita, T., Abe, H., Uchiyama, M., Marumo, S., Takatsuto, S., & Ikekawa, N. (1983). Evidence for plant growth promoting brassinosteroids in leaves of Thea sinensis. Phytochemistry, 22, 1051–1053.
Motegi, C., Takatsuto, S., & Gamoh, K. (1994). Identification of brassinolide and castasterone in the pollen of orange (Citrus sinensis Osbeck) by high-performance liquid chromatography. Journal of Chromatography A, 658, 27–30.
Nakamura, M., Satoh, T., Tanaka, S. I., Mochizuki, N., Yokota, T., & Nagatani, A. (2005). Activation of the cytochrome P450 gene, CYP72C1, reduces the levels of active brassinosteroids in vivo. Journal of Experimental Botany, 56, 833–840.
Nakamura, A., Fujioka, S., Sunohara, H., Kamiya, N., Hong, Z., Inukai, Y., Miura, K., Takatsuto, S., Yoshida, S., Ueguchi-Tanaka, M., Hasegawa, Y., Kitano, H., & Matsuoka, M. (2006). The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiology, 140, 580–590.
Noguchi, T., Fujioka, S., Choe, S., Takatsuto, S., Yoshida, S., Yuan, H., Feldmann, K. A., & Tax, F. E. (1999). Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiology, 121, 743–752.
Noguchi, T., Fujioka, S., Choe, S., Takatsuto, S., Tax, F. E., Yoshida, S., & Feldmann, K. A. (2000). Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiology, 124, 201–209. https://doi.org/10.1104/pp.124.1.201.
Nomura, T., Nakayama, M., Reid, J. B., Takeuchi, Y., & Yokota, T. (1997). Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiology, 113, 31–37.
Nomura, T., Sato, T., Bishop, G. J., Kamiya, Y., Takatsuto, S., & Yokota, T. (2001). Accumulation of 6-deoxocathasterone and 6-deoxocastasterone in Arabidopsis, pea and tomato is suggestive of common rate-limiting steps in brassinosteroid biosynthesis. Phytochemistry, 57, 171–178.
Nomura, T., Jager, C. E., Kitasaka, Y., Takeuchi, K., Fukami, M., Yoneyama, K., Matsushita, Y., Nyunoya, H., Takatsuto, S., Fujioka, S., Smith, J. J., Kerckhoffs, L. H. J., Reid, J. B., & Yokota, T. (2004). Brassinosteroid deficiency due to truncated steroid 5a-reductase causes dwarfism in the lk mutant of pea. Plant Physiology, 135, 2220–2229.
Nomura, T., Kushiro, T., Yokota, T., Kamiya, Y., Bishop, G. J., & Yamaguchi, S. (2005). The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. The Journal of Biological Chemistry, 280, 17873–17879.
Nomura, T., Ueno, M., Yamada, Y., Takatsuto, S., Takeuchi, Y., & Yokota, T. (2007). Roles of brassinosteroids and related mRNAs in pea seed growth and germination. Plant Physiology, 143, 1680–1688.
Ohnishi, T., Nomura, T., Watanabe, B., Ohta, D., Yokota, T., Miyagawa, H., Sakata, K., & Mizutani, M. (2006a). Tomato cytochrome P450 CYP734A7 functions in brassinosteroid catabolism. Phytochemistry, 67, 1895–1906.
Ohnishi, T., Szatmari, A.-M., Watanabe, B., Fujita, S., Bancos, S., Koncz, C., Lafos, M., Shibata, K., Yokota, T., Sakata, K., Szekeres, M., & Mizutani, M. (2006b). C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell, 18, 3275–3288.
Ohnishi, T., Godza, B., Watanabe, B., Fujioka, S., Hategan, L., Ide, K., Shibata, K., Yokota, T., Szekeres, M., & Mizutani, M. (2012). CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. The Journal of Biological Chemistry, 287, 31551–31560.
Oikawa, A., Otsuka, T., Nakabayashi, R., Jikumaru, Y., Isuzugawa, K., Murayama, H., Saito, K., & Shiratake, K. (2015). Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS One, 10, 0131408.
Oklestkova, J., Tarkowska, D., Eyer, L., Elbert, T., Marek, A., Smrzova, Z., …Strnad, M. (2017). Immunoaffinity chromatography combined with tandem mass spectrometry: A new tool for the selective capture and analysis of brassinosteroid plant hormones. Talanta, 170, 432–440. https://doi.org/10.1016/j.talanta.2017.04.044.
Pachthong, C., Supyen, D., Buddhasukh, D., & Jatisatienr, A. (2006). Isolation and characterization of brassinolide and castasterone in the pollen of pumpkin. Chiang Mai Journal of Science, 33, 95–101.
Pachthong, C., Supyen, D., Buddhasukh, D., & Jatisatien, A. (2007). Isolation and characterization of brassinolide and castasterone from mature seeds of smooth loofah (Luffa cylindrica (L.) M.J. Roem). ACGC Chemical Research Communications, 21, 4–8.
Pan, J., Hu, Y., Liang, T., & Li, G. (2012). Preparation of solid-phase microextraction fibers by in-mold coating strategy for derivatization analysis of 24-epibrassinolide in pollen samples. Journal of Chromatography. A, 1262, 49–55.
Pan, J., Huang, Y., Liu, L., Hu, Y., & Li, G. (2013). A novel fractionized sampling and stacking strategy for online hyphenation of solid-phase-based extraction to ultra-high performance liquid chromatography for ultrasensitive analysis. Journal of Chromatography. A, 1316, 29–36.
Park, K. H., Yokota, T., Sakurai, A., & Takahashi, N. (1987). Occurrence of castasterone, brassinolide and methyl 4-chloroindole-3-acetate in immature Vicia faba seeds. Agricultural and Biological Chemistry, 51, 3081–3086.
Park, K. H., Saimoto, H., Nakagawa, S., Sakurai, A., Yokota, T., Takahashi, N., & Shono, K. (1989). Occurrence of brassinolide and castasterone in crown gall cells of Catharanthus roseus. Agricultural and Biological Chemistry, 53, 805–811.
Park, K. H., Park, J. D., Hyun, K. H., Nakayama, M., & Yokota, T. (1994a). Brassinosteroids and monoglycerides in immature seeds of Cassia tora as the active principles in the rice lamina inclination bioassay. Bioscience, Biotechnology, and Biochemistry, 58, 1343–1344.
Park, K. H., Park, J. D., Hyun, K. H., Nakayama, M., & Yokota, T. (1994b). Brassinosteroids and monoglycerides with brassinosteroid like activity in immature seeds of Oryza sativa and Perilla frutescens and in cultured cells of Nicotiana tabacum. Bioscience, Biotechnology, and Biochemistry, 58, 2241–2243.
Park, C. H., Yokota, T., & Kim, S. K. (2009a). 2-Deoxy-25-methyldolichosterone and 3-epi-2-deoxy-25-methyldolichosterone in immature seeds of Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 30, 2422–2424.
Park, C. H., Yokota, T., & Kim, S. K. (2009b). Characterization of 2-epicastasterone from immature seeds of Phaseolus vulgaris. Bulletin of the Korean Chemical Society, 30, 2193–2194.
Pereira-Netto, A. B., Roessner, U., Fujioka, S., Bacic, A., Asami, T., Yoshida, S., & Clouse, S. D. (2009). Shooting control by brassinosteroids: Metabolomic analysis and effect of brassinazole on Malus prunifolia, the Marubakaido apple rootstock. Tree Physiology, 29, 607–620.
Pinto, F. C., Ascenso, J. R., & Ferreira, M. J. U. (2002). A short side chain cycloartane and other triterpenes from Euphorbia tuckeyana. In A. P. Rauter, F. B. Palma, J. Justino, M. E. Araújo, & S. Pina dos Santos (Eds.), Natural products in the new millennium: prospects and industrial application (pp. 73–79).
Plattner, R. D., Taylor, S. L., & Grove, M. D. (1986). Detection of brassinolide and castasterone in Alnus glutinosa (European alder) pollen by mass spectrometry/mass spectrometry. Journal of Natural Products, 49, 540–545.
Pociecha, E., Dziurka, M., Oklestkova, J., & Janeczko, A. (2016). Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. Plant Growth Regulation, 80, 127–135.
Polko, J. K., Pierik, R., van Zanten, M., Tarkowska, D., Strnad, M., Voesenek, L. A. C. J., & Peeters, A. J. M. (2013). Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. Journal of Experimental Botany, 64, 613–624.
Poppenberger, B., Fujioka, S., Soeno, K., George, G. L., Vaistij, F. E., Hiranuma, S., Seto, H., Takatsuto, S., Adam, G., Yoshida, S., & Bowles, D. (2005). The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proceedings of the National Academy of Sciences, 102, 15253–15258.
Pradko, A. G., Litvinovskaya, R. P., Sauchuk, A. L., Drach, S. V., Baranovsky, A. V., Zhabinskii, V. N., Mirantsova, T. V., & Khripach, V. A. (2015). A new ELISA for quantification of brassinosteroids in plants. Steroids, 97, 78–86.
Qian, W., Wu, C., Fu, Y., Hu, G., He, Z., & Liu, W. (2017). Novel rice mutants overexpressing the brassinosteroid catabolic gene CYP734A4. Plant Molecular Biology, 93, 197–208.
Ren, C. G., Chen, Y., & Dai, C. C. (2014). Cross-talk between calcium-calmodulin and brassinolide for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. Journal of Plant Growth Regulation, 33, 285–294.
Roh, H., Jeong, C. W., Fujioka, S., Kim, Y. K., Lee, S., Ahn, J. H., Choi, Y. D., & Lee, J. S. (2012). Genetic evidence for the reduction of brassinosteroid levels by a BAHD acyltransferase-like protein in Arabidopsis. Plant Physiology, 159, 696–709.
Rouleau, M., Marsolais, F., Richard, M., Nicolle, L., Voigt, B., Adam, G., & Varin, L. (1999). Inactivation of brassinosteroid biological activity by a salicylate-inducible steroid sulfotransferase from Brassica napus. The Journal of Biological Chemistry, 274, 20925–20930.
Sakakibara, M., & Mori, K. (1982). Facile synthesis of (22R,23R)-homobrassinolide. Agricultural and Biological Chemistry, 46, 2769–2779.
Sakakibara, M., Okada, K., Ichikawa, Y., & Mori, K. (1982). Synthesis of brassinolide, a plant-growth-promoting steroidal lactone. Heterocycles, 17, 301–304.
Sakamoto, T., Morinaka, Y., Ohnishi, T., Sunohara, H., Fujioka, S., Ueguchi-Tanaka, M., Mizutani, M., Sakata, K., Takatsuto, S., Yoshida, S., Tanaka, H., Kitano, H., & Matsuoka, M. (2006). Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nature Biotechnology, 24, 105–109.
Sakamoto, T., Ohnishi, T., Fujioka, S., Watanabe, B., & Mizutani, M. (2012). Rice CYP90D2 and CYP90D3 catalyze C-23 hydroxylation of brassinosteroids in vitro. Plant Physiology and Biochemistry, 58, 220–226.
Sasse, J. M., Griffiths, P. G., Gaff, D. F., Yokota, T., & Cameron, D. W. (1998). Brassinosteroids of a ressurrection grass. In Abstracts of the 16th international conference on plant growth substances, Chiba, Japan (pp. 13–17).
Schmidt, J., Yokota, T., Adam, G., & Takahashi, N. (1991). Castasterone and brassinolide in Raphanus sativus seeds. Phytochemistry, 30, 364–365.
Schmidt, J., Spengler, B., Yokota, T., & Adam, G. (1993a). The cooccurrence of 24-epi-castasterone and castasterone in seeds of Ornithopus sativus. Phytochemistry, 32, 1614–1615.
Schmidt, J., Tokota, T., Spengler, B., & Adam, G. (1993b). 28-Homoteasterone, a naturally occurring brassinosteroid from seeds of Raphanus sativus. Phytochemistry, 34, 391–392.
Schmidt, J., Kuhnt, C., & Adam, G. (1994). Brassinosteroids and sterols from seeds of Beta vulgaris. Phytochemistry, 36, 175–177.
Schmidt, J., Himmelreich, U., & Adam, G. (1995a). Brassinosteroids, sterols and lup-20(29)-en-2a,3b,28-triol from Rheum rhabarbarum. Phytochemistry, 40, 527–531.
Schmidt, J., Spengler, B., Yokota, T., Nakayama, M., Takatsuto, S., Voigt, B., & Adam, G. (1995b). Secasterone, the first naturally occurring 2,3-epoxybrassinosteroid from Secale cereale. Phytochemistry, 38, 1095–1097.
Schmidt, J., Voigt, B., & Adam, G. (1995c). 2-Deoxybrassinolide – a naturally occurring brassinosteroid from Apium graveolens. Phytochemistry, 40, 1041–1043.
Schmidt, J., Boehme, F., & Adam, G. (1996). 24-epibrassinolide from Gypsophila perfoliata. Zeitschrift fur Naturforschung C: Journal of Biosciences, 51, 897–899.
Schmidt, J., Altmann, T., & Adam, G. (1997). Brassinosteroids from seeds of Arabidopsis thaliana. Phytochemistry, 45, 1325–1327.
Schmidt, J., Porzel, A., & Adam, G. (1998). Brassinosteroids and a pregnane glucoside from Daucus carota. Phytochemical Analysis, 9, 14–20.
Schneider, J. A., Yoshihara, K., Nakanishi, K., & Kato, N. (1983). Typhasterol (2-deoxycastasterone) – a new plant growth regulator from cat-tail pollen. Tetrahedron Letters, 24, 3859–3860.
Schneider, K., Breuer, C., Kawamura, A., Jikumaru, Y., Hanada, A., Fujioka, S., Ichikawa, T., Kondou, Y., Matsui, M., Kamiya, Y., Yamaguchi, S., & Sugimoto, K. (2012). Arabidopsis PIZZA has the capacity to acylate brassinosteroids. PLoS One, 7, 46805.
Sekimoto, H., Hoshi, M., Nomura, T., & Yokota, T. (1997). Zinc deficiency affects the levels of endogenous gibberellins in Zea mays L. Plant & Cell Physiology, 38, 1087–1090.
Shahnejat-Bushehri, S., Tarkowska, D., Sakuraba, Y., & Balazadeh, S. (2016). Arabidopsis NAC transcription factor JUB1 regulates GA/BR metabolism and signalling. Nature Plants, 2, 16013.
Shim, J. H., Kim, I. S., Lee, K. B., Suh, Y. T., & Morgan, E. D. (1996). Determination of brassinolide by HPLC equipped with fluorescence detector in rice (Oriza sativa L.). Journal of the Korean Chemical Society, 39, 84–88.
Shimada, K., Abe, H., Takatsuto, S., Nakayama, M., & Yokota, T. (1996). Identification of castasterone and teasterone from seeds of canary grass (Phalaris canariensis). Recent Research and Development in Chemistry and Pharmaceutical Sciences, 1, 1–5.
Shimada, Y., Goda, H., Nakamura, A., Takatsuto, S., Fujioka, S., & Yoshida, S. (2003). Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiology, 131, 287–297.
Singh, A. P., Fridman, Y., Friedlander-Shani, L., Tarkowska, D., Strnad, M., & Savaldi-Goldstein, S. (2014). Activity of the brassinosteroid transcription factors BRASSINAZOLE RESISTANT1 and BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1/BRASSINAZOLE RESISTANT2 blocks developmental reprogramming in response to low phosphate availability. Plant Physiology, 166, 678–688.
Soeno, K., Asakawa, S., Natsume, M., & Abe, H. (2000a). Reversible conversion between teasterone and its ester conjugates in lily cell cultures. Journal of Pesticide Science, 25, 117–122.
Soeno, K., Kyokawa, Y., Natsume, M., & Abe, H. (2000b). Teasterone-3-O-β-D-glucopyranoside, a new conjugated brassinosteroid metabolite from lily cell suspension cultures and its identification in lily anthers. Bioscience, Biotechnology, and Biochemistry, 64, 702–709.
Soeno, K., Fujioka, S., Hiranuma, S., Seto, H., & Yoshida, S. (2006). Metabolic conversion of castasterone and brassinolide into their glucosides in higher plants. Journal of Plant Growth Regulation, 25, 195–202.
Son, S. H., Youn, J. H., Kim, M. K., & Kim, S. K. (2013). C-26 demethylation of brassinosteroids in Arabidopsis thaliana. Bulletin of the Korean Chemical Society, 34, 259–262.
Sondhi, N., Bhardwaj, R., Kaur, S., Kumar, N., & Singh, B. (2008). Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its antigenotoxicity employing Allium cepa chromosomal aberration assay. Plant Growth Regulation, 54, 217–224.
Sondhi, N., Bhardwaj, R., Kaur, S., Chande, M., Kumar, N., & Singh, B. (2010). Inhibition of H2O2-induced DNA damage in single cell gel electrophoresis assay (comet assay) by castasterone isolated from leaves of Centella asiatica. Health, 2, 595–602.
Spengler, B., Schmidt, J., Voigt, B., & Adam, G. (1995). 6-Deoxo-28-norcastasterone and 6-deoxo-24-epicastasterone – 2 new brassinosteroids from Ornithopus sativus. Phytochemistry, 40, 907–910.
Stirk, W. A., Balint, P., Tarkowska, D., Novak, O., Strnad, M., Oerdoeg, V., & van Staden, J. (2013). Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiology and Biochemistry, 70, 348–353.
Stirk, W. A., Balint, P., Tarkowska, D., Novak, O., Maroti, G., Ljung, K., Tureckova, V., Strnad, M., Oerdoeg, V., & van Staden, J. (2014a). Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiology and Biochemistry, 79, 66–76.
Stirk, W. A., Tarkowska, D., Turecova, V., Strnad, M., & van Staden, J. (2014b). Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. Journal of Applied Phycology, 26, 561–567.
Stirk, W. A., Balint, P., Tarkowska, D., Strnad, M., van Staden, J., & Ordog, V. (2018). Endogenous brassinosteroids in microalgae exposed to salt and low temperature stress. European Journal of Phycology, 53(3), 273–279. https://doi.org/10.1080/09670262.2018.1441447.
Suzuki, Y., Yamaguchi, I., & Takahashi, N. (1985). Identification of castasterone and brassinone from immature seeds of Pharbitis purpurea. Agricultural and Biological Chemistry, 49, 49–54.
Suzuki, Y., Yamaguchi, I., Yokota, T., & Takahashi, N. (1986). Identification of castasterone, typhasterol and teasterone from the pollen of Zea mays. Agricultural and Biological Chemistry, 50, 3133–3138.
Suzuki, H., Fujioka, S., Takatsuto, S., Yokota, T., Murofushi, N., & Sakurai, A. (1993a). Biosynthesis of brassinolide from castasterone in cultured cells of Catharanthus roseus. Journal of Plant Growth Regulation, 12, 101–106.
Suzuki, H., Kim, S. K., Takahashi, N., & Yokota, T. (1993b). Metabolism of castasterone and brassinolide in mung bean explant. Phytochemistry, 33, 1361–1367.
Suzuki, H., Fujioka, S., Takatsuto, S., Yokota, T., Murofushi, N., & Sakurai, A. (1994a). Biosynthesis of brassinolide from teasterone via typhasterol and castasterone in cultured cells of Catharanthus roseus. Journal of Plant Growth Regulation, 13, 21–26.
Suzuki, H., Fujioka, S., Yokota, T., Murofushi, N., & Sakurai, A. (1994b). Identification of brassinolide, castasterone, typhasterol from the pollen of Lilium elegans. Bioscience, Biotechnology, and Biochemistry, 58, 2075–2076.
Suzuki, H., Inoue, T., Fujioka, S., Takatsuto, S., Yanagisawa, T., Yokota, T., Murofushi, N., & Sakurai, A. (1994c). Possible involvement of 3-dehydroteasterone in the conversion of teasterone to typhasterol in cultured cells of Catharanthus roseus. Bioscience, Biotechnology, and Biochemistry, 58, 1186–1188.
Suzuki, H., Fujioka, S., Takatsuto, S., Yokota, T., Murofushi, N., & Sakurai, A. (1995). Biosynthesis of brassinosteroids in seedlings of Catharanthus roseus, Nicotiana tabacum, and Oryza sativa. Bioscience, Biotechnology, and Biochemistry, 59, 168–172.
Suzuki, Y., Saso, K., Fujioka, S., Yoshida, S., Nitasaka, E., Nagata, S., Nagasawa, H., Takatsuto, S., & Yamaguchi, I. (2003). A dwarf mutant strain of Pharbitis nil, Uzukobito (kobito), has defective brassinosteroid biosynthesis. The Plant Journal, 36, 401–410.
Swaczynova, J., Novak, O., Hauserova, E., Fuksova, K., Sisa, M., Kohout, L., & Strnad, M. (2007). New techniques for the estimation of naturally occurring brassinosteroids. Journal of Plant Growth Regulation, 26, 1–14.
Takahashi, N., Yokota, T., Kin, S. (1988). Isolation of brassinosteroids from bean seeds, as plant growth regulators. Japanese Kokkai Tokkyo Koho JP63255297A
Takahashi, N., Nakazawa, M., Shibata, K., Yokota, T., Ishikawa, A., Suzuki, K., Kawashima, M., Ichikawa, T., Shimada, H., & Matsui, M. (2005). shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. The Plant Journal, 42, 13–22.
Takatsuto, S. (1994). Brassinosteroids: Distribution in plants, bioassays and microanalysis by gas chromatography-mass spectrometry. Journal of Chromatography. A, 658, 3–15.
Takatsuto, S., & Makiuchi, K. (2000). Identification of castasterone and sterols in the seeds of Lagenaria siceraria. Journal of Japan Oil Chemists’ Society, 49, 169–171.
Takatsuto, S., Ying, B., Morisaki, M., & Ikekawa, N. (1981). Synthesis of 28-norbrassinolide. Chemical & Pharmaceutical Bulletin, 29, 903–905.
Takatsuto, S., Ying, B., Morisaki, M., & Ikekawa, N. (1982). Microanalysis of brassinolide and its analogs by gas chromatography and gas chromatography-mass spectrometry. Journal of Chromatography, 239, 233–241.
Takatsuto, S., Yazawa, N., Ikekawa, N., Morishita, T., & Abe, H. (1983a). Synthesis of (24R)-28-homobrassinolide analogs and structure-activity relationships of brassinosteroids in the rice-lamina inclination test. Phytochemistry, 22, 1393–1397.
Takatsuto, S., Yazawa, N., Ikekawa, N., Takematsu, T., Takeuchi, Y., & Koguchi, M. (1983b). Structure-activity relationship of brassinosteroids. Phytochemistry, 22, 2437–2441.
Takatsuto, S., Ikekawa, N., Morishita, T., & Abe, H. (1987). Structure-activity relationship of brassinosteroids with respect to the A/B-ring functional groups. Chemical & Pharmaceutical Bulletin, 35, 211–216.
Takatsuto, S., Yokota, T., Omote, K., Gamoh, K., & Takahashi, N. (1989). Identification of brassinolide, castasterone and norcastasterone (brassinone) in sunflower (Helianthus annuus L.) pollen. Agricultural and Biological Chemistry, 53, 2177–2180.
Takatsuto, S., Abe, H., & Gamoh, K. (1990a). Evidence for brassinosteroids in strobilus of Equisetum arvense L. Agricultural and Biological Chemistry, 54, 1057–1059.
Takatsuto, S., Omote, K., Gamoh, K., & Ishibashi, M. (1990b). Identification of brassinolide and castasterone in buckwheat (Fagopyrum esculentum Moench) pollen. Agricultural and Biological Chemistry, 54, 757–762.
Takatsuto, S., Abe, H., Shimada, K., Nakayama, M., & Yokota, T. (1996a). Identification of teasterone and 4-desmethylsterols in the seeds of Ginkgo biloba L. Journal of Japan Oil Chemists’ Society, 45, 1349–1351.
Takatsuto, S., Abe, H., Yokota, T., Shimada, K., & Gamoh, K. (1996b). Identification of castasterone and teasterone in seeds of Cannabis sativa L. Journal of Japan Oil Chemists’ Society, 45, 871–873.
Takatsuto, S., Tsunokawa, E., Noguchi, T., & Fujioka, S. (1999). Identification of sterols and castasterone in the seeds of Amaranthus inamoenus. Journal of Japan Oil Chemists’ Society, 48, 347–349.
Tamiru, M., Takagi, H., Abe, A., Yokota, T., Kanzaki, H., Okamoto, H., Saitoh, H., Takahashi, H., Fujisaki, K., Oikawa, K., Uemura, A., Natsume, S., Jikumaru, Y., Matsuura, H., Umemura, K., Terry, M. J., & Terauchi, R. (2016). A chloroplast-localized protein LESION AND LAMINA BENDING affects defence and growth responses in rice. The New Phytologist, 210, 1282–1297.
Tanabe, S., Ashikari, M., Fujioka, S., Takatsuto, S., Yoshida, S., Yano, M., Yoshimura, A., Kitano, H., Matsuoka, M., Fujisawa, Y., Kato, H., & Iwasaki, Y. (2005). A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell, 17, 776–790.
Taylor, P. E., Spuck, K., Smith, P. M., Sasse, J. M., Yokota, T., Griffiths, P. G., & Cameron, D. W. (1993). Detection of brassinosteroids in pollen of Lolium perenne L. by immunocytochemistry. Planta, 189, 91–100.
Thompson, M. J., Mandava, N., Flippen-Anderson, J. L., Worley, J. F., Dutky, S. R., Robbins, W. E., & Lusby, W. (1979). Synthesis of brassino steroids: New plant-growth-promoting steroids. The Journal of Organic Chemistry, 44, 5002–5004.
Thompson, M. J., Mandava, N. B., Meudt, W. J., Lusby, W. R., & Spaulding, D. W. (1981). Synthesis and biological activity of brassinolide and its 22β,23β-isomer: Novel plant growth-promoting steroids. Steroids, 38, 567–580.
Thompson, M. J., Meudt, W. J., Mandava, N. B., Dutky, S. R., Lusby, W. R., & Spaulding, D. W. (1982). Synthesis of brassinosteroids and relationship of structure to plant growth-promoting effects. Steroids, 39, 89–105.
Turk, E. M., Fujioka, S., Seto, H., Shimada, Y., Takatsuto, S., Yoshida, S., Denzel, M. A., Torres, Q. I., & Neff, M. M. (2003). CYP72B1 inactivates brassinosteroid hormones: An intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiology, 133, 1643–1653.
Turk, E. M., Fujioka, S., Seto, H., Shimada, Y., Takatsuto, S., Yoshida, S., Wang, H. C., Torres, Q. I., Ward, J. M., Murthy, G., Zhang, J. Y., Walker, J. C., & Neff, M. M. (2005). BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. The Plant Journal, 42, 23–34.
Van Meulebroek, L., Vanden Bussche, J., Steppe, K., & Vanhaecke, L. (2012). Ultra-high performance liquid chromatography coupled to high resolution orbitrap mass spectrometry for metabolomic profiling of the endogenous phytohormonal status of the tomato plant. Journal of Chromatography. A, 1260, 67–80.
Verhoef, N., Yokota, T., Shibata, K., de Boer, G. J., Gerats, T., Vandenbussche, M., Koes, R., & Souer, E. (2013). Brassinosteroid biosynthesis and signalling in Petunia hybrida. Journal of Experimental Botany, 64, 2435–2448.
Villiers, F., Jourdain, A., Bastien, O., Leonhardt, N., Fujioka, S., Tichtincky, G., Parcy, F., Bourguignon, J., & Hugouvieux, V. (2012). Evidence for functional interaction between brassinosteroids and cadmium response in Arabidopsis thaliana. Journal of Experimental Botany, 63, 1185–1200.
Vriet, C., Russinova, E., & Reuzeau, C. (2013). From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Molecular Plant, 6, 1738–1757.
Wada, K., Marumo, S., Ikekawa, N., Morisaki, M., & Mori, K. (1981). Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant & Cell Physiology, 22, 323–325. https://doi.org/10.1093/oxfordjournals.pcp.a076173.
Wada, K., Marumo, S., Mori, K., Takatsuto, S., Morisaki, M., & Ikekawa, N. (1983). The rice lamina inclination-promoting activity of synthetic brassinolide analogues with a modified side chain. Agricultural and Biological Chemistry, 47, 1139–1141.
Wang, M. Y., & Lu, D. Z. (2008). Determination of brassinolide in Areca catechu pollen by HPLC. Anhui Nongye Kexue. Journal of Anhui Agricultural Sciences, 36, 1305–1306.
Wang, L., Duan, C., Wu, D., & Guan, Y. (2014). Quantification of endogenous brassinosteroids in sub-gram plant tissues by in-line matrix solid-phase dispersion-tandem solid phase extraction coupled with high performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography. A, 1359, 44–51.
Wang, Q., Cai, W. J., Yu, L., Ding, J., & Feng, Y. Q. (2017). Comprehensive profiling of phytohormones in honey by sequential liquid-liquid extraction coupled with liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry, 65, 575–585.
Watanabe, T., Yokota, T., Shibata, K., Nomura, T., Seto, H., & Takatsuto, S. (2000). Cryptolide, a new brassinolide catabolite with a 23-oxo group from Japanese cedar pollen/anther and its synthesis. Journal of Chemical Research, Synopses, 2000, 18–19. Miniprint 215–236.
Watanabe, T., Noguchi, T., Yokota, T., Shibata, K., Koshino, H., Seto, H., Kim, S. K., & Takatsuto, S. (2001). Synthesis and biological activity of 26-norbrassinolide, 26-norcastasterone and 26-nor-6-deoxocastasterone. Phytochemistry, 58, 343–349.
Wu, C. Y., Trieu, A., Radhakrishnan, P., Kwok, S. F., Harris, S., Zhang, K., Wang, J., Wan, J., Zhai, H., Takatsuto, S., Matsumoto, S., Fujioka, S., Feldmann, K. A., & Pennell, R. I. (2008). Brassinosteroids regulate grain filling in rice. Plant Cell, 20, 2130–2145.
Wu, Q., Wu, D., Shen, Z., Duan, C., & Guan, Y. (2013). Quantification of endogenous brassinosteroids in plant by on-line two-dimensional microscale solid phase extraction-on column derivatization coupled with high performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography. A, 1297, 56–63.
Xin, P., Yan, J., Fan, J., Chu, J., & Yan, C. (2013). An improved simplified high-sensitivity quantification method for determining brassinosteroids in different tissues of rice and Arabidopsis. Plant Physiology, 162, 2056–2066.
Xin, P., Li, B., Fang, S., Yan, C., Chu, J., Yan, J., Fan, J., Tian, H., Shi, Y., & Tian, W. (2016). A comprehensive and effective mass spectrometry-based screening strategy for discovery and identification of new brassinosteroids from rice tissues. Frontiers in Plant Science, 7, 1786.
Xu, F., Zhang, H., Zhang, C. J., Xi, Z. M., & Zhang, Z. W. (2015). Brassinosteroids are involved in controlling sugar unloading in Vitis vinifera ‘cabernet sauvignon’ berries during veraison. Plant Physiology and Biochemistry, 94, 197–208.
Xu, Z., Lei, P., Feng, X., Li, S., & Xu, H. (2016). Analysis of the metabolic pathways affected by poly (γ-glutamic acid) in Arabidopsis thaliana based on genechip microarray. Journal of Agricultural and Food Chemistry, 64, 6257–6266.
Yamamoto, R., Fujioka, S., Demura, T., Takatsuto, S., Yoshida, S., & Fukuda, H. (2001). Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiology, 125, 556–563.
Yamamoto, R., Fujioka, S., Iwamoto, K., Demura, T., Takatsuto, S., Yoshida, S., & Fukuda, H. (2007). Co-regulation of brassinosteroid biosynthesis-related genes during xylem cell differentiation. Plant & Cell Physiology, 48, 74–83.
Yasuta, E., Terahata, T., Nakayama, M., Abe, H., Takatsuto, S., & Yokota, T. (1995). Free and conjugated brassinosteroids in the pollen and anthers of Erythronium japonicum decne. Bioscience, Biotechnology, and Biochemistry, 59, 2156–2158.
Yokota, T., & Takahashi, N. (1988). Isolation of brassinosteroids as plant growth regulators from kidney beans. Japanese Kokai Tokkyo Koho JP 63216896 A.
Yokota, T., Arima, M., & Takahashi, N. (1982a). Castasterone, a new phytosterol with plant-hormone potency, from chestnut insect gall. Tetrahedron Letters, 23, 1275–1278.
Yokota, T., Baba, J., & Takahashi, N. (1982b). A new steroidal lactone with plant growth-regulatory activity from Dolichos lablab seed. Tetrahedron Letters, 23, 4965–4966.
Yokota, T., Arima, M., Takahashi, N., Takatsuto, S., Ikekawa, N., & Takematsu, T. (1983a). 2-Deoxycastasterone, a new brassinolide-related bioactive steroid from Pinus pollen. Agricultural and Biological Chemistry, 47, 2419–2420.
Yokota, T., Baba, J., & Takahashi, N. (1983b). Brassinolide-related bioactive sterols in Dolichos lablab: Brassinolide, castasterone and a new analog, homodolicholide. Agricultural and Biological Chemistry, 47, 1409–1411.
Yokota, T., Morita, M., & Takahashi, N. (1983c). 6-Deoxocastasterone and 6-deoxodolichosterone – putative precursors for brassinolide-related steroids from Phaseolus vulgaris. Agricultural and Biological Chemistry, 47, 2149–2151.
Yokota, T., Baba, J., Koba, S., & Takahashi, N. (1984). Purification and separation of eight steroidal plant-growth regulators from Dolichos lablab seed. Agricultural and Biological Chemistry, 48, 2529–2534.
Yokota, T., Arima, M., Takahashi, N., & Crozier, A. (1985). Steroidal plant-growth regulators, castasterone and typhasterol (2-deoxycastasterone) from the shoots of sitka spruce (Picea sitchensis). Phytochemistry, 24, 1333–1335.
Yokota, T., Kim, S., Kosaka, Y., Ogino, Y., & Takahashi, N. (1987a). Conjugation of brassinosteroids. In K. Schreiber, H. Schütte, & G. Sembdner (Eds.), Conjugated Plant Hormones. Structure, metabolism and function (pp. 288–296). Berlin: VEB Deutscher Verlag der Wissenschaften.
Yokota, T., Kim, S. K., Fukui, Y., Takahashi, N., Takeuchi, Y., & Takematsu, T. (1987b). Brassinosteroids and sterols from a green alga, Hydrodictyon reticulatum: Configuration at C-24. Phytochemistry, 26, 503–506.
Yokota, T., Koba, S., Kim, S. K., Takatsuto, S., Ikekawa, N., Sakakibara, M., Okada, K., Mori, K., & Takahashi, N. (1987c). Diverse structural variations of the brassinosteroids in Phaseolus vulgaris seed. Agricultural and Biological Chemistry, 51, 1625–1631.
Yokota, T., Ogino, Y., Takahashi, N., Saimoto, H., Fujioka, S., & Sakurai, A. (1990a). Brassinolide is biosynthesized from castasterone in Catharanthus roseus crown gall cells. Agricultural and Biological Chemistry, 54, 1107–1108.
Yokota, T., Watanabe, S., Ogino, Y., Yamaguchi, I., & Takahashi, N. (1990b). Radioimmunoassay for brassinosteroids and its use for comparative analysis of brassinosteroids in stems and seeds of Phaseolus vulgaris. Journal of Plant Growth Regulation, 9, 151–159.
Yokota, T., Ogino, Y., Suzuki, H., Takahashi, N., Saimoto, H., Fujioka, S., & Sakurai, A. (1991). Metabolism and biosynthesis of brassinosteroids. In H. G. Cutler, T. Yokota, & G. Adam (Eds.), Brassinosteroids: Chemistry (pp. 86–96). Washington: Bioactivity and Applications. American Chemical Society.
Yokota, T., Nakayama, M., Wakisaka, T., Schmidt, J., & Adam, G. (1994). 3-Dehydroteasterone, a 3,6-diketobrassinosteroid as a possible biosynthetic intermediate of brassinolide from wheat grain. Bioscience, Biotechnology, and Biochemistry, 58, 1183–1185.
Yokota, T., Matsuoka, T., Koarai, T., & Nakayama, M. (1996). 2-Deoxybrassinolide, a brassinosteroid from Pisum sativum seed. Phytochemistry, 42, 509–511.
Yokota, T., Nomura, T., & Nakayama, M. (1997). Identification of brassinosteroids that appear to be derived from campesterol and cholesterol in tomato shoots. Plant & Cell Physiology, 38, 1291–1294.
Yokota, T., Higuchi, K., Takahashi, N., Kamuro, Y., Watanabe, T., & Takatsuto, S. (1998). Identification of brassinosteroids with epimerized substituents and/or the 23-oxo group in pollen and anthers of Japanese cedar. Bioscience, Biotechnology, and Biochemistry, 62, 526–531.
Yokota, T., Sato, T., Takeuchi, Y., Nomura, T., Uno, K., Watanabe, T., & Takatsuto, S. (2001). Roots and shoots of tomato produce 6-deoxo-28-norcathasterone, 6-deoxo-28-nortyphasterol and 6-deoxo-28-norcastasterone, possible precursors of 28-norcastasterone. Phytochemistry, 58, 233–238.
Yokota, T., Ohnishi, T., Shibata, K., Asahina, M., Nomura, T., Fujita, T., Ishizaki, K., & Kohchi, T. (2017). Occurrence of brassinosteroids in non-flowering land plants, liverwort, moss, lycophyte and fern. Phytochemistry, 136, 46–55.
Yoshihara, K., & Katou, N. (1985). A new steroid having plant growth control activity from Typha latifolia pollen. Japanese Kokai Tokkyo Koho JP 60011498 A.
Youn, J. H., Kim, M. K., Son, S. H., Lee, J. E., Jang, M. S., Kim, E. J., Kim, T. W., & Kim, S. K. (2016). ARF7 increases the endogenous contents of castasterone through suppression of BAS1 expression in Arabidopsis thaliana. Phytochemistry, 122, 34–44.
Yu, L., Ding, J., Wang, Y. L., Liu, P., & Feng, Y. Q. (2016). 4-Phenylaminomethyl-benzeneboric acid modified tip extraction for determination of brassinosteroids in plant tissues by stable isotope labeling-liquid chromatography-mass spectrometry. Analytical Chemistry, 88, 1286–1293.
Yu, L., Ye, T., Bai, Y. L., Cai, W. J., Ding, J., Yuan, B. F., & Feng, Y. Q. (2017). Profiling of potential brassinosteroids in different tissues of rape flower by stable isotope labeling-liquid chromatography/mass spectrometry analysis. Analytica Chimica Acta, 1037, 55–62. https://doi.org/10.1016/j.aca.2017.08.038.
Zaki, K., Schmidt, J., Hammouda, F. M., & Adam, G. (1993). Steroidal constituents from pollen grains of Phoenix dactylifera. Planta Med (Supplement Issue), 59, A613–A613.
Zhang, Z., Zhang, Y., Tan, W., Li, G., & Hu, Y. (2010). Preparation of styrene-co-4-vinylpyridine magnetic polymer beads by microwave irradiation for analysis of trace 24-epibrassinolide in plant samples using high performance liquid chromatography. Journal of Chromatography. A, 1217, 6455–6461.
Zhu, W., Wang, H., Fujioka, S., Zhou, T., Tian, H., Tian, W., & Wang, X. (2013). Homeostasis of brassinosteroids regulated by DRL1, a putative acyltransferase in Arabidopsis. Molecular Plant, 6, 546–558.
Zullo, M. A. T. (2018). Brassinosteroids and related compounds. Beau Bassin: Lambert Academic Publishing.
Zullo, M. A. T., & Adam, G. (2002). Brassinosteroid phytohormones: Structure, bioactivity and applications. Brazilian Journal of Plant Physiology, 14, 143–181.
Zullo, M. A. T., & Kohout, L. (2004). Semisystematic nomenclature of brassinosteroids. Plant Growth Regulation, 42, 15–28.
Zullo, M. A. T., Kohout, L., & de Azevedo, M. D. B. M. (2003). Some notes on the terminology of brassinosteroids. Plant Growth Regulation, 39, 1–11.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zullo, M.A.T., Bajguz, A. (2019). The Brassinosteroids Family – Structural Diversity of Natural Compounds and Their Precursors. In: Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A. (eds) Brassinosteroids: Plant Growth and Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6058-9_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-6058-9_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6057-2
Online ISBN: 978-981-13-6058-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)