Abstract
Key message
Moderate overexpression of CYP734A4 improves grain number per main panicle and seed setting rate.
Abstract
Brassinosteroid (BR) homeostasis and signaling are crucial for plant growth and development. CYP734A genes encode cytochrome P450 monooxygenases that control the level of bioactive BRs by degrading BRs. However, fertile plants overexpressing CYP734As have not been reported in rice. Here, we isolated a novel semi-dominant mutant brd3-D, in which T-DNA was inserted approximately 4 kb upstream of the CYP734A4 gene (GenBank Accession AB488667), causing its overexpression. The mutant is characterized by dwarfism, small grains, and erect leaves and is less sensitive to brassinolide-induced lamina joint inclination and primary root elongation. However, increased grain number per main panicle and improved seed setting rate were also found in heterozygous brd3-D. To our knowledge, these traits have not been reported in other BR deficient mutants. Quantitative real-time PCR analysis indicated that phenotypic severity of the brd3-D mutant is positively correlated with the CYP734A4 transcription level. In accordance with the increased expression of CYP734A4, a lower castasterone (a rice BR) content was detected in the brd3-D mutants. Knockout of brd3-D by using the CRISPR/Cas9 system rescued the mutation. In addition, transgenic plants overexpressing CYP734A4 with the 35S enhancer mimicked the brd3-D phenotypes, confirming that moderate overexpression of the CYP734A4 gene can improve grain number per main panicle and the seed setting rate in rice. Further studies showed that overexpression of CYP734A4 influences the expressions of multiple genes involved in the BR pathway, and the expression of CYP734A4 is induced by exogenous brassinolide, confirming the negative regulatory role of CYP734A4 in the BR pathway. CYP734A4 might provide a useful gene resource for developing new high-yielding rice varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassinosteroids (BRs) are plant steroid hormones that are widely distributed in plants. Thus far, more than 69 types of BRs have been isolated from different plants, among which castasterone and brassinolide play roles in many biological processes, including plant growth and development, cell division and elongation, vascular bundle differentiation, photomorphogenesis, male fertility, flowering, senescence and seed germination (Bajguz 2011; Gudesblat and Russinova 2011). They can also improve a plant’s ability to withstand low temperature, high temperature, salt stress and other adverse conditions (Koh et al. 2007).
BR biosynthesis occurs in a complex metabolic network and can be divided into three steps: (1) formation of campestanol from campesterol, (2) formation of castasterone from campestanol, and (3) formation of brassinolide from castasterone. Recently, several rice BR biosynthesis genes functioning in the second step have been isolated. BRD2 is involved in converting 24-methylenecholesterol to campesterol, the precursor of BRs (Hong et al. 2005). D11 and OsDWARF4, which are redundant proteins, function in C-22 hydroxylation, the rate-limiting step in BR biosynthesis (Sakamoto et al. 2006; Tanabe et al. 2005). The BRD1 gene encodes BR-6-oxidase, which regulates multiple C-6 oxidation steps from the late to the early C-6 oxidation pathway (Hong et al. 2002; Mori et al. 2002). The D2 protein catalyzes the conversions from 6-deoxoteasterone to 3-dehydro-6-deoxoteasterone and from teasterone to 3-dehydroteasterone in the late step of the BR biosynthesis pathway (Hong et al. 2003).
The endogenous level of bioactive BRs is also maintained by inactivation of the bioactive forms. In contrast to BR biosynthesis, fewer advances were made in BR catabolism in plants. Several BR catabolic genes, including BAS1, CYP72C1, UGT73C5 and UGT73C6, have been found in Arabidopsis. Overexpression of these genes led to BR-deficient phenotypes (Husar et al. 2011; Nakamura et al. 2005; Neff et al. 1999; Takahashi et al. 2005). However, little is known about rice catabolic genes. Four BAS1 homologues, including CYP734A2, CYP734A4, CYP734A5 and CYP734A6, but not the CYP72C1 ortholog, have been found in rice. These genes appear to be upregulated in response to brassinolide (Thornton et al. 2011; Sakamoto et al. 2011). In vitro analysis showed that these CYP734As were multifunctional and multisubstrate P450s, which inactivate all C-22 hydroxylated BRs, including 6-Deoxocathasterone, Cathasterone, and their downstream products (Sakamoto et al. 2011). Transgenic rice overexpressing CYP734As driven by the rice actin1 promoter showed serious defects in vegetative growth and did not produce floral organs (Sakamoto et al. 2011). Loss of function of CYP734A6 leads to a strongly bending lamina phenotype (Park et al. 2006).
Extensive genetic and biochemical studies in Arabidopsis have identified almost all major BR signaling components. Most components of the BR signaling pathway are conserved between Arabidopsis and rice. However, some BR signaling components in Arabidopsis have not been identified in rice. In addition, a number of rice-specific components, including OsLIC, OsDLT and OsTUD1, have also been identified (Zhang et al. 2014). In rice, OsBRI1 and OsBAK1 coperceive BRs at the plasma membrane (Li et al. 2009; Nakamura et al. 2006; Yamamuro et al. 2000). BR binding to OsBRI1 promotes association with OsBAK1, and inactivates OsGSK1 and OsGSK2, which are orthologues of Arabidopsis BIN2 that functions as a key negative regulator of BR signaling (Koh et al. 2007; Tong et al. 2012). OsGSK1 and OsGSK2 repress the activity of OsBZR1, OsLIC and DLT by phosphorylation. OsBZR1 inhibits the expression of OsLIC and DLT, whereas OsLIC also inhibits the expression of OsBZR1 (Bai et al. 2007; Tong et al. 2012; Wang et al. 2008). OsILI1 and OsIBH1 interact antagonistically with each other and function downstream of OsBZR1. OsBZR1 induces OsILI1, but represses OsIBH1 expression, whereas OsLIC directly represses OsILI1 to oppose the action of OsBZR1 (Zhang et al. 2009, 2012). 14-3-3 proteins bind phosphorylated OsBZR1 to retain OsBZR1 in the cytoplasm (Bai et al. 2007). OsMDP1 play a negative regulatory role in BR signaling. In OsMDP1-deficient plants, expression of OsXTR1 was enhanced and that of OsXTH1 was suppressed. OsXTH1 and OsXTR1 encode xyloglucan endotransglycosylase, which is necessary for cell elongation (Duan et al. 2006). BU1 encoding a helix-loop-helix protein is a positive regulator of BR response (Tanaka et al. 2009). D1 and TUD1 act together to mediate BR signaling; however, the connection between D1 and OsBRI1-mediated BR signaling remains unknown (Hu et al. 2013; Wang et al. 2006). OsGSR1 activates BR synthesis by directly interacting with BRD2 (Wang et al. 2009). OsSPY and RAVL1 coordinate the expression of genes involved in BR signaling and biosynthesis (Je et al. 2010; Shimada et al. 2006).
In this study, a semi-dominant mutant, brd3-D, with a BR-deficient phenotype was identified as the first fertile mutant caused by overexpression of a rice BR catabolic gene, CYP734A4. Different from other BR deficient mutants, the heterozygous brd3-D mutant displayed improved grain number per main panicle and seed setting rate. Knockout of CYP734A4 using the CRISPR/Cas9 system rescued the brd3-D mutant phenotype. Furthermore, transgenic plants overexpressing CYP734A4 with the 35S enhancer mimicked the heterozygous brd3-D phenotype, confirming that moderate overexpression of the CYP734A4 gene can increase grain number per main panicle and the seed setting rate in rice. Our results showed that controlling the BR level by BR catabolism could be useful for increasing grain yield in crop plants.
Materials and methods
Plant materials and phenotypic analysis
The brd3-D mutant with the genetic background of japonica rice cultivar Zhonghua11 was isolated from a T-DNA insertion library containing 15000 transgenic rice lines (Zhu et al. 2001). F2 generation plants from a cross of the mutant and wild type parent Zhonghua11 were used for genetic analysis. All rice plants were grown under natural conditions in the paddy field at the China National Rice Research Institute (119°57′E, 30°03′N). The above-mentioned rice materials were obtained from the State Key Laboratory of Rice Biology, China National Rice Research Institute.
F2 plants from the cross of the brd3-D mutant and its original parent, and T1 plants from NO-1 transgenic lines were used for phenotypic analysis. The plant height, internode length, panicle length, grain length and effective tiller number, grain number per main panicle, seed setting rate, and thousand seed weight were investigated. Data were analyzed with IBM SPSS Statistics 20 software.
Hygromycin resistant assay
To determine whether the mutant phenotype was caused by T-DNA insertion, a hygromycin resistant assay was performed. Fresh blades from the T1 generation of the brd3-D mutant line were sheared into 2–4-cm segments and placed in two Petri dishes containing a solution of 0.5 mg/L 6-BA and 50 mg/L hygromycin. Care was taken to ensure the segments were in direct contact with the hygromycin solution. The experiment was carried out at approximately 26 °C under 12-h light/12-h dark cycles for 3 days (Wang and Waterhouse 1997).
Isolation and sequencing of T-DNA flanking sequence
Thermal asymmetric interlaced PCR (TAIL-PCR) was used to amplify the rice genome sequence flanking the T-DNA insertion site (Liu et al. 1995). Specific primers complementary to the T-DNA left border sequences were designed to amplify the genome sequence flanking the T-DNA insertion site. In addition, four arbitrary degenerate primers were synthesized (Supplemental Table 2). The tertiary TAIL-PCR products were recovered, cloned into a pMD18-T carrier after purification, and then sequenced by the Huada Gene Research Institution (Shenzhen, Guangdong, China).
DNA extraction and PCR-based co-segregation analysis
Fresh leaves of F2 generation plants were used for extracting rice total DNA according to the modified method of Lu and Zheng (Lu and Zhen 1992). Three primers were designed to study whether the brd3-D phenotype co-segregated with the T-DNA insertion (Supplemental Table 2; Fig. 3b). The PCR reaction was carried out as follows: denaturation at 94 °C for 2 min, followed by 30 cycles of 94 °C for 30 s, annealing at 56 °C for 30 s, 72 °C for 1 min, and a final extension step at 72 °C for 7 min.
Quantitative real-time PCR analysis
Total RNA was extracted from various plants using the Qiagen RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). First-strand cDNAs were synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). The cDNAs were assayed by quantitative real-time PCR using SYBR Green or TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on the Applied Biosystems 7900 Real-Time PCR System. The relative expression levels of each transcript were obtained by normalization to the OsACT1 gene. PCR was carried out as follows: preheating at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. No reverse transcription control and no template control were included in all qRT-PCR experiments and no non-specific amplification was seen. The primers and probes used for quantitative real-time PCR analysis are listed in Supplemental Table 2.
Plasmid construction and rice transformation
For obtaining mutants with loss of function of CYP734A4, a CRISPR/Cas9 vector targeting the CYP734A4 gene was constructed. A 20-bp fragment of the CYP734A4 gene was assembled into the intermediate vector SK-gRNA. The gRNA was then inserted into the CRISPR/Cas9 binary vector pC1300-Cas9 (Wang et al. 2015). For the construction of the overexpression vector, two overlapped fragments containing the promoter and whole coding region of CYP734A4 and the 35S enhancer were amplified by PCR and inserted into the p130035SSI-X binary vector (Fig. 6a). The two constructs were introduced into rice using the Agrobacterium tumefaciens-mediated genetic transformation method (Wu et al. 2010).
Lamina joint bending assay
The lamina joint bending assay was performed at the 3-leaf stage. One microliter of ethanol containing 0, 10, 1000 ng of 24-epiBR was spotted on the joint between the lamina and leaf sheath of homozygous brd3-D, the wild type and d1. After incubation for 4 days, the angle between the lamina and leaf sheath was measured. Five plants were measured in each treatment.
The influence of 24-epiBR on primary root elongation
Seeds were soaked in water for 1 day and germinated at 30 °C in darkness for 2 days, and then grown in nutrient solutions with different concentrations (0, 10, 100, 1000 nM) of 24-epiBR. The primary root length was measured after the seedlings were cultured for 4 days at 28 °C under an 18-h photoperiod.
Results
Morphological and genetic characterization of the brd3-D mutant
The brd3-D mutant was identified from a transgenic rice line that displayed a dwarf phenotype typical of BR-deficient mutants. The progeny of brd3-D segregated into discrete groups of wild type, weak, and severe dwarf plants (Fig. 1a), suggesting that the parental dwarf mutants were heterozygous for a semi-dominant mutation. The homozygous mutant was further crossed with its wild type parent Zhonghua11. All F1 generation plants exhibited the mutation phenotype. The separation of wild type, weak, and severe dwarf plants in the F2 generation showed a good fit to a 1:2:1 segregation ratio (normal:weak:severe = 109:206:85, X2 = 1.69 < X2 0.05 = 5.99). The results showed that the mutation was controlled by a single semi-dominant gene.
Phenotype of the brd3-D mutant. a Phenotypes of the wild type and brd3-D heterozygous (brd3-D/+) and homozygous (brd3-D/brd3-D) mutants at the flowering stage. Bar 5 cm. b Panicle morphology and leaf angle of the wild type and brd3-D. Bar 5 cm. c Schematic representation of the elongation patterns of internodes of the wild type and brd3-D. P panicle, I–IV internodes from top to bottom. d Seed morphology of the wild type and brd3-D. Bar 1 mm. e Tiller number from the wild type and brd3-D. f Thousand grain weight of the wild type and brd3-D. g Grain number per main panicle of the wild type and brd3-D. h Seed setting rate of the wild type and brd3-D. Values are means of ten biological replicates (*P < 0.05, **P < 0.01)
The phenotype of the mutant was further characterized using F2 generation plants under natural field conditions. Similar to most BR-related mutants, the brd3-D mutant had crinkly, dark green and erect leaves (Fig. 1a, b). In comparison with the wild type, both homozygous and heterozygous brd3-D mutants exhibited significantly reduced plant height (Fig. 1a, c). The height of the homozygous and heterozygous mutant was reduced on average to about 56.0 and 75.0%, respectively, that of the wild type plants (Fig. 1c, Supplemental Table 1). A further survey showed that all internodes of brd3-D were shortened significantly, but the homozygous brd3-D had little specific reduction in the second internode (Supplemental Table 1, Fig. 1c), which is similar to d61, a BR-insensitive mutant (Yamamuro et al. 2000). Although plant height was substantially reduced, there was no significant difference in panicle length between the homozygous mutant and wild type, and the panicle of the heterozygous mutant was even longer than that of the wild type (Fig. 1c, Supplemental Table 1). The grain of brd3-D was significantly smaller than that of the wild type (Fig. 1d). The number of effective panicles per plant was reduced significantly by 45.6% in the homozygous mutant and 25.5% in the heterozygous mutant (Fig. 1e). The thousand grain weight was reduced by 28.8% in the homozygous mutant and reduced by 18.4% in the heterozygous mutant (Fig. 1f). Remarkably, although there was no significant difference between the homozygous mutant and wild type, the heterozygous mutant had a significantly higher grain number per main panicle (Fig. 1g). We also found that the heterozygous mutant had a slightly higher seed setting rate than the wild type (Fig. 1h). Generally, most traits of the brd3-D mutant were similar to those of BR-deficient mutants; however, improved grain number per main panicle and seed setting rate have not been reported in other BR-deficient mutants.
brd3-D displayed less sensitivity to 24-epiBR
Since some traits of the brd3-D mutant were similar to the BR-deficient mutants, we further examined whether brd3-D was sensitive to BR. Previous studies have shown that a low concentration of BR promotes root elongation, but high concentrations of BR inhibit root growth in rice (Yamamuro et al. 2000). Therefore, we measured the length of the roots under different concentrations of 24-epiBR. The result showed that 1 nM 24-epiBR promoted root elongation, but 10 nM or higher concentrations of 24-epiBR (24-epibrassinolide) inhibited root growth in the wild type and brd3-D. However, the brd3-D mutant showed less sensitivity to high concentrations of 24-epiBR than the wild type (Fig. 2a).
Response of the brd3-D homozygous mutant to 24-epiBR. a Primary root elongation response to 24-epiBR. The wild type and homozygous brd3-D were treated with different concentrations of 24-epiBR (from left to right: 0 nM, 1 nM, 10 nM, 100 nM, 1000 nM). Primary root length was measured on day 4 after germination. Bar 1 cm. b The dose response to 24-epiBR of the bending angle in the wild type and homozygous brd3-D mutants at the 3-leaf stage (from left to right: 0 ng/µL, 10 ng/µL, 1000 ng/µL). Values are the means ± SD of five biological replicates. (*P < 0.05, **P < 0.01)
Lamina joint inclination is one of the most sensitive BR responses in rice (Zhang et al. 2009). To further confirm that brd3-D is a less sensitive BR mutant, a lamina joint inclination assay was performed. When treated with different concentrations of 24-epiBR, both brd3-D and wild type plants showed enhanced bending of the lamina joint in a dose-dependent manner. However, in contrast to the wild type, the degree of lamina joint bending of brd3-D was lower under all treatments. The lamina joint bending of brd3-D at 1000 ng/µL was almost the same as that of the untreated wild type (Fig. 2b). The results further suggest that the brd3-D mutation reduces BR responses in rice.
T-DNA insertion co-segregates with the brd3-D mutant
Transgenic plants expressing the hygromycin phosphotransferase gene can be identified by a hygromycin resistant assay (Wang and Waterhouse 1997). To determine whether the mutation phenotype is caused by T-DNA insertion, 120 T1 generation plants of the brd3-D mutant line were used for co-segregation analysis according to the leaf response to hygromycin. The segments from the brd3-D mutant remained green after 3 days in the hygromycin solution, while all the leaves from the wild type exhibited brown, necrotic, water-soaked lesions under the same condition (Supplemental Fig. 1a). The results suggest that the mutation phenotype could be caused by T-DNA insertion.
Since the mutation could be caused by a T-DNA insertion, we used the TAIL-PCR method to obtain the genomic sequence flanking the T-DNA insertion in the brd3-D mutant (Liu et al. 1995). The tertiary TAIL-PCR products were cloned into pMD18-T vectors and then sequenced (Supplemental Fig. 1b). Sequence analysis indicated that the T-DNA was inserted approximately 4 kb upstream of the CYP734A4 gene of the PAC clone P0457B11, which is located on the sixth chromosome. Both the annotation results of the RiceGAAS system and alignment between the full-length cDNA (002-114-A02) and the genomic sequence of CYP734A4 showed that the CYP734A4 gene contains five exons and four introns (Fig. 3a). The CYP734A4 full-length cDNA encodes a cytochrome P450 family protein of 538 amino acid residues, which inactivates C-22 hydroxylated BRs in vitro (Sakamoto et al. 2011).
Schematic diagram of brd3-D and cosegregation analysis of T-DNA and the brd3-D phenotype. a Schematic diagram of the brd3-D gene. T-DNA was inserted approximately 4 kb upstream of the CYP734A4 gene, which contains five exons and four introns. Exons are indicated with boxes, coding region with filled boxes, and introns with lines between boxes. b Schematic diagrams of the genotyping. P1 forward primer from rice genome flanking the left end of the T-DNA, P2 reverse primer in T-DNA, P3 reverse primer from rice genome flanking the right border of the T-DNA. The P1 and P2 primers produced a 500 bp PCR fragment from the T-DNA insertion DNA template; P1 and P3 primers produced a 623-bp PCR band from the wild type DNA, while no PCR band was obtained from the T-DNA insertion template because the expected fragment was too large to amplify. c Genotyping of F2 plants derived from a cross between brd3-D and its parent Zhonghua11. A1-A7, Homozygous mutants (brd3-D/brd3-D); B1-B7, Heterozygous mutants (brd3-D/+); C1-C7, wild type plants; M marker
Ninety F2 generation plants were genotyped to further study whether the brd3-D phenotype co-segregated with the T-DNA insertion (Fig. 3b). Homozygous mutants were also found to be homozygous for the T-DNA insertion because only the 500-bp bands were amplified by the P1 and P2 primer pairs (Fig. 3c). Normal phenotype plants were found with no T-DNA insertion because only the 623-bp bands were amplified by the P1 and P3 primer pairs (Fig. 3c). Both the 500-bp and 623-bp bands were amplified from heterozygous mutants, which showed their heterozygous nature for the T-DNA insertion (Fig. 3c). These results further suggest that the brd3-D phenotype is caused by the T-DNA insertion.
Expression of the CYP734A4 gene is increased drastically in brd3-D
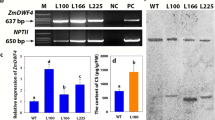
In the brd3-D mutant, T-DNA carrying cauliflower mosaic virus 35S enhancer sequences on its right border, was inserted approximately 4 kb upstream of the CYP734A4 gene. Therefore, we speculated that the 35S enhancer sequence may enhance the transcription of CYP734A4 in brd3-D. The mRNA expression levels of the CYP734A4 gene in different organs of the wild type and brd3-D heterozygous and homozygous mutants were quantified by real-time RT-PCR analysis. In the wild type, the highest expression level of CYP734A4 was found in the leaf blades, followed by roots, leaf sheath, node, panicle and internode (Fig. 4a). Compared with the wild type, the expression levels of CYP734A4 in all organs were significantly increased in the brd3-D mutant. CYP734A4 in the leaf blade was increased by about 38 fold in the brd3-D homozygous mutant and by about 22-fold in the heterozygous mutant (Fig. 4b). Moreover, CYP734A4 in both the brd3-D homozygous and heterozygous mutants showed a similar expression pattern as that in the wild type (Fig. 4a, b). The results suggested that the T-DNA insertion resulted in overexpression of the CYP734A4 gene in brd3-D, and the severity of the brd3-D phenotype was positively correlated with the expression level of CYP734A4 in the transgenic line.
The expression profile of CYP734A4 and BR contents in the wild type and brd3-D. a The expression levels of CYP734A4 in different organs of the wild type. b The transcript levels of CYP734A4 among different organs of the wild type, heterozygous, and homozygous brd3-D. The transcript levels of CYP734A4 in the internode of the wild type were arbitrarily set to 1. Values are the mean ± SD of three biological replicates. c Castasterone and brassinolide contents in the leaves of the wild type and homozygous brd3-D
Because the CYP734A4 gene is involved in BR catabolism, we quantified the contents of castasterone and brassinolide, which are the most potent BRs in plants. As expected, in comparison to the wild type, the castasterone content was reduced by 94.31, 94.32, 94.02% in leaf blade, stem, and panicle in the homozygous brd3-D mutant, respectively (Fig. 4c). A previous study showed that there is no or little brassinolide in rice (Nakamura et al. 2006). Similarly, we did not detect any brassinolide in either the wild type or the homozygous brd3-D mutant.
Knockout of the CYP734A4 gene by CRISPR/Cas9 rescues the brd3-D phenotype
To elucidate the effects of reduced CYP734A4 expression, a CRISPR/Cas9 vector targeting CYP734A4 was constructed (Wang et al. 2015) and transformed into the wild type cultivar Zhonghua11 using the Agrobacterium tumefaciens-mediated genetic transformation method. Five homozygotes with frameshift mutations in the CYP734A4 gene were identified by sequencing. However, none of these plants showed obvious changes in leaf morphology, leaf angles or seed sizes compared with those of the wild type plants (Supplemental Fig. 2). BLASTp analysis showed that CYP734A4 had high identity with CYP734A2 (78%), CYP734A6 (69%) and CYP734A5 (58%) in rice (Supplemental Fig. 3). Therefore, it is suggested that the unaltered phenotype under reduced expression of CYP734A4 could be due to the functional redundancy of CYP734As in rice.
To prove that overexpression of the CYP734A4 gene is responsible for the brd3-D phenotype, the CRISPR/Cas9 construct targeting CYP734A4 was also transformed into homozygous brd3-D mutants. A frameshift mutation in the CYP734A4 gene restored the homozygous brd3-D plant to a wild type phenotype (Fig. 5a, b). We therefore concluded that CYP734A4 is the gene responsible for the brd3-D mutation.
Knockout of the CYP734A4 gene with the CRISPR/Cas9 system rescues the brd3-D phenotype. a Phenotypic differences between the homozygous brd3-D with a frameshift mutation in the CYP734A4 gene (brd3-D-a4) and the homozygous brd3-D plant (brd3-D/brd3-D). Bar 10 cm. b Partial nucleotide sequence of the brd3-D-a4 and wild type alleles. 28: the amino acid number from the translation start site. Amino acid changes in brd3-D-a4 are represented in red in comparison to the reference gene
Overexpressing CYP734A4 with the 35S enhancer mimicked the brd3-D phenotypes
Phenotypic analysis of the brd3-D mutant showed that the moderate overexpression of CYP734A4 improved the grain number per main panicle, panicle length, and seed setting rate. To further confirm the function of CYP734A4, a T-DNA construct containing CYP734A4 with the 35S enhancer was generated and transformed into the rice cultivar Nipponbare (Fig. 6a). One transgenic line with a mild phenotype was selected for detailed analysis. In these transgenic plants, the expression level of CYP734A4 was increased significantly (Fig. 6b). These transgenic plants showed reduced plant height (Fig. 6c) and lamina joint bending (Fig. 6d). These plants also exhibited an increase in total grain number (Fig. 6e) and filled grain number per main panicle (Fig. 6f), panicle length (Fig. 6g), and seed setting rate (Fig. 6h), but a decrease in the thousand grain weight (Fig. 6i, j). These results suggested that manipulating the expression of CYP734A4 can reduce lamina joint angle and improve grain number and seed setting rate in rice.
Overexpression of CYP734A4 under the control of the wild type CYP734A4 promoter together with the CaMV 35S enhancer recapitulates the brd3-D phenotype. a Schematic diagrams of the overexpression construct pN208OX. Pol PolyA terminator, Hpt hygromycin phosphotransferase gene, Nos nos terminator, CYP734A4 cDNA sequence coding for CYP734A4, P734A4 rice CYP734A4 promoter, 35SE 35S enhancer. b Gross morphology of NO-1 (a transgenic positive plant from the T1 generation) and the wild type (a transgenic negative plant from the T1 generation). Bar 10 cm. c Real-time PCR analysis results of the CYP734A4 transcripts in the leaf blade of NO-1 and the wild type. The levels of the CYP734A4 transcript in the wild type plants were arbitrarily set to 1. Values are the mean ± SD of three replicates. d Panicle morphology of NO-1 and the wild type. Bar 5 cm. e Grain number per main panicle of NO-1 and the wild type. f Filled grain number per main panicle of NO-1 and the wild type. g Main panicle length of NO-1 and the wild type. h Seed setting rate of NO-1 and the wild type. i Thousand grain weight of NO-1 and the wild type. j Grain morphology of NO-1 and the wild type. Values are the means of five biological replicates (*P < 0.05, **P < 0.01)
CYP734A4 plays a role in regulating BR homeostasis in vivo
BR homeostasis is controlled by both BR biosynthesis and BR catabolism. Exogenous BR reduces the expression of BR biosynthesis genes, while BR deficiency induces their expression (Tong et al. 2009). However, little is known about the role of BR catabolic genes on BR homeostasis in rice. To investigate the in vivo function of CYP734A4 in regulating BR homeostasis, we monitored changes in CYP734A4 expression in response to 1000 nM 24-epiBR treatment using quantitative real-time RT-PCR. The mRNA levels in the wild type were dramatically increased 8 h after BR treatment and by more than 50 times at 48 h (Fig. 7a). Consistently, expressions of BR biosynthetic genes, including BRD2, D2 and OsDWARF4, were significantly increased and expressions of BR catabolic genes, including CYP734A5 and CYP734A6, were significantly reduced in the brd3-D mutant (Fig. 7b). These results confirmed that CYP734A4 is involved in regulating BR homeostasis in vivo. We also found that the expression of BRD1 was decreased in brd3-D (Fig. 7b), suggesting that BRD1 transcripts could be regulated in a feed-forward manner.
CYP734A4 regulates BR homeostasis in vivo. a Expression of CYP734A4 in response to 24-epiBR in the wild type. 2-month-old rice plants were incubated in solutions of 1000 nM 24-epiBR and leaves were collected at 0, 2, 8, 24, 48, 96 h after treatment. The levels of the CYP734A4 transcript at 0 h were arbitrarily set to 1. b Real-time PCR analysis results of the transcripts of BR biogenesis and BR catabolism-related genes in the leaves of the wild type and homozygous brd3-D. c Real-time PCR analysis results of the transcripts of genes related to BR signaling in the leaves of the wild type and homozygous brd3-D. The transcript levels of all tested genes in the wild type were arbitrarily set to 1. Values were obtained from three independent experiments
Overexpression of CYP734A4 affects expression of BR signaling genes
To further investigate the underlying molecular basis of the brd3-D phenotype, we tested the effects of the brd3-D mutation on the BR signaling genes through quantitative real-time PCR. OsBRI1 and OsBAK1 mediate BR signaling at the plasma membrane (Li et al. 2009; Nakamura et al. 2006). The expression of OsBRI1 and OsBAK1 was increased in brd3-D (Fig. 7c). In rice, the OsBZR1 protein mediates feedback inhibition of BR biosynthetic genes (Bai et al. 2007). Interestingly, a decreased expression of OsBZR1 was found in brd3-D (Fig. 7c). OsBZR1 inhibits the expression of OsLIC. Consistently, expressions of OsLIC were enhanced in brd3-D (Fig. 7c). OsBZR1 induces OsILI1, but represses OsIBH1 expression. In brd3-D, the expression of OsIBH1 was increased (Fig. 7c). It has been reported that the expression of OsXTH1 is decreased and that of OsXTR1 is increased in OsMDP1-deficient plants that exhibited enhanced lamina joint inclination (Duan et al. 2006). Interestingly, the expression of OsXTR1 was decreased in brd3-D (Fig. 7c). We also found elevated levels of 14-3-3 and decreased levels of BU1 (Fig. 7c). These results suggested that a complicated regulation mechanism underlies the BR signaling pathway.
Discussion
Control of BR catabolism and BR homeostasis is important for plant growth and development. However, little is known about BR catabolism, and fertile plants overexpressing BR catabolic genes have not been reported in rice. In the present study, a semi-dominant mutant brd3-D with a BR-related dwarf phenotype was isolated from a rice T-DNA insertion library and the T-DNA was inserted approximately 4 kb upstream of the BR catabolic gene, CYP734A4. Gene expression analysis revealed that the expression of CYP734A4 dramatically increased in the brd3-D mutant, and the severity of the brd3-D phenotype was correlated with the expression level of the CYP734A4 gene. Transgenic brd3-D mutants harboring a frameshift mutation in the CYP734A4 gene rescued the mutation. In addition, transgenic plants overexpressing full-length CYP734A4 cDNA under the control of the wild type CYP734A4 promoter together with the CaMV 35 S enhancer recapitulated the brd3-D phenotype. These results demonstrated that overexpression of the CYP734A4 gene was responsible for the brd3-D phenotype. The expression of CYP734A4 was dramatically increased by treatment with 24-epiBR in the wild type, and the expressions of BR biosynthetic genes were significantly increased (Fig. 7a, b). These results suggest that CYP734A4 plays a role in modulating bioactive BR levels in vivo.
In Arabidopsis, it has been reported that CYP734A1/BAS1 is involved in BR degradation, and overexpression of BAS1 caused a BR-deficient phenotype (Neff et al. 1999; Turk et al. 2005). In rice, four BAS1 homologues, including CYP734A2, CYP734A4, CYP734A5, CYP734A6, have been found. All four rice CYP734As showed high identities with BAS1 (ranging between 50.5 and 60.2%). In vitro analysis showed that rice CYP734As can catalyze inactivation of BRs (Sakamoto et al. 2011). Furthermore, the present study demonstrated that overexpression of the CYP734A4 gene resulted in the brd3-D phenotype, such as dwarfism, small grains, erect and dark green leaves, high grain number per main panicle, and high seed setting rate (Fig. 1). BAS1 and CYP734As exhibit high sequence identity and share a similar function, suggesting that the mechanisms controlling BR inactivation are conserved in monocots and dicots.
Previously, BR was shown to have opposing effects on root meristem size, depending on its site of action (Vragović et al. 2015). Our results showed that the brd3-D mutant, different from other BR-deficient mutants, exhibits increased grain number per main panicle and an improved seed setting rate. It is possible that the spatiotemporal overexpression of CYP734A4 caused specific BR degradation, which resulted in the brd3-D phenotype. In addition, the brd3-D phenotype might also be caused by inactivation of a series of intermediate products for BR biosynthesis. It cannot be excluded that CYP734A4 plays a role in the metabolism of other steroids that affect plant architecture.
Rice BR-related mutants showed distinctive internode elongation features. In the mild BR mutants, including d11, d2, and d61-1, the second internodes were completely stunted but the other internodes were elongated (Hong et al. 2003; Tanabe et al. 2005; Yamamuro et al. 2000). The brd2 and d61-2 mutants with intermediate phenotypes had a specific reduction from the second to the fourth internodes (Hong et al. 2005; Yamamuro et al. 2000). The brd1-1 and brd1-2 mutants with severe phenotypes showed elongation at the neck internode (Hong et al. 2002). In the heterozygous brd3-D mutants, the length of each internode was almost uniformly shortened (Fig. 1c), resulting in an elongation pattern similar to that of the wild type plant. However, the homozygous brd3-D exhibited little specific reduction in the second internode (Fig. 1c; Supplemental Table 1), but the phenotype was far less conspicuous than that of d11, d2, and d61-1. Therefore, the internode elongation pattern of brd3-D is different from those of previously isolated rice BR-related mutants.
Different from mutants deficient in BR biosynthesis, which exhibit supersensitivity to 24-epiBR, the brd3-D mutant was less sensitive to 24-epiBR. Because the brd3-D mutant displayed significantly higher transcription of the CYP734A4 gene, we inferred that an increased CYP734A4 mRNA level produced more CYP734A4 protein in this mutant, which resulted in 24-epiBR degradation and less available 24-epiBR to bind the BR receptor in brd3-D.
Both the overproduction and a mild deficiency of BR can enhance grain yields under certain conditions. Increased BR levels in specific tissues produce more and heavier seed, thus increasing per-plant grain yields (Wu et al. 2008). In contrast, an overall reduction in BR levels results in lower per-plant grain yields due to reduced seed number and size. However, reduced BR levels or sensitivity make leaves more erect. Erect leaves enable planting at higher densities and increase the grain yield per plot (Morinaka et al. 2006; Sakamoto et al. 2006). These results suggest that manipulating BR levels by catabolism can improve crop yield. Our results revealed that overexpression of CYP734A4, a catabolic gene, showed a phenotype with erect leaves in the brd3-D and NO1 mutants. Surprisingly, different from previously reported BR-deficient or insensitive mutants, the heterozygous brd3-D and NO1 mutants also exhibited increased seed setting rate and number of seeds in the main panicle (Figs. 1g, h, 6e, h). These traits are valuable for increasing seed yield. Therefore, CYP734A4 might provide a useful gene resource for developing new high-yielding rice varieties.
References
Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci USA 104:13839–13844
Bajguz A (2011) Brassinosteroids-occurrence and chemical structures in plants. In: Brassinosteroids: a class of plant hormone, 1st edn. Academic Press, New York, pp 1–28
Duan K, Li L, Hu P, Xu SP, Xu ZH, Xue HW (2006) A brassinolide-suppressed rice MADS-box transcription factor, OsMDP1, has a negative regulatory role in BR signaling. Plant J 47:519–531
Gudesblat GE, Russinova E (2011) Plants grow on brassinosteroids. Curr Opin Plant Biol 14(5):530–537
Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, Uozu S, Kitano H, Ashikari M, Matsuoka M (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32:495–508
Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15:2900–2910
Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 17:2243–2254
Hu X, Qian Q, Xu T, Zhang Y, Dong G, Gao T, Xie Q, Xue Y (2013) The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G alpha subunit to regulate Brassinosteroid-mediated growth in rice. PLoS Genet 9:e1003391
Husar S, Berthiller F, Fujioka S, Rozhon W, Khan M, Kalaivanan F, Elias L, Higgins GS, Li Y, Schuhmacher R et al (2011) Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biol 11:51
Je BI, Piao HL, Park SJ, Park SH, Kim CM, Xuan YH, Huang J, Do Choi Y, An G, Wong HL, Fujioka S, Kim MC, Shimamoto K, Han CD (2010) RAV-Like1 maintains brassinosteroid homeostasis via the coordinated activation of BRI1 and biosynthetic genes in rice. Plant Cell 22:1777–1791
Koh S, Lee SC, Kim MK, Koh JH, Lee S, An G, Choe S, Kim SR (2007) T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant mol Biol 65:453–466
Li D, Wang L, Wang M, Xu YY, Luo W, Liu YJ, Xu ZH, Li J, Chong K (2009) Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol J 7:791–806
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463
Lu YJ, Zhen KL (1992) A simple method for DNA extraction of rice. Chin J Rice Sci 6:47–48 (in Chinese)
Mori M, Nomura T, Ooka H, Ishizaka M, Yokota T, Sugimoto K, Okabe K, Kajiwara H, Satoh K, Yamamoto K, Hirochika H, Kikuchi S (2002) Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol 130:1152–1161
Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M (2006) Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol 3:924–931
Nakamura M, Satoh T, Tanaka S, Mochizuki N, Yokota T, Nagatani A (2005) Activation of the cytochrome P450 gene, CYP72C1, reduces the levels of active brassinosteroids in vivo. J Exp Bot 56:833–840
Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, Hasegawa Y, Kitano H, Matsuoka M (2006) The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol 140:580–590
Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J (1999) BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA 96:15316–15323
Park W, Kim HB, Kim WT, Park PB, An G, Choe S (2006) Rice bending lamina 2 (bin2) mutants are defective in a Cytochrome P450 (CYP734A6) gene predicted to mediate brassinesteroid catabolism. J Plant Biol 49(6):469–476
Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, Tanaka H, Kitano H, Matsuoka M (2006) Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24:105–109
Sakamoto T, Kawabe A, Tokida-Segawa A, Shimizu B, Takatsuto S, Shimada Y, Fujioka S, Mizutani M (2011) Rice CYP734As function as multisubstrate and multifunctional enzymes in brassinosteroid catabolism. Plant J 67:1–12
Shimada A, Ueguchi-Tanaka M, Sakamoto T, Fujioka S, Takatsuto S, Yoshida S, Sazuka T, Ashikari M, Matsuoka M (2006) The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J 48:390–402
Takahashi N, Nakazawa M, Shibata K, Yokota T, Ishikawa A, Suzuki K, Kawashima M, Ichikawa T, Shimada H, Matsui M (2005) shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. Plant J 42:13–22
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790
Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang CJ, Dubouzet JG, Kikuchi S, Sekimoto H, Yokota T, Asami T, Kamakura T, Mori M (2009) BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151:669–680
Thornton L, Peng H, Neff M (2011) Rice CYP734A cytochrome P450s inactivate brassinosteroids in Arabidopsis. Planta 234(6):1151–1162
Tong H, Jin Y, Liu W, Li F, Fang J, Yin Y, Qian Q, Zhu L, Chu C (2009) DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J 58:803–816
Tong H, Liu L, Jin Y, Du L, Yin Y, Qian Q, Zhu L, Chu C (2012) DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24:2562–2577
Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Wang H, Torres QI, Ward JM, Murthy G, Zhang J, Walker JC, Neff MM (2005) BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J 42(1):23–34
Vragović K, Sela A, Friedlander-Shani L, Fridman Y, Hacham Y, Holland N, Bartom E, Mockler TC, Savaldi-Goldstein S (2015) Translatome analyses capture of opposing tissue-specific brassinosteroid signals orchestrating root meristem differentiation. Proc Natl Acad Sci USA 112(3):923–928
Wang MB, Waterhouse PM (1997) A rapid and simple method of assaying plants transformed with hygromycin or PPT resistance gene. Plant Mol Biol Rep 15:209–215
Wang L, Xu YY, Ma QB, Li D, Xu ZH, Chong K (2006) Heterotrimeric G protein alpha subunit is involved in rice brassinosteroid response. Cell Res 16:916–922
Wang L, Xu Y, Zhang C, Ma Q, Joo SH, Kim SK, Xu Z, Chong K (2008) OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS One 3:1–12
Wang L, Wang Z, Xu Y, Joo SH, Kim SK, Xue Z, Xu Z, Chong K (2009) OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J 57:498–510
Wang C, Shen L, Fu YP, Yan CJ, Wang KJ (2015) A simple CRISPR/Cas9 system for multiplex genome editing in rice. J Genet Genomics 42(12):703–706
Wu CY, Trieu A, Radhakrishnan P, Kwok SF, Harris S, Zhang K, Wang J, Wan J, Zhai H, Takatsuto S, Matsumoto S, Fujioka S, Feldmann KA, Pennell RI (2008) Brassinosteroids regulate grain filling in rice. Plant Cell 20:2130–2145
Wu C, Fu YP, Hu GC, Si HM, Cheng SH, Liu WZ (2010) Isolation and characterization of a rice mutant with narrow and rolled leaves. Planta 232:313–324
Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12:1591–1606
Zhang LY, Bai MY, Wu J, Zhu JY, Wang H, Zhang Z, Wang W, Sun Y, Zhao J, Sun X, Yang H, Xu Y, Kim SH, Fujioka S, Lin WH, Chong K, Lu TG, Wang ZY (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21:3767–3780
Zhang C, Xu Y, Guo S, Zhu J, Huan Q, Liu H, Wang L, Luo G, Wang X, Chong K (2012) Dynamics of brassinosteroid response modulated by negative regulator LIC in rice. PLoS Genet 8:e1002686
Zhang C, Bai MY, Chong K (2014) Brassinosteroid-mediated regulation of agronomic traits in rice. Plant cell rep 33:683–696
Zhu ZG, Xiao H, Fu YP, Hu GC, Yu YH, Si HM, Zhang JL, Sun ZX (2001) Construction of transgenic rice populations by inserting the maize transponson Ac/Ds and genetic analysis for several mutants. Chinese J Biotech 17:288–292 [In Chinese with English abstract]
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31271686), the Important National Science & Technology Specific Projects for Breeding New Transgenic Varieties in China (2014ZX08001-004 and 2014ZX08010-004) and the Central Public-interest Scientific Institution Basal Research Fund (2012RG002-6).
Author contributions
WL and ZH conceived the project and designed the experiments. WQ, CW, GH, YF and WL performed experiments; WQ and WL analyzed data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wenjing Qian and Chao Wu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qian, W., Wu, C., Fu, Y. et al. Novel rice mutants overexpressing the brassinosteroid catabolic gene CYP734A4 . Plant Mol Biol 93, 197–208 (2017). https://doi.org/10.1007/s11103-016-0558-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0558-4