Abstract
Non-pathogenic Fusarium are spread in different environments such as in soil, in rhizosphere and in planta. Non-pathogenic Fusarium secret many chemically diverse secondary metabolites for competing with other soil microorganisms. The role of secondary metabolites is working together with other modes of action. These mechanisms were comprised of mycoparasitism, antibiotic, competition, induce the resistance and defences plant, and change in plant chemistry, biofertilizer, and production the beneficial enzymes. These features are very helpful in the scope of agriculture. These can be effectively utilized as an eco-friendly alternative to chemical pesticides for the management of phytopathogens. Interestingly, non-pathogenic Fusarium also behaves like an endophyte, entering the host system and inducing the defence response. Finally, the importance of application of non-pathogenic Fusarium (or its secondary metabolites) over chemical pesticides is far outreaching and comparatively more beneficial.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
1 Introduction
Fusarium is a very interesting genus compared with other genera of fungi. Fusarium in the pathogenic state can cause very hazardous diseases in plants and human. Fusarium and other fungi produced very dangerous secondary metabolites such as mycotoxins (Attitalla et al. 2010a, b; Nor Azliza et al. 2014). While the genus can be useful in avirulent state, in this state it is known as non-pathogenic Fusarium. Many species of non-pathogenic Fusarium inhabit the tissues of plants as endophytic fungus or as saprophytes in soil. Non-pathogenic Fusarium can live for a long time in soil, in rhizosphere and in planta (Singh et al. 2016, 2017).
Non-pathogenic Fusarium are endophytes in many crops in agricultural ecosystems (Burgess 1981; Leslie et al. 1990; Kuldau and Yates 2000). Non-pathogenic Fusarium can invade internal plant tissues without causing any symptoms (Burgess 1981). Some non-pathogenic Fusarium isolated from healthy rakkyo roots (Allium chinense) after 97 days including F. fujikuroi, F. solani and F. oxysporum (Honda and Kawakubo 1999). Some non-pathogenic or endophytic Fusarium were isolated from five species of the medicinal plants in the Western Ghats of India (Raviraja 2005). The fungal biomass of non-pathogenic Fusarium strains could differ from other pathogenic F. oxysporum strains present in the root cortex (Validov et al. 2011). Three species of non-pathogenic Fusarium, viz. F. oxysporum, F. solani and F. fujikuroi, were reported to be dormant in the rhizosphere of tomato (Imazaki and Kadota 2015). Non-pathogenic Fusarium are highly diverse in soil, in rhizosphere and in the roots of tomato (Demers et al. 2015). The non-pathogenic Fusarium, viz. F. fujikuroi, F. solani, F. proliferatum and F. polyphialidicum, were among the fungal flora isolated from the roots of banana (Cao et al. 2002; Al-Ani 2017b). Many strains of non-pathogenic F. oxysporum were also isolated from healthy banana (Nel et al. 2006b; Al-Ani 2017b).
Some antimicrobial compounds produced by plants affect the growth of pathogenic Fusarium, exclusively (Mishra et al. 2015). Landa et al. (2002) reported phytoanticipins including biochanin A and tomatine, which inhibit the growth of pathogenic Fusarium, while enhancing the growth of non-pathogenic Fusarium. Also, coumarin could inhibit the growth of both non-pathogenic and pathogenic Fusarium. Different species of non-pathogenic Fusarium could be efficiently used for the management of various phytopathogens by reducing infection of plant-parasitic nematodes, bacteria and fungi (Bisen et al. 2015; Keswani et al. 2016). Indeed, non-pathogenic Fusarium can utilize as biocontrol agent such as other biocontrol agents. Many biocontrol agents with natural products were used in controlling the plant pathogens and pests (Al-Ani 2006; Al-Ani and Salleh 2013b; Mohammed et al. 2011, 2012, 2013, 2014; Al-Ani and Al-Ani 2011; Al-Ani et al. 2012; Al-Ani et al. 2013a, b; AL-Ani 2017a, b; Al-Ani and Albaayit, 2018a, b; Al-Ani et al. 2018; AL-Ani 2018a, b; Al-Ani 2019a, b, c, d, e; Al-Ani et al. 2019). Other methods are including several methods such natural products (Mohammed et al. 2012; Al-Ani et al. 2012). Non-pathogenic Fusarium have been effectively employed for management of Fusarium wilt of many important agricultural crops, including banana (Nel et al. 2006a; Al-Ani 2010, 2017b; Al-Ani et al. 2013a), tomato (Lemanceau and Alabouvette 1991; Larkin and Fravel 1998), chickpea (Hervás et al. 1995), cucumber (Mandeel and Baker 1991; Wang et al. 2013), watermelon (Larkin et al. 1996; Freeman et al. 2002), basil (Fravel and Larkin 2002), celery (Schneider 1984), strawberry (Tezuka and Makino 1991), muskmelon (Freeman et al. 2002), cyclamen (Minuto et al. 1995) and flax (Lemanceau and Alabouvette 1991). Also, non-pathogenic Fusarium is compatible with other biocontrol agents and be very efficiently involved in integrated pest management. Belgrove et al. (2011) used non-pathogenic F. oxysporum with Pseudomonas fluorescens WCS 417 against pathogenic F. oxysporum f. sp. cubense race 4 demonstrating effective suppression and protection of banana cultivar from Panama disease (Fusarium wilt). Also, application of consortia of non-pathogenic Fusarium and Trichoderma proved to be highly effective in reducing the vanilla shoot rot disease (Taufiq et al. 2017).
The efficacy of non-pathogenic Fusarium in the production and secretion of diverse and bioactive secondary metabolites was contributed to the management of phytopathogens (Jayaprakashvel and Mathivanan 2011), while other mechanisms include mycoparasitism, competition and induced resistance in host (Fravel and Larkin 2002; Kaur et al. 2010; Shishido et al. 2005).
2 Secondary Metabolites
Secondary metabolites produced by biocontrol agents are highly effective in controlling phytopathogens. Non-pathogenic Fusarium produces an array of chemically diverse, bioactive secondary metabolites. Non-pathogenic Fusarium secretes low molecular weight volatile organic compounds (VOCs) (Weikl et al. 2016). Non-pathogenic Fusarium produces several secondary metabolites which were absent in its pathogenic counterpart. Nawar (2016) reported that GC-MS analysis of the cultural filtrate of non-pathogenic Fusarium had as many as 30 secondary compounds compared with 22 for the pathogenic isolate.
Two antifungal compounds of F. chlamydosporum were able to inhibit the growth of uredospore of Puccinia arachidis (Mathivanan and Murugesan 1998). α-Pyrones, viz. fusapyrone (FP) and deoxyfusapyrone (DFP), were reported to be produced by F. semitectum (Evidente et al. 1999). DFP and FP inhibited the growth of many filamentous fungi such as Alternaria alternata, Penicillium verrucosum, P. brevicompactum, Ascochyta rabiei, Aspergillus flavus, Cladosporium cucumerinum, Phoma tracheiphila, Botrytis cinerea, Candida albicans, C. glabrata and Cryptococcus neoformans (Altomare et al. 2000; Bartelt and Wicklow 1999; Garret and Robinson 1969; Mathivanan and Murugesan 1999). Non-pathogenic F. oxysporum MSA35 strain produces many VOCs which are highly effective against pathogenic F. oxysporum f. sp. lactucae Fuslat10 (Minerdi et al. 2009). Volatile compounds of MSA35 strain such as α-humulene efficiently reduced the mycelial growth and inhibited the virulence gene of pathogenic Fuslat10 strain (Minerdi et al. 2009). α-Humulene extracted from non-pathogenic MSA35 was effective on pathogenic Fuslat10 strain at 25–100 mM but at 5–20 mM was completely ineffective (Minerdi et al. 2009). The strain CanR-46 of F. oxysporum was producing four VOCs including limonene, octanoic acid, 3,4-2H-dihydropyran and 5-hexenoic acid effective against V. dahliae (Zhang et al. 2015).
For control of the plant parasitic nematodes, non-pathogenic F. solani produced secondary metabolites affecting the juveniles of Meloidogyne javanica (Siddiqui and Shaukat 2003). Two species of endophytic Fusarium such as F. oxysporum and F. solani secrete some secondary metabolites as nematicidal agents against second-stage juveniles of Meloidogyne javanica (Qureshi et al. 2012). Non-pathogenic F. oxysporum produced VOCs against second-stage juveniles of Meloidogyne exigua causing high mortality and immobility after 72 h (Costa 2014). For control of bacterial plant pathogens, endophytic F. oxysporum NRRL26379 in A. thaliana (A) reduced the disease severity of Pseudomonas syringae (Col-0), (B) improved the plant growth and (C) increased salt tolerance by producing volatile compounds (Li and Kang 2018).
For control of the plant parasitic weeds, endophytic Fusarium produces some toxins that can be highly beneficial for field applications. Zonno and Vurro (2002) isolated endophytic Fusarium secreting several toxins such as nivalenol, T-2, neosolaniol, HT-2 and diacetoxyscirpenol that were able to inhibit 100% plant parasitic weed Orobanche ramosa. Two endophytic Fusarium could produce some secondary metabolites as mycoherbicidal agents that are highly effective for growth inhibition of Orobanche aegyptiaca (Egyptian broomrape) of tomato (Cohen et al. 2002a). These species including F. oxysporum produced fusaric acid and fumonisin-like ceramide synthase inhibitors (Cohen et al. 2002a). Beauvericin (as toxin) could significantly improve the secondary metabolite content and plant growth in plant Dioscorea zingiberensis (a traditional Chinese medicinal herb), which was produced by endophytic F. redolens Dzf2 (Campos et al. 2011; Yin et al. 2011). Fusarium sp. KF611679 strain of Brazilian tree Caesalpinia echinata Lam. was secreting a trypanocidal metabolite as beauvericin (Campos et al. 2015). The LC-MS analysis of secondary metabolites for four endophytic Fusarium species such as F. oxysporum, F. solani, F. subglutinans and F. verticillioides isolated from symptomless weeds produced some main compounds comprising beauverin, cyclosporines, enniatins, equisetin, fusaric acid, integracide A and trichosetin (Ilic et al. 2017). Among these compounds equisetin, fusaric acid, beauvericin and enniatins acted as mycotoxins, while trichosetin was an efficient antibacterial compound.
Endophytic Fusarium isolated from the inner bark of Taxus baccata L. was found to be producing some antimicrobial compounds (Tayung and Jha 2010). Endophytic fungi Fusarium were secreting antibacterial compounds active against several pathogenic bacteria including Staphylococcus epidermis, S. aureus, Bacillus subtilis, Klebsiella pneumoniae, Escherichia coli and Shigella flexneri, as well as some antifungal compounds active against pathogenic fungi Candida tropicalis and C. albicans (Tayung and Jha 2010). In addition, endophytic F. oxysporum of plant rhizome Acorus calamus was found to be producing secondary metabolites with antimicrobial activity against many pathogenic microorganisms (Barik et al. 2010). Endophytic F. solani showed high inhibition of six bacteria such as Staphylococcus aureus, S. epidermidis, Bacillus subtilis, Klebsiella pneumoniae, Shigella flexneri and E. coli and two fungi, viz. Candida tropicalis and C. albicans, by secreting antimicrobial secondary metabolites (Tayung et al. 2011a, b). These antimicrobial compounds were analysed using GC-MS for the crud metabolites of F. solani including (1) dodecene, (2) hexylcyclohexane, (3) 1-tetradecene, (4) tetradecane, (5) octylcyclohexane, (6) 10-nonadecanone, (7) 8-pentadecanone and (8) 8-octadecanone (Tayung et al. 2011a). For antibacterial activity, endophytic strain BH-3 of F. oxysporum from the bulbs of Lilium lancifolium produced secondary metabolites as antibacterial against Leuconostoc mesenteroides (Liu et al. 2012).
Several secondary compounds such as ergosterol-5,8-peroxide, triterpene acetate and cerebroside were isolated from endophytic Fusarium (Effendi 2004). Strain K178 of Fusarium maire was able to produce the anticancer compound paclitaxel (Taxol) (Xu et al. 2006). Endophyte F. solani was also reported to produce paclitaxel (Chakravarthi et al. 2008; Deng et al. 2009). Endophyte F. arthrosporioides also secreted a Taxol compound (Li et al. 2008). Two secondary metabolites, viz. camptothecin and 10-hydroxycamptothecin, were produced by two endophytic strains MTCC 9667 and MTCC 9668 of F. solani isolated from plant Apodytes dimidiata (Icacinaceae), and these compounds are used as anticancer drugs, topotecan and irinotecan (Shweta et al. 2010). An anticancer compound rohitukine was secreted by endophytic F. proliferatum (MTCC9690) from plant Dysoxylum binectariferum (Kumara et al. 2012). A Taxol compound with anticancer activity was isolated from F. solani (Tayung et al. 2011a). Endophytic F. oxysporum from mangrove leaves Rhizophora annamalayana was also secreting a Taxol compound (Elavarasi et al. 2012). Endophytic F. oxysporum isolated from the root bark of G. biloba was reported to produce ginkgolide B (Cui et al. 2012). A podophyllotoxin as anticancer was produced by some endophytic F. oxysporum that was isolated from medicinal plant Juniperus recurva (Kour et al. 2008). Endophytic F. solani strain P1 was producing 29.0 μg/g of podophyllotoxin, this strain isolated from roots of Podophyllum hexandrum in Himalayan region (Nadeem et al. 2012). A Taxol compound was secreted by endophytic F. redolens that was isolated from plant Taxus baccata L. subsp. wallichiana (Garyali and Reddy 2013).
In additional, F. oxysporum NFX06 strain from plant Nothapodytes foetida (Musavi et al. 2015), and endophytic F. solani MTCC 9668 (Venugopalan et al. 2016), could produce anticancer compound camptothecin. However, the endophytic strain ZZF60 of Fusarium from mangroves forest secreted several secondary compounds including (1) 5-hydroxy-7-methoxy-40-O-(3-methylbut-2-enyl) isoflavone, (2) vittarin-B, (3) 3,6,7-trihydroxy-1-methoxyxanthone, (4) eriodictyol, (5) cyclo(Phe-Tyr) and (6) 1,3,6-trihydroxy-8-methylxanthone (Huang et al. 2012). Endophytic F. oxysporum could produce anticancer drug vincristine by converting vinblastine to vincristine (Kumar and Ahmad 2013; Kumar et al. 2013). Endophytic Fusarium isolated from the fresh bulbs of Fritillaria unibracteata var. wabensis produced some medicinal compounds such as peiminine and peimisine (Pan et al. 2014). F. redolens 6WBY3 isolated from bulbs of Fritillaria unibracteata var. wabuensis was secreting imperialine-3β-D-glucoside and peimisine (Pan et al. 2015). Five isolates of endophytic Fusarium such as F. oxysporum (one isolate), F. incarnatum (two isolates) and F. solani (two isolates) produced cinchona alkaloids, such as quinine, quinidine, cinchonine and cinchonidine (Hidayat et al. 2016). Weikl et al. (2016) demonstrated the ability of non-pathogenic Fusarium to produce the complex VOCs such as sesquiterpenes.
3 Other Mechanisms
Honda and Kawakubo (1998) used simultaneously two isolates of non-pathogenic Fusarium from healthy root of rakkyo (Allium chinense), viz. F. oxysporum and F. moniliforme against F. oxysporum f. sp. allii causing basal rot of rakkyo. The mode of action for non-pathogenic Fusarium could include several mechanisms including competition for nutrients and infection sites, induced resistance, etc., but the efficacy of these mechanisms depends on the kind of strain and isolates (Fravel et al. 2003).
3.1 Mycoparasitism
Benhamou et al. (2002) reported the ability of non-pathogenic F. oxysporum Fo47 strain to attack other fungal pathogens. The Fo47 strain could inhibit the mycelial growth of Pythium ultimum causing damping-off of cucumber and reported the ability of Fo47 to grow inside the cells of P. ultimum (Benhamou et al. 2002). Non-pathogenic F. oxysporum S6 could attack the sclerotia of Sclerotinia sclerotiorum, considered as a mycoparasite (Rodrıguez et al. 2006). Also, Tsapikounis (2015) reported several isolates of Fusarium able to mycoparasitism on the sclerotia of Sclerotinia sclerotiorum.
3.2 Antibiosis
Non-pathogenic Fusarium produces hydrolytic enzymes and secondary metabolites inhibiting the growth of plant pathogens without direct physical contact. Fo47 strain of non-pathogenic Fusarium secretes some antifungal against P. ultimum (Benhamou et al. 2002). Two strains of non-pathogenic Fusarium such as F. solani CS-1 and F. oxysporum CS-20 were inducing the systemic resistance in some vegetables such as watermelon (Citrullus lanatus) and tomato (Lycopersicon esculentum) against Fusarium wilt (Larkin and Fravel 1999). Endophytic F. equiseti produced two trichothecene compounds, viz. 4,15-diacetoxy-12,13-epoxy-trichothec-9-en-3-ol (diacetoxyscirpenol) and 4,15-diacetoxy-12,13-epoxy-3,7-dihydroxytrichothec-9-en-8-one (4,15-diacetylnivalenol), very effective against Meloidogyne incognita causing the egg-hatching inhibition and immobilization of juveniles at second stage (Nitao et al. 2001).
Additionally, cyclosporine produced by F. oxysporum strain S6 could inhibit the formation of sclerotia of Sclerotinia sclerotiorum (Rodrıguez et al. 2006). Non-pathogenic F. oxysporum was inducing in pepper some bioactive compounds against pathogenic V. dahliae including caffeic acid, ferulic acid and chlorogenic acid (Veloso et al. 2016). High antifungal activity against spore germination of some plant fungal pathogens was detected in the culture filtrate of non-pathogenic F. oxysporum strain F221-B (Thongkamngam and Jaenaksorn 2016). This antifungal of F221-B strain could cause damage to the spores (Thongkamngam and Jaenaksorn 2016). The cell-free culture filtrates of endophytic F. proliferatum I92 showed antifungal activity against fusarium crown and root rot in tomato (Nefzi et al. 2018).
3.3 Competition
Non-pathogenic Fusarium compete with plant pathogens for space and nutrients (Nagao et al. 1990; Couteaudier and Alabouvette 1990; Alabouvette 1990; Larkin and Fravel 1999; Benítez et al. 2004). This competition for nutrients is important for non-pathogenic Fusarium for growth and sporulation. Non-pathogenic Fusarium strain Fo47 could compete for carbon with pathogenic F. oxysporum (Duijff et al. 1998). Larkin and Fravel (1999) demonstrated the ability of non-pathogenic F. oxysporum strain Fo47 for competing for glucose with the pathogenic F. oxysporum. Competition was observed between pathogenic F. oxysporum f. sp. lycopersici and non-pathogenic F. oxysporum for root exudates on the surface of the tomato roots (Olivain and Alabouvette 1999). Non-pathogenic F. oxysporum Fo47 strain was competing for nutrients with pathogenic F. oxysporum f. sp. lycopersici Fo18 strain (Olivain et al. 2006). Two strains ML-5-2 and HK-5b-4-1 of non-pathogenic F. oxysporum were competing with F. oxysporum f. sp. vanillae for nutrients (Xia-Hong 2007).
Gizi et al. (2011) applied the F2 strain of non-pathogenic F. oxysporum against pathogenic Verticillium dahlia that F2 strain reduced 68% of Verticillium wilt disease incidence in eggplant by competing for nutrient and space at the surface and inside the roots. Non-pathogenic Fusarium competed for nutrients with F. oxysporum f. sp. niveum that reduced Fusarium wilt of V. villosa (Himmelstein 2013). Fo47 strain was competing for nutrients with pathogenic V. dahliae on root surface of pepper (Veloso et al. 2016). Some isolates of non-pathogenic Fusarium could produce a siderophore to compete for iron with pathogenic F. oxysporum f. sp. cubense tropical race 4 (FocTR4) LJ27 strain from banana of Palau Penang in Malaysia that lead to high reduction of Fusarium wilt disease of banana (Al-Ani 2017b).
3.4 Induced Plant Resistance
The induced resistance in plants is a mode of action that affects the pathogens indirectly (Al-Ani 2018a). Non-pathogenic Fusarium sp. is able to induce the plant resistance (Benhamou and Garand 2001). The plant resistance is restricted to the proliferation of non-pathogenic Fusarium in the inner roots (Validov et al. 2011).
The Fo47 strain of non-pathogenic F. oxysporum showed high efficacy against F. oxysporum f. sp. lycopersici causing Fusarium wilt of tomato through the split root methods (Fuchs et al. 1997). The split root system includes four methods: (A) benomyl system, (B) split root system, (C) cutting system and (D) layering system (Fuchs et al. 1997). Non-pathogenic F. oxysporum Fo47 strain could induce systemic resistance in tomato plant by accumulating both PR-1 proteins and chitinases (Duijff et al. 1998). The split root is a very interesting method for detecting the ability of non-pathogenic Fusarium isolates to induce plant resistance. Volatile compounds of non-pathogenic Fusarium could induce the plant resistance against Pseudomonas syringae in Arabidopsis thaliana (Bitas and Kang 2012). The process of induced resistance through non-pathogenic F. oxysporum Fo47 strain in cucumber against P. ultimum was demonstrated by Benhamou et al. (2002). The systemic resistance was induced through upregulating some defence-related gene, viz. POX, PIR7A, lectin, PR-3, PAE, PAL, catalase and PR-1, against Radopholus similis through treating banana (Musa spp.) with non-pathogenic F. oxysporum (Paparu et al. 2007).
3.5 Induced Plant Defences
Non-pathogenic Fusarium has the ability to induce plant defence (Paparu et al. 2007). The modulation of phytohormone regulators such as jasmonic acid, ethylene, abscisic acid, salicylic acid and auxin by non-pathogenic F. oxysporum strains leads to induction plant defence network (Di et al. 2016). Olivain et al. (2003) observed non-pathogenic F. oxysporum having the ability to induce the plant defence in flax plant by affecting the host physiology against pathogenic F. oxysporum f. sp. lini Foln3 strain.
Non-pathogenic Fusarium, viz. F. oxysporum, F. moniliforme, F. merismoides and F. solani, induced the plant defences by activating and increasing the polyphenol oxidase and peroxidase content in tomato when (A) directly treated with the spore suspension and (B) extraction of cell wall elicitors (Patil et al. 2011). Many strains of non-pathogenic Fusarium could induce the plant defences enzymes such as phenylalanine ammonia lyase (PAL), β-1,3-glucanase, polyphenol oxidase (PPO), chitinase and peroxidase (POD) that inhibited the pathogenic growth in watermelon (Raghunandan 2013). Fo47 strain of non-pathogenic F. oxysporum could induce the defence genes such as a class II chitinase (CACHI2), basic PR-1 protein (CABPR1) and sesquiterpene cyclase (CASC1) in pepper against Phytophthora capsici and Verticillium dahliae (Veloso and Díaz 2012). Enhancement in the activities for three enzymes, viz. PPO, POD and PAL, in Chinese herbal Dioscorea zingiberensis was observed when treated with three oligosaccharides from endophytic Fusarium oxysporum Dzf17 strain (Li et al. 2012; Li et al. 2014). The gene expression of PR3, LOX1, PAL1, CsCam12, NPR1 and CsCam7 could be induced through inoculation of non-pathogenic F. oxysporum CS-20 in cucumber roots (Pu et al. 2014). Fo47 strain has the ability to induce plant defences through jasmonyl isoleucine and salicylic acid in pepper against the pathogen V. dahliae (Veloso et al. 2016). Three compounds comprising 4-hydroxybenzoic acid, gibepyrone D and indole-3-acetic acid (IAA) produced by non-pathogenic F. oxysporum 162 could induce plant defence against plant pathogenic nematodes (Bogner et al. 2017).
3.6 Induced Changes in Phytochemistry
Non-pathogenic Fusarium induce changes in phytochemistry (Huang et al. 2008). A non-pathogenic Fusarium strain Rs-F-in-11 was observed to be eliciting the metabolic pathway against strain Py71–1 of Pythium ultimum in Lepidium sativum (Ishimoto et al. 2004). Ishimoto et al. (2004) found that strain Rs-F-in-11 induced myrosinase enzyme in roots of L. sativum and this enzyme catalysed the hydrolyzation of glucosinolates to isothiocyanate, leading to the accumulation of isothiocyanates in the roots.
Non-pathogenic Fusarium as endophyte can alter the phenolic profile including ferulic acid, vanillic acid and caffeic acid in the leaves and roots of tomato against pathogenic F. oxysporum f. sp. lycopersici (Panina et al. 2007). The strain 162 (FO162) of non-pathogenic Fusarium could colonize tomato and induce the roots to produce the repellent substance against nematode M. incognita juveniles (Dababat and Sikora 2007). Endophytic F. oxysporum strain Fo162 could induce changes in the proliferation of banana root affecting the growth of nematode Radopholus similis (Kurtz 2010). Three polysaccharides, viz. exopolysaccharide, sodium hydroxide-extracted mycelial polysaccharide and water-extracted mycelial polysaccharide of endophytic F. oxysporum Dzf17, affected the biosynthesis of secondary metabolites and growth for Dioscorea zingiberensis (Li et al. 2011a, b). Some non-pathogenic Fusarium isolates were inducing the free phenol content and total protein content in tomato (Patil et al. 2011) and watermelon (Raghunandan 2013).
3.7 Non-pathogenic Fusarium as Biofertilizers
Non-pathogenic Fusarium enhance the plant growth by producing the gibberellic acid (GA) (Leslie 1996), IAA (Bogner et al. 2017) and siderophores (Al-Ani 2017b) and enhance the nutrient utilization efficiency (Zhang et al. 2012). Louter and Edgington (1990) reported the ability of non-pathogenic Fusarium such as F. oxysporum and F. solani in reducing the tomato root rot and increased the yield. However, endophytic Fusarium such as F. arthrosporioides and F. oxysporum were pathogenic for plant parasitic Orobanche aegyptiaca though affecting the size and number of shoots for O. aegyptiaca by producing IAA (Cohen et al. 2002b). The high production of IAA was for co-transforming two genes both of iaaH and iaaM in Fusarium that was probably increased for suppressing the appressoria formed on infected Orobanche aegyptiaca through attack on tomato (Cohen et al. 2002b). Some strains of non-pathogenic Fusarium can reduce the plant diseases and simultaneously enhance the plant growth. The KGL0401 strain of F. proliferatum was reported to produce several new gibberellins (GAs) (Rim et al. 2005). The conidial suspension 108–109 (spores/ml) of F. oxysporum B6 significantly enhanced various plant growth parameters such as leaf length and leaf area, plant height and root fresh weight (Mennan et al. 2005).
In addition, endophytic Fusarium improved plant growth by secreting gibberellin (GA), indole acetic acid (IAA) and auxin (Dai et al. 2008). Thangavelu and Jayanthi (2009) reported a very effective strain of non-pathogenic F. oxysporum Ro-3 for reducing Fusarium wilt severity of banana. F. oxysporum Ro-3 also increased plant height, petiole length, leaf area, girth and the number of leaves (Thangavelu and Jayanthi 2009). Bitas and Kang (2012) reported an isolate of F. oxysporum producing VOCs that enhanced the plant growth by promoting root and shoot growth in A. thaliana. Also, non-pathogenic Fusarium increased the plant growth of watermelon by producing the IAA and GA with the solubilization for phosphate (Raghunandan 2013). F. oxysporum could enhance the plant growth of A. thaliana and tobacco by producing many volatile compounds (Bitas et al. 2015). Also, LeBlanc (2015) isolated many non-pathogenic Fusarium producing secondary metabolites such as bikaverin (BIK), IAA and GA. F. solani I149 isolate also improved plant growth in axenic cherry plants (Ilic et al. 2017), while endophytic F. proliferatum I92 enhanced plant growth in tomato (Nefzi et al. 2018).
3.8 Secreting the Enzymes
Non-pathogenic Fusarium produce many hydrolytic enzymes. β-D-glucuronidase (GUS) was detected by using a new method in the tomato roots that were treated with 70 T01 strain of non-pathogenic F. oxysporum (Bao and Lazarovits 2002). Myrosinase was produced by non-pathogenic Fusarium strain Ls-F-in-4-1 that inhibited the mycelial growth of P. ultimum strain Py71-1 (Ishimoto et al. 2004). Endophytic F. proliferatum I92 could produce several hydrolytic enzymes, viz. chitinase, lipase, amylase and proteases, that may affect Fusarium crown and root rot in tomato (Nefzi et al. 2018).
4 Conclusion
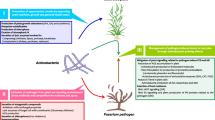
Non-pathogenic Fusarium are soil-borne fungi, as well as an endophyte in the host system. This genus produces a variety of chemically diverse secondary metabolites. Some of the secondary metabolites have been identified but many are yet to be identified. Non-pathogenic Fusarium has other mechanisms that are very useful for agricultural production. Non-pathogenic Fusarium plays a huge role in agriculture through production of bioactive secondary metabolites having diverse functions. Other modes of actions include (1) induction of host defence response, induction of resistance genes and secretion of some plant activators; (2) secretion of various antibiotics against phytopathogens; (3) restricting the nutrient supply to competing microorganisms; and (4) production of hydrolytic enzymes that may be utilized against plant pathogens.
Non-pathogenic Fusarium can be separated from pathogenic Fusarium by pathogenicity/virulence testing. Also, the ability of the non-pathogenic Fusarium for production of mycotoxins should be tested. Finally, the importance of application of non-pathogenic Fusarium over chemical pesticides is far outreaching and comparatively more beneficial.
References
Alabouvette C (1990) Biological control of Fusarium wilt pathogens in suppressive soils. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB International, Wallingford, UK, pp 27–43
Al-Ani LKT (2006) Induce resistance against cucumber mosaic virus by pseudomonas fluorescens migula. MSc Department of Plant Protection, College of Agriculture, University of Baghdad, Baghdad, Iraq, pp 90
Al-Ani LKT (2010) Biological control of Fusarium wilt of banana by non pathogenic Fusarium oxysporum. PPSKH colloquium, Pust Pengajian Sains Kajihayat/School of Biological Sciences, USM, June, p 10
Al-Ani LKT (2017a) PGPR: A good step to control several of plant pathogens. In: Singh HB, Sarma BK, Keswani C (eds) Advances in PGPR Research. CABI, UK, pp 398–410
Al-Ani LKT (2017b) Potential of utilizing biological and chemical agents in the control of Fusarium wilt of banana. PhD, School of Biology Science, Universiti Sains Malaysia, Pulau Pinang, Malaysia, p 259
AL-Ani LKT (2018a) Trichoderma: beneficial role in sustainable agriculture by plant disease management. In: Egamberdieva D, Ahmad P (eds) Plant microbiome: stress response, Microorganisms for sustainability, vol 5. Springer, Singapore, pp 105–126
AL-Ani LKT (2018b) Trichoderma from extreme environments: physiology, diversity, and antagonistic activity. In: Egamberdieva D, Birkeland N-K, Panosyan H, Li W-J (eds) Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications. Microorganisms for Sustainability. Springer, Singapore, pp 388–403
AL-Ani LKT (2019a) The importance of endophytic fungi from the medicinal plant: Diversity, natural bioactive compounds, and control of plant pathogens. In: Egamberdieva D et al (eds) Medically important plant biomes source of secondary metabolites. Springer, Singapore, (In Press)
AL-Ani LKT (2019b) A patent survey on Trichoderma spp. (from 2007-2017). In: Singh HB, Keswani C, Singh SP (eds) Intellectual Property Issues in Microbiology. Springer, Singapore, (In Press)
AL-Ani LKT (2019c) Entomopathogenic fungi in intellectual property and using in biotechnology. In: Singh HB, Keswani C, Singh SP (eds) Intellectual Property Issues in Microbiology. Springer, Singapore, (In Press)
AL-Ani LKT (2019d) Recent Patents on Endophytic Fungi and their International Market. In: Singh HB, Keswani C, Singh SP (eds) Intellectual Property Issues in Microbiology. Springer, Singapore, (In Press)
AL-Ani LKT (2019e) Bioactive secondary metabolites of trichoderma spp. for efficient management of phytopathogens. In: Singh HB, Keswani C, Reddy MS, Royano ES, García-Estrada C (eds) Secondary metabolites of plant growth promoting rhizomicroorganisms - discovery and applications. Springer, Singapore (In Press)
Al-Ani RA, Al-Ani LKT (2011) Induced of systemic resistance in cucumber plants against Cucumber mosaic virus (CMV) by Pseudomonas fluorescens Migula. Arab Journal of Plant Protection 29:36–42
Al-Ani LKT, Albaayit SFA (2018a) Antagonistic of some Trichoderma against Fusarium oxysporum sp. f. cubense tropical race 4 (FocTR4). International conference on Research in Education & Science, ICRES April 28 – May 1, Marmaris, Turkey, pp 271 (Abstract)
Al-Ani LKT, Albaayit SFA (2018b) Antagonistic of some Trichoderma against Fusarium oxysporum sp. f. cubense tropical race 4 (FocTR4). The Eurasia Proceedings of Science. Technology, Engineering & Mathematics (EPSTEM) 2:35–38
Al-Ani LKT, Negim E-S, Mohammed AM, Salleh B, Saleh MI (2012) Antifungal activity of novel Binary grafting polymers. 1st USM – KAZNU International Conference on: Challenges of Teaching and Chemistry Research in Institutions of Higher Learning, 11-13 July, p 44.
Al-Ani LKT, Salleh B, Mohammed AM, Ghazali AHA, Al-Shahwany AW, Azuddin NF (2013a) Biocontrol of Fusarium wilt of Banana by Non-pathogenic Fusarium spp. International symposium on tropical fungi, ISTF, IPB International Convention Center, Bogor, Indonesia; 09/2013, pp 50–51
Al-Ani LKT, Salleh B, Ghazali AHA (2013b) Biocontrol of fusarium wilt of banana by Trichoderma spp. 8th PPSKH colloquium, Pust Pengajian Sains Kajihayat/School of Biological Sciences, USM, 5–6 June.
Al-Ani LKT, Yonus MI, Mahdii BA, Omer MA, Taher JK, Albaayit SFA, Al-Khoja SB (2018) First record of use Fusarium proliferatum fungi in direct treatment to control the adult of wheat flour Tribolium confusum, as well as, use the entomopathogenic fungi Beauveria bassiana. Ecology, Environment and Conservation 24(3):29–34
Al-Ani LKT, Mohammed AM, Ibrahim NF, Azuddin NF, Aguilar-Marcelino L (2019) Biological control of Fusarium oxysporum f. sp. cubense tropical race 4 in vivo by using three species of Trichoderma. Arc Phytopathol Plant Protect (In press)
Altomare C, Perrone G, Zonno MC, Evidente A, Pingue R, Fanti F, Polonelli L (2000) Biological characterization of fusapyrone and deoxyfusapyrone, two bioactive secondary metabolites of Fusarium semitectum. J Nat Prod 63:1131–1135
Attitalla IH, Mansour SE, Mohamed WS, Al-Ani LKT, Mohammed AM, Faturi MY, Balal IAA, El-Maraghy SSM (2010a) Influence of aspergillus flavus and aspergillus terreus on the protein value of the two varieties of peanut grains. International conference, International Mycotoxin Conference, MycoRed, Penang –Malaysia, 1-4 Dec (177)
Attitalla IH, Laith KA, Nasib MA, Balal IAA, Zakaria M, El-Maraghy SSM, Karim SR (2010b). Screening of Fungi Associated With Commercial Grains and Animal Feeds in Al-Bayda Governorate, Libya. World Appl Sci J 9(7):746–756
Bao JR, Lazarovits G (2002) Evaluation of three procedures for recovery of GUS enzyme and colony forming units of a nonpathogenic strain of Fusarium oxysporum 70T01, from inoculated tomato roots. Can J Plant Pathol 24:340–348
Barik BP, Tayung K, Jagadev PN, Dutta SK (2010) Phylogenetic placement of an endophytic fungus Fusarium oxysporum isolated from Acorus calamus rhizomes with antimicrobial activity. Eur J Biol Sci 2:8–16
Bartelt RJ, Wicklow DT (1999) Volatiles from Fusarium verticillioides (sacc.) Nirenb. And their attractiveness to nitidulid beetles. J Agric Food Chem 47:2447–2454
Belgrove A, Steinberg C, Viljoen A (2011) Evaluation of nonpathogenic Fusarium oxysporum and Pseudomonas fluorescens for Panama disease control. Plant Dis 95:951–959
Benhamou N, Garand C (2001) Cytological analysis of defence-related mechanisms induced in pea root tissue in response to colonization by non-pathogenic Fusarium oxysporum Fo47. Phytopathology 91:730–740
Benhamou N, Garand C, Goulet A (2002) Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ultimum infection in cucumber. Appl Environ Microbiol 68(8):4044–4060
Benítez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. International Microbiology 7:249–260
Bisen K, Keswani C, Mishra S, Saxena A, Rakshit A, Singh HB (2015) Unrealized potential of seed biopriming for versatile agriculture. In: Rakshit A, Singh HB, Sen A (eds) Nutrient use efficiency: from basics to advances. Springer, New Delhi, pp 193–206
Bitas V, Kang S (2012) Fusarium oxysporum produces volatile organic compounds that affect the growth and disease defense of Arabidopsis thaliana. APS annual meeting August 4–8 Providence, USA, Poster Session: MPMI-Fungi, p 588
Bitas V, McCartney N, Li N, Demers J, Kim JE, Kim HS, Brown KM, Kang S (2015) Fusarium oxysporum volatiles enhance plant growth via affecting auxin transport and signaling. Front Microbiol 6:1248. https://doi.org/10.3389/fmicb.2015.01248
Bogner CW, Kamdem RST, Sichtermann G, Matthäus C, Hölscher D, Popp J, Proksch P, Grundler FMW, Schoutencorresponding A (2017) Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb Biotechnol 10(1):175–188. https://doi.org/10.1111/1751-7915.12467
Burgess LW (1981) General ecology of the Fusaria. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: diseases, biology, and taxonomy. Pennsylvania State University Press, University Park, pp 225–235
Campos FF, Johann S, Cota BB, Alves TMA, Rosa LH, Caligiorne RB, Cisalpino PS, Rosa CA, Zani CL (2011) Antifungal activity of trichothecenes from Fusarium sp. against clinical isolates of Paracoccidioides brasiliensis. Mycoses 54:122–129
Campos FF, Sales Júnior PA, Romanha AJ, Araújo MSS, Siqueira EP, Resende JMR, Alves TMA, Martins-Filho AO, Santos VL, Rosa CA, Zani CL, Costa BB (2015) Bioactive endophytic fungi isolated from Caesalpinia echinata Lam. (Brazilwood) and identification of beauvericin as a trypanocidal metabolite from Fusarium sp. Mem Inst Oswaldo Cruz 110:65–74. https://doi.org/10.1590/0074-02760140243
Cao LX, Yon JL, Zhao SN (2002) Endophyte fungi from Musa acuminata leaves and roots in South China. World J Microbiol Biotechnol 18:169–171
Chakravarthi BVSK, Das P, Surendranath K, Karande AA, Jayabaskaran C (2008) Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J Biosci 33:259–267
Cohen BA, Amsellem Z, Lev-Yadun S, Gressel J (2002a) Infection of tubercles of the parasitic weed Orobanche aegyptiaca by mycoherbicidal Fusarium species. Ann Bot 90:567–578
Cohen BA, Amsellem Z, Maor R, Sharon A, Gressel J (2002b) Transgenically enhanced expression of indole-3-acetic acid confers hypervirulence to plant pathogens. Phytopathology 92:590–596
Costa LSAS (2014) Volatiles produced by microbiota from Meloidogyne exigua egg masses and plant volatile emission in response to single and dual infestations with spider mite and nematode. Tese (Doutorado em Agronomia/Fitopatologia) – Universidade Federal de Lavras, Lavras, p 94
Couteaudier Y, Alabouvette C (1990) Quantitative comparison of Fusarium oxysporum competitiveness in relation with carbon utilization. FEMS Microbiology 74:261–268
Cui Y, Yi D, Bai X, Sun B, Zhao Y, Zhang Y (2012) Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 83:913–920
Dababat AEA, Sikora RA (2007) Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion. Nematology 9(6):771–776
Dai CC, Yu BY, Li X (2008) Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr J Biotechnol 7(19):3505–3510
Demers JE, Gugino BK, Jiménez-Gasco MM (2015) Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl Environ Microbiol 81:81–90. https://doi.org/10.1128/AEM.02590-14
Deng BV, Liu KH, Chen WQ, Ding XW, Xie XC (2009) Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J Microbiol Biotechnol 25:139–143. https://doi.org/10.1007/s11274-008-987-2
Di X, Takken FL, Tintor N (2016) How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Front Plant Sci 7:170
Duijff BJ, Pouhair D, Olivain C, Alabouvette C, Lemanceau P (1998) Implication of systemic induced resistance in the suppression of Fusarium wilt of tomato by Pseudomonas fluorescens WCS417r and by non-pathogenic Fusarium oxysporum Fo47. Eur J Plant Pathol 104:903–910
Effendi H (2004) Isolation and structure elucidation of bioactive secondary metabolites of sponge-derived fungi collected from the Mediterranean Sea (Italy) and Bali Sea (Indonesia). Doctoral dissertation, Heinrich-Heine-Universität Düsseldorf, pp 106–127
Elavarasi A, Gnanaprakash SR, Murugaiyan K (2012) Taxol producing mangrove endophytic fungi Fusarium oxysporum from Rhizophora annamalayana. Asia Pac J Trop Biomed 2:1081–1085
Evidente A, Amalfitano C, Pengue R, Altomare C (1999) High performance liquid chromatography for the analysis of Fusapyrone and Deoxyfusapyrone, two antifungal a-Pyrones from Fusarium semitectum. Nat Toxins 7:133–137
Fravel DR, Larkin RP (2002) Reduction of Fusarium wilt of hydroponically-grow basil by fusarium oxysporum strain CS-20. Crop Prot 21:539–543
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502
Freeman S, Zveibil A, Vintal H, Maymon M (2002) Isolation of nonpathogenic mutants of Fusarium oxysporum f. sp. melonis for biological control of Fusarium wilt in Cucurbits. Phytopathology 92:164–168
Fuchs JG, Moënne-Loccoz Y, Défago G (1997) Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Dis 81:492–496
Garret MK, Robinson PM (1969) A stable inhibitor of spore germination produced by fungi. Arch Microbiol 67:370–377
Garyali S, Reddy MS (2013) Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J Microbiol Biotechnol 23:1372–1380
Gizi D, Stringlis IA, Tjamos SE, Paplomatas EJ (2011) Seedling vaccination by stem injecting a conidial suspension of F2, a non-pathogenic Fusarium oxysporum strain, suppresses Verticillium wilt of eggplant. Biol Control 58:387–392. https://doi.org/10.1016/j.biocontrol.2011.06.009
Hervás A, Trapero-Casas JL, Jimenez-Diaz RM (1995) Induced resistance against Fusarium wilt of chickpea by nonpathogenic races of Fusarium oxysporum f. sp. ciceris and nonpathogenic isolates of F. oxysporum. Plant Dis 79:1110–1116
Hidayat I, Radiastuti N, Rahayu G, Achmadi S, Okane I (2016) Three Quinine and Cinchonidine producing Fusarium species from Indonesia. Curr Res Environ Appl Mycol 6(1):20–34. https://doi.org/10.5943/cream/6/1/3
Himmelstein JC (2013) Mechanisms of disease suppression by a hairy vetch (Vicia villosa) cover crop on fusarium wilt of watermelon and the efficacy of the biocontrol actinovate. PhD thesis, University of Maryland, USA, p 158
Honda N, Kawakubo Y (1998) Control of Fusarium basal rot of rakkyo by non pathogenic Fusarium moniliforme and Fusarium oxysporum. Soil Microorganisms 51:13–18
Honda N, Kawakubo Y (1999) Isolation of nonpathogenic Fusarium fujikuroi and Fusarium oxysporum from rakkyo tissues and their colonization of rakkyo roots. Soil Microorganisms (Japan) 53:121–128
Huang WY, Cai YZ, Hyde KD, Corke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 33:61–75
Huang Z, Yang J, She Z, Lin Y (2012) A new isoflavone from the mangrove endophytic fungus Fusarium sp. (ZZF60). Nat Prod Res 26(1):11–15. https://doi.org/10.1080/14786419.2010.529444
Ilic J, Cosic J, Vrandecic K, Dugalic K, Pranjic A, Martin J (2017) Influence of endophytic fungi isolated from symptomless weeds on cherry plants. Mycosphere 8(1):18–30. https://doi.org/10.5943/mycosphere/8/1/3
Imazaki I, Kadota I (2015) Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol Ecol 91:fiv098. https://doi.org/10.1093/femsec/fiv098
Ishimoto H, Fukushi Y, Tahara S (2004) Nonpathogenic Fusarium strains protect the seedlings of Lepidium sativum from Pythium ultimum. Soil Biol Biochem 36:409–414
Jayaprakashvel M, Mathivanan N (2011) Management of plant diseases by microbial metabolites. In: Maheshwari DK (ed) Bacteria in agrobiology: plant nutrient management. Springer, Berlin/Heidelberg, pp 237–265
Kaur R, Kaur J, Singh RS (2010) Nonpathogenic Fusarium as a biological control agent. Plant Pathol J 9(3):79–91
Keswani C, Bisen K, Singh V, Sarma BK, Singh HB (2016) Formulation technology of biocontrol agents: present status and future prospects. In: Arora NK, Mehnaz S, Balestrini R (eds) Bioformulations: for sustainable agriculture. Springer, New Delhi, pp 35–52
Kour A, Shawl AS, Rehman S, Sultan P, Qazi PH, Suden P, Khajuria RK, Verma V (2008) Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol 24:1115–1121. https://doi.org/10.1007/s11274-007-9582-5
Kuldau GA, Yates IE (2000) Evidence of Fusarium endophytes in cultivated and wild plants. In: Bacon CW, JJF W (eds) Microbial endophytes. Marcel Dekker Inc., New York, pp 85–117
Kumar A, Ahmad A (2013) Biotransformation of vinblastine to vincristine by the endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. Biocatal Biotransformation 31(2):89–93
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8(9):e71805. https://doi.org/10.1371/journal.pone.0071805
Kurtz A (2010) Endophytic Fusarium oxysporum: Phylogeny and induced defense responses in banana plants against Radopholus similis. PhD dissertation, Rheinischen Friedrich-Wilhems-Universität, Saarbrücken, Deutschland, p 161
Landa BB, Cachinero-Díaz JM, Lemanceau P, Jiménez-Díaz RM, Alabouvette C (2002) Effect of fusaric acid and phytoanticipins on growth of rhizobacteria and Fusarium oxysporum. Can J Microbiol 48:971–985
Larkin RP, Fravel DR (1998) Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis 82:1022–1028
Larkin RP, Fravel DR (1999) Mechanisms of action and dose response relationships governing biological control of Fusarium wilt of tomato by nonpathogenic Fusarium spp. Phytopathology 89:1152–1161
Larkin RP, Hopkins DL, Martin FN (1996) Suppression of Fusarium wilt of watermelon by non-pathogenic Fusarium oxysporum and other microorganisms recovered from disease-suppressive soil. Phytopathology 86:812–819
LeBlanc NR (2015) In uence of plant diversity and perennial plant identity on Fusarium communities in soil. PhD thesis, University of Minnesota, MN, USA, p 108
Lemanceau P, Alabouvette C (1991) Biological control of Fusarium diseases by fluorescent pseudomonas and nonpathogenic Fusarium. Crop Prot 10:279–286
Leslie JF (1996) Genetic problems in some Fusarium species. Sydowia 48(1):32–43
Leslie JF, Pearson CAS, Nelson PE, Toussoun TA (1990) Fusarium spp. from corn, sorghum and soybean fields in the Central and Eastern United States. Phytopathology 80:343–350
Li N, Kang S (2018) Do volatile compounds produced by Fusarium oxysporum and Verticillium dahliae affect stress tolerance in plants. Mycology. https://doi.org/10.1080/21501203.2018.1448009
Li CT, Li Y, Wang QJ, Sung CK (2008) Taxol production by Fusarium arthrosporioides isolated from yew, Taxus cuspidata. J Med Biochem 27(4):454–458. https://doi.org/10.2478/v10011-008-0022-3
Li P, Mou Y, Shan T, Xu J, Li Y, Lu S, Zhou L (2011a) Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules 16:9003–9016. https://doi.org/10.3390/molecules16119003
Li P, Mao Z, Lou J, Li Y, Mou SY, Lu S, Peng Y, Zhou L (2011b) Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum. Molecules 16:10631–10644. https://doi.org/10.3390/molecules161210631
Li P, Lou J, Mou Y, Sun W, Shan T, Zhou L (2012) Effects of oligosaccharide elicitors from endophyitc Fusarium oxysporum Dzf17 on diosgenin accumulation in Dioscorea zingiberensis seedling cultures. J Med Plants Res 6:5128–5134. https://doi.org/10.5897/JMPR12.120
Li P, Haiyu L, Jiajia M, Weibo S, Xiaohan W, Shiqiong L, Youliang P, Ligang Z (2014) Effects of oligosaccharides from endophytic Fusarium oxysporum Dzf17 on activities of defense-related enzymes in Dioscorea zingiberensis suspension cell and seedling cultures. Electron J Biotechnol 17(4):156–161. https://doi.org/10.1016/j.ejbt.2014.04.012
Liu XL, Huang KH, Zhou JZ, Meng L, Wang Y, Zhang LX (2012) Identification and antibacterial characteristics of an endophytic fungus Fusarium oxysporum from Lilium lancifolium. Lett Appl Microbiol 55:399–406
Louter JH, Edgington LV (1990) Indications of cross-protection against fusarium crown and root rot of tomato. Can J Plant Pathol 12:283–288
Mandeel Q, Baker R (1991) Mechanisms involved in biological control of cucumber with strains of non-pathogenic Fusarium oxysporum. Phytopathology 81:462–469
Mathivanan N, Murugesan K (1998) Isolation and purification of an antifungal metabolite from Fusarium chlamydosporum, a mycoparasite to Puccinia arachidis, the rust pathogen of groundnut. Indian J Exp Biol 37:98–101
Mathivanan N, Murugesan K (1999) Isolation and purification of an antifungal metabolite from Fusarium chlamydosporum, a mycoparasite to Puccinia arachidis, the rust pathogen of groundnut. Indian J Exp Biol 37:98–101
Mennan S, Aksoy HM, Ecevit O (2005) Antagonistic effect of Fusarium oxysporum on Heterodera cruciferae. J Phytopathol 153(4):221–225. https://doi.org/10.1111/j.1439-0434.2005.00957.x
Minerdi D, Bossi S, Gullino ML, Garibaldi A (2009) Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ Microbiol 11(4):844–854
Minuto A, Migheli Q, Garibaldi A (1995) Evaluation of antagonistic strains of Fusarium spp. in the biological and integrated control of Fusarium wilt of cyclamen. Crop Prot 14:221–226
Mishra S, Singh A, Keswani C, Saxena A, Sarma BK, Singh HB (2015) Harnessing plant-microbe interactions for enhanced protection against phytopathogens. In: Arora NK (ed) Plant microbe symbiosis–applied facets. Springer, New Delhi, pp 111–125
Mohammed AM, AL-Ani LKT, Bekbayeva L, Salleh B (2011) Biological control of Fusarium oxysporum f. sp. cubense by Pseudomonas fluorescens and BABA in vitro. World Appl Sci J 15(2):189–191
Mohammed AM, Negim E-S, Al-Ani LKT, Salleh B, Saleh MI (2012) Utilization of amino-azines polymers as antifungal activity for banana. 1st USM – KAZNU International Conference on: Challenges of Teaching and Chemistry Research in Institutions of Higher Learning, 11-13 July, p 29
Mohammed AM, Al-Ani LKT, Salleh B (2013) Potential management of Fusarium oxysporum f. sp. cubense, the banana wilt pathogen by using pseudomonas and beta-amino-butyric acid (BABA). International Symposium on Tropical Fungi, ISTF, IPB International Convention Center, Bogor. Indonesia 09(/2013):37
Mohammed AM, Al-Ani LKT, Salleh B, Ghazali, AMA (2014) Determining plant growth promoting and biocontrol factor of bacterial culture media. The 3rd confernce on Pests management, Crop Protection Research Centre, Sudan, 3-4 February, p 103.
Mohana Kumara P, Zuehlke S, Priti V, Ramesha BT, Shweta S, Ravikanth G, Vasudeva R, Santhoshkumar TR, Spiteller M, Umashaanker R (2012) Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Antonie Van Leeuwenhoek 101(2):323–329. https://doi.org/10.1007/s10482-011-9638-2
Musavi SF, Dhavale A, Balakrishnan RM (2015) Optimization and kinetic modeling of cell-associated camptothecin production from an endophytic Fusarium oxysporum NFX06. Prep Biochem Biotechnol 45:158–172. https://doi.org/10.1080/10826068.2014.907177
Nadeem M, Ram M, Alam P, Ahmad MM, Mohammad A, Al-Qurainy F, Khan S, Abdin Z (2012) Fusarium solani, P1, a new endophytic podophyllotoxin-producing fungus from roots of Podophyllum hexandrum. Afr J Microbiol Res 6(10):2493–2499
Nagao H, Coutaudier Y, Alabouvette C (1990) Colonization of sterilized soil and flax roots by strains of Fusarium oxysporum and Fusarium solani. Symbiosis, 9: 343–354
Nawar LS (2016) Phytochemical and SDS-dissociated proteins of pathogenic and nonpathogenic Fusarium oxysporum isolates. Int J Chem Tech Res 9(6):165–172
Nefzi A, Aydi Ben Abdallah R, Jabnoun-Khiareddine H, Ammar N, Somai L, Hamada W, Haouala R, Daami-Remadi M (2018) Investigation on biosuppression of Fusarium crown and root rot of tomato (Solanum lycopersicum L.) and growth promotion using fungi naturally associated to Solanum linnaeanum L. Af J Microbiol Res 12(7):152–170
Nel B, Steinberg C, Labuschagne N, Viljoen A (2006a) The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing Fusarium wilt of banana. Plant Pathol 55(2):216–223
Nel B, Steinberg C, Labuschagne N, Viljoen A (2006b) Isolation and characterization of nonpathogenic Fusarium oxysporum isolates from the rhizosphere of healthy banana plants. Plant Pathol 55(2):207–216
Nitao JK, Meyer SLF, Schmidt WF, Fettinger JC, Chitwood DJ (2001) Nematode antagonistic trichothecenes from Fusarium equiseti. J Chem Ecol 27:859–869
Nor Azliza I, Hafizi R, Nurhazrati M, Salleh B (2014) Production of major mycotoxins by Fusarium Species isolated from Wild Grasses in Peninsular Malaysia. Sains Malaysiana 43(1):89–94
Olivain C, Alabouvette C (1999) Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici discussed in comparison to a non-pathogenic strain. New Phytol 141:497–510
Olivain C, Trouvelot S, Binet MN, Cordier C, Pugin A, Alabouvette C (2003) Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and non-pathogenic strains of Fusarium oxysporum. Appl Environ Microbiolol 69:5453–5462
Olivain C, Humbert C, Nahalkova J, Fatehi J, Haridon FL, Alabouvette C (2006) Colonization of tomato root by pathogenic and nonpathogenic Fusarium oxysporum strains inoculated together and separately into the soil. Appl Environ Microbiol 72(2):1523–1531
Pan F, Hou K, Gao F, Hu B, Chen Q, Wu W (2014) Peimisine and peiminine production by endophytic fungus Fusarium sp. isolated from Fritillaria unibracteata var. wabensis. Phytomedicine 21:1104–1109. https://doi.org/10.1016/j.phymed.2014.04.010
Pan F, Su X, Hu B, Yang N, Chen Q, Wu W (2015) Fusarium redolens 6WBY3, an endophytic fungus isolated from Fritillaria unibracteata var. wabuensis, produces peimisine and imperialine-3β-D-glucoside. Fitoterapia 103:213–221. https://doi.org/10.1016/j.fitote.2015.04.006
Panina Y, Fravel DR, Baker CJ, Shcherbakova LA (2007) Biocontrol and plant pathogenic Fusarium oxysporum-induced changes in phenolic compounds in tomato leaves and roots. J Phytopathol 155:475–481
Paparu P, Dubois T, Coyne D, Viljoen A (2007) Defense-related gene expression in susceptible and tolerant bananas (Musa spp.) following inoculation with non-pathogenic Fusarium oxysporum endophytes and challenge with Radopholus similis. Physiol Mol Plant Pathol 71:149–157
Pu X, Xie B, Li P, Mao Z, Ling J, Shen H, Zhang J, Huang N, Lin B (2014) Analysis of the defence-related mechanism in cucumber seedlings in relation to root colonization by nonpathogenic Fusarium oxysporum CS-20. FEMS Microbiol Lett 355(2):142–151. https://doi.org/10.1111/1574-6968.12461
Qureshi SA, Ruqqia VS, Ara J, Ehteshamul-Haque S (2012) Nematicidal potential of culture filtrates of soil fungi associated with rhizosphere and rhizoplane of cultivated and wild plants. Pak J Bot 44(3):1041–1046
Raghunandan BL (2013) Evaluation of non-pathogenic Fusarium spp. for their biological control efficacy against Fusarium wilt of watermelon [Citrullus lanatus (Thunb.) Matsum and Nakai]. PhD thesis, University of Agricultural Sciences, Bengaluru, p 255
Raviraja NS (2005) Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. J Basic Microbiol 45(3):230–235. https://doi.org/10.1002/jobm.200410514
Rim SO, Lee JH, Choi WY, Hwang SK, Suh SJ, Lee IJ, Rhee IK, Kim JG (2005) Fusarium proliferatum KGL0401 as a new gibberellin-producing fungus. J Microbiol Biotechnol 15:809–814
Rodrıguez A, Cabrera G, Godeas A (2006) Cyclosporine A from a nonpathogenic Fusarium oxysporum suppressing Sclerotinia sclerotiorum. J Appl Microbiol 100(3):575–586. https://doi.org/10.1111/j.1365-2672.2005.02824.x
Schneider RW (1984) Effects of nonpathogenic strains Fusarium oxysporum on celery root infection by F. oxysporum f.sp. apii and a novel use of the Lineweaver-Burke double reciprocal plot technique. Phytopathology 74:646–653
Shishido M, Miwa C, Usami T, Amemiya Y, Johnson KB (2005) Biological control efficiency of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathology 95:1072–1080
Shweta S, Zuehlke S, Ramesha BT, Priti V, Kumar PM, Ravikanth G, Spiteller M, Vasudeva R, Shaanker RU (2010) Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 71:117–122
Siddiqui IA, Shaukat SS (2003) Non-pathogenic Fusarium solani represses the biosynthesis of nematicidal compounds in vitro and reduces the biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett Appl Microbiol 37:109–114
Singh HB, Sarma BK, Keswani C (eds) (2016) Agriculturally important microorganisms: commercialization and regulatory requirements in Asia. Springer, Singapore, p 336. ISBN-13: 978-9811025754
Singh HB, Sarma BK, Keswani C (eds) (2017) Advances in PGPR. CABI, UK, p 408. ISBN-9781786390325
Taufiq E, Hasim Soekarno BP, Surahman M (2017) Keefektifan Trichoderma sp. dan Fusarium non patogenik dalam mengendalikan penyakit busuk pucuk vanili berwawasan lingkungan. J Littri 23(1):18–25. https://doi.org/10.21082/littri
Tayung K, Jha DK (2010) Antimicrobial endophytic fungal assemblages inhabiting bark of Taxus baccata L. of Indo-Burma mega biodiversity hotspot. Indian J Microbiol 50(1):74–81
Tayung K, Barik BP, Jha DK, Deka DC (2011a) Identification and characterization of antimicrobial metabolite from an endophytic fungus, Fusarium solani isolated from bark of Himalayan yew. Mycosphere 2(3):203–213
Tayung K, Barik BP, Jagadev PN, Mohapatra UB (2011b) Phylogenetic investigation of endophytic Fusarium strain producing antimicrobial metabolite isolated from Himalayan Yew Bark. Malays J Microbiol 7(1):1–6. https://doi.org/10.21161/mjm.23810
Tezuka N, Makino T (1991) Biological control of Fusarium wilt of strawberry by nonpathogenic fusarium oxysporum isolated from strawberry. Ann Phytopathol 57:506–511
Thangavelu R, Jayanthi A (2009) RFLP analysis of rDNA-ITS regions of native non-pathogenic Fusarium oxysporum isolates and their field evaluation for the suppression of Fusarium wilt disease of banana. Australas Plant Pathol 38:13–21
Thongkamngam T, Jaenaksorn T (2016) Efficacy of culture filtrate from Fusarium oxysporum F221-B against plant pathogenic fungi in vitro and Fusarium root rot and wilt disease in hydroponics. Int J Environ Agric Res 12(3):609–622
Tsapikounis FA (2015) An integrated evaluation of mycoparasites from organic culture soils as biological control agents of sclerotia of Sclerotinia sclerotiorum in the Laboratory. BAO J Microbiol 1:001
Validov SZ, Kamilova FD, Lugtenberg BJJ (2011) Monitoring of pathogenic and nonpathogenic Fusarium oxysporum strains during tomato plant infection. Microb Biotechnol 4(1):82–88
Veloso J, Díaz J (2012) Fusarium oxysporum Fo47 confers protection to pepper plants against Verticillium dahliae and Phytophthora capsici, and induces the expression of defence genes. Plant Pathol 61:281–288. https://doi.org/10.1111/j.1365-3059.2011.02516.x
Veloso J, Alabouvette C, Olivain C, Flors V, Pastor V, García T, Díaza J (2016) Modes of action of the protective strain Fo47 in controlling verticillium wilt of pepper. Plant Pathol 65(6):997–1007. https://doi.org/10.1111/ppa.12477
Venugopalan A, Potunuru UR, Dixit M, Srivastava S (2016) Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresour Technol 206:104–111. https://doi.org/10.1016/j.biortech.2016.01.079
Wang C, Lin Y, Lin Y, Chung W (2013) Modified primers for the identification of nonpathogenic Fusarium oxysporum isolates that have biological control potential against fusarium wilt of cucumber in Taiwan. PLoS One 8(6):e65093. https://doi.org/10.1371/journal.pone.0065093
Weikl F, Ghirardo A, Schnitzler JP, Pritsch K (2016) Sesquiterpene emissions from Alternaria alternata and Fusarium oxysporum: effects of age, nutrient availability, and co-cultivation. Sci Rep 6:22152
Xia-Hong H (2007) Biocontrol of root rot disease in Vanilla. PhD thesis, University of Wolverhampton, UK, p 224
Xu F, Tao W, Chang L, Guo L (2006) Strain improvement and optimization of the media of taxol-producing fungus Fusarium maire. Biochem Eng J 31:67–73
Yin C, Li P, Li H, Xu L, Zhao J, Shan T, Zhou L (2011) Enhancement of diosgenin production in Dioscorea zingiberensis seedling and cell cultures by beauvericin from the endophytic fungus Fusarium redolens Dzf2. J Med Plants Res 5:6550–6554. https://doi.org/10.5897/JMPR11.921
Zhang X, Lin L, Chen M, Zhu Z, Yang W, Chen B, Yang X, An Q (2012) A nonpathogenic Fusarium oxysporum strain enhances phytoextraction of heavy metals by the hyperaccumulator Sedum alfredii Hance. J Hazard Mater 229–230:361–370. https://doi.org/10.1016/j.jhazmat.2012.06.013
Zhang Q, Yang L, Zhang J, Wu M, Chen W, Jiang D, Li G (2015) Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 392(1):101–114. https://doi.org/10.1007/s11104-015-2448-y
Zonno MC, Vurro M (2002) Inhibition of germination of Orobanche ramosa seeds by fusarium toxins. Phytoparasitica 30:519–524. https://doi.org/10.1007/BF02979757
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Al-Ani, L.K.T. (2019). Secondary Metabolites of Non-pathogenic Fusarium: Scope in Agriculture. In: Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C. (eds) Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms. Springer, Singapore. https://doi.org/10.1007/978-981-13-5862-3_3
Download citation
DOI: https://doi.org/10.1007/978-981-13-5862-3_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5861-6
Online ISBN: 978-981-13-5862-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)