Abstract
Aims
The study aimed at identifying volatile organic compounds (VOCs) produced by the non-pathogenic Fusarium oxysporum (Fo) strain CanR-46, and to determine the anti-fungal spectrum and the control efficacy of the Fo-VOCs.
Methods
The Fo-VOCs were identified by GC-MS. The antifungal activity of the Fo-VOCs was tested using the double-dish method. Soil contaminated with Verticillium dahliae was fumigated with the Fo-VOCs to test the control efficacy. The GFP-tagged derivative strain CanR-46GFP was tested to colonize cotton roots for prevention of infection by V. dahliae.
Results
Nineteen VOCs were identified with eremophila-1 (10),11-diene being the most abundant. The Fo-VOCs inhibited mycelial growth of 14 fungal species including V. dahliae, delayed V. dahliae conidial germination, suppressed V. dahliae germ-tube elongation, and caused V. dahliae hyphal shriveling and collapse. Four synthetic chemicals, 5-hexenoic acid, limonene, octanoic acid and 3,4-2H-dihydropyran, in the Fo-VOCs profile showed antifungal activity against V. dahliae. The Fo-VOCs significantly (P < 0.05) reduced severity of Verticillium wilt of cotton compared to the control. CanR-46GFP extensively colonized the cotton root tissues, thus effectively prevented V. dahliae infection.
Conclusions
The VOC-producing fungus F. oxysporum CanR-46 is a promising biocontrol agent against V. dahliae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) are carbon-based small chemicals with molecular weight usually lower than 300 Da and low polarity, but with high vapor pressure (approximately 0.01 kPa) at room temperatures (Vespermann et al. 2007; Morath et al. 2012). They are ideal infochemicals functioning in above-ground and underground communications between living organisms (Rasmann et al. 2005; McCormick et al. 2012). According to a recent review by Morath et al. (2012), approximately 250 fungal VOCs belonging to hydrocarbons, heterocycles, aldehydes, ketones, alcohols, phenols, thioalcohols, thioesters, benzene derivatives and cyclohexanes have been identified. Previous studies showed that a mixture of VOCs of the endophytic fungus Muscodor albus can be used as a mycofumigant for control of many post-harvest fruit diseases (Mercier and Jiménez 2004; Mercier and Manker 2005; Mercier and Smilanick 2005; Schnabel and Mercier 2006) and soilborne plant diseases (Stinson et al. 2003). The success of M. albus inspired numerous researchers to explore use of VOCs produced by other fungi and bacteria to control plant diseases. Minerdi et al. (2009) reported that the VOCs produced by the non-pathogenic strain MSA 35 of Fusarium oxysporum together with its ectosymbiotic bacterial consortium (e.g., Serratia, Achromobacter, Bacillus and Stenotrophomonas) could inhibit growth of several pathogenic formae speciales of F. oxysporum on agar media.
Two mechanisms, namely direct inhibition of mycelial growth and/or spore germination (Strobel et al. 2001; Huang et al. 2011, 2012), and/or induction of plant resistance (Ryu et al. 2004), have been proposed to be involved in suppression of plant pathogens by the microbial VOCs. Furthermore, previous studies reported that the VOCs of certain bacterial species, including Bacillus subtilis GB03, B. amyloliquefaciens IN937a and E. cloacae JM22 (Ryu et al. (2003); Arthrobacter agilis UMCV2 (Velázquez-Becerra et al. 2011), Proteus vulgaris JBLS202 (Yu and Lee 2013) can promote plant growth. Similarly, Minerdi et al. (2011) found that the VOCs of F. oxysporum MSA 35 can promote growth of lettuce (Lactuca sativa L.).
Verticillium dahliae Kleb. is a destructive soilborne fungus causing Verticillium wilt on many economically important crops including cotton (Gossypium spp.) in temperate and subtropical regions (Pegg and Brady 2002). Stinson et al. (2003) reported that mycofumigation with the VOCs of M. albus and M. roseus effectively reduced inoculum density of V. dahliae in soil, thereby leading to suppression of the severity of Verticillium wilt of eggplant (Solanum melongena L.).
Strain CanR-46 of F. oxysporum is an endophytic fungus isolated from a healthy root of oilseed rape (Brassica napus L.) in our previous study (Zhang et al. 2014). It was stored in China Center for Type Culture Collection (CCTCC) with the accession number of CCTCC M2014440. It was detected to be non-pathogenic on oilseed rape and cotton, and to be able to produce antifungal VOCs inhibitory to growth of the plant pathogenic fungi Botrytis cinerea and Sclerotinia sclerotiorum on agar media (Zhang et al. 2014). However, the chemical components of the VOCs of F. oxysporum CanR-46 and the efficacy of F. oxysporum in suppression of V. dahliae on cotton remain unknown.
The objectives of this study are: (i) to identify the chemical nature of the VOCs produced by F. oxysporum CanR-46; (ii) to determine the antifungal spectrum of the VOCs of F. oxysporum CanR-46; and (iii) to evaluate efficacy of the VOCs of F. oxysporum CanR-46 in suppression of Verticillium wilt of cotton.
Materials and methods
Fungal isolates and cultural media
The endophytic strain CanR-46 of F. oxysporum was originally isolated from a healthy root of oilseed rape grown in Wuhan of central China (Zhang et al. 2014). It was sub-cultured on potato dextrose agar (PDA). Strain CanR-46GFP harboring the green fluorescent protein gene was derived from strain CanR-46 (Supplementary material Fig. S1). Both strains are similar in growth rate on PDA (25 °C), in conidial germination rate on water agar (WA, 25 °C), and in production of the antifungal VOCs on autoclaved wheat grains (AWG). Sixteen plant pathogenic fungal strains were used in this study and their origins were listed in Supplementary material Table S1. Stock cultures of these fungal strains were maintained on PDA and stored at −80 °C in 40 % glycerol (v/v). Working cultures were prepared by transferring mycelia from storage onto PDA. The cultures were incubated in the dark at 20 or 25 °C for 3 to 15 days depending on the mycelial growth rates of the investigated fungal strains.
Three cultural media, including AWG, PDA and water agar (WA), were used in this study. AWG was made of wheat grains (50 % water content, w/w). PDA was made of peeled potato (200 g peeled potato tuber, 20 g glucose, 15 g agar, 1000 ml water). WA contained agar alone in distilled water (1.5 %, w/v).
Analysis of the VOCs of F. oxysporum CanR-46
The chemical components of the VOCs of F. oxysporum CanR-46 were analyzed using GC-MS. Strain CanR-46 was inoculated in a 250-mL Erlenmeyer flask containing 30 g AWG at 3 × 104 microconidia g−1 AWG. The culture was incubated at 20 °C in the dark for 5 days. Preliminary experiments showed that VOC emission reached the plateau in 5 days under such cultural conditions. The VOCs in the flask were collected using the headspace solid-phase microextraction technique (HS-SPME) (Wan et al. 2008). The VOC components were identified using a GC-MS machine (6890N-5975B, Agilent Technologies Inc., CA, USA) following the procedures recommended by the manufacturer. Meanwhile, the VOCs released from the non-inoculated AWG were also collected and identified in the same manner. The experiment was conducted two times. The VOCs appearing in the HS-SPME extract from the AWG cultures of F. oxysporum in both trials, but not appearing in the HS-SPME extract from the non-inoculated AWG, were considered to the components produced by F. oxysporum.
Antifungal activity of selected synthetic VOCs
Five synthetic chemicals (β-caryophyllene, 3,4-dihydro-2H-pyran, (±)-limonene, 5-hexenoic acid, and octanoic acid) were selected based on the VOC profile of F. oxysporum CanR-46, as only these compounds were available from the chemical companies. β-Caryophyllene and 3,4-dihydro-2H-pyran were purchased from TCI (Shanghai) Development Co., Ltd. (Shanghai, China). (±)-Limonene and 5-hexenoic acid were purchased from Tokoyo Chemical Industry Co., Ltd. (Tokyo, Japan). Octanoic acid was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All these synthetic compounds were pure by 90 % or higher. They were individually tested for inhibition of mycelial growth and conidial germination of V. dahliae using the double-dish method described in our previous studies (Wan et al. 2008; Huang et al. 2011). The concentration value for 50 % inhibition of mycelial growth and conidial germination (IC50) expressed as microliters per liter (μL L−1) was inferred from the data about the inhibition percentages and the corresponding VOCs doses applied in the double-dishes assay (Huang et al. 2011).
Antifungal activity of the VOCs of F. oxysporum CanR-46
Inhibition of mycelial growth and conidial germination of V. dahliae by the VOCs of F. oxysporum CanR-46 was tested using the double-dish method as previously described (Wan et al. 2008; Minerdi et al. 2009; Huang et al. 2011). In the mycelial growth experiment, F. oxysporum-inoculated AWG (3 × 104 microconidia g−1 AWG) was transferred to Petri dishes (9 cm in diameter) at 0 (empty dish), 2, 4, 8, 16 or 32 g per dish with three replicated dishes per treatment. Fresh non-inoculated AWG (8 g per dish) served as the control. The dishes with the F. oxysporum culture and the control AWG were incubated at 25 °C in the dark for 3 days to allow fungal growth and production of the VOCs. A bottom dish with PDA was inoculated with a mycelial agar plug (6 mm in diameter) of V. dahliae removed from the colony margin area of a PDA culture. It was placed over facing a bottom dish with the fresh AWG or with the AWG culture of F. oxysporum to create a double-dish set, which was immediately wrapped with parafilms. The double-dish sets were placed at 25 °C for 21 days. Diameter of the colony of V. dahliae was measured on the daily basis and the mycelial growth rate (mm day−1) was calculated. The difference in mycelial growth rate between the control treatment (non-inoculated AWG) and the F. oxysporum treatment (F. oxysporum-inoculated AWG) was used to calculate the percentage of mycelial growth inhibition.
To understand the mechanisms involved in suppression of mycelial growth of V. dahliae by the VOCs of F. oxysporum, morphological characteristics of the hyphae of V. dahliae treated by the VOCs were observed under scanning electron microscope. A double-dish set for the treatment of F. oxysporum-V. dahliae contained 8 g AWG culture of F. oxysporum in one dish and V. dahliae on a cellophane film in the other dish with PDA. A control double-dish set contained 8 g fresh AWG in one dish and V. dahliae on a cellophane film in another PDA dish. The double-dish sets were individually wrapped with parafilms and placed in an incubator at 25 °C in the dark for 7 days. The cellophane film with the colony margin of V. dahliae for each treatment was cut into small pieces (about 1 × 1 mm, length × width) using a sterilized razor blade. The mycelial specimens were immediately fixed, dehydrated, critical-point dried, gold-coated and examined under a scanning electron microscope (JSM-6390/LV, NTC, Tokyo, Japan) using the standard procedures.
In the conidial germination experiment, a double-dish set for the treatment of F. oxysporum-V. dahliae contained 8 g AWG culture of F. oxysporum in one dish and the conidia of V. dahliae (1 × 104 conidia dish−1) on water agar in another dish. A double-dish set for the treatment of V. dahliae alone contained 8 g fresh AWG (control) in one dish and the conidia of V. dahliae on water agar in the other dish. There were 24 double-dish sets for each treatment. The double-dish sets were individually wrapped with parafilms and placed in an incubator at 25 °C in the dark. After incubation for 3, 6, 9, 12, 15, 18, 21 and 24 h, three double-dish sets for each treatment were taken out from the incubator. Germination of the V. dahliae conidia in each dish was observed under a light compound microscope by randomly counting 100 conidia and length of 30 germ tubes were measured. A conidium was regarded germinated when the germ tube length was equal to or longer than the diameter of that conidium. Data about the conidial germination rates or germ-tube length over time were analyzed using Slogistic 1 function in Growth/Sigmoidal Category in the Origin 8.0 software (OriginLab, Northampton, MA, USA).
Antifungal spectrum of the VOCs of F. oxysporum CanR-46
Effects of the VOCs of F. oxysporum CanR-46 on mycelial growth of 15 other fungal strains belonging to nine genera (Supplementary material Table S1) were tested using the above-mentioned double-dish method. V. dahliae was included as reference in this bioassay. The Petri dishes each containing 8 g AWG cultures of F. oxysporum CanR-46 or 8 g fresh AWG (control) and the Petri dishes each containing a tested fungus on PDA were used to set up the double-dish sets. There were three double-dish sets for each tested fungus and V. dahliae. All the double-dish sets were placed in the dark at 20 or 25 °C for 3 to 21 days. Colony diameter of each tested fungus in a double-dish set was measured daily and the mycelial growth rate (mm day−1) was calculated. At the end of the experiment, the viability of the tested fungi was determined by removing the dishes containing F. oxysporum CanR-46 from the double-dish sets. The other dishes with the tested fungi were covered again and placed in the incubator for another 7 days. Appearance of new mycelial growth indicated that the mycelia were still viable after exposure to the VOCs.
Fumigation of soil with the VOCs of F. oxysporum CanR-46
F. oxysporum CanR-46 was inoculated in AWG in Petri dishes, 130 g AWG per dish. The dishes were placed in an incubator at 25 °C in the dark for 3 days. Then, the cover dishes were removed and a bottom dish was placed at the bottom of a desiccator. Meanwhile, autoclaved Organic Culture Mix (Zhengjiang Peilei Organic Manure Manufacturing Co. Ltd., Zheng Jiang, Jiangsu Province, China) was inoculated with V. dahliae to reach the final concentration of 2 × 108 conidia g−1. The culture mix (approximately 40 % water content, w/w) was loaded on a piece of one-layered gauze on the clapboard of that desiccator. For the control treatment, a dish with 130 g fresh AWG was placed at the bottom of a desiccator and the V. dahliae-infested culture mix was loaded on a piece of one-layered gauze on the clapboard of that desiccator. The desiccators were individually covered and placed at 20 °C for 5 days. The culture mix of each treatment was then taken out and loaded in plastic pots (7 × 8 cm, diameter × height), and eight pots for each treatment. Two-true-leaf cotton seedlings (Gossypium hirsutum cv. E Kang Mian No. 12) were individually transplanted into the culture mix in the pots, one seedling per pot. The pots were maintained in a growth chamber at 25 °C under a lighting regime of 16-h light/8-h dark for 20 days. Then, the cotton seedlings were individually rated for disease severity using a 0 to 4 scale (Xu et al. 2012). Disease severity index for each treatment was calculated with the formula described by Xu et al. (2012). The experiment was conducted two times.
Treatment of cotton seedlings with F. oxysporum CanR-46
F. oxysporum CanR-46GFP was used in this experiment to test its efficacy in suppression of Verticillium wilt of cotton and to determine colonization of F. oxysporum in the tissues of cotton roots. V. dahliae strain 4TM6-15 (Xu et al. 2012) and F. oxysporum CanR-46GFP (this study) were incubated on PDA in Petri dishes (25 °C) for 15 days. Conidia of each fungus were harvested washing the cultures with sterile distilled water, and the resulting mixtures were filtered through four-layered cheese cloth to remove the mycelial fragments in the conidial suspension. The conidial concentration was adjusted with sterilized water to 3 × 106 conidia mL−1 for V. dahliae and 3 × 107 microconidia mL−1 for F. oxysporum. Meanwhile, the two-true-leaf stage cotton seedlings were uprooted and the roots were washed under tap water to remove the remaining culture mix. Finally, the cotton seedlings were blotted dry on paper towels and used in the flowing treatments.
There were four treatments in this experiment: F. oxysporum CanR-46GFP + V. dahliae (FoGFP + V. dahliae); V. dahliae alone; F. oxysporum CanR-46GFP alone (FoGFP); and water control (CK). For the treatment of FoGFP + V. dahliae, the conidial suspensions of V. dahliae and F. oxysporum were mixed at an equal volume (Final conidial concentration: F. oxysporum at 1.5 × 107 microconidia mL−1 and V. dahliae at 1.5 × 106 conidia mL−1). For the treatments of FoGFP alone and V. dahliae alone, the conidial suspensions were diluted by 1:1 with sterile water to give the same respective conidial concentrations as those used in the treatment of FoGFP + V. dahliae. Roots of the cotton seedlings (10 per treatment) were dipped for 30 s in the respective conidial suspension, or in water for the control treatment. The seedlings were individually transplanted into a fine clay loam soil in plastic pots (12 × 10 cm, diameter × height), two seedlings per pot. The pots were maintained in a growth chamber at 25 °C under the regime of 16-h light/8-h dark for 10 days. Disease severity on each seedling was scored (Xu et al. 2012). Disease incidence and disease severity index for each treatment was then calculated. The experiment was conducted three times.
Laser scanning confocal microscopy
In order to detect colonization of cotton seedlings by F. oxysporum CanR-46GFP, three cotton seedlings for each treatment were randomly sampled. The tap root of each seedling was cut into slices either transversely or longitudinally using a razor blade. The root slices were examined under ZEISS LSM 510 Meta laser scanning confocal microscope (Carl Zeiss Jena GmbH, Module LSM 510, Germany) equipped with a Diode/Argon/HeNe1-HeNe2 laser. Green fluorescence in the cotton root tissue was excited at 488 nm. Images were analyzed and processed with the LSM Image Browser software (Version 4.2, Southwest Environmental Health Sciences Center, University of Arizona, Tucson, Arizona, USA) and Adobe Photoshop (Adobe Systems). Pure cultures of strains CanR-46 and CanR-46GFP were also examined under the UV light microscope for comparison purposes.
Data analysis
Statistical analyses were carried out using the SAS/STAT® software (SAS Institute, Cary, NC, version 8.0, 1999). Data on germ-tube length and disease severity index for different treatments were directly analyzed using analysis of variance (ANOVA). When significant treatment effects were detected, treatment means were separated using Duncan’s Multiple Range test at P = 0.05. Data on percentage of mycelial growth inhibition, conidial germination rate and disease incidence were individually arcsine-transformed to normalize the variance before ANOVA and Duncan’s Multiple Range test. After analysis, means were individually back-transformed to numerical values.
Results
GC/MS identification of the VOCs of F. oxysporum
GC-MS analysis identified 19 volatile organic compounds (VOCs) in 5-day-old AWG cultures of F. oxysporum CanR-46 based on their mass spectrum properties (Table 1). These compounds fell into classes of alkenes, esters, alkanes, organic acids and alcohols. Most compounds (11/19) belong to alkenes.
Antifungal activity of selected synthetic VOCs
Four (5-hexenoic acid; (±)-limonene; 3,4-2H-dihydropyran; octanoic acid) out of the five synthetic chemicals appearing in the VOC profile of F. oxysporum CanR-46 exhibited inhibitory activity against V. dahliae (Table 2). Among the tested synthetic chemicals, 5-hexenoic acid showed the highest antifungal activity with the lowest 50 % inhibition concentration values (IC50) for growth inhibition (3.5 μL L−1), and for conidial germination (9.7 μL L−1). β-Caryophyllene did not show any detectable inhibitory activity against V. dahliae.
Inhibition of V. dahliae by the VOCs of F. oxysporum
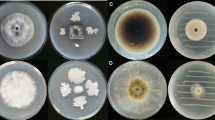
While the VOCs from the fresh AWG did not inhibit growth of V. dahliae, the VOCs from the AWG culture of F. oxysporum effectively inhibited mycelial growth of V. dahliae (Fig. 1). With increase of the dosage of the AWG culture of F. oxysporum from 2 to 32 g per dish, the percentage of growth inhibition gradually increased from 60 to 100 % (25 °C, 21 days). SEM observation showed that while the hyphae of V. dahliae in the control treatment without the F. oxysporum VOCs appeared vigorous and turgid, the hyphae of V. dahliae in the treatment with the F. oxysporum VOCs became shriveled and collapsed (Fig. 2).
Effects of the volatile organic compounds emitted from the AWG culture of Fusarium oxysporum CanR-46 on mycelial growth of Verticillium dahliae. AWG = Autoclaved Wheat Grains. Means ± standard errors (n = 3) labeled with different letters indicate significant difference (P < 0.05) according to Duncan’s Multiple Range Test
Results from the conidial germination experiment indicated that the VOCs of F. oxysporum CanR-46 had suppressive effect both on conidial germination rate and on germ-tube elongation of V. dahliae on WA (Fig. 3). In the absence of the VOCs, the average percentage of germinated conidia reached 26 % at 9 h post incubation (hpi) and 100 % at 21 hpi after incubation (Fig. 3a). The average length of germ tubes reached 37 μm at 9 hpi, and 145 μm at 21 hpi after incubation (Fig. 3b). In the presence of the VOCs, the percentage of germinated conidia was 1 % at 9 hpi and 96 % at 21 hpi (Fig. 3a). The average germ-tube length was lower than 5 μm at 9 hpi and 13 μm at 21 hpi (Fig. 3b).
Antifungal spectrum of the VOCs of F. oxysporum
The VOCs released from the 8 g AWG cultures of F. oxysporum CanR-46 inhibited growth of the 16 investigated fungal strains by 58 to 100 % (Table 3). The fungi belong to belonged to 14 fungal species in ten genera (Aspergillus, Botrytis, Colletotrichum, Fusarium, Magnaporthe, Monilinia, Penicillium, Rhizoctonia, Sclerotinia, Verticillium). Interestingly, the VOCs suppressed F. oxysporum f.sp. vasinfectum and F. oxysporum f.sp. niveum by 58 and 78 %, respectively. The values were significantly lower (P < 0.05) than those of 90 to 100 % for other fungal species (Table 3).
After removal of the dishes with F. oxysporum CanR-46 (the source of the VOCs) from the double-dish sets, 14 out of the 16 fungal strains recovered to grow on PDA at 20 or 25 °C (Table 3) and normal colonies were developed after incubation for 7 days (data not shown), suggesting that some suppressed mycelia were still viable after fumigation by the VOCs. However, M. oryzae WhMO-10 and V. dahliae 4TM6-15 did not grow after incubation for 7 days, suggesting that the hyphae of the two fungal strains were already dead under fumigation of the VOCs.
Suppression of Verticillium wilt of cotton by soil fumigation with the VOCs
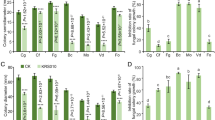
The treatment of soil fumigation with the F. oxysporum VOCs differed significantly from the control treatment (soil fumigated with the VOCs from the fresh AWG) both in disease incidence and in disease severity index. After incubation at 25 °C for 20 days, the disease incidence was 27 % in the treatment of the F. oxysporum VOCs; significantly lower (P < 0.05) than that of 80 % in the control treatment (Fig. 4). The disease severity index was 17 in the treatment of the F. oxysporum VOCs, also significantly lower (P < 0.01) lower than that of 76 in the control treatment (Fig. 4). This result suggests that fumigation of the organic culture mix with the F. oxysporum VOCs of can effectively suppress Verticillium wilt of cotton.
Suppression of Verticillium wilt of cotton by F. oxysporum
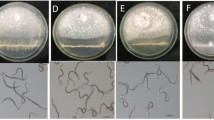
After incubation at 25 °C for 10 days, while all the cotton seedlings in the treatments of water and FoGFP were healthy, some cotton seedlings in the treatments of V. dahliae alone and FoGFP + V. dahliae showed wilt symptoms. The treatment of FoGFP + Vdahliae differed significantly from the treatment of V. dahliae both in disease incidence and in disease severity index. The disease incidence was 18 % in the treatment of FoGFP + V. dahliae, significantly (P < 0.05) lower than that of 94 % in the V. dahliae treatment (Fig. 5). The disease severity index was seven in the treatment of FoGFP + V. dahliae, also significantly (P < 0.05) lower than that of 93 in the V. dahliae treatment. Moreover, vascular discoloration was observed in the V. dahliae treatment, but was not observed in the other three treatments (Fig. 5). These results suggest that treatment of the cotton roots with the conidia of F. oxysporum CanR-46 can effectively control Verticillium wilt of cotton.
Efficacy of Fusarium oxysporum CanR-46GFP in suppression of Verticillium wilt of cotton caused by Verticillium dahliae (25 °C, 10 days post inoculation). a Cotton seedlings treated with water, F. oxysporum CanR-46GFP alone (FoGFP), V. dahliae alone (Vd), and F. oxysporum CanR-46GFP plus V. dahliae (FoGFP + Vd). Stem cross sections of the treated cotton seedlings were shown. Note severe defoliation and vascular discoloration on cotton seedlings treated with V. dahliae alone, but no leaf yellowing, wilting and defoliation, and vascular discoloration on cotton seedlings treated with water, FoGFP and FoGFP + Vd; b Disease incidence and disease severity index of Verticillium wilt of cotton for the four treatments. Means ± standard errors (n = 10) for each treatment labeled with different letters indicate significant differences (P < 0.05) according to Duncan’s Multiple Range Test
The hyphae and the conidia of strain CanR-46GFP of F. oxysporum (FoGFP) exhibited green fluorescent color under UV light, whereas the hyphae and the conidia of the wild type strain CanR-46 (Fo) did not produce any green fluorescent color under the UV light (Supplementary material Fig. S1). Roots of the treated cotton seedlings were randomly sampled and observed under ZEISS LSM 510 Meta laser scanning confocal microscope for endophytic colonization by FoGFP. Results showed that while no signs of green fluorescence in the treatments of water (Figs. 6a and b) and V. dahliae alone (data not shown), green fluorescence was consistently observed in the treatments of FoGFP alone (Figs. 6c and d) and FoGFP + V. dahliae (Fig. 6e and f), indicating that FoGFP colonized the cotton roots. In both treatments, FoGFP colonized both the root epidermis and the vascular bundles (xylem and phloem). FoGFP grew in the intercellular space in most cases. Sometimes, growth of FoGFP was observed inside the root cells.
Laser scanning confocal microscopic micrographs showing endophytic colonization of cotton roots by Fusarium oxysporum CanR-46GFP (25 °C, 10 days post inoculation). a and b Longitudinal and cross sections of the roots treated with water, respectively; c and d Longitudinal and cross sections of the roots inoculated with F. oxysporum CanR-46GFP alone (FoGFP), respectively; e and f Longitudinal and cross sections of the roots inoculated with FoGFP and V. dahliae (FoGFP + Vd), respectively. Green fluorescence color and red fluorescence color indicate hyphae of F. oxysporum CanR-46GFP and cotton root cells, respectively
Discussion
This study revealed that the volatile organic compounds (VOCs) produced by the non-pathogenic strain CanR-46 of F. oxysporum highly inhibited mycelial growth and germ-tube elongation of V. dahliae (Figs. 1,2,3; Table 3). Minerdi et al. (2009) reported that the VOCs produced by the wild type non-pathogenic strain WTMSA 35 of F. oxysporum inhibited growth of several pathogenic formae speciales of F. oxysporum. Freire et al. (2012) showed that VOCs produced by isolates 20a and 21 of F. oxysporum caused 88–96 % mortality of the second stage juveniles of the root-knot nematode Meloidogyne incognita. In this study, we found that the VOCs of F. oxysporum CanR-46 inhibited growth of all the 16 fungal strains belonging to the genera Aspergillus, Botrytis, Collectrichum, Fusarium, Magnaporthe, Molinilia, Rhizoctonia, Sclerotinia and Verticillium (Table 3). These results suggest that the VOCs produced by F. oxysporum may a wide antifungal spectrum.
The VOCs of strain MSA 35 was reported capable of changing surface hydrophobicity to hydrophilic. In this study, the SEM observation showed that hyphae of V. dahliae fumigated by the VOCs of F. oxysporum CanR-46 became shriveled and collapsed (Fig. 2). Ghannoum and Rice (1999) reported that the polyene antifungal substance amphotericin B can damage fungal cell membrane and increase membrane permeability, thus causing cytoplasm leakage. We found that 11 out of 19 VOCs produced by F. oxysporum CanR-46 are alkenes (Table 1). They might be responsible for malformation of the hyphae of V. dahliae.
Strain CanR-46 of F. oxysporum was detected capable of producing caryophyllene (Table 1 ), a bicyclic sesquiterpene compound. Production of this compound by strains of F. oxysporum has been reported previously (Minerdi et al. 2009, 2011; Freire et al. 2012). β-Caryophyllene showed no antifungal activity against V. dahliae in this study (Table 2), and nor against F. oxysporum f. sp. lactucae (FoL) in a previous study (Minerdi et al. 2009). On the other hand, Minerdi et al. (2011) reported that the VOCs of WTMSA 35 could increase growth of lettuce (Lactuca sativa) and expression of the expansin A5 gene in lettuce, and β-caryophyllene was confirmed to be responsible for the plant-growth promotion effect (Minerdi et al. 2011). Yamagiwa et al. (2011) reported that β-caryophyllene significantly enhanced seedlings growth of Brassica campestris and resistance to C. higginsianum, the causal agent for anthracnose disease of brassicaceous plants. Whether or not the VOCs of F. oxysporum CanR-46 can promote plant growth remains unknown and needs further clarification.
Except caryophyllene, other VOC composition produced by the strain CanR-46 of F. oxysporum appears different from the VOC composition of strains WTMSA 35 and 21 of F. oxysporum reported in previous studies (Minerdi et al. 2009; Freire et al. 2012). Minerdi et al. (2009) reported that α-humulene was abundant in the VOCs produced by strain WTMSA 35, but was not detected in the VOCs of strain 21 (Freire et al. 2012). α-Humulene was not detected in the VOC profile produced by strain CanR-46 in this study either (Table 1). Different genetic backgrounds of the strains of F. oxysporum and nutrients/cultural conditions might be responsible for the difference. Although strains CanR-46, WTMSA 35 and 21 were all identified to be F. oxysporum based on selected criteria, they have different origins, strain CanR-46 from a healthy root of oilseed rape in China (Zhang et al. 2014), strain WTMSA 35 from a Fusarium suppressive soil in Italy (Gilardi et al. 2005) and strain 21 from the coffee rhizosphere in Brazil (Freire et al. 2012). Different origins imply that these F. oxysporum strains might be different in niche adaptation, which might be regulated by related genes. Regarding the nutrients/cultural conditions for production of the VOCs, strain CanR-46 was statically incubated in autoclaved wheat grains in this study. However, strains WTMSA 35 and 21 were shake-incubated in liquid media (Minerdi et al. 2009; Freire et al. 2012). Different nutrients in cultural media and in cultural conditions for strain CanR-46 might result in production of the VOCs differing in composition from those produced by strains WTMSA 35 and 21.
Additionally, previous studies conducted by Minerdi et al. (2009, 2011) showed that bacterial ectosymbiosis affected VOC composition produced by F. oxysporum WTMSA 35. Minerdi et al. (2008) reported that the non-pathogenic strain WTMSA 35 harbored a consortium of ectosymbiotic bacteria belonging to the genera Serratia (predominant), Achromobacter, Bacillus and Stenotrophomonas. The bacteria-cured strain CUMSA 35 from WTMSA 35 became pathogenic to lettuce (Minerdi et al. 2008). Minerdi et al. (2009, 2011) reported VOC composition produced by strain WTMSA 35 different from that produced by strain CUMSA 35. For example, sesquiterpenes (e.g., caryophyllene and α-humulene) were detected in the headspace extract of strain WTMSA 35, but was not detected in the headspace extracts of strain CUMSA 35, or in the ectosymbiotic bacteria Achromobacter sp. strain MM1 and Serratia sp. strain DM1 (Minerdi et al. 2009, 2011). These results imply that the bacterial ectosymbiosis may stimulate or induce F. oxysporum WTMSA 35 to produce the sesquiterpene VOCs. In the present study, strain CanR-46 was sub-cultured several times on agar media and ectosymbiotic bacteria were not observed on the hyphae of F. oxysporum CanR-46 under scanning electron microscope (Supplementary material Fig. S2). Thus, production of the VOCs by F. oxysporum CanR-46 is not affected by bacterial ectosymbiosis.
Previous studies have demonstrated that the VOC-producing fungi can be used to treat soil as biofumigants (or mycofumigants) for control of soilborne plant diseases. For example, Stinson et al. (2003) reported that incorporation of the two VOC-producing fungi M. albus and M. roseus into soil resulted in significant reduction of root rot diseases of sugar beet (Beta vulgaris) caused by Aphanomyces cochlioides, P. ultimum and R. solani. A further study conducted by Grimme et al. (2007) showed that a biorational synthetic mixture of organic VOC components mimicking the VOCs of M. albus was more effective than the live culture of this fungus in control of the three fungal pathogens on sugar beet and the root-knot nematode, Meloidogyne incognita, on tomato (Lycopersicon esculentum). In the present study, we found that the VOCs of F. oxysporum CanR-46 inhibited mycelial growth and germ-tube elongation of the soilborne pathogen V. dahliae (Fig. 3, Table 3), and suppressed Verticillium wilt of cotton by fumigation of the organic culture mix (Figs. 4, 5). These results suggest that the mixture of the VOCs produced by F. oxysporum CanR-46 can be used as a biofumigant to control V. dahliae. This study also showed that treatment of cotton roots with the conidia of F. oxysporum CanR-46 effectively prevented the roots from infection by V. dahliae (Fig. 5). These results suggest that F. oxysporum CanR-46 is a promising biocontrol agent of V. dahliae.
Numerous studies reported that non-pathogenic strains of F. oxysporum are effective agents for control of pathogenic formae speciales of F. oxysporum on various crops (Ogawa and Komada 1984; Paulitz et al. 1987; Postrna and Rattink 1992; Larkin et al. 1996; Silva and Belltiol 2005; Panina et al. 2007). Competition for nutrients/space, and induction of systemic resistance have been proposed as mechanisms involved in biocontrol of pathogenic F. oxysporum with non-pathogenic F. oxysporum (Fravel et al. 2003). Minerdi et al. (2009) reported that the VOCs (e.g., α-humulene) of the strain WTMSA 35 of F. oxysporum inhibited growth of several pathogenic formae speciales of F. oxysporum including FoL Fuslat10 and repressed expression of two putative virulence genes coding for a MAP kinase and Class V chitin synthase in FoL Fuslat10. The results about inhibition of V. dahliae on media, in soil and on cotton plants observed in this study provided an example of possible involvement of VOC production in biocontrol of fungal diseases by non-pathogenic F. oxysporum.
Pantelides et al. (2009) and Veloso and Díaz (2012) reported that the non-pathogenic F. oxysporum strains F2 and Fo47, when treated onto roots of eggplant and pepper (Capsicum annuum), respectively, provided effective control of V. dahliae. The two biocontrol agents showed different mechanisms for disease suppression (Gizi et al. 2011; Veloso and Díaz 2012). Strain F2, after being injected into eggplant stems, was capable of colonizing root surface and vascular tissues of eggplant, resulting in a significant reduction of vascular colonization by V. dahliae, suggesting a mechanism of competition for nutrients and space (Pantelides et al. 2009; Gizi et al. 2011). It was detected to be unable to induce resistance against V. dahliae on eggplant (Pantelides et al. 2009). Strain Fo47 was found to be able to induce resistance against V. dahliae on pepper plants (Veloso and Díaz 2012). In the present study, endophytic colonization of the cotton root tissues by F. oxysporum CanR-46 was observed either in the presence of V. dahliae or in the absence of V. dahliae (Fig. 6). This result suggests possible involvement of competition for nutrients and space as a mechanism, in addition to production of antifungal VOCs by F. oxysporum CanR-46. Whether or not F. oxysporum CanR-46 can trigger defense response of cotton against V. dahliae remains unknown and needs further study.
In conclusion, the present study showed that the non-pathogenic endophyte F .oxysporum CanR-46 is a VOC-producing antagonist with a wide spectrum of antifungal activity. The VOCs of F. oxysporum CanR-46 exhibited high efficacy in suppression of mycelial growth and germ-tube elongation of V. dahliae; and in suppression of Verticillium wilt of cotton caused by V. dahliae. Treatment of cotton roots with the conidia of F. oxysporum CanR-46 provided effective control of Verticillium wilt. Therefore, F. oxysporum CanR-46 is a promising biocontrol agent of V. dahliae.
References
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502
Freire ES, Campos VP, Pinho RSC, Oliveira DF, Faria MR, Pohlit AM, Noberto NP, Rezende EL, Pfenning LH, Silva JRC (2012) Volatile substances produced by Fusarium oxysporum from coffee rhizosphere and other microbes affect Meloidogyne incognita and Arthrobotrys conoides. J Nematol 44:321–328
Ghannoum MA, Rice LB (1999) Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517
Gilardi G, Tinivella F, Gullino ML, Garibaldi A (2005) Seed dressing to control Fusarium oxysporum f.sp. lactucae. J Plant Dis Prot 112:240–246
Gizi D, Stringlis IA, Tjamos SE, Paplomatas EJ (2011) Seedling vaccination by stem injecting a conidial suspension of F2, a non-pathogenic Fusarium oxysporum strain, suppresses Verticillium wilt of eggplant. Biol Control 58:387–392
Grimme E, Zidack NK, Sikora RA, Strobel GA, Jacobsen BJ (2007) Comparison of Muscodor albus volatiles with a biorational mixture for control of seedling diseases of sugar beet and root-knot nematode on tomato. Plant Dis 91:220–225
Huang R, Li GQ, Zhang J, Yang L, Che HJ, Jiang DH, Huang HC (2011) Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 101:859–869
Huang R, Che HJ, Zhang J, Yang L, Jiang DH, Li GQ (2012) Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biol Control 62:53–63
Larkin RP, Hopkins DL, Martin FN (1996) Suppression of Fusarium wilt of watermelon by non-pathogenic Fusarium oxysporum and other microorganisms recovered from a disease-suppressive soil. Biol Control 86:812–819
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Mercier J, Jiménez JI (2004) Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol Technol 31:1–8
Mercier J, Manker DC (2005) Biocontrol of soil-borne diseases and plant growth enhancement in greenhouse soilless mix by the volatile-producing fungus Muscodor albus. Crop Prot 24:355–362
Mercier J, Smilanick JL (2005) Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol Control 32:401–407
Minerdi D, Moretti M, Gilardi G, Barberio C, Gullino ML, Garibaldi A (2008) Bacterial ectosymbionts and virulence silencing in a Fusarium oxysporum strain. Environ Microbiol 10:1725–1741
Minerdi D, Bossi S, Gullino ML, Garibaldi A (2009) Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ Microbiol 11:844–854
Minerdi D, Bossi S, Maffei ME, Gullino ML, Garibaldi A (2011) Fusarium oxysporum and its bacterial consortium promote lettuce growth and expansin A5 gene expression through microbial volatile organic compound (MVOC) emission. FEMS Microbiol Ecol 76:342–351
Morath SU, Hung R, Bennett JW (2012) Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev 26:73–83
Ogawa K, Komada H (1984) Biological control of Fusarium wilt of sweet potato by non-pathogenic Fusarium oxysporum. Ann Phytopathol Soc Jpn 50:1–9
Panina Y, Fravel DR, Baker CJ, Shcherbakova LA (2007) Biocontrol and plant pathogenic Fusarium oxysporum-induced changes in phenolic compounds in tomato leaves and roots. J Phytopathol 155:475–481
Pantelides IS, Tjamos SE, Striglis IA, Chatzipavlidis I, Paplomatas EJ (2009) Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using teal time QPCR analysis and biomarker transformation. Biol Control 50:30–36
Paulitz TC, Park CS, Baker R (1987) Biological control of Fusarium wilt of cucumber with nonpathogenic isolates of Fusarium oxysporum. Can J Microbiol 33:346–353
Pegg GF, Brady BL (2002) Verticllium wilts. CABI Publ, Wallingford
Postrna J, Rattink H (1992) Biological control of fusarium wilt of carnation with a nonpathogenic isolate of Fusarium oxysporum. Can J Bot 70:1199–1205
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Schnabel G, Mercier J (2006) Use of a Muscodor albus pad delivery system for the management of brown rot of peach in shipping cartons. Postharvest Biol Technol 42:121–123
Silva JC, Belltiol W (2005) Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Fitopatol Bras 30:409–412
Stinson AM, Zidack NK, Strobel GA, Jacobsen BJ (2003) Mycofumigation with Muscodor albus and Muscodor roseus for control of seedling diseases of sugar beet and Verticillium wilt of eggplant. Plant Dis 87:1349–1354
Strobel GA, Dirkse E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943–2950
Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, Altamirano-Hernández J, Flores-Cortez I, Valencia-Cantero E (2011) A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 339:329–340
Veloso J, Díaz J (2012) Fusarium oxysporum Fo47 confers protection to pepper plants against Verticillium dahliae and Phytophthora capsici, and induces the expression of defence genes. Plant Pathol 61:281–288
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73:5639–5641
Wan MG, Li GQ, Zhang JB, Jiang DH, Huang HC (2008) Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol Control 46:552–559
Xu F, Yang L, Zhang J, Guo X, Zhang X, Li G (2012) Prevalence of the defoliating pathotype of Verticillium dahliae on cotton in central china and virulence on selected cotton cultivars. J Phytopathol 160:369–376
Yamagiwa Y, Inagaki Y, Ichinose Y, Toyoda K, Hyakumachi M, Shiraishi T (2011) Talaromyces wortmannii FS2 emits β-caryphyllene, which promotes plant growth and induces resistance. J Gen Plant Pathol 77:336–341
Yu S-M, Lee YH (2013) Plant growth promoting rhizobacterium Proteus vulgaris JBLS202 stimulates the seedling growth of Chinese cabbage through indole emission. Plant Soil 370(1–2):485–495
Zhang QH, Zhang J, Yang L, Zhang L, Jiang DH, Chen WD, Li GQ (2014) Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control 72:98–108
Acknowledgments
We appreciate the kind help from Dr. Yong-Ju Huang at University of Hertfordshire (Hatfield, UK) for providing the plasmid pCAMBPGFP containing the green fluorescent protein gene.
Compliance with ethical standards
ᅟ
Funding
This research was funded by the Natural Science Foundation of China (Grant No. 31070122) and the R and D Special Fund for Public Welfare Industry (Agriculture) of China (Grant No. 201103016 and 201303025). Conflict of Interest: All the authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Choong-Min Ryu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 563 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Yang, L., Zhang, J. et al. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 392, 101–114 (2015). https://doi.org/10.1007/s11104-015-2448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2448-y