Abstract
Plant growth-promoting bacteria (PGPB) include bacteria that colonize the plant and improve the growth directly by providing growth factors or indirectly by protection against environmental stress. PGPB have the potential to cooperate to the alleviation of drought stress which is among the most critical stress for plants. These beneficial microorganisms convey drought tolerance through the production of exopolysaccharides (EPS), phytohormones, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, and volatile compounds, inducing accumulation of osmolytes and antioxidants, regulation of stress-responsive genes, induction of systemic tolerance, and modification of root morphology toward the adaptation to drought tolerance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
All animals including human depend on plants as they produce oxygen and form the principal food for them. According to an estimation, 98% of the global food requirements are provided by 12 plant species and 14 animal species. Moreover, none of these 14 animals can supply the required substrates without the incorporation of the plants. By another estimation, more than 50% of the world energy intake is affiliated by crops consisting of wheat, rice, and maize. Therefore, reduction in plant productivity immediately affects the growth of a number of species that rely on plants as the nutrition basis (Orhan 2016).
The world food supply must increase considerably to certify food security for the growing population (Hanin et al. 2016). In fact, plant production is substantially affected by multiple environmental factors. Water is one of the most limiting factors for plant development, as well as for all life forms (Kavamura et al. 2013). Drought is a natural phenomenon that affects several parts of the world, causing social, economic, and environmental negative impacts (Kavamura et al. 2013). Water scarcity is among the main constraints on plant productivity worldwide (Delshadi et al. 2017) and is expected to expand with climatic changes (Rapparini and Penuelas 2014). Because drought is a multidimensional stress, plants respond to it at morphological, physiological, biochemical, and molecular levels (Kaur and Asthir 2017; Shrivastava and Kumar 2015; Wang et al. 2016). Thus worldwide, extensive efforts are on the development of the strategies to cope with abiotic stresses such as drought (Grover et al. 2011).
Plants undergo a variety of metabolic and physiological alterations in response to drought (water deficiency) (Kang et al. 2014a, b). Plant growth-promoting bacteria (PGPB) have been recognized to have an essential function in the growth and metabolism of plants to rescue plant growth in stressful conditions (Kang et al. 2014a, b; Bisen et al. 2015; Singh et al. 2016, 2017). Several strategies have been suggested for governing the detrimental effects of drought stress on plants. Among that the selection for tolerant varieties and genetic engineering are the most investigated approaches. Nevertheless, the development of new tolerant varieties is challenging due to the complexity of abiotic stress tolerance mechanisms and genetically modified plants cannot easily be approved based on the most national regulations (Kasim et al. 2012; Timmusk et al. 2014). The priming treatment can be considered as an alternative strategy to induce stress tolerance in the plant by using various chemical and biological agents as the stimulants (Kasim et al. 2012).
Consequently, the importance of exploitation of beneficial bacteria is emerging with the focus on issues such as sustainable agriculture, environmental preservation, and food security (Ilangumaran and Smith 2017). Selection, screening, and use of drought stress-tolerant PGPB to plants can help to overcome productivity restrictions in dry lands (Kaushal and Wani 2015).
The aim of this chapter is to explore the potential of plant-associated bacteria in drought stress protection and meanwhile to overview the possible mechanisms by which PGPB can improve the tolerance to drought stress in plants.
2 Worldwide Water Resource Limitation
By 2050, the world population is expected to reach to 9.2 billion (Rosegrant et al. 2009). As a result of population growth and enhanced demanded protein and energy per capita, global stress on water and land sources is manifold. Worldwide water consumption from irrigation, domestic, industrial, and livestock usages is expected to grow by 21% by 2050. The developing countries are expected to have a more dramatic increase in consumption (up to 25%), compared to the developed regions with an estimated 11% increase (Rosegrant et al. 2009).
Currently, agricultural production is accountable for the majority of global consumptive freshwater use (up to 85%) (Johnson et al. 2010). Although it creates a vast technological need to offer solutions for the effective use of the available water (Timmusk et al. 2013), any efforts to increase the adaptability to the low water activity in plants are a crucial parallel approach. Water scarcity affects all continent and around 2.8 billion people around the world at least 1 month annually (Yu 2016). In other words, almost 40% of the world population and huge area of ecosystems are travailing from water scarcity (Johnson et al. 2010), and more than 1.2 billion population even lack access to clean drinking water (Yu 2016). In a prediction by UN, one in four of the world’s children will be in regions with extremely restricted water resources by 2040 as a result of climate change (Guardian 2017).
Water scarcity decreases crop yields and eventually may cause malnourishment even in the developing world (Johnson et al. 2010). Moreover, worldwide production of biologically derived energy and material sources (e.g., biofuels and biological textiles) is developing and can result in the expansion of the agricultural industry in the future. As a consequence of these pressures, water scarcity and land degradation compete climate change as a main environmental concern in many areas of the world. Hence, there is a strong requirement for precise estimates of available water for future use and linked environmental impacts and for relating these to agricultural tools (Johnson et al. 2010).
Water resources inadequacy is a critical constraint to agriculture in many parts of the world. It often harms the soil through oversaturation and salt accumulation (Rosegrant et al. 2009; Fraiture et al. 2010). It is estimated that there are about 20–30 million hectares of irrigated lands severely affected by salinity on a global scale. An additional 60–80 million hectares are affected to some extent by water logging and salinity (Rosegrant et al. 2009). Hence, saline soils are estimated to extend at a rate of 7% in the world (Orhan 2016).
Despite the fact that drought is more prevalent and devastating than the salinity stress, plants’ adaptation to both is substantially related (Kang et al. 2014b). The water scarcity presents the major challenge in securing enough water to meet human, environmental, social, and economic needs to support sustainable development. This is menacing human health and ecosystems’ integrity; they represent a major concern for the water resource sustainability (International Hydrological Programme). Therefore, in an era of changing climates, there is a critical need for evolving tolerant plants to abiotic stresses specifically drought and salinity (Farrar et al. 2014).
3 Drought Stress in Plants
3.1 Effect of the Drought Stress on Plants
Drought is a multidimensional stress which triggers various plant reactions including morphological, physiological, biochemical to molecular levels (Kaur and Asthir 2017; Shrivastava and Kumar 2015; Wang et al. 2016). Drought stress harbors a decrease in water content, leaf pressure potential, closure of stomata, and a reduction in cell mitosis and in consequence cell elongation and growth. Plant growth is diminished because of the effect of the drought on numerous physiological and biochemical processes mainly photosynthesis, respiration, translocation, phytohormones production, adsorption of ions, sugar and nutrient metabolism, etc. (Farooq et al. 2009; Kaur and Asthir 2017; Reis et al. 2016).
Drought can lead to disturbed flowering process and grain filling that results in smaller and fewer grain production (Kaur and Asthir 2017). In the majority of the plant species, drought is associated with alterations in leaf anatomy and ultrastructure. However, harsh drought condition may cause the obstruction of photosynthesis and disruption of metabolism resulting in the death of plant (Kaur and Asthir 2017). The reactive oxygen species (ROS) such as superoxide radicals and H2O2 production (Kohler et al. 2008) is an initial step of plant defense flow to water stress and acts as a secondary messenger to prompt following defense reaction in plants (Kaur and Asthir 2017). The increased amounts of the ROS can cause extended damage by initiating lipid peroxidation, membrane deterioration, and degrading proteins, lipids, and nucleic acids in plants (Vurukonda et al. 2016). Drought stress can likewise result in misfolding or unfolding of structural and functional proteins leading to denaturation and dysfunction (Kasim et al. 2012).
3.2 Drought Resistance Mechanisms in Plants
The sensitivity of plants to drought is determined by level and duration of stress, plant species, and their growing stages (Kaur and Asthir 2017; Cura et al. 2017). In theory, there are two types of drought avoider plants: (a) water savers which preserve water and (b) water spenders which compensate the transpirational losses with excess absorption. The plant anatomic and morphologic characteristics aid in increased water uptake and reduce water outgoings. Water uptake could be accelerated by a widespread root system with an extensive active surface area and optimum shoot/root ratio. However, water loss through transpiration can be much subjected to adjustment (Timmusk et al. 2013). Drought tolerance capacity of plants can be predicted by applying several drought-related characteristics, including root and leaf traits, osmotic balance capabilities, potential of water content, abscisic acid (ABA) content, and stability of the cell membranes as conventional indicators (Kaur and Asthir 2017).
The reaction of a plant to abiotic stress initiates by a sensation of the extracellular stress signal on receptors of the cell, consequenced by the regulatory networks, comprising signal transduction and expression regulation of stress-responsive genes that cause physiological response of tolerance of the plant to stress (Reis et al. 2016).
The secondary messengers including Ca2+, ROS, ABA, phosphoglycerol, diacylglycerol, and transcriptional regulators are associated with signal-transmitting pathways to react to drought stress (Kaur and Asthir 2017). Furthermore, the plant hormonal apparatus is activated to transduce stress signals during altered osmotic potential (Khan et al. 2013).
At the mophological level, plants may adapt to drought stress by reducing the growth duration and elude the stress with the conservation of high tissue water content either by hindering water deprivation from plants or enhanced water absorption or both mechanisms. Some plants may lessen their surface area by shedding the leaf or generation of smaller leaves (Farooq et al. 2009).
At the molecular levels, numerous genes and transcription factors have been recognized that are involved in drought response, for instance, the dehydration-responsive element-binding gene, dehydrin’s late embryogenesis abundant proteins, aquaporin, and heat shock proteins (Reis et al. 2016; Farooq et al. 2009). To ameliorate protein functionality, a widespread plant protective reaction is to express several heat shock proteins (HSPs) to restore the favorable folding of proteins required for proper structural and functional activity of proteins even during severe stress (Kasim et al. 2012). By moderation of the tissue metabolic activity, osmotic adjustment can act as one of the pivotal mechanisms in plant adaptation to drought as well. The osmotic compounds are also produced under drought condition which include compatible solutes such as glycine betaine, sugars (fructans and sucrose), amino acids (proline, aspartic acid, and glutamic acid), and cyclitols (mannitol and pinitol) (Kaur and Asthir 2017).
In principle, the antioxidant defense system of the plant cell comprises enzymatic and nonenzymatic mechanisms (Farooq et al. 2009). Enzymatic constituents consist of superoxide dismutase, catalase, peroxidase, ascorbate peroxidase, and glutathione reductase (Farooq et al. 2009; Sandhya et al. 2010; Khan et al. 2013). The nonenzymatic components of antioxidant system include cysteine, reduced glutathione, and ascorbic acid (Farooq et al. 2009). The attenuation of ROS production during the drought state can provide plants to encounter water deficiency without extensive injury. The reduction of ROS synthesis highly depends on the effective energy dissipation mechanisms in the mitochondria (Kaur and Asthir 2017).
The plant growth-promoting bacteria (PGPB) or stress homeostasis-regulating bacteria (PSHB) (Sgroy et al. 2009) have the potential to produce the tolerance to drought in plants (Sandhya et al. 2010; Zelicourta et al. 2013). The mechanisms that PGPB provide to act in the mitigation of drought stress in plants are by production of polysaccharides, 1-aminocyclopropane-1-carboxylate deaminase, and phytohormones, inducing accumulation of osmolytes, volatile compounds, and antioxidants, upregulation or downregulation of stress-responsive genes, and modification in morphology of the root (Kaur and Asthir 2017). The early report on the enhancement of plant drought stress resistance by rhizosphere bacteria has been published in 1999, and some Gram-positive bacterial isolates including Paenibacillus sp. and Bacillus sp. were revealed to be effective in enhancing the plant tolerance to drought stress (Timmusk et al. 2013, 2014).
Despite the extensive studies on the plant drought response, there are still no economic practice or technologic tool to boost the crop production under drought (Wang et al. 2016). Therefore, finding efficient low-cost technologies to reduce effects of drought over crops is necessary to the maintenance of crop yields under water deficits, which is the major challenge, faced by agriculture (Furlan et al. 2017). Several strategies have been used in order to decrease the drought stress effects on plant growth, including traditional selection methods, plant genetic engineering, and recently application of plant growth-promoting bacteria (Tapias et al. 2012; Timmusk et al. 2014).
Drought stress tolerance in plant is a complex phenomenon containing by clusters of gene networks involved in drought stress responses which partially has been characterized (Saikia et al. 2018; Timmusk et al. 2014). The suitable phenotypes are also further challenging to be recognized due to plants are exposed to multiple environmental stressors in the field either simultaneously or sequentially (Timmusk et al. 2014).
Furthermore, it is currently not much promising that the gene engineering technology will progress fast enough to fulfill with multiplied food demands in the near future (Timmusk et al. 2013, 2014). Utilization of PGPB has become a promising alternative to withstand abiotic stresses (Tapias et al. 2012; Furlan et al. 2017; Egamberdieva et al. 2017a, b). An existing pattern in the nature, selection, screening, and development of stress-tolerant bacteria, thus, could be a worthwhile approach to neutralize the productivity restrictions of crop plants in stress-prone regions (Meena et al. 2017).
4 Plant Growth-Promoting Bacteria (PGPB)
4.1 Definition and Categorization
Plant growth-promoting bacteria (PGPB) are considered as free-living soil, rhizosphere (soil near the roots), rhizoplane (root surface), endophyte (reside inside the plant) (Bashan and Bashan 2005; Gopalakrishnan et al. 2015), and phyllosphere (the habitat provided by the aboveground parts of plants) (Whipps et al. 2008; Penuelas et al. 2011) bacteria which are beneficial to plants under some conditions (Bashan and Bashan 2005; Gopalakrishnan et al. 2015). The majority of PGPB activities have been investigated in the rhizosphere and to less extent on endophytic which reside in the leaf surface (Bashan and Bashan 2005).
PGPB can promote the plant growth in a direct or an indirect way (Saravanakumar et al. 2011; Sadeghi et al. 2011; Ahemad and Kibret 2014). They directly affect the metabolism of the plants or can be indirectly affected by PGPB by the production of components that are in deficit supply. These bacteria are capable of solubilizing phosphorus and iron, fixing atmospheric nitrogen, and producing plant hormones, such as auxins, gibberellins, cytokinins, ethylene, etc. Exceedingly, such supplementation can improve the plant’s tolerance to other stresses than drought, including salinity, metal, and pesticides. The molecular mechanism that contributes to the plant growth can be a sole combination of mechanisms. The second group of PGPB, known as biocontrol PGPB, promotes indirectly the plant growth by inhibiting the damaging impact of the phytopathogenic microorganisms including the bacteria, fungi, and viruses (Bashan and Bashan 2005; Orhan 2016; Kang et al. 2014b; Ahemad and Kibret 2014). Indirect mechanisms consist of ACC deaminase, cell wall-degrading enzymes, antibiotic production, substrate competition, hydrogen cyanide, induced systemic resistance, siderophore production, and quorum quenching (Olanrewaju et al. 2017; Kang et al. 2014a, b).
The PGPB has been also categorized as extracellular PGPB (ePGPB) and intracellular PGPB (iPGPB). The known ePGPB belong to the genera Bacillus, Pseudomonas, Arthrobacter, Erwinia, Caulobacter, Chromobacterium, Serratia, Micrococcus, Flavobacterium, Agrobacterium, and Hyphomicrobium, and iPGPB consist of the genera Rhizobium, Bradyrhizobium, Mesorhizobium, Sinorhizobium, Azorhizobium, and Allorhizobium (Gopalakrishnan et al. 2015; Vandenberghe et al. 2017).
4.2 Site of Colonization in Plants
The plant-associated bacteria comprise endophytic, phyllospheric, and rhizospheric bacteria (Weyens et al. 2009; Glick 2014).
4.2.1 Endophytic Bacteria
In addition to the rhizosphere populations, diverse communities of microorganisms live in plants with neutralism or commensalism interaction that are broadly referred to as endophytes. Bacterial endophytes have been isolated rather from all tissue types of plant, and they can colonize in specific plant tissues, either inside the cells or in the intracellular fluids. These ancient interactions are not only evolutionary valuable evolved relations while are potential of precious value for sustainable plant production if these interrelationships subject to investigation.
The majority of endophytes exist in both states of free-living and endophytic. These endophytes are considered to represent a group of soil bacteria which colonize the plant without stimulating the host defense reaction. In order to transfer from the soil to the plant, the bacteria must in essence harbor competence in the rhizosphere area, ability to adhere to the root, followed by the establishment in the host plant. Following entering the plant, endophytes may be surrounded by a cell membrane and become either intracellular or remain extracellular. The motility and secretion of various extracellular enzymes mainly cellulases and pectinases are required attributes of bacteria which transform from free-living to endophytic lifestyles. However, endophytic bacteria do not induce detrimental reactions or cellular injury to the plant. Endophytic bacteria compared with pathogens usually have lower population size in the host plant tissues, and this may be a manner by which they skip the plant defenses. In fact, there are types of endophytic bacteria colonizing the host tissue internally, sometimes in high density which leads to the eliciting symptoms of plant damage. In addition to the scape from an immune reaction, useful endophytes partially act by activating the plant-induced systemic resistance (ISR) toward pathogenic bacteria in the site (Farrar et al. 2014).
The cultivation-independent analysis has revealed that a high number of unculturable species colonize plants endophytically and a variety of bacterial species has been isolated from plant tissues, such as seeds, roots, stems, and leaves so far (Sziderics et al. 2007). Major fraction of endophytic bacteria have been shown to have several beneficial effects on their host plant, and the mechanisms involved are probably similar to those have been described for rhizospheric bacteria (Sziderics et al. 2007). It is assumed that the endophyte infection can protect the host from abiotic stresses by improving tolerance to drought, the rate of photosynthesis, and growth (Collemare and Lebrun 2012). Interestingly, microbial functionality seems dependent on the plant colonization compartment (rhizosphere or endosphere), as the endosphere microbiome might harbor significantly more metabolic pathways and PGP phenotypes than those colonizing the rhizosphere (Wang et al. 2017).
In fact, bacterial endophytes have been isolated from virtually all studied plants. Endophytic Bacillus subtilis EPB5, EPB22, EPB 31 have been evaluated for their capacity to induce water stress-related proteins and enzymes in green gram (Vigna radiata) plants (Saravanakumar et al. 2011). However, a far deeper understanding of both the individual components and their interactions is required in order to exploit beneficial bacteria to optimize biomass production (Farrar et al. 2014).
4.2.2 Phyllospheric Bacteria
The phyllosphere is the external parts of the plant that are above the ground, including leaves, stems, blossoms, and fruits (Weyens et al. 2009). The phyllosphere forms the largest biological interface on earth (Penuelas et al. 2011). Considering that the majority of the surface area available for colonization is located on the leaves, this is the dominant tissue of the phyllosphere. The exposure to an extent and rapid fluctuations in temperature, irradiation, and water availability must be tolerated by the symbiont bacteria that reside the phyllosphere (Weyens et al. 2009). Bacteria and fungi in the foliar phyllosphere of Quercus ilex in Mediterranean forest in summer seasons and long-term drought were investigated (Penuelas et al. 2011).
4.2.3 Rhizospheric Bacteria
The area of the contact between root and soil where soil is affected by roots was designated as “rhizosphere” (Tarkka et al. 2008; Kang et al. 2010). The name comes from the Greek rhiza, meaning root (Pujar et al. 2017). The rhizosphere concept was originally described as the narrow zone of soil surrounding the roots where bacterial populations are stimulated by root activities. The original concept has now been amended to include the soil surrounding a root in which physical, chemical, and biological properties have been changed by root growth and activity (Saharan and Nehra 2011). It was proved that the rhizosphere is much richer in bacteria than the surrounding bulk soil. This rhizosphere is supported by a substantial amount of the carbon fixed secreted by the plant, mainly as root exudates (Lugtenberg and Kamilova 2009; Kang et al. 2010).
5 Colonization of PGPB Under Drought Stress
The diversity and population size of the soil bacteria are influenced by the physicochemical conditions including temperature, water activity, and the existence and amount of salt and other chemicals along with the number and types of plants thriving in that soil (Glick 2012). Plant traits determine the conditions for microbial colonization mainly by the organic and inorganic compounds secreted from the roots. A precisely coordinated interaction between the variety of exudates excreted by the plant and individual characteristics of distinct microbial populations is a crucial aspect of driving selection (Moreira et al. 2016; Wang et al. 2017).
The small molecules such as sugars, amino acids, and organic acids that are exuded in large amounts from plant roots (i.e., 5–30% of all fixed carbon during photosynthesis) are usually consumed by bacteria (Olanrewaju et al. 2017).
The “initiation inoculum” of the soil microbiome will be influenced under drought stress by selective choice of desiccation-tolerant taxa, along with indirect transformed soil chemistry and diffusion rates. Like soil bacteria, plants also endure a set of physiological reactions to survive under the drought-induced damages. These responses consist of alterations in root morphology and root exudate profile in principle means by which plants attract bacteria. Therefore, the root microbiome diversity under drought is characterized by how drought shapes both the host plant and neighboring soils. These factors can affect reciprocally. The transformed soil nutrient cycles and subsequent modifications in the type of microbiome under drought can convey an indication for plant health, as plants rely on bacterial activity to make soil nutrients bioavailable. Correspondingly, drought-induced changes in plant exudate can influence the surrounding soil microbiome, by accelerating more alterations to soil geochemistry that sequentially modify magnitude and directionality of soil community levels. As a result of this complication, a comprehensively integrated realization of the influence of drought on the root microbiome is yet not fully revealed (Naylor and Coleman-Derr 2017).
6 Mechanisms of Alleviation of Drought Stress by PGPB or Plant Stress Homeostasis-Regulating Bacteria (PSHB)
Plant growth-promoting bacteria (PGPB) could play a noteworthy role in the mitigation of drought stress in plants. The functionality of PGPB-mediated drought resistance may be related to the interaction between the used PGPB strain and soil type as well as the capability of the plants to accommodate the association of the PGPB populations naturally occurring in the soil. Coarse sandy or gravelly soils can allow the finer roots to grow, which increase soil penetration, and may finally confer the drought tolerance. In addition, the duration and intensity of the stress and stage of the plant’s development at the point of drought exposure may also affect the efficiency of PGPB-mediated drought tolerance (Ngumbi and Kloepper 2016).
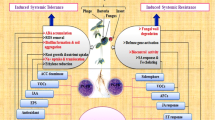
These beneficial bacteria colonize plants and confer drought tolerance by modification in root morphology in acquirement of drought tolerance (Vurukonda et al. 2016); production of exopolysaccharides (EPS) (Sandhya et al. 2009; Kavamura et al. 2013), phytohormones (Fahad et al. 2014), 1-aminocyclopropane-1-carboxylate (ACC) deaminase (Saleem et al. 2007; Reed and Glick 2005; Glick et al. 2007), and volatile compounds (Vurukonda et al. 2016), inducing accumulation of osmolytes (Jha et al. 2011) and antioxidants (Gururani et al. 2013; Wang et al. 2012); and upregulation or downregulation of the genes involved in stress response (Vurukonda et al. 2016) (Fig. 10.1).
6.1 Alteration of Root Morphology in Acquisition of Drought Tolerance
The architecture of the root system is among the important mechanisms adopted by plants to endure the drought situation. Root system structure comprises root system topology, spatial dissemination of primary and lateral roots, and changes in the number and length of root diameters. Root morphological plasticity in response to soil physical conditions provides the plants an available tool to cope with the chemical and physical properties of the soil including the drought conditions. The modification in root features associated with preserving the plant productivity under drought conditions comprises proliferation in the ratio of roots with small diameters and a deeper root length. More numbers of thinner roots allow plants subjected to drought to excess the hydraulic conductance by enhancing the surface area in contact with soil water in parallel rising the extent of soil that can be used for water uptake (Ngumbi and Kloepper 2016).
The incorporation of the PGPB has been shown to accelerate the root growth and to modify its architecture. It has been supposed that the bacterial-induced alterations in root structure often lead to an extent in total root surface area, and subsequently to improved water and nutrient absorption, with an impact on the plant growth (Ngumbi and Kloepper 2016). In a study on the effect of Alcaligenes faecalis (AF3) on maize, 3 weeks after planting of the inoculated seeds, drought-stressed PGPB-treated plants had 10% enhanced root length compared to drought-stressed non-inoculated control group. This could show that alteration of the root net as a result of PGPB treatment results in an improved water uptake, and consequently treated plants show higher tolerance to drought stress (Ngumbi and Kloepper 2016).
It has also been shown that wheat plants treated with Bacillus thuringiensis AZP2 could exhibit two to three times longer root hairs and longer and denser lateral roots following exposed drought stress (Ngumbi and Kloepper 2016).
In addition, plant’s physiological state is controlled by the cell membranes, and rhizobacteria can affect the membrane transportation (Vurukonda et al. 2016; Sahin et al. 2015). Water scarcity changes the phospholipid pattern in the root, rises phosphatidylcholine, and diminishes phosphatidylethanolamine which results in unsaturation, but inoculation with Azospirillum prohibited these variations in wheat seedlings. As a whole, the bacterial elicited changes in the elasticity of the root cell membranes are among the initial responses toward enhanced tolerance to water deficiency (Vurukonda et al. 2016).
6.2 Production of Exopolysaccharides (EPS)
Plants treated with exopolysaccharides (EPS) producing bacteria exhibit increased resistance to water and salinity stress due to improved soil texture (Ledger et al. 2016; Ilangumaran and Smith 2017). Microbial polysaccharides can attach the soil particles to construct microaggregates and macroaggregates. Plant roots and fungal hyphae fit in the pores between microaggregates and contribute to the stabilization of the macroaggregates (Shrivastava and Kumar 2015; Sandhya et al. 2009).
The EPS released by PGPB into the soil as slime materials which can be adsorbed by clay surfaces as a result of cation bridges, hydrogen bonding, Van der Waals forces, and anion adsorption mechanisms. This EPS film creates a protective capsule surrounding the soil aggregates. EPS supply a microenvironment that retains water and desiccate more slowly than the circumstances, thus avoiding the bacteria and plant roots from aridity (Vurukonda et al. 2016;Sandhya et al. 2009). EPS can also absorb the cations such as Na+ therefore making it inaccessible to plants under saline conditions (Shrivastava and Kumar 2015; Upadhyay et al. 2011).
Particularly, the extracellular matrix made by PGPB can offer a range of beneficial macromolecules for plant growth and development. Biofilms have sugars and oligo- and polysaccharides that can also improve water availability in root medium. Additionally, some bacterial polysaccharides have a water retention capacity that can exceed severalfold of their mass (Timmusk et al. 2013, 2014). It has been demonstrated that even small polysaccharide alginate content in the biofilm facilitates the maintenance of hydrated microenvironment (Timmusk et al. 2014).
Accordingly, in a study an EPS-producing strain Pseudomonas putida strain GAP-P45 could form a biofilm on the root surface of sunflower seedlings and impart the plant tolerance to drought stress. The inoculated seedlings showed improved soil aggregation and root-adhering soil and eventually higher relative water content in the leaves (Sandhya et al. 2009; Vardharajula et al. 2011).
6.3 Metabolites with Phytohormone Effects
Phytohormones are synthesized in tissues of plants and are effective in quite a low amount after which are transported to their particular site of action. The hormone upon conveys to the targeted tissues prompts physiological alterations in plants such as lateral root development, flowering, fruit ripening, bud initiation, etc. The plant function is often the net consequence of the antagonistic or synergistic net of several hormones. Plant hormones are classified into five main groups: auxins, gibberellins, ethylene, cytokinins, and abscisic acid (Kang et al. 2014a, b). Phytohormones protect the plants against abiotic stress, and as a result, they can survive under stressful conditions. Additionally, PGPB can synthesize phytohormones that motivate plant cell division and growth and make crops tolerant to the environmental stresses (Vurukonda et al. 2016) (Table 10.1).
6.3.1 Abscisic Acid
Abscisic acid (ABA) is a naturally occurring sesquiterpenoid (Egamberdieva et al. 2017b). The abscisic acid is a stress hormone biosynthesized during water scarcity condition as cellular dehydration. ABA-induced regulates the expression of stress-responsive genes under abiotic stress and interposed signaling, resulting in stronger elicitation of resistance responses. Furthermore, ABA has been assumed to adjust the root development and water quantity under drought stress situations (Egamberdieva et al. 2017b).
In addition to ABA function in signaling, the most significant role of ABA is its action as an antitranspirant by the excitation of stomatal closure and lessening of canopy expansion (Vurukonda et al. 2016; Egamberdieva et al. 2017b; Vacheron et al. 2013). It was revealed that the elevation of ABA content in Arabidopsis inoculated by the PGPB Phyllobacterium brassicacearum strain STM196 could modulate the osmotic stress resistance in inoculated plants, causing reduced leaf transpiration (Vurukonda et al. 2016; Kaushal and Wani 2015).
Additionally, ABA can trigger developing a deeper root system and creating other root changes to intercede optimal water and nutrient attainment in plants exposed to stressful circumstances. Moreover, ABA retains the hydraulic conductivities of shoot and root to efficiently explore environmental water content, resulting in the retention of tissue turgor potential. Furthermore, ABA upregulates the antioxidant system and the accumulation of compatible osmolytes which conserve the relative water content (Egamberdieva et al. 2017b). It has been assumed that ABA conserves the balance of other hormones, including ethylene, causing the preservation of shoot and root growth in Zea mays (Egamberdieva et al. 2017b).
6.3.2 Auxins
A number of identified auxins exist naturally as indole-3-acetic acid (IAA) is the most common (Olanrewaju et al. 2017) that is physiologically the most active auxin in plant growth and development (Vurukonda et al. 2016). In fact, throughout the literature, auxin is often interchanged with IAA. It is estimated that almost 80% of rhizosphere microorganisms have the ability to produce and release the auxin (Olanrewaju et al. 2017). It has been proposed that PGPB may support plants to modulate the abiotic stresses by supplying IAA for plants, which prompts plant growth in spite of the existence of inhibitory material (Glick 2012).
IAA increases the length of root, the root surface area, and the number of root tips, which result in an increased uptake of water and nutrients, therefore supporting plants to adapt with water scarcity (Shrivastava and Kumar 2015; Vurukonda et al. 2016; Kaushal and Wani 2015).
IAA and ACC deaminase enhance the plant growth synergistically. The excluded tryptophan from the roots can be absorbed by PGPB associated with the roots, where it is transformed into IAA. The diffused IAA from the bacterial source absorbed by plant cells and in combination with the plant inherent IAA induces the auxin signal transduction pathway which contains different auxin response factors. The plant cells growth and proliferation are prompted by that, while simultaneously some of the IAA molecules stimulate the expression of the gene encoding the ACC synthase enzyme. The activity of this enzyme leads to a raised level of ACC precursor and finally ethylene synthesized by the enzyme ACC oxidase (Glick 2012; Penrose and Glick 2011).
A number of biotic and abiotic stresses can promote the synthesis of IAA and induce the transcription of the gene for ACC synthase (Glick 2012). A fraction of this ACC may be scavenged by the PGPB which are associated with the plant that has the capability of producing the enzyme ACC deaminase and degraded to ammonia and ǖFC;-ketobutyrate (Nadeem et al. 2014).
Therefore, PGPB that contain genetic information of ACC deaminase can act as a sink for the excess ACC. This root in the fact that, by the impose to an environmental stress, a decreased amount of ethylene is manufactured by the plant and the stress response of the plant is reduced (Glick 2015).
Following the increase in the quantity of ethylene in a plant, the transcription of auxin response factors is suppressed. The ethylene limits the transcription of auxin response factors and as a result restricts both cell growth and proliferation in the absence of bacterial ACC deaminase, while in the presence of ACC deaminase, less ethylene is made. Therefore, when ACC deaminase exists, the transcription of auxin response factors is not repressed, and IAA can prompt cell growth and proliferation without parallel causing the accretion of ethylene. As a result, ACC deaminase reduces the inhibition of plant growth pursued by the ethylene and provides the state that IAA can increase the plant growth, both in the stressful and stressless conditions (Glick 2012).
The enhancement in leaf water content was induced by association of Azospirillum to wheat. This was related to the construction of plant hormones such as IAA by Azospirillum that elevated the root growth and formation of lateral roots, which consequently the water uptake and nutrient absorption of plants increase under the drought stress (Vurukonda et al. 2016).
6.3.3 Cytokinin
Cytokinins such as zeatin (Z) (Sgroy et al. 2009) are compounds with a similar structure to adenine that is termed based on their influence on cytokinesis or cell division in plants. Other than plants, a number of yeast strains and a diversity of soil bacteria, including PGPB are able to synthesize the cytokinins. The overproduction of cytokinins in transgenic plants, particularly during periods of abiotic stress, is considerably protected from the harmful effects of abiotic stresses. The assessment of the protective activity of cytokinin-producing PGPB compared to cytokinin minus mutants will reveal their effect more comprehensively (Glick 2012).
6.3.4 Gibberellins
Gibberellins (GAs) are omnipresent plant hormones that affect different stages of plant growth by regulating numerous physiological functions including seed germination, stem elongation, sex expression, flowering, fruiting, and senescence (Kang et al. 2014b). The exogenous applications of GA3 and GA4 have been shown to reclaim the plant growth and biomass production by countering the abiotic stresses in plants (Kang et al. 2014b). GAs cause improved root length, root surface area, and the number of root tips, causing an enhanced attraction of nutrients, thereby amending plant function under stress environments (Shrivastava and Kumar 2015;Vacheron et al. 2013).
The inoculation of rhizobacterium P. putida H-2–3 which can secrete gibberellins was shown to induce the physiological modifications in soybean plants leading to ameliorated growth under drought environments (Kaushal and Wani 2015). Production of ABA and gibberellins by Azospirillum lipoferum has also been reported to alleviate the drought stress in maize plants (Kaushal and Wani 2015).
6.3.5 Salicylic Acid
Salicylic acid is the main phytohormone with a phenolic nature. It plays an important role in plant stress resistance by the activity regulation of the antioxidative enzyme (Egamberdieva et al. 2017b). SA moderates numerous physiological processes related to plant stress tolerance by stress-activated signal pathways and response mechanisms (Egamberdieva et al. 2017b). It was reported the augmentation of plant by SA increased the plant growth of sesame in drought circumstance (Egamberdieva et al. 2017b).
6.4 Accumulation of Compatible Solutes
Plants adapt to drought stress by the metabolic adjustments that result in the aggregation of osmolytes (compatible solutes) such as proline, betaines, sugars, polyhydric alcohols, polyamines, quaternary ammonium compounds, and other amino acids and water stress proteins like dehydrins. PGPB are able to secrete osmolytes in reaction to drought stress, which works synergistically with inherent plant-produced osmolytes and prompts the plant growth (Vurukonda et al. 2016).
These small, uncharged, soluble molecules do not affect cellular function directly (Cura et al. 2017) while can reduce the hydric potential of cells by trapping water molecules or by retaining the water molecules they are already associated with (Furlan et al. 2017; Cura et al. 2017). In addition, compatible solutes can increase the stability and integrity of membranes and proteins, leading to lessening the cellular damage (Cura et al. 2017).
6.4.1 Proline
Upregulation of proline biosynthesis pathway enhances proline amount which contributes in sustaining cell water station, conserving membranes and proteins from stress (Vurukonda et al. 2016), sweeping hydroxyl radicals, and moderating the NAD/NADH ratio (Marulanda et al. 2009). Higher proline accumulation in inoculated plants correlates with higher plant tolerance to water stress (Ngumbi and Kloepper 2016).
The inoculation of maize plants exposed to drought with PGPB Pseudomonas putida GAP-P45 amended the relative water content and leaf water potential by a concentration of proline (Vurukonda et al. 2016). P. fluorescens enhanced the amount of proline when maize plants were inoculated under drought stress (Vurukonda et al. 2016). Drought tolerance of L. dentate has been attributed to the PGPB B. thuringiensis (Bt) inoculation which was supposed to acquire through enhanced shoot proline accumulation (Vurukonda et al. 2016).
6.4.2 Choline
Choline has a critical importance in plant stress tolerance, principally by increasing glycine betaine (GB) synthesis and aggregation. The investigation of the treatment of B. subtilis GB03 on Arabidopsis and Klebsiella variicola F2, P. fluorescens YX2, and Raoultella planticola YL2 on maize revealed improvements in biosynthesis and accumulation of choline. Choline as a precursor in GB anabolism promotes accumulation of GB; as a result, it elevates the leaf relative water content (RWC) and ultimately the plant biomass (Vurukonda et al. 2016).
A number of PGPB strains can induce accumulation of solutes such as GB under abiotic stress which controls plant stress responses by inhibiting water loss due to osmotic stress. Correspondingly, inoculated plants with PGPB strains such as B. subtilis GB03 and Pseudomonas spp. considerably accumulated higher amounts of GB compared to uninoculated plants under osmotic stress. This might originate from upregulation of GB biosynthesis pathway by appending some key enzyme gene expression as PEAMT (phosphoethanolamine N-methyltransferase) (Vurukonda et al. 2016).
6.4.3 Polyamines
Polyamines are aliphatic nitrogen mixtures which are ubiquitous in bacteria, plants, and animals. They control plant growth and development as well as plant reactions under drought stress by an active function in various metabolic and hormonal pathways (Kaushal and Wani 2015). Increased root growth due to cadaverine (polyamine) production by A. brasilense Az39 could induce the enhanced root growth of Oryza seedlings which caused the mitigation of the osmotic stress (Kaushal and Wani 2015; Vurukonda et al. 2016).
6.4.4 Soluble Sugars
The accumulation of soluble sugars as osmolytes can also be adapted as a contributing mechanism to the osmotic amendment in the drought environment. It was assumed that starch hydrolysis increases the levels of monosaccharides. An enhancement in soluble sugar quantity in drought-stressed plants has been reported. Starch reduction and elevated sugar content were simultaneously detected in grapevine leaves under drought stress (Kaushal and Wani 2015).
Maize seedlings augmented with Bacillus strains inoculation showed elevated sugar content caused by starch degradation, thus made plants to tolerate the drought stress (Kaushal and Wani 2015). The enhanced soluble sugar quantity compared to uninoculated maize was observed in maize seedlings supplemented with Pseudomonas spp., representing that such inoculation causes the hydrolysis of starch, consequently providing sugar of osmotic regulation to alleviate the effect of drought stress (Kaushal and Wani 2015).
6.4.5 Trehalose
Trehalose is a nonreducing disaccharide, consisting of two molecules of ǖFC;-glucose that is widely distributed in bacteria, yeast, fungi, plants, insects, and invertebrates. Trehalose is recognized as a preserver against various abiotic stresses such as drought, high salt, and extreme temperature at high levels of concentration. Trehalose has a high structural stability and is tolerant to high temperature and acidity. Trehalose can form a gel phase as cells dry up, replacing water, consequently facilitate to expel the detriment from drought and salt. Furthermore, trehalose can protect proteins from degradation and aggregation caused by high- and low-temperature stresses (Glick 2012).
Treatments of plants with PGPB which overproduce trehalose have conferred drought (and other stress) tolerance. The inoculated beans with a genetically engineered overproduce of the trehalose (symbiotic Rhizobium etli) conferred the host more nodules, fixed more nitrogen, resulted in higher biomass, and recovered to a greater amount from drought stress than inoculated plants with wild-type R. etli (Glick 2012).
Correspondingly, inoculated maize with the PGPB Azospirillum brasilense (modified to overproduce trehalose) was more drought tolerant and produced more biomass compared to plants treated with wild-type A. brasilense. The use of genetically manipulated PGPB to overproduce trehalose is simpler than engineering plants to achieve the same goal. Another advantage is that using a single engineered bacterial strain may effectively protect a large number of different crop plants (Glick 2012).
6.5 Production of Volatile Compounds
Production of volatiles is induced in plants exposed to a multitude of stresses. The stress-induced volatile compounds act as signals for beginning the systemic responses within the identical and in adjunct plants (Vurukonda et al. 2016).
The augmentation of wheat seedlings with B. thuringiensis AZP2 led to fivefold higher survival under intense drought. This tolerance was caused by a substantial decrease in emissions of volatiles and higher photosynthesis. This support that bacterial inoculation can improve plant drought tolerance by this mechanism. Volatiles are promising candidates of quick noninvasive indicator to evaluate crop drought stress and its alleviation during stress (Vurukonda et al. 2016).
Pseudomonas chlororaphis O6 which colonized root hinders water loss by the production of a volatile metabolite 2R,3R-butanediol. This volatile metabolite mediated stomatal closure. Bacterial volatile 2R,3R-butanediol stimulates the tolerance to drought stress in Arabidopsis. Additionally, Arabidopsis mutants illustrated that induced drought resistance required the signaling pathways of salicylic acid (SA), ethylene, and jasmonic acid. The induced drought resistance and stomatal closure pertained to Aba-1 and OST-1 kinase. Rise in free SA in plants colonized with P. chlororaphis O6 under drought stress and after 2R,3R-butanediol treatment proposes the initial function of SA signaling in induction of tolerance to drought stress. The volatile bacterial metabolite of 2R,3R-butanediol has shown as a major determining factor in promoting tolerance to drought through an SA-dependent mechanism in Arabidopsis (Vurukonda et al. 2016; Liu and Zhang 2015).
VOC treatment increased the level of PEAMT (phosphoethanolamine N-methyltransferase) transcripts. PEAMT is an essential enzyme in the biosynthesis pathway of choline and glycine betaine which mediate the VOC-induced plant tolerance to dehydration (Liu and Zhang 2015).
6.6 Antioxidants Effect to Neutralize the Stress
The systemic exposure of plants to drought stress can cause the generation of reactive oxygen species (ROS), including hydroxyl radicals (OH), superoxide anion radicals (O2−), singlet oxygen (O2) hydrogen peroxide (H2O2), and alkoxy radicals (RO). The reaction of the ROS with proteins, lipids, and deoxyribonucleic acid leads to oxidative damage and impairing the proper functions of plant cells. In order to prevail these consequences, plants have antioxidant defense systems consisting of enzymatic and nonenzymatic components that render to prevent the concentration of ROS and diminish the oxidative damage occurring during drought stress. Enzymatic components consist of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR). Nonenzymatic components include cysteine, glutathione, and ascorbic acid (Vurukonda et al. 2016; Kaushal and Wani 2015).
The consortia of PGPB comprising P. jessenii R62, P. synxantha R81, and A. nitroguajacolicus strainYB3 and strain YB5 enhanced plant growth and induced the stress-associated enzymes (SOD, CAT, peroxidase (POD), APX and lower level of H2O2, malondialdehyde (MDA)) under drought stress compared to control. These studies provide evidence on the influence of PGPB application in increasing the drought resistance of plants by modulating the antioxidants activity under water scarcity environment (Vurukonda et al. 2016).
PGPB species like Azospirillum sp. and Pseudomonas sp. increased the growth and biomass of canola plants by regulating the oxidative stress enzymes under salinity stress (Kang et al. 2014a). Inoculation of lettuce (Lactuca sativa L.) with PGPB Pseudomonas mendocina augmented an antioxidant CAT (catalase) under severe drought conditions, suggesting that they can be used in inoculants to alleviate the oxidative damage elicited by drought condition (Vardharajula et al. 2011).
6.7 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase
Ethylene is an ubiquitous hormone in all higher plants. This subject illustrates its importance in the regulation of normal cell progress and plant growth in addition to its vital role to counteract various levels of stress. Approximately all plant tissues and phases of growth are influenced by ethylene. Ethylene production in a specific plant is related to the existence and content of other plant hormones, temperature, light, gravity, nutrition, and the occurrence of different amounts of biotic/abiotic stress. The concentration of ethylene in plants is enhanced in reaction to the range of stresses including the existence of extreme temperatures, metals, chemicals (both organic and inorganic), extreme amounts of water, ultraviolet light, insect and nematode injury, and fungal and bacterial pathogens along with mechanical damages (Olanrewaju et al. 2017).
ACC oxidase enzyme produces ethylene more than its threshold level in the plant tissues, which can result in “stress ethylene” and influences the root and shoot growth in plants (Olanrewaju et al. 2017). ACC deaminase-producing PGPB favor to relieve “stress ethylene” situation and revive normal plant growth (Mayak et al. 2004b). Rhizospheric and phyllospheric organisms as well as endophytes, all of which can act as a sink for ACC produced as a consequence of plant stress by the synthesizing of ACC deaminase (Saleem et al. 2007). Plant ACC is sequestered and catabolized by ACC deaminase-producing PGPB to nitrogen and energy substrate (Shrivastava and Kumar 2015; Cura et al. 2017).
The “stress ethylene” is being synthesized in two peaks. The first peak is a small portion of the quantity of the second peak. The first little peak which measures hard consumes much of the present 1-aminocyclopropane-1-carboxylate (ACC) in stressed plants and triggers the expression of genes that encode plant defensive/protective proteins. The second, much larger ethylene peak occurs when the level of ACC in response to stress increases. The second peak impairs consequent plant growth and initiates processes in the plant, for instance, senescence, chlorosis, and leaf abscission. The upregulated amount of plant ethylene considerably gets worse the effects of the causing stress that activates the ethylene response. So any treatment that reduces the quantity of the second peak of stress ethylene can also decrease/cease the deleterious effect of stress (Olanrewaju et al. 2017). In this regard, the ACC deaminase-producing bacteria can reduce the detrimental effect of the various stresses on plants by diminishing plant ACC amounts (and consequently plant ethylene levels). The ACC is being catabolized by ACC deaminase to α-ketobutyrate and ammonia in the PGPB (Olanrewaju et al. 2017; Tank and Saraf 2010; Saleem et al. 2007) (Fig. 10.2).
The induction of plant growth by an ACC deaminase-producing PGPB. Stress enhances both IAA and ethylene production within the plant which harbors a reduction in plant biomass production. The ACC deaminase-containing PGPB exhibit decreased levels of ethylene which allows the bacterial IAA to improve plant growth. Therefore, PGPB that synthesize both IAA and ACC deaminase counteract well the growth-limiting environmental stresses. The PGPB can care for plants against the suppressor effects of ethylene-producing stresses including drought, high salt, metal, flooding, temperature extremes and organic pollutants, insect and nematode predation, and both fungal and bacterial phytopathogens. SAM S-adenosylmethionine. (Olanrewaju et al. 2017)
It was previously proposed that PGPB can absorb some of the tryptophan secreted by plants and transform the tryptophan to IAA, which is then exuded by the bacterium and soaked up by the plant. The enhanced amount of IAA can both assist plant growth and activate the expression of the plant enzyme ACC synthase simultaneously, leading to a raise at the level of ACC and therefore the concentration of ethylene within the plant. Consequently, PGPB that produce IAA from plant tryptophan can both stimulate and hinder plant growth (via the act of the ethylene that is ultimately synthesized). Fortunately, ACC deaminase-containing PGPB reduce the level of ACC in the plant by the act of ACC deaminase enzyme. As a consequence, IAA can improve plant growth without considerably inhibiting plant growth. Furthermore, by lessening the amount of ethylene in the plant, ethylene inhibition of auxin signaling pathway is pulled down, and the bacterial auxin enhances further growth of the plant. Therefore, ACC deaminase assists the action of bacterial IAA by the downregulation of plant ethylene amounts. The ACC is finally converted to ammonia and α-ketobutyrate (Olanrewaju et al. 2017). This model is depicted schematically in Fig. 10.2.
ACC deaminase production by endophytic PGPB can alleviate stress-related inhibition to a variety of environmental conditions (Ebels 2015). ACC deaminase-containing PGPB Achromobacter piechaudii ARV8 has revealed that considerably enhanced the fresh and dry weights of both tomato and pepper seedlings and decreased the ethylene construction under drought stress (Vurukonda et al. 2016; Saleem et al. 2007). ACC deaminase-producing Pseudomonas fluorescens prompted the length of roots of Pisum sativum, which resulted in higher absorption of water from soil in drought conditions. Enhanced growth, yield, and water-absorption competency of droughted peas was observed by the inoculation with Variovorax paradoxus (Vurukonda et al. 2016).
6.8 Induction of Stress-Responsive Genes
Cell membrane proteins are at elevated hazard of denaturation because of their direct impose to the circumstance. Dehydration due to water scarcity induces protein aggregation, exposure of hydrophobic areas, modification in tertiary structure, and subsequent inactivation of enzymes or prohibition of their incorporation as structural proteins. HSPs are upregulated upon disposal to drought stress. HSPs, which are also termed chaperones, for instance, GroEL, DnaK, DnaJ, GroES, ClpB, ClpA, ClpX, sHSPs, and proteases, are implicated in responses to multiple of stress. The principal function of these proteins is to regulate the folding and refolding procedure of stress natured proteins. Clp family proteases are implicated in multiple stress reactions, suggesting they are important for ecological fitness of bacteria. Plant small heat shock proteins (sHSPs) act as molecular chaperones that assist native folding of proteins and prevent irreversible aggregation of denatured proteins. Inoculated pepper plants with Bacillus licheniformis K11 exhibited enhanced transcription of genes Cadhn, VA, sHSP, and CaPR-10 during the drought stress (Lim and Kim 2013; Kaushal and Wani 2015).
Some PGPB alter plant gene expression, moderating drought tolerance-associated genes like ERD15 (Early Response to Dehydration15) (Mayak et al. 2004a, b; Bourque et al. 2016) or DREB (Dehydration Responsive Element Protein) (Bourque et al. 2016).
It was shown that elicitation of the drought-responsive gene ERD15 and ABA-responsive gene, RAB18, provides drought resistance in A. thaliana treated with Paenibacillus polymyxa. These genes, well-known as dehydrins (Group II late embryogenesis abundant proteins), are pertaining to drought and cold stresses and are mainly upregulated by the deficiency in cellular water content. Most of the dehydrins are supposed to act by the stabilization of hydrophobic interactions, for example, membrane structures or hydrophobic patches of proteins (Kaushal and Wani 2015).
The unusual expression of 93 genes in sugarcane, comprising drought-responsive genes such as MRB and WRKY transcription factors, is detected under drought stress. Nevertheless, co-treatment of the same plant with Herbaspirillum spp. and Gluconacetobacter diazotrophicus resulted in the induction of stress resistance and salicylic acid biosynthesis genes (Kaushal and Wani 2015).
It was shown that strain B26 of B. subtilis isolated from switch grass can contribute to the drought tolerance in Brachypodium distachyon by the upregulation of expression of drought-responsive genes, moderation of the DNA methylation procedure, and an enhancement in the soluble sugars and starch amount of the leaves. The strain B26 forming a close association with plants was also reported to thrive as a symbiosis strain and to synthesize several well-known lipopeptide toxins and phytohormones (Bourque et al. 2016).
6.9 Induced Systemic Tolerance
The term “induced systemic tolerance” (IST) has been suggested for PGPB-induced physical and chemical alterations that result in enhanced tolerance to abiotic stress (Shrivastava and Kumar 2015; Vardharajula et al. 2011).
The induced systemic resistance (ISR) is a common phenomenon against pathogens in plants that has been intensively investigated with considering the involved signaling pathways along with its potential application in plant protection. Provoked by a local infection, plants reacted with a salicylic-dependent signaling flow that results in the systemic expression of an extensive spectrum and long-standing disease tolerance that is practicable against fungi, bacteria, and viruses. Salicylic acid (SA) has a pivotal impact in the signaling pathway causing ISR. After infection, local and systemic endogenous concentration of SA enhances, and SA amounts rise in the phloem before the occurrence of ISR. The de novo production of SA in non-infected plant parts might contribute to the systemic expression of ISR (Saharan and Nehra 2011).
Compared to pathogens inducing SAR, even the nonpathogenic rhizobacteria inducing ISR can trigger another signal transduction pathway independent of the accumulation of the SA and activation of pathogenesis-related (PR) genes and following the precipitation of ethylene and jasmonic acid (Saharan and Nehra 2011). Interestingly, some of the volatile organic compounds (VOCs) that are emitted from Bacillus subtilis GB03 are recognized as the bacterial agent involved in IST (Vardharajula et al. 2011). In addition, some reports have suggested that some PGPB induce systemic tolerance (IST) in plants through enhanced antioxidant responses at the levels of enzyme activity and metabolite accumulation (Egamberdieva et al. 2017a).
7 Co-inoculation of PGPB for Mitigation of Drought Stress
As well as single strains of PGPB, its mixture with either mycorrhizal fungi or Rhizobium prompts the resistance of the plant to drought. Co-treatment of common bean (P. vulgaris L.) with a combination of Rhizobium tropici CIAT 899, P. polymyxa DSM36, and P. polymyxa-Loutit strains led to greater growth than lone inoculation of Rhizobium. Also, co-treatment showed higher nodulation and nitrogen content of plants inoculated with the sole Rhizobium under drought stress. Inoculated lettuce with a combination of PGPB strain Pseudomonas mendocina and an arbuscular mycorrhizal (AM) fungus (either Glomus intraradices or Glomus mosseae) showed considerably improved root phosphatase activity, proline content, and activities of NR, POD, and CAT in the leaves of lettuce under different levels of drought (Vurukonda et al. 2016).
8 Study of the Plant–Bacteria Interactions
The interactions of plant–bacteria consist of complex mechanisms within the plant cellular system. Currently, the investigation of plant–bacteria cooperation in terms of preservation against abiotic stresses is more critical consistently pressure of increasing climatic changes. Simultaneously, it is also crucial to make deeper understandings of the stress-alleviating mechanisms in crop plants toward the higher productivity (Meena et al. 2017).
It is obvious that any of the compounds manufactured can’t be solely considered responsible for the detected drought stress resistance improvement. It is postulated that a variety of mechanisms are applied in the different growth levels and the drought resistance enhancement of the plants (Timmusk et al. 2013).
The feedback systems act on a diversity of levels: from DNA transcription to a signal transduction pathway within a cell to operate complicated interactions between systems of organisms. Taking advantage of the mathematical and computer modeling to quantify the interactions between constituents of a biological system is among system methods to make known the biological interactions of plant–bacteria. In order to recognize the complex behavior of the association and the processes of PGPB and plant interaction, high-throughput, genome-wide research involving molecular networks along with high-resolution microscopy can also be performed (Timmusk et al. 2013).
Recent progress in “omics” technologies illustrates thoroughly the regulatory networks of stress reactions moderated by the PGPB (Ilangumaran and Smith 2017). Multi-omics technologies consisting of genomics, transcriptomics, proteomics, metabolomics, and phenomics incorporate assessments on the interaction of plants with bacteria and their interaction with peripheral environment and produce multidimensional information that can reflect what is occurring in real time within the cells (Meena et al. 2017).
Recently, meta-omics approaches comprising metagenomics, meta-transcriptomics, and meta-proteomics have been developed as capable techniques to investigate bacterial communities and function at a deeper level within the environment (Meena et al. 2017).
8.1 Genomics
Omics approaches have contributed to acquiring an improved understanding of the mechanisms of established plant–microbe interactions (Meena et al. 2017). The study of the association between the diazotroph Gluconacetobacter diazotrophicus PAL5 and sugarcane under drought stress by Illumina sequencing determined that bacterial treatment stimulated the ABA-dependent signaling genes providing drought tolerance in sugarcane (Vurukonda et al. 2016).
8.2 Metagenomics
The applying of the culture-independent method for the assay of microbial communities provides a comprehensive tool for the resolution of yet uncultured rhizospheric bacterial diversity. High-throughput metagenomic sequencing is demonstrating to be an exceptionally beneficial tool for a better understanding of PGPB populations.
Metagenomics also make known the hidden functional potential of microbial populations with regard to the affluence of the genes that are involved in specific metabolic processes related to stresses or stress mitigation mechanism. In an investigation on endophytes of the potato, two types of ACC deaminase genes (acdS) homologous to that of P. fluorescens for stress mitigation were discovered. Analysis of clones of metagenomic libraries contributed in recognition of whole acdS operon from uncultivated endophyte has shown a transcriptional regulator gene acdR at upstream of acdS. This operon was determined obviously in the genus Burkholderia (Meena et al. 2017).
The physiology of endophytic bacteria that exist inside roots is severely unknown as endophytes which are successfully cultured represent only a portion of the entire bacterial community that resides root interiors. With the aid of metagenomic method, endophytic bacterial inhabitants of rice roots have been defined. Metagenomic sequences acquired from endophytic cell extracts proved that metabolic processes relating to the endophytic functional traits such as quorum sensing and detoxification of ROS are circumvented in enhancing the plant resistance to abiotic stress (Meena et al. 2017). To distinguish the microbiome composition and define its diversity and function, comprehensive methods, namely, metagenomics, meta-transcriptomics, and meta-proteomics, are needed to be implemented (Meena et al. 2017) (Fig. 10.3).
The multi-omics approaches in the investigation of the impact of abiotic stresses or effect of plant–bacteria interactions. (Adapted from Meena et al. 2017)
8.3 Transcriptomics
The transcriptome includes the whole set of transcripts that are expressed in a cell at a specific developing phase or under different environmental situations (Vurukonda et al. 2016). The comparison of transcriptome profiles can indicate the sets of transcripts underlying the alterations between biologically diverse expressions in various conditions. Usage of mRNA sequence survey and microarray to make transcriptome level data is the main molecular approach used in the assessment of plant–microbe interactions (Meena et al. 2017).
Gene expression influenced by drought stress was newly characterized PGPB physiological roles with regard to resistance prompted by PGPB. At the transcriptional level, the positive effect of PGPB on improving plant resistance to drought was shown with the treatment of PGPB Paenibacillus polymyxa B2 on Arabidopsis thaliana. The expression study revealed that an mRNA transcription of a drought-response gene, Early Response to Dehydration15 (ERD15), was amplified in inoculated plants compared with uninoculated controls lacking the PGPB (Vurukonda et al. 2016; Yang et al. 2008).
The gene expression study on Sinorhizobium meliloti indicated the induction of genes for the stress reaction in IAA overproducing strains in comparison to wild-type strain-1021. This investigation compared the transcription profile of two S. meliloti strains. The coding genes of sigma factor RpoH1 and other stress responses were prompted in IAA overproducing strain of S. meliloti (Meena et al. 2017). Upregulation of stress-related genes APX1, SAMS1, and HSP17.8 in the leaves of wheat was recognized by real-time PCR (RT-PCR) analysis. The activity of enzymes of ascorbate–glutathione redox cycle enhanced in wheat when priming with Bacillus amyloliquefaciens 5113 and A. brasilense NO40 awarding drought tolerance to the plant (Vurukonda et al. 2016).
A number of drought signaling response genes were revealed by microarray analysis to downregulate in the P. chlororaphis O6-colonized A. thaliana in comparison to plants without bacterial priming under drought stress. The priming of plants resulted in upregulation of transcripts of the jasmonic acid-marker genes, VSP1 and pdf-1.2; salicylic acid-regulated gene, PR-1; and the ethylene-response gene, HEL (Vurukonda et al. 2016).
The effect of Bacillus amyloliquefaciens NBRISN13 inoculation on growth of the rice plant and expression analysis of related genes under salt stress was evaluated. Expression analysis by semiquantitative reverse transcriptase polymerase chain reaction (SQRT-PCR) revealed at least 14 genes implicating in SN13-mediated salt stress adaptation (Nautiyal et al. 2013). With RNA differential display on parallel RNA preparations from P. polymyxa-treated or untreated plants, changes in gene expression were investigated. From a small number of candidate sequences provided by this approach, one mRNA segment showed an intense inoculation-dependent increase. The corresponding gene was recognized as ERD15, previously identified to be drought stress-responsive (Timmusk and Wagner 1999).
8.4 Proteomics
Proteins play a vital function in expressing plant stress reactions since they directly harbor the phenotypic characteristics. Thus proteomics has become a potent technique for the investigation of physiological metabolism and protein–protein interactions in microorganisms and plants. The implications of proteomics are significant for intra- and inter-microbial species and host–bacterium interplay. Such surveys result in obtaining a comprehensive insight of the regulation of the biological system by determination of several proteins as a signal that alerts the fluctuations in physiological station caused by stress or mitigating factors of stress. Thus, a comparative study of stressed versus non-stressed plants inoculated with bacteria can contribute to the recognization of protein targets and networks (Meena et al. 2017). Six differentially expressed stress proteins were known in pepper plants treated with B. licheniformis K11 in drought environment by 2-D polyacrylamide gel electrophoresis (2D-PAGE) and differential display polymerase chain reaction (DD-PCR). Particular genes of stress proteins (Cadhn, VA, sHSP, and CaPR-10) enhance more than a 1.5-fold in inoculated plants in comparison to the control plant (Vurukonda et al. 2016).
8.5 Metabolomics
Metabolomics implicates the describing of all the metabolites synthesized by an organism under the effect of adjusted environmental situations. Different metabolic pathways of the cell which reflects the presence of corresponding genetic information determine the metabolome of an organism. The metabolome alters largely with variations in the neighboring environment that stimulate direct physiological expression in a cell.
The analogous physiological state is expected in organisms which grow well under particular stress situations. Consequently, it is important to attain comprehensive perception of metabolome of an organism both in normal and under stress conditions for determining the presence/absence of signature metabolites. This will be useful in recognizing changes induced by the pathways and stimulation of typical stress-inducible genes. Metabolomics is progressively being applied for making comprehensive understanding into abiotic stress reactions. Currently, high-throughput advances of molecular recognition techniques have improved the metabolomics analysis that also shows the presence of diverse bioactive substances in plant metabolome (Meena et al. 2017). These facts relate to the findings regarding the identification of diverse signal molecules exuded by plants to attract and activate significant biochemical pathways in the microbial population that colonized plants.
Trichoderma spp. produce auxins which relieve stress and improve plant growth. Trichoderma synthesized two secondary metabolites (harzianolide and 6-pentyl-a-pyrone) which displayed auxin-like effects in pea stem and increase plant growth. Changing of environmental conditions induces variations in plant metabolism, also influences plants’ secretion pattern and composition of secreted molecules, and, as a result, affects the variety and level of root colonization. Molecular signaling mechanisms of microorganisms in the rhizosphere are too influenced in the same way, but this is yet to be discovered (Meena et al. 2017).
Protective metabolites such as trehalose, glycine betaine, IAA, etc. accumulate in plants in reaction to abiotic stress conditions. The mechanisms acting in the microbial cell relate to the conditions of the encompassing environment which affects the metabolome. It is obvious that the same must influence their general performance in the neighboring microenvironment and inside the ecosystem to a greater range in terms of the interactions within and between the residents in the ecosystem. Microbial metabolic products have enhanced plant growth both directly and indirectly. It is verified that various rhizospheric bacteria can synthesize plant growth-motivating biomolecules such as cytokinins, gibberellins, etc. (Meena et al. 2017). Newly the IAA manufactured by Pseudomonas sp., Rhizobium sp., Enterobacter sp., Pantoea sp., Marinobacterium sp., Acinetobacter sp., and Sinorhizobium sp. has been proved to affect the germination and seedling growth of wheat under saline stress (Meena et al. 2017).
The cellular metabolites of plant-colonized bacteria under the impact of stress analyzed by high-throughput mass spectrometry could show the amount of effect of stress source on the whole cellular homeostasis. The interaction between plants and soil microbial population signifies a bilateral process including root exudates and microbial-elaborated signal response molecules.
For rhizosphere supplementation with exogenous bacterial metabolites too, previous understanding on microbial metabolism is demanded. This consists of the balance of cellular richness, biomolecules synthesized in optimal conditions, quantifiable leak, contribution of plant signals in the cascade, and subsequent counter-reaction of microorganisms. The supplementation and enrichment of such biomolecules that are downregulated following the effect of the stressor could be attentive in the rhizosphere. A similar approach can be implemented to the eventual management of stressor-responsive biomolecules influencing the whole communication process between the host and bacteria (Meena et al. 2017). Novel analytical techniques like GC-MS and LC-MS have assisted in the analysis of low amounts of gibberellin in any cultures (Kang et al. 2014b).
9 Phylogenetic Distribution of Bacteria with Effect on Drought Tolerance
Bacterial phylogenetic diversity for soil communities may be dependent on the drought condition. With considering to drought context, a confusing factor that may participate in inconsistency is the absence of standardization of drought treatment which has been executed through a range of means (Naylor and Coleman-Derr 2017).
In contrast to microbial diversity, population composition is considerably influenced by drought. The imposed modifications in the soil microbial community under drought are likely to be a variation in relative amplitude, instead of complete elimination of drought-susceptible taxa and simultaneous emerging of tolerant ones. A commonly observed trend is a rise in the ratio of Gram-positive to Gram-negative bacteria in the drought environment. Definitely, in soils with limited moisture, prevalent relative richness alterations include declines in the most dominant Gram-negative phyla of Proteobacteria, Bacteroidetes, and Verrucomicrobia and increases in the main Gram-positive phyla including Firmicutes and Actinobacteria. These alterations in relative abundance are provoked by one or a few members of a phylum; while relatively few groups change severely, most bacterial groups showed only minor alterations in reaction to drought. In a related study, an excess in Actinobacteria was detected (Naylor and Coleman-Derr 2017). Among the PGPB, the P. fluorescens and endophytic Bacillus subtilis have received special attention throughout because of their catabolic versatility, their excellent root-colonizing ability, and their capacity to produce a wide range of enzymes and secondary metabolites that favor the plant endure under varied biotic and abiotic stress conditions (Saravanakumar et al. 2011).
From the vast phylogenetically diverse microorganisms, Gram-positive bacteria are the most probable to be commercially applied in range of fields with limited water resources due to the endospore-forming ability that boosts the efficient colonization under drought stress conditions (Timmusk et al. 2014). The application of endospore-forming bacteria provides more reproducible results in various environments (Timmusk et al. 2013) (Table 10.2).
10 Biomarkers Used in Evaluation of Bacterial Colonization in Drought-Tolerant Plant
For screening the more stress-adapted resistant strains, assessing stress markers like proline or phytohormone production of bacterial cultures under the exposure to the elevated stress levels may assist (Marulanda et al. 2009). The analysis of the quantity of IAA is a proper indicator of bacterial efficiency principally under osmotic stress (Vardharajula et al. 2011). Significantly, the bacterial trait that is key in efficiency of PGPB in alleviating stress is the production of enzyme ACC deaminase (Glick 2014).
11 Cross-Resistance to the Other Abiotic Stresses
The different abiotic stresses exhibit some common signs, molecular damages, and alleviation strategies. For instance, drought and salinity stresses led to ionic and osmotic imbalance. The expression of drought and salinity tolerance genes both can restore the ionic and osmotic homeostasis via salt-regulated genes pathway or other associated pathways (Kang et al. 2014b). Drought and low temperature cause similar injuries such as the disintegration of the membrane, desiccation, and solute leakage. Crop plants in reaction to both stresses either activate detoxification signaling or induce stress genes which regulate molecular damage and mending of the cell membrane (Shinozak and Shinozaki 2000; Kang et al. 2014b).
12 Successful Cases of the Field Studies
The effect of PGPB on crop productivity varies under laboratory, greenhouse, and field trials (Ahemad and Kibret 2014). The performance of efficient PGPB strains must be evaluated under field conditions where plants are more probable to tolerate cyclic drought rather than the continuous drought in the experiments (Ngumbi and Kloepper 2016).
Although, a small fraction of the studies have been conducted in the field, however, results are inconsistent with those of laboratory or greenhouse studies (Nadeem et al. 2014). With the aid of suitable monitoring systems, the restrictions of applying microbial inoculation in the fields can be significantly resolved. Therefore, the field trials along with the advanced molecular and biochemical monitoring systems suggested to be applied in parallel (Timmusk et al. 2013).
To investigate the impact of plant growth-promoting rhizobacteria (PGPR) on water stress, a field experiment was conducted in Iran during 2010 growing season. The effect of four types of bacterial strain consisting of Pseudomonas sp., Bacillus lentus, Azospirillum brasilense, and a combination of the three mentioned bacteria on proline, soluble carbohydrates, chlorophyll, and mineral content in basil was studied. Results showed water stress and different bacterial strain were substantially affected by proline and soluble carbohydrate accumulations in leaves of plants (Heidari et al. 2011). Also, the effects of PGPB inoculation under the water stress on antioxidant activity and photosynthetic pigments were investigated in basil plants by the field study. Application of rhizobacteria under water stress improved the activity of antioxidant enzymes and photosynthetic pigments in basil plants (Heidari and Golpayegani 2012).
A field experiment was conducted in Iran with soybean to evaluate the performance of different PGPB comprised of Rhizobium japonicum, Azotobacter chroococcum, Azospirillum brasilense, and a mixture of these inoculates on soybean antioxidant enzyme activity subjected to different irrigation regimes in 2012–2013 growing season (Zahedi and Abbasi 2015).
In another field experiment, the effects of selected PGPB including Bacillus megaterium TV 6D and Bacillus subtilis TV 12H on some physiological characteristics, plant growth, yield, and plant nutrient concentration of lettuce were monitored under different irrigation levels in Turkey. The results of the study demonstrated that PGPB inoculations could deduct the detrimental effects of lower irrigation conditions on the growth and yield of lettuce plants (Sahin et al. 2015).
The effect of selected PGPB on the growth, nutrient element content, chlorophyll content, and yield of strawberry plants under salinity condition stress was evaluated in the natural field. Field experiments were undertaken using a randomized complete block design with five PGPB strains consisting of Bacillus subtilis EY2, Bacillus atrophaeus EY6, Bacillus sphaericus GC, Staphylococcus kloosii EY37, and Kocuria erythromyxa EY43 and a control non-PGPB. PGPB inoculations could enhance the chlorophyll content, nutrient element content, and yield of strawberry plants. Priming with PGPB diminished the electrolyte leakage of plants under saline conditions. The leaf relative water content (LRWC) of plants was improved by the inoculation of bacterial cells (Karlidag et al. 2013). Analogous mechanisms can lead to the stronger tolerance of the plant to the water deficiency in the environment. The effect of plant growth-promoting rhizobacteria (Pseudomonas spp.) on asparagus seedlings and germinating seeds imposed to water stress under greenhouse conditions has also been reported (Liddycoat et al. 2009).
A greenhouse study was conducted to assess the effect of biochar in combination with compost and Pseudomonas fluorescens under water deficit stress on the growth of cucumber. The results showed that water deficit stress significantly hindered the growth of cucumber, while the synergistic use of biochar, compost, and PGPB mitigated the negative impact of stress. The synergistic effect of biochar, compost, and PGPB caused remarkable increases in shoot length, shoot biomass, root length, and root biomass that were 88, 77, 89, and 74% more than uninoculated control, respectively (Nadeem et al. 2017).
13 Concluding Remarks
Sustainable food quality and reasonable cost might be a challenge for the increasing population in the next 50 years (Olanrewaju et al. 2017). Further, development of the arable land, insufficient managed water resources, and long-term effects of the climatic change could all contribute to possibly catastrophic consequences (Kasim et al. 2012). Numerous strategies have been introduced for modulating the effects of drought stress in plants and breeding for tolerant varieties, among which genetic engineering is the most focused approach (Ashraf 2010; Kasim et al. 2012). However, the complexity of tolerance mechanisms to abiotic stress makes the task of developing new tolerant varieties highly challenging and genetically modified plants are not adequately accepted in most countries. An alternative strategy is to induce stress tolerance by using various chemical and biological agents in a process known as priming (Kasim et al. 2012). One of the methods that might be subjected is the more extensive application of PGPB, initially in parallel, and possibly eventually in place of the present chemicals used in agriculture (Olanrewaju et al. 2017).
The application of PGPB is an integral component of modern agricultural practices. The agricultural chemicals are relatively inexpensive which has kept the use of PGPB at a limited scale however as a thriving approach in the development of organic agriculture (Glick 2012).
Although microbial inoculants are being extensively used to improve plant growth under controlled condition, the results inferred from these studies often do not attain a reasonable level of efficacy and consistency in natural field conditions that is required for their commercialization on a large scale. This might be due to the soil physicochemical parameters and microbial populations that establish a complex interaction (Keswani et al. 2014; Nadeem et al. 2014).
PGPB have the ability to colonize the roots and promote plant growth directly or through biological control of plant diseases and also involved in abiotic stress tolerance. The major challenges in this area of research lie in the identification of various strains of PGPB and its properties. It is essential to dissect the actual mechanism of PGPB function in their efficacy toward exploitation in sustainable agriculture (Pujar et al. 2017).
Unfortunately, the interaction between associative PGPB and plants can be unstable and temporary. The achieved in vitro results cannot always be expected to be similar to the field conditions. The inconsistency in the performance of PGPB may be because of multiple environmental parameters that may influence their growth and execute their effects on the plant. The major environmental factors consist of soil characteristics, weather conditions, or the composition and activity of the indigenous microflora of the soil. To attain the optimum growth promoting interaction between PGPB and nursery seedlings, it is crucial to discover the mechanisms rhizobacteria putting their effects on the plant and whether the impacts are changed by various environmental factors, such as the activity of other microorganisms. Therefore, the principle challenge is the development of competent strains for the field conditions. Among promising approach is to explore soil microbial diversity for PGPB having a combination of PGP capability and enough adapted to certain drought soil environment (Saharan and Nehra 2011).
14 Future Prospects
Stressful circumstance can impose a negative impact on plant growth and development by causing nutritional and hormonal imbalances. However, the stress-induced detrimental impact on plant growth can be attenuated and/or minimized by the application of free-living microorganisms (Nadeem et al. 2014). In the last 30–40 years, a detailed perception of the way that PGPB increase plants growth is proposed, approaching the extensive application of these organisms more feasible in the near future (Olanrewaju et al. 2017).
However, the more common application of PGPB requires that a number of subjects to be addressed in advance. (A) New and optimized methods for the large-scale cultivation, storage, distribution, formulation, and utilization of stress-protectant bacteria demanding to be developed. (B) Sensible, safe, effective, and constant protocols for their application need to be agreed in all countries by keeping the regulatory obstacles of technology transfer at the minimum possible amount. (C) Broadly based movements of public training noticing the nature of stress-protecting agents (PGPB) must be initiated on safety and obligation of such natural treatments. (D) Following additional fundamental work to acquire a deeper understanding of the biochemistry, genetics, and physiology of these bacteria, it demands to be confirmed that these strains may necessitate some genetic manipulation and the application of such genetically manipulated strains will not make any new threats to humans or the environment. (E) It is expected that diverse crops and varying conditions require the application of bacteria which are either ectophytic or endophytic. It will be essential to define those conditions where either ectophytic or endophytic strains are most proper so that the most efficient mixture of plant and bacteria could be formulated. (F) Regarding that the growth of more than 90% of crop plants is improved by the interaction of plants with mycorrhizae, it is essential to understand the mechanism by which bacteria and mycorrhizae interact in a manner that enhances the plant growth. (G) To the most possible extent, such technology should be developed in the public domain to limit the monopolization of the key know-hows by a few huge companies. Although there is still greatly more basic and applied work to be carried out, use of PGPB has already been effective on a rather small scale, in some countries. If the abovementioned issues are addressed, it is predicted that global agricultural practice can become sustainable and deeply efficient. This paradigm change in agriculture can be a reclaim that assists both the developing and the developed world (Glick 2012; Olanrewaju et al. 2017).
In addition to mentioned issues, for accessing fruitful PGPB in alleviating drought stress effects, the research should focus onto strains which are preferably indigenous from the stress-affected soils that could be applied as bio-inoculants for crops grown under stress conditions (Vurukonda et al. 2016). The future trend needs to be in introducing genetically altered PGPB rather than transgenic plants for propagating plant performance in drought environment, as it is more amenable to transform the bacterial cells instead of complicated higher macroorganisms. Moreover, rather than engineering individual crops, a single, engineered inoculant can be applied for a number of crops, particularly by the implementation of a non-specific genus such as Azospirillum (Kaushal and Wani 2015).
Genetic engineering can be applied to develop PGPB strains that are executable at low formulation doses and under a range of environmental states. It is urgent to develop more operative PGPB strains with longer shelf-lives to achieve sustainable crop production in dry lands (Kaushal and Wani 2015). Applications of bio-nanotechnology could also offer new insights into the development of carrier-based microbial inocula. Application of nano-material may improve the stability of PGPB formulations with regard to desiccation, heat, and UV inactivation (Kaushal and Wani 2015).
Commercial applications of PGPB are under evaluation and have been frequently productive; nevertheless, a more comprehensive perception of the microbial interactions leading to plant growth improvement will impact the success rate of their application in field conditions (Saharan and Nehra 2011). A majority of the mechanisms behind the plant–microbe interactions in the rhizosphere are not completely discovered. Challenges originate mainly in profiling the abundant range of processes existing in microbial communities. The discovery of this signal crosstalk is essential to improve the plant adaptation mechanisms and to enhance the ability of soil strains for stress mitigation in crops (Ngumbi and Kloepper 2016; Vurukonda et al. 2016).
In addition, the mechanism needs to be assessed in phytohormonal regulation (abscisic acid, salicylic acid, jasmonic acid, and gibberellins) during the PGPB interaction with crop host plants enduring abiotic stress, to further evolve strategies for sustainable crop production (Kang et al. 2014b). A very large number of molecular techniques are becoming accessible and being used to describe the molecular bases of the plant–microbiome interactions. In spite of the recent advancement and perspectives emphasized on microbial-facilitated drought resistance in plants, PGPB mechanisms offering drought tolerance to plants are not clearly revealed. However, recent advancement implies that this approach has great potential to provide new awareness for sustainable food production compared to the alternative possible approach (Vurukonda et al. 2016).
With the aid of suitable monitoring systems, the limitations of applying microbial inoculation in fields can be substantially diminished. A spectrum of field trials has to be designed for this purpose, coupled with advanced molecular and biochemical monitoring systems (Timmusk et al. 2013).
Commercial applications need complex prerequisites in the field technology and in the commercial financing and intellectual property of the work. In order to motivate industrial investors, the application technologies of new bacteria are recommended to be patented. However, the academia and research sectors demand to publish the results as their main financial support originates from publications (Timmusk et al. 2013); this should not diminish the trend of the channel to the commercialization of stress-protectant inocula.
It is explicit that the underground resources of the plant rhizosphere could provide advantages associated with global water deficiency and climate change (Timmusk et al. 2013). Rhizospheric bacteria are potential resources for countering such abiotic stresses. Root bacteria perform important functions in retaining soil humidity and water management in arid soils (Daffonchio et al. 2015). Still, challenges must be resolved before the bacterial inoculants could be extensively applied in drought fighting practices. The implementation of the bacterial inoculation technology has multiple of constrictions in the formulation and delivery of the inoculants which should be resolved in case of each bacterial formulation product. As the inoculates are composed of living organisms, there is a specific host range where the growth promotion is more related on the explicit environmental factors such as optimal temperature, moisture, UV radiation, etc. Gram-positive bacteria, in general, are preferred as microbial inoculates as they are amenable to resist the low water activity, irradiation, and chemicals. Additionally, the endospores produced by some groups of them can that can resist under a spectrum of stress conditions in the field, offering higher durable or reproducible protection under the natural conditions. (Timmusk et al. 2013).
Ultimately, integrating assessment of PGPB strains into plant breeding strategies for drought tolerance purposes may aid the agricultural practices adapt to continued warming of the global climate (Ngumbi and Kloepper 2016).
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. JKSUS 26:1–20
Ashraf M (2010) Inducing drought tolerance in plants: recent advances. Biotechnol Adv 28:169–183. https://doi.org/10.1016/j.biotechadv.2009.11.005
Bano Q, Ilyas N, Bano A et al (2013) Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak J Bot 45(S1):13–20
Bashan Y, de Bashan LE (2005) Plant growth-promoting. In: Hillel D (ed) Encyclopedia of soils in the environment. Elsevier, Oxford, pp 103–115
Bisen K, Keswani C, Mishra S, Saxena A, Rakshit A, Singh HB (2015) Unrealized potential of seed biopriming for versatile agriculture. In: Rakshit A, Singh HB, Sen A (eds) Nutrient use efficiency: from basics to advances. Springer, New Delhi, pp 193–206
Bourque FG, Bertrand A, Claessens A (2016) Alleviation of drought stress and metabolic changes in Timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front Plant Sci 7:584. https://doi.org/10.3389/fpls.2016.00584
Bresson J, Varoquaux F, Th B et al (2013) The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol 200:558–569. https://doi.org/10.1111/nph.12383
Collemare J, Lebrun MH (2012) Fungal secondary metabolites: ancient toxins and novel effectors in plant–microbe interactions. In: Martin F, Kamoun S (eds) Effectors in plant–microbe interactions, 1st edn. Wiley, Chichester
Cura JA, Franz DR, Filosofía JE et al (2017) Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms 5:41. https://doi.org/10.3390/microorganisms5030041
Daffonchio D, Hirt H, Berg G (2015) Plant-microbe interactions and water management in arid and saline soils. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-08575-3_27
Delshadi S, Ebrahimi M, Shirmohammadi E (2017) Influence of plant-growth-promoting bacteria on germination, growth and nutrients’ uptake of Onobrychis sativa L. under drought stress. J Plant Interact 12(1):200–208. https://doi.org/10.1080/17429145.2017.1316527
Ebels MA (2015) The use of plant growth promoting bacteria as ‘bio-fertilizers’: crop inoculation to reduce agrochemical devastation. Presented to the Faculty of the Graduate School of The University of Texas at Austin
Egamberdieva D, Davranov K, Wirth S et al (2017a) Impact of soil salinity on the plant-growth – promoting and biological control abilities of root associated bacteria. Saudi J Biol Sci 24:1601–1608
Egamberdieva D, Wirth SJ, Alqarawi AA et al (2017b) Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol 8:2104. https://doi.org/10.3389/fmicb.2017.02104
Fahad S, Hussain S, Bano A et al (2014) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22:4907. https://doi.org/10.1007/s11356-014-3754-2
Farooq M, Wahid A, Kobayashi N et al (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Farrar K, Bryant D, Naomi CS (2014) Understanding and engineering beneficial plant–microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12:1193–1206. https://doi.org/10.1111/pbi.12279
Fraiture C, Molden D, Wichelns D (2010) Investing in water for food, ecosystems, and livelihoods: an overview of the comprehensive assessment of water management in agriculture. Agric Water Manag 97:495–501. https://doi.org/10.1016/j.agwat.2009.08.015
Furlan F, Saatkamp K, Volpiano CG et al (2017) Plant growth-promoting bacteria effect in withstanding drought in wheat cultivars. Revista Scientia Agraria 18(2):104–113
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Hindawi Publishing Corporation, Scientifica 2012, Article ID 963401
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Glick BR (2015) Stress control and ACC deaminase. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-08575-3_27
Glick BR, Cheng Z, Czarny J et al (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339. https://doi.org/10.1007/s10658-007-9162-4
Gopalakrishnan S, Sathya A, Vijayabharathi R (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377. https://doi.org/10.1007/s13205-014-0241-x
Grover M, Ali SZ, Sandhya V et al (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Guardian (2017) World water day: one in four children will live with water scarcity by 2040. https://www.theguardian.com/globaldevelopment/2017/mar/22/world-water-day-one-in-four-children-will-live-with-water-scarcity-by-2040-unicefreport
Gururani MA, Upadhyaya CP, Baskar V et al (2013) Plant growth-promoting Rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 32:245–258. https://doi.org/10.1007/s00344-012-9292-6
Hanin M, Ch E, Ngom M et al (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787. https://doi.org/10.3389/fpls.2016.01787
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11:57–61. https://doi.org/10.1016/j.jssas.2011.09.001
Heidari M, Mousavinik SM, Golpayegani A (2011) Plant growth promoting rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ocimum basilicum L.) under water stress. ARPN J Agric Biol Sci 6(5):6
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768. https://doi.org/10.3389/fpls.2017.01768
International Hydrological Programme. Water Scarcity and Quality. UNESCO. https://en.unesco.org/themes/water-security/hydrology/water-scarcity-and-quality
Jha Y, Subramanian RB, Patel S (2011) Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33:797–802. https://doi.org/10.1007/s11738-010-0604-9
Johnson NC, Wilson GWT, Bowker MA et al (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. PNAS 107(5):2093–2098
Kang BG, Kim WT, Yun HS et al (2010) Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol Rep 4:179–183. https://doi.org/10.1007/s11816-010-0136-1
Kang SM, Khan AL, Waqas M et al (2014a) Plant growth promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9(1):673–682. https://doi.org/10.1080/17429145.2014.894587
Kang SM, Waqas M, Khan AL (2014b) Plant-growth-promoting rhizobacteria: potential candidates for gibberellins production and crop growth promotion. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses, vol 1. Springer, New York. https://doi.org/10.1007/978-1-4614-9466-9_2
Karlidag H, Yildirim E, Turan M et al (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria x ananassa). Hortscience 48(5):563–567
Kasim WA, Osman ME, Omar MN et al (2012) Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul 32:122–130. https://doi.org/10.1007/s00344-012-9283-7
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61(2):201–209
Kaushal M, Wani SP (2015) Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol 66:35. https://doi.org/10.1007/s13213-015-1112-3
Kavamura VN, Santosa SN, JLda S et al (2013) Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol Res 168:183–191
Keswani C, Mishra S, Sarma BK et al (2014) Unravelling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol 98:533–544
Khan AL, Waqas M, Hamayun M et al (2013) Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuum in biomass recovery and osmotic stress mitigation. BMC Microbiol 13:51
Kohler J, Hernandez JA, Caravaca F et al (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol 35:141–151
Ledger T, Rojas S, Timmermann T et al (2016) Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front Microbiol 7:1838. https://doi.org/10.3389/fmicb.2016.01838
Liddycoat SM, Greenberg BM, Wolyn DJ (2009) The effect of plant growth-promoting rhizobacteria on asparagus seedlings and germinating seeds subjected to water stress under greenhouse conditions. Can J Microbiol 55:388–394. https://doi.org/10.1139/W08-144
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol J 29(2):201–208
Liu XM, Zhang H (2015) The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front Plant Sci 6:774. https://doi.org/10.3389/fpls.2015.00774
Liu F, Xing S, Ma H et al (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97:9155–9164. https://doi.org/10.1007/s00253-013-5193-2
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Marulanda A, Barea JM, Azcon R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and Bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124. https://doi.org/10.1007/s00344-009-9079-6
Mayak S, Tirosh T, Glick BR (2004a) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. https://doi.org/10.1016/j.plaphy.2004.05.009
Mayak S, Tirosh T, Glick BR (2004b) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530
Meena KK, Sorty AM, Bitla UM et al (2017) Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci 8:172. https://doi.org/10.3389/fpls.2017.00172
Moreira FS, PBd C, Rd S et al (2016) Functional abilities of cultivable plant growth promoting bacteria associated with wheat (Triticum aestivum L.) crops. Genet Mol Biol 39(1):111–121
Nadeem SM, Ahmad M, Zahir ZA et al (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448
Nadeem SM, Imran M, Naveed M et al (2017) Synergistic use of biochar, compost and plant growth promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J Sci Food Agric 97:5139–5145. https://doi.org/10.1002/jsfa.8393
Nautiyal CS, Srivastava S, Chauhan PS et al (2013) Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 66:1–9
Naveed M, Hussain MB, Zahir ZA (2014) Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul 73:121–131. https://doi.org/10.1007/s10725-013-9874-8
Naylor D, Coleman-Derr D (2017) Drought stress and root-associated bacterial communities. Front Plant Sci 8:2223. https://doi.org/10.3389/fpls.2017.02223
Ngumbi E, Kloepper J (2016) Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol 105:109–125
Niu X, Song L, Xiao Y, Ge W (2018) Drought-tolerant plant growth-promoting Rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front Microbiol 8:2580. https://doi.org/10.3389/fmicb.2017.02580
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33:197. https://doi.org/10.1007/s11274-017-2364-9
Orhan F (2016) Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz J Microbiol 47:621–627
Penrose DM, Glick BR (2011) Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can J Microbiol 47:368–372. https://doi.org/10.1139/cjm-47-4-368
Penuelas J, Rico L, Ogaya R et al (2011) Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol 14:565. https://doi.org/10.1111/j.1438-8677.2011.00532.x
Pujar AM, Handiganoor MG, Hadora R (2017) Influence of plant growth promoting rhizobacteria’s on productivity of crop plants. Adv Res 12(4):1–6., , Article no.air.37479. https://doi.org/10.9734/AIR/2017/37479
Rapparini F, Penuelas J (2014) Mycorrhizal fungi to alleviate drought stress on plant growth. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses, vol 1. Springer, New York. https://doi.org/10.1007/978-1-4614-9466-9_2
Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51:1061–1069. https://doi.org/10.1139/W05-094
Reis SP, Marques DN, Lima AM (2016) Plant molecular adaptations and strategies under drought stress. In: Hossain MA et al (eds) Drought stress tolerance in plants, vol 2. Springer, Cham, pp 91–122. https://doi.org/10.1007/978-3-319-32423-4_4
Rosegrant MW, Ringler C, Zhu T (2009) Water for agriculture: maintaining food security under growing scarcity. Annu Rev Environ Resour 34:205–222
Sadeghi A, Karimi E, Abaszadeh Dahaji P et al (2011) Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J Microbiol Biotechnol 28:1503–1509. https://doi.org/10.1007/s11274-011-0952-7
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 2011:LSMR-21
Sahin U, Ekinci M, Kiziloglu FM et al (2015) Ameliorative effects of plant growth promoting bacteria on water-yield relationships, growth, and nutrient uptake of lettuce plants under different irrigation levels. Hortscience 50(9):1379–1386
Saikia J, Sarma RK, Dhandia R et al (2018) Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci Rep 8:3560. https://doi.org/10.1038/s41598-018-21921-w
Saleem M, Arshad M, Hussain S et al (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648. https://doi.org/10.1007/s10295-007-0240-6
Sandhya V, Ali SKZ, Grover M et al (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26. https://doi.org/10.1007/s00374-009-0401-z
Sandhya V, SkZ A, Grover M et al (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30. https://doi.org/10.1007/s10725-010-9479-4
Saravanakumar D, Kavino M, Raguchander T et al (2011) Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Plant 33:203–209. https://doi.org/10.1007/s11738-010-0539-1
Sgroy V, Cassan F, Masciarelli O et al (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85:371–381. https://doi.org/10.1007/s00253-009-2116-3
Shinozak K, Shinozaki KY (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Singh HB, Sarma BK, Keswani C (eds) (2016) Agriculturally important microorganisms: commercialization and regulatory requirements in Asia. Springer, Singapore
Singh HB, Sarma BK, Keswani C (eds) (2017) Advances in PGPR. CABI, Oxfordshire
Sziderics AH, Rasche F, Trognitz F et al (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53:1195–1202. https://doi.org/10.1139/W07-082
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5(1):51–58
Tapias DR, Galvan AM, Pardo-Díaz S et al (2012) Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl Soil Ecol 61:264–272. https://doi.org/10.1016/j.apsoil.2012.01.006
Tarkka M, Schrey S, Hampp R (2008) Plant associated soil micro-organisms. In: Nautiyal CS, Dion P (eds) Molecular mechanisms of plant and microbe coexistence, Soil biology 15. Springer, Berlin/Heidelberg, p 3. https://doi.org/10.1007/978-3-540-75575-3
Timmusk S, Wagner EGH (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. MPMI 12(11):951–959
Timmusk S, Timmusk K, Behers L (2013) Rhizobacterial plant drought stress tolerance enhancement: towards sustainable water resource management and food security. J Food Secur 1(1):6–9. https://doi.org/10.12691/jfs-1-1-2
Timmusk S, El-Daim IAA, Copolovici L et al (2014) Drought-tolerance of wheat improved by rhizosphere Bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9(5):e96086. https://doi.org/10.1371/journal.pone.0096086
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting Rhizobacteria under salinity condition. Pedosphere 21(2):214–222
Vacheron J, Desbrosses G, Bouffaud ML et al (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:365. https://doi.org/10.3389/fpls.2013.00356
Vandenberghe LPSL, Garcia LMB, Rodrigues C et al (2017) Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol 3(3):629–648. https://doi.org/10.3934/microbiol.2017.3.629
Vardharajula S, Ali SZ, Grover M et al (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6(1):1–14. https://doi.org/10.1080/17429145.2010.535178
Vilchez J, Niehaus K, Dowling DN et al (2018) Protection of pepper plants from drought by Microbacterium SP. 3J1 by modulation of the plants glutamine and ketoglutarate content: a comparative metabolomics approach. Front Microbiol 9:284. https://doi.org/10.3389/fmicb.2018.00284
Vurukonda SSKP, Vardharajula S, Shrivastava M et al (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24
Wang CJ, Yang W, Wang C et al (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One 7(12):e52565. https://doi.org/10.1371/journal.pone.0052565
Wang X, Cai X, Xu C et al (2016) Drought-responsive mechanisms in plant leaves revealed by proteomics. Int J Mol Sci 17:1706. https://doi.org/10.3390/ijms17101706
Wang M, Li E, Ch L et al (2017) Functionality of root-associated bacteria along a salt marsh primary succession. Front Microbiol 8:2102. https://doi.org/10.3389/fmicb.2017.02102
Weyens N, Dvd L, Taghavi S et al (2009) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27(10):591. https://doi.org/10.1016/j.tibtech.2009.07.006
Whipps JM, Hand P, Pink D et al (2008) Phyllosphere microbiology with special reference to diversity and plant genotype. J Appl Microbiol 105:1744–1755. https://doi.org/10.1111/j.1365-2672.2008.03906.x
Yang J, Kloepper JW, ChM R (2008) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1. https://doi.org/10.1016/j.tplants.2008.10.004
Yu X (2016) Water scarcity: fact or fiction? Paper presented at the 3rd International Conference on Education, Management and Computing Technology (ICEMCT), Published by Atlantis Press
Zahedi H, Abbasi S (2015) Effect of plant growth promoting rhizobacteria (PGPR) and water stress on phytohormones and polyamines of soybean. Indian J Agric Res 49(5):427–431. https://doi.org/10.18805/ijare.v49i5.5805
Zelicourta A, Al-Yousif M, Hirt H (2013) Rhizosphere microbes as essential partners for plant stress tolerance. Mol Plant 6(2):242–245
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mohammadipanah, F., Zamanzadeh, M. (2019). Bacterial Mechanisms Promoting the Tolerance to Drought Stress in Plants. In: Singh, H., Keswani, C., Reddy, M., Sansinenea, E., García-Estrada, C. (eds) Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms. Springer, Singapore. https://doi.org/10.1007/978-981-13-5862-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-5862-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5861-6
Online ISBN: 978-981-13-5862-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)