Abstract
Hypertension is a common medical problem which affects almost all organs in the body. Among which, the brain is the major target organ for the adverse effects of untreated hypertension followed by the cardiovascular and renal system. It is a major risk factor for stroke and its related morbidity and also a powerful risk factor for cognitive decline, dementia, and Alzheimer’s disease. Perioperative hypertension is a common event in neurosurgical population due to the activation of sympathetic nervous system activity, renin-angiotensin-aldosterone activity, and interaction between the heart and brain which increases the perioperative morbidity and mortality. The presence of pre-existing hypertension is one of the major risk factors for the development of a postoperative hematoma after craniotomy. Congenital variation in the circle of Willis and vertebral artery leading to medullary ischemia is considered to be the cause for the development of essential hypertension. Pulsatile compression of vessels on the brain stem and cranial nerves can cause neurogenic hypertension. In this chapter, classification of hypertension and its causes, pathophysiology, the brain-heart interaction, preoperative evaluation, various causes of perioperative hypertension, and its management are discussed in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hypertension in neurosurgery
- Neurogenic hypertension
- Cerebrovascular remodeling
- Cerebral autoregulation
- Brain-heart interaction
- Preoperative evaluation
- Perioperative hypertension and its management
1 Introduction
Hypertension is a common medical problem which afflicts 25% of the general population [1]. The World Health Organization has predicted that by 2025, one-third of the world’s population will be hypertensive, and this will be responsible for over 7.1 million deaths per year [2]. High blood pressure can arise from a variety of physiological abnormalities which affects the central nervous, cardiovascular, and renal system [3]. Among which the brain is the major target organ for the adverse effects of untreated hypertension [4] followed by the cardiovascular and renal system [1, 3]. Moreover, hypertension is a major risk factor for stroke and its related morbidity and, also, is one of the powerful risk factors for cognitive decline, dementia, and Alzheimer’s disease (AD) [4, 5].
In the neurosurgical population, perioperative hypertension is a common event due to the interaction between the heart and brain [6, 7]. Direct stimulation of certain regions of the brain termed as CNS trigger zones (hypothalamus, brain stem, cervical spinal cord nuclei) by surgical handling or the presence of blood, (ICH, IVH) thrombus (stroke), infection (meningitis, encephalitis, abscess), or inflammation (TBI) can cause profound sympathetic stimulation which can lead to severe hypertension, neurogenic pulmonary edema, arrhythmias, and myocardial and respiratory failure [6, 8]. Furthermore, pre-existing hypertension in neurosurgical patients is one of the major risk factors for postoperative cerebrovascular and the cardiovascular morbidity [9, 10].
2 Classification of Hypertension
According to the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VIII), hypertension is diagnosed if the systolic blood pressure (SBP) is >140 mmHg and the diastolic blood pressure (DBP) is > 90 mmHg on at least two occasions measured 1–2 weeks apart. It is further classified depending on the value of SBP and the DBP, as given in Table 17.1. The recent 2014 JNC VIII report recommends the desired BP target while treating the hypertensive patients with and without diabetes and chronic kidney disease (CKD) [11]. For instance, patients above 60 years, the goal is to target less than 150/90 mmHg, whereas the target should be reduced to 140/90 mmHg while treating less than 60 years old and in patients with diabetes and chronic kidney disease. Additionally, patients with prehypertension are very prone to develop hypertension, but at this stage, medication is not needed to normalize the BP, instead such patients have to adopt lifestyle modification to control the BP within the normal limit. Lifestyle modification includes physical activity, smoking cessation, reduced alcohol consumption, control of blood sugar and the cholesterol, weight reduction, and diet modification (i.e., dried fruits and vegetables, whole grains, low-fat dairy products), and restrict sodium intake to 2.4 g/day.
3 Hypertensive Crisis
Acute elevation of systolic blood pressure (SBP) more than 180 mmHg and diastolic blood pressure (DBP) more than 110 mmHg is defined as hypertensive crisis, which in turn can be classified into hypertensive emergency and hypertensive urgency, with and without evidence of end-organ injury, respectively [12]. Hypertensive emergencies occur in up to 2% of patients with systemic hypertension [13]. Patients with hypertension frequently have coexisting diabetes, atherosclerosis, hypercholesterolemia, and ischemic heart disease and can present with hypertensive crisis which could lead to numerous adverse complications such as postoperative hematoma, cerebral hemorrhage, cardiac failure, cardiac arrhythmia, pulmonary edema, unstable angina, and myocardial ischemia. The incidence of hypertensive crisis is common during neurosurgery due to the stress and sympathetic activity associated with major neurosurgeries. Patients with limited cardiac reserve are more prone to cardiac complications during the neurosurgical procedure, and long-standing untreated hypertension can cause end-organ damage.
4 Classification of Hypertension Based on the Etiology
Hypertension is classified as primary or essential and secondary hypertension. When the cause of the hypertension is unknown, it is termed as primary hypertension which accounts for about 95% of cases of persistently raised BP. However, genetic factors in combination with environmental factors might play a role in the development of primary hypertension. For example, high sodium intake, physical inactivity, chronic high alcohol, tobacco intake, psychological stress, and low potassium and calcium intake are some of the environmental factors responsible for the development of primary hypertension [1]. Recent animal and cadaveric studies have shown that the brain stem hypoperfusion due to natural variations in vertebral arterial system anatomy could be responsible for a significant number of cases of idiopathic or essential hypertension [14,15,16]. Warnert et al. in their retrospective study using magnetic resonance imaging (MRI) showed a high incidence of vertebral artery hypoplasia (53%) and incomplete circle of Willis (64%) in hypertensive subjects as compared to 27% and 36% respectively in normotensive subjects, While treating hypertension, if the target BP cannot be attained with a diuretic-containing triple drug therapy at a maximum dose, it is termed as resistant hypertension. Before labeling as resistant hypertension, one should rule out the patient’s compliance with medication and all the secondary causes of hypertension. Recently studies have shown an association between posterior circulation hypoperfusion due to congenital variation in the circle of Willis and vertebral artery diameters and the development of essential hypertension [15, 16]. Sandell et al. have shown that pulsatile vertebral artery and the cranial nerve compression on the brain stem can cause hypertension which is often termed as neurogenic hypertension [17].
5 Pathophysiology of Neurogenic Hypertension
The rostral ventrolateral medulla (RVLM) located in the brain stem is the center of the neuronal regulation of BP and heart rate. The sympathoexcitatory C1 neurons are located beneath the surface of the brain stem in the RVLM, so stimulation of this area can cause sympathetic activation [17]. On the other hand, a depressor region in caudal medulla can reduce the sympathetic activity by direct inhibition of the RVLM and by stimulation of medullary parasympathetic centre. Various studies have shown that the neurovascular pulsatile compression (NVPC) of the RVLM by the posterior inferior cerebellar artery or the left vertebral artery was responsible for neurogenic hypertension, with gradual normalisation of BP after neurovascular decompression [18]. Furthermore, pulsatile compression of RVLM and C1 neuron can stimulate the SNS and activates angiotensin II (AngII) production causes vasoconstriction and endothelial dysfunction, which inturn causes overexpression of neural factor leading to vascular inflammation and remodeling. Additionally, an increased expression of ET-1 and NADPH oxidase-derived reactive oxygen species (ROS) results in neurogenic hypertension which is explained in detail in the section on cerebrovascular remodeling.
6 Secondary Hypertension
High blood pressure can arise from a variety of physiological abnormalities affecting the central and autonomic nervous systems and cardiovascular, endocrine, neurohumoral, and renal disturbances. Approximately 5% of hypertensive patients have secondary hypertension and its management depends upon the underlying cause [1].
Box 17.1 Key Points
Neurological causes of hypertension
-
Traumatic brain injury, spinal cord injury
-
Intracranial tumors
-
GH secreting pituitary adenoma—acromegaly
-
ACTH secreting pituitary adenoma—Cushing’s diseases
-
Infection—encephalitis, meningitis, cerebral abscess
-
Brainstem lesion
-
Trigeminal neuralgia
-
Intramedullary spinal cord lesion
-
Sinus venous thrombosis
-
Dysautonomia—Guillain-Barre syndrome
7 Hypertension and Cerebrovascular Remodeling
Hypertension induces several adaptive changes in the cerebrovascular system among which the most important adaptive changes are hypertrophic or eutrophic remodeling and vascular stiffening. In hypertrophic remodeling, smooth muscle cells undergo hypertrophy or hyperplasia and grow inward encroaching into the lumen and reduce the luminal diameter of the artery increasing the wall thickness [4]. In eutrophic remodeling, the smooth muscle cells undergo a rearrangement that leads to a reduction of the vessel lumen without changes in total vascular mass or wall thickness [4, 19].Vascular stiffening is a process in where the collagen content increases together with the rigidity of the vessel wall [19].
Many factors are responsible for cerebrovascular remodeling such as sympathetic overactivity, reduced bioavailability of nitric oxide and endothelial dysfunction. Recently, angiotensin II (AngII) has emerged as a key factor in the mechanisms of cerebrovascular remodeling [4, 20]. Although such adaptive responses reduce the stress on the vessel wall and protect the downstream microvessels from the effect of increased intravascular pressure [19, 20], it increases vascular resistance resulting in vascular insufficiency. Therefore, the duration and magnitude of the blood pressure elevation, as well as the size of blood vessels are important determinants of hypertension-induced alterations in the vascular wall. Hypertension also promotes atherosclerosis and lipohyalinosis leading to vascular occlusion. Brain activity-induced increase in CBF (functional hyperemia) is attenuated in patients with chronic hypertension by altering the endothelium-dependent relaxation of cerebral blood vessels [4, 21]. In addition, hypertension induces oxidative stress in cerebral blood vessels which leads to increased production of reactive oxygen species (ROS) [4, 20]. NADPH oxidase is a multiunit enzyme found in abundance in cerebral blood vessels and has emerged as a major source of the ROS mediated cerebrovascular dysfunction and has been implicated in the cerebrovascular dysfunction induced by angiotensin II (AngII). Another pathway through which AngII could induce vascular dysfunction involves the tissue plasminogen activator (tPA), which contributes to functional hyperemia by regulating the coupling between NMDA receptor activity and neuronal NO production. AngII counteracts the biological effect of tPA by upregulating the expression of its endogenous inhibitor plasminogen activator inhibitor-1 (PAI-1) [22] as explained in Fig. 17.1.
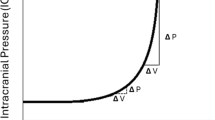
8 Effect of Hypertension on Cerebral Autoregulation
With chronic hypertension, the baroreceptor function is blunted, the autoregulatory range is higher with a rightward shift of the curve, as opposed to normotensives as shown in Fig. 17.2 [23]. Such effects are more pronounced in chronic untreated hypertensive patients, which increases the cerebral oxygen extraction fraction by up to 80%. Therefore, targeting the generally acceptable mean arterial pressure (MAP) of 65 mmHg might result in under perfusion of the brain in such patients. Hypertensives are more prone for ischemia around the periventricular white matter area since it is located at the boundary between two arterial territories such as superficial and the deep arteries. The severity of periventricular white matter injury or leukoaraiosis correlates with the magnitude of autoregulatory dysfunction [24]. The rightward shift of autoregulation indicates that they will tolerate hypertension better as compared to normotensives as presented in Fig. 17.3. However, such impaired autoregulatory response not only leads to more severe ischemia after arterial occlusion but also causes cerebral hyperperfusion during acute severe rise in blood pressure (>180 mmHg) which overwhelms the regulatory capacity disrupting the blood-brain barrier, in turn leading to cerebral edema (hypertensive encephalopathy) and intracerebral hemorrhage (ICH) [19]. Chronic untreated hypertension can cause the development of microaneurysms in small perforating arteries which are <0.9 mm in diameter, and the rupture or leak from this aneurysm could lead to ICH commonly in basal ganglia, thalamus, pons, and cerebellum. Manifestations of hypertension can be observed as end-organ damage in almost every organ system, but more profound effects of untreated hypertension are noted in cerebrovascular system, cardiovascular system, and renal system. In the brain, extraparenchymal arteries and arterioles account for 2/3 of the vascular resistance, while intracerebral arterioles and capillaries account for the remaining 1/3, therefore, vessels residing outside the brain have the greatest impact on parenchymal blood flow. Hypertension potentiates atherosclerosis which in turn potentiates constrictor responses of large cerebral arteries to serotonin and thromboxane contributing to vasospasm and transient ischemic attacks.
Box 17.2 Key Points
Hypertension induced end organ damage
Cardiovascular system
-
Coronary artery disease
-
Left ventricular hypertrophy
-
Diastolic dysfunction
-
Cardiac failure
-
Peripheral vascular disease
-
Atherosclerosis
Neurological
-
Cerebrovascular accident
-
Hypertensive encephalopathy
Renal
-
Glomerulosclerosis
-
Renal tubular ischemia
-
End stage renal disease
Eye
-
Hypertensive retinopathy
9 Pathophysiology of Neurogenic Pulmonary Edema
Neurogenic pulmonary edema (NPE) is a dreadful complication that occurs following various intracranial injuries such as intracranial and subarachnoid hemorrhage, traumatic brain injury, spinal cord injury, and refractory status epilepticus [25,26,27,28,29,30]. This clinical condition usually presents with tachypnea, tachycardia, hypertension, and bilateral basal pulmonary crepitation and even hemoptysis [29, 31]. It is often called as “death rattle” [31] due to its presentation of severe respiratory distress, pulmonary edema with normal jugular venous pressure, and severe hypoxemia [29]. The appearance of bilateral diffuse infiltrates on the chest X-ray within minutes to hours after the CNS injury is the most pathognomonic finding for the diagnosis [29]. The precise mechanism underlying this condition is incompletely understood; however, dissociation of the pulmonary autonomic system from the central vasomotor center and overstimulation of the CNS trigger zone that has been associated with excessive sympathetic discharge are the two commonly proposed mechanisms [32]. Studies have shown that the NPE does not initiate any systemic inflammatory response, as demonstrated by the lack of damage to organs other than lungs, and it is treated with adequate positive end expiratory pressure [32]. The pathophysiology of development of NPE is explained in Fig. 17.2.
10 Brain-Heart Interaction and Hypertension
Modern neuroimaging data, including positron emission tomography and functional magnetic resonance imaging, shows a complex set of neural interactions between the heart and brain, termed the neuro-cardiac axis [7, 33, 34]. The insular cortex, the anterior cingulate cortex, the prefrontal cortex, the amygdala, and the hippocampus are the areas connected to regions involved in autonomic control causing changes in BP and HR.
10.1 Role of Insular Cortex on Hemodynamic Alteration
The insular cortex controls the balance between the parasympathetic and sympathetic tone, and the right insula predominantly regulates sympathetic tone, while the left insula controls parasympathetic tone [35, 36]. During the intraoperative period, stimulation of the rostral posterior insula causes tachycardia, whereas stimulation of the caudal posterior insula causes bradycardia. Also, intraoperative bradycardia or a depressant effect of BP especially diastolic arterial pressure is more frequent with stimulation of the left insular cortex, whereas tachycardia or a pressor effect was elicited while stimulating the right insula. In the setting of cerebrovascular accidents (CVA), involving the left insula can shift the cardiac autonomic balance toward sympathetic predominance, while CVA involving the right insula shifts toward vagal predominance.
10.2 Role of Brainstem on Hemodynamic Changes
It is widely recognized that regulation of cardiac function is dependent on the nucleus of the solitary tract (NST) and the rostral ventrolateral medulla (RVLM) of medulla. The NST receives signals from baroreceptors and vagus nerve. while the RVLM controls the excitatory neurons that are responsible for generation of sympathetic response. Thus stimulation of the RVLM causes sympathetic overactivity, while stimulation of NST can cause parasympathetic overactivity [7, 17].
10.3 Role of Prefrontal Cortex on Hemodynamics
Patients with lesions in the frontal prefrontal cortex or ischemia of the frontal lobe can present with parasympathetic features such as bradycardia and hypotension due to sympathetic activity blockade [7].
10.4 Role of Hippocampus on Hemodynamics
Large hemispheric brain infarcts involving hippocampus insults can result in seizures and are associated with sudden unexpected death due to the intense sympathetic dysfunction resulting in acute MI or heart failure [32].
10.5 Role of Hypothalamus on Hemodynamics
It has been known for a long time that stimulation of the hypothalamus could lead to cardiovascular autonomic disturbances, thus intraoperative stimulation of the lateral hypothalamus produces hypertension and/or rhythm abnrmalities. For instance, stimulation of the anterior hypothalamus produces bradycardia, while stimulation of the posterior hypothalamus results in tachycardia and sympathetic overactivity [7].
11 Effect of Hypertension on the Cardiovascular System
Long-standing hypertension leads to loss of arterial elasticity and compliance in both smaller arterioles and the large conduit arteries resulting in increased myocardial afterload. In order to minimize the wall stress, the cardiac myocytes undergo hypertrophy which leads to left ventricular hypertrophy (Laplace’s law). Such hypertrophy of the cardiac myocytes not only increases the myocardial oxygen demand but also reduces the myocardial compliance resulting in diastolic dysfunction. Furthermore, it also accelerates atherosclerosis which further increases the demand and decreases the supply resulting in subendocardial ischemia and myocardial infarction. A subset of patients with diastolic dysfunction may progress to isolated diastolic heart failure with preserved left ventricular ejection fraction which is often undiagnosed and increases the risk for adverse cardiovascular events during high-risk procedures [37].
12 Hypertension and Alzheimer’s Disease
The Alzheimer’s Disease (AD) is a neurodegenerative condition caused by accumulation of amyloid plaques and neuronal cytoskeletal abnormalities [38]. Studies have shown that mid-life hypertension can promote AD by increasing the production of the amyloid-β peptide. The cerebral autoregulation is impaired by amyloid-β deposition, so hypotension is likely to cause cerebral hypoperfusion, as hypertension can lead to ICH [38].
13 Hypertension in Neurosurgical Emergencies and Its Management
13.1 Acute Ischemic Stroke
Large intracranial vessel occlusion compromises the blood flow to significant portion of the brain, and it is extremely crucial to maintain adequate perfusion to the potentially salvageable penumbric regions surrounding the infarcted tissue [39]. Moreover, the normal protective homeostatic mechanisms are often impaired, and therefore, it is essential to maintain an adequate pressure in the collateral vessels to limit the infarct size. In acute ischemic stroke patients who are eligible for thrombolysis, it is crucial to bring down the BP to 180/105 mmHg before administration of intravenous rtPA and to be maintained it for the first 24 h after rtPA administration to avoid ICH. On the other hand, BP management in patients not undergoing reperfusion strategies remains a challenge, and many patients have spontaneous decline in blood pressure during the first 24 h after onset of stroke. Moreover, the recommendations available in the literature are conflicting and inconclusive. Even in the 2013 ASA/AHA guidelines for the early management of patients with acute ischemic stroke, the benefit of treating arterial hypertension in the setting of acute ischemic stroke is not well established (Class IIb; Level of Evidence C) [40]. However, aggressive reduction of blood pressure is indicated in patients with malignant hypertension associated with other medical emergencies such as MI, aortic dissection, and heart failure to avoid cardiac morbidity and mortality. While in patients, who are not eligible for tPA, the MAP should be reduced only by 15% over a period of 1–2 h while keeping a close watch on neurological status to limit the infarct size.
13.2 Acute Hemorrhagic Stroke
Elevated systolic blood pressure of >140 mmHg is found in almost 75–80% of patients with intracerebral hemorrhage (ICH), as the incidence of secondary hematoma is as high as 30% in the first 24 h which is associated with worse outcomes [39]. At the same time, the associated intracranial hypertension compromises perfusion to normal areas of the brain, and thus while controlling the BP, it is essential to prevent development of cerebral ischemia or re-expansion of cerebral hematoma. According to the recent 2015 ASA/AHA guidelines, in a patient with ICH who presents with the SBP between 150–220 mmHg, acute lowering of SBP to 140 mmHg is considered safe unless there is any other contraindication to acute reduction in SBP (Class I; Level of Evidence A) and can be effective for improving functional outcome (Class Ia; Level of Evidence B), whereas, for ICH with SBP >220 mmHg, it may be reasonable to consider aggressive reduction of BP with a continuous intravenous infusion with frequent BP and neurological monitoring (Class IIb; Level of Evidence C) [41].
13.3 Aneurysmal Subarachnoid Hemorrhage (aSAH)
Subarachnoid hemorrhage (SAH) is a devastating complication associated with hypertensive crisis, often following rupture of an aneurysm which in turn raises ICP and decreases the CPP resulting in medullary ischemia, subsequently resulting in severe sympathetic activation and catecholamine surge. Therefore managing SAH is a challenging task since it is associated with several intracranial and systemic adverse effects which could lead to significant neurological morbidity and mortality (50%). Also patients with a recent aneurysmal SAH are more prone to rebleed (6.9%). So, it is important to optimise cerebral perfusion pressure. maintaining the balance between rebleeding and ischemia. The magnitude of blood pressure control to reduce the risk of rebleeding has not been established. According to 2012 ASA/AHA guidelines for management of a patient with aSAH, a decrease in systolic blood pressure to <160 mmHg is reasonable to prevent rebleeding before securing the aneurysm by clipping or coiling [42].
Cerebral vasospasm and delayed cerebral ischemia (DCI) are the two devastating complications associated with aSAH. Vasospasm occurs due to a combination of cerebral hypoperfusion and autoregulatory dysfunction, which usually peaks between 3 and 14 days following SAH. Also patients with symptomatic vasospasm had been shown to benefit from induced hypertension with use of vasopressors in order to achieve a target increase in blood pressure by 10–15% from the baseline after securing the aneurysm by coiling or clipping. It is reasonable to elevate the systolic blood pressure to 180–220 mmHg while managing the symptomatic vasospasm if there is no contraindication for this acute elevation. (Class II evidence)
13.4 Paroxysmal Sympathetic Hyperactivity (PSH) in Severe Traumatic Brain Injury
The injured brain is at an increased risk of developing secondary damage during episodes of inadequate perfusion, and has been associated with an unfavorable outcome [43, 44]. Also, the injured brain is extremely vulnerable to both global cerebral ischemia and hyperperfusion because of impaired vascular reactivity and autoregulation [45]. So it is very important to maintain an optimal BP to balance the risk between ischemia and hyperperfusion. The recent 2016 Brain Trauma Foundation guidelines for the management of severe traumatic brain injury, could not provide a level I or II recommendation regarding the optimal management of BP [46]. However, it is suggested to maintain the SBP at ≥100 mmHg for patients of 50–69 years old and at ≥110 mmHg for patients 15–49 or over 70 years old to decrease mortality (level III evidence).
Paroxysmal sympathetic hyperactivity (PSH) or central dysautonomia is a clinical condition observed in patients with severe TBI, especially among young males [47, 48]. Episodes of tachycardia, tachypnea, hypertension, and hyperthermia and dystonic postures are common manifestations of this syndrome. Its incidence varies from 7.7 to 33% following TBI, and the available data regarding the management of PSH is limited. The use of alpha-2 agonist clonidine, beta-blockers, bromocriptine, intravenous morphine, midazolam, and intrathecal baclofen all has been tried to treat this condition [47].
14 Perioperative Hypertension
Perioperative hypertension is characterized by an increase in BP by 20% compared to baseline BP. Sympathetic stimulation, activation of the renin-angiotensin-aldosterone pathway, and the metabolic stress associated with cerebral activation, surgical handling of certain areas of the brain (hypothalamus, left insula, brain stem), and cranial nerve manipulation (trigeminal nerve stimulation) are the common causes of perioperative hypertension in neurosurgical patients [1, 9, 49,50,51]. Presence of preoperative hypertension is one of the risk factors for perioperative bradycardia, tachycardia, and hypertension and was found to be the second most common risk factor for surgical morbidity [9, 52]. Marked intraoperative fluctuations in blood pressure are common among hypertensive patients undergoing the neurosurgical procedure under general anesthesia that might result in adverse perioperative outcomes. Longterm hypertension is associated with constricted blood volume, the vasodilation secondary to anesthesia predisposes them to intraoperative hypotension. On the other hand, LVH-induced ventricular diastolic dysfunction can lead to fluid overload and heart failure during the perioperative period [37]. The various causes of perioperative hypertension and its management are listed in Table 17.2.
The most common postoperative complications after the neurosurgical procedure are high blood pressure (25%) and the cardiovascular events (7%). The incidence of acute postoperative hypertension following craniotomy is very high and varies from 54 to 91%, especially after carotid endarterectomy where it is about 9–65% [53]. Postoperative hypertension occurs during the first 20 min of the postoperative period, although their resolution can require up to 3 h, and if left untreated, postoperative hypertension increases the risk of postoperative hematoma and myocardial ischemia [1, 54, 55]. Basali et al., in their retro-spective study, found that the incidence of acute postoperative hypertension is about 57% and also have shown that there is a correlation between postoperative hypertension and the incidence of postoperative hematoma [55]. So, it is extremely important to control the blood pressure for such high-risk cases. The various risk factors for the development of postoperative hematoma are listed in Table 17.3.
15 Emergence Hypertension
Increased sympathetic activity and increased RAA activation during withdrawal of the anesthetics together with the extubation response during coughing and straining on ETT and hypercarbia due to ventilatory insufficiency are the some of the causes for emergence hypertension in neurosurgery. Emergence hypertension may cause intracranial complications such as bleeding and cerebral edema. Postoperative bleeding can be a devastating complication after intracranial surgery, whose incidence varies from 0.9 to 3.5%. Also, emergence hypertension can increase the risk of myocardial ischemia due to an increase in myocardial oxygen demand in patients with high cardiac risk. Studies have shown that there is an association between high systolic BP (>160 mmHg) and intracranial bleeding. So, it is imperative to control the systolic blood pressure between 120 and 150 mmHg during emergence from anesthesia.
16 Perioperative Hypertension in Carotid Endarterectomy and Its Management
The majority of the patients undergoing carotid endarterectomy often have coexisting hypertension. Also, hemodynamic fluctuations are quite frequent during carotid surgeries due to manipulation of the carotid baroreceptors. Thus it is essential to maintain adequate blood flow by keeping the SBP above 180 mmHg during carotid cross-clamping in order to increase the collateral flow. Furthermore, it is extremely crucial to control postoperative hypertension to prevent cerebral hyperperfusion syndrome. Moreover, postoperative carotid sinus dysfunction could manifest as either hyper or hypotension which necessitates close monitoring neurological status with appropriate immediate treatment.
17 Preoperative Evaluation of Hypertensive Patients Coming for the Neurosurgical Procedure
The existing evidence doesn’t show any benefit in postponing an elective surgery if diastolic pressure is <110 mmHg [52, 56, 57]. Similarly, it is not advisable to start a new antihypertensive drug to control BP in the immediate preoperative period, because such treatment could result in marked hemodynamic fluctuations in the intraoperative period. All neurosurgical procedures are considered to be a moderate to high-risk surgeries; therefore it is better to defer the surgery when the DBP is >110 mmHg. It is also recommended that high BP should be reduced slowly over a period of 6–8 weeks to ameliorate the myocardial and cerebrovascular changes related to severe hypertension. The decision to delay surgery for stabilization of BP or to proceed with the surgery after moderate reduction of BP by using antihypertensive drugs depends upon the urgency of the surgical procedure. The recommended target blood pressure in various neurosurgical cases is listed in Table 17.4.
18 Preoperative Evaluation of Hypertensive Patients Coming for Elective Surgery
While evaluating a patient with hypertension, it is essential to know the cause of hypertension (essential or secondary), duration of hypertension, the type of antihypertensive medications, adequacy of blood pressure control, the presence of hypertension-associated end-organ damage, and the presence of coexisting diseases such as diabetes and IHD. The factors that needs to be considered during the preoperative evaluation of neurosurgical patients with hypertension are given in Table 17.6.
19 Preoperative Investigation Needed in Patients with Longstanding Hypertension
Table 17.5 shows the necessary investigation indicated for patients with essential hypertension undergoing neurosurgical procedure, as the extent of the investigations depends on the presence of other comorbidities, end-organ damage, nature and extent of the surgical procedure. The routine preoperative investigation needed for secondary hypertension depends on the cause of secondary hypertension which is beyond the scope of this chapter.
20 Perioperative Oral Antihypertensive Drugs and Its Perioperative Implications
In general, both the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) guidelines for the management of patients with hypertension recommend continuing the routine antihypertensive drugs even on the day of surgery [56, 58] as sudden withdrawal of antihypertensive drugs could result in rebound hypertension during the perioperative period. Moreover, it is reasonable to initiate betablockers prior to surgery in patients with a moderate to high RCRI risk score for major adverse cardiac events, provided it is started well in advance instead of starting immediately before the surgery (Class IIb). On the other hand, the peri-operative management of angiotensin receptor blockers (ARB) and angiotensinogen-converting enzyme inhibitor (ACEI) still remains uncertain, because of the concern of the potentially significant intraoperative hypotension. However, the recent 2017 ACC/AHA task force guidelines on perioperative cardiovascular evaluation suggests to continue them and also to restart in the postoperative period at the earliest possible [58].
21 Management of Perioperative Hypertension Using Parenteral Antihypertensive Drugs
The common causes for intraoperative as well as postoperative hypertension and its treatment are given in Table 17.2. Most of the intraoperative hypertensive episodes are short-lived, and treating these episodes with long-acting antihypertensive drugs can cause an unpredictable drop in BP when the stimulus is ceased and also can lead to wide fluctuation in BP throughout surgery that can increase the perioperative morbidity and mortality. It is better to treat these episodes with short-acting IV anesthetics, analgesics, and antihypertensive drugs, and various patient and surgery related implications should be considered while selecting these drugs. Commonly used parenteral antihypertensive drugs and the recommended doses and their side effects are provided in Table 17.7.
22 Conclusion
Perioperative hypertension is frequently encountered during neurosurgery, and is one of the major risk factors for perioperative cerebrovascular and cardiovascular morbidity. Therefore understanding the various implications of hypertension with proper preoperative evaluation, optimization of antihypertensive agents, meticulous administration of anesthetic and analgesic agents, avoidance of wide fluctuations in BP and strict control of BP during the perioperative period are the essential elements while managing a patient with hypertension.
Key Points
-
Hypertension accelerates atherosclerosis which in turn potentiates the vasoconstrictor responses of large cerebral arteries to serotonin, and thromboxane and lead to several cerebrovascular morbidities, and it is a powerful risk factor for cognitive decline, dementia, and Alzheimer’s disease.
-
Perioperative hypertension and hypertensive crisis are common events in neurosurgical population due to the sympathetic stimulation, activation of the renin-angiotensin-aldosterone pathway and the metabolic stress associated with cerebral activation, surgical handling of certain areas of the brain, and cranial nerve manipulation.
-
It would be prudent to defer the elective surgery when the DBP is >110 mmHg, and the high BP should be controlled gradually over a period of 6–8 weeks to ameliorate the myocardial and cerebrovascular changes related to severe hypertension.
-
In patients in whom the surgery cannot be postponed, it is not advisable to start a new antihypertensive drug to control BP in the immediate preoperative period which can lead to severe hemodynamic instability.
-
Most of the intraoperative hypertensive episodes are short-lived, and treating these episodes with long-acting antihypertensive drugs can cause wide fluctuation in BP throughout the surgery and an undesirable drop in BP when the stimulus is ceased and could add on to morbidity.
-
High blood pressure and adverse cardiovascular events are more common after the neurosurgical procedure. Postoperative hypertension increases the risk of postoperative hematoma which inturn could worsen the postoperative outcome. Therefore, aggressive control of systolic blood pressure between 120 and 150 mmHg during emergence from anesthesia is necessary to reduce the postoperative morbidity.
-
The shift of autoregulation curve to the right in hypertensive patients mandates that the blood pressure should not be dropped more than 15–20% of baseline to maintain the adequate cerebral perfusion pressure.
References
Nadella V, Howell SJ. Hypertension: pathophysiology and perioperative implications. Contin Educ Anaesth Crit Care Pain. 2015;15(6):275–9.
Marik PE, Varon J. Perioperative hypertension: a review of current and emerging therapeutic agents. J Clin Anesth. 2009;21(3):220–9.
Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603.
Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7(6):476–84.
Kelley BJ, Petersen RC. Alzheimer’s Disease and mild cognitive impairment. Neurol Clin. 2018;25(3):577–v.
van der Wall EE. The brain-heart connection: a round trip. Neth Hear J. 2018;19(6):269–70.
Ardell JL, Andresen MC, Armour JA, Billman GE, Chen P-S, Foreman RD, et al. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol. 2016;594(14):3877–909.
Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112(21):3314–9.
Rabelo NN, Filho LJS, dos Passos GS, Junior LAAD, Pereira CU, Dias LAA, et al. Acute arterial hypertension in patients undergoing neurosurgery. Arq Bras Neurocir. 2016;35(4):296–303.
Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265(24):3255–64.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
The fifth report of the Joint National Committee on Detection. Evaluation, and treatment of high blood pressure (JNC V). Arch Intern Med. 1993;153(2):154–83.
Marik PE, Rivera R. Hypertensive emergencies: an update. Curr Opin Crit Care. 2011;17(6):569–80.
Nowicki KW, Jennings R, Sekula RF. Brainstem hypoperfusion as the inciting factor in the development of essential hypertension. Neurosurgery. 2018;82(3):N20–1.
Yavagal DR, Atchaneeyasakul K. Cerebrovascular variants in posterior circulation: a potential cause of essential hypertension. Circ Res. 2016;119(12):1267–9.
Warnert EAH, Rodrigues JCL, Burchell AE, Neumann S, Ratcliffe LEK, Manghat NE, et al. Is high blood pressure self-protection for the brain? Circ Res. 2016;119(12):e140–51.
Sandell T, Holmen J, Eide PK. Hypertension in patients with cranial nerve vascular compression syndromes and comparison with a population-based cohort. J Neurosurg. 2013;119(5):1302–8.
Legrady P, Voros E, Bajcsi D, Fejes I, Barzo P, Abraham G. Observations of changes of blood pressure before and after neurosurgical decompression in hypertensive patients with different types of neurovascular compression of brain stem. Kidney Blood Press Res. 2013;37(4–5):451–7.
Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12(2):89–95.
De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol. 2013;3:484.
Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensive patients is altered by blood pressure treatment. Hypertension. 2008;52(1):65–71.
Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW, et al. Tissue-type plasminogen activator requires a co-receptor to enhance N-Methyl-D-Aspartate receptor function. J Neurochem. 2008;107(4):1091–101.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2(2):161–92.
Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension. 1994;23(5):565–8.
O’Leary R, McKinlay J. Neurogenic pulmonary oedema. Contin Educ Anaesth Crit Care Pain. 2011;11(3):87–92.
Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16(2):212.
Macmillan CSA, Grant IS, Andrews PJD. Pulmonary and cardiac sequelae of subarachnoid haemorrhage: time for active management? Intensive Care Med. 2002;28(8):1012–23.
Bahloul M, Chaari AN, Kallel H, Khabir A, Ayadi A, Charfeddine H, et al. Neurogenic pulmonary edema due to traumatic brain injury: evidence of cardiac dysfunction. Am J Crit Care. 2006;15(5):462–70.
Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 2007;51(4):447–55.
Aneja A, Arora N, Sanjeev R, Semalti K. Neurogenic pulmonary edema following status epilepticus: an unusual case. Int J Clin Pediatr. 2015;4(4):186–8.
Fontes RBV, Aguiar PH, Zanetti MV, Andrade F, Mandel M, Teixeira MJ. Acute neurogenic pulmonary edema: case reports and literature review. J Neurosurg Anesthesiol. 2003;15(2):144–50.
Sedý J, Zicha J, Kunes J, Jendelová P, Syková E. Mechanisms of neurogenic pulmonary edema development. Physiol Res. 2008;57(4):499–506.
Goldstein DS. Sympathetic neuroimaging. Handb Clin Neurol. 2013;117:365–70.
Lizarraga KJ, Gorgulho A, Chen W, De Salles AA. Molecular imaging of movement disorders. World J Radiol. 2016;8(3):226–39.
Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010;4(4):174–82.
Constantinescu V, Detante O, Matei D, Constantinescu I, Arsenescu-Georgescu C, Defaye P, et al. Cortical lateralization and cardiac autonomic control. Insights from insular stroke and epilepsy. Arch Clin Cases. 2017;4(3):154–68.
Pirracchio R, Cholley B, De Hert S, Solal AC, Mebazaa A. Diastolic heart failure in anaesthesia and critical care. Br J Anaesth. 2007;98(6):707–21.
Kelley BJ, Petersen RC. Alzheimer’s disease and mild cognitive impairment. Neurol Clin. 2007;25(3):577–609.. v.
Bösel J. Blood pressure control for acute severe ischemic and hemorrhagic stroke. Curr Opin Crit Care. 2017;23(2):81–6.
Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947.
Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37.
Schubert A. Cardiovascular therapy of neurosurgical patients. Best Pract Res Clin Anaesthesiol. 2007;21(4):483–96.
Prabhakar H, Sandhu K, Bhagat H, Durga P, Chawla R. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. J Anaesthesiol Clin Pharmacol. 2014;30(3):318–27.
Abou El Fadl MH, O’Phelan KH. Management of traumatic brain injury: an update. Neurol Clin. 2017;35(4):641–53.
Brain Trauma Foundation. Guidelines for the management of severe TBI, 4th ed. https://braintrauma.org/guidelines/guidelines-for-the-management-of-severe-tbi-4th-ed#/. Accessed 19 May 2018.
Verma R, Giri P, Rizvi I. Paroxysmal sympathetic hyperactivity in neurological critical care. Indian J Crit Care Med. 2015;19(1):34.
Rabinstein AA. Paroxysmal sympathetic hyperactivity in the neurological intensive care unit. Neurol Res. 2007;29(7):680–2.
Hoxha A, Demneri M, Pilika K, Gjini O, Filipi N, Saraçi M, et al. Postoperative hypertension after craniotomy and catecholamine secretion: A-315. Eur J Anaesthesiol. 2005;22:84.
Olsen KS, Pedersen CB, Madsen JB, Ravn LI, Schifter S. Vasoactive modulators during and after craniotomy: relation to postoperative hypertension. J Neurosurg Anesthesiol. 2002;14(3):171–9.
Chowdhury T, Meuwly C, Sandu N, Cappellani RB, Schaller B. Coronary spasm in neurosurgical patients and role of trigeminocardiac reflex. Neurol Res Int. 2014;2014:974930.
Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–99.
Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93(1):48–54.
Desai VR, Grossman R, Sparrow H. Incidence of intracranial hemorrhage after a cranial operation. Cureus. 2016;8(5):e616.
Seifman MA, Lewis PM, Rosenfeld JV, Hwang PYK. Postoperative intracranial haemorrhage: a review. Neurosurg Rev. 2011;34(4):393–407.
Schonberger RB, Fontes ML, Selzer A. Anesthesia for adult patients with hypertension. UpToDate [Internet]. 2018. https://www.uptodate.com/contents/anesthesia-for-adult-patients-with-hypertension/print. Accessed 18 May 2018.
Howell SJ, Sear JW, Foëx P. Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaesth. 2004;92(4):570–83.
Whelton PK, Carey RM. The 2017 clinical practice guideline for high blood pressure. JAMA. 2017;318(21):2073–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mariappan, R., Arumugam, R. (2019). Co-Existing Hypertension in Neurosurgery. In: Prabhakar, H., Ali, Z. (eds) Textbook of Neuroanesthesia and Neurocritical Care. Springer, Singapore. https://doi.org/10.1007/978-981-13-3387-3_17
Download citation
DOI: https://doi.org/10.1007/978-981-13-3387-3_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3386-6
Online ISBN: 978-981-13-3387-3
eBook Packages: MedicineMedicine (R0)