Abstract
Objective
To review the clinical features, proposed pathophysiology, and the role of medical imaging in the diagnosis and treatment of idiopathic intracranial hypertension and spontaneous intracranial hypotension.
Methods
The authors conducted a narrative review of the current literature on intracranial hypertension and hypotension syndromes, with a focus on imaging findings and role of neurointerventional radiology as a therapeutic option for these pathologies.
Results
Idiopathic intracranial hypertension commonly presents in obese women of childbearing age, being headache and papilledema the main clinical manifestations. Characteristic radiological findings consist of increased cerebrospinal fluid around the optic nerve, partially empty sella turcica and stenosis of the transverse sinuses. Transverse sinus stenting is a treatment alternative that has proven valuable utility in the recent years. Spontaneous intracranial hypotension in most of cases presents with orthostatic headache and has predilection for female population. The typical radiological features in the brain consist of subdural fluid collections, enhancement of the dura, engorgement of the venous structures, pituitary enlargement, and sagging of the brain. In this pathology, a cerebrospinal fluid leak in the spine associated with a defect in the dura, meningeal diverticulum, or a cerebrospinal fluid-venous leak must be actively ruled out.
Conclusions
Neurologic complaints secondary to changes in intracranial pressure exhibit certain clinical features that in combination with fairly specific radiological patterns allow a highly accurate diagnosis. The diverse specialists in neurosciences should be aware of the multiple image modalities in the study of these syndromes as well as the treatment alternatives by neurointerventional radiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Idiopathic intracranial hypertension (IIH) and spontaneous intracranial hypotension (SIH) syndromes are recognized causes of neurological complaints, mainly related to disabling headache, which may be untreatable in severe presentations under conventional medical management.
Despite the pathophysiology of both syndromes is not completely known to the date and several causal theories have been proposed, there are some facts noticeably associated with IIH, such as a female gender predisposition and a higher prevalence among obese population [1]. In the context of suspected SIH, it is recognized that a leakage of cerebrospinal fluid (CSF) (fistula or meningocele) is a potential source of decreased CSF volume, frequently affecting the cervical or thoracic spine [1].
Delayed diagnosis or misdiagnosis of these diseases may affect the clinical outcome, leading to inappropriate therapeutic decisions. The different neuroscience clinicians and subspecialist involved in headache treatment should be familiar with the diverse imaging modalities to guide diagnosis, monitor evolution, and establish adequate treatment.
This review describes the pathophysiology, clinical characteristics, radiological findings, and role of neurointerventional radiology in the treatment of IIH and SIH focused in adult patients.
Methods
A search for articles addressing IIH and SIH was conducted on PubMed, focused on the clinical presentation, imaging assessment, imaging findings, and radiology-guided procedures on adult population. Google Scholar and UpToDate were used as additional sources of information to identify highly cited papers on this field. Free-text terms and Mesh terms were used, including among others the following terms: idiopathic intracranial hypertension, spontaneous intracranial hypotension, MRI with venography, transverse sinus stenting, epidural blood patching. The search did not have a start date limit and covered publications until August 2021. Only English language and peer-review publications were included. All type of studies relevant to the review were considered.
Idiopathic intracranial hypertension
Epidemiology and pathophysiology
Also known as pseudotumor cerebri over the past years, IIH is an entity defined by clinical criteria based on signs and symptoms of elevated intracranial pressure of unknown cause, in the absence of structural abnormalities in the brain parenchyma, meninges, or along the venous structures, also characterized by a normal CSF composition [1].
The estimated annual incidence of IIH is 1–2 per 100,000 general population [2]. Obese women between 15 and 44 years have shown a higher incidence, reaching up to 21 per 100,000 [3]. With the obesity epidemic in the USA and other countries, the incidence and prevalence of IIH have raised during the last decades [4, 5].
Although IIH is by definition idiopathic, a number of medications and exogenous substances have been linked to it, for instance, growth hormone [6], tetracyclines [7], and retinoids [8].
Many mechanisms have been proposed as the cause of IIH over the years, but its pathophysiology remains not completely probed. Currently, the most evaluated theory is in keeping with an elevated intracranial venous pressure [1, 9]. This hypothesis is supported on the idea that patients with elevated intracranial pressure secondary to cerebral venous thrombosis or other abnormalities in the vein outflow may present similar clinical manifestations to those with IIH. Indeed, transverse sinus stenosis is a common finding in IIH, with current controversy if this is a contributing factor or a consequence of raised ICP. It has been reported the restoring of the dural venous sinus caliber after CSF removal or derivation, which may indicate that venous sinus stenosis could be a consequence rather than a cause of IIH. Regarding this topic, it is important to mention that the evidence originates from uncontrolled cases-based series [10, 11].
A recent review emphasizes the role of altered glucocorticoid metabolism in the pathophysiology of IIH [10]. The 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is an intracellular enzyme that converts inactive cortisone into the active cortisol; it is expressed in the choroid plexus and aids in the regulation of CSF production. An in vitro analysis and prospective in vivo cohort study revealed a significant correlation between the decrease in the 11β-HSD1 global activity and reduction of the intracranial pressure [12]. Moreover, the IIH drug trial was a recent randomized controlled trial comparing the selective 11β-HSD1 inhibitor AZD4017 versus placebo, demonstrating significant decrease in lumbar puncture opening pressures in the AZD4017 group compared to the placebo group [13]. The authors therefore have proposed this drug as a potential therapeutic option for IIH.
Alternative theories have also assessed excess of systemic and CSF androgens in IIH, such as testosterone and androstenedione, which can modulate CSF secretion via the choroid plexus. These metabolites were found elevated in IIH patients, and this hormonal cascade can be a potential therapeutic target in the future [14].
Additional theories reported in the literature include increased CSF outflow resistance and increased intraabdominal and intracranial venous pressure related to obesity [15].
Clinical setting
Headache is the most common manifestation of IIH, present in approximately 90% of the patients[1]. Headache attributed to idiopathic intracranial hypertension lacks specific features and depending on the complexity of the presentation can resemble the phenotype of a primary disorder, especially migraine or tension type headache. Nonetheless, papilledema is considered the hallmark of the disease and should be documented to make the diagnosis of IIH [1]. The risk of visual disability and the related visual disturbances makes necessary concurrent ophthalmologic workup for these patients. Additional clinical manifestations of IIH include diplopia secondary to unilateral or bilateral sixth nerve palsy, pulsatile tinnitus, and sporadically neck or back pain [16].
Neuroimaging studies have two main purposes in IIH: firstly, to exclude structural causes of intracranial hypertension and secondly, to identify typical radiologic patterns of IIH. MRI with venography (MRV) would be the non-invasive diagnostic method of choice, particularly in order to rule out a subjacent venous sinus stenosis or thrombosis. Optionally, a computed tomography (CT) study is still considered an alternative for patients with contraindications for MRI. A CT without contrast may detect cases of significant distension of the optic nerve sheaths as well as a partially empty sella turcica. Furthermore, a CT venogram is also an alternative to an MRV when it is contraindicated or not available in a reasonable time frame.
To confirm an elevated intracranial pressure, a lumbar puncture (LP) has to be performed, only after an intracranial mass has been excluded by neuroimages. The recommended technique is with the patient relaxed and lying in the lateral decubitus position with legs extended. It is considered in adult patients pressures greater than 250 mmH2O as abnormal. In the range from 200 to 250 mmH2O, the result has to be read as equivocal [17]. In the latter scenario, the presence or absence of magnetic resonance imaging (MRI) findings compatible with IIH would support the definite diagnosis.
The most widely accepted diagnostic criteria for IIH are the modified Dandy criteria [1] as shown in Table 1.
An entity known as idiopathic intracranial hypertension without papilledema (IIHWOP) includes a subgroup of patients with chronic primary headaches who present increased ICP but no papilledema. Furthermore, these patients may have transverse venous sinus stenosis, which supports the theory of a continuum between typical IIH and IIHWOP. On the other hand, IIHWOP is considered a rare pathology and its diagnosis has to be made with specific diagnostic criteria [17].
Imaging findings
MRV has been postulated as a routine examination in patients with IIH, as it can depict transverse sinus stenosis and can rule out intracranial hypertension secondary to venous sinus thrombosis. MRV without contrast medium, with techniques such as phase contrast, provides appropriate diagnostic accuracy in the assessment of both IIH and venous sinus thrombosis. In cases of doubt, MRV with contrast medium has demonstrated greater diagnostic accuracy [18].
The brain parenchyma and the ventricular system exhibit normal appearance in patients with IIH. A partially empty sella has been described in about 85% of the cases (Fig. 1) [19]. This finding probably reflects a long-standing increased intracranial pressure leading to protrusion of CSF through the diaphragma sellae and subsequent flattening of the pituitary gland [20].
Partially empty sella in IIH. A Sagittal FLAIR image shows widening of the CSF space within the sella turcica and flattening of the pituitary gland (arrow), in a 43-year-old female patient presenting with headache, right 6th and left 3rd cranial nerves palsies. B Corresponding axial T2-weighted sequence shows enlarged CSF space within the sella (asterisk)
Secondary changes can also be found at the level of the eye globes and optic nerves sheaths (Fig. 2), where there is characteristically dilation and increased amount of CSF within the optic nerve sheath, with an incidence up to 95% of IIH cases [20]. Flattening of the posterior contour of the globes is an associated feature as well. Protrusion of the optic nerve heads into the posterior chamber is seen in cases of marked papilledema, clinically referred as reduced visual acuity [21]. Some studies have also documented that there may be vertical or horizontal tortuosity of the intraorbital segments of the optic nerves [22].
Enlargement of other CFS spaces can also occur in IIH, such as enlargement of the Meckel’s cave (Fig. 3), foramen oval and in uncommon presentations, as meningoceles with reported incidence from 9 to 11% [20].
Several observational studies have described the incidence of transverse sinus stenosis in IIH, typically at the junction with the sigmoid sinuses and in some cases also with concomitant sigmoid sinus stenosis, with estimated incidence of 65 to 90% [11, 18] (Fig. 4). Current debate exists regarding dural sinuses stenosis as the cause of this syndrome or if corresponds to the result of persistent elevation of intracranial pressure. A proportion of the literature supports the theory that these changes reflect a consequence rather than a cause of IIH, mainly because when intracranial pressure levels are improved, the caliber of the transverse sinuses also increases spontaneously [23].
Treatment
The treatment of IIH has two main objectives: preserving visual function and improving the symptoms related to intracranial hypertension, especially headache [20].
The first line of treatment consists of measures focused on weight loss, at a rate of approximately 1 pound per week until a 5–10% weight reduction is achieved. In addition to this, sodium restriction in the diet must also be recommended.
Many patients show improvement for an interval of time even after the first diagnostic lumbar puncture, due to decrease in the intracranial pressure.
Some authors recommend pharmacological treatment associated with the aforementioned measures, specifically medication that reduces intracranial pressure. The most widely used drug, which has been evaluated in most clinical trials, is acetazolamide (Diamox), a carbon anhydrase inhibitor that reduces CSF production [24]. Given the side effects of acetazolamide, an alternative to this drug is topiramate [25].
Corticosteroids can be used in a short cycle to rapidly decrease intracranial pressure in those patients with severe symptoms. Long-term use should be avoided [26].
In cases where the therapeutics objectives have not been achieved with pharmacological therapy, surgical alternatives can be used, such as ventricular or lumbar peritoneal shunts, preferably in patients whose main complaint is headache rather than compromised vision. Fenestration of the optic nerve sheath is indicated especially in cases where there is a progressive deterioration of vision despite other conservative measurements. This surgery can also be performed as an emergency measurement in patients with severe vision loss [1].
A recent systematic review evaluating the efficacy of bariatric surgery in the treatment of obese patients with concomitant IIH demonstrated the considerable improvement of the symptoms postoperatively, including headaches, visual symptoms, and papilledema [27]. Bariatric surgery can be considered a therapeutic alternative in patients for whom optimal weight loss is not achieved with conservative management. However, it should be highlighted the limitation of the aforementioned review, which mainly assessed case reports and case series.
Endovascular venous sinus stenting
Venous sinus stenting is a relatively recent alternative in the treatment of IIH, first reported in one patient by Higgings in 2002 [28]. As sinus stenoses have been documented as a frequent finding in patients with IIH, it is presumed that placing a stent will improve venous outflow and thus the reabsorption of CSF (Fig. 5), ultimately lowering intracranial pressure. The existing evidence to the date mainly is based on case series, with the lack of randomized controlled studies evaluating the clinical outcome of this intervention.
Transverse sinus stenting. A 44-year-old male presenting headaches and visual changes, opening pressure of 38 cm/H2O. A Images of conventional angiogram in venous phase show super-selective catheterization of the superior sagittal and right transverse sinuses, noting stenosis at the transverse–sigmoid sinuses junction (arrow). B Images post stenting with marked improvement of the transverse sinus caliber (arrow). C Skull x-rays show radiopaque marks of the stent in correct position (arrow). D CT angiogram follow-up six months post procedure shows patency of the stent, with near complete resolution of the symptoms referred by the patient
The indication for the procedure includes patients who persist with disabling symptoms despite pharmacological management or treatment with CSF shunting or optic nerve sheath fenestration [29].
In general, the studies published on this technique show improvement in the severity of the headache referred by the patients. Information regarding visual impairment has not been consistently described and data on follow-up of patients with visual complaints are limited.
Spontaneous intracranial hypotenison
Epidemiology and pathophysiology
A few decades ago, SIH was also categorized under the term of spontaneous (or idiopathic) low CSF pressure headache or CSF hypovolemia. The pathophysiology of SIH has not been fully identified to the date; however, recent literature supports the concept of decreased intracranial and spinal CSF volume that leads to a decrease in CSF pressure in most patients [30], quantifiable through the opening pressure of a lumbar puncture. It is relevant to convey that a normal opening pressure does not exclude SIH; moreover, several publications have documented normal spinal opening pressure in a significant proportion of patients with diagnosis of SIH [31,32,33], ranging between 43.7 and 90% in the assessed population. Lumbar puncture opening pressure was low enough to meet diagnostic criteria (≤ 60 mmH2O) in only 34% of patients in one study [34]. Most patients had an opening pressure in the low normal to normal range, and 5% had an opening pressure of 200 mmH2O or more. The position of the legs during a lumbar puncture has small impact (approximately 10 mmH2O) on the opening pressure, although the most accurate measurement is produced with the patient relaxed and legs extended [1].
SIH can occur in any group of age, with a peak incidence around the 40 years, affecting women 2:1 compared to men and an annual incidence of 5 per 100,000 people has been estimated [35], being more frequent than IIH.
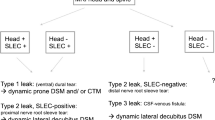
This entity has been related to spontaneous leakages of CSF, typically in the thoracic spine or the cervicothoracic junction. Schievink et al. [36] have classified CSF leaks into four groups based on their underlying cause and the presence of an extradural CFS collection:
-
(1)
Type 1: Leaks secondary to dural tears. More than 90% occur in the ventral region and are associated with bone pathology of the spine, especially with osseous spurs. Most of cases will be associated with a spinal CSF collection.
-
(2)
Type 2: Leaks secondary to meningeal diverticula. It was the most common cause in this study, representing 42% of cases.
-
(3)
Type 3: Direct CSF-venous fistula, secondary to an abnormal communication between the subarachnoid space and an epidural vein. A CSF collection will not be found throughout the spine.
-
(4)
Type 4: No evidence of any of the causes mentioned above. This group consists of patients with clinical and/or brain imaging characteristics of SIH, however without a demonstrable leak.
Clinical setting
The most common symptom in patients with SIH is headache [1], classically described as orthostatic. In most cases, the headache occurs suddenly, increasing in minutes or hours [37]; and the Valsalva maneuver is a well-known headache trigger. It has been described in the literature changes in the headache features over time, with a number of patients evolving to chronic daily headaches without modification during postural changes [1].
Regarding the pathophysiology of headache, it is accepted that the decrease in CSF volume leads to sagging of the brain and consequent traction of pain-sensitive intracranial and meningeal structures [38, 39]. The involvement of the cranial nerves is also explained by the fact that there is traction of them [38, 39].
Other common symptoms are neck pain or stiffness, nausea, vomiting, and complaints related to vestibular-cochlear involvement, such as tinnitus, vertigo, and hypoacusis. Exceptional cases of decreased consciousness or coma have been reported when severe brain sagging occurs [40,41,42].
The most widely known diagnostic criteria for SIH-related headache are that established by the International Classification of Headache Disorders [43], 3 edition (ICHD-3), described in the Table 2. These criteria have high specificity for SIH-related headache; nonetheless, the diverse clinical and radiological manifestations of SIH may limit the diagnostic accuracy.
Role of medical images and technical considerations
Brain images
Brain CT
This is the initial study in a significant proportion of cases.
Brain MRI
This is the ideal modality to assess the intracranial features. The use of paramagnetic contrast medium allows the evaluation of changes secondary to intracranial hypotension with high sensitivity. It is important to note that a brain MRI will be normal in up to 20% of patients with SIH [35].
Spine images
Spinal images are always recommended in conjunction with brain imaging in the study of SIH, in order to determine if an extradural CSF collection is present and to identify the exact level of a CSF leak.
Computed tomography myelography
CTM is the study of choice to identify the CSF leakage site [44, 45]. Among its advantages, we find the high spatial resolution, high contrast resolution between leaked contrast medium and the adjacent tissues, and an excellent assessment of osseous spurs or calcified discs.
Among the technical aspects of this study, special care should be taken in the following points:
-
Adequate concentration of contrast medium within the CSF
-
Early and late images should be obtained of all segments of the spine, as there may be rapid or slow leaks
-
Tailored cuts should be made at the suspected leaking leve
-
Minimize artifacts from breathing movements during the exam
MRI spine/MRI myelography
The use of heavy T2 sequences with fat suppression can increase the sensitivity to detect CSF leaking into the extradural space. Nevertheless, this technique has a relatively decreased sensitivity on the evaluation of associated bone pathology compared to a CT myelography [46]. CSF-Venous fistulae are not visible by this modality.
Several studies have evaluated the utility of MRI myelography for the location of CSF leakage, using a volumetric technique weighted in T2, and showing this technique as a potential alternative to CT myelography [47, 48]. Publications on intrathecal administration of gadolinium as an “off-label” use for the detection of the site of leakage have shown overall diagnostic accuracy between 64 and 100% [49]. Intrathecal gadolinium MRI myelography is reserved for highly selected patients, in whom the site of CSF leak is not demonstrated by other modalities [50,51,52].
Dynamic myelography/digital subtraction myelography
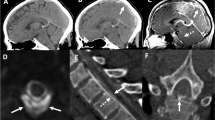
Studies with fluoroscopic vision with or without digital subtraction are an excellent alternative to identify rapid CSF leaks [53,54,55]. One substantial limitation of other diagnostic modalities on the assessment of rapid CSF leaks is the fact that extensive extravasation of contrast medium is present when the images are obtained, reducing the conspicuity of the site of leak. Studies under fluoroscopy have a better temporal resolution and additionally allow the acquisition in different positions, making possible the exposure of lateral or dorsal dural tears [46, 56].
It is important to point out that this modality is the best suited to identify CSF-venous fistulae [46]. Additionally, lateral decubitus during digital subtraction myelography can demonstrate CSF-venous fistulae more frequently (74%) than in prone position (15%) [57].
Dynamic CT myelography
This modality is an option to identify active CSF leaks into the epidural space with better temporal resolution when compared to conventional CT myelography [46].
Radioisotope cysternography (RIC)
RIC has been an imaging modality used for decades to evaluate CSF leaks. Given the superior anatomical resolution of modalities such as MRI and CT, nowadays, RIC is not the first test performed in the SIH study. Among its current uses, some studies have reported a higher sensitivity to reveal intermittent CSF leaks compared to MRI and CT myelography [58,59,60].
The disadvantages of RIC consist primarily in its poor anatomical resolution, which makes it difficult to accurately locate the level of the leak in the spine, as well as the inability to detect the cause of the leak. These limitations are relevant to make treatment decisions.
Imaging findings
Brain findings
The decrease in CSF volume and subsequent decrease in intracranial pressure lead to diverse radiological signs secondary to compensation of such hypotension. Nevertheless, it should be taken into account that 10 to 20% of patients with SIH will have a normal cerebral MRI [30, 61, 62].
The most frequent radiological finding is pachymeningeal enhancement, described to be present in approximately 73% of cases [46, 62, 63] (Fig. 6). It has been proposed that this pachymeningeal enhancement is secondary to dilatation of dural vascular structures in compensation to the decreased CSF volume. Typically, this enhancement is smooth, diffuse, and supra- and infratentorial in extension. In SIH, there is no leptomeningeal enhancement, which is an important feature to differentiate SIH from other conditions.
The venous distension sign has been reported in the literature, consisting of an enlargement of the dominant transverse sinus at its midpoint (Fig. 7), with convex contours, different from the normal triangular or arrowhead configuration. The sensitivity and specificity of this sign are approximately 94% and it is best seen on T1-weighted sagittal imaging of the transverse sinus [64].
As intracranial pressure decreases, the appearance of subdural collections may occur, as a result of fluid transudation from the vascular structures into the subdural space, found in 35% of SIH cases [63]. These collections tend to be bilateral and have density and signal similar to CSF on CT and MRI images respectively (Fig. 8). In certain cases, haemorrhagic content may be visualized, which has been attributed to the rupture of subdural bridging veins [39].
A CT head without contrast shows bilateral slightly hyperdense collections in the frontoparietal convexity bilaterally (asterisk) in a 47-year-old male patient presenting headaches, worse when leaning down. B Axial and C coronal T2-weighted images show mixed fluid-hemorrhagic subdural collections along the cerebral convexity bilaterally (arrows) in the context of SIH
Decreased intracranial CSF volume can lead to brain sagging, present in around 60% of cases (Fig. 9) [46], and both qualitative signs and quantitative measures can be helpful and important in radiologically confirming its presence. Signs that will be found especially on MRI are as follows: effacement of the basal cisterns associated with decreased vertical distance between the mammillary bodies and the pons (mamillopontine distance), descent of the optic chiasm and floor of the third ventricle, flattening of the ventral aspect of the pons, which contacts the clivus. Ectopia of the cerebellar tonsils is commonly found as well. A reduction of optic-nerve sheath subarachnoid space, evident on coronal T2-weighted MRI scan, can be also observed and restored with treatment [65].
Three different patients with several degrees of intracranial hypotension. A Sagittal T1 with contrast image shows engorged and hyperenhancing pituitary gland (arrow). B Sagittal T1 image at the cerebral midline exhibits partially effaced basal cisterns (asterisks) and reduced mamilopontine distance (line). C Low-lying optic chiasm (arrow), inferior displacement of the third ventricle floor (star) and flattening of the ventral aspect of the pons (asterisk) secondary to SIH
An enlarged and hyperenhancing pituitary gland is other distinctive feature of SIH, present in an average of 38% of cases [63], reflecting hypophyseal vascular congestion (Fig. 9A). Thus, the acronym SEEPS synthetizes the cerebral hallmarks of SIH, standing for the following: subdural fluid collections, enhancement of the dura, engorgement of the venous structures, pituitary enlargement, and sagging of the brain.
A nine-point predictive scoring system based on the six most discriminating imaging features of SIH was developed and validated by Dobrocky et al. [66]. The score is based on three major findings (2 points each): pachymeningeal enhancement, engorgement of venous sinus, and effacement of the suprasellar cistern. Three signs are considered minor (1 point each): subdural fluid collection, effacement of the prepontine cistern, and mamillopontine distance of 6.5 mm or less. The score identifies a patient with a high (score > / = 5), intermediate (score 3 to 4), or low (score 2 or lower) probability of having a spinal CSF leak and in whom further invasive myelographic examinations are warranted.
Spine findings
Spinal imaging is performed primarily for two purposes: the first is to identify the presence of fluid collections, identified in 48 to 67% of patients [63], most often at the cervicothoracic junction or in the thoracic spine. The second objective is to determine the exact site of leakage. In cases, of dural-venous fistulae, no CSF collections will be found.
On both CT and MRI, extradural collections, either in the epidural space or paraspinal structures, may be large in extent indicating high-flow or fast leaks (Fig. 10). Digital substraction myelography is an excellent option when a fast CSF leak is suspected (Fig. 11). Small collections, extending no more than one vertebral body, are called low-flow or slow leaks, for which delayed image acquisitions are recommended for their detection.
On contrast-enhanced CT and MR myelography, extension of the contrast medium outside the thecal sac will be found as direct sign of leak (Fig. 12). Some indirect signs may be present also in spinal studies such as dilated epidural venous plexuses and dural enhancement [67].
Treatment
Therapeutic options for SIH depend on the severity and time course of symptoms. Patients with mild symptoms and in the early phase of the disease are usually treated with conservative measurements, such as bed rest, analgesics, oral hydration, and generous administration of caffeine, in order to increase intracranial pressure [68].
The second line of treatment consists of epidural blood patching (EBP). This is indicated on patients with more severe and disabling symptoms, symptoms lasting more than 2 weeks and in whom initial conservative measures have failed [69].
At present, there is lack of recommendations based on randomized clinical trials regarding the regimen and location of EBPs [69]. Retrospective descriptive studies have shown an improvement rate after the first EBP in approximately 30–50% of cases. The need of repeat attempts, up to four or six EBPs, has been reported to achieve improvement in some patients [63, 70]. Based on clinical experience, several authors recommend starting with 10–20 cc of autologous blood targeting the suspected site of leakage. In cases of unknown leak site, patches can be started at the thoracolumbar junction and then placed in the lower lumbar region.
Anecdotal evidence suggests that fibrin patches can be used as an alternative to EBPs, as an option before attempting surgical repair of a dural tear [71].
Microsurgical repair of the dural leak site is reserved for patients in whom non-surgical measures have failed as well as in whom the exact level of the dural tear or nerve root diverticulum has been accurately identified. Additionally, surgical treatment appears at this time as the first option in the management of CFS-venous fistulae [71].
Conclusions
The IIH and SIH syndromes are recognized entities that may lead to significant neurological complaints, especially manifesting as headache, visual disturbances, and altered cranial nerves function. IIH will commonly affect obese women of childbearing age. The distinctive radiological pattern includes increased CSF in the optic nerve sheaths, partially empty sella turcica, and transverse sinus stenosis.
On the other hand, SIH may affect a wider age group and has shown a relatively increased predilection for female patients as well. The underlying abnormality in SIH cases is related to the presence of a dural tear, meningeal diverticulum, or a CSF-venous fistula. Typical radiological manifestations of endocranial hypotension consist of subdural fluid collections, enhancement of the dura, engorgement of the venous structures, pituitary enlargement, and sagging of the brain (summarized in the mnemonic SEEPS).
Interventional radiology has an active role in the treatment of both entities, in transverse sinus stenting for IIH cases and performance of targeted EBP in the SIH management.
Abbreviations
- IIH:
-
Idiopathic intracranial hypertension
- SIH:
-
Spontaneous intracranial hypotension
- CSF:
-
Cerebrospinal fluid
- IIHWOP:
-
Idiopathic intracranial hypertension without papilledema
- LP:
-
Lumbar puncture
- MRI:
-
Magnetic resonance imaging
- MRV:
-
MRI with venography
- CT:
-
Computed tomography
- CTM:
-
Computed tomography myelography
- RIC:
-
Radioisotope cysternography
References
Ducros A, Biousse V. Headache arising from idiopathic changes in CSF pressure. Vol. 14, The Lancet Neurology. Lancet Publishing Group; 2015. p. 655–68.
Radhakrishnan K, Kurland LT, O’fallon WM, Ahlskog JE, Cross SA. Idiopathic intracranial hypertension (pseudotumor cerebri): descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol. 1993 Jan 1;50(1):78–80.
Kesler A, Gadoth N. Epidemiology of idiopathic intracranial hypertension in Israel. J Neuro-Ophthalmology [Internet]. 2001 Mar [cited 2020 May 12];21(1):12–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11315973
Kilgore KP, Lee MS, Leavitt JA, Mokri B, Hodge DO, Frank RD, et al. Re-evaluating the incidence of idiopathic intracranial hypertension in an era of increasing obesity. In: Ophthalmology [Internet]. Elsevier Inc.; 2017 [cited 2020 May 12]. p. 697–700. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28187976
Curry WT, Butler WE, Barker FG (2005) Rapidly rising incidence of cerebrospinal fluid shunting procedures for idiopathic intracranial hypertension in the United States, 1988–2002. Neurosurgery 57(1):97–107
Reeves GD, Doyle DA (2002) Growth hormone treatment and pseudotumor cerebri: Coincidence or close relationship? J Pediatr Endocrinol Metab 15(SUPPL. 2):723–730
Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand [Internet]. 2004 Dec [cited 2020 May 14];110(6):408–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15527455
Friedman DJ. Medication-induced intracranial hypertension in dermatology. Vol. 6, American Journal of Clinical Dermatology. 2005. p. 29–37.
Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. Vol. 83, Journal of Neurology, Neurosurgery and Psychiatry. BMJ Publishing Group; 2012. p. 488–94.
Virdee J, Larcombe S, Vijay V, Sinclair AJ, Dayan M, Mollan SP. Reviewing the recent developments in idiopathic intracranial hypertension. Ophthalmol Ther [Internet]. 2020 Dec 1 [cited 2022 Sep 5];9(4):767. Available from: /pmc/articles/PMC7708542/
Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G et al (2003) Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 60(9):1418–1424
Sinclair AJ, Walker EA, Burdon MA, Van Beek AP, Kema IP, Hughes BA, et al. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: a link between 11-HSD1 and intracranial pressure regulation? jcem.endojournals.org J Clin Endocrinol Metab [Internet]. 2010;(12):95. Available from: http://apps.who.int/bmi/index.jsp
Markey KA, Ottridge R, Mitchell JL, Rick C, Woolley R, Ives N, et al. Assessing the efficacy and safety of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor (AZD4017) in the idiopathic intracranial hypertension drug trial, IIH:DT: clinical methods and design for a phase ii randomized controlled trial. JMIR Res Protoc [Internet]. 2017 Sep 1 [cited 2022 Sep 18];6(9). Available from: /pmc/articles/PMC5625129/
O’Reilly MW, Westgate CSJ, Hornby C, Botfield H, Taylor AE, Markey K, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight [Internet]. 2019 Mar 3 [cited 2022 Sep 19];4(6):125348. Available from: /pmc/articles/PMC6483000/
Sugerman HJ, DeMaria EJ, Felton WL, Nakatsuka M, Sismanis A (1997) Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology 49(2):507–511
Giuseffi V, Wall M, Siegel PZ, Rojas PB (1991) Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology 41(2):239–244
Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Vol. 81, Neurology. 2013. p. 1159–65.
Higgins JNP, Gillard JH, Owler BK, Harkness K, Pickard JD (2004) MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Psychiatry 75(4):621–625
Yuh WTC, Zhu M, Taoka T, Quets JP, Maley JE, Muhonen MG et al (2000) MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging 12(6):808–813
Rehder D. Idiopathic intracranial hypertension: review of clinical syndrome, imaging findings, and treatment. Curr Probl Diagn Radiol [Internet]. 2020 May 1 [cited 2021 Aug 7];49(3):205–14. Available from: https://pubmed.ncbi.nlm.nih.gov/31056359/
Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure [Internet]. Vol. 50, Progress in Retinal and Eye Research. Elsevier Ltd; 2016 [cited 2020 May 17]. p. 108–44. Available from: https://doi.org/10.1016/j.preteyeres.2015.10.001
Brodsky MC, Vaphiades M (1998) Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 105(9):1686–1693
Rohr A, Dörner L, Stingele R, Buhl R, Alfke K, Jansen O (2007) Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. Am J Neuroradiol 28(4):656–659
Friedman DI, Quiros PA, Subramanian PS, Mejico LJ, Gao S, McDermott M et al (2017) Headache in idiopathic intracranial hypertension: findings from the idiopathic intracranial hypertension treatment trial. Headache 57(8):1195–1205
Ko MW (2011) Idiopathic intracranial hypertension. Curr Treat Options Neurol 13(1):101–108
Thurtell MJ, Wall M (2013) Idiopathic intracranial hypertension (Pseudotumor Cerebri): Recognition, treatment, and ongoing management. Curr Treat Options Neurol 15(1):1–12
Sun WYL, Switzer NJ, Dang JT, Gill R, Shi X, de Gara C, et al. Idiopathic intracranial hypertension and bariatric surgery: a systematic review. Can J Surg [Internet]. 2020 [cited 2022 Sep 20];63(2):E123. Available from: /pmc/articles/PMC7828958/
Higgins JNP, Owler BK, Cousins C, Pickard JD (2002) Venous sinus stenting for refractory benign intracranial hypertension. Lancet 359(9302):228–230
Nicholson P, Brinjikji W, Radovanovic I, Hilditch CA, Tsang ACO, Krings T, et al. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. Vol. 11, Journal of NeuroInterventional Surgery. BMJ Publishing Group; 2019. p. 380–5.
Kranz PG, Gray L, Amrhein TJ. Spontaneous intracranial hypotension: 10 myths and misperceptions [Internet]. Vol. 58, Headache. Blackwell Publishing Inc.; 2018 [cited 2020 Aug 16]. p. 948–59. Available from: https://pubmed.ncbi.nlm.nih.gov/29797515/
Griffin⁎ AS, Lu L, Peacock S, Gray L, Kranz PG, Amrhein TJ. CSF volume provocation maneuvers during lumbar puncture as a possible predictive tool for diagnosing spontaneous intracranial hypotension. 2019 [cited 2022 Feb 5]; Available from: https://doi.org/10.1016/j.clineuro.2019.105552
Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia [Internet]. 2016 Nov 1 [cited 2022 Feb 5];36(13):1209–17. Available from: https://pubmed.ncbi.nlm.nih.gov/26682575/
Yao L ling, Hu X yue. Factors affecting cerebrospinal fluid opening pressure in patients with spontaneous intracranial hypotension. J Zhejiang Univ Sci B [Internet]. 2017 Jul 1 [cited 2022 Feb 5];18(7):577–85. Available from: https://pubmed.ncbi.nlm.nih.gov/28681582/
Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goadsby PJ. Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache [Internet]. 2011 Oct [cited 2022 Sep 21];51(9):1442–4. Available from: https://pubmed.ncbi.nlm.nih.gov/21658029/
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension [Internet]. Vol. 295, Journal of the American Medical Association. JAMA; 2006 [cited 2020 Jun 23]. p. 2286–96. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/16705110/
Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology [Internet]. 2016 Aug 16 [cited 2020 Jun 23];87(7):673–9. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/27440149/
Mea E, Chiapparini L, Savoiardo M, Franzini A, Grimaldi D, Bussone G, et al. Application of IHS criteria to headache attributed to spontaneous intracranial hypotension in a large population. Cephalalgia [Internet]. 2009 Apr [cited 2020 Jun 23];29(4):418–22. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/19291244/
Mokri B. Spontaneous low pressure, low csf volume headaches: spontaneous CSF leaks [Internet]. Vol. 53, Headache. Headache; 2013 [cited 2020 Jun 23]. p. 1034–53. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/23808630/
Mokri B. Spontaneous CSF leaks: low CSF volume syndromes [Internet]. Vol. 32, Neurologic Clinics. W.B. Saunders; 2014 [cited 2020 Jun 23]. p. 397–422. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/24703536/
Pleasure SJ, Abosch A, Friedman J, Ko NU, Barbaro N, Dillon W, et al. Spontaneous intracranial hypotension resulting in stupor caused by diencephalic compression. Neurology [Internet]. 1998 [cited 2020 Jun 23];50(6):1854–7. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/9633740/
Kashmere JL, Jacka MJ, Emery D, Gross DW. Reversible coma: a rare presentation of spontaneous intracranial hypotension. Can J Neurol Sci [Internet]. 2004 [cited 2020 Jun 23];31(4):565–8. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/15595268/
Ghavanini AA, Scott CA, Chan DK, Tang-Wai DF. Management of patients with spontaneous intracranial hypotension causing altered level of consciousness: Report of two cases and review of literature [Internet]. Vol. 33, Cephalalgia. Cephalalgia; 2013 [cited 2020 Jun 23]. p. 43–51. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/23144179/
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia [Internet]. 2018 Jan 1 [cited 2021 Jul 27];38(1):1–211. Available from: https://pubmed.ncbi.nlm.nih.gov/29368949/
Mokri B. Spontaneous intracranial hypotension [Internet]. Vol. 21, CONTINUUM Lifelong learning in neurology. Lippincott Williams and Wilkins; 2015 [cited 2020 Jun 24]. p. 1086–108. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/26252593/
Wendl CM, Schambach F, Zimmer C, Förschler A (2012) CT myelography for the planning and guidance of targeted epidural blood patches in patients with persistent spinal CSF leakage. Am J Neuroradiol 33(3):541–544
Kranz PG, Gray L, Malinzak MD, Amrhein TJ. Spontaneous intracranial hypotension: pathogenesis, diagnosis, and treatment. Vol. 29, Neuroimaging Clinics of North America. W.B. Saunders; 2019. p. 581–94.
Tsai PH, Fuh JL, Lirng JF, Wang SJ. Heavily T2-weighted MR myelography in patients with spontaneous intracranial hypotension: a case-control study. Cephalalgia [Internet]. 2007 Aug [cited 2020 Jun 24];27(8):929–34. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/17645756/
Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology [Internet]. 2009 [cited 2020 Jun 24];73(22):1892–8. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/19949036/
Vanopdenbosch LJ, Dedeken P, Casselman JW, Vlaminck SAPA. MRI with intrathecal gadolinium to detect a CSF leak: a prospective open-label cohort study. J Neurol Neurosurg Psychiatry [Internet]. 2011 Apr [cited 2020 Jun 24];82(4):456–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20562454/
Monteith TS, Kralik SF, Dillon WP, Hawkins RA, Goadsby PJ. The utility of radioisotope cisternography in low CSF/volume syndromes compared to myelography. Cephalalgia [Internet]. 2016 Nov 1 [cited 2020 Jun 24];36(13):1291–5. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/26823556/
Albes G, Weng H, Horvath D, Musahl C, Bäzner H, Henkes H. Detection and treatment of spinal CSF leaks in idiopathic intracranial hypotension. Neuroradiology [Internet]. 2012 [cited 2020 Jun 24];54(12):1367–73. Available from: https://pubmed.ncbi.nlm.nih.gov/22766975/
Akbar JJ, Luetmer PH, Schwartz KM, Hunt CH, Diehn FE, Eckel LJ. The role of MR myelography with intrathecal gadolinium in localization of spinal CSF leaks in patients with spontaneous intracranial hypotension. Am J Neuroradiol [Internet]. 2012 Mar [cited 2020 Jun 24];33(3):535–40. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.javeriana.edu.co/22173753/
Farb RI, Nicholson PJ, Peng PW, Massicotte EM, Lay C, Krings T, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. Am J Neuroradiol [Internet]. 2019 [cited 2020 Aug 10];40(4):745–53. Available from: https://pubmed.ncbi.nlm.nih.gov/30923083/
Hoxworth JM, Trentman TL, Kotsenas AL, Thielen KR, Nelson KD, Dodick DW. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. Am J Roentgenol [Internet]. 2012 Sep [cited 2020 Aug 10];199(3):649–53. Available from: https://pubmed.ncbi.nlm.nih.gov/22915407/
Hoxworth JM, Patel AC, Bosch EP, Nelson KD. Localization of a rapid CSF leak with digital subtraction myelography. Am J Neuroradiol [Internet]. 2009 Mar [cited 2020 Aug 10];30(3):516–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18842766/
Martineau P, Chakraborty S, Faiz K, Shankar J. Imaging of the spontaneous low cerebrospinal fluid pressure headache: a review. Can Assoc Radiol J [Internet]. 2020 May 1 [cited 2020 Aug 10];71(2):174–85. Available from: http://journals.sagepub.com/doi/10.1177/0846537119888395
Schievink WI, Maya MM, Moser FG, Prasad RS, Cruz RB, Nuño M, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine [Internet]. 2019 [cited 2022 Sep 22];31(6):902–5. Available from: https://pubmed.ncbi.nlm.nih.gov/31518974/
Chiapparini L, Farina L, D’Incerti L, Erbetta A, Pareyson D, Carriero MR, et al. Spinal radiological findings in nine patients with spontaneous intracranial hypotension. Neuroradiology [Internet]. 2002 [cited 2020 Aug 16];44(2):143–50. Available from: https://pubmed.ncbi.nlm.nih.gov/11942367/
Yoo HM, Kim SJ, Choi CG, Lee DH, Lee JH, Suh DC, et al. Detection of CSF leak in spinal CSF leak syndrome using MR myelography: correlation with radioisotope cisternography. Am J Neuroradiol [Internet]. 2008 Apr [cited 2020 Aug 16];29(4):649–54. Available from: https://pubmed.ncbi.nlm.nih.gov/18202233/
Wiesemann E, Berding G, Goetz F, Windhagen A. Spontaneous intracranial hypotension: correlation of imaging findings with clinical features. Eur Neurol [Internet]. 2006 Dec [cited 2020 Aug 16];56(4):204–10. Available from: https://pubmed.ncbi.nlm.nih.gov/17057379/
Watanabe A, Horikoshi T, Uchida M, Koizumi H, Yagishita T, Kinouchi H. Diagnostic value of spinal MR imaging in spontaneous intracranial hypotension syndrome. Am J Neuroradiol [Internet]. 2009 Jan [cited 2020 Aug 16];30(1):147–51. Available from: https://pubmed.ncbi.nlm.nih.gov/18768717/
Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. In: American Journal of Neuroradiology [Internet]. American Society of Neuroradiology; 2016 [cited 2020 Aug 16]. p. 1374–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26869465/
D’Antona L, Jaime Merchan MA, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA Neurol [Internet]. 2021 Mar 1 [cited 2021 Jun 27];78(3):329–37. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33393980/
Farb RI, Forghani R, Lee SK, Mikulis DJ, Agid R. The venous distension sign: a diagnostic sign of intracranial hypotension at MR imaging of the brain. Am J Neuroradiol [Internet]. 2007 Sep 1 [cited 2021 Mar 31];28(8):1489–93. Available from: www.ajnr.org
Schievink WI. Spontaneous intracranial hypotension. Ropper AH, editor. 101056/NEJMra2101561 [Internet]. 2021 Dec 1 [cited 2022 Sep 21];385(23):2173–8. Available from: https://www.nejm.org/doi/10.1056/NEJMra2101561
Dobrocky T, Grunder L, Breiding PS, Branca M, Limacher A, Mosimann PJ, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol [Internet]. 2019 May 1 [cited 2022 Sep 22];76(5):580–7. Available from: https://pubmed.ncbi.nlm.nih.gov/30776059/
Medina JH, Abrams K, Falcone S, Bhatia RG. Spinal imaging findings in spontaneous intracranial hypotension. Am J Roentgenol [Internet]. 2010 [cited 2021 Jun 14];195(2):459–64. Available from: https://pubmed.ncbi.nlm.nih.gov/20651205/
Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: review and expert opinion [Internet]. Vol. 120, Acta Neurologica Belgica. Springer; 2020 [cited 2021 Jun 17]. p. 9–18. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/31215003/
Perthen JE, Dorman PJ, Morland D, Redfern N, Butteriss DJ. Treatment of spontaneous intracranial hypotension: experiences in a UK regional neurosciences Centre. Clin Med (Northfield Il) [Internet]. 2021 May [cited 2021 Jun 17];21(3):e247–51. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/34001579/
Signorelli F, Caccavella VM, Giordano M, Ioannoni E, Caricato A, Polli FM, et al. A systematic review and meta-analysis of factors affecting the outcome of the epidural blood patching in spontaneous intracranial hypotension [Internet]. Neurosurgical Review. Springer Science and Business Media Deutschland GmbH; 2021 [cited 2021 Jun 27]. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33611638/
Schievink WI, Maya MM, Moser FM. Treatment of spontaneous intracranial hypotension with percutaneous placement of a fibrin sealant: Report of four cases. J Neurosurg [Internet]. 2004 [cited 2021 Jun 27];100(6):1098–100. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/15200130/
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno, M.E., Del Carpio – O’Donovan, R. Neuroimaging in the diagnosis and treatment of intracranial pressure disorders. Neurol Sci 44, 845–858 (2023). https://doi.org/10.1007/s10072-022-06478-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06478-x