Abstract
Lipids are water-insoluble heterogeneous group of compounds, which are soluble in organic solvents. Besides being an important constituent of the diet, lipids have many other uses. A great diversity of lipids occurs in nature, and it is very important to get the right kind of lipid in a diet. Lipids are among the biomolecules required for structure and functions of the cell. Since they are mostly stored in seeds or fruits of some plants, these are the main sources for obtaining dietary lipids. Studying the diversity and the metabolism of lipids is important. A brief introduction to diversity in structure and functions of lipids is taken in this chapter besides learning about the classification and nomenclature of fatty acids. The chapter includes the pathways, the role of enzymes, and the organelles involved in the lipid metabolism. Studying the catabolism of lipids is significant in germinating fatty seeds, since they have primarily oils as the storage form. In fatty seeds the stored lipids are not the source of energy directly. Lipids are metabolized to produce soluble form of sugars in the storage organs, which are translocated to the meristematic regions in the growing seedlings. A study of pathways, enzymes, and organelles involved in lipid metabolism in germinating seeds is also included in the chapter. Though there is similarity in lipid metabolism of plants and animals, a great degree of disparity also exists. In plants lipid metabolism is more complex and involves many cell organelles. Fatty acid synthesis and fatty acid oxidation occur in different subcellular locations and involve different metabolic pathways and different sets of enzymes. There is successive removal of two-carbon units as acetyl-CoA during oxidation of fatty acids. Elongation of hydrocarbon chain also requires, at the time of fatty acid synthesis, addition of two carbons at a time. However, precursors for fatty acid synthesis are three-carbon compounds, malonyl-CoA, except one acetyl-CoA, which is required as a primer. The condensation reaction is coupled with simultaneous release of one carbon as CO2 (Fig. 10.1).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Acyl carrier protein
- β-Oxidation
- Coenzyme A
- Gluconeogenesis
- Glyoxylate cycle
- Malonyl-CoA
- Oleosin
- Phosphatidic acid
- Phosphatidylcholine
- Triglycerides
Lipids are water-insoluble heterogeneous group of compounds, which are soluble in organic solvents. Besides being an important constituent of the diet, lipids have many other uses. A great diversity of lipids occurs in nature, and it is very important to get the right kind of lipid in a diet. Lipids are among the biomolecules required for structure and functions of the cell. Since they are mostly stored in seeds or fruits of some plants, these are the main sources for obtaining dietary lipids. Studying the diversity and the metabolism of lipids is important. A brief introduction to diversity in structure and functions of lipids is taken in this chapter besides learning about the classification and nomenclature of fatty acids. The chapter includes the pathways, the role of enzymes, and the organelles involved in the lipid metabolism. Studying the catabolism of lipids is significant in germinating fatty seeds, since they have primarily oils as the storage form. In fatty seeds the stored lipids are not the source of energy directly. Lipids are metabolized to produce soluble form of sugars in the storage organs, which are translocated to the meristematic regions in the growing seedlings. A study of pathways, enzymes, and organelles involved in lipid metabolism in germinating seeds is also included in the chapter. Though there is similarity in lipid metabolism of plants and animals, a great degree of disparity also exists. In plants lipid metabolism is more complex and involves many cell organelles. Fatty acid synthesis and fatty acid oxidation occur in different subcellular locations and involve different metabolic pathways and different sets of enzymes. There is successive removal of two-carbon units as acetyl-CoA during oxidation of fatty acids. Elongation of hydrocarbon chain also requires, at the time of fatty acid synthesis, addition of two carbons at a time. However, precursors for fatty acid synthesis are three-carbon compounds, malonyl-CoA, except one acetyl-CoA, which is required as a primer. The condensation reaction is coupled with simultaneous release of one carbon as CO2 (Fig. 10.1).
1 Role of Lipids
Lipids are stored as neutral fats in seeds of many plants or are stored in fruits or other vegetative parts of some of the plants. Lipids are integral constituents of all cell membranes. Membrane lipids are amphipathic in nature, i.e., their one end is hydrophilic, and the other one is hydrophobic. This facilitates them to organize in the form of a bilayer with their hydrophilic ends facing toward the aqueous side of the cell (cytosolic side and outside aqueous environment of the cell), and hydrophobic ends face each other. Both hydrophobic interactions between the hydrophobic ends of the lipids and hydrophilic interactions of the polar groups with aqueous environment are important factors for organization of the membranes. Bimolecular layer of lipids along with proteins keeps the membrane structure intact and is key for existence of life. The membrane is responsible for maintaining the optimal chemical environment in the cell and various subcellular compartments for various metabolic reactions to occur by providing limited permeability to selected molecules into and from the cell. Unique structures of thylakoids and those of mitochondria are responsible for carrying out light reactions of photosynthesis and oxidative reactions during respiration, respectively. Cofactors of many enzymes are lipids. Lipoic acid, ubiquinone, and plastoquinone are few of them. Vitamins such as vitamin D, A, E, and K are lipid soluble and are derived from five-carbon isoprene units. Many of the hormones are derived from sterols both in animal and plants. In plants, brassinosteroids are the growth regulators which are steroid in nature, while jasmonates are derived from linolenic acid (Fig. 10.2). Jasmonates are plant hormones which regulates plant development besides being involved in plant defense against insects, herbivorous animals, and many fungal plant pathogens. Pigments such as carotenoids impart colors to plants and animals (bird’s feathers). Colors of feathers of the birds are due to ingestion of carotenoid (such as canthaxanthin and zeaxanthin) containing plant material by them. Besides attracting pollinators, plant pigments also act as secondary photosynthetic pigments and protect chlorophylls from photooxidation. Lipids such as cutin provide protective barrier to the epidermal cells of leaves and being impermeable to water also reduce water loss because of transpiration. Another type of lipid, present in the endodermis of root cells, is suberin which provides barrier to the apoplastic movement of water from the cortex to vascular tissues. Water has to travel through symplasm beyond the endodermis layer in the root cell. Suberin present in epidermal cells protects some of the tubers from infections. In plasma membranes lipids also act as signaling molecules and play an important role in cell signaling. In addition to being membrane lipid, phosphatidylinositol serves as a precursor for secondary messengers, which regulate intracellular concentration of calcium, and in turn regulate cell metabolism (Table 10.1).
2 Diversity in Lipid Structure
A great diversity in the structure is observed in lipids. A quantitative catalogue of all the lipids present in a specific cell type under particular condition is called the lipidome. Application of high-resolution mass spectrometry technique has helped in working out lipidome of a cell. Lipidome of the cell changes under different sets of conditions such as with differentiation or when afflicted with a particular disease or even with the drug treatment. Lipidomics is the science that allows identification and comparison between lipids of different cell types under different sets of conditions. Lipids may be classified on the basis of their structures or functions. These may also be classified as polar and nonpolar lipids. However, in this chapter these are organized under the following categories.
2.1 Fatty Acids
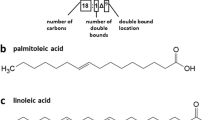
Fatty acids are the structural components of lipids. These are derivatives of hydrocarbons. These consist of hydrocarbon chain and a carboxylic group. Generally fatty acids contain an even number of carbon atoms varying from 4 to 36. However, most common fatty acids contain 12–24 carbon atoms. Hydrocarbon chain may be branched or unbranched. The most common fatty acids have unbranched hydrocarbons. Hydrocarbon chain of fatty acids may not have any double bond and be saturated, while others may be unsaturated because of the presence of double bonds in them. The number and position of double bonds are characteristics of unsaturated fatty acids. If hydrocarbon chain of a fatty acid contains double bonds, two consecutive double bonds are separated by –CH2 (methylene) group. A simplified system for abbreviations has been adopted for fatty acid structures by giving two digits which are separated by colon. The first digit indicates the number of carbon atoms in the hydrocarbon chain, while the number after colon indicates the number of double bonds in the hydrocarbon chain. For example, palmitic acid is abbreviated as 16:0. The first digit 16 indicates that hydrocarbon chain consists of 16 carbons, and the second digit (0) after colon indicates that the number of double bond is zero. Stearic acid is abbreviated as 18:1, which indicates that the hydrocarbon chain of fatty acid is 18 carbons long and it is an unsaturated fatty acid with one double bond being present. The position of double bond may be specified by delta nomenclature according to which structure of stearic acid can be abbreviated as 18:1Δ9 which indicates the presence of double bond in between the ninth and tenth carbon atom, when the carbon of carboxylic group is numbered as one. If two double bonds are present, the number following the colon will be two. The structure of linoleic acid will be written as 18:2Δ9,12. Table 10.2 provides the structure and abbreviations for some of the fatty acids. There is another way of nomenclature for unsaturated fatty acids. Instead of indicating position of double bond from the carboxylic group (as in delta system of nomenclature), the position of double bond is shown with reference to methylene group (-CH3) of fatty acid as omega (ω), which refers to the carbon most distant from carboxylic group and is numbered as carbon 1. This type of nomenclature is significant for polyunsaturated fatty acids (PUFA) in which double-bond near-omega (ω) carbon is of physiological significance. PUFAs with double bond present in between the third and fourth carbon from the methylene group (ω) are called omega-3 (ω-3) fatty acids, while PUFAs with double bonds in between the sixth and seventh carbon atoms are called omega-6 (ω-6) fatty acids. Fatty acids may remain in gel or fluid state which is determined by the surrounding temperature and also by the features of their hydrocarbon chain. Saturated fatty acids with 12–24-carbon-long hydrocarbon chain have waxy consistency at 25 °C, while unsaturated fatty acids remain in oil form. This property of fatty acids is due to the nature of packing of the extended and flexible hydrocarbon chain. The presence of double bonds in cis configuration causes a kink in the hydrocarbon chain. As a result, their compact packing in membranes is not possible, and they remain in fluid consistency. Almost all the naturally occurring unsaturated fatty acids contain double bonds in cis configuration (Box 10.1).
Box 10.1: Adaptation in Lipid Composition of Plant Membranes in Response to the Change in Environment Temperature
At room temperature, fatty acids occur either in waxy consistency or as oils. This property of fatty acids is determined by length, saturation status, and configuration of double bonds in their hydrocarbon chain. The presence of more double bonds will result in reduced melting temperature of fatty acids. Free rotation around carbon atom in fatty acids with saturated hydrocarbons allows their compact packing unlike unsaturated fatty acids where the presence of double bond in the hydrocarbon chain restricts the free rotation. Unsaturated fatty acids can either be trans or cis depending upon the arrangement of hydrogen atoms around double bonds. Compact packing of lipids occurs in membranes when double bonds of hydrocarbon chains of unsaturated lipids are in trans configuration because of the chain being straight as in the case of saturated fatty acids. Compact packing of lipids will restrict their movement resulting in their waxy consistency and affecting permeability of the membrane. Contrary to this, cis- configuration causes a 30° kink in the hydrocarbon chain because of which close packing of lipid is not possible and the membrane will be more fluid. Plants adjust to changing temperature by altering the ratio of lipids with unsaturated fatty acids to lipids with saturated hydrocarbon chains. One way by which plants adapt to freezing temperature (freezing tolerance) is by increasing lipids with unsaturated fatty acids.
Melting temperature of some of the fatty acids:
Name of fatty acid | Abbreviations for the fatty acids | Melting temperature °C |
Lauric acid | 12:0 | 40 |
Palmitic acid | 16:0 | 63 |
Stearic acid | 18:0 | 70 |
Oleic acid | 18:1Δ9 | 13 |
Linoleic acid | 18:2Δ9,12 | −5 |
Linolenic acid | 18:3Δ9,12,15 | −11 |
Arachidonic acid | 20:4Δ5,8,11,14 | −49.5 |
2.2 Storage Lipids (Neutral Fats, Waxes)
Most common form of storage lipids are triglycerides (neutral fats). For some plants, neutral fats are preferred form of storage in seeds, mainly because fats contain more reduced carbon so it will be the source for more energy in comparison to carbohydrates. Secondly, neutral lipids are anhydrous, so they are stored without water unlike carbohydrates which retain hydration of water. Thus, additional weight due to retention of water is not added in case of fats being stored, and seeds remain lighter. This makes them suitable for their dispersal. Triacylglycerol are esters of glycerol in which all three hydroxyl groups of glycerol are esterified with fatty acids. The three hydroxyl groups of a glycerol may be esterified by similar fatty acids or may be esterified by different fatty acids. In case all three hydroxyl groups are esterified by similar fatty acids, these are named according to the fatty acids, e.g., tripalmitin, tristearin, or triolein if fatty acids are palmitic acid, stearic acid, or oleic acid, respectively. In case hydroxyl groups of glycerol are esterified by two or three different fatty acids (mixed), the name and position of each fatty acid are specified. Carbon atoms of glycerol are given stereospecific numbers (sn) with sn1 being the top carbon of the glycerol, sn2 is the middle one, and sn3 is the bottom carbon atom. In plants, synthesis of neutral fats occurs within the endoplasmic reticulum membranes, and these are stored in oil bodies of endosperm cells of the seed. In some cases, triglycerides may be stored in fruits, stems, or roots. Tetraena mongolica Maxim is an example of plant species where triacylglycerols are stored in stem tissues. This plant is also called as “oil firewood.” Almost 9% of the dry weight of plant is triacylglycerol. In Manihot esculenta, triacylglycerol accumulation occurs in roots in which about 25% of the dry weight is oil and 30% is starch, which are accumulated in tubers. Waxes are complex molecules consisting of mixture of long-chain hydrocarbons, alcohols, aldehydes, acids, and esters of long-chain alcohols (C14–C36) with fatty acids having long hydrocarbon chains (C16–C30). These are diverse group of molecules whose composition varies from plant to plant. They have higher melting point (60–100 °C) than triacylglycerols. These are highly hydrophobic and have water-repellent property. Waxes are present within the cutin network as an amorphous substance. They are spread on the cutin surface in leaves of many plants such as those of rhododendrons. It makes cutin highly impermeable to water. As a result, plants are protected from excessive loss of water and also from parasites. Waxes are produced through action of wax synthase in the ER membrane and are exported to the cell wall. Their transport across plasma membrane involves ABC transporters. The mechanism of transfer of wax monomers from epidermal cells to the plant surface is poorly understood. It possibly involves lipid transfer proteins. In aerial organs of plants, a bulk of fatty acids is directed toward synthesis of wax and cutin. Some plant waxes are commercially important, such as carnauba wax, which is found on the surface of Brazilian palm tree. The only angiosperm in which wax is used for storage purpose is jojoba desert plant (Simmondsia chinensis). Liquid wax obtained from the fruits of the plant is used in cosmetic industry.
2.3 Membrane Lipids
Amphipathic nature of membrane lipids makes them different from storage lipids. These are classified as below:
Glycerophospholipids
These lipids have glycerol as the backbone of their structure. Carbons 1 and 2 are esterified with fatty acids, while carbon 3 is linked with phosphate group by phosphoester linkage. This molecule is known as phosphatidic acid (PA). The presence of nonpolar fatty acids at one end of the molecule makes it hydrophobic, while a polar group (an alcohol) linked via phosphodiester linkage at the other end makes it hydrophilic. Depending upon the type of polar group, which are choline, ethanolamine, serine, inositol, or glycerol, these are called as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), or diphosphatidylglycerol, respectively (Fig. 10.3). Phospholipids are present in membranes of bacteria, animals, and plants. Tables 10.3 and 10.4 show distribution of membrane lipids in different cell membranes of a plant cell.
Glycolipids
Plant membranes contain glycolipids and sulfolipids in addition to phospholipids. Glycolipids and sulfolipids constitute major membrane lipids in thylakoids and in cyanobacteria. They have one or two galactose residues attached to carbon 3 of glycerol in place of phosphate. Depending upon the number of galactose residues attached, these are named as monogalactosyldiacylglycerol (MGD) and digalactosyldiacylglycerol (DGD). Sulfolipids (SL) are glycerolipids in which polar group at carbon 3 of diacylglycerol consists of a glucose molecule which is attached to sulfonic acid group at the sixth carbon atom. These are also known as sulfoquinovosyldiacylglycerol (SOD). Galactolipids are the chief constituents of thylakoids and chloroplast envelop. In green plants, 70–80% of the membrane lipids constitute thylakoids. Galactolipids are the most abundant lipids on this earth. This indicates the adaptation of the plants since plants get limited supply of phosphorus and major membrane lipids of plants (both galactolipids and sulfolipids) do not require phosphorus.
Sphingolipids
Sphingolipids are also the membrane lipids which are amphipathic. Instead of glycerol they consist of a long-chain amino alcohol sphingosine, which is derived from serine and fatty acyl-CoA. In place of carbons from glycerol in glycerolipids, the three carbons of sphingolipids are derived from serine. Hydrophobic end of sphingolipids consists of two hydrophobic acyl chains. One of the acyl group linked to C 1 of the molecules is long-chain base (most common chain being 18 carbon). Another acyl group having 14–26 carbon atoms is a N-linked fatty acid which is linked to C-2 of the molecule via an amide linkage. A molecule, with two acyl groups attached to it, is known as ceramide. Hydrophilic end of the molecule consists of a large array of polar head groups which are bound to hydroxyl group at C 3 of ceramide. In plants two major classes of complex sphingolipids have been identified. They are glucosylceramides or glucocerebrosides and glycosylinositol phosphoceramides. Sphingolipids are major components of endomembranes, and ≥40% of plasma membrane also has sphingolipids. They are also present in tonoplast. These are synthesized in the endoplasmic reticulum and Golgi apparatus. In plants sphingolipids are involved in programmed cell death (PCD) associated with defense.
Sterols
Sterols are membrane lipids consisting of four fused rings. Three of the rings consist of six carbons, while the fourth one has five carbons. The presence of hydroxyl group at 3 C position in ring A makes this end of the molecule polar, while a hydrocarbon chain at C 17 makes it nonpolar. As a result, sterols are also amphipathic molecules and are constituents of membrane lipid bilayer with their polar hydrophilic end facing the aqueous sides. Sterols determine the properties of membranes. Plant cell membranes do not contain cholesterol. However, a variety of other sterols are present in the membranes of mitochondria, endoplasmic reticulum, and plasma membrane. Sterols in plants include stigmasterol, while in fungi ergasterol is present. These differ in the hydrocarbon chain which is attached to C 17 of the ring D of the sterane nucleus of the molecule. Bacteria cannot synthesize sterols.
Extracellular Lipids
Cuticle is a hydrophobic layer present on the leaf surface. It restricts water loss in addition to providing protection against pathogens. It also provides skeletal support to plants. Cuticle consists of cutin and cuticular wax. Cutin is characteristics of the plant cells. It is present exterior to leaf epidermal cells and is cross-linked with the cell wall. It is produced by the non-photosynthetic epidermal cells of leaves. Cutin is a polymeric molecule consisting of oxygenated fatty acids with a carbon chain length of 16 or 18. Monomers of cutin are monohydroxy, polyhydroxy, or epoxy fatty acids. Constituent fatty acids of cutin form ester linkages either with glycerol or with primary or secondary hydroxyl groups present in other fatty acids. This results in the formation of inelastic hydrophobic framework. Because of the space in between, water can be lost from the leaf surface. These pores are plugged with cuticular wax. Pathogens need to produce cutinase in order to facilitate their entry into the plant cells. Another extracellular lipid significant for plants is suberin. Suberin is also a polymeric molecule but differs from the cuticle in its composition of fatty acids. In suberin the fatty acids have longer carbon chains of 18–22 carbon than cutin. Unlike in cutin, fatty acids in suberin are not oxygenated. They do not have secondary alcohol or epoxy groups. A high amount of dicarboxylic acids is also present in suberin. Additionally, phenylpropanoids are also present which are partly linked with each other as in lignin. Suberin differs from cutin in getting deposited toward the inner side of the primary cell wall, while cutin is deposited toward the outside of the epidermal cell wall. Deposition of cutin is polar since it is deposited only on one side of the epidermal cells. Cutin is present at the interface of plants and the environment, while suberin is generally deposited all around the plasma membrane of the cells. Casparian strips of endodermis consist of suberized cells which make the cell wall impermeable to water. As a result, water and minerals are not transported to the vasculature of the plant through the apoplast; rather they have to move through the symplast beyond the endodermis in roots. Therefore, water and minerals do not diffuse back from the plant vasculature, thereby contributing to root pressure. In C4 plants, bundle sheath cells have suberin depositions in the cell wall, which makes it impermeable to CO2. Suberin is present in the cork tissue which consists of dead cells surrounded by alternating layers of suberin and wax. Suberin is deposited in response to abiotic stress and mechanical injury. Waxy layer present on pollen grains plays a significant role in pollen-pistil interactions. It is known as exine in which other lipids are also embedded. Exine consists of complex polymer—sporopollenin—which is similar to cutin in its structure. Studies carried out with Arabidopsis mutants have demonstrated that altered lipid composition of exine induces male sterility, indicating significance of exine in pollen-pistil interaction.
3 Fatty Acid Biosynthesis
Unlike carbohydrates, lipid biosynthesis occurs in every cell at the site of their utilization, since the mechanism for the transport of lipids in plants is not known. Unlike in animals where the site for fatty acid (FA) biosynthesis is cytosol, in plants fatty acids having hydrocarbon chain up to 16 or 18 long are synthesized in the chloroplasts of green cells and leucoplasts or chromoplasts in nongreen cells. However, both in plants and animals, further elongation of the hydrocarbon chain occurs in the endoplasmic reticulum. Biosynthesis of fatty acids in plants is quite similar to that in bacteria. Precursors required for FA biosynthesis are acetyl-CoA (required as primer) and malonyl-CoA. Enzymes required are acetyl-CoA carboxylase (ACCase) and fatty acid synthase (FAS).
3.1 Synthesis of Acetyl-CoA
Since synthesis of fatty acids involves condensation of two-carbon fragments, most fatty acids consist of an even number of carbon atoms. Acetyl-CoA is required as the precursor. Since acetyl-CoA is not able to cross the membranes, it is produced in plastids. In chloroplasts, acetyl-CoA is derived from an intermediate of Calvin cycle, i.e., 3-phosphoglycerate, which is converted to pyruvate during glycolysis (plastids also have glycolytic enzymes). Pyruvate dehydrogenase complex (PDC) catalyzes oxidation and decarboxylation of pyruvate to acetyl-CoA. A substantial activity of pyruvate dehydrogenase (PDH), a component of PDC, has been demonstrated in chloroplasts. In nongreen plastids pyruvate is obtained from cytosol of the cell. Pyruvate is produced by the conversion of sucrose to pyruvate in the cytosol through glycolysis. Sucrose is imported from the outside. After being transported to plastids, pyruvate is converted to acetyl-CoA by PDC. It is also produced in plastids from acetate, which is imported either from mitochondria or cytosol. In the cytosol, ATP citrate lyase is responsible for the synthesis of acetyl-CoA from citrate, which has been exported from mitochondria. Acetate is activated to acetyl-CoA in the stroma of plastids by acetyl-CoA synthase (ACS) (Fig. 10.4). In the cytosol, acetyl-CoA is also used as the precursor of malonyl-CoA, which is required for elongation of the fatty acids in the endoplasmic reticulum.
3.2 Synthesis of Malonyl-CoA
Carboxylation of acetyl-CoA produces malonyl-CoA, which is the activated form of acetyl-CoA. Reaction is catalyzed by acetyl-CoA carboxylase (ACCase). This is the first step in biosynthesis of fatty acids and is a rate-limiting step. Cofactor for the enzyme ACCase is biotin, which is covalently linked to ε-amino group of a specific lysine in the enzyme protein. There are two types of ACCase in plant cells. Plastidial ACCase is similar to that of bacteria, while cytosolic ACCase is eukaryotic in origin. Plastidial ACCase catalyzes production of majority of malonyl-CoA required for fatty acid biosynthesis in plants, while malonyl-CoA, produced outside plastids by cytosolic ACCase, is used for elongation of fatty acid chains in the ER, for flavonoid biosynthesis, and for synthesis of aminocyclopropane carboxylic acid, the precursor of ethylene (Fig. 10.5). Plastidial ACCase is a heteromeric protein (HET-ACCase) with a molecular mass 650 kDa. It consists of four subunits: biotin carboxyl carrier protein (BCCP, the subunit to which biotin is covalently linked), biotin carboxylase (BC), and two subunits (α and β) of carboxyl transferase (CT). Three out of the four subunits of plastidial ACCase are encoded in nuclear genome, while β subunit of carboxyl transferase is encoded in plastid genome itself. On the contrary, cytosolic ACCase in plants is similar to ACCase present in animals and fungi. It is a homodimeric protein (HOM-ACCase) which has a molecular mass of 500 kDa, each monomeric unit being 250 kDa. Each monomeric unit is multifunctional and complex. Contrary to plastidial ACCase, which has separate polypeptide subunit for each functional domain, cytosolic ACCase has three functional domains, BCCP, BC, and CT amalgamated in one polypeptide. It is encoded in nuclear genome. However, in the members of grass family (Poaceae), the plastidial form of ACCase is absent, and the homodimeric form of the enzyme with multifunctional domains is present both in the cytosol and plastids (ACCase Fig. 10.6). Activation of acetyl-CoA occurs in two steps and requires ATP:
Biotin serves as temporary CO2 carrier. It is carboxylated at the expense of ATP. Substrate for the enzyme is HCO3 −. NH group of the ureide ring of biotin forms carbamate with HCO3 − at the active site of biotin carboxylase (BC) functional subunit/domain of the multi-subunit/multifunctional enzyme. Long hydrocarbon chain of biotin cofactor is flexible. It moves to carboxyl transferase (CT) subunit/domain of ACCase. The transfer of CO2 from biotin to acetyl-CoA is facilitated by carboxyl transferase, resulting in the formation of malonyl-CoA and releasing biotin which is ready to receive another molecule of HCO3 −. The reaction requires Mg2+ (Fig. 10.7).
Acetyl-CoA carboxylase (ACCase) reaction. 1. Biotin (attached with biotin carboxyl carrier protein, BCCP) activates CO2 by attaching it to the nitrogen ring of biotin. 2. Flexible arm of biotin transfers activated CO2 to carboxyl transferase (CT) 3. CT transfers CO2 to acetyl-CoA converting it to malonyl-CoA. BC biotin carboxylase
3.3 Transfer of Malonyl Moiety from Coenzyme-A to Acyl Carrier Protein (ACP)
After the synthesis of precursors for fatty acid biosynthesis, i.e., acetyl-CoA and malonyl-CoA, the first step involves the transfer of malonyl moiety from malonyl-CoA to ACP resulting in the formation of malonyl-ACP. Reaction is catalyzed by the enzyme malonyl-CoA:ACP transacylase (a serine enzyme). The following reactions include a condensation, two reductions, and a hydration, while the extending acyl group remains attached to the ACP. ACP is a small protein of about 80 amino acids long. The cofactor phosphopantetheine is covalently linked to a serine residue present in almost the center of the protein. It acts like a flexible arm carrying the acyl group from enzyme to enzyme. Similar to ACP, phosphopantetheine group is also present in coenzyme A, but the two differ in the protein being absent in coenzyme A. There is terminal –SH group present in both coenzyme A and ACP. Synthesis of malonyl-ACP from malonyl-CoA occurs in two steps. The first step involves the transfer of malonyl group from coenzyme A to serine residue of the enzyme. In the second step, malonyl group is transferred from the serine residue of the enzyme to sulfhydryl group of ACP.
Once precursors are available, it is followed by (1) condensation of acetyl-CoA with malonyl-ACP, resulting in formation of four-carbon molecule 3-ketobutyryl-ACP, (2) reduction of β-keto group of 3-ketobutyryl-ACP, (3) dehydration reaction, and (4) second reduction. This results in formation of butyryl-S-ACP group. Biosynthesis of fatty acids occurs by addition of two carbons at a time. It requires repeat of four-step sequence of the cycle with the addition of every two carbons to the growing hydrocarbon chain of fatty acid (Figs. 10.8, 10.9, and 10.10).
Fatty acid biosynthesis. Condensation of acetyl-CoA and malonyl-ACP is facilitated by the enzyme ketoacyl synthase (KAS). One carbon of malonyl-ACP is released as CO2 and KAS are released. Condensed acetoacetyl-ACP group enters reduction 1, dehydration, and reduction 2 resulting in synthesis of butyryl-ACP. Butyryl-ACP enters into another cycle of chain elongation in a similar way after butyryl group is transferred to KAS
Role of isoforms of 3-ketoacyl synthase (KAS), i.e., KAS III, KAS I, and KAS II in fatty acid synthesis in plastids. KAS III catalyzes condensation of acetyl-CoA with malonyl-ACP, and KAS I catalyzes condensation of acyl groups that contain carbon chain length from 4 to 16, while KAS II catalyzes extension of carbon chain length of fatty acids from 16 to 18 carbons
Except the initial reaction catalyzed by ACCase, the reactions involving these four steps are catalyzed by a group of enzymes which together are called fatty acid synthase (FAS). There are two types of fatty acid synthases, Type I and Type II. FAS I (Type I) is found in animals and fungi which functions as a homodimer. Single final product is synthesized by FAS I system. When the length of carbon chain reaches 16, the final product (palmate) is released, with no intermediates. Contrary to FAS I, FAS II in plants and bacteria consists of many polypeptides. Each step in the series of reactions is catalyzed independently by individual polypeptides. Intermediates of the reaction can diffuse out and are diverted to other pathways. Fatty acids of variable carbon chain lengths are produced due to the difference in termination sites for extending hydrocarbon chain. The independent catalytic units possibly are associated in supramolecular organization. In such a kind of structural association, efficiency of metabolic pathway is enhanced due to substrate channeling.
3.4 Condensation Reaction
Condensation of acetyl-CoA (primer) with malonyl-ACP results in formation of carbon-carbon bond, and a four-carbon compound, 3-ketobutyryl-ACP, is synthesized. Reaction is catalyzed by ketoacyl-ACP synthase (KAS). KAS has SH groups present at the active site (KAS-SH). Reaction occurs as follows:
Reaction involves condensation of two carbons, donated from acetyl unit of acetyl-CoA, and two carbons from malonyl-ACP. Carboxyl group of malonyl moiety of malonyl-ACP is released as CO2. Release of CO2 makes the reaction irreversible. 3-Ketoacyl-ACP synthase (KAS) is commonly called condensing enzyme. There are three isoforms of the KAS: KAS I, KAS II, and KAS III. They differ in their specificity for the substrate, acyl-ACP, which have hydrocarbon chains with variable carbon numbers. KAS III catalyzes condensation of acetyl-CoA with malonyl-ACP. KAS II prefers a substrate with a longer carbon-chain acyl-ACP (with C10-14/16 long hydrocarbon chain), while KAS I prefers condensation reaction of the acyl-ACP having C4-C14 long hydrocarbon chain. Condensation reaction is initiated by KAS III, hydrocarbon chain is extended by KAS I, and it is completed by KAS II, resulting in fatty acids with C16/C18 long hydrocarbons (Fig. 10.9).
3.5 Reduction of 3-Ketobutyryl-ACP to Butyryl-ACP
Conversion of 3-ketobutyryl-ACP to butyryl-ACP involves two reduction reactions and a dehydration reaction. This set of three reactions is similar to reactions of Krebs cycle in reverse order during which succinate is converted to malate. Krebs cycle involves two oxidations and one hydration reaction, while during fatty acid synthesis, two reduction and one dehydration reactions occur. Reduction requires reducing power mostly in the form of NADPH, which is provided in chloroplasts by the light reaction. In nongreen plastids, the source for NADPH is oxidative pentose phosphate pathway. During reduction 1,3-ketobutyryl-ACP is reduced to 3-hydroxybutyryl-ACP (β-D-hydroxyacyl-ACP). Reaction is catalyzed by 3-ketoacyl-ACP reductase.
Dehydration of 3-hydroxybutyryl-ACP results in formation of 2,3-trans-enoyl-ACP. Reaction is catalyzed by 3-hydroxyacyl-ACP dehydrase.
Second reduction involves reduction of 2, 3-trans-enoyl-ACP to butyryl-ACP, catalyzed by enoyl-ACP reductase which requires reducing power as NAD(P)H/NADH depending upon the isoform of the enzyme which exists in two isoforms.
Flexible arm of ACP shuttles the four-carbon moiety from enzyme to enzyme during the reactions steps.
3.6 Extension of Butyryl-ACP
Extension of hydrocarbon chain in butyryl-ACP (acyl-ACP) occurs through a repeat of similar set of reactions from condensation to reduction 2 in a cyclic manner. In the beginning of the cycle, a growing acyl chain is transferred from ACP to the active site (-SH) of condensing enzyme (KAS-SH). At the time of condensation, the acyl chain is transferred from KAS to ACP, followed by a similar set of reactions as with acyl-ACP (Fig. 10.8). During each cycle of reactions, hydrocarbon chain of acyl group is extended by two carbons (-CH2-CH2) till hydrocarbon chain is extended up to 16- or 18-carbon chain length depending upon the plant (Figs. 10.9 and 10.10).
3.7 Termination of Fatty Acid Synthesis in Plastids
Release of acyl groups from fatty acyl-ACP is catalyzed by ACP thioesterase which is localized in the inner membrane of plastids. After the extension of hydrocarbon chain up to 18-carbon length in plastids, there are three possible fates of the fatty acids:
-
1.
Acyl group is directly transferred from ACP to glycerol in plastids, resulting in the synthesis of glycerolipids which are the precursors for triglycerides to be used for storage or for biogenesis of membrane lipids.
-
2.
A double bond is introduced in the hydrocarbon chain of acyl group by desaturase present in the stroma of plastids, resulting in the formation of oleoyl-ACP (18:1).
-
3.
Release of fatty acids from fatty acyl-ACP is catalyzed by the soluble fatty acyl-ACP thioesterase (Fat). Thioesterase hydrolyzes sulfhydryl bond of acyl group with ACP, releasing ACP and the acyl group (generally fatty acids having hydrocarbons with carbon chain length of 16 or 18). Fatty acids are converted to fatty acyl-CoA at the outer membrane of plastids. Plastidial ACP thioesterase primarily hydrolyzes palmitoyl-ACP (16:0) or oleate acyl-ACP (18:1). Stearate-ACP (18:0) is hydrolyzed to a lesser extent. ACP-thioesterase is localized in the inner envelop of the plastids releasing fatty acyl groups in the stroma. The fatty acyl groups are exported out of plastid and are reactivated to fatty acyl-CoA at the outer envelope by acyl-CoA synthetase (Fig. 10.11). Further elongation of hydrocarbon chain or introduction of double bond in acyl groups occurs as acyl-CoA and not as fatty acyl-ACP in the endoplasmic reticulum.
Role of thioesterase. Acyl-ACP thioesterase catalyzes hydrolysis of acyl moiety from ACP stopping further extension of hydrocarbon chain and yielding fatty acid and ACP. Two types of acyl-ACP thioesterases, fat A and fat B, occur in plants. Fat B is active with shorter chains FA, while fat A is active with 18:1Δ9-ACP. Some of the fatty acids with shorter hydrocarbon chain have commercial significance. This characteristic has been exploited to incorporate ACP-thioesterases in plants to be able to produce FAs with shorter hydrocarbon chains
3.8 Hydrocarbon Chain Elongation in Endoplasmic Reticulum
In plants, fatty acids with C20–C24 carbon atoms are required either for the synthesis of wax (which are esters of long-chain fatty acids having C20–C24 carbon atoms and long-chain acyl alcohols of C26–C32 length) or for the synthesis of sphingolipids, which contain C22–C24 long fatty acids. In some plants, triacylglycerols, containing fatty acids with longer hydrocarbon chain (C20 and C22), are also present. Palmitate (16:0) or oleate (18:1) synthesized in plastids is exported to the endoplasmic reticulum for further extension (Fig. 10.12). They require membrane-bound fatty acid elongase system. It involves condensation with two-carbon unit at a time, followed by reduction, dehydration, and another reduction of the intermediates in sequential order similar to FAS reactions. NADPH is used as the reductant. However, reactions of fatty acid elongation differ from FAS reactions in the following: (1) FAS reactions occur in plastids, while the reaction involving fatty acid elongation occurs in ER membranes. (2) ACP is not involved, and all the intermediates are activated as Coenzyme A esters. (3) The enzyme is elongase 3-ketoacyl-CoA synthase (elongase KCS), which catalyzes condensation with malonyl- CoA. (4) After condensation of two carbon groups from malonyl-CoA with elongating acyl groups of fatty acid, fatty acids undergo another cycle involving two reductions and a dehydration. Fatty acids are also utilized in other pathways of lipid metabolism (Fig. 10.13).
Fatty acid biosynthesis and their further modifications. Before fatty acyl groups are transported out form the plastid, ACP is released from fatty acyl-ACP by action of acyl ACP thioesterase (1) localized in the inner envelope of the plastid. Two and three are acyl-CoA synthetases localized in the outer envelope of the plastids, which catalyze synthesis of fatty acyl-CoA from the released fatty acids. Mostly modifications of the hydrocarbon chain of fatty acids occur via membrane-bound phosphatidylcholine (PC)-specific desaturases or desaturase-like enzymes. Acyl editing occurs via PC-dependent desaturases or desaturase-like enzymes
3.9 Synthesis of Unsaturated Fatty Acids
First double bond in fatty acid molecules is introduced in plastids itself. Stearoyl-ACP (18:0) is desaturated to oleoyl-ACP (18:1), catalyzed by a soluble, plastid localized stearoyl-ACP Δ9-desaturase. However, a family of this enzyme is responsible for introduction of double bonds at various locations of acyl chain, including the one which catalyzes desaturation of palmitic acid at Δ4 and Δ6 locations. Studies with mutants of Arabidopsis have shown the presence of eight genes which encode fatty acid desaturases (FAD). Out of these three have been found to encode for the soluble chloroplast desaturase enzyme and three for the membrane-bound enzyme in the endoplasmic reticulum. Chloroplast enzymes use soluble ferredoxin as the immediate electron donor, and the desaturated fatty acids are esterified as galactolipids, sulfolipids, and phosphatidylglycerol. ER membrane-localized plant desaturases utilize cytochrome b5 instead of ferredoxin and act on fatty acids esterified to phosphatidylcholine and possibly other phospholipids. Ferredoxin and cytochrome b5 are used as intermediate electron donors for the reaction. Except stearoyl-ACP Δ9-desaturase, which is soluble and is present in the stroma of plastids, other desaturases are membrane bound. FAD in ER membrane is responsible for desaturation of acyl groups as phosphatidylcholine. Possible expression of genes encoding desaturases is regulated by temperature in which plants grow or some post-translational modification of the fatty acids is required, resulting in alteration of fatty acid composition of membranes (Box 10.1).
4 Biosynthesis of Membrane Lipids
Unlike storage lipids, which are neutral lipids (triacylglycerol), membrane lipids are amphipathic. Amphipathic nature of membrane lipids makes it possible for them to organize to form bimolecular layer of lipids with their hydrophilic ends facing toward aqueous cytosolic side, while their nonpolar ends face each other creating hydrophobic environment within two layers of lipids. Membrane lipid synthesis, in plastids and in the endoplasmic reticulum, is known as prokaryotic and eukaryotic way of synthesis, respectively. Though each cell synthesizes its own membrane lipids, intracellular transport of lipids occurs between different subcellular compartments. In plastids (prokaryotic way), fatty acids are esterified with C 1 and 2 of glycerol phosphate. Glycerol phosphate is obtained after reduction of ketone group of dihydroxyacetone-P (DHAP). Transfer of acyl group from acyl-ACP is facilitated by acyl-ACP-glycerol-3-phosphate acyltransferases. It is oleoyl group (18:1) which is esterified to C1 hydroxyl group of glycerol phosphate, followed by esterification of C2 hydroxyl group with palmitoyl group (16:0) catalyzed by acyl-ACP transferases. This results in the formation of phosphatidic acid. In eukaryotic way, biosynthesis of membrane lipids occurs in membranes of the endoplasmic reticulum and is catalyzed by membrane-bound enzymes. Unlike in plastids, lipid biosynthesis in the endoplasmic reticulum (eukaryotic way) does not involve acyl-ACP; rather the activated forms of fatty acids are used as acyl-CoA. In the endoplasmic reticulum (eukaryotic way), C2 hydroxyl groups of DHAP is esterified only with unsaturated fatty acids, such as oleoyl acyl (18:1) group. Synthesis of phospholipids containing different polar groups, which are characteristic of membranes in a cell, occurs in different compartments of the cell via CDP-DAG (cytidine diphosphate-diacylglycerol) and DAG (diacylglycerol) pathway (Fig. 10.14). Contribution of each pathway varies among organisms.
At least some of the lipids which are synthesized and modified in the endoplasmic reticulum are exported to plastids to be incorporated in thylakoids. After initial desaturation of stearic group to oleoyl in plastids, further desaturation occurs as acyl groups of phosphatidylcholine in ER membranes. After being transported back to plastids as phosphatidylcholine, fatty acids are transferred to the glycerol backbone to form diacylglycerols. One or two galactose groups are added to diacylglycerol to form galactolipids in the chloroplast envelope which are then inserted in thylakoids. Acyl groups can be further desaturated by FADs present in plastid envelope as well. Galactolipids contain 18:2 and 18:3 acyl groups of the fatty acids. Thylakoids make 80% of the acyl pool of leaf cells, either as monogalactolipids or digalactolipids. In pea and barley leaves, a majority of galactolipids of thylakoids are derived from lipids synthesized in the endoplasmic reticulum by eukaryotic pathway. Phosphatidylglycerol (PG) is the only phospholipid present in thylakoids, which is derived by prokaryotic pathway. However, this (PG) forms only a minor component in thylakoids. In some plants, such as in spinach, a majority of plastid lipids are derived from prokaryotic pathway (Figs. 10.14 and 10.15).
5 Biosynthesis of Storage Lipids in Seeds
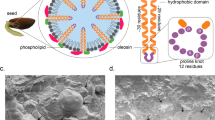
Lipids constitute less than 5% of the total dry weight of most of the vegetative tissues in plants, and only 1% are present as triacylglycerols. Contrary to this, lipids may constitute as much 60% of the dry weight in oil seeds, and 95% of these lipids are stored as triacylglycerols. This shows tissue-specific regulation of metabolism for the synthesis of neutral fats in seeds. Triacylglycerols are synthesized in the endoplasmic reticulum from fatty acids exported out of plastids via specific carriers present in plastid envelope. There are two most important pathways for synthesis of triacylglycerols in plants: (1) Phosphatidate is synthesized by acylation at C1 and C2 carbon atoms of glycerol phosphate. Esterification of hydroxyl group at C1, which results in synthesis of lysophosphatidate, is catalyzed by the enzyme acyl-CoA: glycerol-3-phosphate acyltransferase (GPAT). It is followed by esterification at C2, resulting in the synthesis of phosphatidic acid. The second reaction is catalyzed by acyl-CoA: lysophosphatidate acyltransferase (LPAT). Hydrolysis of phosphate group of phosphatidic acid occurs followed by acylation at C-3 position. Reaction is catalyzed by the enzyme acyl-CoA: diacylglycerol acyltransferase (DGAT) resulting in synthesis of triacylglycerol. (2) The second pathway involves phosphatidylcholine (PC). Membrane-bound fatty acid desaturases (FAD2 & FAD3) catalyze desaturation of acyl residues of PC (18:1Δ9 is desaturated to 18:2Δ9,12 and 18:3Δ9,12,15) in the ER. Acyl groups may also be modified by introduction of hydroxy or epoxy groups by desaturase like enzymes. This is followed by hydrolysis of choline phosphate, resulting in synthesis of diacylglycerol. Reaction is catalyzed by the enzyme phosphatidylcholine: diacylglycerol cholinephosphotransferase (PDCT). Esterification of diacylglycerol at C-3 position, to produce triacylglycerol, is catalyzed by acyl-CoA: diacylglycerol acyltransferase (DGAT) (Fig. 10.16). Relative contribution of these two pathways for in vivo biosynthesis of triacylglycerol in seeds varies in different species. Oleosomes are specialized subcellular structures, which are specifically adapted for storage of neutral fats in oilseeds and pollen grains. Their membrane consists of a monolayer of lipids with hydrophilic ends facing toward cytosolic side and hydrophobic ends facing inside toward the stored triacylglycerols. Oleosin are specific types of proteins present in lipid membranes of oleosomes. These are low-molecular-mass (15–25 kDa) proteins having a central domain consisting of about 70–80 hydrophobic amino acids, which protrudes toward the triacylglycerol core of the oil body. Oleosomes originate on the endoplasmic reticulum membrane. Within the ER membrane bilayer, triacylglycerols are synthesized, and the monolayer of membrane along with triacylglycerols is pinched off resulting in the formation of oil bodies. Diameter of oil bodies varies from 0.5 to 2 μm; however, it may vary from 0.5 to 30 μm. Seeds are extremely dehydrated structures, and oleosins play an important role in stability of the oil body. Oleosins probably also have an important role in maintaining the viability of seeds during dehydration. At the time of seed germination, oleosins play a critical role during extreme hydration associated with imbibition of water. In addition to oleosins, oil bodies also contain caleosins and steroleosins which play a signaling role during oil body mobilization. In fruits such as avocado and olive, oil bodies contain triacylglycerols. Triacylglycerols in seed oil may include unusual acyl groups exhibiting a great diversity. This includes fatty acyl groups with more unsaturated, branched, and longer hydrocarbon chains (Figs. 10.17 and 10.18).
6 Lipid Catabolism
In oil seeds and pollen grains, lipids are stored as neutral lipids which constitute as much as ~60% of dry weight of the seeds. Triglycerides are stored either in the endosperm or in cotyledons of the oil seeds. At the time of germination of oil seeds, triacylglycerides (TAG) are hydrolyzed by lipases resulting in the formation of glycerol and fatty acids. Lipases bind to oleosins of the oil bodies and hydrolyze TAG at the oil/water interface. In castor bean, there are two types of lipases, acid lipases which act on mono-, di-, or triglycerides. These are active in dry seeds also and are localized in oil bodies. On the contrary, alkaline lipases are present in membranes of peroxisomes, which are active only after water is imbibed by the seeds. Alkaline lipases act on monoglycerides.
Glycerol is metabolized in the cytosol of the cell, while fatty acids are transported to peroxisomes (specialized peroxisomes present in nongreen parts are called glyoxysomes). Glycerol is converted to dihydroxyacetone phosphate (DHAP) by the action of glycerol kinase (GLK) and FAD-linked glycerol-3-phosphate dehydrogenase (GDH). DHAP is metabolized through glycolysis. Fatty acids are transported to peroxisomes. A transporter, identified in Arabidopsis, is an ATP-binding cassette (ABC) transporter, which is localized in the membrane of peroxisomes. The peroxisomal ABC transporter comatose (CTS) is required for transporting acyl groups to peroxisomes. Once fatty acyl groups have been transported to peroxisomes, these are activated to form fatty acyl-CoA. This reaction is catalyzed by long-chain fatty acyl-CoA synthetase (LACS), LACS6, and LACS7. ATP and coenzyme A are required for activation of fatty acyl groups to fatty acyl-CoA. Since fats are not transported, these need to be converted to sugars at the time of germination in seeds. During senescence, β-oxidation of degradation products of the membrane lipids occurs in leaves, though at much lower rates. The fatty acids, which are no longer required, are recycled, and the carbohydrates so synthesized are transported to other plant parts. Conversion of fatty acids to sugars involves (1) β-oxidation of fatty acids, (2) glyoxylate cycle, and (3) gluconeogenesis. The organelles involved are peroxisomes (glyoxysomes), mitochondria, and cytosol. Because of the absence of glyoxylate cycle in animals, fats are not converted to sugars (Fig. 10.19).
6.1 β-Oxidation of Fatty Acyl-CoA
β-Oxidation involves breaking down of fatty acyl-CoA with the removal of two-carbon fragment at a time, as acetyl-CoA. Through repeated cycles of β-oxidation, there is complete degradation of fatty acyl-CoA to acetyl-CoA. The number of acetyl-CoA molecules produced depends upon the length of hydrocarbon chain in fatty acids. Unlike in animal cells, where β-oxidation occurs in the mitochondria, in plants it occurs in glyoxysomes. Glyoxysomes are specialized peroxisomes present in endosperm cells of the germinating oil seeds. Glyoxysomes are so called because glyoxylate cycle occurs there. These are found adjacent to oil bodies. β-Oxidation of fatty acids involves the following steps which are similar in plants and animals: (1) Dehydrogenation of fatty acyl-CoA at two- and three-carbon atoms resulting in production of a Δ-2 trans-unsaturated fatty acyl-CoA molecule (trans-Δ2-enoyl-CoA) is catalyzed by acyl-CoA oxidase; (2) hydration of trans-Δ-2-enoyl, which results in formation of ι-hydroxyacyl-CoA, is catalyzed by enoyl-CoA hydratase; (3) oxidation of ι-hydroxyacyl-CoA is catalyzed by β-hydroxyacyl-CoA dehydrogenase resulting in synthesis of a ketone, β-ketoacyl-CoA; (4) thiolytic cleavage of β-ketoacyl-CoA by coenzyme A resulting in the formation of acetyl-CoA and fatty acyl-CoA, which is two carbons shorter. Reaction is catalyzed by acyl-CoA acetyltransferase (thiolase). Similar sets of four steps of β-oxidation of fatty acyl-CoA are repeated in a cyclic manner. During each cycle, a two-carbon fragment would be released as acetyl-CoA, and a two-carbon shorter fatty acyl-CoA is produced. Thus, 16-carbon long palmitoyl-CoA will require 7 cycles of β-oxidation to be completely converted to 8 molecules of acetyl-CoA (Fig. 10.20).
Unlike in animals where enzymes of β-oxidation are separate polypeptides, in plants enzymes catalyzing the second and third step of the pathway are separate domains of a multifunctional protein (MFP), while enzymes catalyzing the first and fourth steps are separate polypeptides. Other two domains of MFP include the auxiliary activities required for oxidation of unsaturated fatty acids (D-3-hydroxyacyl-CoA epimerase and Δ3, Δ2-enoyl-CoA isomerase) (Fig. 10.21). Though mitochondria are the principal sites for β-oxidation in animals, it occurs in peroxisomes also. In first oxidation step during β-oxidation in peroxisomes, two electrons are transferred directly to O2 which is catalyzed by a flavin-linked oxidase, acyl-CoA oxidase. It results in production of H2O2, and energy is released as heat. Instead, in the mitochondria, during first oxidation step, acyl-CoA dehydrogenase catalyzes transfer of the electrons to O2 through electron transport chain, resulting in synthesis of ATP. H2O2 produced in peroxisomes/glyoxysomes is toxic. Two enzyme systems present in peroxisomes/glyoxysomes detoxify H2O2. Catalase, a soluble enzyme, immediately degrades H2O2 to H2O and O2.
In plants, enzymes involved in β-oxidation are present as a complex (MFP); E1, acyl-CoA dehydrogenase; E2, enoyl-CoA hydratase; E3, L-β-hydroxyacyl-CoA dehydrogenase; E4, thiolase; E5, D-3-Hydroxyacyl-CoA epimerase; E6, Δ3, Δ2-enoyl-CoA isomerase; E1 & E4 are separate polypeptides, while E2 and E3 along with other two enzymes which are involved in oxidation of unsaturated fatty acids (E5 & E6) are separate domains of same polypeptide
Second way by which H2O2 is detoxified is by membrane-associated enzymes, ascorbate peroxidase and NADH-dehyroascorbate reductase.
Second oxidation step in β-oxidation involves dehydrogenation of β-ι-hydroxyacylacyl-CoA by the enzyme β-hydroxyacyl-CoA dehydrogenase. This oxidation reaction is coupled with reduction of NAD+ to NADH. NADH needs to be oxidized for continuation of β-oxidation of fatty acids. Since, unlike in the mitochondria, electron transport chain is not present in peroxisomes/glyoxysomes, NADH is oxidized, coupled with reduction of monodehydroascorbate (MDA). The reaction is catalyzed by the enzyme MDAR (MDA reductase). NADH oxidation also occurs by malate dehydrogenase (MDH), which catalyzes reduction of oxaloacetate to malate in peroxisomes. Malate, after being transported to cytosol, is oxidized to oxaloacetate, which is coupled with reduction of NAD+ to NADH. Apparently, reactions of β-oxidation of fatty acids appear to be reversible of the reactions which occur during synthesis of fatty acids. However, a comparison between the two is listed in Table 10.5.
6.2 Glyoxylate Cycle
Since in animal cells, β-oxidation occurs mainly in the mitochondria, acetyl-CoA is metabolized through TCA cycle. In germinating oil seeds, however, β-oxidation occurs in glyoxysomes. Acetyl-CoA, produced during β-oxidation in glyoxysomes, is the source for many of the precursors required at the time of germination of seeds and growth of the seedlings. Acetyl-CoA is also the precursor for sugar biosynthesis. Conversion of acetyl-CoA to sugars involves glyoxylate cycle, TCA cycle, and gluconeogenesis which occur in glyoxysomes, mitochondria, and cytosol, respectively. Two enzymes of glyoxylate pathway, i.e., isocitrate lyase and malate synthase, are specific for glyoxysomes. Isocitrate lyase catalyzes hydrolysis of isocitrate to a four-carbon dicarboxylic acid succinate and a two-carbon compound, glyoxylate. Succinate is exported out of glyoxysomes and is transported to mitochondria where it is converted to OAA through TCA cycle. Oxaloacetate is exported out of mitochondria to the cytosol and is converted into phosphoenolpyruvate (PEP) through the action of phosphoenolpyruvate carboxykinase (PCK). Synthesis of sugars from PEP occurs through gluconeogenesis. Other enzyme, which is specific for glyoxysomes, is malate synthase, which catalyzes synthesis of malate from glyoxylate and acetyl-CoA. Enzymes for two of the reactions of glyoxylate pathway are present in the cytosol. These include aconitase and malate dehydrogenase (MDH), which catalyze synthesis of isocitrate from citrate and dehydrogenation of malate, respectively. In the cytosol MDH catalyzes dehydrogenation of malate resulting in the production of OAA and reduction of NAD+ to NADH. Oxaloacetate is reduced to malate in glyoxysomes after it is transported back which is associated with oxidation of NADH. Oxidation of NADH is required for continuity of oxidation of fatty acids. Entry of β-oxidation product, acetyl-CoA, directly into TCA will result in saving of one carbon since the link reaction is bypassed.
6.3 Gluconeogenesis
Oxaloacetate exported from the mitochondria is converted to sugars in the cytosol. Phosphoenolpyruvate carboxykinase catalyzes the conversion of oxaloacetate to phosphoenolpyruvate (PEP). The reaction is irreversible and requires ATP, and one carbon is lost as CO2.
PEP is converted to sugars by reverse reactions of glycolytic pathway, which are known as gluconeogenesis. Since gluconeogenesis occur in the cytosol along with glycolysis, an understanding of regulation of both pathways is required. Only three carbons of OAA are used for conversion to sugars, since there is a loss of one carbon as CO2 in the reaction catalyzed by PCK. Thus, only 75% of the carbon is utilized for the production of sugars, and there is a loss of 25% of the carbon. Seven out of ten reactions of glycolysis are reversible, while three reactions are irreversible. These include conversion of PEP to pyruvate, fructose-6-phosphate to fructose-1,6-bisphosphate, and glucose to glucose 6-phosphate, catalyzed by PEP kinase, phosphofructokinase, and hexokinase, respectively. During gluconeogenesis, reaction involving conversion of pyruvate to PEP is bypassed, since PEP produced from OAA is available. Conversion of fructose-6-phosphate to fructose-1,6-biphosphate is the main regulatory reaction, which determines whether sugars would be catabolized through glycolysis or sugars will be produced by gluconeogenesis (Fig. 10.22). During glycolysis, the reaction is catalyzed by phosphofructokinase, while another enzyme fructose-1,6-bisphosphatase (FBPase) catalyzes the hydrolysis of phosphate group linked to C-1 of fructose-1,6-bisphosphate, in gluconeogenesis.
Thus, the enzymes catalyzing the reactions are different in gluconeogenesis and glycolysis. Regulation of FBPase and PFK determines the direction of the pathways, whether sugars will be synthesized by gluconeogenesis or will be catabolized through glycolysis. Both pathways occur in the cytosol. Metabolites which regulate activity of FBPase include fructose-2,6-bisphosphate. Low levels of fructose-2,6-bisphosphate (FBP) promote activity of FBPase. As a result, sucrose synthesis will be favored, whereas high concentration of fructose-2,6-bisphosphate inhibits FBPase, inhibiting sucrose synthesis. Intracellular concentration of fructose-2,6-bisphosphate is controlled by a kinase and a phosphatase. Fructose-2,6-bisphospate kinase is responsible for the synthesis of fructose-2,6-bisphosphate, and its degradation is catalyzed by fructose2,6-bisphosphatase. Activity of FBP kinase is promoted by high concentrations of F-6,P and Pi, thereby increasing F2,6-BP concentrations in the cell. This would inhibit gluconeogenesis and promote glycolysis. On the contrary, accumulation of triose phosphates and glycerate 3-phosphate promotes the activity of FBPase, in turn decreasing F2,6-BP concentrations, leading to promotion of gluconeogenesis (Fig. 10.22). Third irreversible step of glycolysis includes conversion of glucose to glucose 6-phosphate. This reaction is also bypassed in gluconeogenesis. Glucose 6-phosphate is converted to glucose 1-phosphate by phosphoglucomutase. Glucose 1-phosphate is the precursor for sucrose biosynthesis. Sucrose thus synthesized is translocated to the growing parts of the seedlings from the storage regions of the germinating seeds, i.e., either from the endosperm (castor seeds) or from cotyledons of the seeds (sunflower). A comparison of lipid metabolism in plants and animals is given in Table 10.6.
6.4 α-Oxidation of Fatty Acids
Generally fatty acids contain an even number of carbon atoms since these are synthesized as a result of addition of two carbon units. However, fatty acids with odd-number carbons also occur in plants and in brain cells of animals. α-Oxidation of fatty acids with an even number of carbon atoms results in the synthesis of fatty acids with odd-number carbon atoms. Generally, fatty acids with C13-C18 are oxidized by α-oxidation which involves sequential removal of α-carbon as CO2 from carboxylic end of the fatty acids. Unlike β-oxidation, there is no energy release and no involvement of CoA-SH intermediates during α-oxidation. α-Oxidation of fatty acids is possibly involved in the degradation of long-chain fatty acids. In humans also, it occurs in peroxisomes and is involved in the oxidation of dietary phytanic acid (a branched-chain fatty acid obtained by humans from dairy products), which cannot undergo β-oxidation because of its methyl branches.
6.5 ω-Oxidation of Fatty Acids
Some fatty acids, which are constituents of cutin and suberin, have hydroxyl group or carboxylic group at the ω-methyl terminal. These are produced as a result of ω-oxidation. Fatty acids having carboxylic groups at both of their ends are dicarboxylic acids which are characteristic of suberin. During ω-oxidation, molecular oxygen is used, and it requires involvement of cytochrome P450. ω-Oxidation occurs in the endoplasmic reticulum.
6.6 Catabolism of Unsaturated Fatty Acids
Besides saturated fatty acids, catabolism of unsaturated fatty acids is also significant both among animals and plants since most of the fatty acids of triacylglycerol and phospholipids are unsaturated. Catabolism of unsaturated fatty acids involves additional enzyme-catalyzed reactions. Most of the unsaturated fatty acids have double bonds present in cis configuration, the first double bond being present between ninenth and tenth carbon atom from the carboxylic end. These double bonds are not acted upon by the enzymes of β-oxidation, which recognize only trans configuration. Thus, catabolism of unsaturated fatty acids requires a change in configuration of double bonds from cis to trans. One of the abundant unsaturated fatty acids, i.e., oleic acid, has the structure 18:1Δ9. Three cycles of β-oxidation will remove 6 carbons as three acetyl-CoA, with a 12-carbon fatty acid which is produced with double bond present at the third carbon atom having a structure 12:1Δ3cis. This intermediate compound is not the substrate for enoyl-CoA hydratase which recognizes the trans double bonds only. An isomerase, Δ3, Δ2-enoyl CoA isomerase will shift the double bond from third to second position and cis form of double bond to trans configuration, resulting in conversion of 12:1, cis-Δ3 enoyl-CoA to 12:l trans-Δ2 enoyl-CoA. This is followed by the action of enoyl-CoA hydratase which results in a hydroxy intermediate, ι-β-hydroxy-CoA. This is the substrate for another dehydrogenase (similar to β-oxidation), followed by subsequent β-oxidation reactions (Fig. 10.23).
6.7 Catabolism of Fatty Acids with Odd Number of Carbon Atoms
Plants have fatty acids with odd numbers of carbons as constituents of lipids. These are oxidized and degraded till propionyl-CoA and acetyl-CoA are produced as a result of the last cycle of β-oxidation, unlike the formation of two molecules of acetyl-CoA. Propionyl-CoA is catabolized due to additional enzymes, i.e., propionyl-CoA carboxylase, methyl malonyl-CoA epimerase, and methyl malonyl-CoA mutase. The reaction catalyzed by propionyl-CoA carboxylase is an ATP-requiring reaction, which requires biotin as the cofactor, and it results in production of D-methyl malonyl-CoA. D-methyl malonyl-CoA is converted to its isomer L-methyl malonyl-CoA by the enzyme methyl malonyl-CoA epimerase. Intramolecular rearrangement of the L-methyl malonyl-CoA molecule is catalyzed by the enzyme methyl malonyl-CoA mutase resulting in the production of succinyl-CoA which is metabolized by the cell (Fig. 10.24).
Complete oxidation of fatty acids with odd-number carbon atoms occurs through β-oxidation similar to fatty acids with an even number of carbon atoms. Last cycle of β-oxidation generates acetyl-CoA and propionyl-CoA instead of two molecules of acetyl-CoA. Propionyl-CoA is catabolized through different pathways which is shown here in this figure
Summary
-
Lipids are important biomolecules having diverse functions. These are stored as neutral fats and waxes. Membrane lipids include glycerophospholipids, glycolipids, sphingolipids, and sterols. Extracellular lipids, such as cutin and suberin, serve to protect plants from water loss by evaporation. Lipids also function as signaling molecules and as cofactors of various enzymes.
-
In plants, fatty acids with carbon atom numbers up to 18 are synthesized in plastids, unlike in animals where fatty acid biosynthesis occurs in the cytosol. However, both in animals and plants, further elongation of the carbon chain and desaturation of fatty acids occur in the endoplasmic reticulum.
-
Precursors for fatty acids biosynthesis are acetyl-CoA and malonyl-CoA. Acetyl Co-A is synthesized from pyruvate which is generated during glycolysis of sugars from either Calvin cycle in plastids or has been imported from the outside in nongreen plastids. Malonyl-CoA is produced as a result of carboxylation of acetyl-CoA catalyzed by acetyl-CoA carboxylase (ACCase), which has biotin as the cofactor. In plants, ACCase consists of four polypeptide subunits, while in animals, the enzyme consists of single polypeptide with separate catalytic domains (multifunctional protein).
-
Biosynthesis of fatty acids is catalyzed by the enzyme fatty acid synthase (FAS). Except ACCase, FAS refers to all other catalytic activities during fatty acid biosynthesis. These include malonyl/acetyl-CoA ACP transferase (MAT), β-ketoacyl-ACP synthase (KAS), β-hydroxyacyl-ACP dehydratase, enoyl-ACP reductase, and β-ketoacyl-ACP reductase. In plants FAS II consists of separate polypeptides for different catalytic activities, while in animals, FAS I is a multifunctional protein having separate catalytic domains for each catalytic activity. Acyl moiety is hydrolyzed from ACP by thioesterase.
-
Fatty acids with a chain length up to 16 carbons or 18 carbons are synthesized in plastids depending on the plant type. Further elongation or desaturation occurs in the endoplasmic reticulum after these are transported out as CoA derivatives. Elongated of fatty acids occurs as CoA derivatives and not as derivatives of ACP. The mechanism of elongation of fatty acid hydrocarbon chains and the enzymes required are similar to those of fatty acid synthesis. Unlike in animals, a soluble desaturase present in the stroma of plastids catalyzes formation of oleoyl-ACP. Other desaturases are present in bound form within membranes of the endoplasmic reticulum.
-
In plants, membrane lipids are synthesized both in plastids (prokaryotic way) and the endoplasmic reticulum (eukaryotic way). At least some of the membrane lipids in plastids are imported from the endoplasmic reticulum.
-
Neutral fats are synthesized in the endoplasmic reticulum by two ways. In one of the ways, glycerol 3-phosphate is acylated first at C1 and C2 carbons followed by hydrolysis of phosphate group and its acylation. Second way includes modification of the acyl groups as phosphatidylcholine followed by hydrolysis of choline phosphate and acylation. Triacylglycerol synthesis occurs within the lipid bilayer of the endoplasmic reticulum. The outer lipid layer is pinched off as oleosomes.
-
Oleosomes are surrounded by a monolayer of lipids in which specific types of proteins including oleosins are present. Oleosins have their hydrophobic domains exposed toward the inside of the oleosomes, while the hydrophilic N- and C-terminal ends are exposed toward the cytosolic side.
-
Lipid catabolism is significant in germinating oil seeds. It involves hydrolysis of fats, β-oxidation of fatty acids, glyoxylate cycle, and gluconeogenesis, which occur in the organelles oleosomes, glyoxysomes, mitochondria, and cytosol, respectively. Sugars thus synthesized are translocated to the meristematic regions of the seedlings.
-
Catalytic domains for β-oxidation of fatty acids in plants are present in separate polypeptides, unlike in animals, where these are present as multifunctional proteins.
Suggested Further Readings
Browsher C, Steer M, Tobin A (eds) (2008) Plant biochemistry. Garland Science, Tailor & Francis Group, New York, pp 303–329
Heldt HW (2005) Plant biochemistry, 3rd edn. Elsevier Academic Press, Burlington, pp 363–396
Nelson DL, Cox MM (2017) Lehninger principles of biochemistry, 7th edn. WH Freeman, MacMillan Learning, New York, pp 649–668
Ohlrogge J, Browse J, Jaworski J, Chris S (2013) Lipids. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. Wiley-Blackwell, Chichester, pp 337–396
Author information
Authors and Affiliations
Multiple-Choice Questions
Multiple-Choice Questions
-
1.
The lipids which form the major component of thylakoids are:
-
(a)
Phospholipids
-
(b)
Sphingolipids
-
(c)
Galactolipids
-
(d)
Neutral lipids
-
(a)
-
2.
In plants fatty acid biosynthesis occurs in:
-
(a)
Plastids
-
(b)
Peroxisomes
-
(c)
Mitochondria
-
(d)
Cytosol
-
(a)
-
3.
First step in fatty acid biosynthesis is catalyzed by:
-
(a)
Acetyl-CoA synthase
-
(b)
Carboxyl transferase
-
(c)
Malonyl-CoA:ACP transacylase
-
(d)
Acetyl-CoA carboxylase
-
(a)
-
4.
At the time of fatty acid synthesis, the cofactor to which acyl group is attached during reactions involving two reductions and dehydration is:
-
(a)
Coenzyme A
-
(b)
Fatty acid synthase
-
(c)
ACP
-
(d)
Ketoacyl-ACP synthase
-
(a)
-
5.
Initial condensation of acetyl CoA with malonyl-ACP resulting in production of acetoacetyl-ACP is catalyzed by:
-
(a)
KAS I
-
(b)
KAS II
-
(c)
KAS III
-
(d)
3-Ketoacyl-ACP reductase
-
(a)
-
6.
In the endoplasmic reticulum, modification of hydrocarbon chain of fatty acids requires:
-
(a)
Fatty acyl-ACP
-
(b)
Fatty acyl-CoA
-
(c)
Thioesterase
-
(d)
FAS
-
(a)
-
7.
In a prokaryotic way, synthesis of membrane lipids involves:
-
(a)
Esterification of C1 hydroxyl group of glycerol phosphate with 16:0 FA and C2 hydroxyl group with 18:0 FA
-
(b)
Esterification of C1 hydroxyl group of glycerol phosphate with 18:1 FA and C2 hydroxyl group with 16:0 FA
-
(c)
Esterification of both C1 and C2 of glycerol phosphate with 18:1 FA
-
(d)
Esterification of both C1 and C2 of glycerol phosphate with 16:0 FA
-
(a)
-
8.
Conversion of stearoyl-ACP to oleoyl-ACP (tick the correct answer):
-
(a)
Is catalyzed by a soluble desaturase present in the stroma of plastids
-
(b)
Is catalyzed by desaturase present in the envelope of the plastid
-
(c)
Is catalyzed by desaturase present in the ER membrane
-
(d)
Is catalyzed by a soluble desaturase present in the cytosol
-
(a)
-
9.
In plants β-oxidation of fatty acids occurs in the:
-
(a)
Mitochondria
-
(b)
Cytosol
-
(c)
Oleosomes
-
(d)
Glyoxysomes
-
(a)
-
10.
Which of the following statements is correct?
-
(a)
In plants acyl-CoA oxidase catalyzes transfer of electrons O2 during first oxidation step in β-oxidation of fatty acids.
-
(b)
Acyl-ACP is transported out of plastids to be used for further modifications in the hydrocarbon chain of fatty acids.
-
(c)
Oleosin is the membrane lipid of oleosomes.
-
(d)
Glyoxylate cycle occurs both in plants and animals.
-
(a)
Answers
1. c | 2. a | 3. d | 4. c | 5. c | 6. b | 7. b |
8. a | 9. d | 10. a |
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
A. Lal, M. (2018). Lipid Metabolism. In: Plant Physiology, Development and Metabolism. Springer, Singapore. https://doi.org/10.1007/978-981-13-2023-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-2023-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2022-4
Online ISBN: 978-981-13-2023-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)