Abstract
The geology of the Lesser Caucasus is complex, owing to accretion of terrains through plate-tectonic processes and to ongoing tectonic activity and volcanism. Numerous geothermal springs of different geotectonic origins and with different physicochemical properties are found on the territory of the Lesser Caucasus. Despite intensive microbiological studies on terrestrial geothermal springs in various regions of the globe, very little is known about microbial diversity of similar ecosystems in the Lesser Caucasus. Recently the phylogenetic diversity of the prokaryotic community thriving in some geothermal springs located on the territory of Armenia, Georgia, and Nagorno-Karabakh has been explored following both cultivation-based and culture-independent approaches. Despite previous efforts, a comprehensive census of the microbial communities in the Lesser Caucasus hot springs is still lacking. This chapter contains a review of the results of microbial diversity analyses of 11 geothermal springs of the Lesser Caucasus with special emphasis to its distribution, ecological significance, and biotechnological potential.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Lesser Caucasus
- Geothermal springs
- Microbial diversity
- Thermophiles
- Culture-dependent and culture-independent techniques
4.1 Introduction
Natural geothermal springs, including terrestrial hot springs, are widely distributed in various regions of our planet and are primarily associated with tectonically active zones in areas where the Earth’s crust is relatively thin. These habitats have attracted broad interest since they are analogs for primitive Earth (Stan-Lotter and Fendrihan 2012). Geothermal springs offer a new source of fascinating microorganisms with unique properties well adapted to these extreme environments (Hreggvidsson et al. 2012; Deepika and Satyanarayana 2013). The adaptation to these harsh habitats makes thermophiles and their thermostable proteins suitable for various industrial and biotechnological applications (Raddadi et al. 2015; DeCastro et al. 2016).
The scientific interest in the microbial diversity of these exotic niches has increased during the last decades. With time, the tools used for microbial exploration have improved. Initially, studies were incepted with culture-based approaches. In recent time, culture-independent techniques (16S rRNA gene-based clone library analysis, denaturing gradient gel electrophoresis (DGGE), pyrosequencing, metagenomics, and metatranscriptomics) are mostly being used (Bhaya et al. 2007; Liu et al. 2011; López-López et al. 2013; DeCastro et al. 2016). This has shifted the cultivation-based narrow view into a more detailed and holistic insight of hot spring microbial habitats in terms of diversity, adaptation, functions, and ecological significance. Using a combination of several approaches of traditional microbiology with state-of-the-art molecular biology techniques has substantially increased our understanding of the structural and functional diversity of the microbial communities. Such approaches has been extensively used to study microbiota of the geothermal springs located in Iceland (Krebs et al. 2014), Azores (Sahm et al. 2013), the United States (Meyer-Dombard et al. 2005; Bowen De León et al. 2013), Bulgaria (Stefanova et al. 2015), Russia (Kublanov et al. 2009), China (Hedlund et al., 2012; Hou et al. 2013), India (Singh and Subudhi 2016; Saxena et al. 2017; Poddar and Das 2017), Malaysia (Chan et al. 2015), Argentina (Urbieta et al. 2015), Turkay (Cihan et al. 2011), Italy (Maugeri et al. 2009), Thailand (Portillo et al. 2009), New Zeland (Hetzer et al. 2007), Tunisia (Sayeh et al. 2010), Marocco (Aanniz et al. 2015) Romania (Coman et al. 2013), Spain (López-López et al. 2015) and other parts of world.
Thermal springs located in the Lesser Caucasus still represent a challenge for exploring biodiversity and searching of undescribed biotechnological resource. The geology of the region where Armenia, Georgia, and Nagorno-Karabakh are situated is complex, owing to accretion of terrains through plate-tectonic processes and to ongoing tectonic activity and volcanism (Henneberger et al. 2000; Badalyan 2000). Numerous geothermal springs with different geochemical properties are found on the territory of Lesser Caucasus. Despite a wide distribution of hot springs throughout Lesser Caucasus with hints of intrinsic scientific interest, limited attention has been paid toward microbiological analysis of these hot springs. With the best of information available, it was noted that data of microbial communities of several hot springs distributed on the territory of Armenia and Nagorno-Karabakh were published to date (Panosyan 2010; Hedlund et al. 2013; Panosyan and Birkeland 2014; Panosyan 2017; Panosyan et al. 2017). Despite these previous efforts, a comprehensive census of the microbial communities in Lesser Caucasus hot springs is still lacking.
The primary objective of this chapter is to review the findings of microbiological studies of several geothermal springs in the Lesser Caucasus and to summarize investigations on relationships between thermophilic microbial communities and geochemical conditions of their habitats. The results of this study expand the current understanding of the microbiology of hot springs in Lesser Caucasus and provide a basis for comparison with other geothermal systems around the world.
4.2 Geographical Distribution and Physiochemical Profiling of Geothermal Springs
The Caucasus Mountains include the Greater Caucasus in the north and Lesser Caucasus in the south (Stokes 2011). The Lesser Caucasus Mountains are formed predominantly of the Paleogene rocks with a smaller portion of the Jurassic and Cretaceous rocks. The formation of the Caucasus began from the Late Triassic to the Late Jurassic during the Cimmerian orogeny at the active margin of the Tethys Ocean while the uplift of the Greater Caucasus is dated to the Miocene during the Alpine orogeny. The Caucasus Mountains formed largely as the result of a tectonic plate collision between the Arabian plate moving northwards with respect to the Eurasian plate. This collision caused the uplift and the Cenozoic volcanic activity in the Lesser Caucasus Mountains. This region is regularly subjected to strong earthquakes from this activity (Reilinger et al. 1997). While the Greater Caucasus Mountains have a mainly folded sedimentary structure, the Lesser Caucasus Mountains are largely of volcanic origin (Philip et al. 1989). The geology of the region is complex, owing to accretion of exotic terranes through plate-tectonic processes and to ongoing tectonic activity and volcanism which have taken place more or less continuously since Lower Pliocene or Upper Miocene time.
The distribution of natural geothermal springs, including terrestrial hot springs (with water temperature higher than 21.1 °C), in various regions of our planet are primarily associated with tectonically active zones in areas where the Earth’s crust is relatively thin. On the territory of the Lesser Caucasus, where traces of recently active volcanic processes are still noticeable, many geothermal springs with different geotectonic origins and physicochemical properties are found (Mkrtchyan 1969, Kapanadze et al. 2010).
Although no high-temperature geothermal resources have been identified in Armenia, numerous low-temperature resource areas (cooler than 100 °C) are present. Geothermal springs distributed on the territory of Armenia have been catalogued and described, and hundreds of shallow wells have been drilled to investigate mineral water sources throughout the country (Mkrtchyan 1969).
Three main heat flow zones (northeastern, central, and southwestern) have been distinguished on the basis of heat flow and temperature gradients (Fig. 4.1). The central zone (Zone II), which coincides closely with the belt of Quaternary volcanoes, has highest heat flow (75 to more than 90 mW/m2) and elevated temperature gradients (generally greater than 50 °C/km). The Zone I is considered to have no significant potential for geothermal resources. In Zone III there are scattered occurrences of thermal water, despite the overall low heat flow in this region (Karakhanian et al. 1997; Henneberger et al. 2000).
Contour of heat flow with deduced heat flow zones in Armenia. (From Henneberger et al. 2000)
Nagorno-Karabakh is located in the southeastern part of the Lesser Caucasus. It is typically mountainous, embracing the eastern part of the Karabakh Plateau with the Artsakh valley, forming the great part of the Kura-Araks lowland. The Artsakh plateau like all Armenian plateaus is characterized by seismic activity. Volcanic rocks that appeared in ancient times are gaining ground: limestone and other sedimentary rocks from the Jurassic and Cretaceous period. Numerous geothermal springs at high elevations with different physicochemical properties are found also on the territory of Nagorno-Karabakh.
Georgia is located in the central and western parts of the Trans-Caucasus and lies between the Euro-Asiatic and Afro-Arabian plates. Apart from the Precambrian and Paleozoic formations that cover a smaller area, Mesozoic and Cenozoic rock assemblages mainly make up the geological structure of Georgia (Moores and Fairbridge 1998). Three major tectonic units can be distinguished according to the geologic development of Georgia: (1) the Greater Caucasus fold system, which represents a marginal sea in the geological past, (2) the Trans-Caucasus intermountain area which marks the northern part of the Trans-Caucasus island arc, and (3) the Lesser Caucasus fold system, the southern part of the ancient Trans-Caucasus island arc. The amount of thermal flow for the main parts of Georgia can be listed as follows:
-
1.
The south flank of Caucasus Mountains, 100 mWm2
-
2.
Plate of Georgia:
-
(a)
For the west zone 40 mWm2
-
(b)
For the east zone 30mWm2
-
(a)
-
3.
Adjara-Trialeti folded system:
-
(a)
Central part 90 mWm2
-
(b)
East zone 50 mWm2
-
(a)
-
4.
Artvin-Bolnisi platform 60 mWm (Achmadova 1991)
The maximum heat flow is observed for the central zone of folded part of Georgia and the minimum for the plate, while the Adjara-Trialeti folded system is characterized by the middle range (Bunterbart et al. 2009).
Physical conditions, especially temperature, are regarded as a key factor for correlating microbial abundance and diversity of a spring (Everroad et al. 2012). Hot springs in the Lesser Caucasus could be grouped into three categories based on intrinsic temperature: warm springs (20–37 °C), moderately hot or mesothermal springs (37–50 °C), and hot springs (>50 °C). Using a cutoff temperature of 20 °C to distinguish thermal from nonthermal waters, several thermal areas are known to exist in Armenia (Mkrtchyan 1969). Hot springs at Uyts have the lowest temperature (25.8 °C). The highest temperature has been recorded for hot springs at Jermuk (>53 °C) and Karvachar (70 °C) (Fig. 4.2). The studies of some higher-temperature geothermal springs (for instance, Jermuk spring, located in the Karabakh Upland along Armenia’s eastern border) using various geophysical surveys indicated that temperature at deeper levels (from 600 to 1000 m) can reach up to 99 °C (Karakhanian et al. 1997; Henneberger et al. 2000).
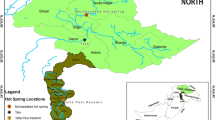
Map of the locations of microbiologically explored terrestrial geothermal springs in the Lesser Caucasus. Closeup photographs of some geothermal springs. (1) Samtredia (2) Tbilisi sulfur spring (3) Akhurik (4) Hankavan (5) Bjni (6) Arzakan (7) Jermuk (8) Tatev (9) Uyts (10) Karvachar (11) Zuar. The source of the map is http://www.geocurrents.info/place/russia-ukraine-and-caucasus/where-is-the-caucasus
Geothermal springs found on the territory of Nagorno-Karabakh are also mainly classified as springs with moderate temperature. Two of Nagorno-Karabakh geothermal springs located in Karvachar (≥70 °C) and Zuar (42 °C) are characterized with higher water temperature (Fig. 4.2).
Up to 250 natural thermal springs and artificial wells are known in Georgia with water temperature ranging between 30–108 °C (Fig. 4.3) (Kapanadze et al. 2010). The lowest water temperature geothermal springs (30–35 °C) are distributed all over the territory of Georgia but are mainly found in Borjomi, Tsikhisjvari, Tskaltubo, and Saberio areas, while the highest water temperatures (78–108 °C) have been recorded for the waters from the artificial wells and boreholes in West Georgia, such as the Zugdidi-Tsaishi, Kvaloni, and Kindgi regions (Tsertsvadze et al. 1998).
Distribution of thermal waters in Georgia (Kapanadze et al. 2010)
All studied Armenian and Nagorno-Karabakhian hot springs are neutral, moderately alkaline, or alkaline in nature. Most of the spring samples have neutral pH (7–7.5), but hot springs at Tatev, Ajhurik, and Uyts have pH lower than 7. The hot springs in Georgia range from alkaline to acidic, but most of them are close to neutral or weak alkaline. The highly acidic hot spring in Georgia with pH 2.2 is located in Vani region, village Tsikhesulori, while the alkaline springs are found in Tbilisi area with pH 9.7 (Tsertsvadze et al. 1998).
Compared to physical analysis, limited attention has been paid to chemical profiling of hot spring water or sediment samples. Hot spring water usually has high concentrations of various elements owing to mineralization of dissolved solid elements from the adjacent areas. The composition of hot water is mainly determined by chemical interactions with reservoir rocks and rock-forming minerals along the ascent path, which may cause the spring water to be acidic or alkaline. All of the Armenian and Nagorno-Karabakhian thermal waters studied have mixed-cation mixed-anion compositions. Total dissolved solids contents tend to be less than about 0.5 mg/l but are occasionally higher. As is typically the case, the hotter and more saline samples tend to have higher ratios of (Na + K)/(Ca + Mg) and relatively high ratios of chloride to bicarbonate (Cl/HCO3) or sulfate to bicarbonate (SO4/HCO3). The cooler waters tend to be higher in Ca + Mg and bicarbonate (Mkrtchyan 1969; Henneberger et al. 2000). For a few springs’ major and minor elements, anions were analyzed by ionic coupled plasma optical emission spectrometry (ICP-OES; Thermo Iris), by mass spectrometry (ICP-MS; Thermo Element 2), and by ion chromatography (IC; Metrohm). Analyses of major and minor elements in the water sampled from the Arzakan geothermal spring revealed the following composition (in ppm): Na, 1183; Ca, 153; K, 108; Si, 47; Mg, 29; B, 15; Sr, 2.3; As, 1.6; Li, 1.3; Mn, 0.12; Fe, 0.72; Ba, 0.09; Cl, 297; and SO4 2, 200. Nitrate was not detected (<2 ppm). For trace elements, the following concentrations were obtained (in ppb): Cr, 0.28; Co, 0.49; Cu, 0.82; and Zn, 6.73 (Panosyan and Birkeland 2014). The Georgian hot springs are characterized by diverse chemical composition, with mineralization ranging from 0.2 mg/L (Borjomi region) to 11.3 mg/L (Aspindza region). Similar to Armenian and Nagorno-Karabakhian region, the Georgian thermal waters also have mixed-cation and mixed-anion ratios mainly composed of hydrocarbonate, chloride, sulfate, sodium, potassium, magnesium, and calcium ions (Tsertsvadze et al. 1998). All studied springs are rich in heavy metals. Some of the springs contain gasses such as hydrogen sulfide, methane, nitrogen, and carbon dioxide (Mkrtchyan 1969; Tsertsvadze et al. 1998; Melikadze et al. 2010).
Most of the studies were focused on the hot springs at higher altitude and with high temperature. A majority of the hot springs found in the Lesser Caucasus are anthropogenically influenced and often used by tourists and local people for bath. Some of the geothermal springs are used for balneology (Mkrtchyan 1969; Melikadze et al. 2010).
The geographical locations, physicochemical profiling, and brief characteristic of main geothermal springs distributed on the territory of the Lesser Caucasus are summarized in Table 4.1.
4.3 Microbiological Analysis
Only a small fraction of the microorganisms found in a natural habitat can be cultivated under laboratory conditions and subsequently isolated. The knowledge of environmental microbial diversity has been largely aided by the development of culture-independent molecular phylogenetic techniques (Amann et al. 1995; DeLong and Pace 2001; Amann and Ludwig 2000; Zhou 2003; Bhaya et al. 2007; Liu et al. 2011; López-López et al. 2013; DeCastro et al. 2016). Using a combination of several approaches of traditional microbiology with state-of-the-art molecular biology techniques has substantially increased our understanding of the structural and functional diversity of microbial communities. Both culture-based and not culture-independent approaches have been used for addressing microbial diversity associated with geothermal springs. It has been reported that hot springs are inhabited by a variety of microbes belonging to the Bacteria and Archaea domains that tolerate environmental extremes and could have some yet undescribed biotechnological potential (Antranikian and Egorova 2007). Here we have summarized data of the phylogenetic diversity of the prokaryotic communities thriving in some of the geothermal springs in the Lesser Caucasus based on molecular- and culture-based methods (Tables 4.2 and 4.3).
4.3.1 Cultivation-Independent Studies
Up to date, two Armenian, two Georgian, and two thermal springs from Nagorno-Karabakh region have been analyzed using cultivation-independent approaches. Studies based on sequence analysis of 16S rRNA gene clone libraries from the mixed water and sediment sampled from the Arzakan (Armenia) geothermal spring have been done recently (Panosyan and Birkeland 2014). It was the first microbiological investigation on any hot spring in the Lesser Caucasus. The study indicated a predominance of Alphaproteobacteria (8%), Betaproteobacteria (22%), Gammaproteobacteria (13%), Epsilonproteobacteria (9%), Firmicutes (9%), Bacteroidetes (48%), and Cyanobacteria (35%). In addition, DGGE was employed to reveal the microbial profile of sediments of this hot spring. The authors reported an abundance of bacterial populations related to Proteobacteria (affiliated with the Beta-, Epsilon-, and Gammaproteobacteria), Bacteroidetes, and Cyanobacteria based on the DGGE profile, which was in good agreement with the clone library results. The sequence of dominating DGGE bands showed affiliation to Rhodoferax sp., a phototrophic, purple non-sulfur betaproteobacterium and to Sulfurimonas sp., a hydrogen-oxidizing chemolithoautotrophic bacterium isolated from a rearing tank with dissolved hydrogen (Panosyan and Birkeland 2014; Panosyan et al. 2017).
Samples from the Arzakan spring were screened also with advanced metagenomic approaches. Amplification of small-subunit rRNA genes using “universal” primers followed by pyrosequencing (pyrotags) on 454 GS FLX platform also revealed highly diverse microbial communities in Arzakan mat samples (Hedlund et al. 2013).The spring in Arzakan was colonized by a photosynthetic mat dominated by Cyanobacteria, in addition to Proteobacteria, Bacteroidetes, Chloroflexi, Spirochaeta, and a diversity of other Bacteria. It was shown that in Arzakan spring, relatively few (16%) of the total pyrotags could be assigned to known genera, underscoring the novelty of these ecosystem and the need for continued efforts to cultivate and describe microorganisms in geothermal systems.
The phylogenetic analysis of Bacteria identified the dominant phylotypes as members of Proteobacteria. The phylogeny for Proteobacteria revealed considerable diversity. While it is not possible to predict their metabolism from environmental sequences alone, the closest phylogenetic affiliations were to aerobic and anaerobic heterotrophs and methanotrophs (within the Proteobacteria lineage). It was established that the primary production of the Arzakan geothermal system supports by a complex microbial community composed of chemolithotrophs (hydrogen- and sulfide-oxidizing Epsilonproteobacteria and methanotrophic Gammaproteobacteria) and phototrophs (Cyanobacteria and purple non-sulfur anoxygenic phototrophic Betaproteobacteria). The most abundant Cyanobacteria OTUs were confidently assigned to the genera Spirulina, Stanieria, Leptolyngbya, and Rivularia/Caldithrix.
To study bacterial diversity of the hot spring in Jermuk (Armenia), 454 GS FLX pyrosequencing of V4–V8 variable regions of the small-subunit rRNA was applied. As reported, the most abundant phyla represented in the pyrotag dataset from Jermuk were the Proteobacteria, Bacteroidetes, and Synergistetes (Hedlund et al. 2013). Several abundant Proteobacteria OTUs were related to obligate or facultative chemolithoautotrophs capable of using sulfur compounds, Fe2+, and/or H2 as electron donors, including the genera Thiobacillus, Sulfuricurvum, Sideroxydans, and Hydrogenophaga, suggesting the importance of chemolithotrophy in primary productivity (Kampfer et al. 2005; Kellermann and Griebler 2009; Kodama and Watanabe 2004; Liu et al. 2012). The gross morphology of the mat was consistent with iron precipitation at the spring source as ferrous iron supplied from the subsurface is oxidized as the spring water becomes oxygenated. The Bacteroidetes were diverse, and many OTUs could not be assigned to known genera. An exception was an abundant OTU assigned to the genus Lutibacter, which contains chemoorganotrophs most commonly found in marine environments (Lee et al. 2006). Other Bacteroidetes and the Synergistetes in Jermuk are likely involved in heterotrophic processing of mat exudates and biomass.
DGGE analysis of the partial bacterial 16S rRNA gene PCR amplicons also was used to profile bacterial populations inhabiting the sediment and water fractions in the Jermuk geothermal spring. The sequence analysis of DGGE bands showed affiliation with Epsilonproteobacteria, Bacteroidetes, Spirochaetes, Ignavibacteriae, and Firmicutes. The sequences obtained from bands were related to anaerobic or facultatively anaerobic organotrophic or H2-utilizing and thiosulfate-/sulfur-reducing bacteria. Heterotrophic microorganisms detected in the DGGE profile clustered among fermentative microorganisms, which are actively involved in C-cycle (Panosyan 2017).
Culture-independent technique with an emphasis on members of the Archaea was used to determine the composition and structure of microbial communities inhabiting microbial mats in the source pools of two geothermal springs, Arzakan and Jermuk. Based on an analysis of near full-length small-subunit rRNA genes amplified using Archaea-specific primers, it was shown that these springs are inhabited by a diversity of methanogens, including Methanomicrobiales and Methanosarcinales and relatives of Methanomassiliicoccus luminyensis, close relatives of the ammonia-oxidizing archaeon (AOA) “Candidatus Nitrososphaera gargensis,” and the yet-uncultivated Miscellaneous Crenarchaeotal Group and Deep Hydrothermal Vent Crenarchaeota group 1 (Fig. 4.4) (Hedlund et al. 2013). Archaeal sequences were present at low abundance in both pyrotag datasets, with six archaeal pyrotags in three OTUs in Arzakan and nine pyrotags in six OTUs in Jermuk. The Methanosarcinales were represented in both pyrotag datasets, with Methanomethylovorans detected in Jermuk, and Methanosaeta and a sequence that could not be classified below the order level were detected in Arzakan. Close relatives of Methanospirillum hungatei, in the order Methanomicrobiales, were inferred to be abundant in both springs. In addition, two phylotypes in Arzakan were related to the genus Methanoregula, also in the Methanomicrobiales. Members of both genera use H2/CO2 and/or format as methanogenic substrates; however, their presence in the geothermal systems was somewhat surprising since they are not reported to grow above 37 °C. The other order of methanogens present in both springs was Methanosarcinales, represented by Methanosaeta and Methanomethylovorans. Methanosaeta was abundant in Jermuk and includes obligate acetoclastic species known to grow up to 60 °C (Liu and Whitman 2008).
Maximum-likelihood phylogeny depicting relationships between near-complete archaeal 16S rRNA genes recovered from Arzakan (red) and Jermuk (blue) and closely related sequences, including well-studied microbial isolates. Percent values for each OTU represent the percent abundance of the OTU in the clone library. Bootstrap support is indicated at major nodes for maximum-likelihood (ML; 100 replicates), parsimony (P; 1000 replicates), and distance (neighbor-joining, NJ; 1000 replicates) methods. Taxonomic designations for major phylogenetic groups are shown at the right (Hedlund et al. 2013)
Recently, Illumina HiSeq2500 paired-end sequencing of metagenomic DNA also was used to analyze water/sediment samples of the Jermuk hot spring. Taxonomic analyses of the metagenomic rRNA sequences revealed a prevalence of Proteobacteria Firmicutes and Bacteroidetes. However, many of the largest contigs represented uncharacterized or poorly characterized groups such as candidate division WS6 and candidate phylum Ignavibacteria. The archaeal community, constituting a minor fraction (~1%) of the community, was dominated by Euryarchaeota, followed by Crenarchaeota, unclassified groups, and a minor fraction of Thaumarchaeota. The functional composition based on metagenomics sequence information indicated a dominance of heterotrophic types of metabolism (Poghosyan 2015).
For investigation of the bacterial composition of sediment and water samples from the Zuar geothermal spring (Nagorno-Karabakh), only a bacterial clone library based on 16S rRNA genes was constructed. It was shown that clones obtained from the Zuar geothermal spring originated from phyla Proteobacteria (42.3%), Firmicutes (19.2%), Bacteroidetes (15.4%), Cyanobacteria (3.8%), Tenericutes (3.8%), and yet-unclassified phylotypes (15.4% for Zuarr) (Saghatelyan et al. 2014).
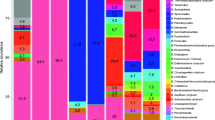
According to the recent report of sequence analysis of clones obtained from bacterial 16S rRNA gene libraries, the presence of Proteobacteria (48.6%), Cyanobacteria (29.7%), Bacteroidetes (5.4%), Chloroflexi (5.4%), Verrucomicrobia (2.7%), and Planctomycetes (2.7%) in sediment and water samples in Karvachar (Nagorno-Karabakh) hot spring (Fig. 4.5) was indicated (Saghatelyan and Panosyan 2015). The dominating bacterial group was the phylum Proteobacteria. A few phylotypes belonging to the phylum Bacteroidetes were obtained. One of the dominating groups was Cyanobacteria, representatives of which dominate especially on top layer of microbial mats and are the most important primary producers in hot spring ecosystems (Roeselers et al. 2007).
Representatives of phylum Firmicutes were not detected in the clone library, while DGGE profiling of the same samples indicated presence of Firmicutes (genus Geobacillus) as a one of the major components in bacterial community of Karvachar geothermal spring (Panosyan 2017). This has been confirmed later by metagenome analysis of the Karvachar hot spring samples.
Based on recent data (unpublished data) obtained from the whole-genome shotgun sequencing of sediment samples of Karvachar, using an Illumina HiSeq 2500 platform, 580 bacterial sequences were aligned to reference genes (NCBI RefSeq), belonging to the following bacterial taxonomical groups: Actinobacteria; Alpha-, Beta-, Delta-, Epsilon-, and Gammaproteobacteria; Bacteroidetes/Chlorobi; Firmicutes; Chlamydiae; Cyanobacteria/Melainabacteria; Fusobacteria; and Synergistia. Among these groups, Proteobacteria (Alpha-, Beta-, and Gammaproteobacteria) and Firmicutes were the major components in the total bacterial sequence reads (Fig. 4.5). The sequences affiliated with Gammaproteobacteria were predominant (48.96% of Proteobacteria, 235 out of 480), and most of them were closely (98–100%) related to cultured Gammaproteobacteria. Representative of the groups of Porphyrobacter, Paracoccus, and Oceanibaculum was predominate Alphaproteobacteria found in study samples. The majority of sequences derived from spring were closely related (95–99% identity) to Porphyrobacter cryptus, a slightly thermophilic, aerobic, bacteriochlorophyll a-containing species isolated from a hot spring at Alcafache in Central Portugal (Rainey et al. 2003).
Betaproteobacterial-related sequences were the third major group of obtained bacterial sequences (20.6% of Proteobacteria, 99 out of 480). The majority of the obtained sequences showed 92–100% similarity to Caldimonas taiwanensis, an aerobic amylase-producing heterotrophic bacterium isolated from a hot spring located in Taiwan (Chen et al. 2005) and 94–99% of similarity to representatives of genus Tepidimonas, particularly to the species T. taiwanensis, T. thermophilus, and T. fonticaldi, isolated from hot springs in Taiwan and India (Chen et al. 2013; Poddar et al. 2014). Forty sequences (6.9%, 40 out of 580) were affiliated with Firmicutes. Around 10.3% (60 out of 580) of the total bacterial clone sequences were affiliated with some minor groups, such as Actinobacteria, Bacteroidetes/Chlorobi, Chlamydia, Cyanobacteria/Melainabacteria, Fusobacteria, and Synergistia. Most of these sequences were closely (98–99%) related to clones retrieved from water environments and different habitats (Anil Kumar et al. 2010; Yoon et al. 2009). Phototrophic bacteria belonging to genera Neosynechococcus, Pseudanabaena, and Fischerella represented the three most abundant and metabolically active primary producers of the analyzed community. Most Cyanobacteria detected were related to others previously reported in thermophilic environments (Portillo et al. 2009). Representatives of genus Rhodobacter (purple non-sulfur anoxygenic phototrophs) and other phototrophic microbes were found to share these environments with the cyanobacteria.
The sequence reads from the Samtredia geothermal spring (Georgia) water sample, obtained from the whole-genome shotgun sequencing on Illumina HiSeq 2500 platform, showed high similarity (>90%) to 938 bacterial and 15 archaeal reference sequences (Fig. 4.6). The majority of bacterial sequence reads were affiliated with the Firmicutes (33%) and Gammaproteobacteria (32%), followed by Actinobacteria (15.5%), Betaproteobacteria (9.1%), Alphaproteobacteria (2.9%), Chlamydia (1.6%), and Bacteroidetes (1.5%). Other groups of Prokaryotes (Aquificae, Deinococcus-Thermus, Deltaproteobacteria, Epsilonproteobacteria, Acidithiobacillia, Planctomycetes, Cyanobacteria/Melainabacteria group) comprised a minority, less than 1% of the communities. Archaeal sequence reads were affiliated with Crenarchaeota (1.4%) and Euryarchaeota (0.2%) (unpublished data).
The most dominant phylum, Firmicutes, was represented by genera Streptococcus, Enterococcus, Clostridioides, Bacillus, and Listeria. The majority of these bacteria can be recovered from a wide range of habitats. Firmicutes representatives considered as inhabitants of thermal waters include genera such as Geobacillus, Thermoanaerobacter, Desulfotomaculum, and Desulfovirgula have been revealed in the Samtredia hot spring. Similarly to other above described thermal waters, Proteobacteria were largely represented in the sequence reads. Four hundred and twenty two sequences (45%, 422 out of 938) were affiliated with Proteobacteria, belonging to following subgroups: Alpha-, Beta-, Gamma-, and Deltaproteobacteria. The sequences affiliated with Gammaproteobacteria were predominant (72.5% of Proteobacteria, 306 out of 422). The dominant groups were Escherichia, Acinetobacter, Pseudomonas, Salmonella, and Legionella. Surprisingly most gammaproteobacterial sequences were Escherichia-related sequences. These microbes are not autochthons for hot springs and could be considered as contaminants.
Betaproteobacterial-related sequences were the second major group of obtained proteobacterial sequences (20.6% of Proteobacteria, 87 out of 422). The genera of Caldimonas and Tepidiphilus, representing the hot spring microbiota, were one of the minor groups of Betaproteobacteria found in the study samples. Alphaproteobacterial-related sequences comprised 6.6% of Proteobacteria and were represented mainly by nonindigenous bacteria. Actinobacteria accounted for a significant portion of bacteria, composing 15.8% (148 out of 938) of total bacterial populations dominated by Mycobacteria, while the Deinococcus-Thermus group was mainly represented by thermophilic bacteria belonging to the genus Thermus. Aquificales accounted for 0.8% of the reads, affiliated to facultatively anaerobic, hydrogen- or sulfur-/thiosulfate-oxidizing, thermophilic bacteria belonging to genus Sulfurihydrogenibium. Less than 5% of the total bacterial sequences were aligned with some other minor groups, such as Acidithiobacillia, Bacteroidetes/Chlorobi, Chlamydia, Cyanobacteria/Melainabacteria, and Planctomycetes. Most of these sequences were closely related to clones retrieved from water and soil environments.
The microbial diversity of the Tbilisi sulfur spring (Georgia) was analyzed using whole-genome shotgun sequencing using Illumina MiSeq platform (unpublished data). The sequences obtained from metagenomic DNA showed high similarity (>90%) to 1090 RefSeq database reference sequences, revealing 240 species. The thermal water was dominated by Gammaproteobacteria (46.4% of total reads) followed by Firmicutes (20.6%), Betaproteobacteria (16.4%), Actinobacteria (6.1%), Alphaproteobacteria (5.7%), Chlamydiae (1.7%), and Bacteroidetes (1.5%). Deinococcus-Thermus, delta/epsilon subdivisions, Acidithiobacillus Cyanobacteria/Melainabacteria group, and Synergistia comprised a minority of the prokaryotic populations accounting for less than 1% of total reads for each group (Fig. 4.7). Archaeal sequence reads were also in minority, belonging to the Euryarchaeota and comprising 0.2% of total reads.
Gammaproteobacteria were represented by 35 bacterial genera, dominated by Escherichia (30%), Pseudomonas (24%), Xanthomonas (11%), Legionella (5%), Salmonella (3.5%), and Acinetobacter (2.8%). Some of these bacteria are found in diverse habitats and may also cause diseases in humans. Interestingly, Pseudomonas, Legionella, and Acinetobacter were reported in a variety of geothermal springs (Lin et al. 2007; Petursdottir et al. 2009; Saxena et al. 2017). The sulfur spring also harbored Silanimonas lenta (3,5%), belonging to moderately thermophilic alkaliphilic bacteria isolated from a hot spring in Korea (Lee et al. 2005), purple sulfur bacteria Ectothiorhodospira, and thermophilic bacterium Thermomonas hydrothermalis isolated from a hot spring in Central Portugal (Alves et al. 2003).
The second most abundant group of bacteria was Firmicutes, including representatives belonging to the Bacillus and related genera such Geobacillus and Tepidibacillus.
Betaproteobacteria were dominated by Neisseria (29%), presumably allochthonous bacteria. The other two most prevalent betabacteria inhabiting the studied spring were amylase-producing Caldimonas taiwanensis (22%) and alkaline-protease-producing Tepidimonas taiwanensis (14%), thermophilic bacteria reported in geothermal springs in Taiwan (Chen et al. 2005; Chen et al. 2006) and, as described above, have been found in the Karvachar hot spring as well.
Actinobacteria were represented by 12 genera dominated by Streptomyces and Mycobacteria that may inhabit thermal spring environments. Streptomyces spp. are known to produce various enzymes and biological active compounds, including antimicrobials, and can be readily isolated from geothermal environments (Al-Dhabi et al. 2016). Mycobacteria, with potential to cause diseases in humans, have been also found and isolated from the sulfur hot springs (Lee et al. 2015).
Alphaproteobacteria of the sulfur spring comprised 18 genera dominated by Mesorhizobium (23% of total Alphaproteobacteria reads) and Thalassobius (16%) species. Though these bacteria were not described in the hot springs, they have been found in diverse environments such as marine waters and soils (Arahal et al. 2005; Yuan et al. 2016), indicating possibility of their presence in thermal waters as well.
The Bacteroidetes/Chlorobi group was represented by 12 genera with 25% and 12% of sequence reads aligned to Bacteroides and Pedobacter reference genes, respectively. Bacteroides spp. have not been described in geothermal waters and can be considered as a contaminant, while the presence of Pedobacter has been reported in an alkaline hot spring in Thermopolis (Buckingham et al. 2013).
The delta/epsilon subdivision comprised a minority of the sulfur spring microbial population represented only with five genera, including the sulfur-reducing microaerophilic bacterium Sulfurospirillum that could be considered as natural inhabitant of this geothermal spring. The Deinococcus-Thermus group was also in minority, represented by Meiothermus taiwanensis, aerobic, thermophilic, non-sporulating, filamentous bacteria reported in a hot spring in Taiwan (Chen et al. 2002).
The sulfur spring was inhabited by two methanogenic Euryarchaeota species, Methanolacinia paynteri and Methanosarcina mazei. The optimum growth conditions for Methanolacinia paynteri are pH 6.6–7.2, temperature 40 °C, and the sulfide may serve as the sulfur source (Zellner et al. 1989), thus presence of this archaeon in the sulfur spring is not surprising. Methanosarcina spp. can survive in a variety of habitats, including extreme environments and may use different metabolic pathways to produce methane (Assis das Graças et al. 2013).
In addition to whole metagenomic DNA sequencing, the microbial diversity of the sulfur spring was also analyzed using a PCR/DGGE approach. The majority of DGGE bands were affiliated with Betaproteobacteria involved in sulfur cycle, such as species belonging to the genera Sulfurisoma, Thiobacillus, and oxalotrophic bacterium Oxalicibacterium faecigallinarum.
The study has also revealed the presence of the methanogen Methanosaeta harundinacea, belonging to Euryarchaeota, confirming that the methanogenic Euryarchaeota dominate archaeal populations of the sulfur spring.
4.3.2 Cultivation-Dependent Studies
Cultivable approaches have been used for analysis of microbial diversity associated with hot springs. Several studies have been performed on the description of novel genera, species and strains, characterization of different bio-resources, and whole-genome analysis of a few isolates from geothermal springs in the Lesser Caucasus. Many thermostable enzymes, including lipase, protease, amylase, DNA polymerase, aspartase, aminoacylase, glucose isomerase and inulinase, producers of EPS, protein and vitamins, enrichments of nitrite-oxidizing bacteria (NOB), and methylotrophic, acetoclastic, and hydrogenotrophic methanogens with potential biotechnological applications have been reported by several authors (Table 4.3).
Overall, all isolates of bacteria and Archaea from the Lesser Caucasus belong to more than 40 distinct species of 21 different genera, namely, Bacillus, Geobacillus, Anoxybacillus, Paenibacillus, Brevibacillus, Aeribacillus, Ureibacillus, Thermoactinomyces, Sporosarcina, Thermus, Rhodobacter, Thiospirillum, Thiocapsa, Rhodopseudomonas, Methylocaldum, Desulfomicrobium, Desulfovibrio, Treponema, Arcobacter, Nitrospira, and Methanoculleus. The members of phylum Firmicutes were most dominant among the identified bacteria isolated from all thermal springs. Culture-dependent studies indicate that Bacillus and related genera were ubiquitous and predominant in harsh environments of high temperatures. Representatives of the genera Geobacillus and Anoxybacillus are the most highly distributed obligate thermophiles in the Lesser Caucasus hot springs. All isolates from the hot springs that belonged to the genus Bacillus were thermotolerant microorganisms among which B. licheniformis appeared as the dominating species. All studied springs demonstrated significantly lower content of species belonging to genera Brevibacillus, Ureibacillus, Paenibacillus, Thermoactinomyces, and Sporosarcina.
Bacteria belonging to the genera Bacillus and Thermus were mostly reported as aerobic, heterotrophic thermophiles and found in thermal systems with neutral to alkaline pH (Spanevello and Patel 2004). Although Thermus spp. may be predominant heterotrophs in many hot springs (Hjorleifsdottir et al. 2001), they were isolated only from the Karvachar hot spring.
Several strains representing potentially novel species were reported from the Akhurik, Jermuk, and Karvachar geothermal springs. Two novel strains belonging to genera Anoxybacillus and Treponema were reported from the hot spring at Jermuk. A novel species belonging to genus Anoxybacillus and a new strain belonging to Thermus scotoductus were reported from the Karvachar spring (Saghatelyan et al. 2015; Hovhannisyan et al. 2017). 16S rRNA gene sequences of a methanotrophic isolate from Akhurik geothermal spring showed that it was a new gammaproteobacterial methanotroph, forming a separate clade in the Methylococcaceae family. It fell into a cluster with thermotolerant and mesophilic methanotrophs, comprising the genera Methylocaldum-Methylococcus-Methyloparacoccus-Methylogaea. The genes pmoA, mxaF, cbbL, and nifH were detected, but no mmoX gene was found. The strain probably represents a novel methanotrophic genus (Islam et al. 2015).
Whole-genome analysis of the hot spring isolates was a major thrust area of investigation. Whole-genome shotgun sequencing of novel species isolated from hot springs at Jermuk (Treponema thermophilum sp. nov) and Karvachar (Anoxybacillus sp. strain K103) was performed (Poghosyan 2015; Hovhannisyan et al. 2017). Similarly, the whole-genome sequence of Thermus scotoductus K1 was reported following its isolation from the Karvachar spring (Saghatelyan et al. 2015).
Attention was also paid to the bioprospecting of geothermal spring’s microbes with an intention of using these resources for commercial applications. In total, 135 thermophilic and thermotolerant bacilli strains were isolated under aerobic conditions at 55–65 °C and identified based on 16S rRNA gene sequence analysis as representatives of genera Bacillus, Geobacillus, Anoxybacillus, Paenibacillus, Brevibacillus, Aeribacillus, Ureibacillus, Thermoactinomyces, and Sporosarcina. These thermophilic bacilli were tested for hydrolytic enzyme production capacities, and biotechnologically valuable enzyme producers were selected (Panosyan 2017). The majority of the studies focused on hydrolytic enzymes like lipase (Vardanyan et al. 2015; Shahinyan et al. 2017), amylase (Hovhannisyan et al. 2016), and protease (Panosyan 2017).
Some phototrophic bacteria isolated from Armenian hot springs were good producers of enzymes such as aspartase, aminoacylase, glucose isomerase, and inulinase, as well as sources of protein, carbohydrates, and vitamins (Paronyan 2003). Two isolates belonging to the genus Geobacillus are able to produce heteropolymeric EPSs with high molecular weight (Panosyan et al. 2014).
Prospective microbes from hot springs offer a major advantage of preserving those strains for future studies and exploring them in due course for potential biotechnological applications in medical, industrial, and agricultural processes.
4.4 Correlation Between Geophysiology and Microbiology of the Hot Springs in the Lesser Caucasus
Understanding the microbial community structure in hot springs with different ecologies is important to elucidate community functions and their importance for the maintenance of hot spring ecosystems.
In general, microbial diversity was inversely correlated with temperature, and temperature has been shown to be a key factor in controlling the microbial diversity in hot springs (Wang et al. 2013). Thermophilic or hyperthermophilic Bacteria are commonly present in high-temperature hot springs (>75 °C) (Hou et al. 2013). When temperature is suitable for photosynthesis (<75 °C), moderately thermophilic and mesophilic phototrophic Bacteria are important members in terrestrial thermal springs, such as Cyanobacteria, Chloroflexi, and phototrophic representatives of Proteobacteria (Cox et al. 2011). In addition to Bacteria, members of the archaeal phyla Crenarchaeota, Euryarchaeota, and Thaumarchaeota are also commonly detected in geothermal systems (Ochsenreiter et al. 2003; Zhang et al. 2008).
The comparison of microbial species abundance and diversity in the Lesser Caucasus hot springs with those available internationally displays similar patterns. It was shown earlier that there is a negative correlation between spring temperature and diversity of microbes (Wang et al. 2013; Poddar and Das 2017).
Prokaryotic diversity was found to be low at high-temperature springs in contrast to low-temperature springs. Temperature has also been shown to drive phylum diversity in hot springs. Most of the studied hot springs in the Lesser Caucasus have a temperature below 50 °C and harbor bacterial species pertaining to phyla Firmicutes, Proteobacteria, Bacteroidetes, and Cyanobacteria, although with varying abundance between springs. Springs with higher temperatures also contained thermophiles belonging to Actinobacteria, Deinococcus-Thermus, and Aquificae. Representatives of the phylum Firmicutes were most versatile in the investigated hot springs and could populate hot springs with a wide range of temperatures. These observations are in accordance with many global studies indicating that thermophilic bacteria belonging to phyla Aquificae, Deinococcus-Thermus, and Firmicutes were abundant in the hot springs with high temperatures, whereas mesophilic bacterial members of Cyanobacteria, Chloroflexi, and Proteobacteria mostly occupy mesothermal hot springs (Wang et al. 2013). Cyanobacteria are the most commonly reported microbial group in these types of environments and are considered to be the major primary producers in these habitats (Castenholtz 1973). It was shown earlier that moderate-temperature geothermal systems cool enough to permit phototrophy at the source with neutral or alkaline pH are often colonized by visible microbial growth that forms laminated mats or streamers dominated by phototrophic bacteria (Klatt et al. 2011). Relatively low-temperature (>75 °C) and neutral pH in all studied springs can support growth of phototrophic bacteria due to obvious light effect in the outlet of the spring.
A comparison of the optimum growth temperature of the closest cultivated relatives of the microorganisms detected in the clone libraries, DGGE profiles, or pyrotags suggested that most of the microorganisms, including microorganisms representing some of the most dominant groups, are likely able to grow at reservoir temperature and, therefore, should not be regarded as contaminants. The bacterial metagenomic DNA sequences also affiliated with taxa that are not described in the literature as being associated with geothermal environments. This can be explained by the presence of contamination from surrounding soils. Although most of the retrieved sequences were most similar to environmental sequences representing uncultured bacteria from various habitats, some of them were phylogenetically associated with environmental clones obtained from similar habitats.
Archaea appeared to be a minority in the prokaryotic community. High-temperature environments were previously generally believed to be the realm of Archaea (Li et al. 2015; Urbieta et al. 2015; Chan et al. 2017). However, recent studies applying molecular methods have revealed that bacteria rather are the predominant prokaryotic communities in such environments (Badhai et al. 2015; López-López et al. 2015). The factors that allow bacteria to dominate in high-temperature habitats are not well understood.
All reported Lesser Caucasus springs have circumneutral pH and, therefore, harbor a microbial community different from acidic hot springs environments (Purcell et al. 2007; Poddar and Das 2017). Acidic springs have been reported to contain chemolithotrophic acidophiles belonging to genera Acidithiobacillus, Sulfobacillus, Hydrogenobaculum, Acidobacteria, Acidimicrobium, etc. that participate in Fe and sulfur oxidation in those environments (Burgess et al. 2012; Urbieta et al. 2015; Skirnisdottir et al. 2000). Acidophiles were hardly detected in Lesser Caucasian hot springs. Bacterial species isolated from the studied hot springs exhibited optimal growth at neutral pH and could not grow at low pH conditions. It was shown by many investigators that Firmicutes and Proteobacteria are the phyla consistently present in circumneutral hot springs. Results obtained from Lesser Caucasus geothermal springs also are in line with observations of microbial assemblages distributed in hot springs with pH ≥ 7 globally (Nakagawa and Fukui 2002; Wang et al. 2013). In general, Archaea are not dominant in circumneutral hot springs, which is in agreement with several recent reports with similar pH ranges (Wang et al. 2013; Merkel et al. 2017).
Environmental conditions and the nutritional status in a natural habitat may drive the development of a particular microbial group or population. The set of abiotic factors allow natural selection of a few species that can dominate and multiply in the ecologically relevant niche. Limited carbon and nitrogen sources and high temperature of the springs located in the Lesser Caucasus allowed also the development of a unique population dominated by a large number of bacilli including Geobacillus and Anoxybacillus spp.
Besides temperature and pH, the limiting factor for microbial diversity and biomass could be a combination of abiotic factors including dissolved gasses (H2, CO2, H2S, CH4) and high mineralization. The geothermal systems of the Lesser Caucasus are known to contain high concentrations of minerals, and thus, the mineralization may also have a strong influence on the community composition. Recent studies have also highlighted that other factors, such as biogeography and geological history, can be important in determining the thermophilic diversity of geothermal springs (Whitaker et al. 2003; Takacs-Vesbach et al. 2008).
4.5 Conclusion
Investigations of the geothermal springs’ microbiome are important for understanding the microbe-mediated biogeochemical cycles and ecosystem functioning as well as exploring the biotechnological potency of thermophilic isolates. This is the first comprehensive census of the microbial communities thriving in 11 geothermal springs of the Lesser Caucasus. Firmicutes, Proteobacteria, Bacteroidetes, and Cyanobacteria were the signature phyla in all 11 hot springs that along with the presence of site-specific taxa contributed to the uniqueness of each spring. Archaea appeared to be a minority in the prokaryotic community composing less than 1% of all microbial population. Overall, microbial diversity and richness were negatively affected by increasing temperature. Other influential factors shaping the microbiota of the studied Lesser Caucasus circumneutral geothermal springs appear to be pH and mineralization. Biogeography and geological history should not be ignored in microbial ecology studies, as all abiotic factors collectively contribute to the dynamics of the microbial populations. Many new thermophilic microbes mainly belonging to the Bacillus and related genera have been isolated, identified, and evaluated taking into account their biotechnological potency.
The present work, therefore, extends the previous sphere of information regarding the thermophilic bacterial diversity of thermal springs in the Lesser Caucasus.
References
Aanniz TM, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M, Amar M (2015) Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Braz J Microbiol 46(2):443–453
Achmadova FR (1991) Rasprostranenie termofilnikh bakterii vidov Bacillus и Тhermus v gorjachikh istochnikov Azerbadjanskoj SSR (Distribution of bacteria belonging to the Bacillus and Тhermus in hot springs of Azerbadjan SSR). Dissertation, Timiriazev KA Agriculture Academy, Moscow (in Russian)
Al-Dhabi NA, Esmail GA, Duraipandiyan V, Valan Arasu M, Salem-Bekhit MM (2016) Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 20:79–90
Alves MP, Rainey FA, Nobre MF, da Costa MS (2003) Thermomonas hydrothermalis sp. nov., a new slightly thermophilic gamma-proteobacterium isolated from a hot spring in Central Portugal. Syst Appl Microbiol 26:70–75
Amann R, Ludwig W (2000) Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol Rev 24:555–565
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in-situ detection of individual microbial-cells without cultivation. Microbiol Rev 59:143–169
Anil Kumar P, Srinivas TN, Madhu S, Manorama R, Shivaji S (2010) Indibacter alkaliphilus gen. nov., sp. nov., an alkaliphilic bacterium isolated from a haloalkaline lake. Int J Syst Evol Microbiol 60(4):721–726
Antranikian G, Egorova K (2007) Extremophiles, a unique resource of biocatalysts for industry. In: Gerday C, Glansdorff N (eds) Physiology and biochemistry of extremophiles. ASM Press, Washington, DC, pp 361–406
Arahal DR, Macian MC, Garay E, Pujalte MJ (2005) Thalassobius mediterraneus gen. nov., sp. nov., and reclassification of Ruegeria gelatinovorans as Thalassobius gelatinovorus comb. nov. Int J Syst Evol Microbiol 55:2371–2376
Assis das Graças D, Thiago Jucá Ramos R, Vieira Araújo AC, Zahlouth R, Ribeiro Carneiro A, Souza Lopes T et al (2013) Complete genome of a Methanosarcina mazei strain isolated from sediment samples from an Amazonian flooded area. Genome Announc 1:e00271–13
Badalyan M (2000) Geothermal features of Armenia: a country update. In: Proceedings world geothermal congress, Kyushu-Tohoku, Japan, 28 May–10 June, vol 2000, pp 71–75
Badhai J, Ghosh TS, Das SK (2015) Taxonomic and functional characteristics of microbial communities and their correlation with physicochemical properties of four geothermal springs in Odisha, India. Front Microbiol 6:1166. https://doi.org/10.3389/fmicb.2015.01166
Bhaya D, Grossman AR, Steunou A-S, Khuri N et al (2007) Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J 1:703–713
Bowen De León K, Gerlach R, Peyton BM, Fields MW (2013) Archaeal and bacterial communities in three alkaline hot springs in Heart Lake Geyser Basin, Yellowstone National Park. Front Microbiol 4:330. https://doi.org/10.3389/fmicb.2013.00330
Buckingham AR, Law RM, Holley GA, Yeomans NS and Smaglik SM (2013) An intergrated study of a hot springs microbial community in Thermopolis, WY. In: The Geological Society of America abstracts with programs of the 125th anniversary annual meeting&expo, Denver, Colorado, USA, 27–30 October 2013. vol 45(7), p 582
Bunterbart G, Kapanadze N, Kobzev G, Melikadze G (2009) Re-assessment of the geothermal potential of Tbilisi region: the hydrodynamic digital model project. In: Environment and resources, Association of Academics of Science in Asia Workshop, Izmir, 25–27 September, vol 2009, pp 154–161
Burgess AE, Unrine JM, Mills GL, Romanek CS, Wiegel J (2012) Comparative geochemical and microbiological characterization of two thermal ponds in the Uzon Caldera, Kamchatka, Russia. Microb Ecol 63:471–489
Castenholtz RW (1973) Ecology of blue-green algae in hot springs. In: Carr NG, Whitton BA (eds) The biology of blue-green algae. University of California Press, Los Angeles, pp 379–414
Chan CS, Chan KG, Tay YL, Chua YH, Goh KM (2015) Diversity of thermophiles in a Malaysian hot spring determined using 16S rRNA and shotgun metagenome sequencing. Front Microbiol 6:177. https://doi.org/10.3389/fmicb.2015.00177
Chan CS, Chan KG, Ee R, Hong KW, Urbieta MS, Donati ER, Shamsir MS, Goh KM (2017) Effects of physiochemical factors on prokaryotic biodiversity in Malaysian Circumneutral Hot Springs. Front Microbiol 8:1252. https://doi.org/10.3389/fmicb.2017.01252
Chen MY, Lin GH, Lin YT, Tsay SS (2002) Meiothermus taiwanensis sp. nov., a novel filamentous, thermophilic species isolated in Taiwan. Int J Syst Evol Microbiol 52:1647–1654
Chen WM, Chan JS, Chiu CH, Chang SC, Chen WC, Jiang CM (2005) Caldimonas taiwanensis sp. nov., α-amylase producing bacterium isolated from a hot spring. Syst Appl Microbiol 28:415–420
Chen TL, Chou YJ, Che WM, Arun B, Young CC (2006) Tepidimonas taiwanensis sp. nov., a novel alkaline-proteaseproducing bacterium isolated from a hot spring. Extremophiles 10(1):35–40
Chen WM, Huang HW, Chang JS, Han YL, Guo TR, Sheu SY (2013) Tepidimonas fonticaldi sp. nov., a slightly thermophilic betaproteobacterium isolated from a hot spring. Int J Syst Evol Microbiol 63(5):1810–1816
Cihan AC, Ozcan B, Tekin N, Cokmus C (2011) Phylogenetic diversity of isolates belonging to genera Geobacillus and Aeribacillus isolated from different geothermal regions of Turkey. World J Microbiol Biotechnol 27:2683–2696
Coman C, Drugă B, Hegedus A, Sicora C, Dragoş N (2013) Archaeal and bacterial diversity in two hot spring microbial mats from a geothermal region in Romania. Extremophiles 17(3):523–534. https://doi.org/10.1007/s00792-013-0537-5
Cox A, Shock EL, Havig JR (2011) The transition to microbial photosynthesis in hot spring ecosystems. Chem Geo 280:344–351
DeCastro ME, Rodríguez-Belmonte E, González-Siso MI (2016) Metagenomics of thermophiles with a focus on discovery of novel thermozymes. Front Microbiol 7:1521. https://doi.org/10.3389/fmicb.2016.01521
Deepika M, Satyanarayana T (2013) Diversity of hot environments and thermophilic microbes. In: Satyanarayana T, Littlechild J, Kawarabayasi Y (eds) Thermophilic microbes in environmental and industrial biotechnology biotechnology of thermophiles. Springer, Dordrecht/Heidelberg/New York/London, pp 3–60
DeLong EE, Pace NR (2001) Environmental diversity of bacteria and archaea. Syst Biol 50:470–478
Edwards TA, Calica NA, Huang DA, Manoharan N, Hou W, Huang L, Panosyan H, Dong H, Hedlund BP (2013) Cultivation and characterization of thermophilic Nitrospira species from geothermal springs in the U.S. Great Basin, China, and Armenia. FEMS Microbiol Ecol 85(2):283–292
Everroad RC, Otaki H, Matsuura K, Haruta S (2012) Diversification of bacterial community composition along a temperature gradient at a thermal spring. Microbes Environ 27:374–381
Hedlund BP, Cole JK, Williams AJ, Hou W, Zhou E, Li WJ, Dong H (2012) A review of the microbiology of the Rehai geothermal field in Tengchong, Yunnan Province, China. Geosci Front 3(3):273–288
Hedlund BP, Dodsworth JA, Cole JK, Panosyan HH (2013) An integrated study reveals diverse methanogens, Thaumarchaeota, and yet-uncultivated archaeal lineages in Armenian hot springs. Antonie Van Leeuwenhoek 104(1):71–82
Henneberger R, Cooksley D, Hallberg J (2000) Geothermal resources of Armenia: in: proceedings world geothermal congress, Kyushu-Tohoku, Japan, 28 May–10 June 2000, pp 1217–1222
Hetzer A, Morgan HW, McDonald IR, Daughney CJ (2007) Microbial life in champagne pool, a geothermal spring in Waiotapu, New Zealand. Extremophiles 11(4):605–614
Hjorleifsdottir S, Skirnisdottir S, Hreggvidsson G, Holst O, Kristjansson J (2001) Species composition of cultivated and noncultivatedbacteria from short filaments in an Icelandic hot spring at 88 °C. Microb Ecol 42:117–125
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, Huang Q, Huang L, Wu G, Zhi X, Li W, Dodsworth JA, Hedlund BP, Zhang C, Hartnett HE, Dijkstra P, Hungate BA (2013) A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan province China using 16S rRNA gene pyrosequencing. PLoS One 8(1):e53350
Hovhannisyan P, Turabyan A, Panosyan H, Trchounian A (2016) Thermostable amylase production bacilli isolated from Armenian geothermal springs. Biol J Armenia 68:6–15
Hovhannisyan P, Panosyan H, Trchounian A, Birkeland NK (2017) Amplification and cloning of an alpha-amylase gene from Anoxybacillus flavithermus K103 isolated from an Armenian geothermal spring. In: Abstracts of the 7th congress of European microbiologists (the FEMS 2017), Valencia, Spain, 9–13 July, 2017
Hreggvidsson GO, Petursdottir SK, Bjornsdottir SH, Fridjonsson OH (2012) Microbial speciation in the geothermal ecosystem. In: Stan H, Fendrihan LS (eds) Adaption of microbial life to environmental extremes: novel research results and application. Springer, Wien, pp 37–68
Islam T, Larsen Ø, Torsvik V, Øvreås L, Panosyan H, Murrell C, Birkeland NK, Bodrossy L (2015) Novel methanotrophs of the family Methylococcaceae from different geographical regions and habitats. Microorganisms 3:484–499
Kampfer P, Schulze R, Jackel U, Malik KA, Amann R, Spring S (2005) Hydrogenophaga defluvii sp. nov. and Hydrogenophaga atypica sp. nov., isolated from activated sludge. Int J Syst Evol Microbiol 55:341–344
Kapanadze N, Melikadze GI, Janelidze P (2010) Estimation of Zugdidi and Tbilisi thermal water deposits. In: Weller A, Melikadze G, Kapanadze N (eds) Exploration and exploitation of groundwater and thermal water systems in Georgia. Tbilisi, 2010
Karakhanian AS, Trifonov VG, Azizbekian OG, Hondkarian DG (1997) Relationship of late Quaternary tectonics and volcanism in the Khanarassar active fault zone, the Armenian Upland. Terra Nova 16:131–134
Kellermann C, Griebler C (2009) Thiobacillus thiophilus sp. nov., a chemolithoautotrophic, thiosulfate-oxidizing bacterium isolated from contaminated aquifer sediments. Int J Syst Evol Microbiol 59:583–588
Klatt CG, Wood JM, Rusch DB, Bateson MM, Hamamura N, Heidelberg JF, Grossman AR, Bhaya D, Cohan FM, Kühl M, Bryant DA, Ward DM (2011) Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J 5:1262–1278
Kodama Y, Watanabe K (2004) Sulfuricurvum kujiense gen. nov., sp. nov., a facultatively anaerobic, chemolithoautotrophic, sulfur-oxidizing bacterium isolated from an underground crude-oil storage cavity. Int J Syst Evol Microbiol 54:2297–2300
Krebs JE, Vaishampayan P, Probst AJ, Tom LM, Marteinsson VT, Andersen GL, Venkateswaran K (2014) Microbial community structures of novel Icelandic hot spring systems revealed by PhyloChip G3 analysis. Astrobiology 14(3):229–240
Kublanov IV, Perevalova AA, Slobodkina GB, Lebedinsky AV, Bidzhieva SK, Kolganova TV, Kaliberda EN, Rumsh LD, Haertle T, Bonch-Osmolovskaya EA (2009) Biodiversity of thermophilic prokaryotes with hydrolytic activities in hot springs of Uzon caldera, Kamchatka (Russia). Appl Environ Microbiol 75(1):286–291
Lee EM, Jeon CO, Choi I, Chang KS, Kim CJ (2005) Silanimonas lenta gen. nov., sp. nov., a slightly thermophilic and alkaliphilic gammaproteobacterium isolated from a hot spring. Int J Syst Evol Microbiol 55:385–389
Lee SY, Lee MH, Oh TK, Yoon JH (2006) Lutibacter aestuarii sp. nov., isolated from a tidal flat sediment, and emended description of the genus Lutibacter Choi and Cho. Int J Syst Evol Microbiol 62(2):420–424
Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR (2015) Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21(9):1638–1646
Li H, Yang Q, Li J, Gao H, Li P, Zhou H (2015) The impact of temperature on microbial diversity and AOA activity in the Tengchong geothermal field, China. Sci Rep 5:17056. https://doi.org/10.1038/srep17056
Lin YE, Lu WM, Huang HI, Huang WK (2007) Environmental survey of Legionella pneumophila in hot springs in Taiwan. J Toxicol Environ Health A 70(1):84–87
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125:171–189
Liu Z, Klatt CG, Wood JM, Rusch DB, Ludwig M et al (2011) Metatranscriptomic analyses of chlorophototrophs of a hotspring microbial mat. ISME J 5:1279–1290
Liu J, Wang Z, Belchik SM, Edwards MJ, Liu C, Kennedy DW, Merkley ED, Lipton MD, Butt JN, Richardson DJ, Zachara JM, Fredrickson JK, Rosso KM, Shi L (2012) Identification and characterization of MtoA: a decaheme c-type cytochrome of the neutrophilic Fe(II)-oxidizing bacterium Sideroxydans lithotrophicus ES-1. Front Microbiol 3:37. https://doi.org/10.3389/fmicb.2012.00037
López-López O, Cerdán ME, González-Siso MI (2013) Hot spring metagenomics. Life 3(2):308–320
López-López O, Knapik K, Cerdán ME, González-Siso MI (2015) Metagenomics of an alkaline hot spring in Galicia (Spain): microbial diversity analysis and screening for novel lipolytic enzymes. Front Microbiol 6:1291. https://doi.org/10.3389/fmicb.2015.01291
Margaryan A, Panosyan H, Popov YG (2010) Isolation and characterization of new metallotolerant bacilli strains. Biotechnol Biotechnol Equip 24(2):450–454
Maugeri TL, Lentini V, Gugliandolo C, Italiano F, Cousin S, Stackebrandt E (2009) Bacterial and archaeal populations at two shallow hydrothermal vents off Panarea Island (Eolian Islands, Italy). Extremophiles 13(1):199–212
Melikadze G, Tsertsvadze L, Tsertsvadze N, Vardigoreli O, Barabadze T (2010) Country update from Georgia. In: Proceedings of world geothermal congress, Bali, Indonesia, 25–29 April 2010
Merkel AY, Pimenov NV, Rusanov II, Slobodkin AI, Slobodkina GB, Tarnovetckii IY et al (2017) Microbial diversity and autotrophic activity in Kamchatka hot springs. Extremophiles 21(2):307–317
Meyer-Dombard DR, Shock EL, Amend JP (2005) Archaeal and bacterial communities in geochemically diverse hot springs of Yellowstone National Park, USA. Geobiology 3:211–227
Mkrtchyan S (ed) (1969) Geology of Armenian SSR. Publishing house of AS of ASSR, Yerevan (in Russian)
Moores EM, Fairbridge RW (eds) (1998) Encyclopedia of European and Asian Regional Geology. Springer Netherlands
Nakagawa T, Fukui M (2002) Phylogenetic characterization of microbial mats and streamers from a Japanese alkaline hot spring with a thermal gradient. J Gen Appl Microbiol 48:211–222
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5:787–797
Panosyan H (2010) Phylogenetic diversity based on 16S rRNA gene sequence analysis of aerobic thermophilic endospore-forming bacteria isolated from geothermal springs in Armenia. Biol J Armenia 62(4):73–80
Panosyan HH (2017) Thermophilic bacilli isolated from Armenian geothermal springs and their potential for production of hydrolytic enzymes. Int J Biotech Bioeng 3(8):239–244
Panosyan H, Birkeland NK (2014) Microbial diversity in an Armenian geothermal spring assessed by molecular and culture-based methods. J Basic Microbiol 54(11):1240–1250
Panosyan H, Anzelmo G, Nicolaus B (2014) Production and characterization of exopolysaccharadies synthesized by geobacilli isolated from an Armenian geothermal spring. FEBS J 281(1):667
Panosyan HH, Margaryan AA, Trchounian AH (2017) Denaturing gradient gel electrophoresis (DGGE) profiles of the partial 16S rRNA genes defined bacterial population inhabiting in Armenian geothermal springs. Biol J Armenia 68(3):102–109
Paronyan A (2002a) Ecology and biodiversity of phototrophic bacteraia in various ecosystems of Armenia. Biol J Armenia 54(1–2):91–98 (in Russion)
Paronyan AK (2002b) Consumption of organic carbon sources and biosynthesis of lactic acid by the photosynthetic bacterium Rhodobacter sp. D-4. Appl Biochem Microbiol 38(1):53–58
Paronyan AKh (2003) Ekologia, biologicheskie osobennosti fototrofnikh bakterii Armenii i perspektiwi ikh ispolzovanija. (Ecology, biological pecularities of phototrophic bacteria of Armenia and prospects its application). Disertation, Institute of Microbiology NAS of RA
Paronyan A (2007) Ecophisiological charachteristics of phototrophic bacteria Rhodopseudomonas palustris isolated from mineral geothermal Jermuk. Biol J Armenia 59(1–2):73–77 (in Russion)
Petursdottir SK, Bjornsdottir SH, Hreggvidsson GO, Hjorleifsdottir S, Jakob KK (2009) Analysis of the unique geothermal microbial ecosystemof the Blue Lagoon. FEMS Microbiol Ecol 70(3):425–432
Philip H, Cisternas A, Gvishiani A, Gorshukov A (1989) The Caucasus: an actual example of the initial stages of continental collision. Tectonophysics 161(1–2):1–21
Poddar A, Das SK (2017) Microbiological studies of hot springs in India: a review. Arch Microbiol 200:1. https://doi.org/10.1007/s00203-017-1429-3
Poddar A, Lepcha RT, Das SK (2014) Taxonomic study of the genus Tepidiphilus: transfer of Petrobacter succinatimandens to the genus Tepidiphilus as Tepidiphilus succinatimandens comb. nov., emended description of the genus Tepidiphilus and description of Tepidiphilus thermophilus sp. nov., isolated from a terrestrial hot spring. Int J Syst Evol Microbiol 64(1):228–235
Poghosyan L (2015) Prokaryotic diversity in an Armenian geothermal spring using metagenomics, anaerobic cultivation and genome sequencing. Master thesis, University of Bergen
Portillo MC, Sririn V, Kanoksilapatham W, Gonzalez JM (2009) Differential microbial communities in hot spring mats from Western Thailand. Extremophiles 13(2):321–331
Purcell D, Sompong U, Lau CY, Barraclough TG et al (2007) The effects of temperature, pH and sulphide on community structure of hyperthermophilic streamers in hot springs of northern Thailand. FEMS Microbiol Ecol 60(3):456–466
Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99(19):7907–7913
Rainey FA, Silva J, Nobre MF, Silva MT, da Costa MS (2003) Porphyrobacter cryptus sp. nov., a novel slightly thermophilic, aerobic, bacteriochlorophyll a-containing species. Int J Syst Evol Microbiol 53(1):35–41
Reilinger RE, McClusky SC, Oral MB, King RW, Toksoz MN, Barka AA, Kinik I, Lenk O, Sanli I (1997) Global Positioning System measurements of present-day crustal movements in the Arabia-Africa-Eurasia plate collision zone. J Geophys Res 102(B5):9983–9999
Roeselers G, Norris T, Castenholz R, Rysgaard S, Glud R, Kuhl M, Muyzer G (2007) Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ Microbiol 9(1):26–38
Saghatelyan A, Panosyan H (2015) Study of bacterial diversity of Karvachar geothermal spring using culture-independent methods. In: Collection of scientific articles of YSU SSS: materials of the scientific session dedicated to the 95th anniversary of YSU. Yerevan State University, Yerevan, pp 13–18
Saghatelyan A, Panosyan H, Trchounian A, Birkeland NK (2014) Investigation of microbial diversity of geothermal springs in Nagorno-Karabakh based on metagenomic and culture-based approaches. In: Abstracts book of international scientific workshop trends in microbiology and microbial biotechnology, YSU, Yerevan, Armenia, 5–8 October 2014
Saghatelyan A, Poghosyan L, Panosyan H, Birkeland NK (2015) Draft genome sequence of Thermus scotoductus strain K1, isolated from a geothermal spring in Karvachar, Nagorno Karabakh. Genome Announc 3(6):e01346-15. https://doi.org/10.1128/genomeA.01346-15
Saghatelyan A, Panosyan H, Trchounian A, Birkeland NK (2016) Amplification and cloning of DNA polymerase 1 (pol1) of Thermus scotoductus K1 isolated from an Armenian geothermal spring. FEBS J 283(1):165–166
Sahm K, John P, Nacke H, Wemheuer B, Grote R, Daniel R et al (2013) High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 17(4):649–662
Saxena R, Dhakan DB, Mittal P, Waiker P, Chowdhury A, Ghatak A, Sharma VK (2017) Metagenomic analysis of hot springs in Central India reveals hydrocarbon degrading thermophiles and pathways essential for survival in extreme environments. Front Microbiol 7:2123. https://doi.org/10.3389/fmicb.2016.02123
Sayeh R, Birrien JL, Alain K, Barbier G, Hamdi M, Prieur D (2010) Microbial diversity in Tunisian geothermal springs as detected by molecular and culture-based approaches. Extremophiles 14(6):501–514
Shahinyan GS, Margaryan AA, Panosyan HH, Trchounian AH (2015) Isolation and characterization of lipase-producing thermophilic bacilli from geothermal springs in Armenia and Nagorno-Karabakh. Biol J Armenia 67(2):6–15
Shahinyan G, Margaryan AA, Panosyan HH, Trchounian AH (2017) Identification and sequence analysis of novel lipase encoding novel thermophilic bacilli isolated from Armenian geothermal springs. BMC Microbiol 17:103. https://doi.org/10.1186/s12866-017-1016-4
Singh A, Subudhi E (2016) Profiling of microbial community of Odisha hot spring based on metagenomic sequencing. Genomics Data 7:187–188
Skirnisdottir S, Hreggvidsson GO, Hjorleifsdottir S, Marteinsson VT, Petursdottir SK, Holst O, Kristjansson JK (2000) Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol 66:2835–2841
Spanevello MD, Patel BK (2004) The phylogenetic diversity of Thermus and Meiothermus from microbial mats of an Australian subsurface aquifer runoff channel. FEMS Microbiol Ecol 50:63–73
Stan-Lotter H, Fendrihan S (2012) Adaption of microbial life to environmental extremes. Springer, Wien
Stefanova K, Tomova I, Tomova A, Radchenkova N, Atanassov I, Kambourova M (2015) Archaeal and bacterial diversity in two hot springs from geothermal regions in Bulgaria as demonstrated by 16S rRNA and GH-57 genes. Int Microbiol 18:217–223
Stokes CR (2011) Caucasus Mountains. In: Singh VP, Singh P, Haritashya UK (eds) Encyclopedia of snow, ice and glaciers. Springer, Dordrecht, pp 127–128
Takacs-Vesbach C, Mitchell K, Jakson-Weaver O, Reysenbach AL (2008) Volcanic calderas delineate biogeographi provinces among Yellowstone thermophiles. Environ Microbiol 10(7):1681–1689
Tsertsvadze N, Buachidze G, Vardigoreli O, Vashakidze B, Inaishvili T, Kotrikadze N, Tsertsvadze L (1998) Thermal water of Georgia. Georgian Geothermal Association, Tbilisi
Urbieta MS, Gonzalez-Toril E, Bazan AA, Giaveno MA, Donati E (2015) Comparison of the microbial communities of hot springs waters and the microbial biofilms in the acidic geothermal area of Copahue (Neuquen, Argentina). Extremophiles 19(2):437–450
Vardanyan G, Margaryan A, Panosyan H (2015) Isolation and characterization of lipase-produvting bacilli from Tatev geothermal spring (Armenia). In: Collection of scientific articles of YSU SSS: materials of the scientific session dedicated to the 95th anniversary of YSU. Yerevan State University, Yerevan, pp 33–36
Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G et al (2013) Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS One 8:e62901. https://doi.org/10.1371/journal.pone.0062901
Whitaker RJ, Grogan DW, Taylor JW (2003) Geographical barriers isolate endemic population of hyperthermophilic archaea. Science 301:976–978
Yoon HS, Aslam Z, Song GC, Kim SW, Jeon CO, Chon TS, Chung YR) (2009) Flavobacterium sasangense sp. nov., isolated from a wastewater stream polluted with heavy metals. Int J Syst Evol Microbiol 59(P5):1162–1166
Yuan CG, Jiang Z, Xiao M, Zhou EM, Kim CJ, Hozzein WN, Park DJ, Zhi XY, Li WJ (2016) Mesorhizobium sediminum sp. nov., isolated from deep-sea sediment. Int J Syst Evol Microbiol 66:4797–4802
Zellner G, Messner P, Kneifel H, Tindall BJ, Winter J, Stackebrandt E (1989) Methanolacinia gen. nov., incorporating Methanomicrobium paynteri as Methanolacinia paynteri comb. nov. J Gen Appl Microbiol 35:185–202
Zhang CL, Ye Q, Huang Z, Li W, Chen J et al (2008) Global occurrence of archaeal amoA genes in terrestrial hot springs. Appl Environ Microbiol 74:6417–6426
Zhou JH (2003) Microarrays for bacterial detection and microbial community analysis. Curr Opin Microbiol 6:288–294
Acknowledgments
This work was supported by grants from the EURASIA Program of the Norwegian Center for International Cooperation in Education (CPEA-2011/10081, CPEA-LT-2016/10095) and partially supported by the RA MES State Committee of Science, in the frames of the research project №15 T-1F399, Armenian National Science and Education Fund based in New York, USA, to HP (ANSEF-NS-microbio 2493, 3362 and 4676) and Project CRDF/GRDF/DTRA A60920.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Panosyan, H. et al. (2018). Microbial Diversity of Terrestrial Geothermal Springs in Lesser Caucasus. In: Egamberdieva, D., Birkeland, NK., Panosyan, H., Li, WJ. (eds) Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications. Microorganisms for Sustainability, vol 8. Springer, Singapore. https://doi.org/10.1007/978-981-13-0329-6_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-0329-6_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0328-9
Online ISBN: 978-981-13-0329-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)