Abstract
Flowering time is one of the most important agronomic traits in rice (Oryza sativa L.) and is primarily controlled by quantitative trait loci (QTLs) that are associated with a photoperiodic response, particularly in short-day (SD) plants such as rice. Since the early twentieth century, rice breeders and researchers have been interested in clarifying the genetic control of flowering time because its modification is important for regional adaptation. The sequencing of the rice genome has facilitated genome-wide mapping of loci and gene cloning; thus, more progress has been made in elucidating the genetic control pathways of flowering. In this chapter, we provide an overview of the studies investigating rice flowering.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Flowering is the dramatic transition from the vegetative phase to reproductive development and is predominantly regulated by genetic control pathways that integrate internal and external signals. The ability of plant species to initiate flowering at the most favourable time for reproduction depends primarily on their accurate measurement of seasonal changes in day length and temperature (Thomas and Vince-Pure 1997; Song et al. 2015).
The flowering time (often termed the heading date) is important for regional adaptability and is easy to observe; therefore, its variations among rice varieties (Oryza sativa L.) have been known for a long time. Studies investigating the inheritance of rice flowering time date back to the 1910s. Hoshino (1915) suggested that multiple loci were involved in the inheritance of the flowering time based on the segregation patterns in progeny from experimental crosses between early- and late-flowering varieties. Since the 1920s, due to the development of the chromosome theory of inheritance and the discovery of genetic linkage, the linkage relationship between the flowering time genes and known loci (for other phenotypic traits) has been investigated (Chao 1928; Jodon 1940; Chandraratna 1953).
After the discovery of photoperiodism in plants (i.e. the response of an organism to the relative length of the day and night) by Garner and Allard (1920, 1923), many researchers have measured the flowering responses in rice varieties by day length. These studies revealed that rice is a facultative short-day (SD) plant as follows: its flowering is promoted under SD conditions, and the difference in the photoperiod response among rice varieties results in extensive variations in the flowering time (Vergara and Chang 1985).
The development of DNA markers in the 1990s dramatically enhanced the determination of the chromosomal location of genes/quantitative trait loci (QTLs) that are involved in the flowering time, and after decoding the genome sequences, map-based cloning strategies have facilitated the molecular cloning of these genes (Hori et al. 2016) (see also Chaps. 8 and 9 and those from Chaps. 11, 12, 13, 14, 15, 16, and 17).
In this chapter, we summarise the findings of many studies investigating rice flowering time using forward- and reverse-genetic approaches based on genomic information from rice. The studies performed over the last two decades have clearly shown that the use of a combination of these approaches has enhanced our understanding of the genetic control pathways of flowering in rice.
10.2 Mapping of the QTLs Responsible for Flowering Time
In the 1990s, the development of DNA markers allowed researchers to clarify the number and effects of the genes underlying the flowering time using QTL analyses (Li et al. 1995; Xiao et al. 1996; Yano et al. 1997). In particular, using several types of progeny derived from a single cross between the rice varieties ‘Nipponbare’ (ssp. japonica) and ‘Kasalath’ (ssp. indica), our group detected several QTLs responsible for the flowering time. Five of these QTLs (Hd1–Hd5) were mapped by performing a QTL analysis of an F2 population (Yano et al. 1997), and Hd7, Hd8, and Hd11 were detected in the BC1F5 lines (Lin et al. 1998). Other loci were detected only when advanced backcross progeny, such as BC3F2 or BC4F2, was used (Yamamoto et al. 2000; Lin et al. 2002). The results of these QTL mapping studies are summarised by Yano et al. (2001). Since then, many research groups have detected the QTLs for flowering time using different cross combinations. More comprehensive genetic analyses revealed that more than 100 loci, including major and minor effects, might be involved in flowering time control (Hori et al. 2016).
10.3 Molecular Cloning of Flowering Time Genes
Efforts detecting QTL above mentioned have led to the map-based cloning of the genes responsible for flowering time and improved our understanding of the function of these genes at the molecular level and the genetic pathways controlling flowering in rice.
Hd1 was the first rice flowering time gene cloned using natural variation (Table 10.1; Yano et al. 2000). Using more than 9000 recombinants, we defined the Hd1 region within 12 kb on chromosome 6. This region contained one candidate gene with a high similarity to Arabidopsis CONSTANS (CO). Comparison of the candidate gene in ‘Nipponbare’ and ‘Kasalath’ revealed many sequence variations, including a 36-bp insertion and a 33-bp deletion (in exon 1) and a 2-bp deletion (in exon 2) in ‘Kasalath’. A small genomic fragment of ‘Nipponbare’ containing the Hd1 candidate gene was transferred into the near isogenic line of ‘Nipponbare’ carrying Hd1 from ‘Kasalath’ and was found to promote flowering under SD conditions. These results clearly indicated that the candidate gene homologous to Arabidopsis CO was Hd1.
At least 14 flowering time QTLs have been isolated using map-based cloning strategies assessing natural variation (Yano et al. 2000; Takahashi et al. 2001; Kojima et al. 2002; Doi et al. 2004; Xue et al. 2008; Wei et al. 2010; Bian et al. 2011; Matsubara et al. 2012; Gao et al. 2013; Hori et al. 2013; Koo et al. 2013; Ogiso-Tanaka et al. 2013; Wu et al. 2013; Shibaya et al. 2016). Rice mutants have also been used to isolate flowering time genes and investigate their functions (Izawa et al. 2000; Lee et al. 2004; Matsubara et al. 2008, 2011; Saito et al. 2012; Dai and Xue 2010; Yang et al. 2013; Yokoo et al. 2014; Yoshitake et al. 2015). Other flowering time genes have been identified, and their functions have been investigated using forward- and reverse-genetic approaches, such as overexpression or knockdown of a target gene. For example, the functions of RFT1 and OsTrx1 were revealed by knocking down these genes, whereas the functions of OsMADS50, OsMADS56, and OsMADS15 were verified by overexpressing these genes (Komiya et al. 2008; Ryu et al. 2009; Lu et al. 2012; Choi et al. 2014).
10.4 Genetic Pathways Controlling Flowering Revealed by Molecular Cloning
Under SD conditions, rice flowering is promoted by the expression of Hd3a, which is activated by Hd1 and Ehd1 (Table 10.1; Yano et al. 2000; Kojima et al. 2002; Doi et al. 2004; Tamaki et al. 2007) (Fig. 10.1). Hd3a acts as a mobile flowering signal (florigen) (Tamaki et al. 2007). The expression of Ehd1 is upregulated by DTH3, Ehd2, Ehd3, Ehd4, and OsMADS51 (Kim et al. 2007; Matsubara et al. 2008, 2011; Bian et al. 2011; Gao et al. 2013) and downregulated by OsCOL4 and OsCOL10 (Lee et al. 2010; Tan et al. 2016).
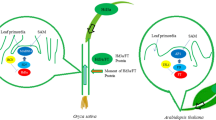
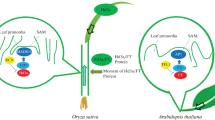
A schematic representation of the genetic pathways controlling flowering in rice. The clocks at the top show the circadian clock. Genes with no obvious Arabidopsis counterparts are shown in red. Orange ovals show physical interactions between genes. Genes with a natural allelic variation are underlined. X indicates an unknown gene. SD short-day conditions, LD long-day conditions. Arrows upregulation, bars downregulation. Phys phytochromes
The transcriptional activation of Hd3a is lower under long-day (LD) conditions than under SD conditions; consequently, flowering is suppressed (Fig. 10.1). Although Hd1 activates the expression of Hd3a under SD conditions, Hd1 represses the expression of Hd3a under LD conditions (Fig. 10.1). This functional conversion of Hd1 is caused by phytochrome-mediated signalling (Hayama and Coupland 2004; Izawa 2007). The Hd1 repressor function under LD conditions is enhanced by the kinase activity of Hd6 and is mediated by unknown genes (Takahashi et al. 2001; Ogiso et al. 2010). In addition to Hd1, Ghd7 also represses the expression of Hd3a by repressing Ehd1 under LD conditions (Xue et al. 2008). Based on genetic analyses, it was originally believed that rice photoperiodic flowering is controlled by the following two independent signalling pathways: the Hd1–Hd3a pathway, which is evolutionarily related to the Arabidopsis CO–FT pathway, and the Ghd7-Ehd1–Hd3a pathway, which has no Arabidopsis counterpart (Doi et al. 2004; Xue et al. 2008). However, a physical interaction was recently demonstrated between Hd1 and Ghd7 in vivo (Nemoto et al. 2016). The protein complex of Hd1 and Ghd7 specifically binds to a cis-regulatory region in Ehd1 and represses its expression, suggesting that the two pathways are integrated into Ehd1 and repress flowering under LD conditions (Fig. 10.1).
Due to the progress in our understanding of the core pathways, many genes underlying rice flowering under LD conditions have been discovered during the last decade (Fig. 10.1). RFT1, which is located within 11.5 kb of Hd3a, is an Hd3a paralog (Kojima et al. 2002; Komiya et al. 2008). The expression of RFT1 increases under LD conditions, and RFT1 moves from the leaves to the shoot apical meristem, indicating that the control of the flowering time in rice involves two florigen genes, Hd3a and RFT1, under LD conditions (Komiya et al. 2008, 2009). The expression of RFT1 is promoted by Ehd1 and DTH2 but is repressed by Se14 (Doi et al. 2004; Wu et al. 2013; Yokoo et al. 2014). The expression of Ehd1 is induced by DTH3, OsMADS51, OsMADS56, Ehd2, and Ehd4 (Kim et al. 2007; Matsubara et al. 2008; Ryu et al. 2009; Bian et al. 2011; Gao et al. 2013) but is repressed by DTH8, OsCOL4, OsCOL10, OsLFL1, and OsPRR37 (Peng et al. 2008; Lee et al. 2010; Wei et al. 2010; Yan et al. 2011; Gao et al. 2014; Tan et al. 2016). Recently, DTH8 has been reported to form a complex with Hd1 to control flowering (Chen et al. 2014; Zhu et al. 2017). DTH3 and OsMADS56 form a complex that regulates Ehd1 (Ryu et al. 2009). OsMADS51 is upregulated by Hd18 and induces the expression of Ehd1 (Kim et al. 2007; Shibaya et al. 2016). OsLFL1 is induced by OsVIL2 and has been proposed to downregulate the expression of Ehd1 (Peng et al. 2008; Yang et al. 2013). Ehd2 is downregulated by Se13 and induces the expression of Ehd1 (Matsubara et al. 2008; Yoshitake et al. 2015). OsCOL4 is a constitutive repressor upstream of Ehd1 (Lee et al. 2010). OsCOL10 downregulates the expression of Ehd1 and is upregulated by Ghd7 (Tan et al. 2016). The expression of Ghd7 is induced by Hd17 and is repressed by the Ehd3 and OsTrx1 complex (Choi et al. 2014). Ghd7 activity is increased by phosphorylation by Hd16 (Hori et al. 2013). Hd16 also phosphorylates OsPRR37, which represses the expression of Hd3a either directly or through Ehd1 (Hori et al. 2013; Koo et al. 2013; Gao et al. 2014).
Thus, under LD conditions, most flowering time gene signals (by both repressors and promoters) are transmitted to rice florigen genes through Ehd1 in flowering rice (Fig. 10.1).
The shared regulation by flowering time genes downstream of Hd3a and RFT1 is an underlying mechanism under both SD and LD conditions (Fig. 10.1). Hd3a interacts with GF14C, and then the Hd3a-GF14C complex interacts with OsFD (Taoka et al. 2011; Tsuji et al. 2013). The resultant protein complex induces the expression of floral meristem identity genes (e.g. OsMADS15) to initiate the floral transition in the shoot apex.
An additional description of the genes described above is provided in Table 10.1.
10.5 Circadian Clock Genes Control the Expression of Flowering Time Genes
The expression of many rice flowering time genes depends on the day length (Izawa 2007; Itoh et al. 2010). For example, Hd3a and Ehd1 are expressed in the morning under SD conditions, whereas Ghd7 is expressed in the morning under LD conditions. The expression of OsGI shows daily circadian oscillations with a peak at the end of the light period, and the expression of OsGI is regulated by the circadian clock and activates the expression of Hd1 (Table 10.1; Hayama et al. 2002, 2003) (Fig. 10.1). OsGI also activates the expression of Ehd1 either directly or via OsMADS51 (Kim et al. 2007; Itoh et al. 2010). The expression levels of certain flowering time genes are regulated by the circadian gating of light responses through phytochromes (red-light receptors) and cryptochromes (blue-light receptors) (Itoh et al. 2010). The expression of Ehd1 is induced by blue light in an OsGI-dependent manner regardless of day length; however, the expression is repressed by Ghd7 under LD conditions. The expression of Ghd7 is induced by phytochrome signalling, and the sensitivity to red light is gated at the beginning of the light period under LD conditions.
Recently, Matsuzaki et al. (2015) developed a statistical model of the expression of multiple genes with phase setting by sunlight and the circadian clock under field conditions. The integration of the expression patterns of individual flowering time genes can accurately estimate the internal biological time determined by both the circadian clock and the actual physical time of day.
To date, the control of flowering by the circadian clock in rice remains largely unknown compared to that in Arabidopsis likely because experiments in the laboratory are difficult to perform due to its large plant size. However, field experiments, such as those performed by Matsuzaki et al. (2015), will provide a better understanding of the role of the circadian clock in rice flowering.
10.6 Genetic Architecture of the Natural Variations in Flowering Time
To clarify the natural allelic variations in flowering time, we carried out QTL analyses in 12 F2 populations derived from crosses of ‘Koshihikari’ (ssp. japonica), which is an elite Japanese variety that is commonly used as a parental line, with varieties originating in various regions in Asia (Ebana et al. 2011; Shibaya et al. 2011). A limited number of loci with large effects that corresponded to Hd1, Hd2, Hd6, RFT1, Ghd7, DTH8, and Hd16 accounted for some varietal differences, but additional QTLs are likely to be involved in the flowering variation in these populations.
To detect QTLs with small effects, we analysed advanced backcross progeny derived from each cross combination by Ebana et al. (2011) and Shibaya et al. (2011) and detected a total of 255 QTLs widely distributed across the genome (Hori et al. 2015). We detected 128 QTLs with a relatively large effect, which corresponded to the genomic positions of previously detected flowering time genes, such as Hd1, Hd2, Hd6, RFT1, Ghd7, DTH8, and Hd16. The sequence analyses revealed that the chromosomal positions of the large-effect QTLs mainly corresponded to those of different alleles of the flowering time genes in 12 rice varieties. The other 127 QTLs were detected in chromosomal regions other than those of the flowering time genes and had relatively small effects. These results indicate that much of the variation in the flowering time can be explained by combinations of alleles in large- and small-effect QTLs.
Genome-wide association studies have also supported the hypothesis that allelic variations at multiple QTLs play an important role in the differences in the flowering time among rice varieties (Zhao et al. 2011; Huang et al. 2012; Yano et al. 2016). Zhao et al. (2011) detected ten genomic regions that were significantly associated with the flowering time variation, although only Hd1 was detected as a major QTL. These genomic regions explained less than 50% of the flowering time variation. Huang et al. (2012) found 14 significant genomic regions: 5 regions surrounding Hd1, Ghd7, RCN1, OsGI, and Hd3a and 9 newly discovered regions. The detected regions explained 36% of the flowering time variation. More recently, a genome-wide association study revealed that two novel QTLs on chromosomes 1 and 11 contributed to the flowering time variation in japonica rice varieties (Yano et al. 2016). The above-mentioned studies suggest that QTLs that have not yet been discovered are associated with the natural variation in the flowering time in rice varieties.
10.7 Regional Adaptation Based on Allelic Differences in Flowering Time Genes
Early flowering conferred by deficient alleles in the flowering time genes is important for expanding the range of rice cultivation to high latitudes (LD conditions) (Izawa 2007; Shrestha et al. 2014), where early heading and maturity are required for seed production. A sequence analysis of the known flowering time genes, including Hd1, Ghd7, DTH8, Hd16, OsPRR37, DTH2, and Ehd4, indicated that allelic differences contribute to regional adaptation (Takahashi et al. 2009; Naranjo et al. 2014; Gómez-Ariza et al. 2015; Zheng et al. 2015; Goretti et al. 2017). The functional alleles of Hd1 are associated with late flowering, and its non-functional alleles are associated with early flowering under natural day-length conditions; the geographical distribution of the Hd1 alleles suggests that favourable alleles have been selected by breeders to enhance rice productivity and adaptability in each region (Takahashi et al. 2009; Fujino et al. 2010; Ebana et al. 2011; Takahashi and Shimamoto 2011; Naranjo et al. 2014; Gómez-Ariza et al. 2015; Goretti et al. 2017). The deficient or weak alleles of Ghd7, DTH8, DTH2, Hd16, and OsPRR37 are distributed in cultivation areas at high latitudes (Xue et al. 2008; Wei et al. 2010; Fujino et al. 2013; Hori et al. 2013; Koo et al. 2013; Wu et al. 2013; Kwon et al. 2014; Goretti et al. 2017), suggesting that these alleles must be involved in the expansion of rice cultivation areas.
10.8 Conclusions and Perspectives
During the last two decades, tremendous progress in genome sequencing has improved our understanding of the genetic and molecular mechanisms that control the flowering time in rice. For example, Ehd1 is an important integrator in genetic control pathway, the putative homolog has found in sorghum (SD plant); on the other hand, it has not been found in Arabidopsis and wheat (LD plants) (Brambilla et al. 2017). Such a finding in rice provides valuable suggestion about the genetic control of flowering time in sorghum; additionally, it plays an important role in understanding of the diversity and evolution of flowering time control in plants.
This progress was due not only to genomic approaches, such as QTL analyses and map-based cloning, but also to the large number of rice accessions (including wild relatives) and genetic mapping populations derived from artificial crosses. Most genes with major effects on flowering time have already been identified during the last 20 years. Although some genes with minor effects have also been analysed, additional new QTLs with minor effects need to be further examined. In fact, genome-wide association studies could be an effective method for detecting new chromosomal regions (QTLs) responsible for the flowering time (Yano et al. 2016). The verification of the allelic effects of these QTLs with minor effects must be performed using experimental populations derived from single crosses. This approach will lead a more in-depth understanding of the genetic control of flowering time in rice. Information derived from these analyses has also been applied to the modulation of the flowering time of cultivars for regional adaptation and cropping system (Takeuchi et al. 2006; Takeuchi 2011; Hori et al. 2016).
Recently, genome-wide prediction models of the flowering time in rice have been tested and demonstrated high prediction accuracy by adding environmental variables (Nakagawa et al. 2005; Onogi et al. 2016; Spindel et al. 2016). Furthermore, it appears that these models can predict the flowering time using genome-wide genotypes and trait values in various types of populations, such as experimental biparental populations or an array of varieties (Spindel et al. 2016). Further progress using these approaches will enhance the fine-tuning of flowering time in rice breeding programmes.
References
Bian XF, Liu X, Zhao ZG et al (2011) Heading date gene, dth3 controlled late flowering in O. glaberrima Steud. by down-regulating Ehd1. Plant Cell Rep 30:2243–2254
Brambilla V, Gomez-Ariza J, Cerise M et al (2017) The importance of being on time: regulatory networks controlling photoperiodic flowering in cereals. Front Plant Sci 8:665
Chandraratna MF (1953) A gene for photoperiod sensitivity in rice linked with apiculus colour. Nature 171:1162–1163
Chao LF (1928) Linkage studies in rice. Genetics 13:133–169
Chen J, Li X, Cheng C et al (2014) Characterization of epistatic interaction of QTLs LH8 and EH3 controlling heading date in rice. Sci Rep 4:4263
Choi SC, Lee S, Kim SR et al (2014) Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol 164:1326–1337
Dai C, Xue HW (2010) Rice early flowering1, a CKI, phosphorylates DELLA protein SLR1 to negatively regulate gibberellin signalling. EMBO J 29:1916–1927
Doi K, Izawa T, Fuse T et al (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18:926–936
Ebana K, Shibaya T, Wu J et al (2011) Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor Appl Genet 122:1199–1210
Fujino K, Wu J, Sekiguchi H et al (2010) Multiple introgression events surrounding the Hd1 flowering-time gene in cultivated rice, Oryza sativa L. Mol Gen Genomics 284:137–146
Fujino K, Yamanouchi U, Yano M (2013) Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet 126:611–618
Gao H, Zheng XM, Fei G et al (2013) Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet 9:e1003281
Gao H, Jin M, Zheng X-M et al (2014) Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci 111:16337–16342
Garner WW, Allard HA (1920) Agricultural United States Department of Agriculture and for the association. J Agric Res XVIII:553–606
Garner WW, Allard HA (1923) Further studies in photoperiodism, the response of the plant to relative length of day and night. J Agric Res XXIII:871–920
Gómez-Ariza J, Galbiati F, Goretti D et al (2015) Loss of floral repressor function adapts rice to higher latitudes in Europe. J Exp Bot 66:2027–2039
Goretti D, Martignago D, Landini M et al (2017) Transcriptional and post-transcriptional mechanisms limit heading date 1 (Hd1) function to adapt rice to high latitudes. PLoS Genet 1:1–22
Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and Rice. Plant Physiol 135:677–684
Hayama R, Izawa T, Shimamoto K (2002) Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant Cell Physiol 43:494–504
Hayama R, Yokoi S, Tamaki S et al (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422:719–722
Hori K, Ogiso-Tanaka E, Matsubara K et al (2013) Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J 76:36–46
Hori K, Nonoue Y, Ono N et al (2015) Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol 15:1–16
Hori K, Matsubara K, Yano M (2016) Genetic control of flowering time in rice: integration of Mendelian genetics and genomics. Theor Appl Genet 129:2241–2252
Hoshino Y (1915) On the inheritance of the flowering time in peas and rice. J Coll Agric Tohoku Imp Univ Sapporo, Japan 6:229–288
Huang X, Zhao Y, Wei X et al (2012) Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet 44:32–39
Itoh H, Nonoue Y, Yano M et al (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42:635–638
Izawa T (2007) Daylength measurements by rice plants in photoperiodic short-day flowering. In: Kwang WJ (ed) International review of cytology. Academic, Cambridge, pp 191–222
Izawa T, Oikawa T, Tokutomi S et al (2000) Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J 22:391–399
Jodon NE (1940) Inheritance and linkage relationships of a chlorophyll mutation in rice. Agron J 32:342–346
Kim SL, Lee S, Kim HJ et al (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145:1484–1494
Kojima S, Takahashi Y, Kobayashi Y et al (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105
Komiya R, Ikegami A, Tamaki S et al (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135:767–774
Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136:3443–3450
Koo BH, Yoo SC, Park JW et al (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6:1877–1888
Kwon CT, Yoo SC, Koo BH et al (2014) Natural variation in Early flowering1 contributes to early flowering in japonica rice under long days. Plant Cell Environ 37:101–112
Lee S, Kim J, Han JJ et al (2004) Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J 38:754–764
Lee YS, Jeong DH, Lee DY et al (2010) OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J 63:18–30
Li Z, Pinson SRM, Stansel JW et al (1995) Identification of quantitative trait loci (QTLs) for heading date and plant height in cultivated rice (Oryza sativa L.) Theor Appl Genet 91:374–381
Lin SY, Sasaki T, Yano M (1998) Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theor Appl Genet 96:997–1003
Lin H, Ashikari M, Yamanouchi U et al (2002) Identification and characterization of a quantitative trait locus, Hd9, controlling heading date in rice. Breed Sci 52:35–41
Lu SJ, Wei H, Wang Y et al (2012) Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.) Plant Mol Biol Rep 30:1461–1469
Matsubara K, Yamanouchi U, Wang ZX et al (2008) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene promotes flowering by up-regulating Ehd1. Plant Physiol 148:1425–1435
Matsubara K, Yamanouchi U, Nonoue Y et al (2011) Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 66:603–612
Matsubara K, Ogiso-Tanaka E, Hori K et al (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53:709–716
Matsuzaki J, Kawahara Y, Izawa T (2015) Punctual transcriptional regulation by the rice circadian clock under fluctuating field conditions. Plant Cell 27:633–648
Nakagawa H, Yamagishi J, Miyamoto N et al (2005) Flowering response of rice to photoperiod and temperature: a QTL analysis using a phenological model. Theor Appl Genet 110:778–786
Naranjo L, Talón M, Domingo C (2014) Diversity of floral regulatory genes of japonica rice cultivated at northern latitudes. BMC Genomics 15:101
Nemoto Y, Nonoue Y, Yano M et al (2016) Hd1, a CONSTANS orthlog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J 86:221–233
Ogiso E, Takahashi Y, Sasaki T et al (2010) The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol 152:808–820
Ogiso-Tanaka E, Matsubara K, Yamamoto S et al (2013) Natural variation of the RICE FLOWERING LOCUS T 1 contributes to flowering time divergence in rice. PLoS One 8:e75959
Onogi A, Watanabe M, Mochizuki T et al (2016) Toward integration of genomic selection with crop modelling: the development of an integrated approach to predicting rice heading dates. Theor Appl Genet 129:805–817
Peng LT, Shi ZY, Li L et al (2008) Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J Plant Physiol 165:876–885
Ryu CH, Lee S, Cho LH et al (2009) OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ 32:1412–1427
Saito H, Ogiso-Tanaka E, Okumoto Y et al (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short-and long-day conditions. Plant Cell Physiol 53:717–728
Shibaya T, Nonoue Y, Ono N et al (2011) Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theor Appl Genet 123:1133–1143
Shibaya T, Hori K, Ogiso-Tanaka E et al (2016) Hd18, encoding histone acetylase related to arabidopsis flowering locus D, is involved in the control of flowering time in rice. Plant Cell Physiol 57:1828–1838
Shrestha R, Gómez-Ariza J, Brambilla V et al (2014) Molecular control of seasonal flowering in rice, arabidopsis and temperate cereals. Ann Bot 114:1445–1458
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol 66:441–464
Spindel JE, Begum H, Akdemir D et al (2016) Genome-wide prediction models that incorporate de novo GWAS are a powerful new tool for tropical rice improvement. Heredity 116:395–408
Takahashi Y, Shimamoto K (2011) Heading date 1 (Hd1), an ortholog of Arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes Genet Syst 86:175–182
Takahashi Y, Shomura A, Sasaki T et al (2001) Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc Natl Acad Sci U S A 98:7922–7927
Takahashi Y, Teshima KM, Yokoi S et al (2009) Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci U S A 106:4555–4560
Takeuchi Y (2011) Developing isogenic lines of Japanese rice cultivar ‘Koshihikari’ with early and late heading. Jpn Agric Res 45:15–22
Takeuchi Y, Ebitani T, Yamamoto T et al (2006) Development of isogenic lines of rice cultivar Koshihikari with early and late heading by marker-assisted selection. Breed Sci 56:405–413
Tamaki S, Matsuo S, Wong HL et al (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Tan J, Jin M, Wang J et al (2016) OsCOL10, a CONSTANS-like gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant Cell Physiol 57:798–812
Taoka K, Ohki I, Tsuji H et al (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476:332–335
Thomas B, Vince-Pure D (1997) Photoperiodism in plants, 2nd edn. Academic, San Diego
Tsuji H, Nakamura H, Taoka K (2013) Functional diversification of FD transcription factors in rice, components of florigen activation complexes. Plant Cell Physiol 54:385–397
Vergara BS, Chang TT (1985) The flowering response of the rice plant to photoperiod, 4th edn. IRRI, Manila
Wei X, Xu J, Guo H et al (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153:1747–1758
Wu W, Zheng XM, Lu G et al (2013) Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proc Natl Acad Sci U S A 110:2775–2780
Xiao J, Li J, Yuan L et al (1996) Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from a subspecific rice cross. Theor Appl Genet 92:230–244
Xue W, Xing Y, Weng X et al (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yamamoto T, Lin H, Sasaki T et al (2000) Identification of heading date quantitative trait locus Hd6 and characterization of its epistatic interactions with Hd2 in rice using advanced backcross progeny. Genetics 154:885–891
Yan WH, Wang P, Chen HX et al (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4:319–330
Yang J, Lee S, Hang R et al (2013) OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. Plant J 73:566–578
Yano M, Harushima Y, Nagamura Y et al (1997) Identification of quantitative trait loci controlling heading date in rice using a high-density linkage map. Theor Appl Genet 95:1025–1032
Yano M, Katayose Y, Ashikari M et al (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473–2484
Yano M, Kojima S, Takahashi Y et al. (2001) Genetic control of flowering time in rice, a short day plant. Plant Physiol 127: 1425–1429
Yano K, Yamamoto E, Aya K et al (2016) Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat Genet 48:927–936
Yokoo T, Saito H, Yoshitake Y et al (2014) Se14, encoding a JmjC domain-containing protein, plays key roles in long-day suppression of rice flowering through the demethylation of H3K4me3 of RFT1. PLoS One 9:e96064
Yoshitake Y, Yokoo T, Saito H et al (2015) The effects of phytochrome-mediated light signals on the developmental acquisition of photoperiod sensitivity in rice. Sci Rep 5:7709
Zhao K, Tung CW, Eizenga GC et al (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467
Zheng XM, Feng L, Wang J et al (2015) Nonfunctional alleles of long-day suppressor genes independently regulate flowering time. J Integr Plant Biol 58:540–548
Zhu S, Wang J, Cai M et al (2017) The OsHAPL1-DTH8-Hd1 complex functions as the transcription regulator to repress heading date in rice. J Exp Bot 68:553–568
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Matsubara, K., Yano, M. (2018). Genetic and Molecular Dissection of Flowering Time Control in Rice. In: Sasaki, T., Ashikari, M. (eds) Rice Genomics, Genetics and Breeding. Springer, Singapore. https://doi.org/10.1007/978-981-10-7461-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-7461-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7460-8
Online ISBN: 978-981-10-7461-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)