Abstract
Key message
Integration of previous Mendelian genetic analyses and recent molecular genomics approaches, such as linkage mapping and QTL cloning, dramatically strengthened our current understanding of genetic control of rice flowering time.
Abstract
Flowering time is one of the most important agronomic traits for seed production in rice (Oryza sativa L.). It is controlled mainly by genes associated with photoperiod sensitivity, particularly in short-day plants such as rice. Since the early twentieth century, rice breeders and researchers have been interested in elucidating the genetic basis of flowering time because its modification is important for regional adaptation and yield optimization. Although flowering time is a complex trait controlled by many quantitative trait loci (QTLs), classical genetic studies have shown that many associated genes are inherited in accordance with Mendelian laws. Decoding the rice genome sequence opened a new era in understanding the genetic control of flowering time on the basis of genome-wide mapping and gene cloning. Heading date 1 (Hd1) was the first flowering time QTL to be isolated using natural variation in rice. Recent accumulation of information on rice genome has facilitated the cloning of other QTLs, including those with minor effects on flowering time. This information has allowed us to rediscover some of the flowering genes that were identified by classical Mendelian genetics. The genes characterized so far, including Hd1, have been assigned to specific photoperiod pathways. In this review, we provide an overview of the studies that led to an in-depth understanding of the genetic control of flowering time in rice, and of the current state of improving and fine-tuning this trait for rice breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the rediscovery of the Mendelian concept of heredity in 1900, many genetic studies were performed to elucidate genes controlling plant phenotypes. Flowering is a profound transition from vegetative to reproductive development, and is determined largely by genetic pathways that integrate endogenous and exogenous signals. The ability of many plant species to initiate flowering at a time most favorable for their reproduction depends mainly on the accurate measurement of seasonal changes in daylength and temperature (Thomas and Vince-Pure 1997; Hayama et al. 2003).
In this review, we summarize the findings from many studies of rice flowering time based on classical Mendelian genetics and on molecular genetics and genomics. Studies performed over a century clearly showed that integration of these approaches dramatically improves our understanding of the genetic control of flowering time in rice.
Mendelian genetics of flowering time in rice
Flowering time is important for regional adaptability and is easy to observe; its variability among rice varieties has been known for a long time. Studies of the inheritance of rice flowering time date back to the 1910s. On the basis of segregation patterns in progeny from experimental crosses between early- and late-flowering varieties, Hoshino (1915) suggested the involvement of at least three loci.
Since the discovery of photoperiodism in plants (the response of an organism to the relative length of day and night) by Garner and Allard (1920, 1923), many researchers investigated flowering responses of rice varieties to daylength. These studies revealed that rice is a short-day (SD) plant: its flowering is promoted under SD conditions, and the difference in photoperiod sensitivity among varieties results in extensive variation in flowering time (reviewed in Chandraratna 1964; Vergara and Chang 1985).
To investigate the genetic control of flowering time in rice, early- and late-flowering varieties were crossed (Nomura and Yamazaki 1925; Jones 1928, 1933; Fuke 1955; Sampath and Seshu 1961), and the number of genes and their allelic effects were estimated from segregation patterns in F2 plants and their F3 progeny selected on the basis of flowering time (for example, early-, intermediate-, and late-flowering segregants). In F2 plants, the distribution of flowering time varied from continuous unimodal to clearly bimodal; the distribution depended on the photoperiod sensitivity of the parents used and on the environment (latitude and season) where the segregating population was grown. In several crosses, the ratio of late flowering to early flowering in F2 plants and their F3 progeny (3:1 or 15:1) was similar to the ratio expected from Mendel’s law (Nomura and Yamazaki 1925; Jones 1928, 1933; Jones et al. 1935; Fuke 1955; Sampath and Seshu 1961). These results showed that one or two Mendelian genes likely play a major role, yet in some crosses, the presence of transgressive segregants among F2 plants suggested the contribution of additional gene(s) (Jones 1928; Jones et al. 1935). Moreover, segregation patterns suggesting a complementary effect of two genes (9 late-flowering: 7 early-flowering F2 plants) were also reported (Jones et al. 1935; Fuke 1955). These early studies eventually led to the identification of underlying genes, which are described in the following section.
Classical Mendelian genes for flowering time in rice
From the 1920s, with the development of the chromosome theory of inheritance and the discovery of genetic linkage, the linkage relationship between flowering time genes and known loci (for other phenotypic traits) was investigated, and thereby the possibility of a connection between phenotype and genotype in rice was reinforced (Chao 1928; Jodon 1940).
Chandraratna (1953, 1955) found bimodal segregation in F2 plants from crosses between varieties insensitive and sensitive to photoperiod, suggesting control by a Mendelian gene. A dominant allele at the locus resulted in late flowering. The gene (called Se) was linked to a locus for apiculus color (Table 1). To explain variation in flowering time among Japanese varieties, Fuke (1955) distinguished six genetic factors, Z, M, K, G, O, and F. Factor K had the largest effect on photoperiod sensitivity and was linked to a locus for apiculus color. Yokoo and Fujimaki (1971) reported that a late-flowering gene (designated Lm) is closely linked to a blast resistance gene in crosses between the O. sativa subspecies japonica and indica (Table 1). Yokoo and Kikuchi (1977) found that different alleles at the Lm locus determine photoperiod sensitivity. The gene for late flowering was also linked to a locus for apiculus color (called C) on chromosome 6. On the basis of linkage relationships among these flowering time loci (Se, K, and Lm) and the apiculus color locus, Yokoo et al. (1980) suggested that they are the same locus (Table 1), and that the Se/K/Lm gene contributes to flowering time variation among both japonica and indica varieties.
Syakudo and Kawase (1953) and Syakudo et al. (1954) proposed that the loci E1 and E2 are the main determinants of the segregation patterns of flowering time in the progeny of a cross between Japanese rice varieties (Table 1). Syakudo et al. (1954) suggested three additional loci (E3, E4, and E5), to interpret segregation of flowering time in crosses between other varieties, but E4 and E5 were later shown to be the same as E1 and E2, respectively (Okumoto et al. 1991). Of the three genes, E1 had the largest effect on flowering time and was investigated in detail; a recessive allele at the E1 locus reduces photoperiod sensitivity (Yamagata et al. 1986). In addition, the allelic distribution of E1, E2, and E3 among Japanese varieties was estimated from the results of crosses with tester lines, in which the genotypes for these genes had been defined. The dominant E1 allele was distributed among varieties grown in southwestern Japan; the recessive e1 allele was distributed among varieties grown in northern regions and was probably associated with early flowering (Okumoto et al. 1991, 1996). Linkage analyses of a trisomic line with phenotypic markers revealed that E1 is located on chromosome 7 and is linked to the rolled fine strip and slender glume genes (Okumoto and Tanisaka 1997).
To delineate the effect of earliness genes carried by northern varieties, Tsai and Oka (1966) developed near-isogenic lines (NILs) for early flowering by introducing earliness genes into the background of a photoperiod-insensitive variety by successive backcrossing. Genetic analysis of these NILs revealed that the genes E and M are involved in early flowering; a dominant allele at the E locus and a recessive allele at the M locus (m) resulted in early flowering (Table 1). The effect of the E allele was enhanced in the presence of the m allele, implying epistasis between E and M (Tsai 1976). Recently, this epistasis was confirmed by Uwatoko et al. (2008). Independently of Tsai (1976), Sato et al. (1988) developed early-flowering NILs and identified the dominant earliness allele Ef1 (Table 1) on chromosome 10 by examining linkage to phenotypic markers and cytological interchange breakpoints on the chromosome. An allelism test performed by crossing NILs for Ef1 or E, which had the same background, placed these genes at the same locus (Sato et al. 1988). Okumoto et al. (1992) suggested that M is identical to E1 by crossing NILs for the m allele (developed by Tsai 1976) with tester lines for E1, E2, or E3 (Table 1).
Yokoo and Okuno (1993) found an early-flowering mutant among X-ray-irradiated plants. The mutant showed a significant reduction of photoperiod sensitivity. The mutated gene, designated se5, was located on chromosome 6 but was distinct from Se/K/Lm.
Thus, in the above studies, the chromosomal locations and allelic relationships of genes were evaluated from their linkage relationships to known phenotypic markers and using experimental crosses with tester lines or NILs for flowering time.
In addition to the six major genes described above (Table 1), to our knowledge, at least 15 genes have been reported so far (Se2, Yu and Yao 1968; Se3, Poonyarit et al. 1989; Ef2, Ef3, and Ef4, Tsai 1991; Efx and Efy, Sato et al. 1992; Se4, Ohshima et al. 1993; Se6 and Se7, Tsai 1995; Se9, Ichitani et al. 1998a; Se10 and Se11, Tsai 1999; Ef5, Khun et al. 2004; Ef6, Khun et al. 2006). However, little is known about the allelic relationships among these genes and their precise positions in the genome.
Mendelizing flowering time in rice on the basis of QTL analysis
In the 1990s, the development of DNA markers allowed researchers to clarify the number and effects of the genes underlying flowering time by using quantitative trait locus (QTL) analysis (Li et al. 1995; Xiao et al. 1996; Yano et al. 1997).
Using several types of progeny derived from a single cross between the rice cultivars ‘Nipponbare’ (japonica) and ‘Kasalath’ (indica), our group identified 15 QTLs for flowering time (Fig. 1). Five of them (Hd1–Hd5) were mapped by QTL analysis of an F2 population (Yano et al. 1997), and Hd7, Hd8, and Hd11 were detected with BC1F5 lines (Lin et al. 1998). Other loci were detected only when we used advanced backcross progeny such as BC3F2 or BC4F2 (Yamamoto et al. 2000; Lin et al. 2002).
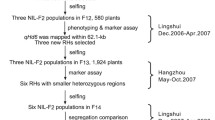
Plant materials used for QTL mapping of flowering time in rice. Mapping populations were derived from a cross between the cultivars ‘Nipponbare’ and ‘Kasalath’. QTL mapping was performed by using F2, BC1F5, BC3F2, and BC4F2 lines. Fine mapping and selection of NILs were conducted by using advanced backcross progeny. QTLs are shown next to the mapping populations in which they were detected. MAS marker-assisted selection, SSD single seed descent. Modified from Fig. 1 in Yano et al. (2001)
The development of NILs by marker-assisted selection (MAS) provided advantages for the genetic analysis of flowering time in rice. For example, QTL-NILs can be used to characterize epistatic interactions. This approach was used to clarify epistasis between Hd1 and Hd3 (Lin et al. 2000). Lin et al. (2000) suggested that the ‘Kasalath’ allele of Hd3 does not affect photoperiod sensitivity but enhances the effect of the ‘Nipponbare’ alleles of the photoperiod sensitivity QTLs Hd1 and Hd2. A significant epistatic interaction between Hd2 and Hd6 was detected in the analysis of advanced progeny in which the effect of the ‘Kasalath’ allele of Hd6 was observed only in the presence of the ‘Nipponbare’ allele of Hd2 (Yamamoto et al. 2000).
QTL-NILs can also be used for fine mapping of target QTLs. In such advanced backcross progenies, Hd1–Hd3, Hd6, and Hd9 were fine-mapped on a genetic linkage map as single Mendelian factors (Yamamoto et al. 1998, 2000; Lin et al. 2002). High-resolution mapping dissected two tightly linked loci, Hd3a and Hd3b, in the Hd3 region (Monna et al. 2002). The ‘Kasalath’ allele of Hd3a promotes flowering under SD conditions, whereas that of Hd3b delays flowering under long-day (LD) and natural-field conditions (Monna et al. 2002). These efforts have led to map-based cloning of genes for flowering time and thereby improved our understanding of the function of these genes at the molecular level and of the gene regulatory network for flowering time in rice, as described in the following section.
Molecular cloning of flowering time genes in rice
Hd1 was the first rice flowering time gene cloned using natural variation (Yano et al. 2000). Using more than 9000 BC3F3 plants, we defined the Hd1 region within 12 kb on chromosome 6. This region contained one candidate gene highly similar to Arabidopsis CONSTANS (CO). Comparison of the candidate gene in ‘Nipponbare’ and ‘Kasalath’ revealed many sequence variations, including a 36-bp insertion and a 33-bp deletion (in exon 1) and a 2-bp deletion (in exon 2) in ‘Kasalath’. A 7.1-kb fragment of ‘Nipponbare’ containing the Hd1 candidate gene was transferred into a NIL and was found to promote flowering under SD conditions. These results clearly indicated that the candidate gene homologous to Arabidopsis CO was Hd1.
More than 70 rice genes associated with flowering time have been isolated by using mutants, transgenic plants, and natural variation (Table 1; see also the OGRO database, Yamamoto et al. 2012). In segregating populations derived from crosses between rice varieties that differ in flowering time, at least 14 flowering time QTLs have been identified using natural variation and subsequently isolated by map-based cloning strategies (Table 1; Yano et al. 2000; Takahashi et al. 2001; Kojima et al. 2002; Doi et al. 2004; Xue et al. 2008; Wei et al. 2010; Bian et al. 2011; Matsubara et al. 2012; Gao et al. 2013; Hori et al. 2013; Koo et al. 2013; Ogiso-Tanaka et al. 2013; Wu et al. 2013; Shibaya et al. 2016). Rice mutants have also been used to isolate flowering time genes and investigate their functions (Izawa et al. 2000; Lee et al. 2004; Matsubara et al. 2008b; Kuromori et al. 2009; Saito et al. 2009, 2012; Dai and Xue 2010; Yang et al. 2013; Yokoo et al. 2014; Yoshitake et al. 2015). Other flowering time genes have been identified and their functions have been investigated by forward- and reverse-genetics approaches such as experiments using overexpression or knockdown transgenic plants. For example, the functions of OsMADS34 and OsTrx1 were revealed using knockdown transgenic plants, whereas the functions of OsMADS50, OsMADS56, and OsMADS15 were identified using overexpressing transgenic plants (Ryu et al. 2009; Lu et al. 2012; Choi et al. 2014; Lin et al. 2014).
It should be noted that even in such a modern genomics era, classical Mendelian genetics is still in an important position in the analysis of the genetic control of flowering time in rice. For example, in map-based cloning, an association between phenotype and molecular marker is often examined based on the Mendel’s law. The law of dominance gives us a useful estimation about gene function; recessive phenotype may be caused by loss-of-function mutation of gene of interest.
Relationships between genes identified by classical Mendelian genetics and molecular genomics
Unfortunately, in the almost studies on rice flowering time genes, different parental varieties or mutant lines have been used in classical Mendelian genetics and recent genomics approaches. Nevertheless, sequence comparison of isolated genes in rice varieties and mutant lines provided critical information about the connection between genes identified by the two approaches. For example, Hd1 was isolated in the segregating populations of ‘Nipponbare’ and ‘Kasalath’, and sequence comparisons of Hd1 in the two varieties revealed multiple sequence variations, such as insertions and deletions (Yano et al. 2000). In the same study, they analyzed sequences of the Hd1 gene in the Se1 mutants of HS66 and HS110 and their progenitor variety ‘Ginbozu’, in which the Se1 gene had been considered as a different allele at the Hd1 locus based on the chromosomal location on linkage maps (Tamura et al. 1998; Yamamoto et al. 1998). By sequence comparisons with the ‘Ginbozu’, HS66 had a 43-bp deletion and HS110 had a 433-bp insertion in their Hd1 genes (Yano et al. 2000). Both polymorphisms in HS66 and HS110 were estimated to cause the nonfunctional protein of Hd1. These results could lead to the conclusion that Hd1 is allelic to Se1. Other isolated genes have been also defined clear relationships with some classical Mendelian genes (Table 1). Tsai and Oka (1966) reported that a Taiwan variety ‘Taichung 65’ showed a long vegetative growth period and had a recessive allele of Ef1. Sequencing analysis of Ehd1 in various rice varieties revealed that the nonfunctional Ehd1 allele had been identified only in Taiwan varieties including ‘Taichung 65’ (Saito et al. 2009), showing that Ehd1 is allelic to Ef1. In the same way, it could be estimated that E1 (M), E2 and E3 are allelic to Ghd7, Hd17 and Hd6, respectively (Ichitani et al. 1998b; Matsubara et al. 2008a; Monden et al. 2009; Saito et al. 2012). Thus, molecular genomics approaches including map-based cloning and sequencing analysis have unraveled correspondence between classical Mendelian genes and isolated genes.
Gene regulatory network for flowering time in rice
Map-based cloning revealed a regulatory network of flowering time genes in rice. Rice photoperiodic flowering is controlled by two independent signaling pathways. The OsGI–Hd1–Hd3a pathway is evolutionarily related to the Arabidopsis GI–CO–FT pathway, whereas the other pathway, which includes Ehd1, Ghd7, Ehd2, Ehd3, and Ehd4, has no Arabidopsis counterpart.
Under SD conditions, rice flowering is promoted by the transcription of Hd3a, which is activated independently by Hd1 and Ehd1 (Yano et al. 2000; Kojima et al. 2002; Doi et al. 2004; Tamaki et al. 2007) (Fig. 2). Hd3a acts as a mobile flowering signal (florigen) (Tamaki et al. 2007). OsGI expression is regulated by the circadian clock and activates Hd1 expression (Hayama et al. 2003). Ehd1 expression is upregulated by the products of Ehd2, Ehd3, Ehd4, and OsMADS51 and downregulated by the product of OsCOL4. Ehd2 is a homolog of maize indeterminate1 and is a strong promoter of flowering (Matsubara et al. 2008b). Ehd3 encodes a putative transcriptional regulator with two plant homeodomain finger motifs (Matsubara et al. 2011). Ehd4 encodes a zinc finger CCCH domain–containing protein, which is an Oryza-specific regulator of photoperiodic flowering (Gao et al. 2013). OsGI also strongly activates Ehd1 expression, either directly or via OsMADS51 (Itoh et al. 2010).
Under LD conditions, transcriptional activation of Hd3a is lower than under SD conditions; consequently, flowering is suppressed. Although Hd1 activates Hd3a expression under SD conditions, it represses Hd3a expression under LD conditions (Fig. 2); this functional conversion of Hd1 is caused by phytochrome-mediated signaling (Hayama and Coupland 2004; Izawa 2007). The Hd1 repressor function under LD conditions is enhanced by the activity of a kinase encoded by Hd6 and is mediated by unknown genes (Takahashi et al. 2001; Ogiso et al. 2010); it is also regulated by a complex of Hd1 and Ghd7 (Nemoto et al. 2016). RFT1, located within 11.5 kb of Hd3a, is an Hd3a paralog (Komiya et al. 2008). RFT1 expression increases under LD conditions, and RFT1 moves from the leaves to the shoot apical meristem. These results strongly indicate that flowering time control in rice involves two florigen genes, Hd3a and RFT1 (Komiya et al. 2008; 2009). RFT1 expression is promoted by the product of Ehd1 and DTH2, but is repressed by the product of Se14 (Doi et al. 2004; Wu et al. 2013; Yokoo et al. 2014). Ehd1 expression is induced by the products of DTH3, OsMADS51, OsMADS56, Ehd2, and Ehd4, but is repressed by the products of DTH8, OsCOL4, OsCOL10, and OsLFL1. DTH8 might form a complex with Hd1 to control flowering (Wei et al. 2010; Yan et al. 2011). DTH3 and OsMADS56 form a complex that regulates Ehd1 (Ryu et al. 2009). OsMADS51 is upregulated by Hd18 and induces Ehd1 expression (Kim et al. 2007; Shibaya et al. 2016). OsLFL1 is induced by the product of OsVIL2, and has been proposed to downregulate Ehd1 expression (Peng et al. 2008; Yang et al. 2013). Ehd2 is downregulated by Se13, and induces Ehd1 expression (Matsubara et al. 2008b; Yoshitake et al. 2015). OsCOL4 and OsCOL10 are members of the CONSTANS-like family. OsCOL4 is a constitutive repressor upstream of Ehd1 (Lee et al. 2010). OsCOL10 downregulates the expression of Ehd1 and is upregulated by the product of Ghd7 (Tan et al. 2016). Ghd7 expression is induced by the products of Ehd3 and Hd17 and is repressed by OsTrx1, which is a chromatin-remodeling factor having histone methyltransferase activity (Choi et al. 2014). Ghd7 activity is increased by phosphorylation by the casein kinase I–like protein Hd16 (Hori et al. 2013). Hd16 also phosphorylates the product of OsPRR37 (likely allelic to Hd2), which encodes a pseudo-response regulator protein (Koo et al. 2013). Regulation of flowering time genes downstream of Hd3a and RFT1 is the same under both SD and LD conditions. Hd3a interacts with the 14-3-3 protein OsFD to initiate floral transition in the shoot apex (Purwestri et al. 2009). OsFD and GF14c induce the expression of OsMADS14 and OsMADS15 that control the phase transition of the SAM from vegetative to reproductive (Lim et al. 2000).
Circadian clock genes control the expression of flowering time genes in rice
The expression of many rice flowering time genes is strictly controlled by a critical daylength (Izawa 2007; Itoh et al. 2010; Matsuzaki et al. 2015). For example, Hd3a and Ehd1 are expressed in the morning under SD conditions, whereas Ghd7 is expressed in the morning under LD conditions. OsGI expression shows daily circadian oscillation with a peak at the end of the light period (Hayama et al. 2002). Expressions of some flowering time genes are regulated by the circadian gating of light responses through phytochromes (red light receptors) and cryptochromes (blue light receptors) (Itoh et al. 2010). Ghd7 expression is induced through phytochrome signaling, and sensitivity to red light is gated at the beginning of the light period under LD conditions. Ehd1 expression confers clear gating responses of blue light pulses (Itoh et al. 2010). Recently, Matsuzaki et al. (2015) developed a statistical model for the expression of multiple genes with phase setting by sunlight and the circadian clock. The integration of the expression patterns of individual flowering time genes can accurately estimate both the internal biological time determined by the circadian clock and actual physical time of day (Matsuzaki et al. 2015). However, additional molecular genetics and physiological analyses are needed to understand the circadian clock and the gating mechanisms of flowering time pathways in rice.
Genetic architecture of variation in flowering time among rice varieties
To date, more than 700 additional QTLs for flowering time have been detected on all 12 chromosomes by using segregating populations derived from crosses among rice varieties (Yonemaru et al. 2010; Youens-Clark et al. 2011). To comprehensively characterize natural allelic variations in flowering time, we carried out QTL analyses in 12 F2 populations derived from crosses of ‘Koshihikari’ (japonica), a widely grown elite Japanese cultivar commonly used as a parental line, with varieties originating from various regions in Asia (Ebana et al. 2011; Shibaya et al. 2011). A limited number of loci that coincided with Hd1, Hd2, Hd6, RFT1, Ghd7, DTH8, and Hd16 explained some varietal differences, but additional QTLs are likely to be involved in the phenotypic variation in these populations.
To detect such “hidden” QTLs, we analyzed advanced backcross progeny (BC4F2) derived from each cross combination of Ebana et al. (2011) and Shibaya et al. (2011) and detected a total of 255 QTLs distributed widely across the genome (Hori et al. 2015). We detected relatively large additive effects of 128 of these QTLs, which corresponded to the genomic positions of previously identified flowering time genes such as Hd1, Hd2, Hd6, RFT1, Ghd7, DTH8, and Hd16. Sequence analyses revealed that the chromosomal positions of the large-effect QTLs corresponded in the main to those of different alleles of the flowering time genes in 12 rice varieties. The other 127 QTLs were detected in chromosomal regions other than those of flowering time genes, and had relatively small additive effects. These results indicate that much of the phenotypic variation in flowering time can be explained by combinations of large- and small-effect QTLs.
Genome-wide association studies have also supported the hypothesis that allelic variations play an important role in the differences in flowering time among rice varieties (Zhao et al. 2011; Huang et al. 2012). Zhao et al. (2011) detected 10 genomic regions significantly associated with flowering time variation, although only Hd1 was detected as a major QTL. These genomic regions explained fewer than 50 % of the phenotypic variations (Zhao et al. 2011). Huang et al. (2012) found 14 significant genomic regions: 5 around Hd1, Ghd7, RCN1, OsGI, and Hd3a and 9 newly discovered regions. The identified regions explained 36 % of the phenotypic variation in flowering time. The above studies suggest that multiple QTLs are associated with natural variation in flowering time in rice varieties.
Regional adaptation conferred by allelic differences in flowering time genes
Early flowering conferred by deficient alleles of flowering time genes has been important for expanding the range of rice cultivation to high latitudes (Izawa 2007; Shrestha et al. 2014; Zheng et al. 2015), where early heading and maturity coincide with a period of optimal climatic conditions. Sequence analysis of the known flowering time genes, including Hd1, Ghd7, DTH8, Hd16, OsPRR37, DTH2, and Ehd4, indicates that allelic differences contribute greatly to regional adaptation (Takahashi et al. 2009; Shrestha et al. 2014; Gómez-Ariza et al. 2015; Zheng et al. 2015). Functional alleles of Hd1 are associated with late flowering and its nonfunctional alleles with early flowering under natural-daylength conditions; the geographic distribution of Hd1 alleles suggests that favorable alleles have been selected by breeders to enhance rice productivity and adaptability for each region (Takahashi et al. 2009; Fujino et al. 2010; Ebana et al. 2011; Takahashi and Shimamoto 2011; Gómez-Ariza et al. 2015). Deficient or weak alleles of Ghd7, DTH8, DTH2, Hd16, and OsPRR37 are distributed in cultivation areas at high latitudes (Xue et al. 2008; Wei et al. 2010; Fujino et al. 2013; Hori et al. 2013; Koo et al. 2013; Wu et al. 2013; Kwon et al. 2014), strongly suggesting that these alleles must be involved in the expansion of rice cultivation areas.
Fine-tuning of flowering time by gene introgression into rice varieties
Information about additive effects of individual flowering time genes and their epistatic interactions allows the estimation of phenotypic effects, such as the degree of alteration in flowering time, when flowering time genes are introgressed into a particular rice variety. Rice breeders have used this information to develop novel rice varieties. Modulation of rice flowering time helps to avoid flowering and ripening during periods with high risk of drought, flooding, and extreme temperatures in each cultivation area (Bentley et al. 2013). Plants can, thus, avoid severe stresses during the most sensitive developmental stages. Thus, the fine-tuning of flowering time is a major objective of rice breeding programs to adapt cultivars to regional cultivation systems and environmental conditions.
To facilitate modification of flowering time for use in commercial cultivars, we used MAS to develop a set of NILs of ‘Koshihikari’ with different alleles of flowering time genes derived from ‘Kasalath’ (Takeuchi et al. 2006; Takeuchi 2011). The set included NILs with a loss-of-function allele at Hd1 (designated ‘Koshihikari Kanto HD1’) and gain-of-function alleles at Hd4 (Ghd7) (‘Wakei 370’), DTH8 (‘Kanto HD2’), and Hd6 (‘Kanto IL5’) (Fig. 3). Each NIL contained less than 650 kb of the ‘Kasalath’ genome. ‘Koshihikari Kanto HD1’ flowered earlier than ‘Koshihikari’, whereas ‘Wakei 370’, ‘Kanto HD2’, and ‘Kanto IL5’ flowered later. Most agronomic traits of the NILs were similar to those of ‘Koshihikari’, although culm length and some other traits differed (Takeuchi et al. 2006; Takeuchi 2011). A recent large-scale QTL analysis of agronomic trait selected in rice breeding programs (Hori et al. 2012) demonstrated that flowering time genes can have pleiotropic effects on morphological traits (such as culm length and panicle length) and eating quality. Therefore, it is important to consider these pleiotropic effects to modify flowering time by introgression of corresponding genes.
Development of a series of isogenic lines of the Japanese elite cultivar ‘Koshihikari’ with different flowering times. a Graphical genotypes of the developed lines. Introgressed chromosome segments with target flowering time genes are indicated in red. b Phenotypes of the same lines and ‘Koshihikari’ at flowering stage (color figure online)
Our results demonstrate that MAS for alteration of flowering time can be effective in rice. We started to develop novel NIL series in genetic backgrounds of elite rice varieties grown in many regions of Japan. These NILs could have high agronomic performance in terms of high grain yield and optimum flowering time. Moreover, similar MAS strategies could be used to develop NILs of rice varieties grown in subtropical areas, where plants are at risk of both high-temperature stress and drought stress during flowering and ripening stages.
Because not only single genes but also interactions between genes affect flowering time (Lin et al. 2000; Yamamoto et al. 2000; Shibaya et al. 2011; Hori et al. 2013), one has to consider the genetic background of a rice variety when selecting the genes to introgress, combine, and pyramid for alteration of flowering time. Cultivation conditions also need to be considered, because the effects of flowering time genes often depend on daylength. For example, rice varieties with strong photoperiod sensitivity flower late in temperate areas (LD conditions during the growing season) and early in tropical areas (SD conditions). Accumulation of knowledge about flowering time genes and their regulatory network would facilitate the development of new rice varieties.
Conclusions and perspectives
In the last two decades, tremendous progress in genome sequencing has improved our understanding of genetic and molecular mechanisms that control flowering time in rice. This progress was made possible not only by genomics approaches, such as QTL analysis and map-based cloning, but also the large number of rice accessions (including wild relatives) and genetic mapping populations derived from artificial crosses. Before the development of genomics, classical genetics based on the Mendelian concept had already shown that several genes and their interactions are involved in the control of flowering time in rice. A combination of expanding genome sequence information with appropriate tools and plant materials has dramatically enhanced our understanding of the genetic control mechanism. Eventually, this progress has contributed to rice breeding because it allows us to fine-tune flowering time by combining several QTLs.
Unlike flowering time, some other agriculturally and economically important rice traits, such as yield performance and stress tolerance, are difficult to measure and are strongly affected by environmental conditions. To improve them to satisfy the demand for food supply for still increasing world population under climate change, we have to understand the genetic complexity of these traits at a fundamental level. In this situation, survey of genetic resources and development of plant materials will also be inevitable to strengthen our understanding of these traits.
Author contribution statement
MY, KH and KM conceived, designed and wrote this review manuscript.
References
Bentley A, Jensen E, Mackay I et al (2013) Flowering time. In: Kole C (ed) Genomics and breeding for climate-resilient crops, vol 2. Springer-Verlag, Berlin, pp 1–66
Bian XF, Liu X, Zhao ZG et al (2011) Heading date gene, dth3 controlled late flowering in O. glaberrima Steud. by down-regulating Ehd1. Plant Cell Rep 30:2243–2254
Chandraratna MF (1953) A gene for photoperiod sensitivity in rice linked with apiculus colour. Nature 171:1162–1163
Chandraratna MF (1955) Genetics of photoperiod sensitivity in rice. J Genet 53:215–223
Chandraratna MF (1964) Flowering in rice with particular reference to photoperiod control. In: Chandraratna MF (ed) Genetics and breeding of rice. Longmans, London, pp 181–214
Chao LF (1928) Linkage studies in rice. Genetics 13:133–169
Chen J, Li X, Cheng C et al (2014) Characterization of epistatic interaction of QTLs LH8 and EH3 controlling heading date in rice. Sci Rep 4:4263
Choi SC, Lee S, Kim SR et al (2014) Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol 164:1326–1337
Dai C, Xue HW (2010) Rice early flowering1, a CKI, phosphorylates DELLA protein SLR1 to negatively regulate gibberellin signalling. EMBO J 29:1916–1927
Dai X, Ding Y, Tan L et al (2012) LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice (Oryza rufipogon). J Integr Plant Biol 54:790–799
Doi K, Izawa T, Fuse T et al (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18:926–936
Ebana K, Shibaya T, Wu J et al (2011) Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor Appl Genet 122:1199–1210
Fujino K, Wu J, Sekiguchi H et al (2010) Multiple introgression events surrounding the Hd1 flowering-time gene in cultivated rice, Oryza sativa L. Mol Genet Genom 284:137–146
Fujino K, Yamanouchi U, Yano M (2013) Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet 126:611–618
Fuke Y (1955) On the genes controlling the heading time of leading rice varieties in Japan and their specific response to day length and temperature. Bull Nat Inst Agric Sci Ser D 5:1–71 (in Japanese with English summary)
Gao H, Zheng XM, Fei G et al (2013) Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet 9:e1003281
Garner WW, Allard HA (1920) Agricultural United States Department of Agriculture and for the Association. J Agric Res XVIII:553–606
Garner WW, Allard HA (1923) Further studies in photoperiodism, the response of the plant to relative length of day and night. J Agric Res XXIII:871–920
Gómez-Ariza J, Galbiati F, Goretti D et al (2015) Loss of floral repressor function adapts rice to higher latitudes in Europe. J Exp Bot 66:2027–2039
Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and Rice. Plant Physiol 135:677–684
Hayama R, Izawa T, Shimamoto K (2002) Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant Cell Physiol 43:494–504
Hayama R, Yokoi S, Tamaki S et al (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422:719–722
Hori K, Kataoka T, Miura K et al (2012) Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breed Sci 62:223–234
Hori K, Ogiso-Tanaka E, Matsubara K et al (2013) Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J 76:36–46
Hori K, Nonoue Y, Ono N et al (2015) Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol 15:1–16
Hoshino Y (1915) On the inheritance of the flowering time in peas and rice. J Coll Agric Tohoku Imp Univ Sapporo, Japan 6:229–288
Huang X, Zhao Y, Wei X et al (2012) Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet 44:32–39
Ichitani K, Okumoto Y, Tanisaka T (1998a) Genetic analyses of low photoperiod sensitivity of rice varieties from the northernmost regions of Japan. Plant Breed 117:543–547
Ichitani K, Okumoto Y, Tanisaka T (1998b) Genetic analysis of the rice cultivar Kasalath with special reference to two photoperiod sensitivity loci, E1 and Se-1. Breed Sci 48:51–57
Itoh H, Nonoue Y, Yano M et al (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42:635–638
Izawa T (2007) Daylength measurements by rice plants in photoperiodic short-day flowering. In: Kwang WJ (ed) International Review of Cytology. Academic Press, Cambridge, pp 191–222
Izawa T, Oikawa T, Tokutomi S et al (2000) Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J 22:391–399
Jodon NE (1940) Inheritance and linkage relationships of a chlorophyll mutation in rice. Agron J 32:342–346
Jones JW (1928) Inheritance of earliness and other agronomic characters in rice. J Agric Res 36:581–601
Jones JW (1933) Inheritance of characters in rice. J Agric Res 47:771–782
Jones JW, Adair CR, Beachell HM et al (1935) Inheritance of earliness and length of kernel in rice. J Amer Soc Agron 27:910–921
Khun LH, Hiraiwa M, Sato S et al (2004) Location of new gene for late heading in rice, Oryza sativa L. using interchange homozygotes. Breed Sci 54:259–263
Khun LH, Miyaji S, Motomura K et al (2006) Trisomic analysis of new gene for late heading in rice, Oryza sativa L. Euphytica 151:235–241
Kim SL, Lee S, Kim HJ et al (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145:1484–1494
Kojima S, Takahashi Y, Kobayashi Y et al (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43:1096–1105
Komiya R, Ikegami A, Tamaki S et al (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135:767–774
Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136:3443–3450
Koo BH, Yoo SC, Park JW et al (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6:1877–1888
Kuromori T, Takahashi S, Kondou Y et al (2009) Phenome analysis in plant species using loss-of-function and gain-of-function mutants. Plant Cell Physiol 50:1215–1231
Kwon CT, Yoo SC, Koo BH et al (2014) Natural variation in Early flowering1 contributes to early flowering in japonica rice under long days. Plant, Cell Environ 37:101–112
Lee S, Kim J, Han JJ et al (2004) Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J 38:754–764
Lee YS, Jeong DH, Lee DY et al (2010) OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J 63:18–30
Li Z, Pinson SRM, Stansel JW et al (1995) Identification of quantitative trait loci (QTLs) for heading date and plant height in cultivated rice (Oryza sativa L.). Theor Appl Genet 91:374–381
Lim J, Moon YH, An G et al (2000) Two rice MADS domain proteins interact with OsMADS1. Plant Mol Biol 44:513–527
Lin SY, Sasaki T, Yano M (1998) Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theor Appl Genet 96:997–1003
Lin HX, Yamamoto T, Sasaki T et al (2000) Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theor Appl Genet 101:1021–1028
Lin H, Ashikari M, Yamanouchi U et al (2002) Identification and characterization of a quantitative trait locus, Hd9, controlling heading date in rice. Breed Sci 52:35–41
Lin X, Wu F, Du X et al (2014) The pleiotropic SEPALLATA-like gene OsMADS34 reveals that the ‘empty glumes’ of rice (Oryza sativa) spikelets are in fact rudimentary lemmas. New Phytol 202:689–702
Lu SJ, Wei H, Wang Y et al (2012) Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Mol Biol Rep 30:1461–1469
Matsubara K, Kono I, Hori K et al (2008a) Novel QTLs for photoperiodic flowering revealed by using reciprocal backcross inbred lines from crosses between japonica rice varieties. Theor Appl Genet 117:935–945
Matsubara K, Yamanouchi U, Wang ZX et al (2008b) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene promotes flowering by up-regulating Ehd1. Plant Physiol 148:1425–1435
Matsubara K, Yamanouchi U, Nonoue Y et al (2011) Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 66:603–612
Matsubara K, Ogiso-Tanaka E, Hori K et al (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53:709–716
Matsuzaki J, Kawahara Y, Izawa T (2015) Punctual transcriptional regulation by the rice circadian clock under fluctuating field conditions. Plant Cell 27:633–648
Monden Y, Naito K, Okumoto Y et al (2009) High potential of a transposon mPing as a marker system in japonica × japonica cross in rice. DNA Res 16:131–140
Monna L, Lin HX, Kojima S et al (2002) Genetic dissection of a genomic region for a quantitative trait locus, Hd3, into two loci, Hd3a and Hd3b, controlling heading date in rice. Theor Appl Genet 104:772–778
Nemoto Y, Nonoue Y, Yano M et al (2016) Hd1, a CONSTANS orthlog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J 86:221–233
Nomura M, Yamazaki R (1925) A study of the inheritance of the shooting time in rice. Jpn J Genet 3:112–130 (in Japanese with English summary)
Ogiso E, Takahashi Y, Sasaki T et al (2010) The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol 152:808–820
Ogiso-Tanaka E, Matsubara K, Yamamoto S et al (2013) Natural variation of the RICE FLOWERING LOCUS T 1 contributes to flowering time divergence in rice. PLoS ONE 8:e75959
Ohshima I, Kikuchi F, Watanabe Y et al (1993) Analysis of heading time in a cross between two Indica varieties with inhibitor genes for photoperiod sensitivity. Jpn J Breed 43:101–106 (in Japanese with English summary)
Okumoto Y, Tanisaka T (1997) Trisomic analysis of a strong photoperiod-sensitivity gene E1 in rice (Oryza sativa L.). Euphytica 95:301–307
Okumoto Y, Tanisaka T, Yanagata H (1991) Heading-time genes of the rice varieties grown in south-west-warm region in Japan. Jpn J Breed 41:135–152 (in Japanese with English summary)
Okumoto Y, Yoshimura A, Tanisaka T et al (1992) Analysis of a rice variety Taichung 65 and its isogenic early-heading lines for late-heading genes E 1 , E 2 and E 3 . Jpn J Breed 42:415–429 (in Japanese with English summary)
Okumoto Y, Ichitani K, Inoue H et al (1996) Photoperiod insensitivity gene essential to the varieties grown in the northern limit region of paddy rice (Oryza sativa L.) cultivation. Euphytica 92:63–66
Peng LT, Shi ZY, Li L et al (2008) Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J Plant Physiol 165:876–885
Poonyarit M, Mackill DJ, Vergara BS (1989) Genetics of photoperiod sensitivity and critical daylength in rice. Crop Sci 29:647–652
Purwestri YA, Ogaki Y, Tamaki S et al (2009) The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol 50:429–438
Ryu CH, Lee S, Cho LH et al (2009) OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant, Cell Environ 32:1412–1427
Saito H, Yuan Q, Okumoto Y et al (2009) Multiple alleles at Early flowering 1 locus making variation in the basic vegetative growth period in rice (Oryza sativa L.). Theor Appl Genet 119:315–323
Saito H, Ogiso-Tanaka E, Okumoto Y et al (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short-and long-day conditions. Plant Cell Physiol 53:717–728
Sampath S, Seshu DV (1961) Genetics of photoperiod response in rice. Indian J Genet Plant Breed 21:38–42
Sato S, Sakamoto I, Shirakawa K et al (1988) Chromosomal location of an earliness gene Ef 1 of rice, Oryza sativa L. Jpn J Breed 38:385–396
Sato S, Ogata K, Shinjyo C (1992) Thermo-sensitive action of an earliness gene Ef-x in rice, Oryza sativa L. Jpn J Genet 67:473–482
Shibaya T, Nonoue Y, Ono N et al (2011) Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theor Appl Genet 123:1133–1143
Shibaya T, Hori K, Ogiso-Tanaka E et al (2016) Hd18, encoding histone acetylase related to Arabidopsis FLOWERING LOCUS D, is involved in the control of flowering time in rice. Plant Cell Physiol. doi:10.1093/pcp/pcw105
Shrestha R, Gómez-Ariza J, Brambilla V et al (2014) Molecular control of seasonal flowering in rice, arabidopsis and temperate cereals. Ann Bot 114:1445–1458
Syakudo K, Kawase T (1953) Studies on the quantitative inheritance (11) A. Rice (Oryza sativa L.) (d) Inheritance of the heading duration and the quantitative function of the causal genes E 1 , E 2 and D 1 . Jpn J Breed 3:6–12 (in Japanese with English summary)
Syakudo K, Kawase T, Yoshino K (1954) Studies on the quantitative inheritance (13) A. Rice (Oryza sativa L.) (d) Inheritance of the heading period and the quantitative function of the causal genes in its determination. (2) On the quantitative function of the genes E 3 , E 4 and E 5 . Jpn J Breed 4:83–91 (in Japanese with English summary)
Takahashi Y, Shimamoto K (2011) Heading date 1 (Hd1), an ortholog of Arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes Genet Syst 86:175–182
Takahashi Y, Shomura A, Sasaki T et al (2001) Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc Natl Acad Sci USA 98:7922–7927
Takahashi Y, Teshima KM, Yokoi S et al (2009) Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA 106:4555–4560
Takeuchi Y (2011) Developing isogenic lines of Japanese rice cultivar ‘Koshihikari’ with early and late heading. Jpn Agric Res 45:15–22
Takeuchi Y, Ebitani T, Yamamoto T et al (2006) Development of isogenic lines of rice cultivar Koshihikari with early and late heading by marker-assisted selection. Breed Sci 56:405–413
Tamaki S, Matsuo S, Wong HL et al (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Tamura K, Nomura K, Oshima I et al (1998) Identification of restriction fragment length polymorphism markers tightly linked to a major photoperiod sensitivity gene, Se1, and to a blast resistance gene, Piz-t, in rice. SABRAO J Breed Genet 30:61–67
Tan J, Jin M, Wang J et al (2016) OsCOL10, a CONSTANS-like gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant Cell Physiol 57:798–812
Thomas B, Vince-Pure D (1997) Photoperiodism in plants, 2nd edn. Academic Press, San Diego
Tsai KH (1976) Studies on earliness genes in rice, with special reference to analysis of isoalleles at the E locus. Jpn J Genet 51:115–128
Tsai KH (1991) Genes for late heading and their interaction in the background of Taichung 65. In: Rice Genetics II, Proceedings of International Rice Genetics Symposium, IRRI, Manila, pp 211–215
Tsai KH (1995) Genetic analysis for heading time in wild rice strains. Jpn J Genet 70:555–562
Tsai KH (1999) Genetic analysis for heading time in tropical rice strains. Rice Genet Newslett 16:55–57
Tsai KH, Oka HI (1966) Genetic study of yielding capacity and adaptability in crop plants. 2. Analysis of genes controlling heading time in Taichung 65 and other rice varieties. Bot Bull Acad Sin 7:54–70
Uwatoko N, Onishi A, Ikeda Y et al (2008) Epistasis among the three major flowering time genes in rice: coordinate changes of photoperiod sensitivity, basic vegetative growth and optimum photoperiod. Euphytica 163:167–175
Vergara BS, Chang TT (1985) The flowering response of the rice plant to photoperiod, 4th edn. IRRI, Manila
Wei X, Xu J, Guo H et al (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153:1747–1758
Wu W, Zheng XM, Lu G et al (2013) Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proc Natl Acad Sci USA 110:2775–2780
Xiao J, Li J, Yuan L et al (1996) Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from a subspecific rice cross. Theor Appl Genet 92:230–244
Xue W, Xing Y, Weng X et al (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yamagata H, Okumoto Y, Tanisaka T (1986) Analysis of genes controlling heading time in Japanese rice. In: Rice Genetics, Proceedings of International Rice Genetics Symposium, IRRI, Manila, pp 351–359
Yamamoto T, Kuboki Y, Lin SY et al (1998) Fine mapping of quantitative trait loci Hd-1, Hd-2 and Hd-3, controlling heading date of rice, as single Mendelian factors. Theor Appl Genet 97:37–44
Yamamoto T, Lin H, Sasaki T et al (2000) Identification of heading date quantitative trait locus Hd6 and characterization of its epistatic interactions with Hd2 in rice using advanced backcross progeny. Genetics 154:885–891
Yamamoto E, Yonemaru J, Yamamoto T et al (2012) OGRO: the overview of functionally characterized genes in rice online database. Rice 5:26
Yan WH, Wang P, Chen HX et al (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4:319–330
Yang J, Lee S, Hang R et al (2013) OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. Plant J 73:566–578
Yano M, Harushima Y, Nagamura Y et al (1997) Identification of quantitative trait loci controlling heading date in rice using a high-density linkage map. Theor Appl Genet 95:1025–1032
Yano M, Katayose Y, Ashikari M et al (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473–2484
Yano M, Kojima S, Takahashi Y et al (2001) Genetic control of flowering time in rice, a short day plant. Plant Physiol 127:1425–1429
Yokoo M, Fujimaki H (1971) Tight linkage of blast-resistance with late maturity observed in different indica varieties of rice. Jpn J Breed 23:35–39 (in Japanese with English summary)
Yokoo M, Kikuchi F (1977) Multiple allelism of the locus controlling heading time of rice, detected using linkage with blast-resistance. Japan J Breed 27:123–130
Yokoo M, Okuno K (1993) Genetic analysis of earliness mutations induced in the rice variety Norin 8. Japan J Breed 43:1–11
Yokoo M, Kikuchi F, Nakane A et al (1980) Genetical analysis of heading time by aid of close linkage with blast resistance. Bull Nat Inst Agric Sci Ser D 31:95–126 (in Japanese with English summary)
Yokoo T, Saito H, Yoshitake Y et al (2014) Se14, encoding a JmjC domain-containing protein, plays key roles in long-day suppression of rice flowering through the demethylation of H3K4me3 of RFT1. PLoS ONE 9:1–9
Yonemaru J, Yamamoto T, Fukuoka S et al (2010) Q-TARO: QTL annotation rice online database. Rice 3:194–203
Yoshitake Y, Yokoo T, Saito H et al (2015) The effects of phytochrome-mediated light signals on the developmental acquisition of photoperiod sensitivity in rice. Sci Rep 5:7709
Youens-Clark K, Buckler E, Casstevens T et al (2011) Gramene database in 2010: updates and extensions. Nucleic Acids Res 39:D1085–D1094
Yu CJ, Yao YT (1968) Genetische Studien beim Reis. II. Die Koppelung des Langhüllspelzengens mit dem Photoperiodizitätsgen. Bot Bull Acad Sin 9:34–35
Yuan Q, Saito H, Okumoto Y et al (2009) Identification of a novel gene ef7 conferring an extremely long basic vegetative growth phase in rice. Theor Appl Genet 119:675–684.Zhao J, Huang X, Ouyang X et al (2012) OsELF3-1, an ortholog of Arabidopsis EARLY FLOWERING 3, regulates rice circadian rhythm and photoperiodic flowering. PLoS One 7:e43705
Zhao K, Tung CW, Eizenga GC et al (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467
Zheng XM, Feng L, Wang J et al (2015) Nonfunctional alleles of long-day suppressor genes independently regulate flowering time. J Integr Plant Biol. doi:10.1111/jipb.12383
Acknowledgments
The research activities at the Institute of Crop Science, NARO (formally National Institute of Agrobiological Sciences), have been supported mainly by the Ministry of Agriculture, Forestry and Fisheries of Japan and the Program for the Promotion of Basic Research Activities for Innovative Biosciences, and partly by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Bürstmayr and J. Vollmann.
Rights and permissions
About this article
Cite this article
Hori, K., Matsubara, K. & Yano, M. Genetic control of flowering time in rice: integration of Mendelian genetics and genomics. Theor Appl Genet 129, 2241–2252 (2016). https://doi.org/10.1007/s00122-016-2773-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2773-4