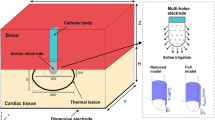
Abstract
Radiofrequency ablation (RFA) as an alternative treatment to the conventional open surgery is the most popular minimally invasive thermal therapy, and it is widely used in clinic today. One of the most important limits for the RFA in clinic is the difficulty to deal with the heat-sink effect of blood vessels, as it causes the difficulty of control the RFA process and consequently the coagulation size of RFA is decreased considerably (empirically, the coagulation size is less than 3 cm with a single RFA electrode). This paper reviews the literature of the current solution for the heat-sink effect due to large blood vessels and suggests future work for finding more effective solutions.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
RFA, as a heat-mediated modality, has become an accepted treatment option for focal primary and secondary malignancies in some organs including the liver, lung, kidney, bone, and adrenal glands [1, 2]. So far, RFA treatment has achieved a competitive success rate compared to the conventional surgery treatment for small tumors (<3 cm in diameter) [3]. One of the major limits with RFA is the small size of coagulation [4]. It is noted that the coagulation zone also includes the 0.5 to 1-cm margin area of the healthy tissue adjacent to the tumor for the purpose of eliminating microscopic foci of disease and off-setting the possibility of incomplete tumor destruction [5]. The coagulation size is affected by heterogeneity of the tissue composition, which causes differences in the tumor tissue density and subsequently differences in the electrical and thermal conductivity; certainly, heterogeneity causes less coagulation [6]. Reduction of the coagulation size is further caused by the proximity of tumors to large blood vessels, as large blood vessels serve as a heat sink [7]. Goldberg et al. and Poch et al. [8, 9] demonstrated that the blood flow in blooded vessels is highly responsible for reduction of the coagulation size. It is noted that the electrical conductivity of blood is about five times higher than that of the hepatic tissue, which means a low-resistance pathway to diffuse away heat [10].
This paper aims to provide a comprehensive review of the current solutions to the large blood vessel problem with RFA and to propose some new solution to the problem if the current solutions are found not satisfactory.
2 The Heat Transfer Principle of RFA with Large Blood Vessels
A general configuration of the RFA system is: an RF generator, an RF electrode (active tip and insulated shaft), several grounding pads, and several electrical wires [11]. With this configuration, a current field is established between the RF electrode and the grounding pads and an alternating current (375–500 kHz) is produced by the RF generator [12]. In the current field, ions (sodium, potassium, and chloride etc.) inside the biological tissue around the RF electrode are forced to interact with each other and to move back and forth rapidly along the direction of the alternating current. Then, the friction-induced heat will be generated due to the interaction between the ions and the electrons. Tissues around the RF electrode are heated to the temperature of approximately 50–100 \( ^\circ {\text{C}} \), and the temperature in this range causes irreversible damages of the protein coagulation of cells [13].
The heat transfer inside the target tissue during the RFA procedure is governed by the so-called Pennes’ bio-heat transfer equation with an outside energy source, which is expressed by [14,15,16]:
where \( \uprho \left( {{\text{kg}}/{\text{m}}^{3} } \right) \) is the density, \( {\text{c }}\left( {{\text{J}}/{\text{kg}}\cdot{\text{K}}} \right) \) is the specific heat, \( {\text{T}}\left( {{\mathbf{x}},{\text{t}}} \right) \left( {\text{K}} \right) \) is the temperature, \( {\text{k }}\left( {{\text{W}}/{\text{m}}\cdot{\text{K}}} \right) \) is the thermal conductivity, \( \uprho\left( {{\text{kg}}/{\text{m}}^{3} } \right) \) is the blood density, \( {\text{c}}_{\text{b}} \left( {{\text{J}}/{\text{kg}}\cdot{\text{K}}} \right) \) is the specific heat of the blood, \( \upomega_{\text{b}} \left( {1/{\text{s}}} \right) \) is the blood perfusion, \( {\text{T}}_{\text{b}} \left( {\text{K}} \right) \) is the temperature of the blood entering the tissue, \( {\mathbf{x}} = \left\{ {{\text{x}}, {\text{y}}, {\text{z}}} \right\} \) in the Cartesian coordinate system, \( \wedge \) denotes the analyzed spatial domains, \( {\text{Q}}_{\text{m}} \left( {{\text{x}},{\text{t}}} \right) \left( {{\text{W}}/{\text{m}}^{3} } \right) \) is the energy generated due to metabolic processes, and \( {\text{Q}}_{\text{hs}} \left( {{\text{x}},{\text{t}}} \right) \left( {{\text{W}}/{\text{m}}^{3} } \right) \) is the spatial heat generated by the RF electrical current. It is to be noted that Eq. (1) does not capture the heat transfer of the tissue in the presence of large blood vessels in vicinity to the tissue [17].
For the situation in the presence of large blood vessels, the heat transfer of blood flow in the vessels may not be neglected. To take into account the large blood vessel in vicinity to the tissue, a convection boundary to the tissue should be considered based on the Newton law of cooling as Eq. (2) [18].
where hb is the convection heat transfer coefficient of the blood to the tissue.
A constant heat transfer coefficient between vessel and tissue was assumed under the fully developed flow in the vessel, and the coefficient is expressed by [18, 19]:
where \( {\text{Nu}}_{\text{D}} \) is the local Nusselt number, \( {\text{k}}_{\text{b}} \) is the thermal conductivity of blood, \( {\text{D}} \) is the vessel diameter.
For \( {\text{Nu}}_{\text{D}} \), it can be approximated, within 3.5%, by [20]:
where \( {\text{Re}} \) is the Reynolds number, \( {\text{Pr }} \) is the Prandtl number, \( {\text{L}} \) is the vessel length, \( \mu \) is the viscosity of blood, and \( {\text{V}}_{\text{b}} \) is the average blood velocity.
3 The Influence of the Heat-Sink Effect
Based on studies, the thermal coagulation obtained ex vivo is larger than those in vivo [21]. The principle of causing smaller coagulation in vivo is that the blood flow in vessels is able to bring heat quantities transferred from active tip away which aggravates the heat lost. The act of heat convection between the tissue and the blood flow is termed the heat-sink effect [8]. Lu et al. and Shih et al. [22, 23] demonstrated that the heat-sink effect starts to occur with a blood vessel (<2 mm in diameter) and obviously occurs with a blood vessel (<3 mm in diameter), whereas, when vessel diameter was less than 2 mm, the short-duration and high-intensity heating scheme could overcome the heat-sink effect of the blood vessels. Therefore, the vessels exceeding 3 mm in diameter are defined as medium or large vessels. Pillai et al. [24] demonstrated the differences between the heat-sink and the heat-sink absent and the results are tabulated in Table 1. A huge influence of the heat-sink effect was illustrated, which causes the dramatic decrease of coagulation size. Furthermore, the recurrence rate in the vicinity of medium or large liver vessels is 36.5% compared to 6.3% without neighboring vessels [25]. Besides, the heat-sink effect can cause an irregular shape of coagulation zone closed to large vessels, which enhances the difficulty in ablating tumors completely and the early local recurrence in the tissue near the vessels [26].
4 The Current Physical Solution to the Heat-Sink Effect
For the heat-sink effect, an efficient method to solve this problem is still missing. In clinic, one of solution is the mechanical occlusion. And another way is to design an appropriate electrode, because a suitable structure of the electrodes is able to reduce the heat-sink effect effectively [24].
4.1 New Designed Electrode
As the development of the technique of RFA, some new designed electrodes were invented for enlarging the size of coagulation, such as internally cooled electrode [27], expandable electrode [28], cluster (or multiple) electrode [29], perfusion electrode [30], and bipolar electrode [25]. All of those new designed electrodes, although, had enlarged the coagulation size (Table 3), their special characteristics also need to be further investigated to show the capability to overcome the heat-sink effect. For example, when an electrode abuts to a medium or large vessel, it is necessary to know the optimal characters about the relative position between the electrode and the vessel for increasing coagulation size [9] (Table 2).
Besides, Rossi et al. [36] discovered the reason avoiding the heat-sink effect efficiently by the hook tips is that expandable electrode can retract or redeploy the hooks after slightly rotating the electrode to change the hook position, or by completely repositioning the electrode until hook tips reach the killing temperature. Unfortunately, there is a challenge for an expandable electrode system to precisely deploy multiple electrodes simultaneously during percutaneous procedures and to modify the overall survival rate or the disease-free survival rate with less treatment sessions [37].
There is a tendency to design a novel electrode with a dynamic geometry, such as expandable electrode and spiral bipolar electrode, because it is able to adjust the geometry to contact a wider range of tissue and avoid or reduce the heat-sink effect, such as multitined expandable electrode with difference structures, bipolar-expandable electrode etc. [21, 38, 39]. Therefore, electrode structure is a main factor affecting the RFA treatment, because a pretty electrode structure can deliver more energy and far away. Taking into consideration the medium or large vessels during the process of electrode design seems to be able to reduce the heat-sink effect efficiently [38]. It is meaningful to design a new electrode for the purpose of reducing the heat-sink effect in future.
4.2 Temporary Vessel Occlusions by the Mechanical Occlusion
Temporary vessel occlusion as an alternative method for reducing the effect of heat sink is also used in the current procedure of RFA [40]. In modern clinical trials, surgeons usually prefer the occlusion with inflatable balloons to the Pringle maneuver to assist the RFA procedure [41]. Because, compared with the Pringle maneuver, the balloon occlusion is simple, safe, and highly feasible to avoid the effect of heat-sink [42]. However, for the method of inflatable balloon, some hepatic vessels cannot be occluded completely such as variant vascular anatomy, irregular shape, or stenosis of hepatic artery. Those problems can be solved by injecting a mixture sponge particles or contrast material [43].
Therefore, the hepatic vessel occlusion weakens the influence of the heat-sink effect for achieving a larger coagulation size and a more regular coagulation shape [44]. For the temporary vessel occlusion, the safety of mechanical occlusion had been approved in clinical practice and one-year survival rate was 44%, which was better than hepatectomy (28%) [45]. But, the difficulty and complexity of RF procedure are increased dramatically after the vessel occlusion [46].
In conclusion, all of those solutions obviously enlarged the size of coagulation (exceed the restriction of 3 cm in diameter) for liver tumors abut to medium or large vessels. The principle of mechanical occlusion is to decrease the heat loss. However, the mechanical occlusion increases the difficulty and invasiveness of RFA [46]. For the development of RF electrode, it aims to increase power deposition for enlarging coagulation, especially for that under the heat-sink effect of medium and large vessels. But, it is still difficult for an RF electrode having those characters simultaneously: (1) it can be inserted into body easily; (2) it is able to be expanded to maintain maximum contact with tissue; and (3) it is able to avoid the heat sink effect as much as possible [38].
5 RFA Computer Model with the Heat-Sink Effect
RFA computer models are often used to assist clinical treatments recently. RFA procedures are simulated with computer models for planning, evaluating, and optimizing RFA therapies. Meanwhile, RFA computer models also benefit to investigating the complex processes of RFA and to minimizing treatment risks preoperatively [47]. For example, a counter current blood vessels model analyzes the robustness of the power deposition scheme [48]. Especially, the heat-sink effect as a main factor of higher tumor recurrence is added into RFA computer model for investigating and optimizing clinical treatments [7]. Therefore, many RFA computer models with the heat-sink effect were proposed to predict the temperature, tissue properties change, ablation time, and ablation results within the organ intraoperatively for improving protocol of tumor ablation as well as reducing the damage of surrounding normal tissue [15]. Huang et al. [7] simulated the influence of position relationship contained direction and relative distance between vessel and RF electrode. Haemmerich et al. [20] simulated the results of RF ablation abutting large vessels with different RF electrodes. Nonetheless, those models, until now, did not include all variables among tumors, electrodes and medium or large vessels to simulate. For example, the variable of blood flow is excluded in Haemmerich’s model. RFA procedures is also hard to plan and interventionally guide due to the heat-sink effect of medium and large vessels [49].
6 Future Work
During RFA treatment, the heat-sink effect of blood vessels remarkably narrows the coagulation size and raises mortality. To address this problem, great efforts should be made in all aspects of RFA technique, especially in the following areas:
First, further understanding of the surrounding characters of RFA is needed. Specially, the vessel structure, vessel types, and blood characteristics are needed to improve the accuracy and veridicality of RFA computer model. For example, O’Rourke et al. [50] demonstrated the performance of heat-sink effect in cirrhotic livers. Nevertheless, an RFA computer model requires complete surrounding information to achieve the predictability and controllability of RFA procedures with the heat-sink effect. Therefore, mass scientific experiments are necessary to obtain the surroundings characters. Correspondingly, the RFA model based on surrounding information is able to optimize the parameters of tumor ablation procedure for weakening the heat-sink effect.
Second, improving RFA procedure control protocol avoids the heat-sink effect of medium and large vessels. The current approach to heat dissipation is to block blood vessels or slow down blood flow, but this increases the complexity of the RFA procedure and introduces some other complications. A RFA procedure control protocol achieving the decrease of heat-sink effect is necessary. Nevertheless, it is also lack of a standard control protocol, which can achieve the largest coagulation in RFA procedure and meanwhile decrease the heat-sink effect. Adding the condition of medium and large vessels into RFA control protocol is able to avoid or overcome the effect of heat-sink. Meanwhile, the control protocols taking into consideration the heat-sink effect of blood vessels need to be further studied to find an optimal method.
Third, designing a new electrode is another tendency to overcome the heat-sink effect of medium or large vessels. The development of RF electrode from structure, polar principle, and control method promotes efficiency and safety of tumor treatment. Until now, the design of RF electrodes, such as expandable, cooled cluster, or other combination designs, is still hard to obtain a large coagulation zone (>3 cm in diameter) abutting medium or large vessels [51]. Because all designs of RF electrodes just purely aimed at enlarging the coagulation zone in the tumor and ignored the influence of medium and large vessels. Only very few studies talking about the new design of RF electrodes considering the heat-sink effect can be found in literature [52]. The heat-sink effect initially is set into the design process as a restriction in conceptual design level. Then the new design of electrode possibly overcome the problem of heat-sink effect.
7 Conclusion
Radiofrequency ablation technique is very safe and effective for small tumor (<3 cm in diameter). One of the reasons causing the failure of RFA in the treatment of large tumors is the heat-sink effect with medium and large vessels. The small coagulation, lower survival rate and incomplete ablations have been demonstrated in vivo due to the heat-sink effect of blood vessels. Therefore, the further development of RFA is necessary to overcome the heat-sink effect for promoting RFA to be a more favorable tumor ablation modality in clinic.
References
Ahmed, M., Goldberg, S.N.: Principles of radiofrequency ablation. J. Interv. Oncol., 23–37 (2012). Springer
Ni, Y., et al.: A review of the general aspects of radiofrequency ablation. Abdom. Imaging 30, 381–400 (2005)
Yang, W., et al.: Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J. Gastroenterol. 22, 2993 (2016)
Zhang, B., et al.: A review of radiofrequency ablation: Large target tissue necrosis and mathematical modelling. Phys. Med. 32, 961–971 (2016)
Lee, J.M., et al.: Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest. Radiol. 42, 163–171 (2007)
Rhim, H., et al.: Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics 21, S17–S35 (2001)
Huang, H.-W.: Influence of blood vessel on the thermal lesion formation during radiofrequency ablation for liver tumors. Med. Phys. 40, 073303 (2013)
Goldberg, S., et al.: Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology 209, 761–767 (1998)
Poch, F.G., et al.: The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int. J. Hyperth., 1–8 (2016)
Dodd III, G.D., et al.: Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 267, 129–136 (2013)
Zhang, B., et al.: Evaluation of the current radiofrequency ablation systems using axiomatic design theory. Proc. Inst. Mech. Eng. H 228, 397–408 (2014)
Ahmed, M., et al.: Image-guided tumor ablation: Standardization of terminology and reporting criteria—a 10-year update. J. Vasc. Interv. Radiol. 25, 1691–1705, e1694 (2014)
Goldberg, S.N.: Radiofrequency tumor ablation: principles and techniques. Eur. J. Ultrasound 13, 129–147 (2001)
Zhang, B., et al.: Study of the relationship between the target tissue necrosis volume and the target tissue size in liver tumours using two-compartment finite element RFA modelling. Int. J. Hyperth. 30, 593–602 (2014)
Zhang, B., et al.: Numerical analysis of the relationship between the area of target tissue necrosis and the size of target tissue in liver tumours with pulsed radiofrequency ablation. Int. J. Hyperth. 31, 715–725 (2015)
Zhang, B., et al.: A new approach to feedback control of radiofrequency ablation systems for large coagulation zones. Int. J. Hyperth. 33, 367–377 (2017)
Hariharan, P., et al.: Radio-frequency ablation in a realistic reconstructed hepatic tissue. J. Biomech. Eng. 129, 354–364 (2007)
Consiglieri, L., et al.: Theoretical analysis of the heat convection coefficient in large vessels and the significance for thermal ablative therapies. Phys. Med. Biol. 48, 4125 (2003)
Nakayama, A., Kuwahara, F.: A general bioheat transfer model based on the theory of porous media. Int. J. Heat Mass Transf. 51, 3190–3199 (2008)
Haemmerich, D., et al.: Hepatic bipolar radiofrequency ablation creates coagulation zones close to blood vessels: a finite element study. Med. Biol. Eng. Comput. 41, 317–323 (2003)
Yu, J., et al.: A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur. J. Radiol. 79, 124–130 (2011)
Lu, D.S., et al.: Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the “heat sink” effect. AJR Am. J. Roentgenol. 178, 47–51 (2002)
Shih, T.-C., et al.: Cooling effect of thermally significant blood vessels in perfused tumor tissue during thermal therapy. Int. Commun. Heat Mass 33, 135–141 (2006)
Pillai, K., et al.: Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine 94, e580 (2015)
Lehmann, K.S., et al.: Minimal vascular flows cause strong heat sink effects in hepatic radiofrequency ablation ex vivo. J. Hepatobiliary Pancreat. Sci. 23, 508–516 (2016)
de Baere, T., et al.: Radiofrequency ablation of lung metastases close to large vessels during vascular occlusion: preliminary experience. J. Vasc. Interv. Radiol. 22, 749–754 (2011)
Cha, J., et al.: Radiofrequency ablation using a new type of internally cooled electrode with an adjustable active tip: An experimental study in ex vivo bovine and in vivo porcine livers. Eur. J. Radiol. 77, 516–521 (2011)
Ito, N., et al.: Bipolar radiofrequency ablation: development of a new expandable device. Cardiovasc. Intervent. Radiol. 37, 770–776 (2014)
Yoon, J.H., et al.: Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: Evaluation of the in vivo efficiency. Korean J. Radiol. 15, 235–244 (2014)
Lee, J., et al.: Radiofrequency ablation in pig lungs: in vivo comparison of internally cooled, perfusion and multitined expandable electrodes. Br. J. Radiol. (2014)
Goldberg, S.N., et al.: Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad. Radiol. 2, 399–404 (1995)
Cha, J., et al.: Radiofrequency ablation zones in ex vivo bovine and in vivo porcine livers: Comparison of the use of internally cooled electrodes and internally cooled wet electrodes. Cardiovasc. Intervent. Radiol. 32, 1235–1240 (2009)
Pereira, P.L., et al.: Radiofrequency ablation: In vivo comparison of four commercially available devices in pig livers. Radiology 232, 482–490 (2004)
Hirakawa, M., et al.: Randomized controlled trial of a new procedure of radiofrequency ablation using an expandable needle for hepatocellular carcinoma. Hepatol. Res. 43, 846–852 (2013)
Lee, E.S., et al.: Multiple-electrode radiofrequency ablations using Octopus® electrodes in an in vivo porcine liver model. Br. J. Radiol. 85, e609–e615 (2014)
Rossi, S., et al.: Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am. J. Roentgenol. 170, 1015–1022 (1998)
Choi, D., et al.: Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann. Surg. Oncol. 14, 2319–2329 (2007)
Lau, L., Han, Y.-L.: Exploring a novel heating probe design for tumor ablation. J. Med. Device 10, 030930 (2016)
Huo, Y.R., et al.: “Edgeboost”: a novel technique to extend the ablation zone lateral to a two-probe bipolar radiofrequency device. Cardiovasc. Intervent. Radiol. 39, 97–105 (2016)
Šubrt, Z., et al.: Temporary liver blood-outflow occlusion increases effectiveness of radiofrequency ablation: an experimental study on pigs. Eur. J. Surg. Oncol. 34, 346–352 (2008)
Sobczyński, R., et al.: Transoesophageal echocardiography reduces invasiveness of cavoatrial tumour thrombectomy. Wideochir Inne Tech Maloinwazyjne 9, 479 (2014)
de Baere, T., et al.: Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion 1. Radiology 248, 1056–1066 (2008)
Rossi, S., et al.: Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 217, 119–126 (2000)
Ahmed, M., et al.: Principles of and advances in percutaneous ablation. Radiology 258, 351–369 (2011)
Yamada, R., et al.: Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 148, 397–401 (1983)
Goldberg, S.N., et al.: Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 9, 101–111 (1998)
Rossmann, C., et al.: Platform for patient-specific finite-element modeling and application for radiofrequency ablation. Visual Image Proc. Comput. Biomed. 1 (2012)
Huang, H.-W., et al.: A robust power deposition scheme for tumors with large counter-current blood vessels during hyperthermia treatment. Appl. Therm. Eng. 89, 897–907 (2015)
Audigier, C., et al.: Challenges to validate multi-physics model of liver tumor radiofrequency ablation from pre-clinical data. Comput. Biomech. Med., 29–40 (2015)
O’Rourke, A.P., et al.: Current status of liver tumor ablation devices. Expert Rev. Med. Devices 4, 523–537 (2007)
Lim, D., et al.: Effect of input waveform pattern and large blood vessel existence on destruction of liver tumor using radiofrequency ablation: Finite element analysis. J. Biomech. Eng. 132, 061003 (2010)
Wang, Z., et al.: Bi-component conformal electrode for radiofrequency sequential ablation and circumferential separation of large tumours in solid organs: development and in-vitro evaluation. IEEE Trans. Biomed. Eng. 64, 699–705 (2017)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Fang, Z., Zhang, B., Zhang, W. (2017). Current Solutions for the Heat-Sink Effect of Blood Vessels with Radiofrequency Ablation: A Review and Future Work. In: Fei, M., Ma, S., Li, X., Sun, X., Jia, L., Su, Z. (eds) Advanced Computational Methods in Life System Modeling and Simulation. ICSEE LSMS 2017 2017. Communications in Computer and Information Science, vol 761. Springer, Singapore. https://doi.org/10.1007/978-981-10-6370-1_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-6370-1_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6369-5
Online ISBN: 978-981-10-6370-1
eBook Packages: Computer ScienceComputer Science (R0)