Abstract
Recently, newer treatments have been introduced for autosomal dominant polycystic kidney disease (ADPKD) patients. Since cysts grow and renal function declines over a long period of time, the evaluation of treatment effects in ADPKD has been very difficult. Therefore, there has been a great interest to find out the “better” surrogate marker or biomarker which reflects disease progression. Biomarkers in ADPKD should have three clinical implications: (1) They should reflect disease severity, (2) they should distinguish patients with poor versus good prognosis to select those who will benefit better from the treatment, and (3) they should be easy to evaluate short-term outcome after treatment, which will demonstrate hard outcome. Herein, we will discuss currently available surrogate biomarkers including the volume of total kidney and urinary molecular markers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease, which is characterized by progressive multiple cyst formation, proliferation, and apoptosis, finally causing interstitial fibrosis and end-stage renal disease (ESRD) (Torres et al. 2007). It is caused by genetic mutation on either PKD1 or PKD2 gene; however, it is not a congenital disease since phenotypic presentation only begins in young adulthood. Furthermore, renal function remains stable at the early stage because glomerular hyperfiltration compensates for the progressive loss of healthy glomeruli, which result in renal failure only after several decades from diagnosis. Interestingly, even within a family, individual members show different prognosis. Therefore, it is not a pure ‘genetic’ disease but a ‘genetic-environmental’ disease.
Recently, several novel therapies have been introduced to slow the rate of disease progression in ADPKD. Since ADPKD progresses slowly after cyst formation, typical hard outcomes such as time to ESRD or patient survival are often not useful in studying ADPKD treatment effect. Therefore, there has been a great interest to develop alternative endpoints or surrogate biomarkers for patient and renal outcomes. Surrogate biomarkers should fulfill following conditions. Firstly, they should reflect disease severity. Second, they should predict rapid disease progression, which will distinguish high-risk patients who will benefit from the treatment. Lastly, they should be easy and convenient to evaluate disease progression in a short-term interval. Herein, we will discuss diagnostic evaluation of currently available surrogate biomarker s in the patients with ADPKD.
2 Biomarker as a Predictor of Rapid Disease Progression
Since renal function declines only at the late stage of the disease, early biomarkers to predict renal disease progression need to be clarified. This part will seek for markers used to assess prognosis in ADPKD (Table 8.1).
2.1 Genetic Factors
ADPKD is hereditary disease which involves mutations in PKD1 and/or PKD2. Recent advances in molecular diagnosis have led us to more precisely predict the renal disease progression in ADPKD patients. Not only type of genes but also type or location of mutations would make a difference in prognosis. We will discuss current understanding of genetic prognostic factors.
2.1.1 Locus Heterogeneity
ADPKD is genetically heterogeneous, in which cystic phenotype is caused by mutations in genes at different chromosomal loci: PKD1 (chromosome 16p 13.3) and PKD2 (chromosome 4q21). This is called locus heterogeneity . Previously, a possibility of third gene locus has been proposed and searched since five pedigrees could not find pathologic mutations from either PKD1 or PKD2 by linkage analysis -->(Paterson and Pei 1998). However, reanalysis of clinical data and genetic samples from ‘PKD3 pedigree’ by mutation screening revealed that ‘PKD3’ is unlikely to exist (Paul et al. 2014). Therefore, currently it is widely accepted that only PKD1 and PKD2 genes are responsible for ADPKD development.
Patients with PKD1 mutations generally show a more severe form of ADPKD than those with PKD2 mutation, showing earlier onset of hypertension, earlier onset of diagnosis, larger kidneys, faster renal function decline, and earlier onset of ESRD. In the European PKD1-PKD2 cohort study, PKD1 pedigree showed earlier onset of ESRD or death compared to PKD2 pedigree (53.0 years vs. 69.1 years) (Hateboer et al. 1999). The incidence of other clinical manifestations such as hypertension, hematuria or urinary tract infection was also less frequent in PKD2 pedigree. Similarly, patients with PKD1 mutation have more cysts in kidneys resulting in larger kidney size compared to patients with PKD2 mutation (Harris et al. 2006).
2.1.2 Allelic Heterogeneity
The PKD1 and PKD2 genes present a high level of allelic heterogeneity . Within each gene, different type and location of mutations can cause cyst formation. These variable mutations are collected and reported in the ADPKD Mutation Database (PKDB, http://pkdb.mayo.edu/). To date, 1273 pathogenic mutations in PKD1 and 202 pathogenic mutations in PKD2 have been described in the database. Recently, the role of allelic heterogeneity in modulating disease severity has been studied. The Consortium for Radiologic Imaging Study of PKD (CRISP) researchers found that missense mutation in PKD1 presents with mild phenotype similar to PKD2 phenotype (Pei et al. 2012). Another study from Genkyst cohort compared renal survival among 387 patients with PKD1 truncating mutation, 184 patients with PKD1 non-truncating mutation, and 133 patients with PKD2 mutation (Cornec-Le Gall et al. 2013). As the result, the patients with PKD1 non-truncating mutation (in-frame mutation or missense mutation) demonstrated 12-year delayed onset of ESRD compared to PKD1 truncating mutation (68 years vs. 56 years, p < 0.0001). Interestingly, recent study suggested that PKD1 non-truncating mutations can show heterogeneous renal prognosis according to gene dosage (Hwang et al. 2015). For example, some PKD1 missense mutations result in milder form of disease similar to PKD2 mutation, while other PKD1 missense mutations can show deleterious outcome such as PKD1 truncating mutation. The allelic effect according to mutation location within PKD1 non-truncating mutations should be elucidated further.
2.1.3 Hypomorphic Allele
A mutation that reduces but doesn’t eliminate a gene’s functionality is hypomorphic. Hypomorphic alleles in ADPKD can be demonstrated as a mild phenotype, but they can result in a severe disease when they occur together with a pathogenic mutation on the second allele (Rossetti et al. 2009; Bergmann et al. 2011). This phenomenon can explain why some members from the family of mild phenotype show extremely severe disease form. Segregation study may be useful to find out hypomorphic allele effect within the family (Hwang et al. 2015).
2.1.4 Modifier Genes
Intra-familial variability is another feature of ADPKD. Locus heterogeneity and allelic heterogeneity can explain variable disease severity among different pedigrees. However, within the same pedigree, family members may show variable renal disease progression (Barua et al. 2009). This can be partially explained with modifier gene effect . Several gene polymorphism s such as angiotensin converting enzyme (ACE) (Perez-Oller et al. 1999), Dickkopf 3 (DKK3) (Liu et al. 2010), endothelial nitric oxide synthase (Persu et al. 2002) may be associated with poorer prognosis.
2.1.5 Epigenetic Modification
Recently, epigenetic modification effect on ADPKD phenotype has become focus of scientific research. Epigenetic regulation is defined as the modification of external or environmental factors that switch genes on and off and affect how cells read genes instead of changing DNA sequence (Park et al. 2011). Epigenetic modulation includes DNA methylation, histone modification, and gene modification by microRNAs. ADPKD patients showed hyper-methylation on PKD1 gene and other cystogenesis-related genes and subsequent downregulation of PKD1 expression (Woo et al. 2014). Moreover, histone deacetylases (HDACs) are known to be activated in ADPKD kidneys, which result in dysregulated PKD1 gene expression, disrupted fluid-flow regulated calcium signal regulation in renal epithelia, and subsequent cyst formation and progression (Li 2011). Recently, there is increasing evidence that microRNA dysregulation is associated with the pathogenesis of cystic kidney disease (Woo and Park 2013; Lakhia et al. 2015; Sun et al. 2015) and urinary microRNAs can be a potential biomarker for ADPKD progression (Ben-Dov et al. 2014). Therefore, epigenetic modification is discussed as a possible therapeutic target of ADPKD.
2.2 Demographic and Clinical Factors
Apart from the culprit gene affecting ADPKD, non-modifiable and modifiable factors affect ADPKD renal disease progression. Since genetic analysis is not the routine diagnostic work up at the clinic, collecting demographic and clinical factors and family history would be most helpful to determine prognosis and to counsel each patient.
2.2.1 Age at Diagnosis
Children with early severe disease showed faster renal volume growth and faster decline of renal function on follow up (Fick-Brosnahan et al. 2001). Another study showed that children who were diagnosed with PKD < 18 months demonstrated larger kidneys and severe hypertension compared to those who were diagnosed after 18 months of age (Shamshirsaz et al. 2005). In a similar vein, patients with PKD1 genotype showed more cysts than PKD2 genotype, but growth rates were not different between two genotypes (Harris et al. 2006). Altogether, these results suggest that diagnosis of ADPKD at the early age is likely to mean severe genotype.
2.2.2 Male Gender
Men is known to have faster renal function decline, larger kidneys, and hypertension in ADPKD resulting in earlier onset of ESRD. Gabow et al. described that men had a faster decline of renal function compared to female counterpart (Gabow et al. 1992b). Another retrospective study of 1215 ADPKD patients, male gender showed earlier onset of ESRD compared to female gender (52 vs. 56 years) (Johnson and Gabow 1997).
2.2.3 Family History of ESRD
As we described above, genetic factor is the strongest determinant of renal prognosis. The problem is that we cannot perform genetic analysis in every case due to practical and economic issue. One study group performed the interesting study to show that family history of ESRD onset can predict mutated gene (Barua et al. 2009). In this study, they examined 484 affected members from 90 pedigrees whose pathogenic mutations are already known. The researcher found that we can predict gene locus by collecting the information of ESRD onset from family members. If any affected member shows ESRD onset < 55 years, this family is highly likely to have PKD1 gene mutation (positive predictive value 100 %, sensitivity 72 %). On the other hand, if none of family members experience ESRD until 70 years old, this family is highly likely to have PKD2 gene mutation (positive predictive value 100 %, sensitivity 74 %). This study emphasizes the importance of history taking and collecting family history to predict prognosis.
2.2.4 Early Onset and Severity of Hypertension
Elevated blood pressure is the most common comorbidity in ADPKD patients. It is the most common early manifestation and also the major contributor to renal disease progression. Hypertension is also associated with other risk factors such as hematuria , proteinuria , and cardiovascular outcomes. Intrarenal renin-angiotensin system (RAS) has been suggested as the major contributor to blood pressure elevation in ADPKD (Schrier 2011). Of interest, primary hypertension or essential hypertension in unaffected parent is also related to renal progression and early onset of hypertension in ADPKD offsprings (Geberth et al. 1995; Schrier et al. 2003). Therefore, there is a debate whether hypertension and related biomarkers are specific to ADPKD or they are general risk factors for chronic kidney disease progression.
Nonetheless, ADPKD patients with early onset hypertension demonstrate poorer renal outcome. One survival analysis demonstrated that patients with early hypertension < 35 years showed faster ESRD progression compared to those with late onset hypertension (ESRD onset 51 vs. 65 years) (Johnson and Gabow 1997). In addition, severity of hypertension is also related to renal progression rate. Recent large scale, randomized prospective study showed that low blood pressure target (95/60 to 110/75 mmHg) group demonstrated attenuated renal cyst growth, reduced albuminuria , reduced left ventricular mass index compared to standard blood pressure target (120/70 to 130/80 mmHg) group (Schrier et al. 2014). Therefore, early onset hypertension and severity of hypertension both contribute as predictors of rapid disease progression.
2.2.5 Gross Hematuria
Gross hematuria may occur in as much as 40 % of ADPKD patients at some time during the course of the disease. Many of them can experience recurrent episode of gross hematuria or cyst rupture . A retrospective study demonstrated that patients with gross hematuria had larger kidneys and those with recurrent gross hematuria tended to have faster renal function decline (Gabow et al. 1992a). Another survival analysis showed that patients with gross hematuria before age 30 had a worse renal outcome compared to those with later onset (Idrizi et al. 2009). It was postulated that cyst rupture and subsequent gross hematuria may be the sign of faster growth of cyst and related to poor outcome (Kistler et al. 2009b). Others also claim that gross hematuria may release and deposit iron and heme promoting inflammation around kidney tissue (Tracz et al. 2007).
2.2.6 Proteinuria
Overt proteinuria (>300 mg/day) was associated with faster decline of renal function and early onset of ESRD in several studies. In prospective cohort study of 323 ADPKD patients with mean follow up duration of 8 ~ 9years, baseline proteinuria was significantly correlated with the declining rate of renal function (Ozkok et al. 2013). Secondary subgroup analysis from Modification of Diet in Renal Disease (MDRD) study revealed that higher degree of proteinuria was associated with faster decline of renal function (Klahr et al. 1995). Microalbuminuria (30 ~ 300 mg/day) also was strongly associated with larger kidneys faster growth of renal volume, and higher blood pressure (Kistler et al. 2009b). Whether proteinuria is the result of cyst growth and subsequent renal tissue injury of the cause of renal progression is not clearly defined at this moment.
2.2.7 Recurrent Urinary Tract Infection or Pyuria
Retrospective studies showed that urinary tract infection (UTI) is very common among ADPKD patients and female was more prone to overt UTI (Idrizi et al. 2011). Recent study also showed that asymptomatic pyuria is prevalent in ADPKD patients and chronic recurrent pyuria itself is associated with faster decline of renal function (Hwang et al. 2013). However, this single-center retrospective study has a major limitation in which culture study was not performed in most cases of pyuria. Pyuria as a potential biomarker should be elucidated further in prospective studies.
3 Biomarker as a Tracer of Disease Progression
ADPKD has a typically long period of preserved renal function at the early stage and renal function only begins to decline after kidneys are filled with enormous cysts. Moreover, at the early stage of disease, glomerular hyperfiltration compensates for the progressive loss of healthy glomeruli. Therefore, traditional markers such as serum creatinine or glomerular filtration rate (GFR) do not always reflect the disease progression. Hence, the following surrogate biomarkers to identify and trace the disease progression have been introduced (Table 8.2).
3.1 Decline Rate of GFR
GFR describes the flow rate of filtered fluid through the kidney and presents renal function. It is used instead of blood urea nitrogen or serum creatinine because plasma concentration of these waste substances will not be raised above the normal range until kidney function falls below 60 % of total kidney function. GFR can be measured by calculating the clearance of exogenous materials such as inulin or iothalamate which are neither reabsorbed nor secreted by the kidney after glomerular filtration. Since it is not practical to measure GFR at each clinic visit, estimated GFR (eGFR) is calculated by equation. There is still debates whether eGFR can be used instead of measured GFR (mGFR) as a tracer of renal progression in clinical trials. Early studies showed that mGFR may be superior to eGFR early in chronic kidney disease (CKD) (Rule et al. 2006; Ruggenenti et al. 2012). The eGFR measured by MDRD or Cockcroft-Gault equation may underestimate GFR in cohort with normal renal function > 60 mL/min/1.73 m2. However, recent studies suggested that eGFR may perform relatively well compared to mGFR and reliably reflect GFR change in the patients with ADPKD (Spithoven et al. 2013; Chapman et al. 2015). Among eGFR equation, CKD-EPI equation should be considered then the trial is designed to include patients with normal renal function (Orskov et al. 2010).
Since eGFR measurement is not as accurate as mGFR, small changes in eGFR may not reflect a true decline in renal function. Therefore, data of repeated measurement of eGFR over a long period should be available to calculate the decline rate of eGFR. Recent review paper suggested to define rapid progressor as a confirmed annual eGFR decline ≥ 5 mL/min/1.73 m2/year or by an average annual eGFR decline ≥ 2.5 mL/min/1.73 m2/year over 5 years (Gansevoort et al. 2016). However, absolute slopes (mL/min/year or mL/min/1.73 m2/year) may not accurately reflect the true decline rate of eGFR. For example, a rise in serum creatinine from 0.9 to 1.0 mg/dL or from 3.3 to 6.0 mg/dL represents a decline in eGFR of 10 mL/min/1.73 m2 for a 60-year old man by MDRD equation. In another words, small increase in serum creatinine may be interpreted as a large decrement of eGFR in early CKD. Therefore, logarithmic slopes (% per year) rather than absolute slopes (mL/min/1/73 m2/year) should be considered in the early CKD stages.
3.2 Total Kidney Volume
Cysts develop as early as in utero but renal function declines very slowly thereafter until kidney fails in fifth or sixth decades. Therefore, GFR measurement often do not reflect disease progression in the early stage of the disease. Measurement of total kidney volume or cyst volume is considered excellent surrogate marker to reflect cyst growth and to predict further disease progression.
Renal volume can be measured either by ultrasonography , computed tomography (CT) , or magnetic resonance imaging (MRI) . Ultrasonography is an easy and safe screening method to diagnose and evaluate kidney volume in ADPKD. One can calculate renal volume by ellipsoid formula: height × transverse width × thickness × π/6. However, it is an operator-dependent, less reproducible, and underestimates the true value. Therefore, it is not suitable for short-term follow up of renal progression. On the other hands, CT and MRI can detect as small cyst as 2 mm, reliably measure volume progression in a short interval, and are highly reproducible (Bae and Grantham 2010). Therefore, they are more suitable as surrogate markers for clinical trials. Similar to eGFR, kidney volume can be estimated by formula instead of measured TKV (mTKV). Recently, some researchers suggested that estimated TKV (eTKV) by ellipsoid formula or midslice method can be conveniently and reliably used instead of mTKV (Spithoven et al. 2015). Therefore, both mTKV and eTKV can be used as a tracer for disease progression in ADPKD.
However, there can still be intra-observer and intra-individual variability in renal volume measurement. Therefore, it is recommended to at least measure renal volume more than three times with 6–12 month apart to assess volume change (Gansevoort et al. 2016). It is well documented in the previous study that mTKV by MRI imaging can detect cyst growth within 6 months (Kistler et al. 2009b). When previous data or repeated measurements of renal volume are not available, however, we can still make prediction by single TKV value. Chapman et al. showed that height-adjusted TKV (HtTKV) ≥ 600 mL/m predict the risk of developing CKD stage 3 within 8 years (Chapman et al. 2012). Irazabal et al. developed risk prediction tool from a cohort of 590 ADPKD patients and classified them into five groups according to HtTKV ranges for age (1A-1E, in the order of increasing risk of ESRD development) (Irazabal et al. 2015). The risk of ESRD development within 10 years was increased from 2.4 to 66.9 % according to subclasses (1A vs. 1E). In summary, single and repeated mTKV or eTKV can be used as a useful surrogate biomarker for renal disease progression in ADPKD.
3.3 Serum and Urinary Biomarkers
3.3.1 General Biomarkers of Kidney Disease
ADPKD is initiated from cystic transformation of a few percent of nephrons leading to increased cell proliferation and apoptosis, interstitial inflammation, fibrosis, and finally ESRD. Therefore, ADPKD kidney may show the whole spectrum of acute kidney injury and chronic kidney disease (CKD) along the disease course. Hence, various serum and urinary biomarkers of kidney disease have been studies as a surrogate marker of disease progression. Since serum creatinine based GFR is considered of limited use and measurement of TKV may be time-consuming and expensive, serum and urinary biomarkers can be easy and inexpensive method to trace disease progression in the future.
3.3.1.1 Biomarkers of Acute Tubular Damage
Since cysts initiate from tubules, the potential markers of tubular damage were studied widely to seek the relationship with renal function change. In one cross-sectional study with 107 ADPKD patients from CRISP cohort, urinary neutrophil gelatinase-associated lipocalin (NGAL) and Interleukin-18 (Il-18) were stably increased but was not associated with either TKV or eGFR (Parikh et al. 2012). Another cross-sectional study revealed that markers of proximal tubular damage (NGAL, β2-microglobulin) and the marker of distal tubular damage (heart-type fatty acid binding protein, H-FABP) were negatively associated with mGFR. NGAL was also positively associated with TKV together with kidney injury molecule 1 (KIM-1) (Meijer et al. 2010). However, whether these potential tubular damage markers can predict disease progression needs to be further elucidated. One prospective study showed that N-acetyl-β-D glucosaminidase (NAG) is better associated with eGFR compared to KIM-1, β2-microglobulin, and NGAL. However, urinary NAG was failed to predict renal function decline in 1 year (Park et al. 2012).
3.3.1.2 Biomarkers of Inflammation
Inflammation and oxidative stress is evident from early stage of disease. Urinary biomarkers to measure intrarenal inflammation and oxidative stress have been studied. Previous cross-sectional studies showed that monocyte chemoattractant protein-1 (MCP-1) was elevated in the urine of ADPKD patients and it was associated with decreased renal function (Zheng et al. 2003; Parikh et al. 2012). Another study demonstrated that the level of asymmetric dimethylarginine (ADMA) , an inhibitor of nitric oxide synthase, is increased in patients with early ADPKD (Wang et al. 2008). Most recently, proteomic analysis of urinary vesicle revealed that the level of complements 3 and 9 (C3 and C9) were elevated in the early stage of ADPKD and urinary plakins were elevated in the later stage of the disease (Salih et al. 2016). Both complements and plakin levels were associated with TKV. This study suggests that inflammation process takes an important role in disease progression in the early stage of ADPKD.
3.3.1.3 Biomarkers of Fibrosis
ADPKD kidneys eventually change to fibrotic kidneys similar to other types of CKD. However, unlike the CKD of other causes, fibrosis starts from the epithelial change from cyst formation, which drives changes in the peri-cystic interstitium and fibroblasts (Norman 2011). Previous studies tried to discover urinary biomarker reflecting the early fibrotic process in the polycystic kidneys. One researcher group performed proteomic analyses showing that collagen fragments take most part of excreted peptide in the urine (Kistler et al. 2009a, 2013). The results suggested that fibrotic process takes the important role in ADPKD progression.
Apelin , an endogenous ligand of the G-protein-coupled receptor APJ, has been recently identified as a main regulator of organ fibrosis. In kidneys, Apelin-APJ axis has been known to protect kidneys from fibrosis (Huang et al. 2016). One recent study compared the excretion levels of fibrotic markers, apelin and transforming growth factor-β1 (TGF-β1), in the urine samples from 45 ADPKD patients and 28 healthy controls (Kocer et al. 2016). The results showed that urinary apelin level was lower and TGF-β1 level was higher in ADPKD cohort compared to the healthy control. In one prospective cohort study of 52 ADPKD patients, apelin has been investigated for its predictive value (Lacquaniti et al. 2013). Of 52 patients, 33 patients reached primary outcome (combined measurement of decreased GFR and increased TKV by 5 % per year). Apelin independently predicted the renal progression in ADPKD patients. Apelin and its role as a biomarker should be evaluated in a larger cohort with longer period.
3.3.1.4 Biomarkers of Hypertension
High blood pressure is one of the early complications of ADPKD. It is well known that intrarenal RAS contributes to the rise of blood pressure in ADPKD (Schrier 2011). Because blood pressure begins to rise as early as the stage with a few cyst, biomarkers that reflect intrarenal RAS activity and hypertension have been studied.
Urinary angiotensinogen (AGT) has been suggested as an effective biomarker to reflect intrarenal RAS activity and hypertension (Kobori et al. 2009). Since AGT cannot be filtered through glomeruli, urinary AGT solely reflect intrarenal RAS activity. In addition, urinary AGT is well known to be strongly associated with the activity of angiotensin-II, a main player in intrarenal RAS. In our previous study, urinary AGT was associated with eGFR and TKV. Hypertensive patients showed higher level of urinary AGT compared to normotensive patients (Park et al. 2015). In addition, polycystic kidneys showed strong expression of AGT along the cyst-lining epithelium as early as CKD stage II (Fig. 8.1). However, the study failed to show the association of urinary AGT with decline rate of GFR or ESRD progression.
Components of intrarenal RAS in polycystic kidneys (Reproduced from Park et al. 2015)
On the other hands, serum uric acid has recently been recognized as a marker of endothelial dysfunction, hypertension, and predictor of renal disease progression in ADPKD. One cross-sectional study evaluated the association of serum uric acid and endothelial dysfunction in early normotensive ADPKD patients (Kocyigit et al. 2013). Endothelial dysfunction was measured by flow-mediated vasodilation (FMD) in the forearm. The group of higher serum uric acid (>7.0 for males and >6.0 for females) showed higher ADMA levels (1.19 ± 0.2 vs. 1.47 ± 0.3, p < 0.001) and lower FMD rates (8.1 ± 1.3 vs. 6.8 ± 0.7, p < 0.001). Another retrospective analysis of a prospective cohort showed that higher serum uric acid level was associated with hypertension development, larger TKV, and risk of developing ESRD (Helal et al. 2013). This study demonstrated that the risk of ESRD increases in the 4th and 3rd quartiles of uric acid compared with the 1st [4.8 (2.6–8.9; p < 0.001)] and 2.9 [(1.6–5.3; p < 0.001)].
3.3.2 Biomarkers Reflecting Specific Pathway Activity
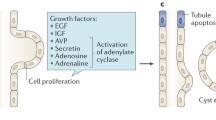
3.3.2.1 Biomarker of cAMP Pathway: Copeptin
Copeptin is the carboxy-terminal portion of AVP and serves as a marker for endogenous AVP levels. Because AVP activates cAMP pathway to stimulate cyst growth, plasma and urinary copeptin may be a valuable marker for renal disease progression. In a cross-sectional study, plasma copeptin level was associated with mGFR, albuminuria, and TKV (Meijer et al. 2011). In the following prospective cohort study, plasma level of copeptin was not only associated with baseline eGFR but also with decline rate of eGFR during follow up duration of mean 11.2 years (standard B −0.345, p < 0.01) (Boertien et al. 2012). In addition, 8 out of 9 patients who developed ESRD during follow up showed higher than median value of plasma copeptin at the baseline. In a longitudinal observational study of CRISP cohort, plasma copeptin concentration was independently associated with change in TKV (p > 0.001) and mGFR (p = 0.09) (Boertien et al. 2013). Urinary copeptin was also investigated recently and researchers found that urinary copeptin was positively associated with TKV and negatively associated with eGFR (Nakajima et al. 2015). The predictive role of urinary copeptin should be further elucidated in the future study.
3.3.2.2 Biomarker of Wnt Pathway: sFRP4
The abnormal Wnt signaling has been suggested to have a role in the pathogenesis of ADPKD. Secreted Frizzled-related protein 4 (sFRP4) is a secreted molecule that antagonizes Wnt signaling pathway. The sFRP4 was well documented to be upregulated in human ADPKD and in 4 different PKD animal models (Romaker et al. 2009). Cyst fluid from ADPKD kidneys activated the sFRP4 production in renal tubular epithelial cell lines. Recent study showed that higher serum level of sFRP4 at the baseline may predict renal function decline in 24 months (Zschiedrich et al. 2016). The patients with sFRP4 level below 5 ng/mL at baseline experienced an average eGFR decline of 3.2/mL/min/1.73 m2/year while those with sFRP4 level above 30 ng/mL at baseline experienced an average eGFR decline of 4.5 mL/min/1.73 m2/year (p = 0.0063). Whether urinary sFRP4 can predict disease progression in ADPKD needs to be further elucidated.
4 Identifying High-Risk Patients for Treatment
Not all the patients diagnosed with ADPKD reach end-stage. The variable disease courses make clinicians difficult to select the treatment boundary or guideline. Before several therapeutic agents became available, the goal of ADPKD management was similar to that of CKD – lowering blood pressure and treating complications. However, recent advances in molecular diagnosis and treatment strategy made us available to slow down the rate of disease progression. Therefore, selecting patients who are most likely to benefit from novel treatment should be considered before launching clinical trials.
Recent review paper well summarized the algorithm to select high-risk patients or rapid progressors for clinical trials (Gansevoort et al. 2016) (Fig. 8.2). First of all, clinicians should consider baseline age and renal function before prescribing novel treatment. For example, a middle aged ADPKD patient in CKD stage 1 is less likely to progress rapidly in his life time. In the same manner, PKD2 family member with preserved renal function is less likely to progress in a few years. Therefore, we should evaluate baseline age and renal function before patient selection. Next, we should define rapid progressors according to previous mentioned risk factors – decline rate of eGFR, TKV change in recent years, baseline TKV, PKD genotype, and family history of ESRD. By following this algorithm, we can expect the optimal outcome with minimal harm to the ADPKD subjects.
Algorithm to assess indications for initiation of treatment in ADPKD (Reproduced from Gansevoort et al. 2016)
5 Conclusions
On the basis of improved knowledge in molecular pathophysiology, a large number of novel therapies have been proposed for ADPKD patients. As a clinician, selecting the patients who will benefit the most and monitoring disease progression are as important as initiating treatment. Various biomarkers described in this chapter will help us to define rapid progressor and to trace disease progression to get the best result from novel treatment.
References
Bae KT, Grantham JJ (2010) Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6(2):96–106. doi:10.1038/nrneph.2009.214
Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, Parfrey P, Pei Y (2009) Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol JASN 20(8):1833–1838. doi:10.1681/ASN.2009020162
Ben-Dov IZ, Tan YC, Morozov P, Wilson PD, Rennert H, Blumenfeld JD, Tuschl T (2014) Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS One 9(1):e86856. doi:10.1371/journal.pone.0086856
Bergmann C, von Bothmer J, Ortiz Bruchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K (2011) Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol JASN 22(11):2047–2056. doi:10.1681/ASN.2010101080
Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, Struck J, Bakker SJ, Peters DJ, de Jong PE, Gansevoort RT (2012) Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27(11):4131–4137. doi:10.1093/ndt/gfs070
Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, Gansevoort RT, Torres VE, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease C (2013) Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am J Kidney Dis Off J Nat Kidney Found 61(3):420–429. doi:10.1053/j.ajkd.2012.08.038
Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ (2012) Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7(3):479–486. doi:10.2215/CJN.09500911
Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC, Conference P (2015) Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 88(1):17–27. doi:10.1038/ki.2015.59
Cornec-Le Gall E, Audrezet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Ferec C, Le Meur Y (2013) Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol JASN 24(6):1006–1013. doi:10.1681/ASN.2012070650
Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA (2001) Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 59(5):1654–1662. doi:10.1046/j.1523-1755.2001.0590051654.x
Gabow PA, Duley I, Johnson AM (1992a) Clinical profiles of gross hematuria in autosomal dominant polycystic kidney disease. Am J Kidney Dis Off J Nat Kidney Found 20(2):140–143
Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH (1992b) Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41(5):1311–1319
Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, Devuyst O, Drechsler C, Eckardt KU, Emma F, Knebelmann B, Le Meur Y, Massy ZA, Ong AC, Ortiz A, Schaefer F, Torra R, Vanholder R, Wiecek A, Zoccali C, Van Biesen W (2016) Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and European renal best practice. Nephrol Dial Transplant 31(3):337–348. doi:10.1093/ndt/gfv456
Geberth S, Stier E, Zeier M, Mayer G, Rambausek M, Ritz E (1995) More adverse renal prognosis of autosomal dominant polycystic kidney disease in families with primary hypertension. J Am Soc Nephrol JASN 6(6):1643–1648
Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP (2006) Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol JASN 17(11):3013–3019. doi:10.1681/ASN.2006080835
Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D (1999) Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353(9147):103–107
Helal I, McFann K, Reed B, Yan XD, Schrier RW, Fick-Brosnahan GM (2013) Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant 28(2):380–385. doi:10.1093/ndt/gfs417
Huang S, Chen L, Lu L, Li L (2016) The apelin-APJ axis: a novel potential therapeutic target for organ fibrosis. Clin Chim Acta 456:81–88. doi:10.1016/j.cca.2016.02.025
Hwang JH, Park HC, Jeong JC, Ha Baek S, Han MY, Bang K, Cho JY, Yu SH, Yang J, Oh KH, Hwang YH, Ahn C (2013) Chronic asymptomatic pyuria precedes overt urinary tract infection and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol 14:1. doi:10.1186/1471-2369-14-1
Hwang YH, Conklin J, Chan W, Roslin NM, Liu J, He N, Wang K, Sundsbak JL, Heyer CM, Haider M, Paterson AD, Harris PC, Pei Y (2015) Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol JASN. doi:10.1681/ASN.2015060648
Idrizi A, Barbullushi M, Petrela E, Kodra S, Koroshi A, Thereska N (2009) The influence of renal manifestations to the progression of autosomal dominant polycystic kidney disease. Hippokratia 13(3):161–164
Idrizi A, Barbullushi M, Koroshi A, Dibra M, Bolleku E, Bajrami V, Xhaferri X, Thereska N (2011) Urinary tract infections in polycystic kidney disease. Med Arh 65(4):213–215
Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE, Investigators C (2015) Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol JASN 26(1):160–172. doi:10.1681/ASN.2013101138
Johnson AM, Gabow PA (1997) Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol JASN 8(10):1560–1567
Kistler AD, Mischak H, Poster D, Dakna M, Wuthrich RP, Serra AL (2009a) Identification of a unique urinary biomarker profile in patients with autosomal dominant polycystic kidney disease. Kidney Int 76(1):89–96. doi:10.1038/ki.2009.93
Kistler AD, Poster D, Krauer F, Weishaupt D, Raina S, Senn O, Binet I, Spanaus K, Wuthrich RP, Serra AL (2009b) Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int 75(2):235–241. doi:10.1038/ki.2008.558
Kistler AD, Serra AL, Siwy J, Poster D, Krauer F, Torres VE, Mrug M, Grantham JJ, Bae KT, Bost JE, Mullen W, Wuthrich RP, Mischak H, Chapman AB (2013) Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One 8(1):e53016. doi:10.1371/journal.pone.0053016
Klahr S, Breyer JA, Beck GJ, Dennis VW, Hartman JA, Roth D, Steinman TI, Wang SR, Yamamoto ME (1995) Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol JASN 5(12):2037–2047
Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG (2009) Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 53(2):344–350. doi:10.1161/HYPERTENSIONAHA.108.123802
Kocer D, Karakukcu C, Ozturk F, Eroglu E, Kocyigit I (2016) Evaluation of fibrosis markers: apelin and transforming growth factor-beta1 in autosomal dominant polycystic kidney disease patients. The Apher Dial Off Peer-reviewed J Int Soc Apher Jpn Soc Apher Jpn Soc Dial Ther. doi:10.1111/1744-9987.12412
Kocyigit I, Yilmaz MI, Orscelik O, Sipahioglu MH, Unal A, Eroglu E, Kalay N, Tokgoz B, Axelsson J, Oymak O (2013) Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin Pract 123(3–4):157–164. doi:10.1159/000353730
Lacquaniti A, Chirico V, Lupica R, Buemi A, Loddo S, Caccamo C, Salis P, Bertani T, Buemi M (2013) Apelin and copeptin: two opposite biomarkers associated with kidney function decline and cyst growth in autosomal dominant polycystic kidney disease. Peptides 49:1–8. doi:10.1016/j.peptides.2013.08.007
Lakhia R, Hajarnis S, Williams D, Aboudehen K, Yheskel M, Xing C, Hatley ME, Torres VE, Wallace DP, Patel V (2015) MicroRNA-21 aggravates cyst growth in a model of polycystic kidney disease. J Am Soc Nephrol JASN. doi:10.1681/ASN.2015060634
Li X (2011) Epigenetics and autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812(10):1213–1218. doi:10.1016/j.bbadis.2010.10.008
Liu M, Shi S, Senthilnathan S, Yu J, Wu E, Bergmann C, Zerres K, Bogdanova N, Coto E, Deltas C, Pierides A, Demetriou K, Devuyst O, Gitomer B, Laakso M, Lumiaho A, Lamnissou K, Magistroni R, Parfrey P, Breuning M, Peters DJ, Torra R, Winearls CG, Torres VE, Harris PC, Paterson AD, Pei Y (2010) Genetic variation of DKK3 may modify renal disease severity in ADPKD. J Am Soc Nephrol JASN 21(9):1510–1520. doi:10.1681/ASN.2010030237
Meijer E, Boertien WE, Nauta FL, Bakker SJ, van Oeveren W, Rook M, van der Jagt EJ, van Goor H, Peters DJ, Navis G, de Jong PE, Gansevoort RT (2010) Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis Off J Nat Kidney Found 56(5):883–895. doi:10.1053/j.ajkd.2010.06.023
Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, Gansevoort RT (2011) Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6(2):361–368. doi:10.2215/CJN.04560510
Nakajima A, Lu Y, Kawano H, Horie S, Muto S (2015) Association of arginine vasopressin surrogate marker urinary copeptin with severity of autosomal dominant polycystic kidney disease (ADPKD). Clin Exp Nephrol 19(6):1199–1205. doi:10.1007/s10157-015-1101-7
Norman J (2011) Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta 1812(10):1327–1336. doi:10.1016/j.bbadis.2011.06.012
Orskov B, Borresen ML, Feldt-Rasmussen B, Ostergaard O, Laursen I, Strandgaard S (2010) Estimating glomerular filtration rate using the new CKD-EPI equation and other equations in patients with autosomal dominant polycystic kidney disease. Am J Nephrol 31(1):53–57. doi:10.1159/000256657
Ozkok A, Akpinar TS, Tufan F, Kanitez NA, Uysal M, Guzel M, Caliskan Y, Alisir S, Yazici H, Ecder T (2013) Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol 17(3):345–351. doi:10.1007/s10157-012-0706-3
Parikh CR, Dahl NK, Chapman AB, Bost JE, Edelstein CL, Comer DM, Zeltner R, Tian X, Grantham JJ, Somlo S (2012) Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int 81(8):784–790. doi:10.1038/ki.2011.465
Park EY, Woo YM, Park JH (2011) Polycystic kidney disease and therapeutic approaches. BMB Rep 44(6):359–368. doi:10.5483/BMBRep.2011.44.6.359
Park HC, Hwang JH, Kang AY, Ro H, Kim MG, An JN, In Park J, Kim SH, Yang J, Oh YK, Oh KH, Noh JW, Cheong HI, Hwang YH, Ahn C (2012) Urinary N-acetyl-beta-D glucosaminidase as a surrogate marker for renal function in autosomal dominant polycystic kidney disease: 1 year prospective cohort study. BMC Nephrol 13:93. doi:10.1186/1471-2369-13-93
Park HC, Kang AY, Jang JY, Kim H, Han M, Oh KH, Kim SH, Noh JW, Cheong HI, Hwang YH, Ahn C (2015) Increased urinary Angiotensinogen/Creatinine (AGT/Cr) ratio may be associated with reduced renal function in autosomal dominant polycystic kidney disease patients. BMC Nephrol 16:86. doi:10.1186/s12882-015-0075-8
Paterson AD, Pei Y (1998) Is there a third gene for autosomal dominant polycystic kidney disease? Kidney Int 54(5):1759–1761. doi:10.1046/j.1523-1755.1998.00166.x
Paul BM, Consugar MB, Ryan Lee M, Sundsbak JL, Heyer CM, Rossetti S, Kubly VJ, Hopp K, Torres VE, Coto E, Clementi M, Bogdanova N, de Almeida E, Bichet DG, Harris PC (2014) Evidence of a third ADPKD locus is not supported by re-analysis of designated PKD3 families. Kidney Int 85(2):383–392. doi:10.1038/ki.2013.227
Pei Y, Lan Z, Wang K, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T (2012) A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int 81(4):412–417. doi:10.1038/ki.2011.370
Perez-Oller L, Torra R, Badenas C, Mila M, Darnell A (1999) Influence of the ACE gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease. Am J Kidney Dis Off J Nat Kidney Found 34(2):273–278. doi:10.1053/AJKD03400273
Persu A, Stoenoiu MS, Messiaen T, Davila S, Robino C, El-Khattabi O, Mourad M, Horie S, Feron O, Balligand JL, Wattiez R, Pirson Y, Chauveau D, Lens XM, Devuyst O (2002) Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum Mol Genet 11(3):229–241
Romaker D, Puetz M, Teschner S, Donauer J, Geyer M, Gerke P, Rumberger B, Dworniczak B, Pennekamp P, Buchholz B, Neumann HP, Kumar R, Gloy J, Eckardt KU, Walz G (2009) Increased expression of secreted frizzled-related protein 4 in polycystic kidneys. J Am Soc Nephrol JASN 20(1):48–56. doi:10.1681/ASN.2008040345
Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van’t Hoff WG, Niaudet P, Torres VE, Harris PC (2009) Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75(8):848–855. doi:10.1038/ki.2008.686
Ruggenenti P, Gaspari F, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Prandini S, Ene-Iordache B, Diadei O, Perico N, Ondei P, Pisani A, Buongiorno E, Messa P, Dugo M, Remuzzi G, Group G-AS (2012) Measuring and estimating GFR and treatment effect in ADPKD patients: results and implications of a longitudinal cohort study. PLoS One 7(2):e32533. doi:10.1371/journal.pone.0032533
Rule AD, Torres VE, Chapman AB, Grantham JJ, Guay-Woodford LM, Bae KT, Klahr S, Bennett WM, Meyers CM, Thompson PA, Miller JP, Consortium C (2006) Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. J Am Soc Nephrol JASN 17(3):854–862. doi:10.1681/ASN.2005070697
Salih M, Demmers JA, Bezstarosti K, Leonhard WN, Losekoot M, van Kooten C, Gansevoort RT, Peters DJ, Zietse R, Hoorn EJ, Consortium D (2016) Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J Am Soc Nephrol JASN. doi:10.1681/ASN.2015090994
Schrier RW (2011) Hypertension and autosomal dominant polycystic kidney disease. Am J Kidney Dis Off J Nat Kidney Found 57(6):811–813. doi:10.1053/j.ajkd.2011.02.379
Schrier RW, Johnson AM, McFann K, Chapman AB (2003) The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int 64(5):1792–1799. doi:10.1046/j.1523-1755.2003.00264.x
Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB, Investigators H-PT (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371(24):2255–2266. doi:10.1056/NEJMoa1402685
Shamshirsaz AA, Reza Bekheirnia M, Kamgar M, Johnson AM, McFann K, Cadnapaphornchai M, Nobakhthaghighi N, Schrier RW (2005) Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int 68(5):2218–2224. doi:10.1111/j.1523-1755.2005.00678.x
Spithoven EM, van Gastel MD, Messchendorp AL, Casteleijn NF, Drenth JP, Gaillard CA, de Fijter JW, Meijer E, Peters DJ, Kappert P, Renken RJ, Visser FW, Wetzels JF, Zietse R, Gansevoort RT, Consortium D (2015) Estimation of total kidney volume in autosomal dominant polycystic kidney disease. Am J Kidney Dis Off J Nat Kidney Found 66(5):792–801. doi:10.1053/j.ajkd.2015.06.017
Spithoven EM, Meijer E, Boertien WE, Sinkeler SJ, Tent H, de Jong PE, Navis G, Gansevoort RT (2013) Tubular secretion of creatinine in autosomal dominant polycystic kidney disease: consequences for cross-sectional and longitudinal performance of kidney function estimating equations. Am J Kidney Dis Off J Nat Kidney Found 62(3):531–540. doi:10.1053/j.ajkd.2013.03.030
Sun L, Zhu J, Wu M, Sun H, Zhou C, Fu L, Xu C, Mei C (2015) Inhibition of MiR-199a-5p reduced cell proliferation in autosomal dominant polycystic kidney disease through targeting CDKN1C. Med Sci Mon Int Med J Exp Clin Res 21:195–200. doi:10.12659/MSM.892141
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369(9569):1287–1301. doi:10.1016/S0140-6736(07)60601-1
Tracz MJ, Alam J, Nath KA (2007) Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol JASN 18(2):414–420. doi:10.1681/ASN.2006080894
Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS (2008) Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis Off J Nat Kidney Found 51(2):184–191. doi:10.1053/j.ajkd.2007.09.020
Woo YM, Park JH (2013) microRNA biomarkers in cystic diseases. BMB Rep 46(7):338–345
Woo YM, Bae JB, Oh YH, Lee YG, Lee MJ, Park EY, Choi JK, Lee S, Shin Y, Lyu J, Jung HY, Lee YS, Hwang YH, Kim YJ, Park JH (2014) Genome-wide methylation profiling of ADPKD identified epigenetically regulated genes associated with renal cyst development. Hum Genet 133(3):281–297. doi:10.1007/s00439-013-1378-0
Zheng D, Wolfe M, Cowley BD Jr, Wallace DP, Yamaguchi T, Grantham JJ (2003) Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol JASN 14(10):2588–2595
Zschiedrich S, Budde K, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hoerl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Lindner TH, Faerber L, Wimmer P, Stork R, Eckardt KU, Walz G (2016) Secreted frizzled-related protein 4 predicts progression of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 31(2):284–289. doi:10.1093/ndt/gfv077
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Park, H.C., Ahn, C. (2016). Diagnostic Evaluation as a Biomarker in Patients with ADPKD. In: Park, J., Ahn, C. (eds) Cystogenesis. Advances in Experimental Medicine and Biology, vol 933. Springer, Singapore. https://doi.org/10.1007/978-981-10-2041-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-2041-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2040-7
Online ISBN: 978-981-10-2041-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)