Abstract
Autosomal dominant polycystic kidney disease (ADPKD), the most common hereditary renal disease , is characterized by continuous and progressive growth of innumerable cysts in both kidneys. Despite gross enlargement of the kidneys, renal function is usually maintained within the normal range for decades through hyperfiltration of the remaining nephrons. Once renal function starts to decline, it usually deteriorates rapidly resulting in end-stage renal disease (ESRD) within a few years. Until recently, the treatment of ADPKD has been exclusively symptomatic, but based on accumulating mechanistic insights, several potential targeted pharmacological therapeutic approaches have now been emerging during the last decade. This development has led to an urgent need for biomarkers to allow early detection of patients at risk for accelerated progression and as surrogate markers for interventional trials. Total kidney volume (TKV) assessed by magnetic resonance imaging (MRI) or computed tomography (CT) is the most extensively studied measure of disease severity and correlates with future renal function loss. In addition, a few biochemical markers as well as proteomic and metabolomic patterns have been described that characterize patients with ADPKD. While some of these markers are promising, their use to reliably predict prognosis has yet to be proven. A major obstacle to the discovery and validation of biomarkers for ADPKD is the slowly progressive nature of the disease and the still limited follow-up information on well-characterized patient cohorts. As longitudinal information on well-characterized patients continues to accumulate, the discovery and validation of disease progression markers will be greatly facilitated. This chapter summarizes both established progression markers of ADPKD (i.e., kidney and cyst volume) and recently used approaches to the discovery of novel biochemical markers and discusses general aspects relevant to biomarker discovery for ADPKD.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts
-
ADPKD is the most common hereditary kidney disease, characterized by continuous development and growth of cysts in both kidneys.
-
End-stage renal disease (ESRD) is a stage of advanced kidney failure that requires treatment by renal replacement therapy, i.e., dialysis or kidney transplantation.
-
Overall kidney function is best quantified by the glomerular filtration rate (GFR), i.e., the amount of primary urine that is produced every minute by filtration of blood in the kidney glomeruli.
-
ADPKD leads to ESRD in many patients at a median age of 55 years, but the age at which ESRD occurs is highly variable and difficult to predict.
-
The three-dimensional structure of kidneys can be visualized by three different imaging methods (ultrasound, computed tomography, and magnetic resonance imaging), which each has their own advantages and disadvantages.
-
Capillary electrophoresis-mass spectrometry (CE-MS) is a very reproducible method used to simultaneously quantify several thousands of peptides (protein fragments) present in human urine by direct coupling two separation methods based on the physicochemical characteristics of the peptides.
Definitions of Words and Terms
Chronic Kidney Disease (CKD)
Abnormalities of kidney structure or function that have implications for health and are present for more than 3 months. CKD is graded into stages according to GFR and urinary albumin excretion.
End-Stage Renal Disease (ESRD)
Chronic impairment of renal function that has critical impacts on health and necessitates renal replacement therapy (i.e., dialysis or kidney transplantation).
Glomerular Filtration Rate (GFR)
The amount of primary filtrate produced per minute by glomerular filtration in the kidney. Currently used as the main parameter to quantify renal function.
Glomerulus
Glomeruli are the basic filtration units of the kidney consisting of a tuft of capillaries with highly selective permeability, surrounded by a capsule (Bowman’s capsule), which collects the primary filtrate of the kidney.
Nephron
A basic functional unit of the kidney which consists of a glomerulus (see above) and a renal tubule with a complex architecture of specific segments, in which the primary glomerular filtrate is further modified, primarily by reabsorption of water and solutes.
Support Vector Machine (SVM)
Learning models that are used to classify data into two categories by constructing a virtual hyperplane between data points in a virtual space that belong to two different categories.
Introduction: Epidemiology, Genetics, and Clinical Course of ADPKD
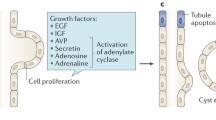
ADPKD is the most common hereditary renal disease with an incidence between 1:400 and 1:1,000 of all life births (Dalgaard 1957; Iglesias et al. 1983). It accounts for an estimated 5–10 % of patients requiring renal replacement therapy, i.e., dialysis or kidney transplantation. Genetically, ADPKD is heterogeneous, being caused by mutations in either the PKD1 gene (chromosome 16p13.3, ~85 % of cases) or the PKD2 gene (chromosome 4p21, ~15 % of cases) (Harris and Rossetti 2010). Phenotypically, the hallmark of ADPKD is the continuous development and growth of innumerable cysts in both kidneys, leading to their progressive enlargement with distortion of the normal renal architecture (Wilson 2004). These cysts branch from renal tubuli of all nephron segments, with a predominance of the collecting ducts. During their continuous enlargement, most cysts lose connection to the urine collection system and continue to grow by a combination of proliferative and secretory mechanisms (Arnaout 2001). Progressive growth of innumerable cysts ultimately leads to grotesque enlargement of both kidneys, which can exceed volumes of 4,000 ml per kidney (Fig. 1). Extrarenal manifestations of ADPKD include cyst growth in other organs, such as the liver, pancreas, and seminal vesicle, as well as connective tissue abnormalities, including cardiac valve defects, intracerebral aneurysms, diverticuli, and abdominal herniae (Pirson 2010). However, renal disease dominates the clinical picture in the majority of patients. Diagnosis of ADPKD is usually based on the imaging of patients at risk by ultrasound with established diagnostic criteria that reach very high accuracy in adult patients with a positive family history (Pei et al. 2009). In clinical routine, genetic testing is reserved for special situations, such as atypical disease, negative family history, or the need to exclude disease with certainty in young patients (e.g., potential living kidney donors) (Pei 2011).
Cyst burden in advanced ADPKD. Left side: T2-weighed MRI of a 42-year-old patient with a high cyst burden in advanced ADPKD. Note the multiple cysts also present in the liver rendering a delineation of the right upper kidney boundary difficult. Right side: nephrectomy specimen of the same patient shown on the left, who was nephrectomized after kidney transplantation because of recurrent cyst infections. The scale bar next to the kidney measures 10 cm
Despite progressive and eventually gross enlargement of the kidneys, patients often remain asymptomatic for a considerable amount of time. The most common early disease manifestations are arterial hypertension as well as urological symptoms such as flank pain, urinary tract and cyst infections, kidney stones, and recurrent macrohematuria (Grantham 2008). Renal function remains within the normal range for decades in most patients before an accelerated loss of glomerular filtration rate (GFR) usually ensues between the fourth and sixth decade of life. The exact mechanisms leading to renal failure in ADPKD remain incompletely defined, but mechanical factors most likely play a key role (Grantham et al. 2011): expanding cysts lead to compression of neighboring distal tubuli and collecting ducts and cause local obstruction; distortion of the renal architecture causes vascular compression thus compromising renal perfusion. Although these processes do not primarily involve glomerular filtration, the obstruction and obliteration of tubuli surrounding cysts can be clearly demonstrated even at early stages and lead to loss of the entire corresponding nephrons (Grantham et al. 2011). Thus, a considerable amount of nephrons are irreversibly damaged well before GFR decline becomes clinically apparent. It is generally being assumed that GFR is initially maintained in ADPKD through compensatory hyperfiltration of the remaining nephrons (Grantham et al. 2011), and a clinically apparent decline of GFR usually indicates that compensatory mechanisms are exhausted leading to an accelerated loss of renal function and progression to end-stage renal disease (ESRD) within a few years.
The rate of progression and the age at which patients reach ESRD are highly variable in ADPKD with cases of ESRD occurring as early as in the second decade of life, while others survive to their 80s without clinically significant renal impairment. Until recently, relatively few strong risk factors for accelerated progression have been identified apart from the type of mutation. Individuals with PKD1 mutations reach ESRD at a median age of 53–58 years, whereas patients with PKD2 mutations usually show a much milder disease course and reach ESRD 20 years later at a median age of 72–80 years (Torra et al. 1996; Hateboer et al. 1999; Cornec-Le Gall et al. 2013). However, the phenotypes of PKD1 and PKD2 mutations overlap considerably. Data on whether and how the position and type of a PKD1 mutation affects outcome are conflicting (Rossetti et al. 2003; Cornec-Le Gall et al. 2013). The high variability of disease severity even within a family (Rossetti et al. 2002) suggests a relevant influence of environmental factors and modifier genes. The importance of the latter is supported by the analysis of age variability at ESRD in genetically identical twins vs. siblings (Persu et al. 2004), but the involved genes and polymorphisms remain elusive. Apart from genotype, other identified prognostic factors include sex (which was associated with age at ESRD in most (Gabow et al. 1992; Choukroun et al. 1995; Johnson and Gabow 1997; Orskov et al. 2012; Cornec-Le Gall et al. 2013) but not all (Hateboer et al. 1999; Rossetti et al. 2002) studies) and age at the occurrence of hypertension, gross hematuria, proteinuria, urinary tract infections, urine sodium excretion, urine osmolality, dyslipidemia, and left ventricular hypertrophy (Gabow et al. 1992; Chapman et al. 1994; Choukroun et al. 1995; Johnson and Gabow 1997; Torres et al. 2011; Panizo et al. 2012). However, these factors all have limited predictive power, and many of them are relatively late signs of advanced disease. Thus, it remains a challenge to predict prognosis in young ADPKD patients.
Emerging Therapeutic Options for ADPKD Call for Biomarkers of Disease Severity and Progression
Until recently, the utility to predict prognosis in young ADPKD patients would have been largely limited to informing them and aiding family and career planning given the lack of specific therapeutic options for ADPKD. Treatment has been symptomatic, targeting complications, such as hypertension, flank pain, urinary tract infections, and ultimately renal replacement therapy, with no proven benefit for early interventions. This situation, however, has been changing recently. Rapidly accumulating molecular insights into disease pathogenesis and progression have resulted in the development of several targeted treatment approaches aiming to retard cyst growth, reduce symptoms, and delay the onset of renal failure (Chang and Ong 2012). Some of these approaches have been tested in randomized controlled trials (RCT) with variable success, while several others are being evaluated in ongoing clinical studies or are in preclinical testing. A few principles are common to all specific therapeutic approaches for ADPKD: (1) Since kidney function loss becomes apparent only once massive structural damage to the kidneys has occurred and compensatory mechanisms are exhausted, treatment initiation in patients with significantly reduced GFR is considered unlikely to affect outcomes to a relevant degree. (2) Considering the slowly progressive nature of disease and the need for early initiation of therapy, any specific treatment will likely need to be given over extended periods of time (i.e., decades), and treatment benefits therefore need to be weighed against potential long-term side effects as well as cost. (3) Given the large variability of disease severity, treatment decisions will need to be individualized: some patients might not require specific therapy at all, while in others, early aggressive treatment is warranted. Therefore, risk stratification of patients at early disease stages would be important. (4) To evaluate a therapy that is initiated during early disease stages, GFR is not a particularly useful measure of outcome given its late decline. To detect an effect of treatment on future GFR decline and ultimately on age at ESRD (i.e., on hard endpoints), unfeasibly long observation periods would be required. Thus, alternative measures of disease progression (i.e., surrogate markers) are needed for the design of clinical studies and to follow individual patients.
As a consequence of these principles, the development of specific treatment approaches for ADPKD has led to an urgent need for biomarkers that would allow for both risk stratification in order to select the appropriate patients for treatment and monitoring progression during early stages of disease.
Biomarkers for ADPKD: General Considerations
Generally speaking, an ADPKD biomarker may be used for either diagnostic purposes, for risk stratification, or to monitor disease progression and response to treatment. It is unlikely that one particular biomarker fulfills all these purposes simultaneously. As outlined above, diagnosis of ADPKD is usually easily established by ultrasound imaging with few exceptions, and biomarkers would be particularly required for the latter two purposes, i.e., predicting outcomes and monitoring disease course. Given the slowly progressive nature of ADPKD, a prognostic biomarker might either reflect established damage or the activity of the underlying process driving progression of disease (i.e., cyst growth and renal fibrosis). A marker of damage will aid to predict the risk of clinical outcomes (e.g., age at ESRD) in a given patient and aid treatment decisions, but it is not expected to respond to treatment. Hence, such a parameter will need to be measured repeatedly over time, and its change over time will need to be assessed as an outcome parameter for, e.g., clinical trials. Furthermore, markers of damage are less useful during very early disease stages. In contrast, a marker of disease activity may theoretically help to predict prognosis even in young patients with little established damage, and it will more immediately reflect response to treatment, but its reliability to predict long-term outcomes may be limited by the potential of fluctuating disease activity over time. Finally, a biomarker may be relatively specific for ADPKD, or it may just reflect renal damage independent of the underlying process (e.g., proteinuria).
Any prognostic biomarker will need to be validated against a clinically relevant outcome as the “gold standard” for disease progression. The most clinically relevant outcome in ADPKD is certainly age at ESRD. Unfortunately, as outlined above, extremely long observation periods are required in order for a significant proportion of patients to reach this endpoint. Thus, for the same reason why reliable biomarkers of ADPKD would be of great importance to predict outcomes and detect early response to treatments, few, if any, prognostic biomarkers of ADPKD have been prospectively validated against hard clinical endpoints.
Imaging Parameters as Biomarkers of ADPKD
According to the biomarker definition of the NIH Biomarkers Definitions Working Group as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group 2001), any quantifiable biological characteristic may serve as a biomarker, including imaging results. Given that ADPKD is morphologically characterized by the continuous growth of cysts in both kidneys, which is readily detectable by several imaging modalities, imaging parameters reflecting cyst burden are obvious candidate biomarkers for ADPKD. Based on the current pathophysiological understanding of the disease, suggesting that cyst expansion is the basic mechanism responsible for renal function deterioration in ADPKD, it is reasonable to assume that the rate of kidney volume increase caused by a progressive cyst burden directly reflects disease progression. Two parameters have been used to quantify cyst burden in ADPKD patients: total cyst volume (TCV) is defined as the sum of the volume of all cysts in both kidneys and is theoretically the most accurate measure of overall cyst burden but is difficult to assess, requiring segmentation of kidney tissue into cysts vs. parenchymal volume. Total kidney volume (TKV) is defined as the sum of the right and left kidney volume (cystic and non-cystic). TKV is much easier to assess compared to TCV, and because cystic volume clearly dominates over parenchymal volume in ADPKD except at very early stages, TKV correlates well with TCV and reflects overall cyst burden.
Kidney volume can be determined by ultrasonography, CT, or MRI. Renal ultrasonography is readily available and inexpensive and can be performed as a bedside procedure. It has emerged as the standard diagnostic modality for ADPKD (Barua and Pei 2010). Ultrasound-based kidney volume determinations are relatively imprecise, however, due to operator dependence as well as the facts that volume determinations with ultrasound must be approximated by the ellipsoid formula (Bakker et al. 1999) and that the enlarged ADPKD kidneys do not usually fit on one single ultrasound image. Thus, renal ultrasonography is useful for screening and initial evaluation of patients but is of limited utility for quantifying volume changes over relatively short intervals of time (Bae and Grantham 2010). CT and MRI allow for precise measurement of kidney volumes using one of the several available more or less automated approaches to image analysis. CT provides accurate and precise images with low acquisition times but involves ionizing radiation, which is of concern when used repeatedly in young adults. MRI does not involve ionizing radiation, provides an excellent tissue contrast even without the use of contrast agents (Kistler et al. 2009a) (Fig. 1, left), and is superior to ultrasound and CT for the visualization of small cysts (Nascimento et al. 2001). Therefore, MRI has been evolving as the standard modality for quantitative imaging of polycystic kidneys.
For measuring TKV and TCV based on MR or CT images, fully automated image analysis and kidney volume quantification would be highly desirable but have proven to be very challenging. The variable composition of cyst fluid results in cysts appearing either hyper- or hypointense on MRI, and the massively enlarged kidneys distort the anatomy of surrounding tissues and might be difficult to be discriminated from adjacent structures such as the liver, which often also contains numerous cysts (Fig. 1, left). Thus, TKV measurement in ADPKD usually relies on visual distinction between renal and nonrenal tissue aided by a number of more or less automated approaches to image segmentation. Such methods include stereology (Bae et al. 2000) as well as computer-aided manual contour tracing (Kistler et al. 2009a), the latter being potentially more precise but clearly more time consuming. TCV determination usually relies on region-based threshold methods and is much less accurate than TKV determination.
A growing number of studies have serially measured TKV in cohorts of ADPKD patients with one of the methods mentioned above either as part of observational studies or using TKV as an outcome variable in clinical trials (Bae and Grantham 2010). Most of these studies have found a strong correlation between baseline TKV and signs and symptoms of disease, such as GFR, hypertension, and gross hematuria. More importantly, an accumulating body of evidence establishes TKV as a robust predictor of future GFR decline. Patients with TKV >1,500 ml have a significantly more negative GFR slope during follow-up (Grantham et al. 2006), and TKV adjusted to patient height (htTKV) correlates with the risk of developing stage 3 chronic kidney disease (CKD), i.e., GFR <60 ml/min/1.73 m2, over the following 8 years (Chapman et al. 2012). When plotting TKV over time for individual patients, the majority of patients exhibit an exponential-like progression of their renal volume with a patient-specific growth rate, resulting in their TKV progressing along “percentiles” (Grantham et al. 2006). Hence, comparing a single TKV measurement of a given patient at a certain age to available data from large cohorts allows a prediction of future kidney growth and of expected outcomes (Fig. 2). However, some points must be noted regarding TKV as a prognostic factor: First, there remains considerable variability regarding renal outcomes even among patients of similar baseline TKV. Second, TKV is a less reliable prognostic marker in young patients, because cystic volume contributes less to TKV at early disease stages. The prognostic value of TKV, although established in patients with relatively preserved GFR at baseline, is based on cohorts that contain few very young patients. Most patients from these cohorts, who developed relevant renal functional impairment during follow-up, started with large kidneys at baseline, whereas there is still insufficient prospective follow-up of patients included at very early stages for them to experience hard endpoints. Thus, the value of baseline TKV to predict reaching a certain CKD stage within a certain amount of time might just reflect the fact that starting out with advanced disease is certainly a risk for further deterioration, rather than helping to identify high-risk patients before significant damage has occurred.
Total polycystic kidney volume according to age. MRI-based TKV measures in the SUISSE ADPKD cohort are shown according to patient age and sex. Two measurements in each patient, 6 months apart, are shown, connected by straight lines (Kistler et al. 2009a). The patient-specific TKV growth rate can be estimated based on the baseline TKV according to age, and patients can be divided into slow, rapid, or intermediate progressors, based on their TKV according to age
In addition to serving as a prognostic marker when assessed once, TKV can be followed over time to monitor disease progression. A time interval as short as 6 months has been demonstrated to be useful to assess disease progression by MRI volumetry (Kistler et al. 2009a). However, with short time intervals, even small measurement errors of TKV negatively affect the precision of volume progression estimates. Furthermore, actual TKV changes might occur with some fluctuations, and asymptomatic rupture or involution of cysts has been shown to significantly affect short-term TKV changes (Kistler et al. 2009a). Therefore, overall observation periods of at least 1–2 years and preferably longer are likely required for a reliable assessment of kidney volume progression in ADPKD. Given that polycystic kidneys follow an exponential-like growth characteristic (Grantham et al. 2008), TKV change over time is best expressed as percentage change per year rather than in terms of absolute volume change, since only the former is relatively constant over time in a given patient. Percentage annual TKV change correlates with several measures of disease severity (Grantham et al. 2006; Kistler et al. 2009a), including GFR slope. Based on the abovementioned pathophysiological considerations, cyst growth precedes measurable GFR decline, and therefore, TKV growth rate likely predicts the rate of future GFR loss. This association, however, has not yet been rigorously tested in prospective studies. Importantly, effective treatments are likely to both reduce TKV change and maintain GFR, and TKV change over time has therefore been used as main clinical outcome parameter for clinical trials. One caveat is that the correlation of cyst growth with GFR decline might be uncoupled in certain instances, i.e., a pharmacological treatment might decrease cyst volume while at the same time negatively affecting GFR. Nevertheless, the bulk of evidence from animal data suggests that those treatments which reduce kidney volume growth do also exhibit a protective effect on renal function. Major limitations to the use of serial TKV measurements in clinical practice are the need for expensive imaging acquisition and for time-consuming image analysis and quantification as well as a relative lack of standardization of either part of the procedure.
Conventional Serum and Urine Biomarkers of ADPKD
The common but unspecific biochemical measures of renal damage, such as serum creatinine/eGFR and proteinuria, are late signs of disease in ADPKD, as outlined in the introduction, and they will not be further considered here. Aiming to identify markers that reflect early damage and might have predictive potential, several markers of tubular damage conventionally used as biomarkers of acute kidney injury have been tested for their association with disease severity in ADPKD. Monocyte chemoattractant protein-1 (MCP-1), neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-d-glucosaminidase (NAG), kidney injury molecule-1 (KIM-1), interleukin-18, and β2-microglobulin have all been evaluated in ADPKD. Most or all of them are elevated in ADPKD and some may correlate with disease severity (Zheng et al. 2003; Bolignano et al. 2007; Meijer et al. 2010). However, data evaluating a value of these markers to predict changes in GFR or kidney volume are either lacking, negative, or controverse (Boertien et al. 2012a; Parikh et al. 2012; Park et al. 2012).
Plasma copeptin is so far probably the only ADPKD-specific individual biomarker that has been evaluated regarding its prognostic utility. Experimental data indicate a major role for vasopressin in the progression of ADPKD via its action on V2 receptors and stimulation of cAMP, and a vasopressin antagonist has been shown to slow disease progression and ameliorate symptoms in ADPKD (Devuyst and Torres 2013). Endogenous vasopressin levels might therefore serve as markers of disease activity in ADPKD. However, vasopressin is mostly bound to platelets and is unstable in isolated plasma, and assays are of limited sensitivity. Copeptin, the c-terminal portion of the vasopressin precursor, is more stable and serves as a clinically useful measure of endogenous vasopressin secretion. Plasma copeptin levels have been shown to correlate with some measures of disease severity in ADPKD (Meijer et al. 2011). More importantly, copeptin levels had some predictive value for the estimation of TKV change and GFR decline during follow-up in two independent longitudinal studies (Boertien et al. 2012b, 2013). The correlation with TKV and GFR changes over time, and thus, the predictive power for prognosis, however, was limited.
Proteomic and Metabolomic Approaches to Biomarker Discovery in ADPKD
In addition to the abovementioned hypothesis-driven approaches to biomarker identification, proteomic methods have been used to identify novel biomarkers and biomarker patterns for ADPKD. Proteome analysis of easily accessible body fluids allows for unbiased identifications of novel biomarkers. Urine is particularly suited for clinical proteome analysis (Fliser et al. 2007): it is easily accessible and abundantly available. In contrast to blood, urine contains no relevant protease activity and is normally largely free of cellular elements and highly abundant proteins (such as albumin and immunoglobulins in plasma or serum) which would obscure the detection of low abundant proteins. Thus, preanalytical sample handling can be minimized, and urine is particularly suited for use with automated high-throughput methods which allow for the reproducible simultaneous detection of hundreds to thousands of proteins. Rather than using “classical” proteomic techniques to identify individual biomarkers, such high-throughput methods can be used for proteomic profiling, i.e., to identify biomarker patterns that characterize a certain disease state. As an additional advantage, clinical proteomic profiling can be used to differentiate among several potential diagnoses and may provide additional, e.g., prognostic information using one single sample analysis.
Capillary electrophoresis coupled online to mass spectrometry (CE-MS) (Mischak et al. 2009) is a particularly useful method for proteomic profiling, utilizing CE to separate small proteins according to their electrophoretic characteristics, directly followed by MS analysis by electron spray ionization (ESI) and time-of-flight mass spectrometry (TOF-MS). Thus, every detected peptide is unambiguously characterized by the migration time in CE and the molecular mass determined by MS (Fig. 3). CE-MS offers several advantages over other frequently used methods for clinical proteome analysis: (1) it provides fast separation and high resolution; (2) it uses inexpensive capillaries instead of expensive columns required for liquid chromatography; (3) it is compatible with most buffers and salts; (4) it provides a stable constant flow, thus avoiding elution gradients that may otherwise interfere with MS detection; and (5) it is therefore robust and highly reproducible. Using CE-MS , a total of over 100,000 different peptides have been detected in human urine, and 5,000 of those are detectable in at least 20 % of urine samples (Coon et al. 2008). Appropriate statistical methods can be used to combine distinct biomarkers into a proteomic score, which may largely increase sensitivity and specificity for a certain disease state in comparison to single markers. Over 20,000 human urine samples from patients suffering from a variety of diseases as well as from healthy controls have been analyzed to date in a comparative way using this technique (Stalmach et al. 2013), and disease-specific biomarker patterns have been identified for many renal and nonrenal diseases, including ANCA vasculitides, diabetic nephropathy, Fabry’s disease, prostate cancer, vesicoureteral reflux, coronary artery disease, and many more. Deposited in a large database, these data provide an enormous amount of information that can be used for the detection of disease-specific patterns.
Principle of capillary electrophoresis-mass spectrometry. Top: schematic representation of the setup. Low-molecular-weight proteins contained in a urine sample are separated according to their mass and charge properties and the eluting fractions are directly subjected to mass spectrometry following electron spray ionization (ESI). Data from each individual sample are stored in a large database. Raw data (bottom left) are normalized by internal calibration, relevant signals are identified, and mass/charge ratio data are converted into molecular mass data by identifying signals corresponding to equal mass with different charge states after ESI (bottom middle). Finally, signal intensity is depicted as peak height in a three-dimensional illustration for easier visibility (bottom right). By calculating average peak intensities for a defined group of patients, compiled patterns representing a particular patient group can be generated
Comparing CE-MS analyses of urine samples from ADPKD patients to those of healthy controls, a biomarker pattern could be identified that accurately differentiated between cases and controls. In an initial study (Kistler et al. 2009b), comparison of urine from 17 ADPKD patients to that of 86 age-matched healthy controls led to the identification of 197 proteins with significantly different excretion after adjustment for multiple testing (Fig. 4). Thirty-eight of the 197 differentially excreted peptides could be identified by sequencing. A majority of them represented collagen fragments, consistent with an important role of extracellular matrix reorganization during cyst expansion, and fragments of uromodulin, which has recently been implicated in a growing number of interstitial kidney diseases (Rampoldi et al. 2011). A support vector machine (SVM)-based biomarker score combining the most consistently altered proteins was extensively validated in an independent dataset of 24 ADPKD patients, 224 healthy controls, 150 patients with a variety of other renal diseases, 113 patients with renal cell carcinoma, 112 patients with bladder carcinoma, and 127 elderly patients. The biomarker score showed a high diagnostic accuracy in the independent validation cohort (area under the receiver-operating characteristics curve, AUC 0.95) and was specific for ADPKD vs. other renal and urological diseases. In a subsequent study (Kistler et al. 2013), this proteomic pattern was further validated in a large independent ADPKD cohort (n = 224) vs. 86 healthy controls, where it yielded a slightly lower but still acceptable accuracy. By increasing the number of ADPKD patients in the development cohort (n = 41 vs. 189 age-matched healthy controls), the number of identified proteins with altered excretion in ADPKD could be increased to 657, and a refined SVM-based model was developed. Similar to ultrasound diagnostic criteria, the sensitivity of the biomarker model was lower in younger patients and in those with PKD2 mutations indicating that the proteomic changes reflected by the model likely correlate with cyst burden.
Given that ADPKD can be readily diagnosed using ultrasound in most cases (see above), the fundamental question is whether urinary proteomic alterations in ADPKD can be used as predictors of disease outcome. By correlating proteomic changes with disease extent as defined by height-adjusted TKV, a pattern could be defined that associated with disease severity (Kistler et al. 2013). However, the correlation was relatively modest, and it has not yet been possible to develop a biomarker model that reliably predicts GFR decline. Apart from a still relatively limited follow-up time in the cohorts that have been analyzed, current limitations to the identification of prognostic markers include the heterogeneity of patients at baseline, making it difficult to separate markers of damage from markers of progression and age-specific markers.
A similar statistical approach, including the generation of an SVM-based model for pattern identification, has been applied to the analysis of urine from ADPKD patients using a very different analytical methodology, focusing on urinary metabolites in general rather than just on peptides (Gronwald et al. 2011). The comparison of NMR-spectrometric analysis of urine from 54 ADPKD patients to that of 46 healthy controls resulted in the identification of 51 features that appeared altered in ADPKD, including several proteins, formate, citrate, and methanol. An SVM-based model could be used to distinguish ADPKD patients from controls with reasonable diagnostic accuracy (AUC 0.91) using internal nested cross-validation, and this model was specific for ADPKD as compared to other diseases (renal transplant recipients and diabetes mellitus). The model, however, has not yet been validated in an independent patient cohort, and it is unknown whether any of the metabolites correlate with disease severity or, more importantly, with prognosis.
Future Directions
In summary, several biomarkers of disease burden and/or progression of ADPKD have been identified, the most widely accepted certainly being TKV and its change over time. Several recently established large cohorts of ADPKD patients are continuously being followed, hence generating a growing body of outcome data to which potential biomarkers can be correlated. Several of these cohorts include serial MRI of the kidneys as well as biobanking of serum and urine. A major limitation of currently available data is that follow-up times are still rather limited and hard renal outcomes (i.e., ESRD) are few. Thus, ironically, the development of reliable prognostic biomarkers is hampered by the same facts that have prompted the quest for their identification, namely, the long lag time between the initiation of pathophysiologic processes and the occurrence of adverse outcomes. Furthermore, even those cohorts focusing on patients with relatively preserved renal function at baseline mostly include patients with a wide range of age, GFR, and TKV (typically age ca. 16–45 years, GFR 60 ml/min/1.73 m2 to >140 ml/min/1.73 m2, and TKV from as low as 200 ml to over 3,000 ml at baseline). Thus, when testing is done on individual parameters for their utility to predict outcomes, other variables must be corrected for. This is not trivial, given the nonlinearity and interaction of many of these variables (Fig. 5). Furthermore, careful consideration must be given to the selection of adequate endpoints, again, given the nonlinear and age-dependent disease course. For example, reaching stage 3 CKD at age 30 years has different implications as compared to its occurrence at the age of 50 years, and a GFR loss of 30 ml/min/1.73 m2 over a period of 5 years might reflect much more aggressive disease if occurring at an age of 25 years (where GFR is usually still relatively stable) as compared to its occurrence at an age of 65 years, where it might reflect the final accelerated phase of GFR loss after relatively stable disease. The continued follow-up of patients included in the mentioned cohorts will be of great value. Combination of a large number of patients from several cohorts that have been followed over extended periods of time (>10 years) will be required to gain detailed knowledge on the natural history of the disease and can then be used to validate previously suggested biomarkers. In particular, it will be important to accumulate long-term follow-up data on patients that have been included into cohorts at young ages. Archived (i.e., biobanked) materials of patients in these cohorts may serve for the identification of novel biomarkers. If available data from ongoing cohorts will be used in collaborative efforts, there is justified hope that the next decade will advance our knowledge regarding ADPKD progression and lead to the identification of novel clinically useful biomarkers as well as to the validation of previously identified markers.
Schematic drawing of key parameters influencing potential ADPKD biomarkers and their complex interrelations. Influences between parameters are drawn in blue: both genetic and likely also environmental factors determine disease progression rate or activity. Disease activity in turn leads to accumulation of cyst burden and chronic tissue damage over time, which then finally results in a decline in GFR. A decline in GFR is the clinically most relevant outcome, once it reaches a critical degree, and thus, it is the primary outcome which candidate prognostic biomarkers should be able to predict (red arrow); however, given the limited follow-up time in many studies, measures of disease burden (e.g., TKV) or disease activity (e.g., TKV change over time) are often used as outcomes to which biomarkers are correlated (dashed red lines). Potential biomarkers may be directly influenced by these outcomes they are supposed to predict. In addition, however, all factors that affect outcomes (such as age, genetic background, environmental factors, etc.) may have direct effects on biomarkers, independent of their effect on the relevant outcome parameters
Summary Points
-
In autosomal dominant polycystic kidney disease (ADPKD), cyst growth progresses slowly but relentlessly in both kidneys, while kidney function is maintained within normal limits until at late disease stages, when a rapid decline of kidney function leads to renal failure.
-
Diagnosis of ADPKD is currently based on ultrasound imaging and mostly straight forward – in contrast, prediction of the highly variable disease course is very difficult, particularly at early stages of the disease.
-
Treatment of ADPKD has been symptomatic by now, but as novel potential targeted treatments evolve, biomarkers are urgently needed to identify high-risk patients requiring treatment and to evaluate therapeutic effects.
-
Currently, total kidney volume is considered to be the best measure of disease severity at earlier stages of disease, when renal function is not yet significantly impaired.
-
ADPKD is characterized by distinct urinary patterns of protein and metabolite excretion that distinguish affected patients from controls.
-
Attempts to develop reliable prognostic biomarkers or biomarker patterns have not yet been successful but are ongoing.
-
A major obstacle to identify relevant biomarkers is the slowly progressive nature of the disease requiring very extended follow-up times for relevant outcomes to occur and the heterogeneity of patients in most cohorts with respect to their baseline characteristics.
-
With a recently growing interest in the disease, several large patient cohorts have been initiated and continue to be followed that will ultimately provide the data needed for the identification and validation of disease biomarkers.
Abbreviations
- ADPKD:
-
Autosomal Dominant Polycystic Kidney Disease
- CKD:
-
Chronic Kidney Disease
- CT:
-
Computed Tomography
- ESRD:
-
End-stage Renal Disease
- GFR:
-
Glomerular Filtration Rate
- htTKV:
-
Height-Adjusted Total Kidney Volume
- MRI:
-
Magnetic Resonance Imaging
- RCT:
-
Randomized Controlled Trials
- SVM:
-
Support Vector Machine
- TCV:
-
Total Cyst Volume
- TKV:
-
Total Kidney Volume
References
Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123.
Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:96–106.
Bae KT, Commean PK, Lee J. Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr. 2000;24:614–9.
Bakker J, Olree M, Kaatee R, et al. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology. 1999;211:623–8.
Barua M, Pei Y. Diagnosis of autosomal-dominant polycystic kidney disease: an integrated approach. Semin Nephrol. 2010;30:356–65.
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95.
Boertien WE, Meijer E, Gansevoort RT. Urinary biomarkers in autosomal dominant polycystic kidney disease: is there no prognostic value? Kidney Int. 2012a;82:361.
Boertien WE, Meijer E, Zittema D, et al. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2012b;27:4131–37.
Boertien WE, Meijer E, Li J, et al. Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am J Kidney Dis. 2013;61:420–9.
Bolignano D, Coppolino G, Campo S, et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–8.
Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120:c25–34; discussion c35.
Chapman AB, Johnson AM, Gabow PA, et al. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1349–54.
Chapman AB, Bost JE, Torres VE et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86.
Choukroun G, Itakura Y, Albouze G, et al. Factors influencing progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;6:1634–42.
Coon JJ, Zurbig P, Dakna M, et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964.
Cornec-Le Gall E, Audrezet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24:1006–13.
Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl. 1957;328:1–255.
Devuyst O, Torres VE. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:459–70.
Fliser D, Novak J, Thongboonkerd V, et al. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18:1057–71.
Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–9.
Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–85.
Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30.
Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–16.
Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–66.
Gronwald W, Klein MS, Zeltner R, et al. Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011;79:1244–53.
Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:197–206.
Hateboer N, van Dijk M, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–7.
Iglesias CG, Torres VE, Offord KP, et al. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis. 1983;2:630–9.
Johnson AM, Gabow PA. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997;8:1560–7.
Kistler AD, Poster D, Krauer F, et al. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009a;75: 235–241.
Kistler AD, Mischak H, Poster D, et al. Identification of a unique urinary biomarker profile in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2009b;76:89–96.
Kistler AD, Serra AL, Siwy J, et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One. 2013;8:e53016.
Meijer E, Boertien WE, Nauta FL, et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis. 2010;56:883–95.
Meijer E, Bakker SJ, van der Jagt EJ, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:361–8.
Mischak H, Coon JJ, Novak J, et al. Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spectrom Rev. 2009;28:703–24.
Nascimento AB, Mitchell DG, Zhang XM, et al. Rapid MR imaging detection of renal cysts: age-based standards. Radiology. 2001;221:628–32.
Orskov B, Christensen KB, Feldt-Rasmussen B, et al. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int. 2012;81:919–24.
Panizo N, Goicoechea M, Garcia de Vinuesa S, et al. Chronic kidney disease progression in patients with autosomal dominant polycystic kidney disease. Nefrologia. 2012;32:197–205.
Parikh CR, Dahl NK, Chapman AB, et al. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int. 2012;81:784–90.
Park HC, Hwang JH, Kang AY, et al. Urinary N-acetyl-beta-d glucosaminidase as a surrogate marker for renal function in autosomal dominant polycystic kidney disease: 1 year prospective cohort study. BMC Nephrol. 2012;13:93.
Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2011;118:c19–30.
Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–12.
Persu A, Duyme M, Pirson Y, et al. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004;66:2132–6.
Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173–80.
Rampoldi L, Scolari F, Amoroso A, et al. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80:338–47.
Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol. 2002;13:1230–7.
Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–201.
Stalmach A, Albalat A, Mullen W, et al. Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis. 2013;34:1452–64.
Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7:2142–51.
Torres VE, Grantham JJ, Chapman AB, et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:640–7.
Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–64.
Zheng D, Wolfe M, Cowley Jr BD, et al. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:2588–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Kistler, A.D. (2015). Traditional and Proteomic Biomarkers of Autosomal Dominant Polycystic Kidney Disease (ADPKD). In: Preedy, V., Patel, V. (eds) General Methods in Biomarker Research and their Applications. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7696-8_48
Download citation
DOI: https://doi.org/10.1007/978-94-007-7696-8_48
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7695-1
Online ISBN: 978-94-007-7696-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences